Submitted:
27 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
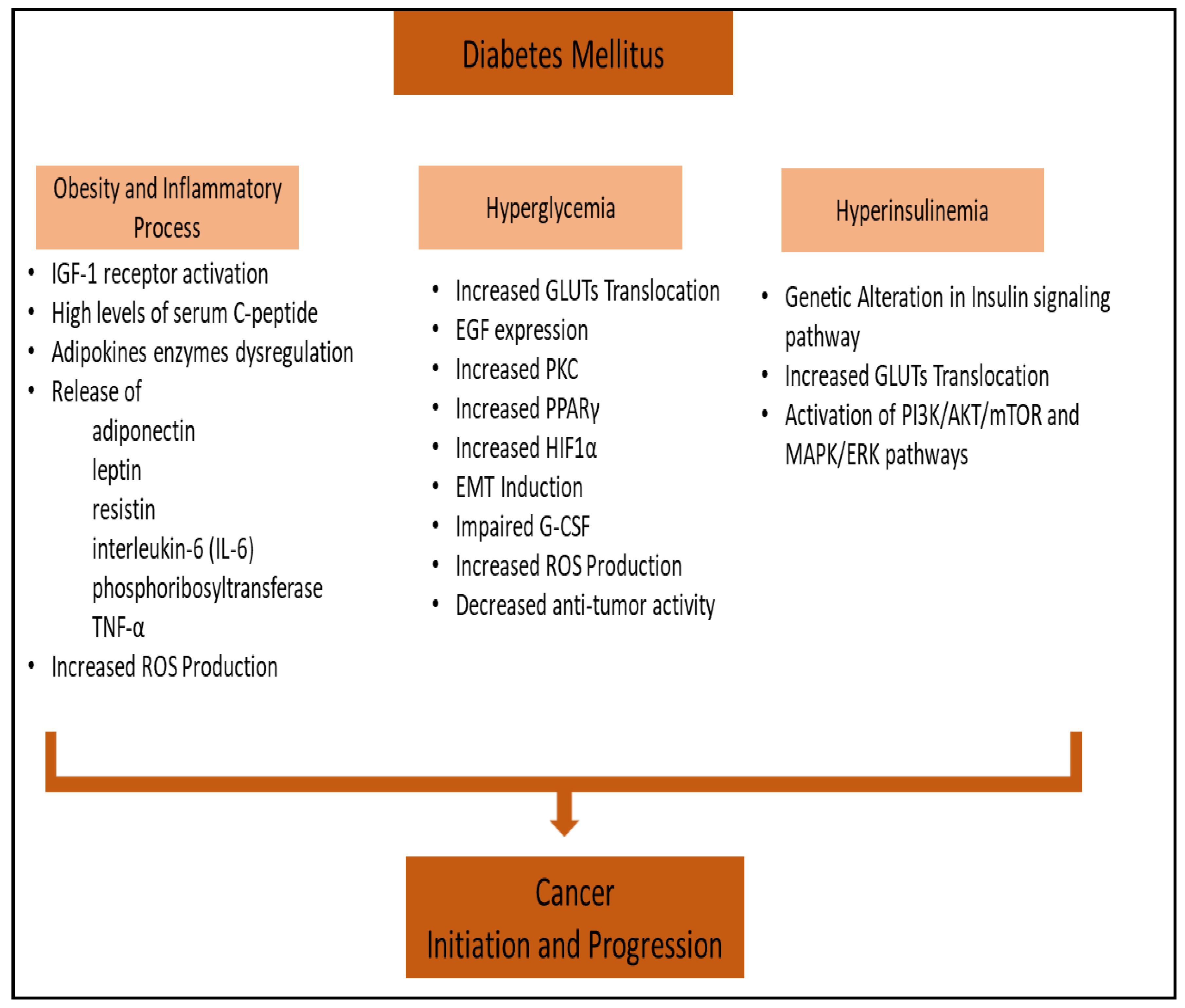
2. Obesity-Associated Diabetes and Inflammatory Process in Cancer
3. Hyperglycemia and Cancer
3.1. Cancer Cells Proliferation
3.2. Apoptosis and Cancer
3.3. Cancer Metastasis
4. Hyperinsulinemia and Cancer
4.1. PI3K/AKT/mTOR Signaling Pathway in Cancer
4.2. RAS/MAPK/ERK Signaling Pathway in Cancer
5. Insulin-Mediated PI3K Pathway Inhibition in Cancer
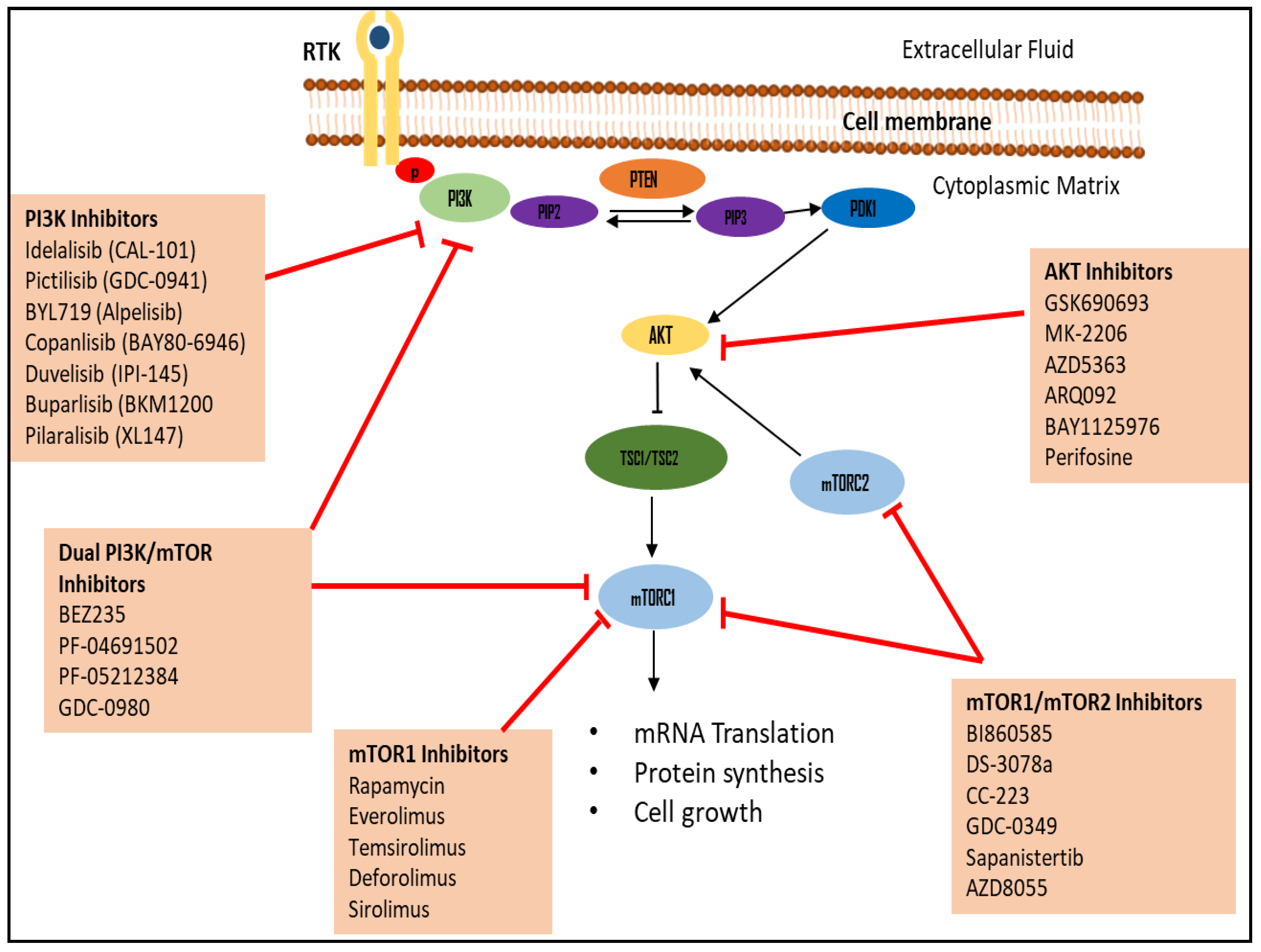
5.1. PI3K Inhibitors
5.2. Akt Inhibitors
5.3. mTOR Inhibitors
6. Antidiabetic Drugs and Their Anti-Cancer Effects
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olatunde, A. et al. (2021) ‘Cancer and diabetes: The interlinking metabolic pathways and repurposing actions of Antidiabetic Drugs’, Cancer Cell International, 21(1). [CrossRef]
- Goyal, R. (2023) Type 2 diabetes, StatPearls [Internet]. Available at: https://www.ncbi.nlm.nih.gov/books/NBK513253/ (Accessed: 19 February 2024).
- Sameer, A., Banday, M. and Nissar, S. (2020) ‘Pathophysiology of diabetes: An overview’, Avicenna Journal of Medicine, 10(4), p. 174. [CrossRef]
- Facts & figures (2024) International Diabetes Federation. Available at: https://idf.org/about-diabetes/diabetes-facts-figures/ (Accessed: 19 February 2024).
- Ling, S. et al. (2020) ‘Association of type 2 diabetes with cancer: A meta-analysis with bias analysis for unmeasured confounding in 151 cohorts comprising 32 million people’, Diabetes Care, 43(9), pp. 2313–2322. [CrossRef]
- Abudawood, M. (2019) ‘Diabetes and cancer: A comprehensive review’, Journal of Research in Medical Sciences, 24(1), p. 94. [CrossRef]
- Tsilidis, K.K. et al. (2015) ‘Type 2 diabetes and cancer: Umbrella Review of Meta-analyses of observational studies’, BMJ, 350(jan02 1). [CrossRef]
- Hu, Y. et al. (2020) ‘Incident type 2 diabetes duration and cancer risk: A prospective study in two US cohorts’, JNCI: Journal of the National Cancer Institute, 113(4), pp. 381–389. [CrossRef]
- Ediriweera, M. K., & Jayasena, S. (2023). The Role of Reprogrammed Glucose Metabolism in Cancer. Metabolites, 13(3), 345. [CrossRef]
- Zhu, B. and Qu, S. (2022) ‘The relationship between diabetes mellitus and cancers and its underlying mechanisms’, Frontiers in Endocrinology, 13. [CrossRef]
- Suh, S. and Kim, K.-W. (2019) ‘Diabetes and cancer: Cancer should be screened in routine diabetes assessment’, Diabetes & Metabolism Journal, 43(6), p. 733. [CrossRef]
- Xu, C.-X. (2014) ‘Diabetes and cancer: Associations, mechanisms, and implications for medical practice’, World Journal of Diabetes, 5(3), p. 372. [CrossRef]
- Zhang, A.M.Y. et al. (2021) ‘Hyperinsulinemia in obesity, inflammation, and cancer’, Diabetes & Metabolism Journal, 45(4), pp. 622–622. [CrossRef]
- Jiang, N. et al. (2020) ‘Role of PI3K/Akt pathway in cancer: The framework of malignant behavior’, Molecular Biology Reports, 47(6), pp. 4587–4629. [CrossRef]
- Shahid, R.K. et al. (2021) ‘Diabetes and cancer: Risk, challenges, management and outcomes’, Cancers, 13(22), p. 5735. [CrossRef]
- Renehan, A.G. and Howell, A. (2005) ‘Preventing cancer, cardiovascular disease, and diabetes’, The Lancet, 365(9469), pp. 1449–1451. [CrossRef]
- Lega, I.C. and Lipscombe, L.L. (2019) ‘Review: Diabetes, obesity, and cancer—pathophysiology and clinical implications’, Endocrine Reviews, 41(1), pp. 33–52. [CrossRef]
- Chandrasekaran, P. and Weiskirchen, R. (2024) ‘The role of obesity in type 2 diabetes mellitus—an overview’, International Journal of Molecular Sciences, 25(3), p. 1882. [CrossRef]
- Becker, S., Dossus, L. and Kaaks, R. (2009) ‘Obesity related hyperinsulinaemia and hyperglycaemia and cancer development’, Archives of Physiology and Biochemistry, 115(2), pp. 86–96. [CrossRef]
- Mesquita, L. de, et al. (2023) ‘Obesity, diabetes, and cancer: Epidemiology, Pathophysiology, and potential interventions’, Archives of Endocrinology and Metabolism, 67(6). [CrossRef]
- Nam, S. et al. (1997) ‘Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and Growth Hormone’, International Journal of Obesity, 21(5), pp. 355–359. [CrossRef]
- Renehan, A.G., Frystyk, J. and Flyvbjerg, A. (2006) ‘Obesity and Cancer Risk: The role of the insulin–igf axis’, Trends in Endocrinology & Metabolism, 17(8), pp. 328–336. [CrossRef]
- Ma, J. et al. (2008) ‘Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: A long-term survival analysis’, The Lancet Oncology, 9(11), pp. 1039–1047. [CrossRef]
- Gallagher, E.J. and LeRoith, D. (2015) ‘Obesity and diabetes: The increased risk of cancer and cancer-related mortality’, Physiological Reviews, 95(3), pp. 727–748. [CrossRef]
- Kim, J.W., Kim, J.H. and Lee, Y.J. (2024) ‘The role of adipokines in tumor progression and its association with obesity’, Biomedicines, 12(1), p. 97. [CrossRef]
- Renehan, A.G., Zwahlen, M. and Egger, M. (2015) ‘Adiposity and cancer risk: New mechanistic insights from epidemiology’, Nature Reviews Cancer, 15(8), pp. 484–498. [CrossRef]
- Parida, S., Siddharth, S. and Sharma, D. (2019) ‘Adiponectin, obesity, and cancer: Clash of the bigwigs in health and disease’, International Journal of Molecular Sciences, 20(10), p. 2519. [CrossRef]
- Taliaferro-Smith, L. et al. (2009) ‘LKB1 is required for adiponectin-mediated modulation of AMPK–S6K axis and inhibition of migration and invasion of breast cancer cells’, Oncogene, 28(29), pp. 2621–2633. [CrossRef]
- Saxena, N.K. and Sharma, D. (2010) ‘Metastasis suppression by adiponectin’, Cell Adhesion & Migration, 4(3), pp. 358–362. [CrossRef]
- Chung, S.J. et al. (2017) ‘ADIPOQ/adiponectin induces cytotoxic autophagy in breast cancer cells through STK11/LKB1-mediated activation of the AMPK-ulk1 axis’, Autophagy, 13(8), pp. 1386–1403. [CrossRef]
- Sánchez-Jiménez, F. et al. (2019a) ‘Obesity and breast cancer: Role of leptin’, Frontiers in Oncology, 9. [CrossRef]
- Mhaidat, N. et al. (2020) ‘High levels of leptin and non-high molecular weight-adiponectin in patients with colorectal cancer: Association with chemotherapy and common genetic polymorphisms’, Biomedical Reports, 14(1), pp. 1–1. [CrossRef]
- Stattin, P. et al. (2003) ‘Obesity and colon cancer: Does leptin provide a link?’, International Journal of Cancer, 109(1), pp. 149–152. [CrossRef]
- Pan, H. et al. (2018) ‘Association between serum leptin levels and breast cancer risk’, Medicine, 97(27). [CrossRef]
- Gui, Y. et al. (2017) ‘The association between obesity related adipokines and risk of breast cancer: A meta-analysis’, Oncotarget, 8(43), pp. 75389–75399. [CrossRef]
- Stone, T.W., McPherson, M. and Gail Darlington, L. (2018) ‘Obesity and cancer: Existing and new hypotheses for a causal connection’, eBioMedicine, 30, pp. 14–28. [CrossRef]
- Wellen, K.E. and Thompson, C.B. (2010) ‘Cellular metabolic stress: Considering how cells respond to nutrient excess’, Molecular Cell, 40(2), pp. 323–332. [CrossRef]
- Lee, J.Y. et al. (2001) ‘Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through toll-like receptor 4’, Journal of Biological Chemistry, 276(20), pp. 16683–16689. [CrossRef]
- Ramos, E.J.B. et al. (2003) ‘Is obesity an inflammatory disease?’, Surgery, 134(2), pp. 329–335. [CrossRef]
- Ramteke, P. et al. (2019) ‘Hyperglycemia associated metabolic and molecular alterations in cancer risk, progression, treatment, and mortality’, Cancers, 11(9), p. 1402. [CrossRef]
- Galicia-Garcia, U. et al. (2020) ‘Pathophysiology of type 2 diabetes mellitus’, International Journal of Molecular Sciences, 21(17), p. 6275. [CrossRef]
- Ruze, R. et al. (2023) ‘Obesity and type 2 diabetes mellitus: Connections in epidemiology, pathogenesis, and treatments’, Frontiers in Endocrinology, 14. [CrossRef]
- Ryu, T.Y., Park, J. and Scherer, P.E. (2014) ‘Hyperglycemia as a risk factor for cancer progression’, Diabetes & Metabolism Journal, 38(5), p. 330. [CrossRef]
- Hanahan, D. and Weinberg, R.A. (2011) ‘Hallmarks of cancer: The next generation’, Cell, 144(5), pp. 646–674. [CrossRef]
- Warburg, O. (1956) ‘On the origin of Cancer Cells’, Science, 123(3191), pp. 309–314. [CrossRef]
- Masur, K. et al. (2010) ‘Diabetogenic glucose and insulin concentrations modulate transcriptom and protein levels involved in tumour cell migration, adhesion and proliferation’, British Journal of Cancer, 104(2), pp. 345–352. [CrossRef]
- Hahn, T. et al. (1998) ‘Hyperglycemia regulates the glucose-transport system of clonal choriocarcinoma cellsin vitro. A potential molecular mechanism contributing to the adjunct effect of glucose in tumor therapy’, International Journal of Cancer, 78(3), pp. 353–360. [CrossRef]
- Han, L. et al. (2011) ‘High glucose promotes pancreatic cancer cell proliferation via the induction of EGF expression and transactivation of EGFR’, PLoS ONE, 6(11). [CrossRef]
- Duan, W. et al. (2014) ‘Hyperglycemia, a neglected factor during cancer progression’, BioMed Research International, 2014, pp. 1–10. [CrossRef]
- Okumura, M. et al. (2002) ‘Leptin and high glucose stimulate cell proliferation in MCF-7 human breast cancer cells: Reciprocal involvement of PKC-α and PPAR expression’, Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, 1592(2), pp. 107–116. [CrossRef]
- Ways, D.K. et al. (1995) ‘MCF-7 breast cancer cells transfected with protein kinase C-alpha exhibit altered expression of other protein kinase C isoforms and display a more aggressive neoplastic phenotype.’, Journal of Clinical Investigation, 95(4), pp. 1906–1915. [CrossRef]
- Yu, T., Robotham, J.L. and Yoon, Y. (2006) ‘Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology’, Proceedings of the National Academy of Sciences, 103(8), pp. 2653–2658. [CrossRef]
- Weinberg, F. et al. (2010) ‘Mitochondrial metabolism and Ros generation are essential for Kras-mediated tumorigenicity’, Proceedings of the National Academy of Sciences, 107(19), pp. 8788–8793. [CrossRef]
- Li, W. et al. (2019) ‘Effects of hyperglycemia on the progression of tumor diseases’, Journal of Experimental & Clinical Cancer Research, 38(1). [CrossRef]
- Pothiwala, P., Jain, S.K. and Yaturu, S. (2009) ‘Metabolic syndrome and cancer’, Metabolic Syndrome and Related Disorders, 7(4), pp. 279–288. [CrossRef]
- Lopez, R. et al. (2013) ‘Hyperglycemia enhances the proliferation of non-tumorigenic and malignant mammary epithelial cells through increased leptin/IGF1R signaling and activation of AKT/mTOR’, PLoS ONE, 8(11). [CrossRef]
- Luo, J. et al. (2018) ‘High glucose-induced ROS production stimulates proliferation of pancreatic cancer via inactivating the JNK pathway’, Oxidative Medicine and Cellular Longevity, 2018, pp. 1–10. [CrossRef]
- Hanahan, D. and Weinberg, R.A. (2011) ‘Hallmarks of cancer: The next generation’, Cell, 144(5), pp. 646–674. [CrossRef]
- Allen, D.A. et al. (2003) ‘High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases’, The FASEB Journal, 17(8), pp. 1–21. [CrossRef]
- Ho, F.M. et al. (2006) ‘High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-ΚB and C-jun NH2-terminal kinase pathway and prevented by PI3K/AKT/enos pathway’, Cellular Signalling, 18(3), pp. 391–399. [CrossRef]
- Vaughn, A.E. and Deshmukh, M. (2008) ‘Glucose metabolism inhibits apoptosis in neurons and cancer cells by redox inactivation of cytochrome c’, Nature Cell Biology, 10(12), pp. 1477–1483. [CrossRef]
- Rudlowski, C. et al. (2004) ‘Glut1 mRNA and protein expression in ovarian borderline tumors and cancer’, Oncology, 66(5), pp. 404–410. [CrossRef]
- Walenta, S. and Mueller-Klieser, W.F. (2004) ‘Lactate: Mirror and motor of tumor malignancy’, Seminars in Radiation Oncology, 14(3), pp. 267–274. [CrossRef]
- Kim, J. and Dang, C.V. (2006) ‘Cancer’s molecular sweet tooth and the Warburg effect’, Cancer Research, 66(18), pp. 8927–8930. [CrossRef]
- Kondoh, H. (2008) ‘Cellular life span and the Warburg effect’, Experimental Cell Research, 314(9), pp. 1923–1928. [CrossRef]
- Gatenby, R.A. and Gillies, R.J. (2004) ‘Why do cancers have high aerobic glycolysis?’, Nature Reviews Cancer, 4(11), pp. 891–899. [CrossRef]
- Semenza, G.L. (1999) ‘Regulation of mammalian O2 homeostasis by hypoxia-inducible factor 1’, Annual Review of Cell and Developmental Biology, 15(1), pp. 551–578. [CrossRef]
- Lee, J.-W. et al. (2004) ‘Hypoxia-inducible factor (hif-1)α: Its protein stability and biological functions’, Experimental & Molecular Medicine, 36(1), pp. 1–12. [CrossRef]
- Catrina SB, Okamoto K, Pereira T, Brismar K, Poellinger L. Hyperglycemia regulates hypoxia-inducible factor-1alpha protein stability and function. Diabetes 2004;53:3226-32.
- Wu, Y. and Zhou, B.P. (2008) ‘New insights of epithelial-mesenchymal transition in cancer metastasis’, Acta Biochimica et Biophysica Sinica, 40(7), pp. 643–650. [CrossRef]
- Kauppi, J. et al. (2013) ‘Cause of death during long-term follow-up for superficial esophageal adenocarcinoma’, Annals of Surgical Oncology, 20(7), pp. 2428–2433. [CrossRef]
- Walters, S. et al. (2013) ‘Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: A population-based study, 2004–2007’, Thorax, 68(6), pp. 551–564. [CrossRef]
- Walters, S et al. (2013) ‘Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: A population-based study’, British Journal of Cancer, 108(5), pp. 1195–1208. [CrossRef]
- Li, W. et al. (2018) ‘Hyperglycemia aggravates microenvironment hypoxia and promotes the metastatic ability of pancreatic cancer’, Computational and Structural Biotechnology Journal, 16, pp. 479–487. [CrossRef]
- Li, W. et al. (2011) ‘Hyperglycemia enhances the invasive and migratory activity of pancreatic cancer cells via hydrogen peroxide’, Oncology Reports, 25(5). [CrossRef]
- Li, W. (2012) ‘Hyperglycemia as a mechanism of pancreatic cancer metastasis’, Frontiers in Bioscience, 17(1), p. 1761. [CrossRef]
- Kang, X. et al. (2015) ‘High glucose promotes tumor invasion and increases metastasis-associated protein expression in human lung epithelial cells by upregulating heme oxygenase-1 via reactive oxygen species or the TGF-β1/PI3K/akt signaling pathway’, Cellular Physiology and Biochemistry, 35(3), pp. 1008–1022. [CrossRef]
- Pickup, M.W. et al. (2017) ‘Development of aggressive pancreatic ductal adenocarcinomas depends on granulocyte colony stimulating factor secretion in carcinoma cells’, Cancer Immunology Research, 5(9), pp. 718–729. [CrossRef]
- Gallagher, E.J. and LeRoith, D. (2020) ‘Hyperinsulinaemia in cancer’, Nature Reviews Cancer, 20(11), pp. 629–644. [CrossRef]
- Gunter, M.J. et al. (2015) ‘Breast cancer risk in metabolically healthy but overweight postmenopausal women’, Cancer Research, 75(2), pp. 270–274. [CrossRef]
- Hirose, K. et al. (2003) ‘Physical exercise reduces risk of breast cancer in Japanese women’, Cancer Science, 94(2), pp. 193–199. [CrossRef]
- Ma, J. et al. (2004) ‘A prospective study of plasma C-peptide and colorectal cancer risk in men’, JNCI Journal of the National Cancer Institute, 96(7), pp. 546–553. [CrossRef]
- Hammarsten, J. and Högstedt, B. (2005) ‘Hyperinsulinaemia: A prospective risk factor for lethal clinical prostate cancer’, European Journal of Cancer, 41(18), pp. 2887–2895. [CrossRef]
- Gunter, M.J. et al. (2008) ‘A prospective evaluation of insulin and insulin-like growth factor-I as risk factors for endometrial cancer’, Cancer Epidemiology, Biomarkers & Prevention, 17(4), pp. 921–929. [CrossRef]
- Loftfield, E. et al. (2016) ‘Higher glucose and insulin levels are associated with risk of liver cancer and chronic liver disease mortality among men without a history of diabetes’, Cancer Prevention Research, 9(11), pp. 866–874. [CrossRef]
- Walraven, I. et al. (2013) ‘Fasting proinsulin levels are significantly associated with 20 year cancer mortality rates. The Hoorn Study’, Diabetologia, 56(5), pp. 1148–1154. [CrossRef]
- Frasca, F. et al. (2008) ‘The role of insulin receptors and IGF-I receptors in cancer and other diseases’, Archives of Physiology and Biochemistry, 114(1), pp. 23–37. [CrossRef]
- BAXTER, R.C., BRYSON, J.M. and TURTLE, J.R. (1980) ‘Somatogenic receptors of rat liver: Regulation by insulin*’, Endocrinology, 107(4), pp. 1176–1181. [CrossRef]
- Le, T.K. et al. (2023) ‘Insulin signaling and its application’, Frontiers in Endocrinology, 14. [CrossRef]
- Petersen, M.C. and Shulman, G.I. (2018) ‘Mechanisms of insulin action and insulin resistance’, Physiological Reviews, 98(4), pp. 2133–2223. [CrossRef]
- Gallagher, E.J. and LeRoith, D. (2010) ‘The proliferating role of insulin and insulin-like growth factors in cancer’, Trends in Endocrinology & Metabolism, 21(10), pp. 610–618. [CrossRef]
- Boucher, J., Kleinridders, A. and Kahn, C.R. (2014) ‘Insulin receptor signaling in normal and insulin-resistant states’, Cold Spring Harbor Perspectives in Biology, 6(1). [CrossRef]
- Savova, M.S. et al. (2023) ‘Targeting PI3K/akt signaling pathway in obesity’, Biomedicine & Pharmacotherapy, 159, p. 114244. [CrossRef]
- Tsay, A. and Wang, J.-C. (2023) ‘The role of PIK3R1 in metabolic function and insulin sensitivity’, International Journal of Molecular Sciences, 24(16), p. 12665. [CrossRef]
- Brachmann, S.M. et al. (2005) ‘Phosphoinositide 3-kinase catalytic subunit deletion and regulatory subunit deletion have opposite effects on insulin sensitivity in mice’, Molecular and Cellular Biology, 25(5), pp. 1596–1607. [CrossRef]
- Clarke, J.F. et al. (1994) ‘Inhibition of the translocation of GLUT1 and GLUT4 in 3T3-L1 cells by the phosphatidylinositol 3-kinase inhibitor, Wortmannin’, Biochemical Journal, 300(3), pp. 631–635. [CrossRef]
- Asano, T. et al. (2007) ‘Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions’, Biological and Pharmaceutical Bulletin, 30(9), pp. 1610–1616. [CrossRef]
- Carracedo, A. and Pandolfi, P.P. (2008) ‘The PTEN–PI3K pathway: Of feedbacks and cross-talks’, Oncogene, 27(41), pp. 5527–5541. [CrossRef]
- Stokoe, D. et al. (1997) ‘Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B’, Science, 277(5325), pp. 567–570. [CrossRef]
- Mîinea, C.P. et al. (2005) ‘AS160, the AKT substrate regulating GLUT4 translocation, has a functional Rab GTPase-activating protein domain’, Biochemical Journal, 391(1), pp. 87–93. [CrossRef]
- Manning, B.D. and Cantley, L.C. (2007) ‘AKT/PKB signaling: Navigating downstream’, Cell, 129(7), pp. 1261–1274. [CrossRef]
- Okoro, D.R., Rosso, M. and Bargonetti, J. (2012) ‘Splicing up MDM2 for cancer proteome diversity’, Genes & Cancer, 3(3–4), pp. 311–319. [CrossRef]
- Zafar, A., Khan, M.J. and Naeem, A. (2023) ‘Mdm2- an indispensable player in tumorigenesis’, Molecular Biology Reports, 50(8), pp. 6871–6883. [CrossRef]
- Cheng, X. et al. (2010) ‘Activation of murine double minute 2 by Akt in mammary epithelium delays mammary involution and accelerates mammary tumorigenesis’, Cancer Research, 70(19), pp. 7684–7689. [CrossRef]
- Haeusler, R.A., McGraw, T.E. and Accili, D. (2017) ‘Biochemical and cellular properties of insulin receptor signalling’, Nature Reviews Molecular Cell Biology, 19(1), pp. 31–44. [CrossRef]
- Trajkovic-Arsic, M., Kalideris, E. and Siveke, J.T. (2013) ‘The role of insulin and IGF system in pancreatic cancer’, Journal of Molecular Endocrinology, 50(3). [CrossRef]
- Draznin, B. (2009) ‘Mitogenic action of insulin: Friend, foe or “frenemy”?’, Diabetologia, 53(2), pp. 229–233. [CrossRef]
- Bedinger, D.H. and Adams, S.H. (2015) ‘Metabolic, anabolic, and mitogenic insulin responses: A tissue-specific perspective for insulin receptor activators’, Molecular and Cellular Endocrinology, 415, pp. 143–156. [CrossRef]
- Padma, V.V. (2015) ‘An overview of targeted cancer therapy’, BioMedicine, 5(4). [CrossRef]
- Huemer, F., Bartsch, R. and Gnant, M. (2014) ‘The PI3K/AKT/MTOR signaling pathway: The role of PI3K and AKT inhibitors in breast cancer’, Current Breast Cancer Reports, 6(2), pp. 59–70. [CrossRef]
- Vanhaesebroeck, B. et al. (2010) ‘The emerging mechanisms of isoform-specific PI3K signalling’, Nature Reviews Molecular Cell Biology, 11(5), pp. 329–341. [CrossRef]
- Alzahrani, A.S. (2019) ‘PI3K/AKT/mtor inhibitors in cancer: At the bench and bedside’, Seminars in Cancer Biology, 59, pp. 125–132. [CrossRef]
- Courtney, K.D., Corcoran, R.B. and Engelman, J.A. (2010) ‘The PI3K pathway as drug target in human cancer’, Journal of Clinical Oncology, 28(6), pp. 1075–1083. [CrossRef]
- Pons-Tostivint, E., Thibault, B. and Guillermet-Guibert, J. (2017) ‘Targeting PI3K signaling in combination cancer therapy’, Trends in Cancer, 3(6), pp. 454–469. [CrossRef]
- Akinleye, A. et al. (2013) ‘Phosphatidylinositol 3-kinase (PI3K) inhibitors as cancer therapeutics’, Journal of Hematology & Oncology, 6(1). [CrossRef]
- Pirali, T. et al. (2017) ‘Identification of a potent phosphoinositide 3-kinase PAN inhibitor displaying a strategic carboxylic acid group and development of its prodrugs’, ChemMedChem, 12(18), pp. 1542–1554. [CrossRef]
- Ando, Y. et al. (2019) ‘Phase I study of alpelisib (BYL719), an α-specific PI3K inhibitor, in Japanese patients with advanced solid tumors’, Cancer Science, 110(3), pp. 1021–1031. [CrossRef]
- Fruman, D.A. and Rommel, C. (2014) ‘PI3K and cancer: Lessons, challenges and opportunities’, Nature Reviews Drug Discovery, 13(2), pp. 140–156. [CrossRef]
- Fritsch, C. et al. (2014) ‘Characterization of the novel and specific pi3kα inhibitor NVP-BYL719 and development of the patient stratification strategy for clinical trials’, Molecular Cancer Therapeutics, 13(5), pp. 1117–1129. [CrossRef]
- Juric, D. et al. (2018) ‘Phosphatidylinositol 3-kinase α–selective inhibition with Alpelisib (BYL719) in pik3ca-altered solid tumors: Results from the first-in-human study’, Journal of Clinical Oncology, 36(13), pp. 1291–1299. [CrossRef]
- Lampson, B.L. and Brown, J.R. (2017) ‘Pi3kδ-selective and PI3Kα/δ-combinatorial inhibitors in clinical development for B-cell non-Hodgkin Lymphoma’, Expert Opinion on Investigational Drugs, 26(11), pp. 1267–1279. [CrossRef]
- Nagaraj, G. et al. (2012) ‘A phase I study of BKM120, a novel oral selective phosphatidylinositol-3-kinase (PI3K) inhibitor, in combination with fulvestrant in postmenopausal women with estrogen receptor positive metastatic breast cancer.’, Journal of Clinical Oncology, 30(15_suppl). [CrossRef]
- Gopal, A.K. et al. (2014) ‘PI3Kδ inhibition by Idelalisib in patients with relapsed indolent lymphoma’, New England Journal of Medicine, 370(11), pp. 1008–1018. [CrossRef]
- Herman, S.E. et al. (2010) ‘Phosphatidylinositol 3-kinase-δ inhibitor CAL-101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals’, Blood, 116(12), pp. 2078–2088. [CrossRef]
- Yang, J. et al. (2019) ‘Targeting PI3K in cancer: Mechanisms and advances in clinical trials’, Molecular Cancer, 18(1). [CrossRef]
- Commissioner, O. of the (2017) FDA approves new treatment for adults with relapsed follicular lymphoma, U.S. Food and Drug Administration. Available at: https://www.fda.gov/news-events/press-announcements/fda-approves-new-treatment-adults-relapsed-follicular-lymphoma (Accessed: 23 July 2024).
- Center for Drug Evaluation and Research (2018) Duvelisib (COPIKTRA, Verastem, inc.) for adult patients with relapsed, U.S. Food and Drug Administration. Available at: https://www.fda.gov/drugs/resources-information-approved-drugs/duvelisib-copiktra-verastem-inc-adult-patients-relapsed-or-refractory-chronic-lymphocytic-leukemia (Accessed: 23 July 2024).
- Juric, D. et al. (2014) ‘Convergent loss of PTEN leads to clinical resistance to a PI(3)KA inhibitor’, Nature, 518(7538), pp. 240–244. [CrossRef]
- Haddadi, N. et al. (2018) ‘PTEN/PTENP1: “regulating the regulator of RTK-dependent PI3K/Akt signalling”, new targets for cancer therapy’, Molecular Cancer, 17(1). [CrossRef]
- Aziz, S.A. et al. (2010) ‘Vertical targeting of the phosphatidylinositol-3 kinase pathway as a strategy for treating melanoma’, Clinical Cancer Research, 16(24), pp. 6029–6039. [CrossRef]
- Calero, R. et al. (2017) ‘Synergistic anti-tumor effect of 17AAG with the PI3K/mtor inhibitor NVP-bez235 on human melanoma’, Cancer Letters, 406, pp. 1–11. [CrossRef]
- Wang, Y. et al. (2014) ‘Activation of AR sensitizes breast carcinomas to NVP-bez235’s therapeutic effect mediated by PTEN and KLLN upregulation’, Molecular Cancer Therapeutics, 13(2), pp. 517–527. [CrossRef]
- Martinelli, E. et al. (2013) ‘Antitumor activity of pimasertib, a selective MEK 1/2 inhibitor, in combination with PI3K/mtor inhibitors or with multi-targeted kinase inhibitors in pimasertib-resistant human lung and colorectal cancer cells’, International Journal of Cancer, 133(9), pp. 2089–2101. [CrossRef]
- YANG, X. et al. (2016) ‘Autophagy inhibition enhances colorectal cancer apoptosis induced by dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor NVP-bez235’, Oncology Letters, 12(1), pp. 102–106. [CrossRef]
- Cho, D.C. et al. (2010) ‘The efficacy of the novel dual PI3-kinase/mtor inhibitor NVP-Bez235 compared with rapamycin in renal cell carcinoma’, Clinical Cancer Research, 16(14), pp. 3628–3638. [CrossRef]
- Cheng, H. et al. (2010) ‘Discovery of the highly potent PI3K/mtor dual inhibitor PF-04691502 through structure based drug design’, MedChemComm, 1(2), p. 139. [CrossRef]
- Yuan, J. et al. (2011) ‘PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity’, Molecular Cancer Therapeutics, 10(11), pp. 2189–2199. [CrossRef]
- [137] Mallon, R. et al. (2011) ‘Antitumor efficacy of PKI-587, a highly potent dual PI3K/mtor kinase inhibitor’, Clinical Cancer Research, 17(10), pp. 3193–3203. [CrossRef]
- Colombo, I. et al. (2021) ‘Phase I dose-escalation study of the dual PI3K-mtorc1/2 inhibitor Gedatolisib in combination with paclitaxel and carboplatin in patients with advanced solid tumors’, Clinical Cancer Research, 27(18), pp. 5012–5019. [CrossRef]
- Herzog, A. et al. (2013) ‘PI3K/mtor inhibitor PF-04691502 antitumor activity is enhanced with induction of wild-type TP53 in human xenograft and murine knockout models of head and neck cancer’, Clinical Cancer Research, 19(14), pp. 3808–3819. [CrossRef]
- Yuan, J. et al. (2011a) ‘PF-04691502, a potent and selective oral inhibitor of PI3K and mTOR kinases with antitumor activity’, Molecular Cancer Therapeutics, 10(11), pp. 2189–2199. [CrossRef]
- Soares, H.P. et al. (2015) ‘Dual PI3K/mTOR inhibitors induce rapid overactivation of the MEK/ERK pathway in human pancreatic cancer cells through suppression of mtorc2’, Molecular Cancer Therapeutics, 14(4), pp. 1014–1023. [CrossRef]
- Freitag, H. et al. (2016) ‘Inhibition of mtor’s catalytic site by PKI-587 is a promising therapeutic option for gastroenteropancreatic neuroendocrine tumor disease’, Neuroendocrinology, 105(1), pp. 90–104. [CrossRef]
- Shariff, A.I. et al. (2019) ‘Novel cancer therapies and their association with diabetes’, Journal of Molecular Endocrinology, 62(2). [CrossRef]
- Hopkins, B.D. et al. (2018) ‘Suppression of insulin feedback enhances the efficacy of PI3K inhibitors’, Nature, 560(7719), pp. 499–503. [CrossRef]
- Świderska, E. et al. (2020) ‘Role of PI3K/Akt pathway in insulin-mediated glucose uptake’, Blood Glucose Levels [Preprint]. [CrossRef]
- Goncalves, M.D., Hopkins, B.D. and Cantley, L.C. (2018) ‘Phosphatidylinositol 3-kinase, growth disorders, and cancer’, New England Journal of Medicine, 379(21), pp. 2052–2062. [CrossRef]
- Nair, K.S. and Cheson, B. (2016) ‘The role of idelalisib in the treatment of relapsed and refractory chronic lymphocytic leukemia’, Therapeutic Advances in Hematology, 7(2), pp. 69–84. [CrossRef]
- Staal, S.P. (1987) ‘Molecular cloning of the AKT oncogene and its human homologues akt1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma.’, Proceedings of the National Academy of Sciences, 84(14), pp. 5034–5037. [CrossRef]
- He, Y. et al. (2021) ‘Targeting PI3K/AKT signal transduction for cancer therapy’, Signal Transduction and Targeted Therapy, 6(1). [CrossRef]
- Nunnery, S.E. and Mayer, I.A. (2019) ‘Management of toxicity to isoform α-specific PI3K inhibitors’, Annals of Oncology, 30, pp. x21–x26. [CrossRef]
- West, K.A., Sianna Castillo, S. and Dennis, P.A. (2002) ‘Activation of the PI3K/Akt pathway and chemotherapeutic resistance’, Drug Resistance Updates, 5(6), pp. 234–248. [CrossRef]
- Wilson, J. M., Kunnimalaiyaan, S., Gamblin, T. C., & Kunnimalaiyaan, M. (2014). MK2206 inhibits hepatocellular carcinoma cellular proliferation via induction of apoptosis and cell cycle arrest. Journal of Surgical Research, 191(2), 280–285. [CrossRef]
- Papadimitrakopoulou, V. (2012) ‘Development of PI3K/AKT/mtor pathway inhibitors and their application in personalized therapy for non–small-cell lung cancer’, Journal of Thoracic Oncology, 7(8), pp. 1315–1326. [CrossRef]
- Hirai, H. et al. (2010) ‘MK-2206, an allosteric AKT inhibitor, enhances antitumor efficacy by standard chemotherapeutic agents or molecular targeted drugsin vitroandin vivo’, Molecular Cancer Therapeutics, 9(7), pp. 1956–1967. [CrossRef]
- Konopleva, M.Y. et al. (2014) ‘Preclinical and early clinical evaluation of the oral akt inhibitor, MK-2206, for the treatment of acute myelogenous leukemia’, Clinical Cancer Research, 20(8), pp. 2226–2235. [CrossRef]
- Yap, T.A. et al. (2010) ‘First-in-class phase I trial of a selective AKT inhibitor, MK2206 (MK), evaluating alternate day (QOD) and once weekly (QW) doses in Advanced cancer patients (PTS) with evidence of target modulation and antitumor activity.’, Journal of Clinical Oncology, 28(15_suppl), pp. 3009–3009. [CrossRef]
- Davies, B.R. et al. (2012) ‘Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background’, Molecular Cancer Therapeutics, 11(4), pp. 873–887. [CrossRef]
- ZHANG, Y. et al. (2016) ‘A novel AKT inhibitor, AZD5363, inhibits phosphorylation of Akt downstream molecules, and activates phosphorylation of mTOR and SMG-1 dependent on the liver cancer cell type’, Oncology Letters, 11(3), pp. 1685–1692. [CrossRef]
- Li, J. et al. (2013) ‘The AKT inhibitor AZD5363 is selectively active in PI3KCA mutant gastric cancer, and sensitizes a patient-derived gastric cancer xenograft model with PTEN loss to taxotere’, Journal of Translational Medicine, 11(1). [CrossRef]
- Davies, B.R. et al. (2012) ‘Preclinical pharmacology of AZD5363, an inhibitor of AKT: Pharmacodynamics, antitumor activity, and correlation of monotherapy activity with genetic background’, Molecular Cancer Therapeutics, 11(4), pp. 873–887. [CrossRef]
- Lai, Y.-C. et al. (2012) ‘A novel PKB/AKT inhibitor, MK-2206, effectively inhibits insulin-stimulated glucose metabolism and protein synthesis in isolated rat skeletal muscle’, Biochemical Journal, 447(1), pp. 137–147. [CrossRef]
- Laplante, M. and Sabatini, D.M. (2012) ‘MTOR signaling in growth control and disease’, Cell, 149(2), pp. 274–293. [CrossRef]
- Popova, N.V. and Jücker, M. (2021) ‘The role of mTOR signaling as a therapeutic target in cancer’, International Journal of Molecular Sciences, 22(4), p. 1743. [CrossRef]
- Liu, Y. et al. (2019) ‘Rapamycin: A bacteria-derived immunosuppressant that has anti-atherosclerotic effects and its clinical application’, Frontiers in Pharmacology, 9. [CrossRef]
- Edwards, S.R. and Wandless, T.J. (2007) ‘The rapamycin-binding domain of the protein kinase mammalian target of rapamycin is a destabilizing domain’, Journal of Biological Chemistry, 282(18), pp. 13395–13401. [CrossRef]
- Stanfel, M.N. et al. (2009) ‘The tor pathway comes of age’, Biochimica et Biophysica Acta (BBA) - General Subjects, 1790(10), pp. 1067–1074. [CrossRef]
- de Braud, F. et al. (2020) ‘A phase 1 study of mtorc1/2 inhibitor BI 860585 as a single agent or with Exemestane or paclitaxel in patients with advanced solid tumors’, Cancers, 12(6), p. 1425. [CrossRef]
- Capelan, M. et al. (2013) ‘Abstract C173: A first-in-human phase I study of DS-3078A, an oral torc1/2 inhibitor, in patients with advanced solid tumors: Preliminary results.’, Molecular Cancer Therapeutics, 12(11_Supplement). [CrossRef]
- Yang, H. et al. (2020) ‘GDC-0349 inhibits non-small cell lung cancer cell growth’, Cell Death & Disease, 11(11). [CrossRef]
- Voss, M.H. et al. (2020) ‘Phase 1 study of mtorc1/2 inhibitor sapanisertib (TAK-228) in advanced solid tumours, with an expansion phase in renal, endometrial or bladder cancer’, British Journal of Cancer, 123(11), pp. 1590–1598. [CrossRef]
- Wolin, E. et al. (2019) ‘A phase 2 study of an oral mtorc1/mtorc2 kinase inhibitor (CC-223) for non-pancreatic neuroendocrine tumors with or without carcinoid symptoms’, PLOS ONE, 14(9). [CrossRef]
- Chresta, C.M. et al. (2010) ‘AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity’, Cancer Research, 70(1), pp. 288–298. [CrossRef]
- Murugan, A.K., Liu, R. and Xing, M. (2019) ‘Identification and characterization of two novel oncogenic mTOR mutations’, Oncogene, 38(26), pp. 5211–5226. [CrossRef]
- Kwiatkowski, D.J. et al. (2016) ‘Mutations in TSC1, TSC2, and MTOR are associated with response to Rapalogs in patients with metastatic renal cell carcinoma’, Clinical Cancer Research, 22(10), pp. 2445–2452. [CrossRef]
- Wagle, N. et al. (2014) ‘Response and acquired resistance to Everolimus in anaplastic thyroid cancer’, New England Journal of Medicine, 371(15), pp. 1426–1433. [CrossRef]
- Murugan, A.K. et al. (2015) ‘Absence of somatic mutations of the mTOR gene in differentiated thyroid cancer’, Meta Gene, 6, pp. 69–71. [CrossRef]
- Hassan, B. et al. (2014) ‘Catalytic mTOR inhibitors can overcome intrinsic and acquired resistance to allosteric mTOR inhibitors’, Oncotarget, 5(18), pp. 8544–8557. [CrossRef]
- Bahar, M.E., Kim, H.J. and Kim, D.R. (2023) ‘Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies’, Signal Transduction and Targeted Therapy, 8(1). [CrossRef]
- Li, Q. et al. (2022) ‘Targeting the PI3K/AKT/mtor and RAF/MEK/ERK pathways for cancer therapy’, Molecular Biomedicine, 3(1). [CrossRef]
- Vergès, B. and Cariou, B. (2015) ‘MTOR inhibitors and diabetes’, Diabetes Research and Clinical Practice, 110(2), pp. 101–108. [CrossRef]
- Fraenkel, M. et al. (2008) ‘MTOR inhibition by rapamycin prevents β-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes’, Diabetes, 57(4), pp. 945–957. [CrossRef]
- Yim, C. et al. (2021) ‘Current cancer therapies and their influence on glucose control’, World Journal of Diabetes, 12(7), pp. 1010–1025. [CrossRef]
- Chresta, C.M. et al. (2010) ‘AZD8055 is a potent, selective, and orally bioavailable ATP-competitive mammalian target of rapamycin kinase inhibitor with in vitro and in vivo antitumor activity’, Cancer Research, 70(1), pp. 288–298. [CrossRef]
- Kleinert, M. et al. (2014) ‘Acute mtor inhibition induces insulin resistance and alters substrate utilization in vivo’, Molecular Metabolism, 3(6), pp. 630–641. [CrossRef]
- Rachdi, L. et al. (2008) ‘Disruption of TSC2 in pancreatic β cells induces β cell mass expansion and improved glucose tolerance in a torc1-dependent manner’, Proceedings of the National Academy of Sciences, 105(27), pp. 9250–9255. [CrossRef]
- Asahara, S. et al. (2022) ‘Roles of mtor in the regulation of pancreatic β-cell mass and insulin secretion’, Biomolecules, 12(5), p. 614. [CrossRef]
- Dąbrowski, M. (2021) ‘Diabetes, antidiabetic medications and cancer risk in type 2 diabetes: Focus on SGLT-2 inhibitors’, International Journal of Molecular Sciences, 22(4), p. 1680. [CrossRef]
- Soranna, D. et al. (2012) ‘Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: A meta-analysis’, The Oncologist, 17(6), pp. 813–822. [CrossRef]
- Noto, H. et al. (2012) ‘Cancer risk in diabetic patients treated with metformin: A systematic review and meta-analysis’, PLoS ONE, 7(3). [CrossRef]
- Currie, C.J., Poole, C.D. and Gale, E.A. (2009) ‘The influence of glucose-lowering therapies on cancer risk in type 2 diabetes’, Diabetologia, 52(9), pp. 1766–1777. [CrossRef]
- Evans, J.M. et al. (2005) ‘Metformin and reduced risk of cancer in diabetic patients’, BMJ, 330(7503), pp. 1304–1305. [CrossRef]
- Nathan, D.M. et al. (2008) ‘Medical management of hyperglycaemia in type 2 diabetes mellitus: A consensus algorithm for the initiation and adjustment of therapy’, Diabetologia, 52(1), pp. 17–30. [CrossRef]
- Bailey, C.J. (2017) ‘Metformin: Historical overview’, Diabetologia, 60(9), pp. 1566–1576. [CrossRef]
- Quinn, B.J. et al. (2013) ‘Repositioning metformin for cancer prevention and treatment’, Trends in Endocrinology & Metabolism, 24(9), pp. 469–480. [CrossRef]
- Alimova, I.N. et al. (2009) ‘Metformin inhibits breast cancer cell growth, colony formation and induces cell cycle arrest in vitro’, Cell Cycle, 8(6), pp. 909–915. [CrossRef]
- Buzzai, M. et al. (2007) ‘Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth’, Cancer Research, 67(14), pp. 6745–6752. [CrossRef]
- Zakikhani, M. et al. (2006) ‘Metformin is an AMP kinase–dependent growth inhibitor for breast cancer cells’, Cancer Research, 66(21), pp. 10269–10273. [CrossRef]
- Heckman-Stoddard, B.M. et al. (2017) ‘Repurposing metformin for the Prevention of Cancer and cancer recurrence’, Diabetologia, 60(9), pp. 1639–1647. [CrossRef]
- Morgillo, F. et al. (2013) ‘Metformin in lung cancer: Rationale for a combination therapy’, Expert Opinion on Investigational Drugs, 22(11), pp. 1401–1409. [CrossRef]
- Zhou, G. et al. (2001) ‘Role of AMP-activated protein kinase in mechanism of metformin action’, Journal of Clinical Investigation, 108(8), pp. 1167–1174. [CrossRef]
- Cerezo, M. et al. (2013) ‘Metformin blocks melanoma invasion and metastasis development in AMPK/p53-dependent manner’, Molecular Cancer Therapeutics, 12(8), pp. 1605–1615. [CrossRef]
- Lv, Z. and Guo, Y. (2020) ‘Metformin and its benefits for various diseases’, Frontiers in Endocrinology, 11. [CrossRef]
- Algire, C. et al. (2012) ‘Metformin reduces endogenous reactive oxygen species and associated DNA damage’, Cancer Prevention Research, 5(4), pp. 536–543. [CrossRef]
- Soccio, R.E., Chen, E.R. and Lazar, M.A. (2014) ‘Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes’, Cell Metabolism, 20(4), pp. 573–591. [CrossRef]
- Fröhlich, E. and Wahl, R. (2015) ‘Chemotherapy and chemoprevention by thiazolidinediones’, BioMed Research International, 2015, pp. 1–14. [CrossRef]
- Nath, M., Nath, S. and Choudhury, Y. (2021) ‘The impact of thiazolidinediones on the risk for prostate cancer in patients with type 2 diabetes mellitus: A review and meta-analysis’, Meta Gene, 27, p. 100840. [CrossRef]
- Xiao, Y. et al. (2010) ‘3T3-L1 adipocyte apoptosis induced by thiazolidinediones is peroxisome proliferator-activated receptor-γ-dependent and mediated by the caspase-3-dependent apoptotic pathway’, The FEBS Journal, 277(3), pp. 687–696. [CrossRef]
- Lv, S. et al. (2019) ‘PPARΓ activation serves as therapeutic strategy against bladder cancer via inhibiting PI3K-akt signaling pathway’, BMC Cancer, 19(1). [CrossRef]
- Ciaramella, V. et al. (2019) ‘Activity and molecular targets of pioglitazone via blockade of proliferation, invasiveness and bioenergetics in human NSCLC’, Journal of Experimental & Clinical Cancer Research, 38(1). [CrossRef]
- Sola, D. et al. (2015) ‘State of the art paper sulfonylureas and their use in clinical practice’, Archives of Medical Science, 4, pp. 840–848. [CrossRef]
- Pasello, G. et al. (2013) ‘Effects of sulfonylureas on tumor growth: A review of the literature’, The Oncologist, 18(10), pp. 1118–1125. [CrossRef]
- Payen, L. et al. (2001) ‘The sulphonylurea glibenclamide inhibits multidrug resistance protein (MRP1) activity in human lung cancer cells’, British Journal of Pharmacology, 132(3), pp. 778–784. [CrossRef]
- Avery, M. et al. (2008) ‘Type 2 diabetes and oral antihyperglycemic drugs’, Current Medicinal Chemistry, 15(1), pp. 61–74. [CrossRef]
- Ashcroft, F.M. (2005) ‘ATP-sensitive potassium channelopathies: Focus on insulin secretion’, Journal of Clinical Investigation, 115(8), pp. 2047–2058. [CrossRef]
- Núñez, M. et al. (2013) ‘Glibenclamide inhibits cell growth by inducing G0/G1 arrest in the human breast cancer cell line MDA-MB-231’, BMC Pharmacology and Toxicology, 14(1). [CrossRef]
- Wondergem, R. et al. (1998) ‘Membrane potassium channels and human bladder tumor cells: II. growth properties’, Journal of Membrane Biology, 161(3), pp. 257–262. [CrossRef]
- Abdul, M. and Hoosein, N. (2002) ‘Expression and activity of potassium ion channels in human prostate cancer’, Cancer Letters, 186(1), pp. 99–105. [CrossRef]
- Malhi, H. et al. (2000) ‘KATP channels regulate mitogenically induced proliferation in primary rat hepatocytes and human liver cell lines’, Journal of Biological Chemistry, 275(34), pp. 26050–26057. [CrossRef]
- Schneider, E. (1998) ‘ATP-binding-cassette (ABC) Transport Systems: Functional and structural aspects of the ATP-hydrolyzing subunits/domains’, FEMS Microbiology Reviews, 22(1), pp. 1–20. [CrossRef]
- Zhang, Y.-K. et al. (2015) ‘Multidrug resistance proteins (mrps) and cancer therapy’, The AAPS Journal, 17(4), pp. 802–812. [CrossRef]
- Lautier, D. et al. (1996) ‘Multidrug resistance mediated by the multidrug resistance protein (MRP) gene’, Biochemical Pharmacology, 52(7), pp. 967–977. [CrossRef]
- Xu, K. et al. (2019) ‘Glibenclamide targets sulfonylurea receptor 1 to inhibit p70s6K activity and upregulate KLF4 expression to suppress non-small cell lung carcinoma’, Molecular Cancer Therapeutics, 18(11), pp. 2085–2096. [CrossRef]
- Qian, X. et al. (2008) ‘Glibenclamide exerts an antitumor activity through reactive oxygen species–C-jun nh(2)-terminal kinase pathway in human gastric cancer cell line MGC-803’, Biochemical Pharmacology, 76(12), pp. 1705–1715. [CrossRef]
- Lin, Y. et al. (2024) ‘Pharmacological targets of SGLT2 inhibition on prostate cancer mediated by circulating metabolites: A drug-target Mendelian randomization study’, Frontiers in Pharmacology, 15. [CrossRef]
- Wang, L. et al. (2024) ‘GLP-1 receptor agonists and colorectal cancer risk in drug-naive patients with type 2 diabetes, with and without overweight/obesity’, JAMA Oncology, 10(2), p. 256. [CrossRef]
| Target | Inhibitor | Disease Condition | Sample Size | Clinical Phase | Clinical Trial ID* |
|---|---|---|---|---|---|
| PI3K | Idelalisib (CAL-101) | Non-Hodgkin’s Lymphomas | 125 | II | NCT01282424 |
| PI3K | Pictilisib (GDC-0941) | Non-Hodgkin’s Lymphoma | 60 | I | NCT00876122 |
| PI3K | BYL719 (Alpelisib) | Advanced Solid Malignancies | 221 | I | NCT01219699 |
| PI3K | Copanlisib (BAY80-6946) | Advanced Cancer | 57 | I | NCT00962611 |
| PI3K | Duvelisib (IPI-145) | Advanced Hematologic Malignancies | 210 | I | NCT01476657 |
| PI3K | Buparlisib (BKM120) | Relapsed or Refractory Non-Hodgkin Lymphoma | 7 | I | NCT01719250 |
| PI3K | Pilaralisib (XL147) | Endometrial Carcinoma | 67 | II | NCT01013324 |
| Dual PI3K/mTOR | BEZ235 | Advanced Solid Tumors | 33 | I | NCT01343498 |
| Dual PI3K/mTOR | PF-04691502 PF-05212384 |
Recurrent Endometrial Cancer | 67 | II | NCT01420081 |
| Dual PI3K/mTOR | GDC-0980 | Refractory Solid Tumors or Non-Hodgkin’s Lymphoma | 121 | I | NCT00854152 |
| mTOR | AZD8055 | Gliomas | 22 | I | NCT01316809 |
| mTOR | Temsirolimus | Breast Neoplasms | 108 | II | NCT00062751 |
| mTOR | Sirolimus | Advanced Cancers | 40 | I | NCT00707135 |
| mTOR | BI860585 | Advanced and/or Metastatic Solid Tumors | 90 | I | NCT01938846 |
| mTOR | DS-3078a | Advanced Solid Tumors or Lymphomas | 32 | I | NCT01588678 |
| mTOR | GDC-0349 | Advanced or Metastatic Solid Tumors or Non-Hodgkin’s Lymphoma | 10 | I | NCT01356173 |
| mTOR | Sapanisertib(MLN0128) | Advanced Malignancies | 198 | I | NCT01058707 |
| mTOR | CC-223 | Advanced Solid Tumors, Non-Hodgkin Lymphoma or Multiple Myeloma | 226 | I/II | NCT01177397 |
| mTOR | Everolimus | Advanced solid tumors | 30 | II | NCT02201212 |
| mTOR | Ridaforolimus | Refractory or Advanced Malignancies | 147 | I | NCT00112372 |
| mTOR | Deforolimus (AP23573) | Relapsed or Refractory Hematologic Malignancies | 57 | II | NCT00086125 |
| AKT | GSK690693 | Solid Tumors and Lymphoma | 70 | I | NCT00493818 |
| AKT | MK-2206 | Metastatic Neuroendocrine Tumors | 11 | II | NCT01169649 |
| AKT | AZD5363 | Advanced Solid Tumors | 12 | I | NCT03310541 |
| AKT | ARQ092 | Advanced Solid Tumors and Recurrent Malignant Lymphoma | 120 | I | NCT01473095 |
| AKT | BAY1125976 | Neoplasm | 79 | I | NCT01915576 |
| AKT | Perifosine | Recurrent/Progressive Malignant Gliomas | 32 | II | NCT00590954 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





