Submitted:
26 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- Human Papilloma virus and associated cancers
- HPV vaccine and vaccination rates in the US
- HPV disease burden and vaccinations in Tennessee
- HPV vaccination rates by race/ethnicity, insurance coverage, and urbanicity in Tennessee.
- HPV vaccination rates for individuals 27-45 years old (mid-adults)
- Disparities in HPV vaccine health literacy
- Strategies that could be implemented to improve HPV vaccine confidence and uptake in Tennessee
3. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Villiers, E.M. Cross-roads in the classification of papillomaviruses. Virology 2013, 445, 2–10. [Google Scholar] [CrossRef]
- Strauss, M.J.; Shaw, E.W. Crystalline virus-like particles from skin papillomas characterized by intranuclear inclusion bodies. Proceedings of the Society for Experimental Biology and Medicine. Society for Experimental Biology and Medicine (New York, N.Y.) 1949, 72, 46–50. [Google Scholar] [CrossRef]
- zur Hausen, H. Human papillomaviruses and their possible role in squamous cell carcinomas. Current topics in microbiology and immunology 1977, 78, 1–30. [Google Scholar]
- Lehoux, M.; D'Abramo, C.M.; Archambault, J. Molecular mechanisms of human papillomavirus-induced carcinogenesis. Public health genomics 2009, 12, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Burd, E.M. Human papillomavirus and cervical cancer. Clin Microbiol Rev. 2003, 16, 1–17. [Google Scholar] [CrossRef]
- Petca, A.; Borislavschi, A.; Zvanca, M.E.; Petca, R.-C.; Sandru, F.; Dumitrascu, M.C. Non-Sexual HPV Transmission and Role of Vaccination for a Better Future (Review). Exp. Ther. Med. 2020, 20, 186. [Google Scholar]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Valier, M.R.; Fredua, B.; Crowe, S.J.; DeSisto, C.L.; Stokley, S.; Singleton, J.A. (). Vaccination Coverage Among Adolescents Aged 13-17 Years - National Immunization Survey-Teen, United States, 2022. MMWR. Morbidity and mortality weekly report 2023, 72, 912–919. [Google Scholar] [CrossRef] [PubMed]
- ACIP HPV Vaccine Recommendations | CDC [Internet]. 2019 [cited 2021 Jun 12]. Available from: https://www.cdc.gov/vaccines/hcp/acip-recs/vaccspecific/hpv.html.
- Jendoubi-Ferchichi, M.; Satouri, L.; Ghoul, F.; Malek-Mellouli, M.; Derbel, A.M.; Makni, M.K.; Reziga, H.; Baba, A.; Zili, M.; Segondy, M.; et al. Phylogeny and Classification of Human Papillomavirus (HPV)16 and HPV18 Variants Based on E6 and L1 genes in Tunisian Women with Cervical Lesions. Asian Pac. J. Cancer Prev. 2018, 19, 3361–3366. [Google Scholar] [CrossRef]
- McBride, A.A. Human papillomaviruses: Diversity, infection and host interactions. Nat. Microbiol. 2021. [Google Scholar] [CrossRef]
- Wang, J.W.; Roden, R.B. L2, the minor capsid protein of papillomavirus. Virology 2013, 445, 175–86. [Google Scholar] [CrossRef] [PubMed]
- Yim, E.K.; Park, J.S. The role of HPV E6 and E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer research and treatment 2005, 37, 319–324. [Google Scholar] [CrossRef]
- Rusan, M.; Li, Y.Y.; Hammerman, P.S. Genomic landscape of human papillomavirus-associated cancers. Clinical cancer research: an official journal of the American Association for Cancer Research 2015, 21, 2009–2019. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Jones, T.E.; Jiang, W.; Austin, M.; He, Y.; Li, L.; Tong, L.; Wang, C.; Yang, K.; Yin, R.; Zhao, C. Extended human papillomavirus genotype distribution in cervical intraepithelial neoplasia and cancer: Analysis of 40 352 cases from a large academic gynecologic center in China. Journal of medical virology 2023, 95, e28302. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Shi, L.W.; Li, K.; Huang, L.R.; Li, J.B.; Dong, Y.L.; et al. Comparison of the safety and persistence of immunogenicity of bivalent HPV16/18 vaccine in healthy 9-14-year-old and 18–26-year-old Chinese females: A randomized, double-blind, non-inferiority clinical trial. Vaccine 2023, 41, 7212–7219. [Google Scholar] [CrossRef]
- Food and Drug Administration. Prescribing information [package insert]. Gardasil 9 (human papillomavirus 9-valent vaccine, recombinant). Silver Spring, MD: US Department of Health and Human Services, Food and Drug Administration; 2018. https://www.fda. 9006.
- Petrosky, E.; Bocchini, J.A.; Jr Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E.; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR. Morbidity and mortality weekly report 2015, 64, 300–304. [Google Scholar]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; Markowitz, L.E.; Valier, M.R.; Fredua, B.; Crowe, S.J.; DeSisto, C.L.; Stokley, S.; Singleton, J.A. Vaccination Coverage Among Adolescents Aged 13-17 Years - National Immunization Survey-Teen, United States, 2022. MMWR. Morbidity and mortality weekly report 2023, 72, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, A.F.; Delinger, R.L.; Eisenberg, M.C.; Campredon, L.P.; Walline, H.M.; Carey, T.E.; Meza, R. HPV vaccination has not increased sexual activity or accelerated sexual debut in a college-aged cohort of men and women. BMC public health 2019, 19, 821. [Google Scholar] [CrossRef]
- National Foundation for Infectious Diseases https://www.nfid.org/vaccines-save-lives-what-is-driving-vaccine-preventable-disease-outbreaks/ access March 4, 2019.
- U.S. Cancer Statistics Working Group. U.S. Cancer Statistics Data Visualizations Tool, based on 2022 submission data (1999-2020): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute; https://www.cdc.gov/cancer/dataviz, released in June 2023.
- HPV (Human Papillomavirus) Vaccine Requirements for Secondary School https://www.immunize.org/official-guidance/state-policies/vaccine-requirements/hpv-secondary-2024/.
- New Tennessee law requires parental consent for all vaccinations https://www.wbir.com/article/news/health/tennessee-lawadding-vaccine-requirement/51-d6a22096-d206-4c72-a671-0cde8f13fb88 access on July 26, 2023.
- CDC HPV Vaccination Recommendations https://www.cdc.gov/vaccines/vpd/hpv/hcp/recommendations.html#:~:text=HPV%20vaccine%20is%20recommended%20for,not%20adequately%20vaccinated%20when%20younger.accessed November 16, 2021.
- Tennessee HPV Teen Coverage Rate Dashboard: Immunization Coverages rates for 11-17 year olds in Tennessee, as reported by TennIIS for HPV in 2023 https://data.tn.gov/t/Public/views/Teen_Dash_Q2_for_publication/TeenStory?%3Aembed=y&%3Atabs=no&%3Atoolbar=no.
- Tennessee HPV Teen Coverage Rate Dashboard: Immunization Coverages rates for 18-26 year olds in Tennessee, as reported by TennIIS for HPV in 2023 https://data.tn.gov/t/Public/views/Adult_Dash_Q2_for_publication/Adult_Dash?%3Aembed=y&%3Atabs=no&%3Atoolbar=no.
- Sinard, D. HPV Vaccination Roundtable of the Southeast: Tennessee State Profile. HPV Cancer Prevention Program. St. Jude Children’s Research Hospital. https://stjude.scene7.com/is/content/stjude/hpv-seminar-tennessee-042723.
- U.S. FOOD & DRUG. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old (2018). Available online at: https://www.fda.gov/news-events/press-announcements/fda-approves-expanded-use-gardasil-9-include-individuals-27-through-45-years-old. (accessed on 5 December 2023).
- Munoz ˜, N.; Manalastas, R., Jr.; Pitisuttithum, P.; et al. Safety, immunogenicity, and efficacy of quadrivalent human papillomavirus (types 6, 11, 16, 18) recombinant vaccine in women aged 24-45 years: a randomised, double-blind trial. Lancet. 2009, 373, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Castellsagu´e, X.; Munoz ˜, N.; Pitisuttithum, P.; et al. End-of-study safety, immunogenicity, and efficacy of quadrivalent HPV (types 6, 11, 16, 18) recombinant vaccine in adult women 24-45 years of age. Br. J. Cancer 2011, 105, 28–37. [Google Scholar] [CrossRef]
- Meites, E.; Szilagyi, P.G.; Chesson, H.W.; Unger, E.R.; Romero, J.R.; Markowitz, L.E. . Human papillomavirus vaccination for adults: updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2019, 68, 698–702. [Google Scholar] [CrossRef]
- Ellingson, M.K.; Sheikha, H.; Nyhan, K.; Oliveira, C.R.; Niccolai, L.M. Human papillomavirus vaccine effectiveness by age at vaccination: a systematic review. Hum. Vaccin. Immunother. 2023, 19, 2239085. [Google Scholar] [CrossRef]
- Kasting, M.L.; Giuliano, A.R.; Christy, S.M.; Rouse, C.E.; Robertson, S.E.; Thompson, E.L. Human papillomavirus vaccination prevalence among adults aged 19-45 years: an analysis of the 2017 National Health Interview Survey. Am. J. Prev. Med. 2020, 59, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.K.; Mann, A.K.; Lee, D.; et al. Human papillomavirus vaccination trends and disparities in the United States: who is getting left behind? Sex. Transm. Dis. 2021, 48, 714–719. [Google Scholar] [CrossRef]
- Wheldon, C.W.; Eaton, L.A.; Watson, R.J. Predisposing, enabling, and need-related factors associated with human papillomavirus vaccination intentions and uptake among Black and Hispanic sexual and gender diverse adults in the USA. J. Racial Ethn. Health Disparities 2023, 10, 237–243. [Google Scholar] [CrossRef]
- Reiter, P.L.; Bustamante, G.; McRee, A.L. HPV vaccine coverage and acceptability among a national sample of sexual minority women ages 18-45. Vaccine 2020, 38, 4956–4963. [Google Scholar] [CrossRef]
- Chan, J.K.; Mann, A.K.; Lee, D.; et al. Human papillomavirus vaccination trends and disparities in the United States: who is getting left behind? Sex. Transm. Dis. 2021, 48, 714–719. [Google Scholar] [CrossRef]
- Akpan, I.N.; Taskin, T.; Wheldon, C.W.; Rossheim, M.E.; Thompson, E.L. Human papillomavirus vaccination uptake among 27-to-45-year-olds in the United States. Preventive medicine 2024, 182, 107951. [Google Scholar] [CrossRef] [PubMed]
- Pingali, C.; Yankey, D.; Elam-Evans, L.D.; et al. Vaccination coverage among adolescents aged 13–17 years – National Immunization Survey-Teen, United States, 2022. MMWR Morb. Mortal. Wkly Rep. 2023, 72, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Mercer, C.H.; Keeling, M.J. Capturing sexual contact patterns in modelling the spread of sexually transmitted infections: Evidence using Natsal-3. PloS one 2018, 13, e0206501. [Google Scholar] [CrossRef]
- Lewis, R.M.; Markowitz, L.E.; Gargano, J.W.; Steinau, M.; Unger, E.R. Prevalence of Genital Human Papillomavirus Among Sexually Experienced Males and Females Aged 14-59 Years, United States, 2013-2014. The Journal of infectious diseases 2018, 217, 869–877. [Google Scholar] [CrossRef]
- Trottier, H.; Ferreira, S.; Thomann, P.; Costa, M.C.; Sobrinho, J.S.; Prado, J.C.; Rohan, T.E.; Villa, L.L.; Franco, E.L. Human papillomavirus infection and reinfection in adult women: the role of sexual activity and natural immunity. Cancer research 2010, 70, 8569–8577. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, G.; Zhang, Z.; Wang, L.; Wang, D.; Dai, J. Reinfection of Nine-Valent Human Papillomavirus Vaccine Types Among HIV-Negative Men Who Have Sex With Men: A Prospective Cohort Study. Frontiers in public health 2022, 10, 896479. [Google Scholar] [CrossRef] [PubMed]
- Chesson, H.W.; Meites, E.; Ekwueme, D.U.; Saraiya, M.; Markowitz, L.E. Cost-effectiveness of HPV vaccination for adults through age 45 years in the United States: Estimates from a simplified transmission model. Vaccine 2020, 38, 8032–8039. [Google Scholar] [CrossRef]
- Daniels, V.; Prabhu, V.S.; Palmer, C.; Samant, S.; Kothari, S.; Roberts, C.; Elbasha, E. Public health impact and cost-effectiveness of catch-up 9-valent HPV vaccination of individuals through age 45 years in the United States. Human vaccines & immunotherapeutics 2021, 17, 1943–1951. [Google Scholar]
- Laprise, J.F.; Chesson, H.W.; Markowitz, L.E.; Drolet, M.; Martin, D.; Bénard, É.; Brisson, M. Effectiveness and Cost-Effectiveness of Human Papillomavirus Vaccination Through Age 45 Years in the United States. Annals of internal medicine 2020, 172, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J.; Simms, K.T.; Killen, J.; Smith, M.A.; Burger, E.A.; Sy, S.; Regan, C.; Canfell, K. Human papillomavirus vaccination for adults aged 30 to 45 years in the United States: A cost-effectiveness analysis. PLoS medicine 2021, 18, e1003534. [Google Scholar] [CrossRef]
- Hurley, L.P.; O’Leary, S.T.; Markowitz, L.E.; et al. US primary care physicians’viewpoints on HPV vaccination for adults 27 to 45 years. J. Am. Board Fam. Med. 2021, 34, 162–170. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Kengwoung-Keumo, J.J.; Eby, W.M.; Bae, S.; Guemmegne, J.T.; Manne, U.; Fouad, M.; Partridge, E.E.; Singh, K.P. Disparities in cervical cancer mortality rates as determined by the longitudinal hyperbolastic mixed-effects type II model. PloS one 2014, 9, e107242. [Google Scholar] [CrossRef]
- Hirth, J. Disparities in HPV vaccination rates and HPV prevalence in the United States: a review of the literature. Human vaccines & immunotherapeutics 2019, 15, 146–155. [Google Scholar]
- Morales, C.G.; Jimenez, N.R.; Herbst-Kralovetz, M.M.; Lee, N.R. Novel Vaccine Strategies and Factors to Consider in Addressing Health Disparities of HPV Infection and Morales, C.G., Jimenez, N.R., Herbst-Kralovetz, M.M., & Lee, N.R. Novel Vaccine Strategies and Factors to Consider in Addressing Health Disparities of HPV Infection and Cervical Cancer Development among Native American Women. Medical sciences (Basel, Switzerland). 2022, 10, 52. [Google Scholar]
- Baliga, S.; Mitchell, D.; Yildiz, V.O.; Gogineni, E.; Konieczkowski, D.J.; Grecula, J.; Blakaj, D.M.; Liu, X.; Gamez, M.E. Disparities in survival outcomes among Black patients with HPV-associated oropharyngeal cancer. Journal of medical virology 2023, 95, e28448. [Google Scholar] [CrossRef] [PubMed]
- Le, D.; Kim, H.J.; Wen, K.Y.; Juon, H.S. Disparities in awareness of the HPV vaccine and HPV-associated cancers among racial/ethnic minority populations: 2018 HINTS. Ethnicity & health 2023, 28, 586–600. [Google Scholar]
- Adjei Boakye, E.; Tobo, B.B.; Rojek, R.P.; Mohammed, K.A.; Geneus, C.J.; Osazuwa-Peters, N. Approaching a decade since HPV vaccine licensure: Racial and gender disparities in knowledge and awareness of HPV and HPV vaccine. Human vaccines & immunotherapeutics 2017, 13, 2713–2722. [Google Scholar]
- King, L.M.; Lewnard, J.A.; Niccolai, L.M. Clinical and Public Health Considerations for HPV Vaccination in Midadulthood: A Narrative Review. Open forum infectious diseases 2023, 10, ofad004. [Google Scholar] [CrossRef]
- Thompson, E.L.; Rosen, B.L.; Maness, S.B. Social Determinants of Health and Human Papillomavirus Vaccination Among Young Adults, National Health Interview Survey 2016. Journal of community health 2019, 44, 149–158. [Google Scholar] [CrossRef]
- Brandt, H.M.; Vanderpool, R.C.; Pilar, M.; Zubizarreta, M.; Stradtman, L.R. A narrative review of HPV vaccination interventions in rural U.S. communities. Preventive medicine 2021, 145, 106407. [Google Scholar] [CrossRef]
- Bigaard, J.; Franceschi, S. Vaccination against HPV: boosting coverage and tackling misinformation. Molecular oncology 2021, 15, 770–778. [Google Scholar] [CrossRef]
- Galvin, A.M.; Garg, A.; Griner, S.B.; Moore, J.D.; Thompson, E.L. Health Literacy Correlates to HPV Vaccination Among US Adults Ages 27-45. Journal of cancer education: the official journal of the American Association for Cancer Education 2023, 38, 349–356. [Google Scholar] [CrossRef] [PubMed]
- Zahnd, W.E.; Rodriguez, C.; Jenkins, W.D. Rural-Urban Differences in Human Papillomavirus-associated Cancer Trends and Rates. The Journal of rural health : official journal of the American Rural Health Association and the National Rural Health Care Association 2019, 35, 208–215. [Google Scholar] [CrossRef]
- CDC and Advisory Committee on Immunization Practices: ACIP Shared Clinical Decision-Making Recommendations https://www.cdc.gov/vaccines/acip/acip-scdm-faqs.html accessed September 29, 2023.
- Senkomago, V.; Henley, S.J.; Thomas, C.C.; Mix, J.M.; Markowitz, L.E.; Saraiya, M. Human Papillomavirus-Attributable Cancers - United States, 2012-2016. MMWR. Morbidity and mortality weekly report 2019, 68, 724–728. [Google Scholar] [CrossRef]
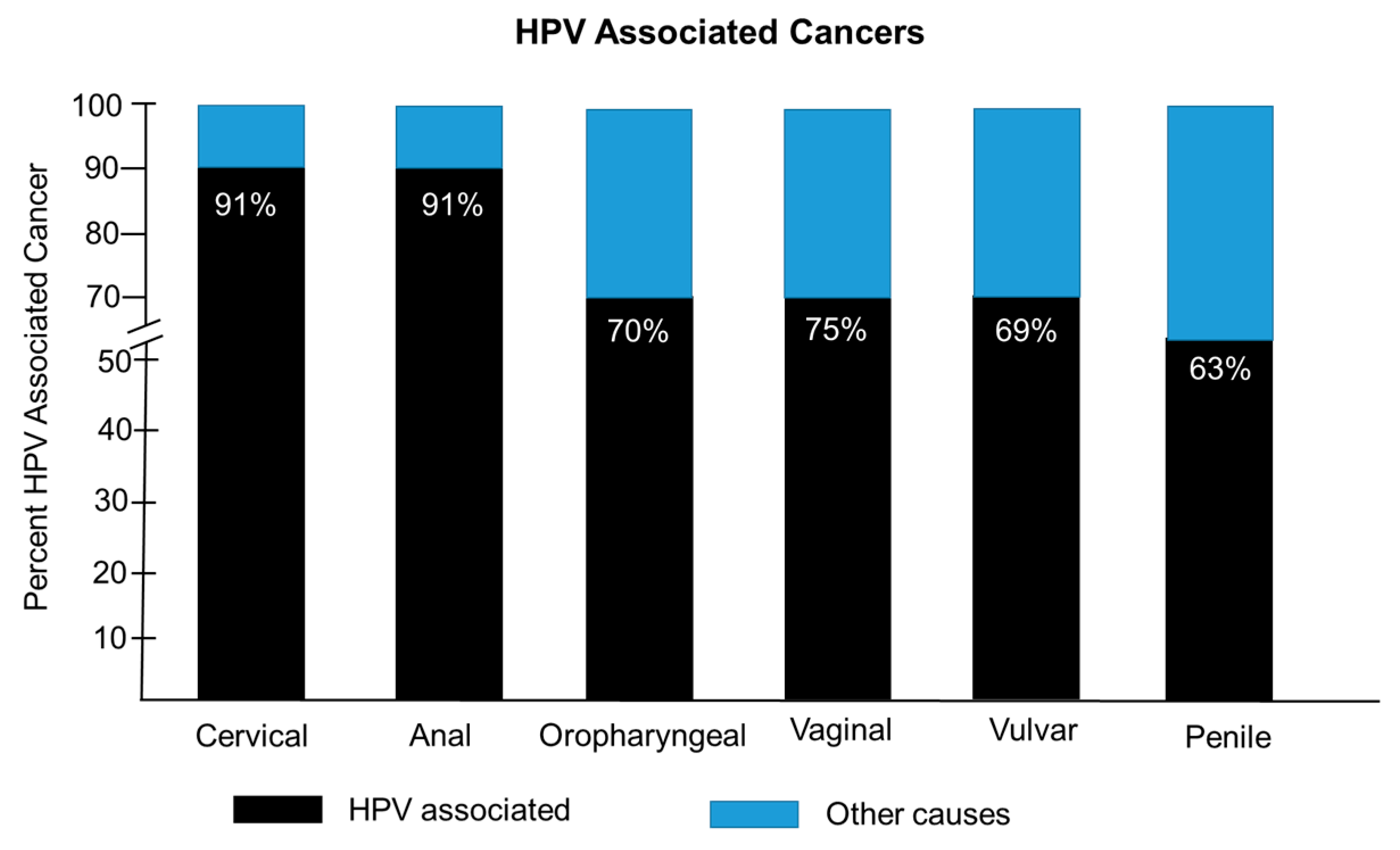





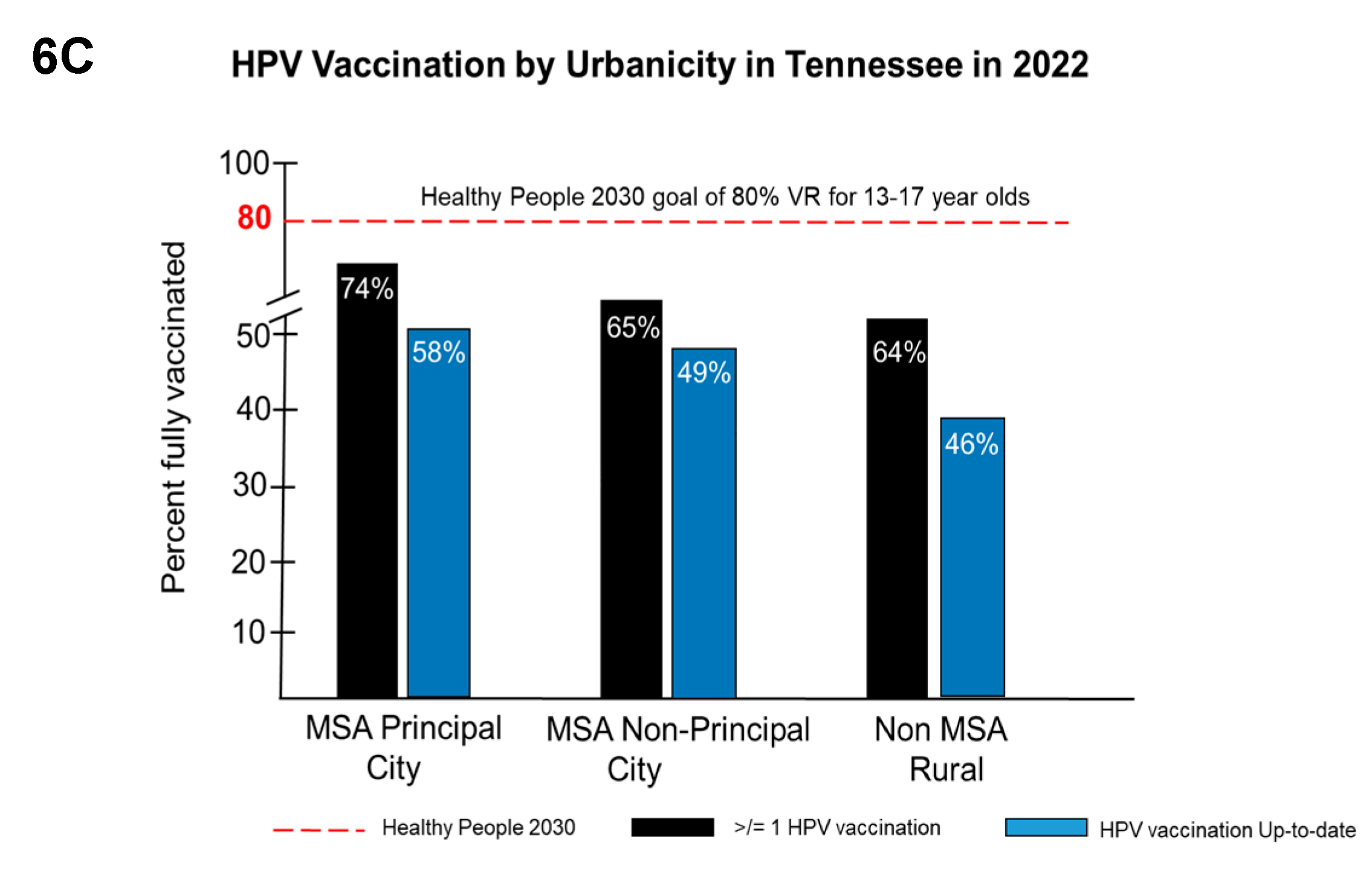

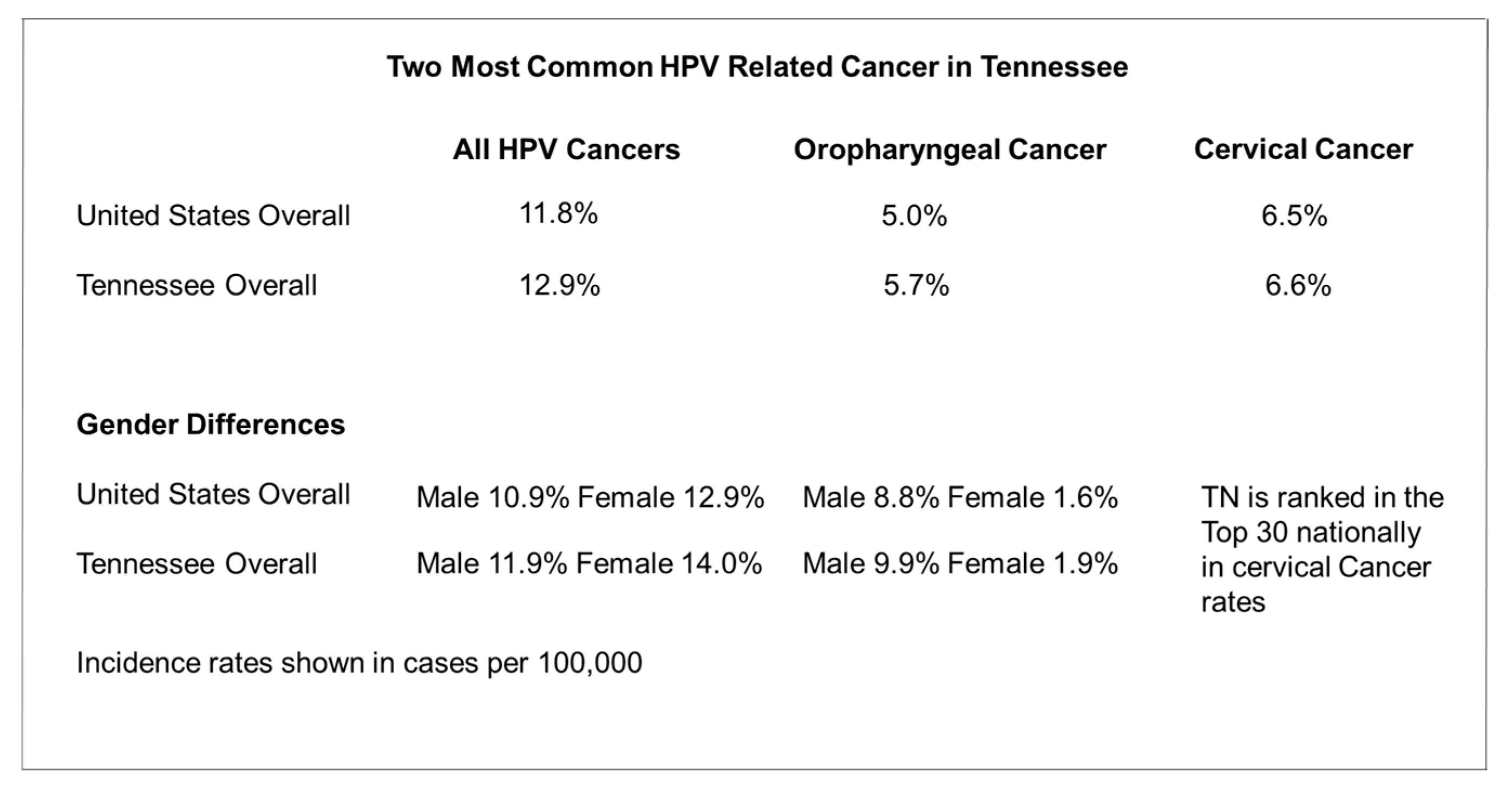 |
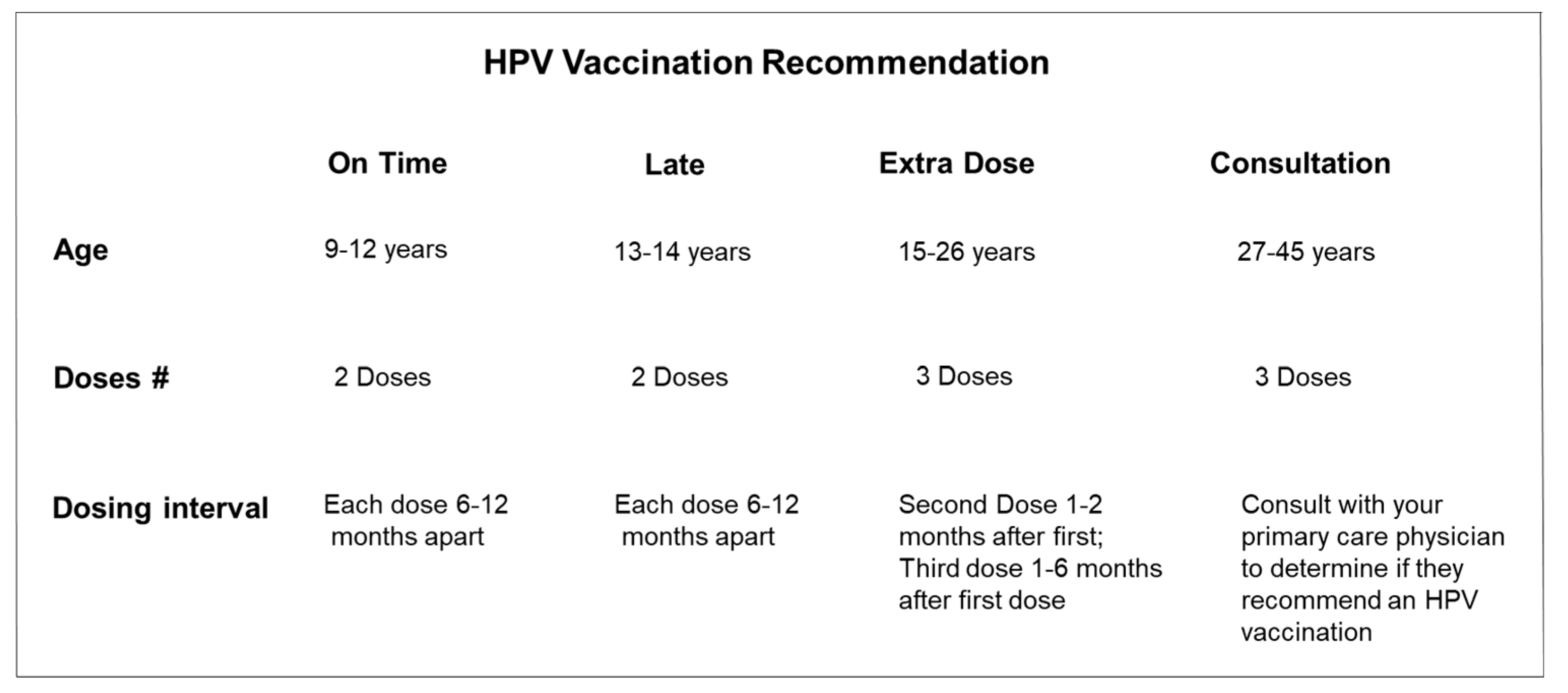 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





