Submitted:
26 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ultra-High Pressure Homogenisation of Musts
2.2. Yeast Strains and Growing Media
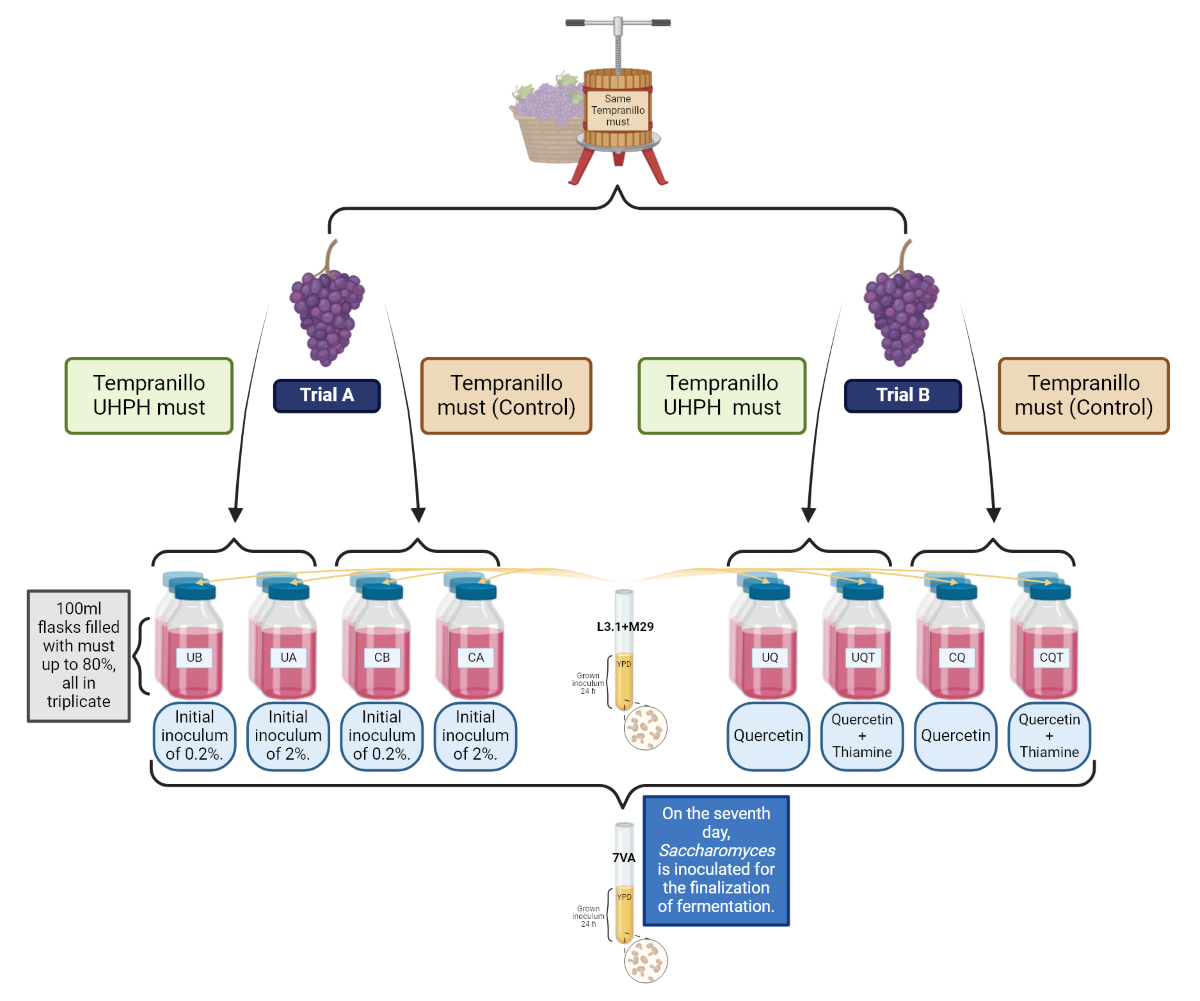
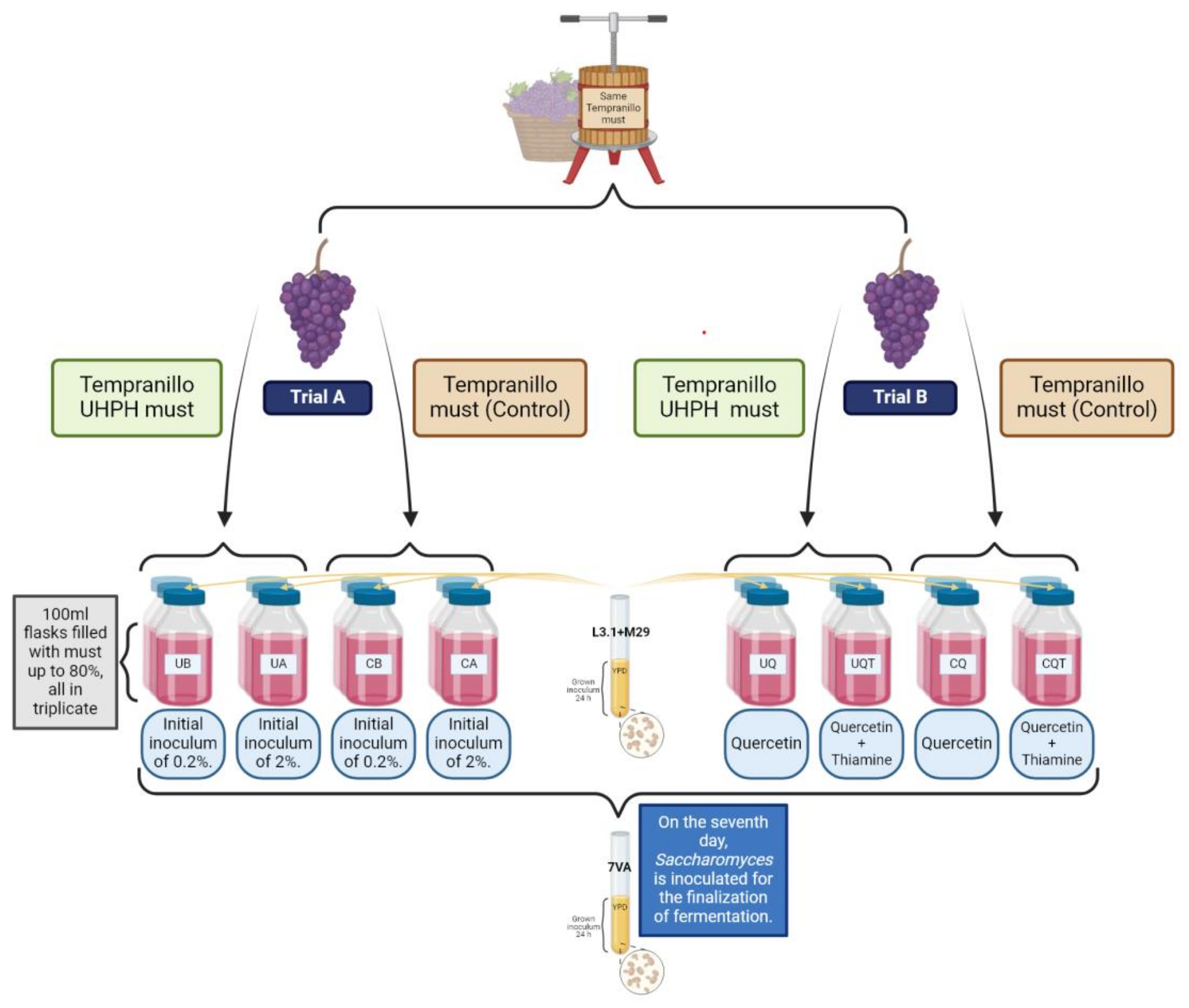
2.3. Micro-Fermentation Trials
2.4. Oenological Parameters
2.5. Aroma Volatile Compounds
2.6. Colour Parameters
2.7. Statistics
3. Results and Discussion
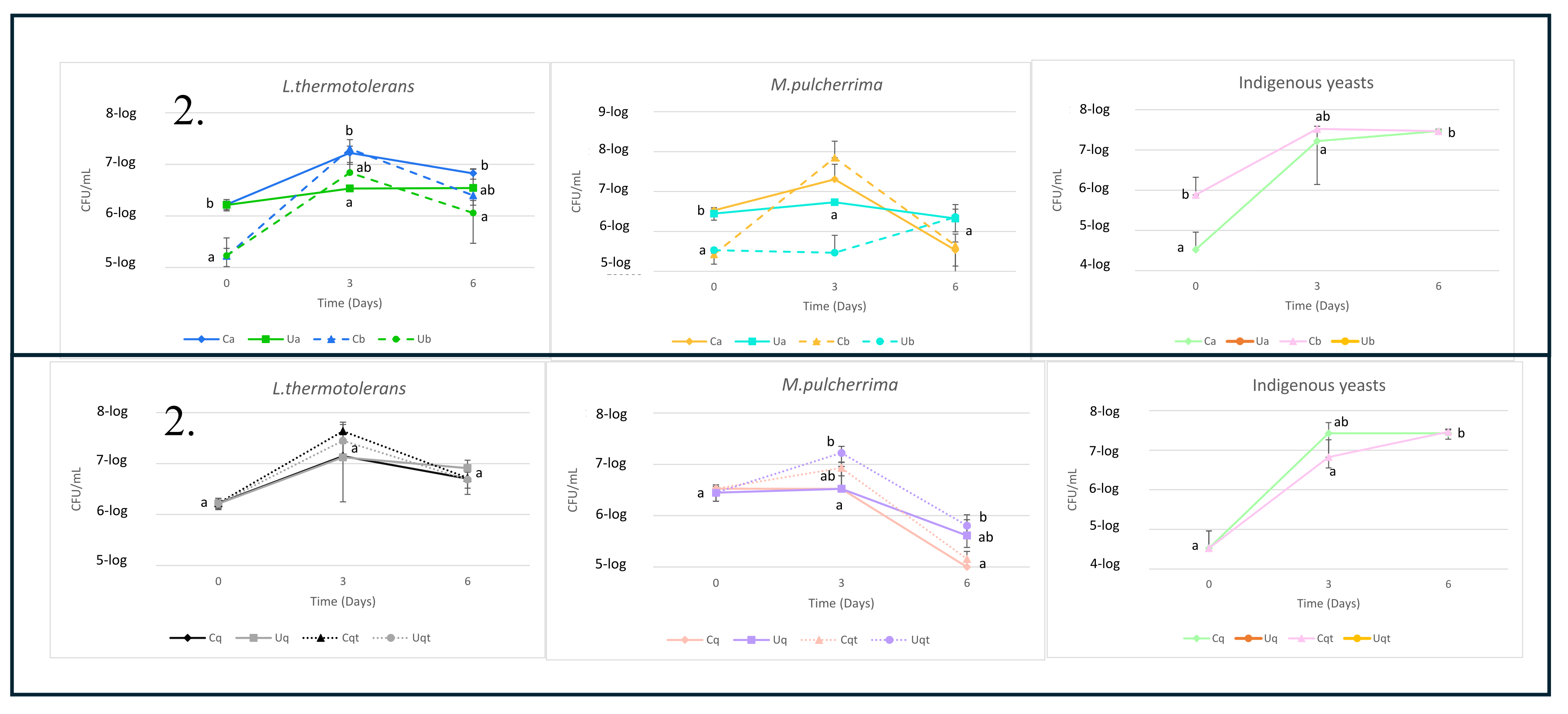
3.1. Evolution of the Inoculated Population
3.2. Oenological Parameters
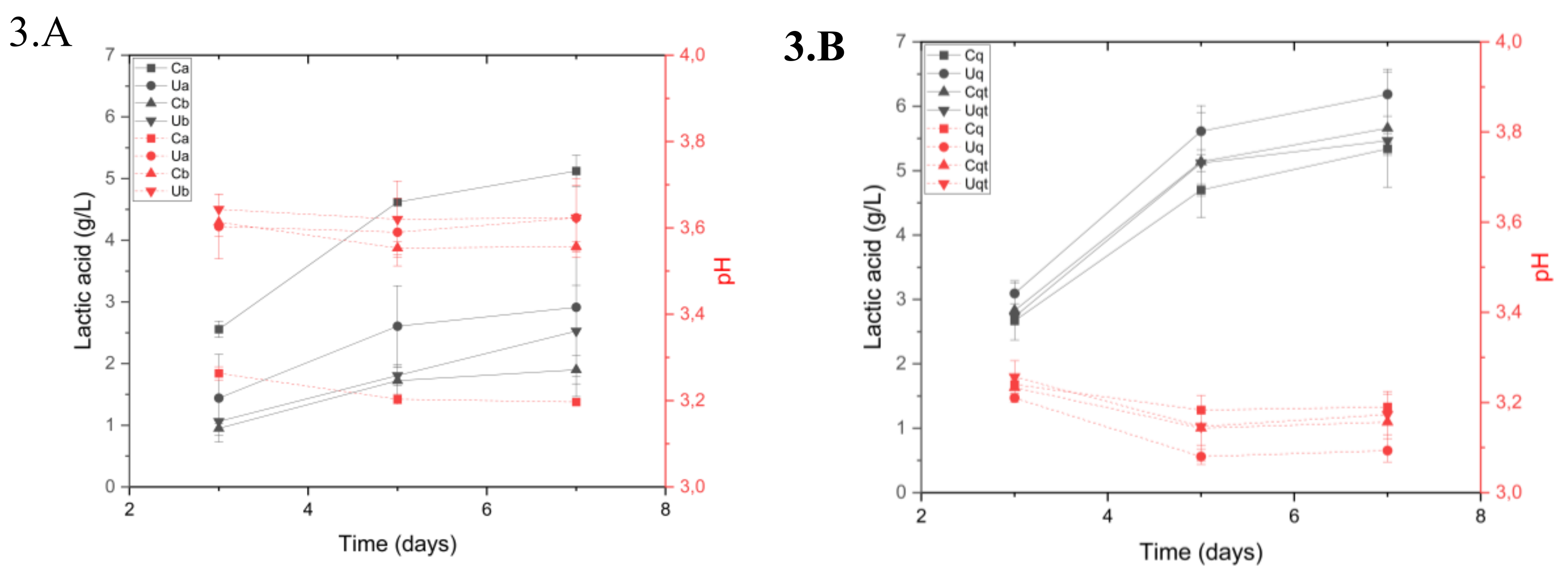
3.3. Lactic Acidity and pH
3.4. Colour Assessment
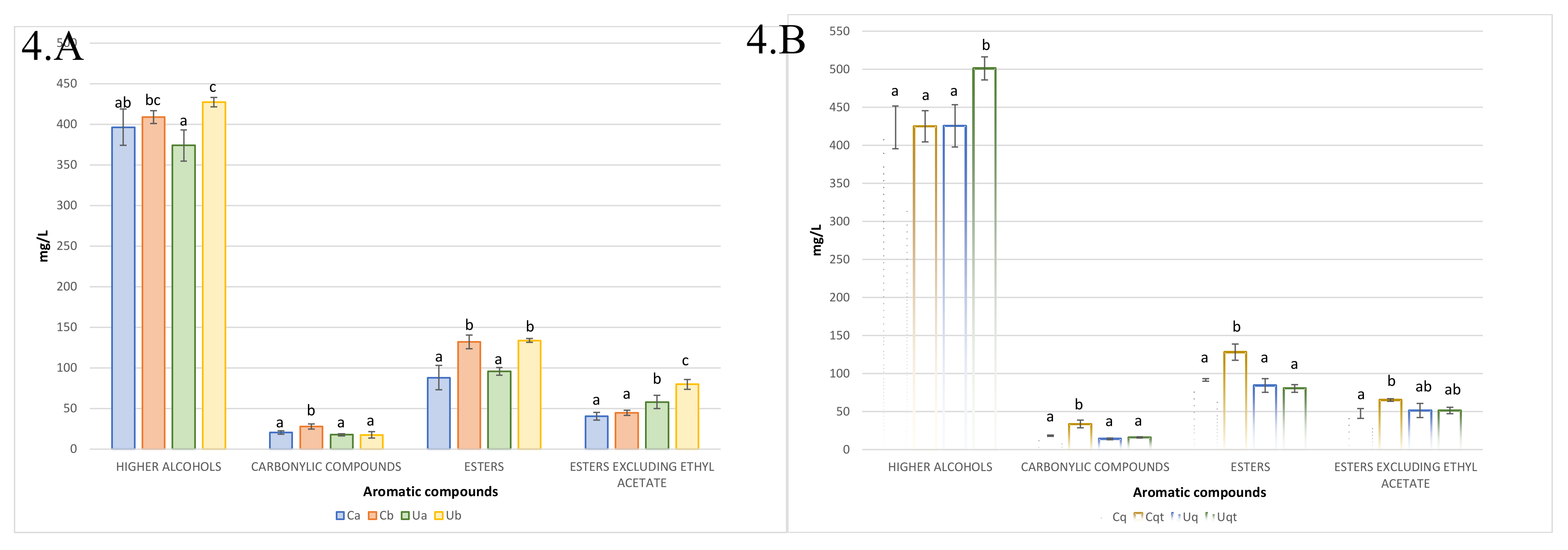
3.5. Aromatic Profile
4. Conclusions
Author Contributions
Funding
References
- Civa V, Chinnici F, Picariello G, Tarabusi E, Bosaro M, Mannazzu I, et al. Non-Saccharomyces yeast derivatives: characterization of novel potential bio-adjuvants for the winemaking process. Curr Res Food Sci [Internet]. 2024 May 22 [cited 2024 May 27];100774. Available online: https://linkinghub.elsevier.com/retrieve/pii/S266592712400100X.
- Yang B, Liu S, Zang H, Dai Y, Zhang S, Lin X, et al. Flavor profile and quality of strawberry wine are improved through sequential fermentation with indigenous non-Saccharomyces yeasts and Saccharomyces cerevisiae. Food Biosci [Internet]. 2024 Jun 1 [cited 2024 May 27];59:104021. Available online: https://linkinghub.elsevier.com/retrieve/pii/S2212429224004516.
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl Microbiol Biotechnol [Internet]. 2016 Dec 27;100(23):9861–74. [CrossRef]
- Jolly NP, Augustyn OPH, Pretorius IS. The Effect of Non-Saccharomyces Yeasts on Fermentation and Wine Quality. South African J Enol Vitic [Internet]. 2003 May [cited 2022 May 28];24(2):55–62. Available online: https://www.journals.ac.za/index.php/sajev/article/view/2638.
- Vaquero C, Loira I, Heras JM, Carrau F, González C, Morata A. Biocompatibility in Ternary Fermentations With Lachancea thermotolerans, Other Non-Saccharomyces and Saccharomyces cerevisiae to Control pH and Improve the Sensory Profile of Wines From Warm Areas. Front Microbiol [Internet]. 2021 Apr 29 [cited 2021 Apr 29];12. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2021.656262/full.
- Sadineni V, Kondapalli N, Obulam VSR. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann Microbiol [Internet]. 2012 Dec 22 [cited 2022 Jun 18];62(4):1353–6. Available online: https://annalsmicrobiology.biomedcentral.com/articles/10.1007/s13213-011-0383-6.
- Vaquero C, Escott C, Heras JM, Carrau F, Morata A. Co-inoculations of Lachancea thermotolerans with different Hanseniaspora spp.: Acidification, aroma, biocompatibility, and effects of nutrients in wine. Food Res Int [Internet]. 2022 Nov 1 [cited 2022 Sep 21];161:111891. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0963996922009498.
- Hranilovic A, Gambetta JM, Schmidtke L, Boss PK, Grbin PR, Masneuf-Pomarede I, et al. Oenological traits of Lachancea thermotolerans show signs of domestication and allopatric differentiation. Sci Rep [Internet]. 2018 Oct 4 [cited 2023 Jan 20];8(1):14812. Available online: https://www.nature.com/articles/s41598-018-33105-7.
- Vaquero C, Loira I, Bañuelos MA, Heras JM, Cuerda R, Morata A. Industrial Performance of Several Lachancea thermotolerans Strains for pH Control in White Wines from Warm Areas. Microorganisms [Internet]. 2020 Jun 1 [cited 2020 Jun 3];8(6):830. Available online: https://www.mdpi.com/2076-2607/8/6/830.
- Zhang M, Zhong T, Heygi F, Wang Z, Du M. Effects of inoculation protocols on aroma profiles and quality of plum wine in mixed culture fermentation of Metschnikowia pulcherrima with Saccharomyces cerevisiae. LWT [Internet]. 2022 May 1 [cited 2024 Aug 20];161:113338. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0023643822002730.
- Kántor A, Hutková J, Petrová J, Hleba L, Kačániová M. Antimicrobial activity of pulcherrimin pigment produced by Metschnikowia pulcherrima against various yeast species. J Microbiol Biotechnol food Sci [Internet]. 2015 Dec 1 [cited 2022 May 30];5(3):282–5. Available online: https://www.researchgate.net/publication/285310898.
- González-Royo E, Pascual O, Kontoudakis N, Esteruelas M, Esteve-Zarzoso B, Mas A, et al. Oenological consequences of sequential inoculation with non-Saccharomyces yeasts (Torulaspora delbrueckii or Metschnikowia pulcherrima) and Saccharomyces cerevisiae in base wine for sparkling wine production. Eur Food Res Technol [Internet]. 2015 May 7;240(5):999–1012. Available online: http://link.springer.com/10.1007/s00217-014-2404-8.
- Sadoudi M, Tourdot-Maréchal R, Rousseaux S, Steyer D, Gallardo-Chacón JJ, Ballester J, et al. Yeast-yeast interactions revealed by aromatic profile analysis of Sauvignon Blanc wine fermented by single or co-culture of non-Saccharomyces and Saccharomyces yeasts. Food Microbiol [Internet]. 2012;32(2):243–53. [CrossRef]
- ŽENIŠOVÁ K, CABICAROVÁ T, SIDARI R, KOLEK E, PANGALLO D, SZEMES T, et al. Effects of co-fermentation with Lachancea thermotolerans or Metschnikowia pulcherrima on concentration of aroma compounds in Pinot Blanc wine. Food Nutr Res [Internet]. 2021 [cited 2024 May 30];60(1):87. Available online: https://openurl.ebsco.com/EPDB%3Agcd%3A2%3A21148786/detailv2?sid=ebsco%3Aplink%3Ascholar&id=ebsco%3Agcd%3A149520655&crl=c.
- Vaquero C, Escott C, Loira I, Guamis B, del Fresno JM, Quevedo JM, et al. Cabernet Sauvignon Red Must Processing by UHPH to Produce Wine Without SO2: the Colloidal Structure, Microbial and Oxidation Control, Colour Protection and Sensory Quality of the Wine. Food Bioprocess Technol [Internet]. 2022 Mar 4 [cited 2022 Nov 4];15(3):620–34. Available online: https://link.springer.com/article/10.1007/s11947-022-02766-8.
- Escott C, Vaquero C, del Fresno JM, Bañuelos MA, Loira I, Han S, et al. Pulsed Light Effect in Red Grape Quality and Fermentation. Food Bioprocess Technol [Internet]. 2017 Aug 5 [cited 2021 Sep 6];10(8):1540–7. Available online: https://link.springer.com/article/10.1007/s11947-017-1921-4.
- Vaquero C, Loira I, Raso J, Álvarez I, Delso C, Morata A. Pulsed Electric Fields to Improve the Use of Non-Saccharomyces Starters in Red Wines. Foods [Internet]. 2021 Jun 25 [cited 2022 Jan 11];10(7):1472. Available online: https://www.mdpi.com/2304-8158/10/7/1472/htm.
- Bañuelos MA, Loira I, Escott C, Del Fresno JM, Morata A, Sanz PD, et al. Grape processing by high hydrostatic pressure: Effect on use of non-Saccharomyces in must fermentation. Food Bioprocess Technol [Internet]. 2016;9(10):1769–78. [CrossRef]
- Bañuelos MA, Loira I, Guamis B, Escott C, Del Fresno JM, Codina-Torrella I, et al. White wine processing by UHPH without SO2. Elimination of microbial populations and effect in oxidative enzymes, colloidal stability and sensory quality. Food Chem. 2020 Dec 1;332:127417.
- Morata A, Loira I, Bañuelos MA, Puig-Pujol A, Guamis B, González C, et al. Use of Ultra High Pressure Homogenization to sterilize grape must. Roca P, editor. BIO Web Conf [Internet]. 2019 Oct 23;15:02035. Available online: https://www.bio-conferences.org/10.1051/bioconf/20191502035.
- Sadineni V, Kondapalli N, Obulam VSR. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann Microbiol [Internet]. 2012 Dec 22 [cited 2024 Jun 12];62(4):1353–60. Available online: https://link.springer.com/articles/10.1007/s13213-011-0383-6.
- Puškaš VS, Miljić UD, Djuran JJ, Vučurović VM. The aptitude of commercial yeast strains for lowering the ethanol content of wine. Food Sci Nutr [Internet]. 2020 Mar 1 [cited 2024 Aug 16];8(3):1489–98. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/fsn3.1433.
- Escott C, Vaquero C, del Fresno JM, Topo A, Comuzzo P, Gonzalez C, et al. Effect of processing Verdejo grape must by UHPH using non- Saccharomyces yeasts in the absence of SO2. Sustain Food Technol [Internet]. 2024 Mar 21 [cited 2024 Aug 17];2(2):437–46. Available online: https://pubs.rsc.org/en/content/articlehtml/2024/fb/d3fb00226h.
- Labuschagne P, Divol B. Thiamine: a key nutrient for yeasts during wine alcoholic fermentation. Appl Microbiol Biotechnol [Internet]. 2021 Feb 6 [cited 2023 Jan 20];105(3):953–73. Available online: https://link.springer.com/article/10.1007/s00253-020-11080-2.
- Bayliak MM, Burdylyuk NI, Lushchak VI. Quercetin increases stress resistance in the yeast Saccharomyces cerevisiae not only as an antioxidant. Ann Microbiol [Internet]. 2016 Jun 14 [cited 2024 Aug 17];66(2):569–76. Available online: https://link.springer.com/articles/10.1007/s13213-015-1136-8.
- Ristic R, Hranilovic A, Li S, Longo R, Pham D-T, Qesja B, et al. Alcohol: Integrated strategies to moderate the alcohol content of wines. Wine Vitic J [Internet]. 2016;31(5):33–8. Available online: https://search.informit.org/doi/10.3316/informit.511380340406896.
- Ivit NN, Longo R, Kemp B. The Effect of Non-Saccharomyces and Saccharomyces Non-Cerevisiae Yeasts on Ethanol and Glycerol Levels in Wine. Fermentation [Internet]. 2020 Jul 30 [cited 2022 Jun 3];6(3):77. Available online: https://www.mdpi.com/2311-5637/6/3/77/htm.
- Gonzalez R, Quirós M, Morales P. Yeast respiration of sugars by non-Saccharomyces yeast species: A promising and barely explored approach to lowering alcohol content of wines. Trends Food Sci Technol. 2013;29(1):55–61.
- Gobbi M, Comitini F, Domizio P, Romani C, Lencioni L, Mannazzu I, et al. Lachancea thermotolerans and Saccharomyces cerevisiae in simultaneous and sequential co-fermentation: A strategy to enhance acidity and improve the overall quality of wine. Food Microbiol [Internet]. 2013;33(2):271–81. [CrossRef]
- Binati RL, Innocente G, Gatto V, Celebrin A, Polo M, Felis GE, et al. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res Int [Internet]. 2019;122:432–42. [CrossRef]
- Vaquero C, Escott C, Heras JM, Carrau F, Morata A. Co-inoculations of Lachancea thermotolerans with different Hanseniaspora spp.: Acidification, aroma, biocompatibility, and effects of nutrients in wine. Food Res Int [Internet]. 2022 Nov 4 [cited 2022 Mar 1];161:111891. Available online: https://link.springer.com/article/10.1007/s11947-022-02766-8.
- Escott C, Vaquero C, Loira I, López C, González C, Morata A. Synergetic Effect of Metschnikowia pulcherrima and Lachancea thermotolerans in Acidification and Aroma Compounds in Airén Wines. Foods [Internet]. 2022 Nov 21 [cited 2022 Nov 22];11(22):3734. Available online: https://www.mdpi.com/2304-8158/11/22/3734/htm.
- Moreira, PF, Giestas L, Yihwa C, Vautier-Giongo C, Quina FH, Maçanita AL, et al. Ground- and Excited-State Proton Transfer in Anthocyanins: From Weak Acids to Superphotoacids. J Phys Chem A [Internet]. 2003 May 1 [cited 2024 Aug 22];107(21):4203–10. Available online: https://pubs.acs.org/doi/abs/10.1021/jp027260i.
- Jiang X, Lu Y, Liu SQ. Effects of different yeasts on physicochemical and oenological properties of red dragon fruit wine fermented with Saccharomyces cerevisiae, Torulaspora delbrueckii and Lachancea thermotolerans. Microorganisms. 2020;8(3):1–14.
- Lee SB, Banda C, Park HD. Effect of inoculation strategy of non-Saccharomyces yeasts on fermentation characteristics and volatile higher alcohols and esters in Campbell Early wines. Aust J Grape Wine Res. 2019;25(4):384–95.
- Saberi S, Cliff MA, van Vuuren HJJ. Impact of mixed S. cerevisiae strains on the production of volatiles and estimated sensory profiles of Chardonnay wines. Food Res Int [Internet]. 2012;48(2):725–35. [CrossRef]
- Antón-Díaz MJ, Suárez Valles B, Mangas-Alonso JJ, Fernández-García O, Picinelli-Lobo A. Impact of different techniques involving contact with lees on the volatile composition of cider. Food Chem [Internet]. 2016 Jan;190:1116–22. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814615008961.
- González Álvarez M, González-Barreiro C, Cancho-Grande B, Simal-Gándara J. Relationships between Godello white wine sensory properties and its aromatic fingerprinting obtained by GC–MS. Food Chem [Internet]. 2011 Dec;129(3):890–8. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0308814611007291.
- Carrau FM, Medina K, Farina L, Boido E, Henschke PA, Dellacassa E. Production of fermentation aroma compounds by Saccharomyces cerevisiae wine yeasts: effects of yeast assimilable nitrogen on two model strains. FEMS Yeast Res [Internet]. 2008 Nov;8(7):1196–207. Available online: https://academic.oup.com/femsyr/article-lookup/doi/10.1111/j.1567-1364.2008.00412.x.
- Rogerson FSS, Castro H, Fortunato N, Azevedo Z, Macedo A, De Freitas VAP. Chemicals with Sweet Aroma Descriptors Found in Portuguese Wines from the Douro Region: 2,6,6-Trimethylcyclohex-2-ene-1,4-dione and Diacetyl. J Agric Food Chem [Internet]. 2001 Jan 1;49(1):263–9. Available online: https://pubs.acs.org/doi/10.1021/jf000948c.
- Peinado RA, Moreno J, Medina M, Mauricio JC. Changes in volatile compounds and aromatic series in sherry wine with high gluconic acid levels subjected to aging by submerged flor yeast cultures. Biotechnol Lett [Internet]. 2004 May;26(9):757–62. Available online: http://link.springer.com/10.1023/B:BILE.0000024102.58987.de.
- Morata A, Bañuelos MA, Vaquero C, Loira I, Cuerda R, Palomero F, et al. Lachancea thermotolerans as a tool to improve pH in red wines from warm regions. Eur Food Res Technol [Internet]. 2019 Apr 16;245(4):885–94. Available online: http://link.springer.com/10.1007/s00217-019-03229-9.
- Perestrelo R, Silva C, Câmara JS. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules [Internet]. 2019 Aug 21;24(17):3028. Available online: https://www.mdpi.com/1420-3049/24/17/3028.
- Zea L, Serratosa MP, Mérida J, Moyano L. Acetaldehyde as Key Compound for the Authenticity of Sherry Wines: A Study Covering 5 Decades. Compr Rev Food Sci Food Saf [Internet]. 2015 Nov 1 [cited 2024 Jun 17];14(6):681–93. Available online: https://onlinelibrary.wiley.com/doi/full/10.1111/1541-4337.12159.
- Li E, Mira de Orduña R. Acetaldehyde kinetics of enological yeast during alcoholic fermentation in grape must. J Ind Microbiol Biotechnol [Internet]. 2017 Feb 1 [cited 2024 Jun 17];44(2):229–36. [CrossRef]
- Wang S, Li S, Zhao H, Gu P, Chen Y, Zhang B, et al. Acetaldehyde released by Lactobacillus plantarum enhances accumulation of pyranoanthocyanins in wine during malolactic fermentation. Food Res Int [Internet]. 2018 Jun 1 [cited 2024 Jun 17];108:254–63. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0963996918302084.
| Residual sugars (g/L) | Ethanol (% v/v) | Total acidity1 (g/L) | Volatile acidity2 (g/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Trial/Days | 3 | 25 | 3 | 25 | 3 | 25 | 3 | 25 |
| Ca | 154.40 ± 2.23a | 0.00 ± 0.00a | 4.07 ± 0.15c | 12.25 ± 0.32a | 7.65 ± 0.03c | 8.27 ± 0.26c | 0.35 ± 0.01c | 0.44 ± 0.05c |
| Ua | 208.77 ± 6.43c | 2.07 ± 0.90b | 2.03 ± 0.15a | 14.09 ± 0.15c | 5.15 ± 0.46b | 6.41 ± 0.49b | 0.30 ± 0.02b | 0.29 ± 0.02b |
| Cb | 170.77 ± 3.10b | 0.00 ± 0.00a | 3.70 ± 0.26b | 13.52 ± 0.34b | 5.21 ± 0.09b | 5.30 ± 0.11a | 0.28 ± 0.02ab | 0.20 ± 0.02a |
| Ub | 216.30 ± 2.08d | 1.87 ± 0.49b | 1.93 ± 0.06a | 14.54 ± 0.15c | 4.30 ± 0.24a | 6.10 ± 0.52b | 0.26 ± 0.02a | 0.25 ± 0.06ab |
| Residual sugar (g/L) | Ethanol (% v/v) | Total acidity1 (g/L) | Volatile acidity2 (g/L) | |||||
|---|---|---|---|---|---|---|---|---|
| Trial/Days | 3 | 25 | 3 | 25 | 3 | 25 | 3 | 25 |
| Cq | 154.43 ± 4.31a | 0.00 ± 0.00a | 4.17 ± 0.23a | 12.89 ± 0.17a | 7.71 ± 0.13c | 8.36 ± 0.24a | 0.35 ± 0.01a | 0.39 ± 0.03bc |
| Uq | 172.13 ± 4.88a | 0.30 ± 0.17b | 3.60 ± 0.26a | 13.56 ± 0.85a | 7.32 ± 0.13b | 9.20 ± 0.18b | 0.35 ± 0.02a | 0.30 ± 0.02a |
| Cqt | 163.93 ± 18.89a | 0.00 ± 0.00a | 3.67 ± 0.93a | 13.04 ± 0.26a | 7.71 ± 0.13c | 8.52 ± 0.31a | 0.34 ± 0.02a | 0.46 ± 0.07c |
| Uqt | 168.37 ± 2.47a | 0.47 ± 0.25b | 3.90 ± 0.10a | 13.56 ± 0.06a | 7.07 ± 0.07a | 8.74 ± 0.13a | 0.33 ± 0.02a | 0.32 ± 0.03ab |
| COLOR INTENSITY (Absorbance units) | TONALITY (Adimensional) |
TPI (Absorbance unirts) |
||
|---|---|---|---|---|
| Trial A | Ca | 2.13 ± 0.01b | 6.46 ± 0.01a | 15.23 ± 0.25a |
| Ua | 1.78 ± 0.09a | 10.26 ± 0.10c | 17.19 ± 0.72b | |
| Cb | 1.64 ± 0.01a | 7.63 ± 0.01b | 15.76 ± 0.15a | |
| Ub | 1.74 ± 0.01a | 10.39 ± 0.01c | 17.06 ± 0.51b | |
| Trial B | Cq | 2.00 ± 0.05b | 6.53 ± 0.05a | 14.95 ± 0.15a |
| Uq | 1.48 ± 0.05a | 10.22 ± 0.06b | 16.03 ± 0.64ab | |
| Cqt | 2.10 ± 0.03b | 6.52 ± 0.04a | 15.10 ± 0.15a | |
| Uqt | 1.56 ± 0.10a | 10.79 ± 0.13b | 16.40 ± 1.00b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





