Introduction
Corneal allogenic intrastromal ring segments (CAIRS) were introduced by Soosan Jacob1,2 in 2015 as an alternative to intracorneal synthetic ring segment implantation for keratoconus (KC) patients, aiming to reinforce and reshape the corneal surface, reduce high-order aberrations, and improve visual acuity.1,2 This therapy involves the transplantation of allogenic donor cornea arcuate stromal segments (of different shapes and angular degrees of thickness) into the mid-peripheral intrastromal layer through manual or femtosecond laser-assisted methods. CAIRS avoids adverse events common with plastic intrastromal corneal ring segments (ICRS) such as migration, exposure, stromal melt, necrosis, extrusion, neovascularization, infectious keratitis, etc.3,4
Research indicates that CAIRS segments allow the passage of oxygen and nutrients unlike synthetic segments,5,6 promoting better tolerance in the recipient cornea as these are made of allogenic tissue such as donor stroma, which can be easily integrated into the host tissue.1 Being allogenic and less rigid, CAIRS can be suitable for more advanced KC cases with thinner corneas (between 300 and 400 µm) as they do not require the minimum thickness of 400 µm to prevent stromal necrosis and melt.1 CAIRS offers more customizability2,5 in terms of arc length, thickness, optical zone and depth of implantation. Additionally, CAIRS recipients experience less glare compared to patients with synthetic segments due to the absence of reflections.6
To simplify the learning curve, different methods have been reported beyond femtosecond laser-assisted preparation, such as varying extents of dehydration and trypan blue staining, particularly for segment insertion.7-10 Corneal collagen crosslinking (CXL) has been used for 20 years to stop or delay corneal ectasia progression by inducing stromal stiffening, which increases collagen fibers’ covalent bonds and diameter, while reducing interfibrillar spacing.12 CXL also enhances resistance to stromal enzymatic digestion by inhibiting collagenase activity.13-15
This study presents the first pilot ex-vivo study and first in-vivo clinical application of AFXL CAIRS (crosslinked all-femtosecond laser-cut corneal allogenic intracorneal ring segments) using donor human corneas, which were provided by the Veneto Eye-Bank Foundation, Mestre, Italy for experimental use. The aim of the preliminary ex-vivo study was to determine if pre-implantation Riboflavin-UVA induced crosslinking enhances the surgical technique by increasing corneal rigidity, maintaining its shape and thickness and thus facilitating CAIRS insertion into recipent tunnels.
Methods
Phase 1: Ex-Vivo Preclinical Study
After joint approval of the pilot study protocol by the Siena Crosslinking Center, Siena, and Vampolieri Eye Clinic, Acicastello, Catania, Italy, Institutional Review Boards (IRB), six human donor eye-bank corneas, unsuitable for corneal transplant due to low endothelial cell count and polymorphism, were used for the preclinical ex-vivo human study. The corneas were mounted on a single-use testing anterior chamber (Moria, Bourbon, France) and subjected to tomographic analysis with the MS 39 OCT Tomographer (CSO, Florence, Italy) to evaluate total, epithelial and stromal minimum corneal thickness, anterior and posterior curvatures, and aberrations. Three donor corneas (D1, D2, D3) were used for AFXL CAIRS, cut and prepared with the IntraLase™ femtosecond laser (Johnson & Johnson, NJ, USA) and a software the authors (CM, MZ) specifically modified and developed. A raster cut of 9 mm was performed to an adequate depth based on the tomographic characteristics of the recipient cornea, relative to the thickness of the ring to be constructed. This was followed by double spiral cuts at diameters of 6.5 and 8 mm, respectively, to obtain a 1.5 mm wide, 300µm thick (without epithelium), 160° arch length allogenic ring, and specific external and internal inclination angles for trapezoid shape customization were utilized, as shown in
Table 1,
Figure 1.
Preparation of the donor ring was performed from the epithelial side to preserve the integrity of Bowman’s lamina. The epithelium was manually removed by a blunt metal spatula. The allogenic tissue rings were immediately crosslinked before implantation using the Riboflavin-UV-A accelerated crosslinking (ACXL) protocol,10 employing a 0.1% HPMC Riboflavin isotonic solution (Vibex Rapid, Glaukos-Avedro, Burlington, USA) and the new KXL UV-A emitter (Glaukos-Avedro, Burlington, USA).10 All the CAIRS were imbibed by immersion in a riboflavin 0.1% plus HPMC 1% bath for 5 minutes and, after BSS rinsing (10 seconds), irradiated for 3, 6 and 9 minutes with an irradiance of 9mW/5.4J/cm2.
Three donor corneas were used as recipients (R1, R2, R3) of the AFXL CAIRS. The femtosecond laser-created 2 mm diameter and 160° arc length tunnels in the recipients for all corneas. The depth of the ACXL CAIRS implants varied from 50% to 70% to verify the feasibility of ring insertion and the variations in corneal curvature of the recipient corneas. Beyond the all femtosecond laser-cut segment creation, the main objective of the pilot study was to evaluate the effect of pre-insertion crosslinking on the allogenic corneal segments in terms of increased resistance, viscoelastic consistency, ease of insertion into the recipient tunnels, maintaining the planned shape and volume for the purpose of take a further step towards the future surgical technique standardization.
An anterior testing chamber was used for pre-and post-operative tomographic analysis of all corneas. The three corneas with the greatest thicknesses were chosen as donors (D1,D2,D3) for the preparation of the 300 µm (without epithelium),1,5 mm wide, 160° arch legnth CAIRS, while the three thinnest corneas were designated for implantation as recipients. Coincidentally, tomographic analysis revealed the presence of a keratoconus in the thinnest donor cornea. The stiffening effect of ACXL and the riboflavin-induced yellowish coloration renders the allogenic ring stronger, less deformable, and more malleable, improving grip and visibility, as shown in
Figure 2 (b), compared to the non-crosslinked, soft, and dehydrated segments
Figure 2 (a, c).
The addition of crosslinking improved the rigidity and the viscoelastic consistency of the corneal allogenic rings while maintaining the physical characteristics and volume of CAIRS (1.5 mm width, 300µm thickness without epithelium, 160° arch length). This facilitated insertion into the recipient's tunnels and consequently flattened the learning curve of the technique,
Figure 3.
Phase 1 Ex Vivo Preclinical Study Outcomes
The test was successful, independent of the implant depth (50, 60 and 70%), as shown in
Figure 4.
The crosslinked ring maintained its shape and volume (trapezoid, 1.5 mm wide, 300 µm thick) with no observed differences pre- and post-CXL, as shown in
Figure 2(b). In contrast, the non-crosslinked ring was soft, bent easily and lost its original volume and shape,
Figure 2 (a, c).
Crosslinking the segments enhanced manipulation and accurate insertion into the tunnels, significantly improving the surgical maneuver. The process did not alter the thickness and volume of the segment compared to the non-crosslinked, dehydrated segment. Additionally, the yellowish coloration of the crosslinked segment facilitated visibility and positioning,
Figure 3.
The anterior testing chamber allowed for pre-and post-operative tomographic analysis of all corneas. This enabled us to select the 3 thickest samples for the preparation of the 300 µm (without epithelium), 160° arch legnth trapezoid rings, and the three thinnest corneas to receive the implants. Furthermore, the tomographic analysis facilitated decisions on ring thickness, internal and external angle cut inclinations, and recepient cornea implantation depth, as shown in
Figure 5. The recipient corneas with the thinnest thickness and with keratoconus (
Figure 5a) showed corneal profile regularization after ACXL CAIRS implantation, as can be seen from the postoperative scan and differential tangential map (
Figure 5b).
The ex-vivo changes in corneal thickness, maximum corneal curvature (K max) and high-order aberrations (HOAs) values following ACXL CAIRS implantation are reported in
Table 2.
The overall data on corneal thickness (both central and at the implant site) and higher-order aberration (HOA) demonstrate the high potential of this additive technique in keratoconus in the preclinical ex-vivo study. It induces a centripetal tissue shift, similar to a tissue wave, capable of moving over 13 diopters of curvature with a standard deviation of 5.62 D, as shown in
Table 3.
Phase 2: Pilot Clinical Study in Italy
Patient 1: Single ACXL CAIRS Implant
A 51-year-old male intolerant to rigid gas permeable contact lens (RGL CL) with stage II duck-shape asymmetric KC, who had undergone a deep anterior lamellar keratoplasty in the left eye, was scheduled for the first AFXL CAIRS implantation in Italy in his right eye. Preop uncorrected distance visual acuity (UCVA) at 0.1 decimal equivalents (d. eq), and best spectacles corrected visual acuity with 0.3 d. eq. with 3 D sphere = -7cylinder axis 20°. The Italian Pilot study patient received unanimous and joint approval from the Institutional Review Board of the Siena Crosslinking Center, Siena, and the Vampolieri Eye Clinic, Acicastello, Catania, Italy. The patient signed an informed consent form.
This patient was selected for implantation of a single ACXL CAIRS in the right eye according to the MS39 integrated software nomogram.
16-19 This was based on the ICRS Jose F. Alfonso studies
16-19 which had been adapted by the authors personalized nomogram based on the direction of the vector axis in the direction of the apex of the cone, according to preimplant tomographic parameters of the recipient. The relative crosslinking protocol was adapted by the authors based on CAIRS thickness according to M nomogram ACXL.
20 The AFXL CAIRS MZ (Mazzotta-Zagari) nomogram for ACXL CAIRS is displayed in
Table 4.
The segment was prepared by all femtosecond laser-cut procedure from a donor cornea mounted on an artificial anterior chamber whose parameters are displayed in
Table 5.
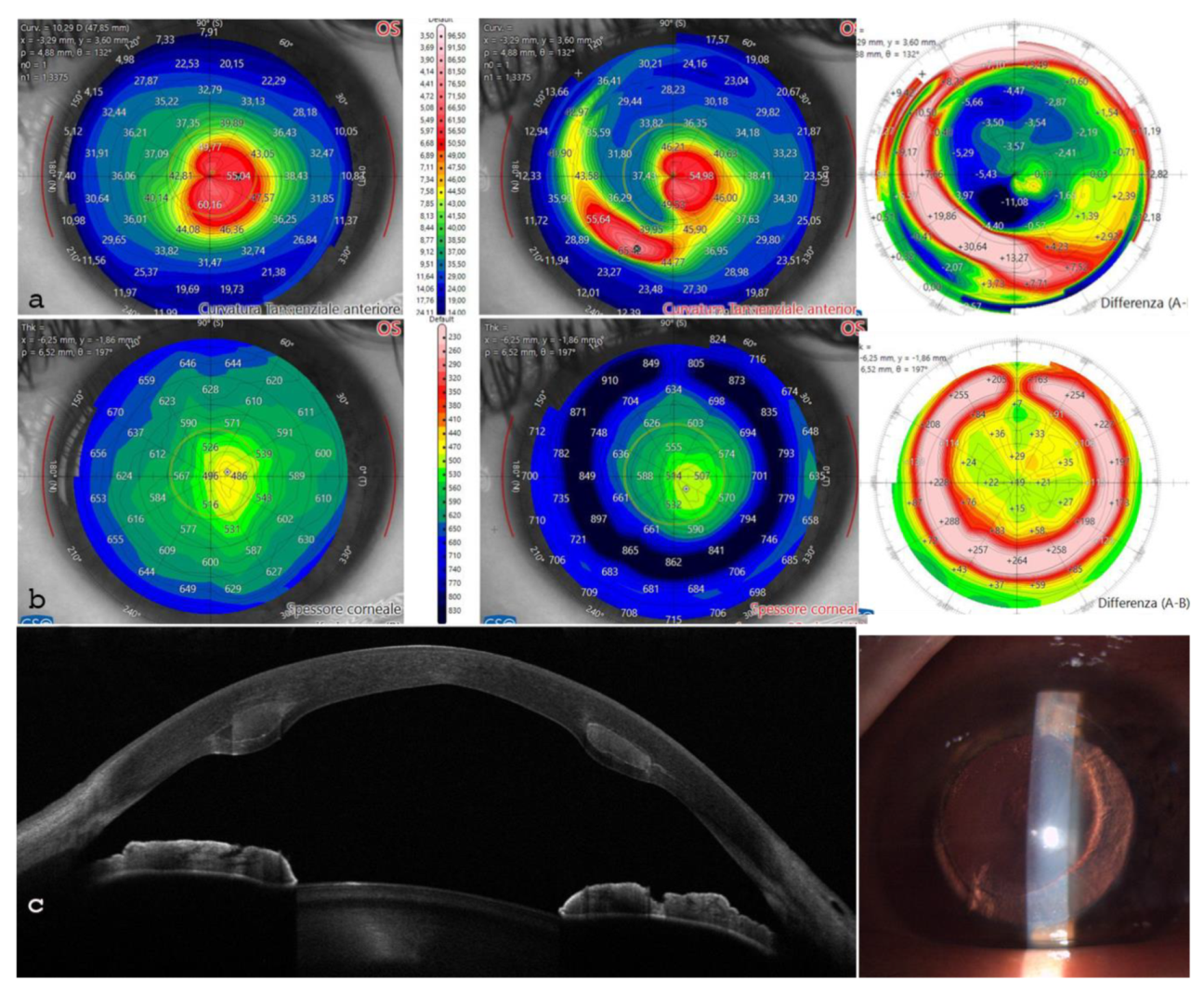
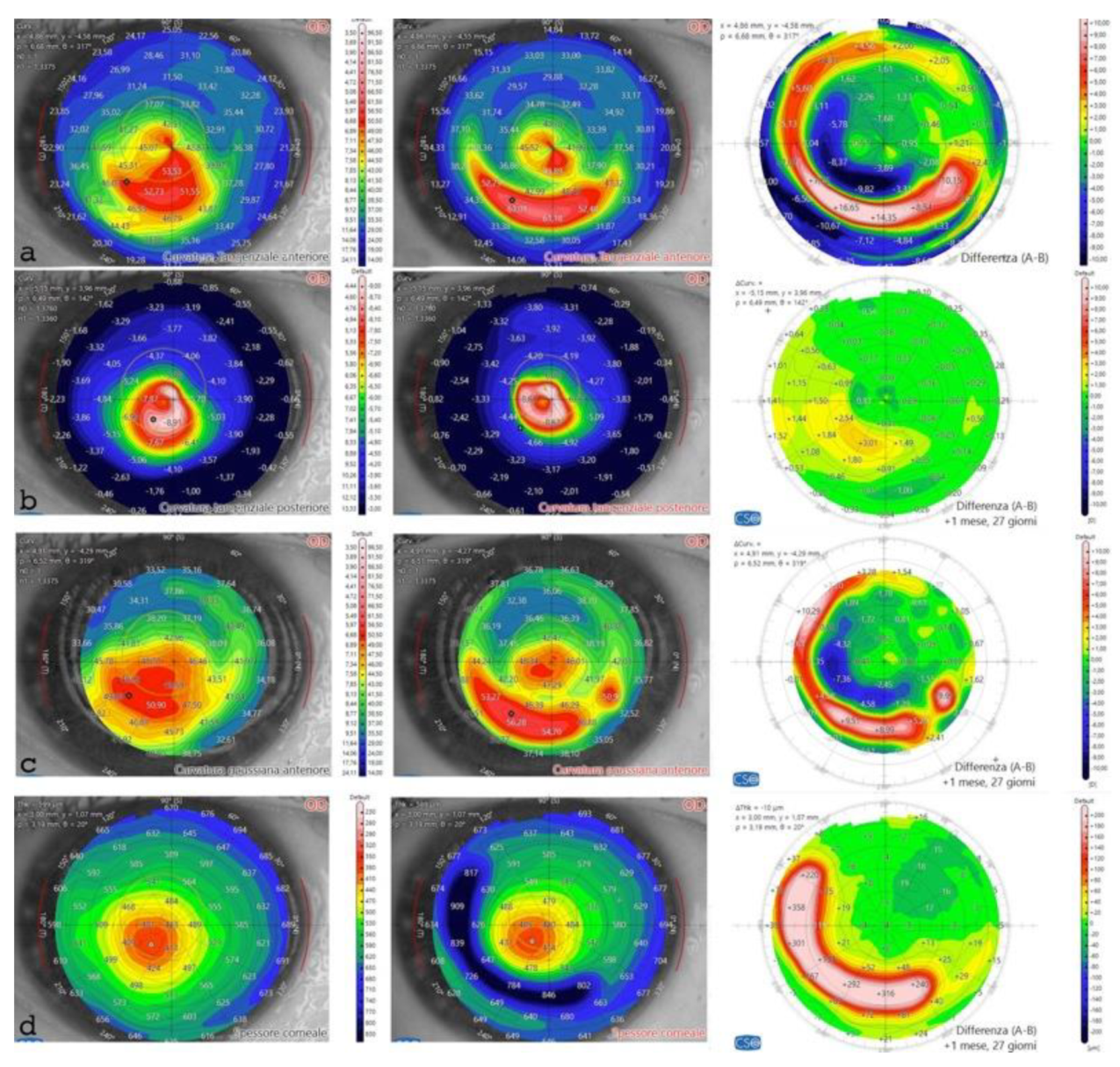
The post-operative analysis revealed the changes in the patient's corneal profile. Specifically, the maximum Sim K value decreased from 50.00 D to 47.6 D. The corneal wavefront analysis showed a reduction in coma aberration, with values decreasing from 1.49 diopters equivalent (D eq.) axis 90° pre-operatively to 0.19 D eq. axis 165° post-operatively. A differential anterior tangential map revealed a flattening of K max, with a reduction from 53.53 D to 49.89 D,
Figure 7 a. The posterior tangential map algorithm showed a significant increase in curvature of +3.01 D,
Figure 7 b.
The anterior Gaussian differential map showed a flattening of the anterior surface by approximately 7.36 D at the steepest point, Figure 7 c. The minimum corneal thickness after ACXL CAIRS tissue addition increased from 362 μm to 378 μm, Figure 7 d.
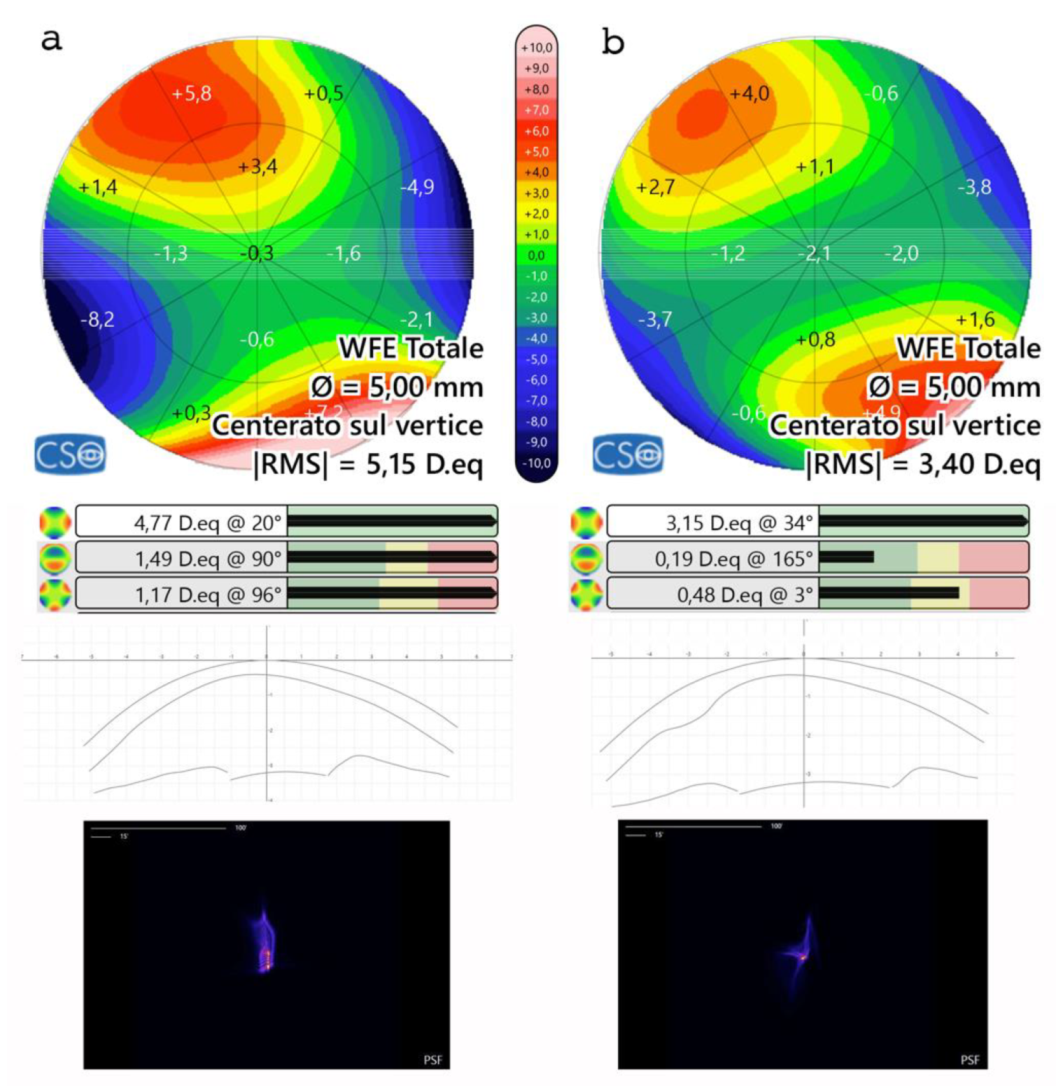
Aberrometric analysis including point spread function (PSF) and Coma values showed a reduction of High Order Aberrations (HOA) with coma and trefoil improvement,
Figure 8. Tomographic astigmatism decreased from 4.77D to 3.15D. Additionally, it is noteworthy that the root mean square (RMS) varies from 5.15 D. eq to 3.40 D. eq,
Figure 8.
The tissue displacement leads to a regularization of the relationship between the anterior and posterior surfaces.
The tissue displacement leads to a regularization of the relationship between the anterior and posterior corneal surfaces. Patient BSCVA passed from 0.3 decimal equivalents (d. eq) with a refraction of -3 sphere = -7cylinder axis 20°to 0.8 d. eq. with a refraction of -2.5 sphere = - 1.5 D cylinder axis 20° and uncorrected distance visual acuity (UDVA) improved from 0.1 to 0.5 d. eq.
Patient 2: Double ACXL CAIRS Implant
The second Italian patient scheduled for a double ACXL CAIRS implant was a 46-year-old male patient with stage IV, central symmetric “bow-tie” KC in the left eye. He underwent a penetrating keratoplasty (PKP) in the right eye 15 years prior. The left eye exhibited an UDVA of 0.1 d eq. and a BSCVA of 0.3 d. eq. 3/10 with -6.50 axis 180°. Pre and postoperative data are displayed in
Table 7.
The patient was selected for an AFCXL CAIRS implantation using a personalized Mazzotta-Zagari ACXL CAIRS Wire-Assisted (WA) push-and-pull surgical technique. This involved the insertion of two customized trapezoid 160° arcs ACXL CAIRS with a thickness of 400 µm, an 80° inner angle and 110° outer angle cut, Bowman’s lamina face-up and a size of 1.5 mm.
The patient achieved a BSCVA of 0.6 d eq. with a refraction of +2 sphere = -5.00 @ 180°. There was a significant reduction in K max from 62.4 to 54 D, accompanied by corneal remodeling and regularization of the tomographic profile. The thinnest point increased from 443 to 486 µm and the Sim K steep values were adjusted from 58.3 to 45.7 D as reported in
Table 7.
Figure 10.
10 a: the anterior tangential topographic map shows corneal flattening and profile regularization. 10 b: the differential pachymetry map indicates the correct placement of the ACXL CAIRS. 10 c.: AS-OCT and slit-lamp images reveal the allogenic segments positioned at 65% planned stromal depth. .
Figure 10.
10 a: the anterior tangential topographic map shows corneal flattening and profile regularization. 10 b: the differential pachymetry map indicates the correct placement of the ACXL CAIRS. 10 c.: AS-OCT and slit-lamp images reveal the allogenic segments positioned at 65% planned stromal depth. .
Conclusions
CAIRS represents a new frontier in the refractive additive therapy of keratoconus, offering the possibility of reducing complications and the demand for plastic rings, and further reducing the need for transplantation as reported in the literature.1,2 One of the most important aspects of this technique is the use of human corneal tissues with low endothelial cell counts, since they are not suitable for elective corneal transplantation they can be utilized for this procedure, thus respecting and utilizing the donation.
The preparation and insertion of the crosslinked CAIRS using all femtosecond laser-cut technique allows for better accuracy and customization by eliminating the difficulties associated with the manual technique. In particular, the addition of pre-implantation accelerated crosslinking could, in our opinion, represent a turning point in the so called AFXL CAIRS technique. This enhancement increases the resistance and viscoelastic consistency of the donor corneal tissue, thus facilitating manipulation and insertion while maintaining the original shape and volume with the planned applanating effect, leading to better efficacy due to their improved strength.
Further, one of the significant benefits of crosslinking the CAIRS lies in its microbicidal activity, which could reduce the chance of post-implantation infectious keratitis.11 However, this aspect requires additional studies. As for the clinical results of the pilot study in Italy with CAIRS and the first international experience with the use of preimplant ACXL, we firmly believe this is a milestone and the crosslinking of allogeneic rings is an added value to be included in the surgical technique in all cases for the purposes of standardization. It would further help mimic the insertion of synthetic segments, therefore making it easier for transitioning surgeons. The remodeling capacity of CAIRS on the shape of irregular corneas, combined with the possibility of implanting the allogenic rings in corneas thinner than 400 µm and with maximum curvatures greater than 55 D up to 75 D, as reported in the literature1,2, significantly broadens the indications of non-replacement therapy of keratoconus. This includes more advanced stages that have historically been reserved for deep anterior lamellar keratoplasty (DALK), or at least rigid gas permeable contact lenses (RGP CL). Furthermore, the possibility of increasing corneal thickness and decreasing warping and aberrations, as this pilot study has demonstrated by showing an increase in corneal thickness in all cases of implantation and a remodeling of the corneal profile with reduction of corneal curvature, high order aberrations and improvement in minimum corneal thickness, can pave the way for future refractive refinement using ray-tracing-based excimer laser corneal remodeling.20 Limitations of the pilot study are represented by the small sample and the short follow-up lacking blinding and controls. However, recent literature and these early results demonstrate the possibility of reshaping the cornea even in more advanced KC cases where excimer laser-guided corneal reshaping and synthetic intrastromal rings are not applicable.
What Was Known
Corneal allogenic intrastromal ring segments (CAIRS) represent a new therapeutic, corneal transplant sparing approach for the treatment of keratoconus.
Corneal allogenic intrastromal ring segments (CAIRS) can be an alternative to synthetic ring segments, eliminating their complications and being implantable in more advanced cases.
All-femtosecond laser-cut crosslinked corneal allogenic intracorneal ring segments (AF-CAIRS) facilitate customization and improve clinical results in corneal reshaping.
What Paper Adds
All femtosecond laser-cut crosslinked corneal allogenic intrastromal ring segments (AFXL CAIRS) further facilitates the manipulation and insertion of the segments, increasing their resistance and viscoelastic consistency, thereby flattening the learning curve of this technique.
Crosslinking of CAIRS can be an added value in the surgical technique, aiding in its future standardization.
Funding
No Funding/Support.
Conflicts of Interest
Authors declare no conflict of interest/nothing to disclose.
References
- Jacob, S.; Patel, S.R.; Agarwal, A.; Ramalingam, A.; Saijimol, A.I.; Raj, J.M. Corneal Allogenic Intrastromal Ring Segments (CAIRS) Combined With Corneal Cross-linking for Keratoconus. J. Refract. Surg. 2018, 34, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Agarwal, A.; Awwad, S.T.; Mazzotta, C.; Parashar, P.; Jambulingam, S. Customized corneal allogenic intrastromal ring segments (CAIRS) for keratoconus with decentered asymmetric cone. Indian J. Ophthalmol. 2023, 71, 3723–3729. [Google Scholar] [CrossRef] [PubMed]
- Coskunseven, E.; Kymionis, G.D.; Tsiklis, N.S.; Atun, S.; Arslan, E.; Siganos, C.S.; Jankov, M.; Pallikaris, I.G. Complications of intrastromal corneal ring segment implantation using a femtosecond laser for channel creation: a survey of 850 eyes with keratoconus. Acta Ophthalmol. 2009, 89, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Bteich, Y.; Assaf, J.F.; Mrad, A.A.; Jacob, S.; Hafezi, F.; Awwad, S.T. Corneal Allogenic Intrastromal Ring Segments (CAIRS) for Corneal Ectasia: A Comprehensive Segmental Tomography Evaluation. J. Refract. Surg. 2023, 39, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Bteich, Y.; Assaf, J.F.; Gendy, J.E.; Müller, F.; Jacob, S.; Hafezi, F.; Awwad, S.T. Asymmetric All-Femtosecond Laser–Cut Corneal Allogenic Intrastromal Ring Segments. J. Refract. Surg. 2023, 39, 856–862. [Google Scholar] [CrossRef] [PubMed]
- Parker JS, Dockery PW, Parker JS. Flattening the curve: Manual method for corneal allogenic intrastromal ring segment implantation. J Cataract Refract Surg 2021;47:e31-3.
- Parker JS, Dockery PW, Jacob S, Parker JS. Preimplantation dehydration for corneal allogenic intrastromal ring segment implantation. J Cataract Refract Surg 2021;47:e37-9.
- Parker JS, Dockery PW, Parker JS. Trypan blue-assisted corneal allogenic intrastromal ring segment implantation. J Cataract Refract Surg 2021;47:127.
- Awwad, S.T.; Jacob, S.; Assaf, J.F.; Bteich, Y. Extended Dehydration of Corneal Allogenic Intrastromal Ring Segments to Facilitate Insertion: The Corneal Jerky Technique. Cornea 2023, 42, 1461–1464. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Raiskup, F.; Hafezi, F.; A Torres-Netto, E.; Balamoun, A.A.; Giannaccare, G.; Bagaglia, S.A. Long term results of accelerated 9 mW corneal crosslinking for early progressive keratoconus: the Siena Eye-Cross Study 2. Eye Vis. 2021, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, D.; Mazzotta, C.; Hafezi, F. PACK-CXL: Corneal cross-linking in infectious keratitis. Eye Vis. 2016, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Spoerl, E.; Seiler, T. Stress-strain measurements of human and porcine corneas after riboflavin–ultraviolet-A-induced cross-linking. J. Cataract. Refract. Surg. 2003, 29, 1780–1785. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, E.; Wollensak, G.; Seiler, T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr. Eye Res. 2004, 29, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Raiskup F, Spoerl E. Corneal crosslinking with riboflavin and ultraviolet A. I. Principles. Ocul Surf. 2013 Apr;11(2):65-74.
- Beshtawi, I.M.; O’donnell, C.; Radhakrishnan, H. Biomechanical properties of corneal tissue after ultraviolet-A–riboflavin crosslinking. J. Cataract. Refract. Surg. 2013, 39, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.F.; Lisa, C.; Fernández-Vega, L.; Madrid-Costa, D.; Montés-Micó, R. Intrastromal corneal ring segment implantation in 219 keratoconic eyes at different stages. Graefe's Arch. Clin. Exp. Ophthalmol. 2011, 249, 1705–1712. [Google Scholar] [CrossRef] [PubMed]
- Cueto, L.F.-V.; Lisa, C.; Madrid-Costa, D.; Merayo-Lloves, J.; Alfonso, J.F. Long-Term Follow-Up of Intrastromal Corneal Ring Segments in Paracentral Keratoconus with Coincident Corneal Keratometric, Comatic, and Refractive Axes: Stability of the Procedure. J. Ophthalmol. 2017, 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vega-Cueto, L.; Lisa, C.; Poo-López, A.; Alfonso, J.F.; Madrid-Costa, D. Three-year follow-up of intrastromal corneal ring segment implantation in central keratoconus with regular astigmatism: 'Bow-tie' shape. Eur. J. Ophthalmol. 2020, 30, 643–649. [Google Scholar] [CrossRef] [PubMed]
- Alfonso, J.F.; Cueto, L.F.-V.; Baamonde, B.; Merayo-Lloves, J.; Madrid-Costa, D.; Montés-Micó, R. Inferior Intrastromal Corneal Ring Segments in Paracentral Keratoconus With No Coincident Topographic and Coma Axis. J. Refract. Surg. 2013, 29, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Mazzotta, C.; Stojanovic, A.; Romano, V.; Addabbo, G.; Borroni, D.; Balamoun, A.A.; Ferrise, M. Ray-Tracing Transepithelial Excimer Laser Central Corneal Remodeling Plus Pachymetry-Guided Accelerated Corneal Crosslinking for Keratoconus. Cornea 2023, 43, 285–294. [Google Scholar] [CrossRef] [PubMed]
Figure 1.
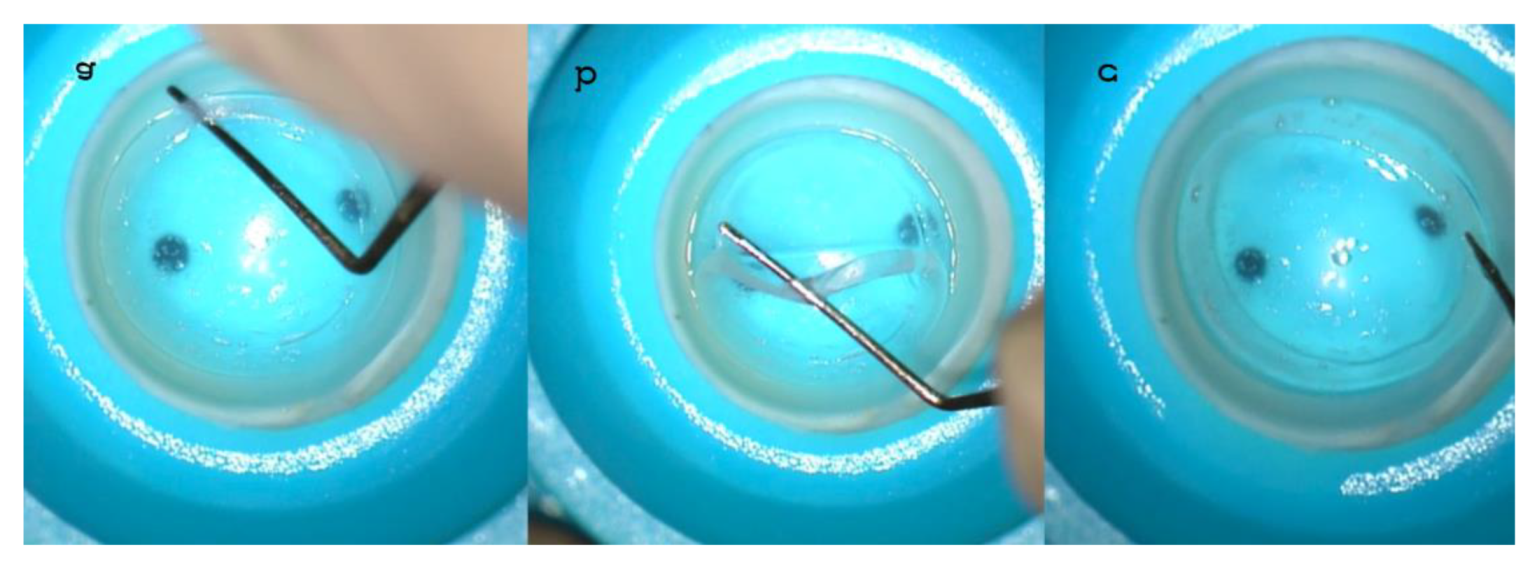
IntraLase™ (Johnson & Johnson, NJ, USA) all-femtosecond laser-cut CAIRS extraction from the donor cornea using a blunt spatula (a, b, c). A raster cut of 9 mm was planned to an adequate depth in the donor tissue in relation to the thickness of the full 360° ring to be constructed and based on the tomographic characteristics of the recipient cornea. This was followed by all femtosecond laser-cut double spiral cuts, 1.5 micro-Joules (μJ) energy at 6.5 and 8 mm in diameters, respectively, with different angles of inclination. In all cases, 1.5 mm wide, 300µm thick (without epithelium), 160° arch length allogenic rings were prepared after full-ring extraction.
Figure 1.
IntraLase™ (Johnson & Johnson, NJ, USA) all-femtosecond laser-cut CAIRS extraction from the donor cornea using a blunt spatula (a, b, c). A raster cut of 9 mm was planned to an adequate depth in the donor tissue in relation to the thickness of the full 360° ring to be constructed and based on the tomographic characteristics of the recipient cornea. This was followed by all femtosecond laser-cut double spiral cuts, 1.5 micro-Joules (μJ) energy at 6.5 and 8 mm in diameters, respectively, with different angles of inclination. In all cases, 1.5 mm wide, 300µm thick (without epithelium), 160° arch length allogenic rings were prepared after full-ring extraction.
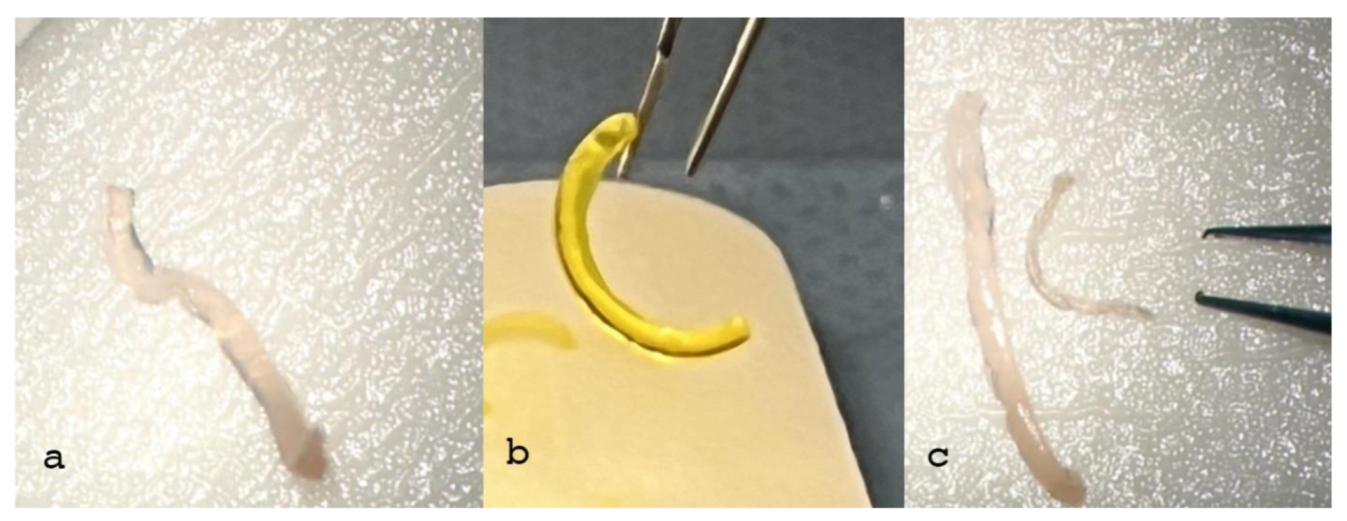
Figure 2.
Non-crosslinked CAIRS (a) demonstrates softness and deformability, resulting in poor consistency, grip and visibility. In contrast, the crosslinked allogenic ring (b) shows better consistency, shape retention (trapezoid in shape, 1.5 mm wide, 300 µm thick) and significantly improved surgical grip for insertion maneuvers. Although ring dehydration (c) increases consistency it reduces ring volume, altering the original shape, and it does not improve visibility which consequently requires additional coloring, potentially resulting in poorer results.
Figure 2.
Non-crosslinked CAIRS (a) demonstrates softness and deformability, resulting in poor consistency, grip and visibility. In contrast, the crosslinked allogenic ring (b) shows better consistency, shape retention (trapezoid in shape, 1.5 mm wide, 300 µm thick) and significantly improved surgical grip for insertion maneuvers. Although ring dehydration (c) increases consistency it reduces ring volume, altering the original shape, and it does not improve visibility which consequently requires additional coloring, potentially resulting in poorer results.
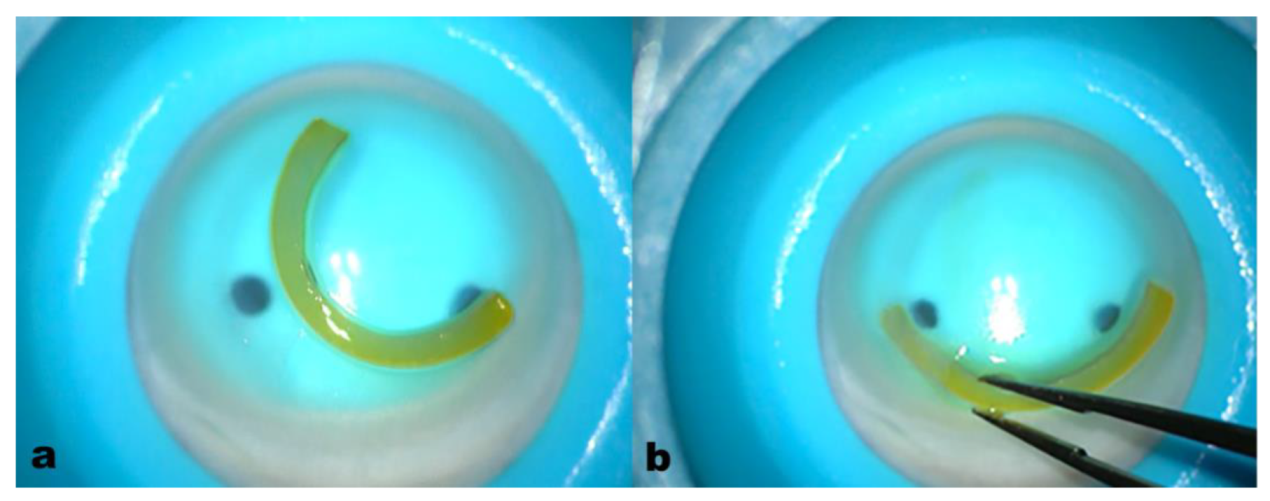
Figure 3.
All femtosecond laser-cut ACXL CAIRS before insertion (a) and during the insertion maneuver (b). The crosslinking of the segments produced stronger tissue with better grip and visibility, facilitating more precise and easier manipulation and insertion into the 2 mm diameter recipient tunnels. This significantly improved the maneuver without altering the thickness and volume of the segment (1.5 mm wide, 300µm thick) and the yellowish riboflavin-induced coloration enhanced AFXL CAIRS visibility without needing additional coloring.
Figure 3.
All femtosecond laser-cut ACXL CAIRS before insertion (a) and during the insertion maneuver (b). The crosslinking of the segments produced stronger tissue with better grip and visibility, facilitating more precise and easier manipulation and insertion into the 2 mm diameter recipient tunnels. This significantly improved the maneuver without altering the thickness and volume of the segment (1.5 mm wide, 300µm thick) and the yellowish riboflavin-induced coloration enhanced AFXL CAIRS visibility without needing additional coloring.
Figure 4.
Recipient corneas with Crosslinked All femtosecond laser-cut CAIRS (AFXL CAIRS) implants at different depths, well-visualized by high-resolution (HR) Anterior Segment OCT Tomography performed with the MS 39 (CSO, Florence, Italy) at 50% (a), 60 % (b) and 70% (c).
Figure 4.
Recipient corneas with Crosslinked All femtosecond laser-cut CAIRS (AFXL CAIRS) implants at different depths, well-visualized by high-resolution (HR) Anterior Segment OCT Tomography performed with the MS 39 (CSO, Florence, Italy) at 50% (a), 60 % (b) and 70% (c).
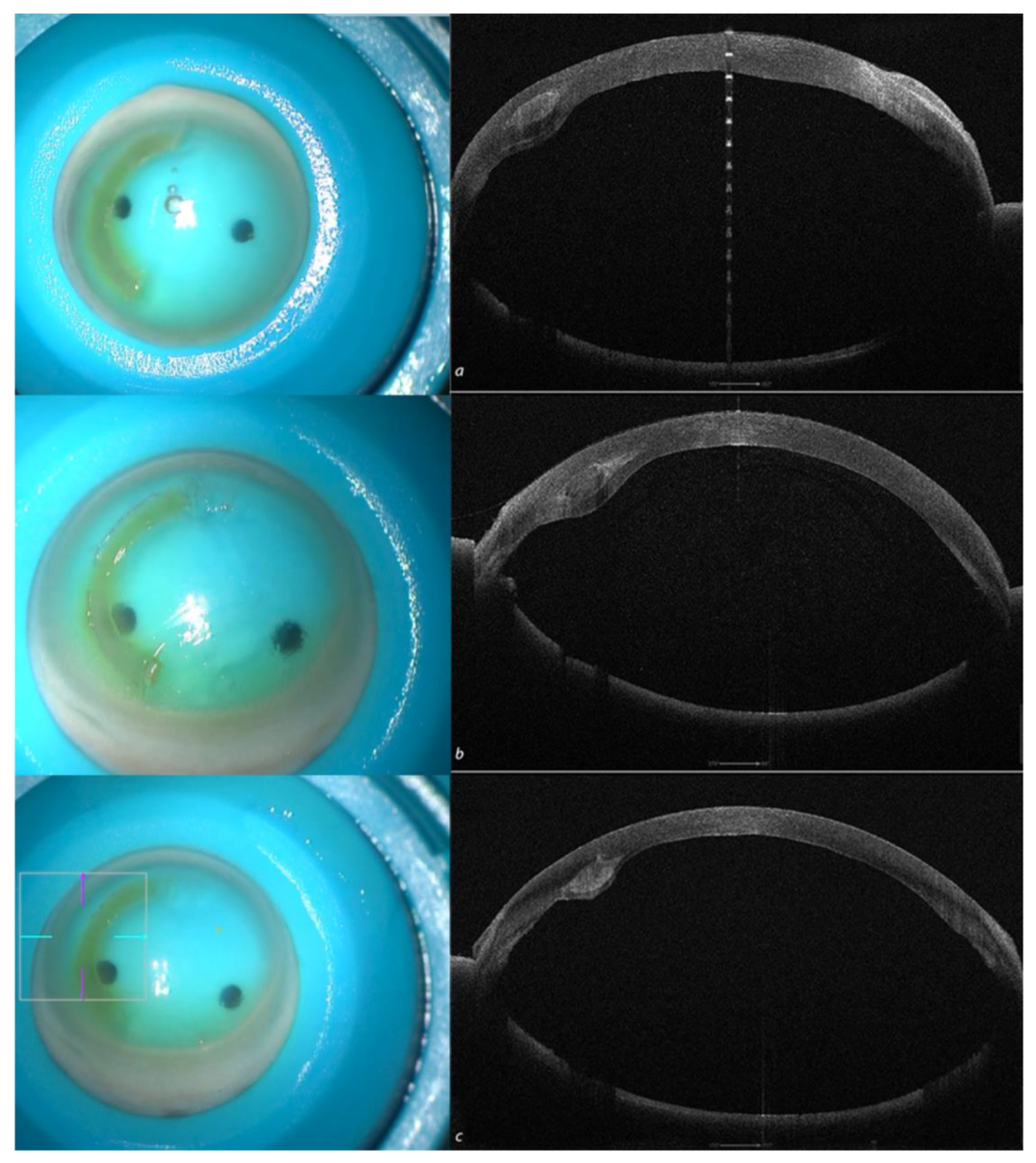
Figure 5.
Human donor corneas were mounted on a single-use testing anterior chamber and subjected to tomographic analysis with the MS 39 OCT Tomographer (CSO, Florence, Italy), as shown in the bottom left image. Incidentally, the tomographic analysis revealed the presence of a Keratoconus (a) in one of the recipient corneas and the implantation of the cross-linked allogenic ring allowed reshaping of the corneal curvature, as documented by the postoperative scan (b) and differential tangential map (c).
Figure 5.
Human donor corneas were mounted on a single-use testing anterior chamber and subjected to tomographic analysis with the MS 39 OCT Tomographer (CSO, Florence, Italy), as shown in the bottom left image. Incidentally, the tomographic analysis revealed the presence of a Keratoconus (a) in one of the recipient corneas and the implantation of the cross-linked allogenic ring allowed reshaping of the corneal curvature, as documented by the postoperative scan (b) and differential tangential map (c).
Figure 6.
Surgical Steps: Preoperative anterior segment (AS) High-Resolution OCT highlighted the regular cuts with pre-set angles in the crosslinked tissue (a). Surgical technique involved a recipient tunnel formation, segment insertion with a “push-and-pull technique” and the geometric alignment along the major coma aberration axis (b). Early postoperative follow-up slit lamp and AS-OCT and high-resolution (HR) images confirmed the correct position of the ACXL CAIRS at the predetermined depth tomographic location and thickness (c).
Figure 6.
Surgical Steps: Preoperative anterior segment (AS) High-Resolution OCT highlighted the regular cuts with pre-set angles in the crosslinked tissue (a). Surgical technique involved a recipient tunnel formation, segment insertion with a “push-and-pull technique” and the geometric alignment along the major coma aberration axis (b). Early postoperative follow-up slit lamp and AS-OCT and high-resolution (HR) images confirmed the correct position of the ACXL CAIRS at the predetermined depth tomographic location and thickness (c).
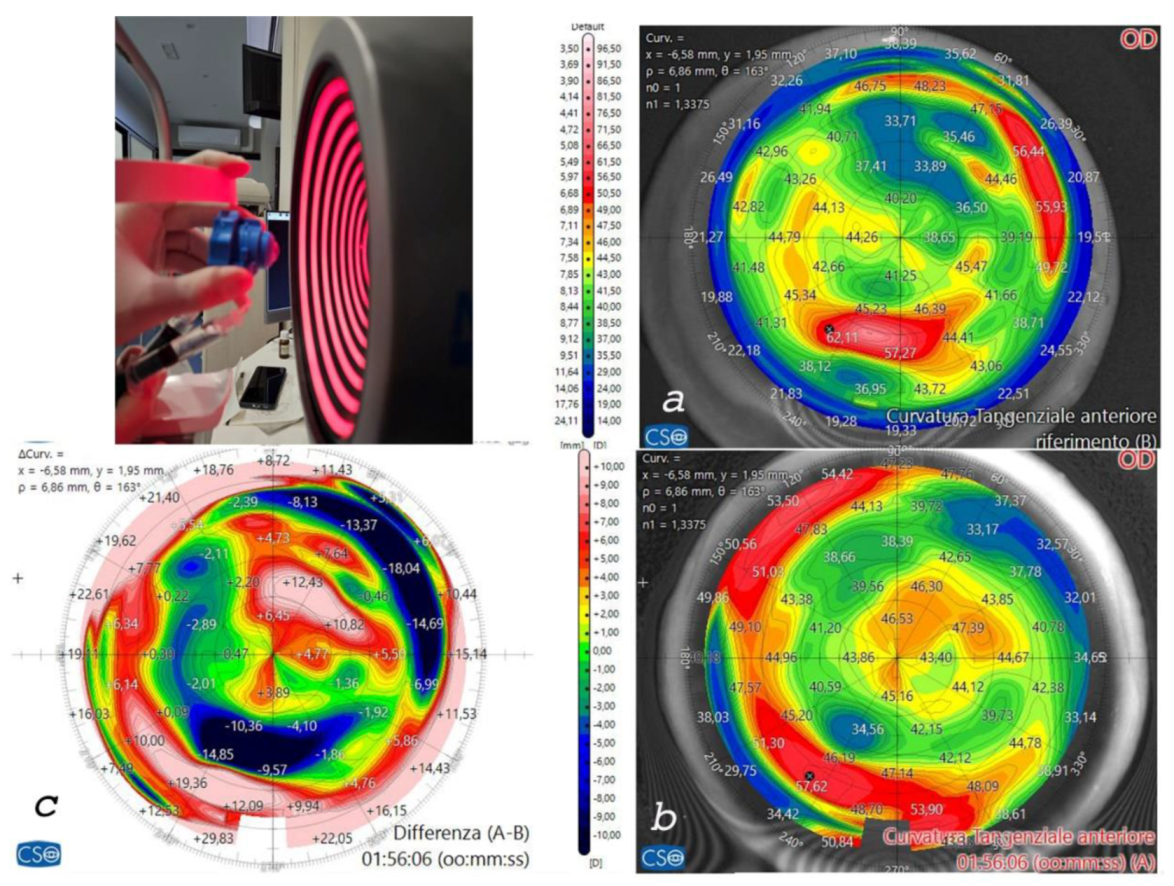
Figure 7.
Differential Tomography maps after single ACXL CAIRS implant in the first patient in Italy: Anterior Tangential Map: Shows flattening of K max from 53.53 D to 49.89 D and topographic hourglass regularization (a). Posterior Tangential Map displays a significant change of +3.01 D postoperatively (b). Anterior Gaussian Map: Demonstrates centralization of aberrations with anterior surface flattening of approximately 7.36 D at the most curved point (c). Pachymetry Differential Map indicates increased thickness due to the inserted tissue and stability over the 6-month follow-up (d).
Figure 7.
Differential Tomography maps after single ACXL CAIRS implant in the first patient in Italy: Anterior Tangential Map: Shows flattening of K max from 53.53 D to 49.89 D and topographic hourglass regularization (a). Posterior Tangential Map displays a significant change of +3.01 D postoperatively (b). Anterior Gaussian Map: Demonstrates centralization of aberrations with anterior surface flattening of approximately 7.36 D at the most curved point (c). Pachymetry Differential Map indicates increased thickness due to the inserted tissue and stability over the 6-month follow-up (d).
Figure 8.
Aberrometric analysis pre (a) and post (b) ACXL CAIRS implantation in the first patient in Italy. The comparative images show the improvement of point spread function (PSF) after ACXL CAIRS implantation and an overall reduction of High Order Aberrations (HOAs), including coma aberrations and vertical trefoil. Tomographic astigmatism also decreases from 4.77D to 3.15D. Additionally, it is noteworthy that the RMS varies from 5.15 D. eq to 3.40 D. eq. This data indicates that ACXL CAIRS affects not only coma-like aberrations but also other HOAs.
Figure 8.
Aberrometric analysis pre (a) and post (b) ACXL CAIRS implantation in the first patient in Italy. The comparative images show the improvement of point spread function (PSF) after ACXL CAIRS implantation and an overall reduction of High Order Aberrations (HOAs), including coma aberrations and vertical trefoil. Tomographic astigmatism also decreases from 4.77D to 3.15D. Additionally, it is noteworthy that the RMS varies from 5.15 D. eq to 3.40 D. eq. This data indicates that ACXL CAIRS affects not only coma-like aberrations but also other HOAs.
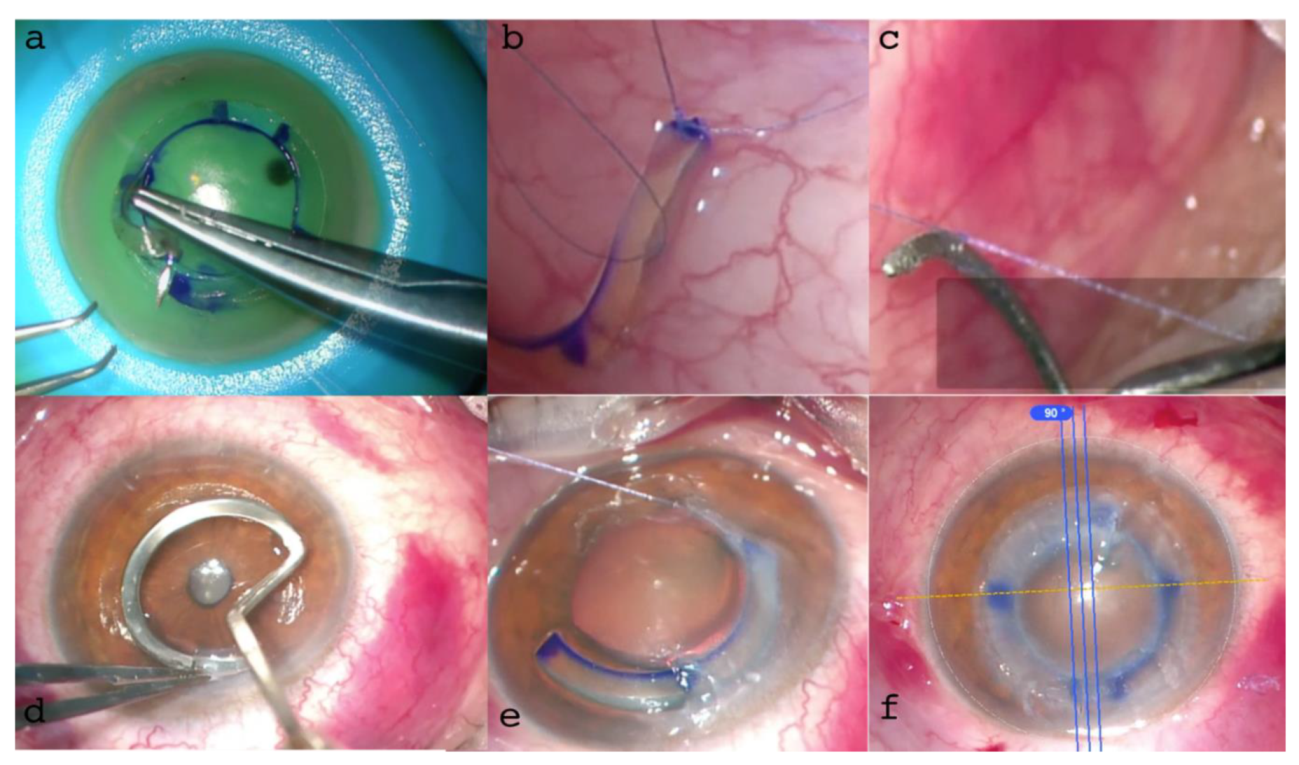
Figure 9.
ACXL CAIRS MZ (Mazzotta Zagari) “Wire-Assisted Push-and-Pull Technique”. 9 a: suturing of the ACXL CAIRS with 8-0 Vicryl. 9 b: knotting the suture end to the modified pocket former. 9 c: formation of the corneal pocket. d: introduction of the CAIRS using the personalized Mazzotta-Zagari “wire-assisted push-and-pull technique”. 9 e: alignment of the ACXL CAIRS.
Figure 9.
ACXL CAIRS MZ (Mazzotta Zagari) “Wire-Assisted Push-and-Pull Technique”. 9 a: suturing of the ACXL CAIRS with 8-0 Vicryl. 9 b: knotting the suture end to the modified pocket former. 9 c: formation of the corneal pocket. d: introduction of the CAIRS using the personalized Mazzotta-Zagari “wire-assisted push-and-pull technique”. 9 e: alignment of the ACXL CAIRS.
Table 1.
Phase 1 Ex Vivo Study: AFXL CAIRS characteristics. Legend: AFXL CAIRS: Crosslinked All Femtosecond laser-cut corneal allogenic ring segments; HPMC: hydroxypropyl methylcellulose (HPMC); UV-A: Ultraviolet A rays.
Table 1.
Phase 1 Ex Vivo Study: AFXL CAIRS characteristics. Legend: AFXL CAIRS: Crosslinked All Femtosecond laser-cut corneal allogenic ring segments; HPMC: hydroxypropyl methylcellulose (HPMC); UV-A: Ultraviolet A rays.
| AFXL CAIRS |
Thickness (µm) |
Diameter (mm) |
Arch lenght (°) |
Total Crosslinking time (min) |
Riboflavin 0.1% HPMC soaking time (min) |
UV-A irradiation (min) |
Depth of implant (%) |
Recipient tunnel diameter (mm) |
Donor
central corneal thickness
(µm) |
| 1 |
350 |
1.5 |
160 |
8 |
5 |
3 |
50 |
2 |
600 |
| 2 |
350 |
1.5 |
160 |
10 |
5 |
5 |
60 |
2 |
530 |
| 3 |
350 |
1.5 |
160 |
15 |
5 |
10 |
70 |
2 |
517 |
Table 2.
Changes in corneal thickness, maximum corneal curvature (K max) and high-order aberration (HOA) values following ACXL CAIRS implantation. Legend: High-Order Aberrations (HOA), Diopters (D), Recipient Corneas (R).
Table 2.
Changes in corneal thickness, maximum corneal curvature (K max) and high-order aberration (HOA) values following ACXL CAIRS implantation. Legend: High-Order Aberrations (HOA), Diopters (D), Recipient Corneas (R).
| Recipient Corneas |
Average central thickness pre µm |
Average central thickness
post µm |
Thickness
in the site of implant
pre µm |
Thickness
in the site of implant post µm |
K max
pre D |
K max post D |
HOA pre
D
6mm |
HOA
post
D 6mm |
| R1 |
405 |
405 |
500 |
800 |
50.43 |
59.38 |
0.31 |
2.51 |
| R2 |
459 |
553 |
456 |
806 |
51.65 |
69.76 |
0.47 |
3.49 |
| R3 |
462 |
473 |
463 |
813 |
53.58 |
68.3 |
0.99 |
7.30 |
Table 3.
Average maximum corneal curvature pre and post ACXL-CAIRS implantation. The average and standard deviation (SD) of K max pre- and post-implantation showed a change in the corneal shape through a centripetal wave movement of corneal tissue of 13.93 D (±5.62 SD).
Table 3.
Average maximum corneal curvature pre and post ACXL-CAIRS implantation. The average and standard deviation (SD) of K max pre- and post-implantation showed a change in the corneal shape through a centripetal wave movement of corneal tissue of 13.93 D (±5.62 SD).
| K max pre (D) |
Pre (D) |
Post (D) |
Δ K (D) |
| K max (average) |
51.89 |
65.81 |
13.93 |
| K max (Standard Deviation) |
1.59 |
5.62 |
|
Table 4.
MZ (Mazzotta-Zagari) CAIRS personalized nomogram used for AFXL CAIRS. Legend: CLASS: ectasia classification; MMP D: Mean Pupil Power Diopters; CAIRS T: CAIRS Thickness; A/P ELr: Anterio/Posterior Elevation ratio; IA CUT: Internal Angle femtosecond laser-cut; EA CUT: External Angle femtosecond laser-cut; RS m: Riboflavin soaking minutes; CXLF: Crosslinking Fluence; UVP/T: UVA Power/Time of exposure minutes.
Table 4.
MZ (Mazzotta-Zagari) CAIRS personalized nomogram used for AFXL CAIRS. Legend: CLASS: ectasia classification; MMP D: Mean Pupil Power Diopters; CAIRS T: CAIRS Thickness; A/P ELr: Anterio/Posterior Elevation ratio; IA CUT: Internal Angle femtosecond laser-cut; EA CUT: External Angle femtosecond laser-cut; RS m: Riboflavin soaking minutes; CXLF: Crosslinking Fluence; UVP/T: UVA Power/Time of exposure minutes.
| CLASS |
MMP
D |
CAIRS T
μm |
AP ELr μm |
ARC
Degrees |
IA CUT
Degrees |
EA CUT
degrees |
RS mins |
CXLF
Joules |
UVP/T
mW/mins |
| 0 |
≤ 45 |
≤ 250 |
< 20 |
160±20 |
60-90 |
90-120 |
5 |
5.4 |
30/6 |
| 1 |
46-48 |
250-350 |
20-30 |
160±20 |
60-90 |
90-120 |
7 |
5.4 |
18/10 |
| 2 |
49-53 |
350-450 |
30-40 |
160±20 |
60-90 |
90-120 |
8 |
5.4 |
15/12 |
| 3 |
>53 |
450-550 |
>40 |
160±20 |
60-90 |
90-120 |
10 |
5.4 |
9/10 |
Table 5.
AFCXL-CAIRS all femtosecond laser-cut parameters.
Table 5.
AFCXL-CAIRS all femtosecond laser-cut parameters.
| Parameters |
Donor |
Recipient |
| Depth in cornea (µm) |
400 |
382 (65%) |
| Diameter (mm) |
9.2 |
160° (arc) |
| Inner diameter (mm) |
6.0 |
5.8 |
| Inner angle cut (°) |
70 |
- |
| Outer diameter (mm) |
7.5 |
7.8 |
| Outer angle cut (°) |
110 |
- |
| Energy (micro J) |
1.50 |
1.50 |
Table 6.
Pre and Postoperative Parameters.
Table 6.
Pre and Postoperative Parameters.
| Parameter |
Preoperative |
Postoperative |
| SimK (K flat) |
42.74 D |
42.08 D |
| SimK (K steep) |
53.53 D |
49.89 D |
| Astigmatism |
-7.25 D@25° |
-3.71 D@30° |
| Coma Aberrations |
1.49 D eq. @90 |
0.19 D @165° |
Thinnest Point (µm)
|
362 |
378
|
Table 7.
Pre and Postoperative data of double ACXL CAIRS.
Table 7.
Pre and Postoperative data of double ACXL CAIRS.
| Parameter |
Preoperative |
Postoperative |
| SimK (K flat) |
49.78 D |
54.51 D |
| SimK (K steep) |
58.30 D |
45.75 D |
| Astigmatism |
-7.25 D axis 25° |
4,75D axis 30° |
| K Max |
62.41 D |
54.01 D |
| Coma Aberrations |
1.49 D eq. Axis 90 |
D axis 165° |
Thinnest Point (µm)
|
443 |
486
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).