1. Introduction
Despite daily exposure to external environmental stresses, skin cells (keratinocytes, fibroblasts, dermal stem cells, etc.) cooperate to maintain the extracellular matrix environment, ensuring an ongoing stress response. The major contributors to skin aging include photoaging (UV radiation), glycation (advanced glycation end-product [AGE] stimulation), and oxidation (H
2O
2 stimulation). Resistance to these stresses is one way of combating skin aging, with the inhibition of intracellular reactive oxygen species (ROS) accumulation being crucial [
1,
2]. Recently, in vivo cultured stem cells from regenerative medicine research have been incorporated in skin anti-aging research, and the skin anti-aging effects of extracellular vesicles, such as mesenchymal stem cell-derived exosomes (hMSC-Exo), have been investigated [
3,
4].
Human placental extracts (hPLas) have been widely used in traditional medicine, including Chinese medicine, for wound healing [
5,
6]. hPLa is considered a “natural reservoir” because it contains numerous bioactive materials, including vitamins, amino acids, peptides, growth factors, and trace elements [
7]. Subcutaneous and intramuscular hPLa injections have been used clinically to treat hepatic diseases and menopausal disorders [
8]. Moreover, hPLa is used clinically as an anti-wrinkle agent, mainly in Asian countries, due to its antioxidant, cellular growth, and collagen synthesis effects [
9,
10]. However, since the use of hPLa as a medicinal product is limited in some countries, such as Japan, porcine placental extracts (Pla-Ext) have been recently developed as a substitute for hPLa and is used as a raw material in healthy foods and cosmetics [
11]. Pla-Ext reportedly has antioxidant, immunomodulatory, and immunopotentiating effects [
12,
13,
14]. Evidence suggests that Pla-Ext stimulation increases cell numbers and collagen and elastin production in human dermal keratinocytes, and Pla-Ext can penetrate epidermal tissue to reach fibroblasts and skin stem cells in a three-dimensional skin model (data being prepared for publication). hMSC-Exos have also been demonstrated to repair fibroblast damage under H
2O
2 water stress [
15].
Here, we evaluated the response elicited by the Pla-Ext treatment of cultured human dermal fibroblasts (HDFs) to verify its efficacy against human skin aging and whether it confers resistance to damage when HDFs are subjected to several types of aging stress. To determine the effects of the control (purified water [DW]), hMSC-Exo alone, Pla-Ext and hMSC-Exo combined, and hMSC-Exo (Pla/MSC-Exo) recovered from the culture broth containing Pla-Ext added to hMSCs (preconditioning), HDFs were subjected to photoaging (UV radiation), saccharification (AGE stimulation), and oxidation (H2O2 stimulus).
Dermal fibroblasts have been shown to be capable of synthesizing collagen, elastin, and dermal hyaluronan as indicators for several stress responses. Therefore, the mRNA levels of these proteins were measured in the present study [
16,
17]. This study aimed to determine the mechanism of action underlying the anti-aging effect of Pla-Ext on human skin. In addition, we also investigated whether the mechanism of action was altered by MSC-Exo obtained from preconditioning, wherein Pla-Ext acts directly on hMSC-Exo.
2. Results
2.1. Effects on Normal HDFs
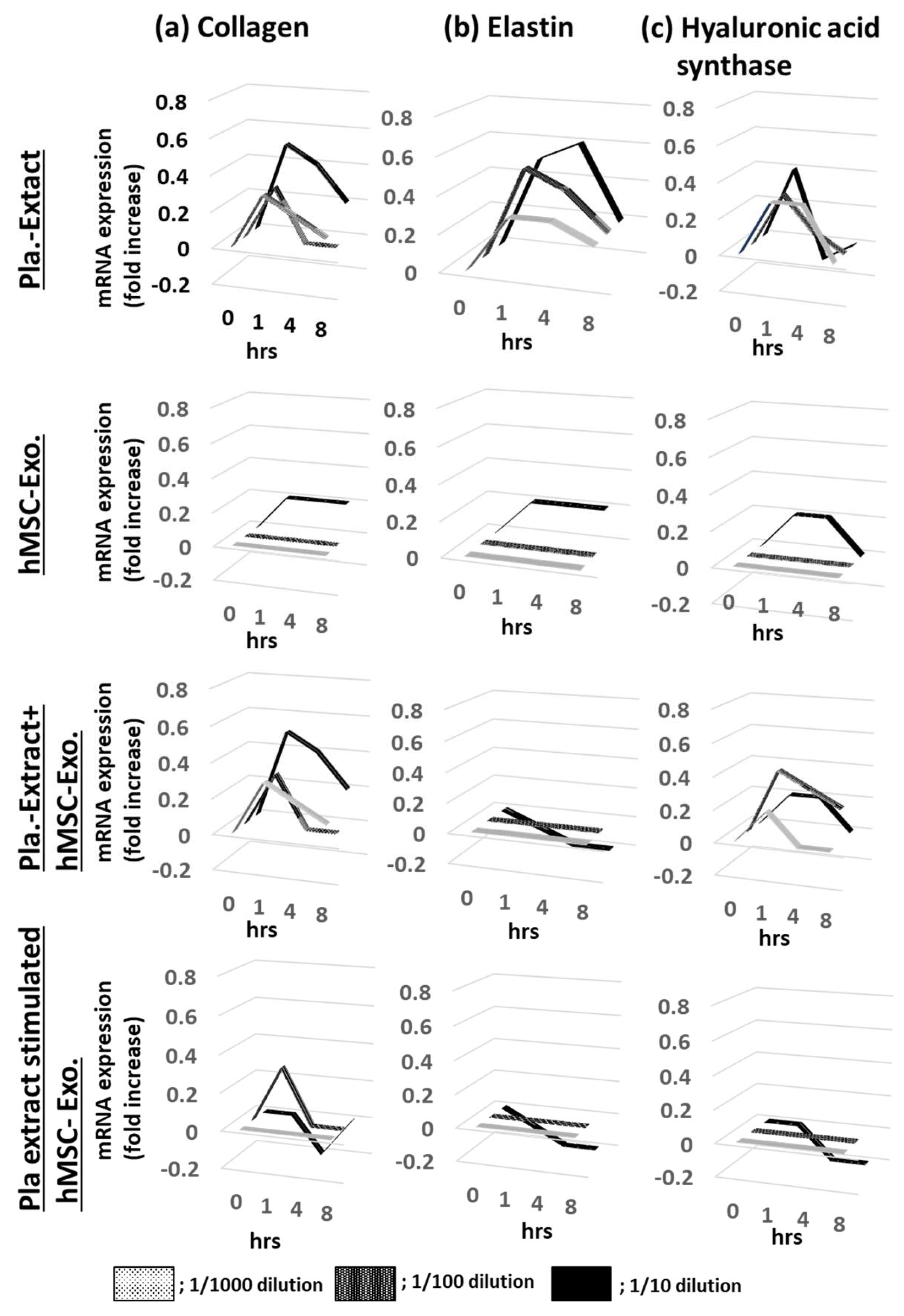
HDFs were treated with five additives, and the mRNA expression levels for collagen, elastin, and hyaluronic acid synthase were assessed over time (
Figure 1a,
Figure 1b and
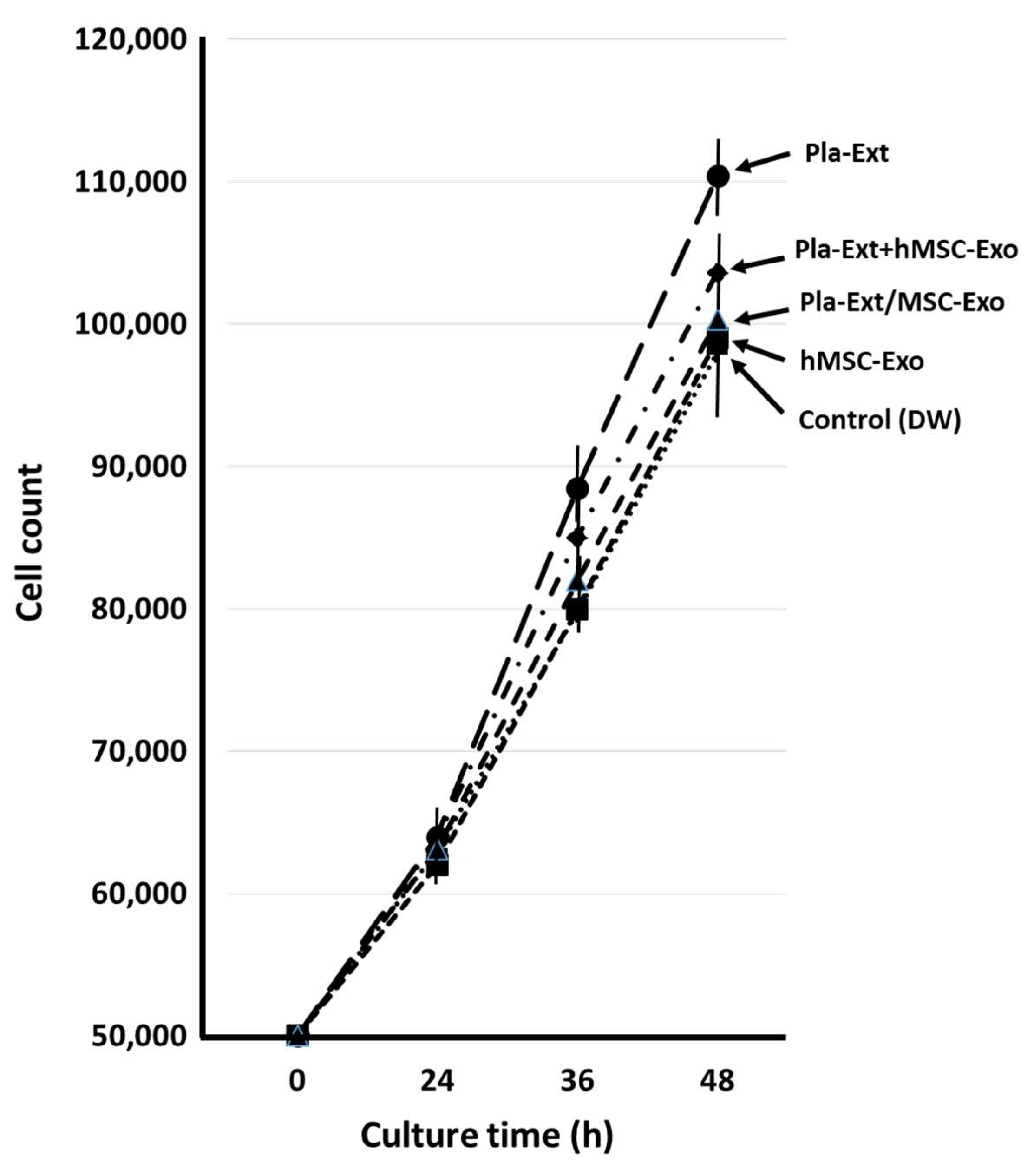
Figure 1c). After adding Pla-Ext, mRNA expression levels tended to increase in a concentration-dependent manner from 1 to 4 h, but none significantly increased. No mRNA variations were observed in the group treated with hMSC-Exo alone. In addition, mRNA levels following Pla-Ext and hMSC-Exo combination stimulation or hMSC-supplement exosome treatment of normal skin cells did not significantly vary. Furthermore, the effect of the five treatments on cell proliferation was examined. All treatment groups exhibited higher proliferation than that of the controls, with the Pla-Ext-treated group demonstrating the highest growth potential (13% increase compared with the controls at 48 h;
P < 0.05) (
Figure 2).
2.2. Damage Resistance in HDFs Subjected to Photoaging Stimuli (UV Radiation)
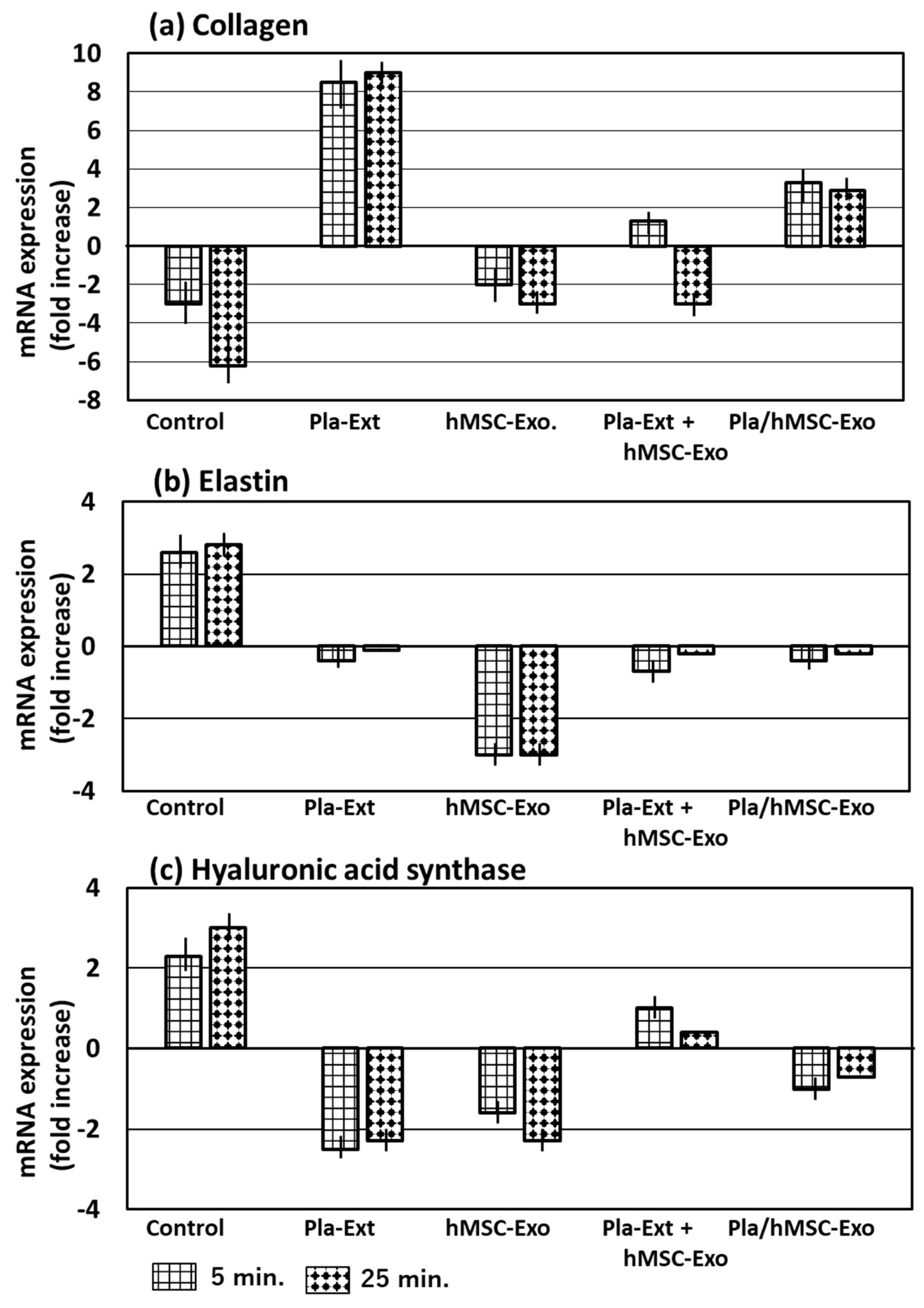
Collagen, elastin, and hyaluronic acid synthase mRNA levels were measured following treatment with the five additives and UV radiation for 5 or 25 min (
Figure 3). UV irradiation decreased collagen mRNA expression levels, considering irradiation time; however, it increased elastin and hyaluronic acid synthase mRNA levels. Pla-Ext increased collagen expression to counteract the response but decreased elastin and hyaluronic acid synthase expression. hMSC-Exo was not resistant to collagen but was resistant to elastin and hyaluronic acid synthase in response to UV radiation. However, co-treatment with Pla-Ext and hMSC-Exo resulted in weakened resistance. Furthermore, exosomes obtained by stimulating hMSCs with Pla-Ext were resistant to the three parameters.
2.3. Damage Resistance in HDFs Subjected to Glycation Stress
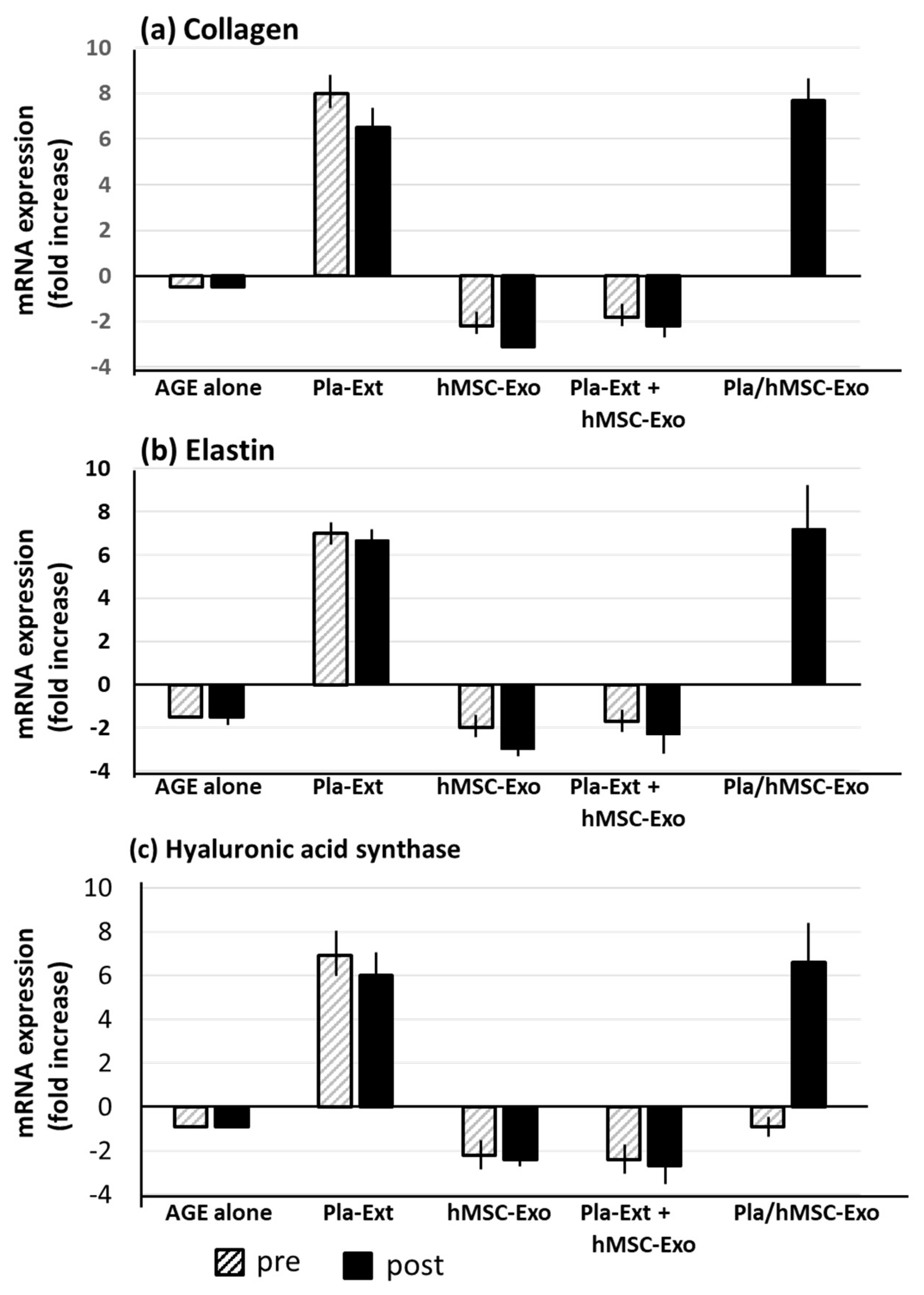
HDFs tended to display reduced collagen, elastin, and hyaluronic acid synthase mRNA expression levels following treatment with AGEs. This effect was reversed by Pla-Ext treatment, which elevated the expression levels of all three proteins. hMSC-Exo stimulated by Pla-Ext also showed a similar effect (
Figure 4).
2.4. Damage Resistance in HDFs Subjected to Oxidative Stress
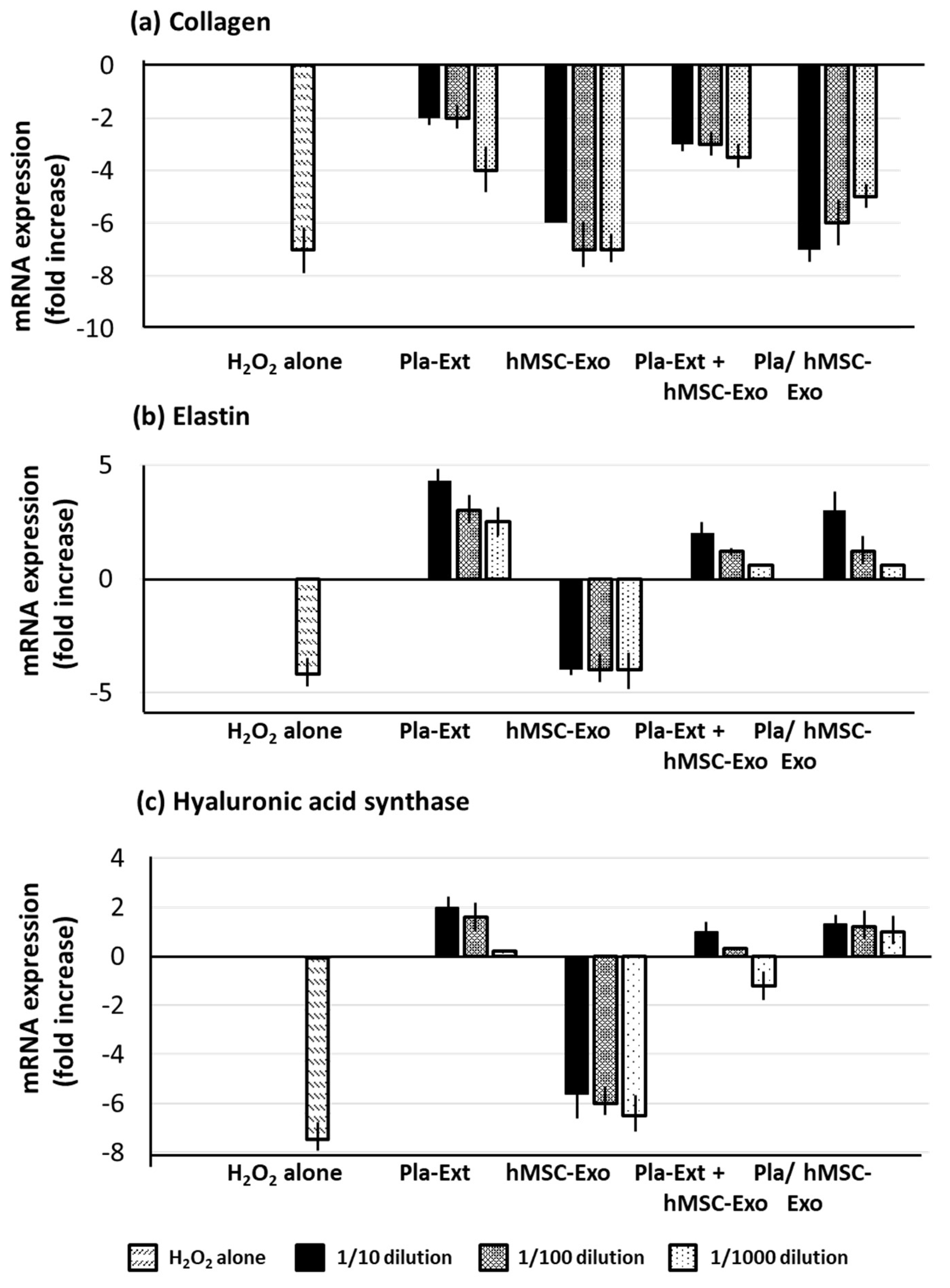
H
2O
2 stress significantly reduced collagen, elastin, and hyaluronic acid synthase in HDFs. Pla-Ext mitigated collagen damage and compensated for the reduction in elastin and hyaluronic acid synthase, surpassing untreated control levels. hMSC-Exo alone did not confer resistance. However, Pla-Ext-stimulated hMSC-Exo conferred resistance (
Figure 5).
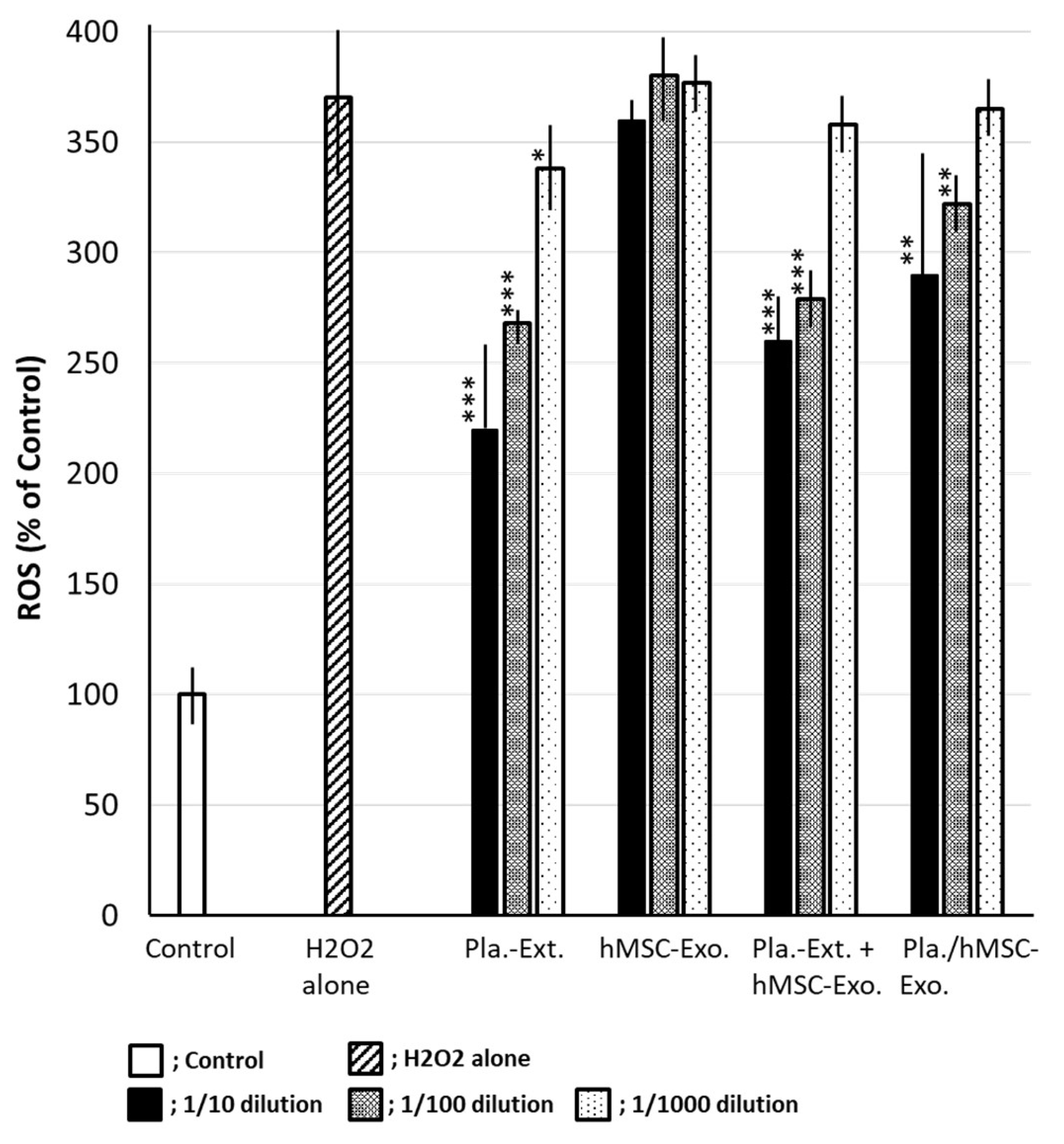
2.5. Inhibitory Efficacy against Intracellular ROS Accumulation in HDFs Subjected to Oxidative Stress
H
2O
2 stress results in ROS accumulation in HDFs, with an increase of up to 370% when the control is set at 100%. Pla-Ext reduced ROS in a concentration-dependent manner. The effect of Pla-Ext seemed to become apparent upon combination with exosomes. Although hMSC-Exo alone had no inhibitory effect, a suppressive effect on ROS production was observed following treatment with Pla-Ext-stimulated hMSC-Exo (
Figure 6).
3. Discussion
To verify the efficacy of Pla-Ext against human skin aging, we evaluated the response following Pla-Ext treatment of cultured HDFs and determined whether HDFs resist damage when stressed by photoaging and glycation or H
2O
2 stimuli. hPLas reportedly have growth-promoting effects on skin cells. However, cell proliferation has not been directly observed [
11,
18]. In the present study, Pla-Ext did not significantly affect intracellular parameters in normal skin fibroblasts, but a pro-proliferative effect was observed (
Figure 1 and
Figure 2). Pla-Ext conferred HDFs with resistance to UV irradiation and glycation and H
2O
2 stimulation. In the present study, we examined three parameters—collagen, elastin, and hyaluronic acid synthase. The results demonstrated that stress-induced damage was repaired beyond the original condition, and the treatment conferred resistance to processes related to cellular senescence. Although a combined effect with hMSC-Exo was not established, we observed that preconditioned stem cell-derived exosomes, stimulated by Pla-Ext, conferred resistance to senescence-related stress (
Figure 3,
Figure 4 and
Figure 5). hMSC-Exo did not inhibit H
2O
2-induced ROS accumulation; however, an inhibitory effect was observed with hMSC-Exo derived from preconditioning with Pla-Ext alone and Pla-Ext (
Figure 6).
This suggests that Pla-Ext treatment may have influenced changes in the surface molecules or content (such as mRNA) of hMSC-secreted exosomes. Several studies have demonstrated that stem cell preconditioning improves the capacity of MSCs, as well as exosome secretion and function, indicating the enhanced biological effects of pretreated MSC exosomes and improved therapeutic effects against various diseases [
19,
20,
21,
22,
23,
24]. Although Pla-Ext had no specific effect on cultured skin fibroblasts in vivo, Pla-Ext is thought to have some effect when applied to the non-stressed skin of living organisms [
3]. In the future, it may be possible to infer the anti-skin aging mechanism at the molecular level by analyzing exosome surface molecules and even their cargo (mRNA, etc.).
An increasing number of studies have demonstrated that exosomes alone or in combination with Pla-Ext and preconditioned stem cell-derived exosomes can secrete a range of trophic factors, including cytokines, growth factors, and chemokines, from MSCs. Therefore, MSCs are considered a paracrine tool [
25,
26]. Future studies should examine the effects of stem cell-derived secretome stimulation.
4. Materials and Methods
4.1. Porcine Placental Extracts (Pla-Ext)
Pla-Exts were fed through a GMP-grading cosmetic manufacturer (Sapporo, Japan). At the manufacturing plant, raw placentas were supplied by a pig farm and stored frozen for several months before entering the manufacturing process after confirming the absence of infectious disease at the pig farm. Certificates ensuring the safety of the extracts, including sterility tests and the absence of residual estrogens, were issued. The extracts were approved as cosmetic preparations.
4.2. Cell
HDFs and hMSCs were purchased from PromoCell (Heidelberg, Germany) and subcultured in a 5% CO2 incubator at 37°C in a designated dedicated medium for experimental use.
4.3. Isolation of Cultured Cell-Derived Exosomes
hMSC-derived exosomes or Pla/MSC-Exo from hMSC and Pla-Ext-treated hMSC cultures were recovered using a miRCURY Exosome Isolation Kit (Product#: 300102; Exiqon, Hovedstaden, Denmark).
4.4. Photoaging (UV) Stimulation
Various cell types were cultured in a 24-well culture plate. Culturing was continued by quickly returning the plate to a CO2 incubator at 37°C after each well was irradiated for 5 or 25 min at a constant distance from the UV lamp. The UV radiation doses were set at 2 J/cm2 for 5 min and 10 J/cm2 for 25 min using a UV-intensity meter.
4.5. AGE Generation
AGE treatment of bovine serum albumin (BSA, Fraction V; Sigma-Aldrich, St. Louis, MO, USA) was performed according to the methods of Maeda et al. [
27]. Briefly, BSA(25 mg/mL) was reacted with 0.2 M phosphate buffer (pH 7.4) for 7 days. Subsequently, glyceraldehyde that did not bind to BSA was removed using PD-10 gel-filtration columns equilibrated with phosphate-buffered saline. The glycated BSA in the obtained samples were quantified using a Glyceraldehyde-derived AGE-ELISA KIT and subjected to experimentation.
4.6. Oxidative Stress Reagent
H
2O
2 (FUJIFILM Wako Pure Chemical Corporation, Osaka, Japan) was added to the culture solution by diluting the stock solution with purified water to a final concentration of 0.2 mM [
28].
4.7. Intracellular Total RNA Extraction
Intracellular total RNA was extracted using a Trizol reagent (Ambion, Austin, TX, USA) according to the manufacturer’s instructions.
4.8. RT-qPCR
Collagen [
29], elastin [
30], and hyaluronic acid synthase [
29] mRNA expression levels were determined using a one-step RT-qPCR method with the respective specific primers (
Table 1). Specifically, one-step PCR was performed in the same tubing using a Luna Universal One-Step qRT-PCR Kit (New England Biolabs, Ipswich, MA, USA) and Thermal Cycler Dice Real Time System II (Takara Bio, Shiga, Japan). The reaction was performed according to the reagent kit manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase [
29], a constant-expression gene, was used to calculate the delta Ct [
31] with internal standards. Differences from the experimental controls were determined using the delta-delta Ct method and expressed as fold changes in mRNA expression.
4.9. Determination of Intracellular Reactive Oxygen Species
Intracellular ROS production was determined by a fluorometric assay using dichlorofluorescein-diacetate (DCF-DA; Invitrogen, Carlsbad, CA, USA). After labeling the cells in the experimental groups with 10 mM DCF-DA at 37°C for 20 min, intracellular ROS was assessed by measuring the excitation wavelength fluorescence intensity (525 nm) using an Agilent Microplate Reader (Agilent Technologies, Santa Clara, CA, USA).
4.10. Experimental Protocol
4.10.1. Effects on Normal HDFs
HDFs were treated with purified water (DW), Pla-Ext alone, hMSC-Exo alone, exosomes (Pla/MSC-Exo) secreted from hMSCs stimulated by P-Ext + hMSC-Exo, and Pal-Ext, which were serially diluted (1/100, 1/1,000, 1/10,000 dilutions) to extract intracellular total RNA. Collagen, elastin, and hyaluronic acid synthase mRNA expression levels at 1, 4, and 8 h were determined. In addition, we also determined the number of viable cells after the course of the respective stimuli after 24, 36, and 48 h in the five groups.
4.10.2. Resistance to Damage in HDFs Stimulated by Photoaging
HDFs were subjected to the five treatments for 2 h. Subsequently, the treated HDFs were irradiated with UV for 5 or 25 min and further cultured for 2 h. Thereafter, total HDF RNA was extracted, and collagen, elastin, and hyaluronic acid synthase mRNA levels were determined using RT-qPCR.
4.10.3. AGE Receptor mRNA Expression in HDFs Subjected to Glycation Stress
HDFs were subjected to glycation stress by AGE treatment (100 mg/mL) for 2 h. After subjection to the five treatments for 4 h before or after stress, total RNA in HDFs was extracted, and collagen, elastin, and hyaluronic acid synthase mRNA levels were determined using the RT-qPCR method.
4.10.4. Damage Resistance in HDFs Subjected to Oxidative Stress
HDFs were subjected to H2O2 oxidative stress for 2 h. After subjecting the HDFs to the five treatments for 4 h before or after stress, total HDF RNA was extracted, and collagen, elastin, and hyaluronic acid synthase mRNA levels were determined using the RT-qPCR method. The efficiency of H2O2 treatment in inhibiting intracellular ROS accumulation was also determined.
4.11. Statistical Analysis
All experiments were performed in triplicate, and the data are presented as mean ± SD. Student’s t-test was performed, and statistical significance was considered at P < 0.05. According to RT-qPCR, differences were considered significant when they differed from delta-delta Ct by more than 2 (≧4-fold difference at mRNA levels).
5. Conclusions
In this study, we demonstrated that applying Pla-Ext to skin tissue could enhance resistance to aging stress by directly affecting fibroblasts. Additionally, this resistance is also achieved through the secretion of exosomes, which, unlike conventional methods, reach and act directly on dermal stem cells.
Author Contributions
All authors contributed to this work. T Matsuoka, K. Dan, and A. Ogino designed the research. T Matsuoka, K. Dan, K Takanashi carried out the experiments and analyzed the results. All authors interpreted the results and designed the research strategy. T Matsuoka prepared the manuscript.
Funding
This research was funded by OMOTESANDO HELENE CLINIC and STEMCELL Co. Ltd.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
We encourage all authors of articles published in MDPI journals to share their research data. In this section, please provide details regarding where data supporting reported results can be found, including links to publicly archived datasets analyzed or generated during the study. Where no new data were created, or where data is unavailable due to privacy or ethical restrictions, a statement is still required. Suggested Data Availability Statements are available in section “MDPI Research Data Policies” at
https://www.mdpi.com/ethics.
Acknowledgments
We would like to thank Editage (
www.editage.jp) for English language editing.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shin, S.H.; Lee, Y.H.; Rho, N.K.; Park, K.Y. Skin aging from mechanisms to interventions: focusing on dermal aging. Front. Physiol. 2023, 14, 1195272, Review. [Google Scholar] [CrossRef]
- Wang, Y.; Branicky, R.; Noë, A.; Hekimi, S. Superoxide dismutases: Dual roles in controlling ROS damage and regulating ROS signaling. J. Cell Biol. 2018, 217, 1915–1928. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yang, S.; Lv, M.; Lv, J.; Sui, Y.; Guo, S. Protective roles of mesenchymal stem cells on skin photoaging: a narrative review. Tissue Cell 2022, 76, 101746. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Sun, Y.; Zhang, J.; Zhu, Q.; Yang, Y.; Niu, X.; Deng, Z.; Li, Q.; Wang, Y. Human embryonic stem cell-derived exosomes promote pressure ulcer healing in aged mice by rejuvenating senescent endothelial cells. Stem Cell Res. Ther. 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Lee, W.J.; Hahn, S.B.; Kim, B.J.; Lew, D.H. The effect of human placenta extract in a wound healing model. Ann. Plast. Surg. 2010, 65, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Nath, S.; Bhattacharyya, D. Cell adhesion by aqueous extract of human placenta used as wound healer. Indian, J. Exp. Biol. 2007, 45, 732–738. [Google Scholar] [PubMed]
- Pan, S.Y.; Chan, M.; Wong, M.; Klokol, D.; Chernykh, V. Placental therapy: an insight to their biological and therapeutic properties. J. Med. Ther. 2017, 1, 12. [Google Scholar]
- Kong, M.H.; Lee, E.J.; Lee, S.Y.; Cho, S.J.; Hong, Y.S.; Park, S.B. Effect of human placental extract on menopausal symptoms, fatigue, and risk factors for cardiovascular disease in middle-aged Korean women. Menopause 2008, 15, 296–303. [Google Scholar] [CrossRef]
- Togashi, S.; Takahashi, N.; Iwama, M.; Watanabe, S.; Tamagawa, K.; Fukui, T. Antioxidative collagen-derived peptides in human-placenta extract. Placenta 2002, 23, 497–502. [Google Scholar] [CrossRef]
- Yoshikawa, C. Effect of porcine placental extract on collagen production in human skin fibroblasts in vitro. Gynecol. Obstet. 2013, 03, 0–4. [Google Scholar] [CrossRef]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef]
- Takuma, K.; Mizoguchi, H.; Funatsu, Y.; Kitahara, Y.; Ibi, D.; Kamei, H.; Matsuda, T.; Koike, K.; Inoue, M.; Nagai, T.; Yamada, K. Placental extract improves hippocampal neuronal loss and fear memory impairment resulting from chronic restraint stress in ovariectomized mice. J. Pharmacol. Sci. 2012, 120, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Park, H.J.; Seo, H.G.; Kim, J.H.; Lim, G.S.; Lee, W.Y.; Kim, N.H.; Kim, J.H.; Lee, J.H.; Jung, H.S.; Sung, S.H.; Song, H. Immune modulation effect of porcine placenta extracts in weaned the pig. J. Anim. Sci. 2013, 91, 2405–2413. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.Y.; Kim, S.W.; Kim, B.; Lee, H.N.; Kim, S.J.; Song, M.; Kim, S.; Kim, J.; Kim, Y.B.; Kim, J.H.; Cho, S.G. Alpha-fetoprotein, identified as a novel marker for the antioxidant effect of placental extract, exhibits synergistic antioxidant activity in the presence of estradiol. PLOS ONE 2014, 9, e99421. [Google Scholar] [CrossRef]
- Matsuoka, T.; Takanashi, K.; Dan, K.; Yamamoto, K.; Tomobe, K.; Shinozuka, T. Effects of mesenchymal stem cell-derived exosomes on oxidative stress responses in skin cells. Mol. Biol. Rep. 2021, 48, 4527–4535. [Google Scholar] [CrossRef] [PubMed]
- Eckes, B.; Mauch, C.; Hüppe, G.; Krieg, T. Differential regulation of transcription and transcript stability of pro-alpha 1 (I) collagen and fibronectin in activated fibroblasts derived from patients with systemic scleroderma. Biochem. J. 1996, 315, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.A.; Yi, B.R.; Choi, K.C. Molecular mechanisms and in vivo mouse models of skin aging associated with dermal matrix alterations. Lab. Anim. Res. 2011, 27, 1–8. [Google Scholar] [CrossRef]
- Pal, P.; Roy, R.; Datta, P.K.; Dutta, A.K.; Biswas, B.; Bhadra, R. Hydroalcoholic human placental extract: skin pigmenting activity and gross chemical composition. Int. J. Dermatol. 1995, 34, 61–66. [Google Scholar] [CrossRef]
- Phan, J.; Kumar, P.; Hao, D.; Gao, K.; Farmer, D.; Wang, A. Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J. Extracell. Vesicles 2018, 7, 1522236. [Google Scholar] [CrossRef]
- Cha, J.M.; Shin, E.K.; Sung, J.H.; Moon, G.J.; Kim, E.H.; Cho, Y.H.; Park, H.D.; Bae, H.; Kim, J.; Bang, O.Y. Efficient scalable production of therapeutic microvesicles derived from human mesenchymal stem cells. Sci. Rep. 2018, 8, 1171. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.A.; Gonçalves, R.M. Mesenchymal stromal cell secretome: influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef] [PubMed]
- Pendse, S.; Kale, V.; Vaidya, A. Extracellular vesicles isolated from mesenchymal stromal cells primed with hypoxia: novel strategy in regenerative medicine. Curr. Stem Cell Res. 2021, 16, 243.e61. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Kim, H.W.; Gong, M.; Wang, J.; Millard, R.W.; Wang, Y.; Ashraf, M.; Xu, M. Exosomes secreted from GATA-4 overexpressing mesenchymal stem cells serve as a reservoir of anti-apoptotic microRNAs for cardioprotection. Int. J. Cardiol. 2015, 182, 349–360. [Google Scholar] [CrossRef]
- Liang, L.; Zheng, D.; Lu, C.; Xi, Q.; Bao, H.; Li, W.; Gu, Y.; Mao, Y.; Xu, B.; Gu, X. Exosomes derived from miR-301a-3p-overexpressing adipose-derived mesenchymal stem cells reverse hypoxia-induced erectile dysfunction in rat models. Stem Cell Res. Ther. 2021, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Mazini, L.; Rochette, L.; Admou, B.; Amal, S.; Malka, G. Hopes and limits of adipose-derived stem cells (ADSCs) and mesenchymal stem cells (MSCs) in wound healing. Int. J. Mol. Sci. 2020, 21, 1306. [Google Scholar] [CrossRef]
- Gnecchi, M.; He, H.; Noiseux, N.; Liang, O.D.; Zhang, L.; Morello, F.; Mu, H.; Melo, L.G.; Pratt, R.E.; Ingwall, J.S.; Dzau, V.J. Evidence supporting paracrine hypothesis for Akt-modified mesenchymal stem cell-mediated cardiac protection and functional improvement. F.A.S.E.B. J. 2006, 20, 661–669. [Google Scholar] [CrossRef]
- Maeda, S.; Matsui, T.; Ojima, A.; Takeuchi, M.; Yamagishi, S.I. Sulforaphane inhibits advanced glycation end product-induced pericyte damage by reducing expression of receptor for advanced glycation end products. Nutr. Res. 2014, 34, 807–813. [Google Scholar] [CrossRef]
- Kartal, B.; Akçay, A.; Palabiyik, B. Oxidative stress upregulates the transcription of genes involved in thiamine metabolism. Turk. J. Biol. 2018, 42, 447–452. [Google Scholar] [CrossRef]
- Liu, W.; Ma, C.; Li, H.Y.; Chen, L.; Yuan, S.S.; Li, K.J. MicroRNA-146a downregulates the production of hyaluronic acid and collagen I in Graves’ ophthalmopathy orbital fibroblasts. Exp. Ther. Med. 2020, 20, 38. [Google Scholar]
- Deslee, G.; Woods, J.C.; Moore, C.M.; Liu, L.; Conradi, S.H.; Milne, M.; Gierada, D.S.; Pierce, J.; Patterson, A.; Lewit, R.A.; Battaile, J.T.; Holtzman, M.J.; Hogg, J.C.; Pierce, R.A. Elastin expression in very severe human COPD. Eur. Respir. J. 2009, 34, 324–331. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase mRNA expression levels in normal human dermal fibroblasts. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 1.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase mRNA expression levels in normal human dermal fibroblasts. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 2.
Effects of Pla-Ext on normal human dermal fibroblast proliferation. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 2.
Effects of Pla-Ext on normal human dermal fibroblast proliferation. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 3.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase mRNA expression levels in UV-treated human dermal fibroblasts (5 or 25 min). hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 3.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase mRNA expression levels in UV-treated human dermal fibroblasts (5 or 25 min). hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 4.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase mRNA expression levels in human dermal fibroblasts treated with advanced glycation end products (AGE). hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 4.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase mRNA expression levels in human dermal fibroblasts treated with advanced glycation end products (AGE). hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 5.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase in human dermal fibroblasts treated with H2O2. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 5.
Effects of Pla-Ext on (a) collagen, (b) elastin, and (c) hyaluronic acid synthase in human dermal fibroblasts treated with H2O2. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 6.
Effects of Pla-Ext on the generation of reactive oxygen species (ROS) in human dermal fibroblasts treated with H2O2. *P < 0.05, **P < 0.01, ***P < 0.001 vs. H2O2 alone. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Figure 6.
Effects of Pla-Ext on the generation of reactive oxygen species (ROS) in human dermal fibroblasts treated with H2O2. *P < 0.05, **P < 0.01, ***P < 0.001 vs. H2O2 alone. hMSC-Exo, human mesenchymal stem-cell-derived exosomes; Pla-Ext, placental extracts.
Table 1.
Primers for RT-qPCR.
Table 1.
Primers for RT-qPCR.
| Gene |
Primer |
Ref. |
| Collagen 1 A2 |
Forward: CTGGACCTCCAGGTGTAAGC |
[29] |
| Reverse: TGGCTGAGTCTCAAGTCACG |
| Elastin |
Forward: GGCCATTCCTGGTGGAGTTCC |
[30] |
| Reverse: AACTGGCTTAAGAGGTTTGCCTCCA |
| Hyaluronic acid synthase |
Forward: CACGTAACGCAATTGGTCTTGTCC |
[29] |
| Reverse: CCAGTGCTCTGAAGGCTGTGTAC |
| GAPDH |
Forward: GACATGCCGCCTGGAGAAAC |
[29] |
| Reverse: AGCCCAGGATGCCCTTTAGT |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).