Section 1.1 ADHD Neuroanatomy
Structural Abnormalities
Numerous structural magnetic resonance imaging (sMRI) studies have
identified various neuroanatomical abnormalities in individuals with ADHD. These abnormalities primarily involve alterations in brain volume, cortical thickness, and surface area (Cortese et al., 2012).
Total Brain Volume: Meta-analyses have consistently reported reduced total brain volume in individuals with ADHD compared to typically developing controls (Valera et al., 2007; Nakao et al., 2011). This reduction is estimated to be approximately 3-5%, with the most significant differences observed in children (Castellanos et al., 2002).
Specific brain regions have been implicated in ADHD, with the most consistent findings pointing to volume reductions in the prefrontal cortex (PFC), basal ganglia, corpus callosum, and cerebellum (Valera et al., 2007; Nakao et al., 2011).
Prefrontal Cortex (PFC): The PFC is involved in executive functions such as attention, working memory, and impulse control. Structural imaging studies have reported reduced volume and cortical thickness in the PFC of individuals with ADHD, particularly in the dorsolateral PFC (DL-PFC) and orbitofrontal cortex (OFC) (Shaw et al., 2006; Almeida et al., 2010). Basal Ganglia: The basal ganglia, comprising the caudate nucleus, putamen, and globus pallidus, play a crucial role in motor control, learning, and executive functions. Volumetric reductions in the basal ganglia, particularly the caudate nucleus, have been consistently reported in individuals with ADHD (Valera et al., 2007; Nakao et al., 2011).
Corpus Callosum: The corpus callosum is the largest white matter tract in the brain, connecting the left and right cerebral hemispheres. Structural imaging studies have reported reduced volume and altered morphology of the corpus callosum in individuals with ADHD, suggesting impaired interhemispheric communication (Valera et al., 2007; Hutchinson et al., 2008).
Cerebellum: The cerebellum is involved in motor coordination, balance, and cognitive functions such as attention and working memory (see image 1).
Structural imaging studies have reported reduced volume in the cerebellum, particularly the vermis, in individuals with ADHD (Valera et al., 2007; Nakao et al., 2011).
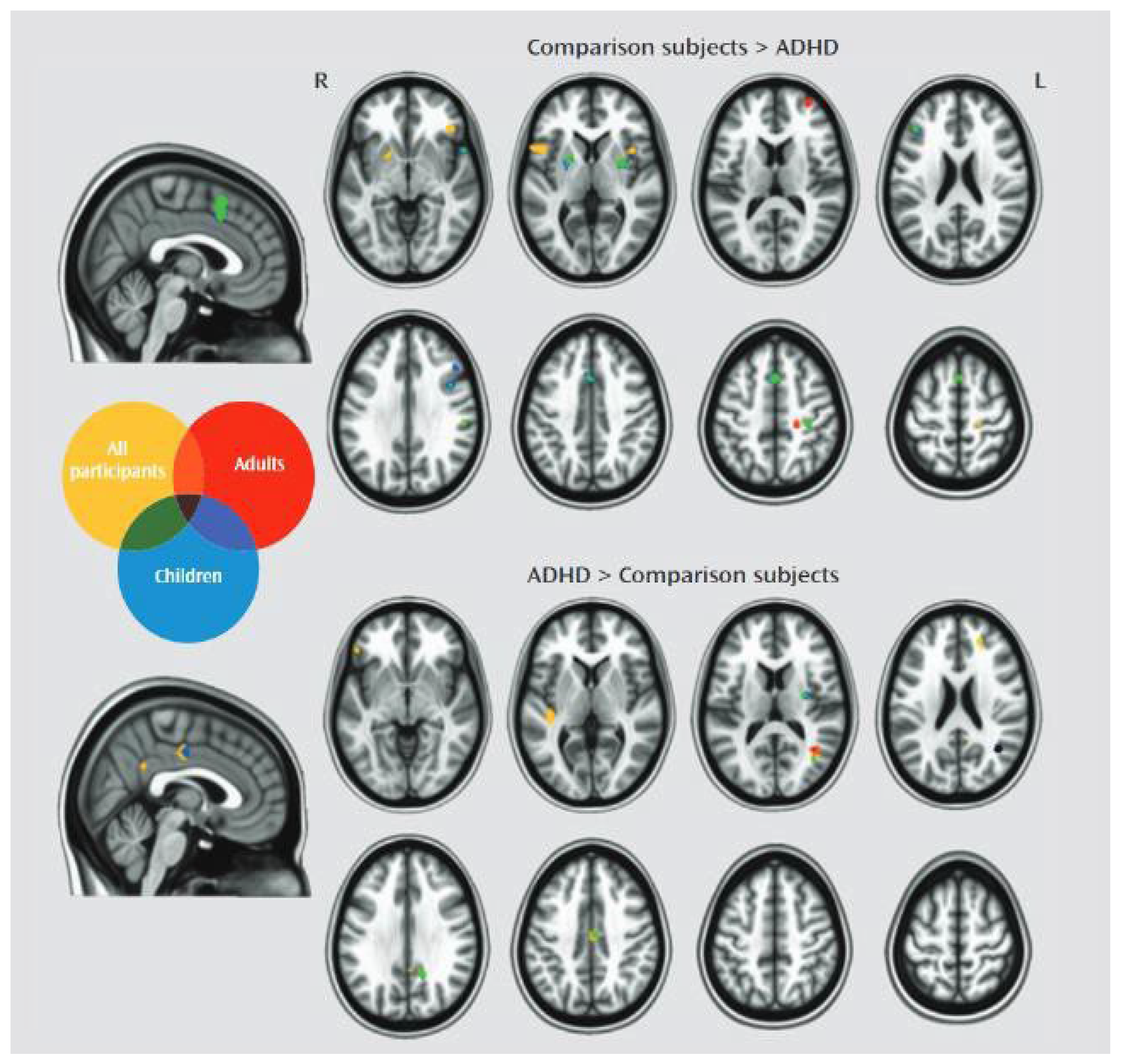
Image 1.
Functional MRI comparing activity in different brain areas, described in the text, adults and children with ADHD and the general population. Source: CORTESE, KELLY, CHABERNAUD, ET AL.(2012). American Journal of Psychiatry_vol 169_issue10.
Image 1.
Functional MRI comparing activity in different brain areas, described in the text, adults and children with ADHD and the general population. Source: CORTESE, KELLY, CHABERNAUD, ET AL.(2012). American Journal of Psychiatry_vol 169_issue10.
White Matter Integrity
Diffusion tensor imaging (DTI) studies have investigated white matter integrity in individuals with ADHD, focusing on fractional anisotropy (FA) as a measure of white matter organization and connectivity (Cortese et al., 2012).
Several studies have reported reduced FA in various brain regions in individuals with ADHD, including the corpus callosum, internal capsule, and long-range white matter tracts connecting the frontal, temporal, and parietal lobes (Ashtari et al., 2005; Konrad & Eickhoff, 2010).
Reduced FA in individuals with ADHD suggests altered connectivity within and between brain networks, particularly those involved in attention, executive functions, and motor control (Cortese et al., 2012).
Delayed Cortical Maturation
Longitudinal structural imaging studies have provided evidence for delayed cortical maturation in individuals with ADHD, particularly in the PFC (Shaw et al., 2007; Shaw et al., 2013). Individuals with ADHD exhibit delayed peak cortical thickness in the PFC, with a delay of approximately 2-5 years compared to typically developing controls (Shaw et al., 2007; Shaw et al., 2013). Delayed cortical maturation in ADHD is also evident in the surface area of the PFC, with individuals with ADHD reaching peak surface area later than typically developing controls (Shaw et al., 2012).
Section 2. Discussion
Section 2.1 Functional Aspects of ADHD ADHD Brain Wave Characteristics
Electroencephalography (EEG) Studies
Electroencephalography (EEG) has been extensively used to investigate brain wave characteristics in individuals with ADHD. EEG studies have focused on spectral power analysis, event-related potentials (ERPs), and connectivity measures to identify neural correlates of ADHD symptoms (Loo & Barkley, 2005; Barry et al., 2003).
Spectral power analysis involves quantifying the power of different frequency bands in the EEG signal. The most consistent findings in ADHD include increased theta power (4-8 Hz), decreased beta power (13-30 Hz), and increased theta/beta ratio (TBR) (Barry et al., 2003; Loo & Barkley, 2005)
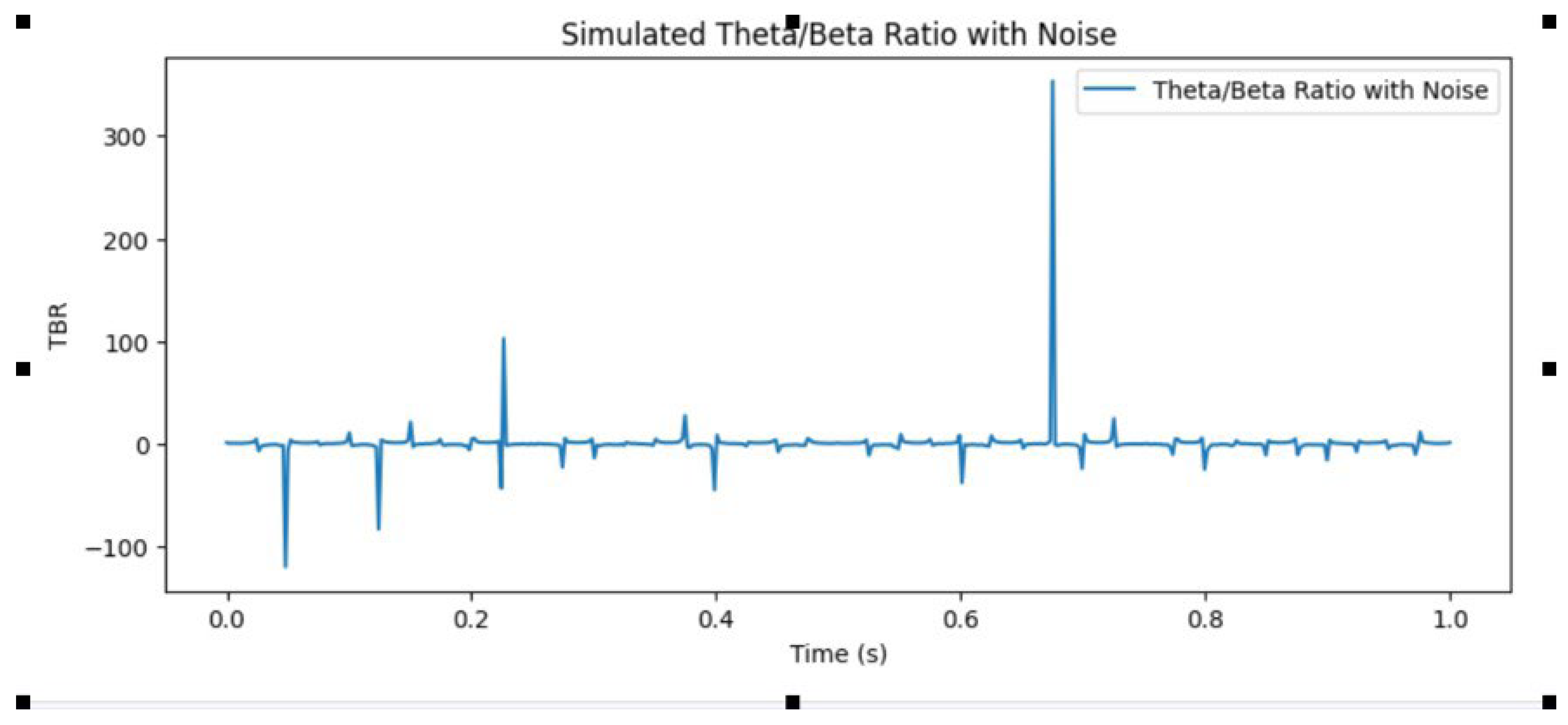
Image 2.
Theta/Beta Ratio, commonly used as a complementary exam indicator of ADHD. Source: Author.
Image 2.
Theta/Beta Ratio, commonly used as a complementary exam indicator of ADHD. Source: Author.
Increased theta power in individuals with ADHD is most prominent in the frontal and central regions and has been associated with inattention and cognitive impairment (Barry et al., 2003; Loo & Barkley, 2005).
Decreased beta power in individuals with ADHD has been linked to impulsivity and hyperactivity, with the most significant differences observed in the frontal and central regions (Barry et al., 2003; Loo & Barkley, 2005).
Theta/Beta Ratio (TBR): The TBR is a widely used metric in ADHD research, with numerous studies reporting increased TBR in individuals with ADHD compared to typically developing controls (Barry et al., 2003; Loo & Barkley, 2005). The TBR has been proposed as a potential biomarker for ADHD,
including The Federal Drug Administration (FDA) of The United States, with some studies suggesting that it may be useful in predicting treatment response (Arns et al., 2008; Snyder & Hall, 2006). Several recent studies have been published by Richard Murdoch Montgomery, a neurologist with a mathematical pen dour, which enlighten brain dynamics of ADHD with interesting conclusions, not all of them of negative, deficitary qualities, as mentioned by the author in several articles. ADHD patients seem to have an evolutionary root and are enabled, at least a part of the spectrum, with notably increased habilities in creativity and cognitive functions. Several prominent figures of our time had recently admitted having ADHD and the relief its treatment brought to them. Those personalities are far from underdeveloped. Montgomery´s insists also in the neurodevelopmental aspect of ADHD, which makes the patients brain structurally different, with difficult direct and straightforward comparisons with typical brains (Montgomery R. M., 2023, 2024a, 2024b, 2024c).
ERPs are time-locked brain responses to specific events or stimuli. ERP studies in ADHD have focused on various components, including the P300, contingent negative variation (CNV), and error-related negativity (ERN) (Banaschewski & Brandeis, 2007; Johnstone et al., 2013).
The P300 is a positive deflection in the ERP waveform that occurs approximately 300 ms after the onset of a rare or task-relevant stimulus.
Reduced P300 amplitude and prolonged latency have been consistently reported in individuals with ADHD, suggesting impaired attention allocation and cognitive processing (Banaschewski & Brandeis, 2007; Johnstone et al., 2013).
The CNV is a slow negative wave that occurs during the interval between a warning stimulus and an imperative stimulus. Reduced CNV amplitude has been reported in individuals with ADHD, indicating impaired response preparation and motor inhibition (Banaschewski & Brandeis, 2007; Johnstone et al., 2013).
The ERN is a negative deflection in the ERP waveform that occurs approximately 50-100 ms after an erroneous response. Reduced ERN amplitude has been observed in individuals with ADHD, suggesting impaired error monitoring and performance adjustment (Banaschewski & Brandeis, 2007; Johnstone et al., 2013).
EEG connectivity studies in ADHD have focused on coherence and phase synchrony as measures of functional connectivity between different brain regions (Barry et al., 2005; Coben et al., 2008).
Coherence is a measure of the linear correlation between two EEG signals in the frequency domain. Altered coherence patterns have been reported in individuals with ADHD, with the most consistent findings pointing to
increased theta coherence and decreased beta coherence, particularly in the frontal and central regions (Barry et al., 2005; Coben et al., 2008). Phase synchrony is a measure of the temporal coordination between two EEG signals in the time domain. Altered phase synchrony patterns have been observed in individuals with ADHD, suggesting impaired functional connectivity within and between brain networks (Barry et al., 2005; Coben et al., 2008).
Section 2.3 Magnetoencephalography (MEG) Studies
Magnetoencephalography (MEG) is a non-invasive neuroimaging technique that measures the magnetic fields generated by neural activity. MEG studies in ADHD have provided valuable insights into the spatiotemporal dynamics of brain activity and connectivity (Wilson et al., 2013; Doesburg et al., 2013).
MEG studies have replicated and extended the findings of EEG studies, reporting increased theta power and decreased beta power in individuals with ADHD (Wilson et al., 2013; Doesburg et al., 2013).
MEG connectivity studies have investigated functional and effective connectivity in individuals with ADHD, focusing on measures such as coherence, phase synchrony, and Granger causality (Wilson et al., 2013; Doesburg et al., 2013).
Altered functional connectivity patterns have been reported in individuals with ADHD, with the most consistent findings pointing to reduced connectivity within the default mode network (DMN) and increased connectivity between the DMN and task-positive networks (Doesburg et al., 2013; Wilson et al., 2013).
Effective connectivity studies in ADHD have investigated the directional influences between brain regions, revealing altered causal interactions within and between brain networks (Wilson et al., 2013; Doesburg et al., 2013).
Section 2.4 Neurotransmitter Systems in ADHD
The neuroanatomical and brain wave characteristics of ADHD are closely linked to alterations in neurotransmitter systems, particularly dopamine and norepinephrine (Genro et al., 2010; Del Campo et al., 2011). Dopamine is a catecholamine neurotransmitter involved in various cognitive and motor functions, including attention, working memory, reward processing, and motor control. The mesocorticolimbic dopamine system, which originates in the ventral tegmental area (VTA) and projects to the PFC, striatum, and other brain regions, has been extensively implicated in ADHD (Genro et al., 2010; Del Campo et al., 2011).
The Dopamine Transporter DAT is a membrane-spanning protein responsible for the reuptake of dopamine from the synaptic cleft. Molecular imaging studies have reported increased DAT density in the striatum of individuals with ADHD, suggesting altered dopamine signaling (Genro et al., 2010; Del Campo et al., 2011). Alterations in dopamine receptor density and function have also been implicated in ADHD. For example, molecular imaging studies have reported reduced D2/D3 receptor availability in the striatum of individuals with ADHD (Genro et al., 2010; Del Campo et al., 2011).
Norepinephrine is a catecholamine neurotransmitter involved in various cognitive and autonomic functions, including attention, arousal, and stress response. The locus coeruleus-norepinephrine (LC-NE) system, which originates in the LC and projects to various brain regions, has been implicated in ADHD (Berridge & Waterhouse, 2003; Arnsten, 2011).
The NET is a membrane-spanning protein responsible for the reuptake of norepinephrine from the synaptic cleft. Genetic studies have identified polymorphisms in the NET gene (SLC6A2) that are associated with an increased risk of ADHD (Bobb et al., 2005; Kim et al., 2006).
Alpha-2 adrenergic receptors are involved in the regulation of norepinephrine release and have been implicated in ADHD. For example, genetic studies have identified polymorphisms in the alpha-2A adrenergic receptor gene (ADRA2A) that are associated with an increased risk of ADHD (Roman et al., 2003; Deupree et al., 2006).
Section 2.5 Neural Networks and Models of ADHD
The neuroanatomical and brain wave characteristics of ADHD have been integrated into various neural network models, aiming to explain the underlying neural mechanisms and their relationship to clinical symptoms (Castellanos & Proal, 2016; Cortese, 2012).
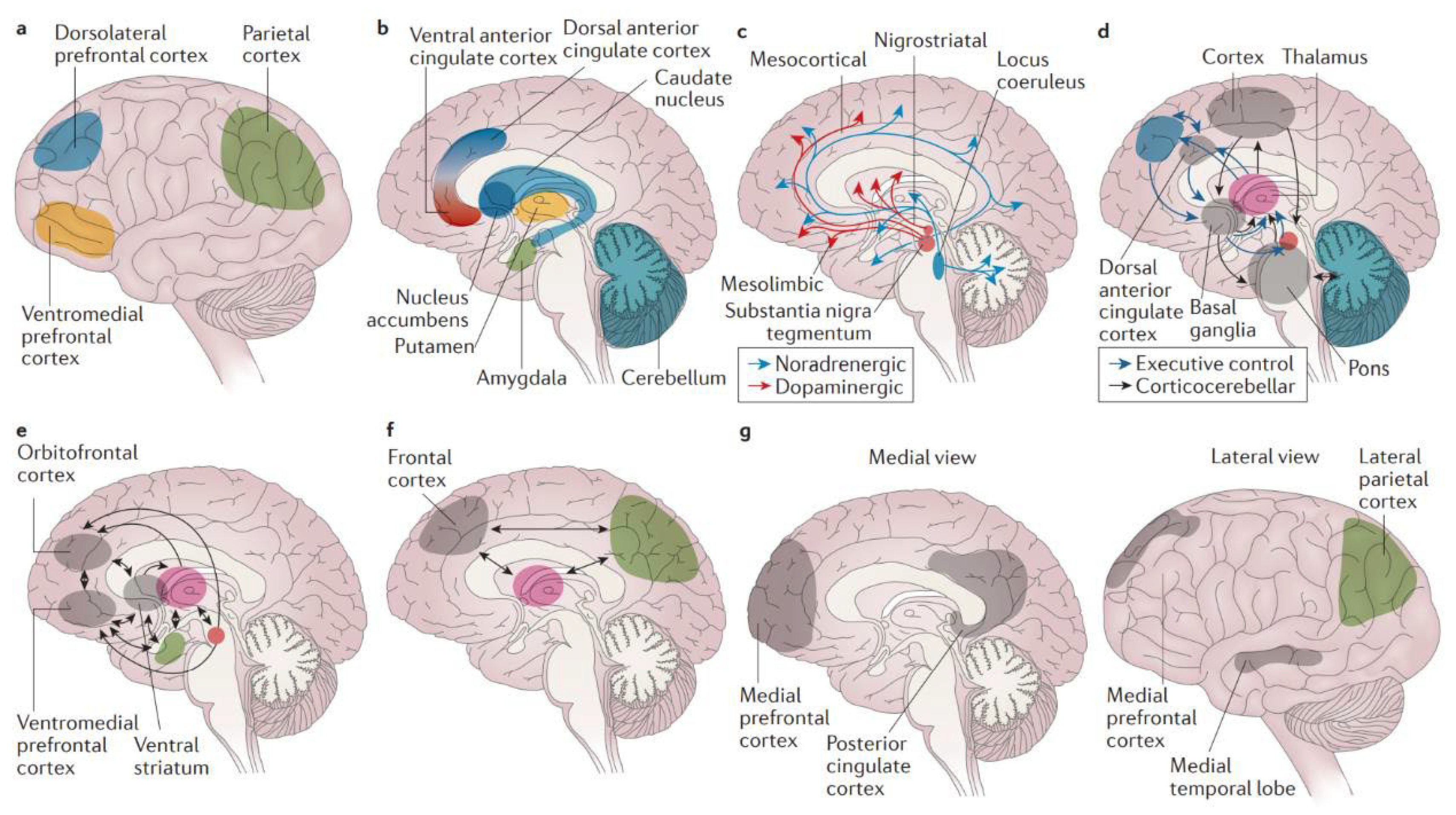
Image 3.
Brain mechanisms in attention-deficit/hyperactivity disorder. a | The cortical regions (lateral view) of the. brain have a role in attention-deficit/hyperactivity disorder (ADHD). The dorsolateral prefrontal cortex is linked to working memory, the ventromedial prefrontal cortex to complex decision making and strategic planning, and the parietal cortex to orientation of attention. b | ADHD involves the subcortical structures (medial view) of the brain. The ventral anterior cingulate cortex and the dorsal anterior cingulate cortex subserve affective and cognitive components of executive control. Together with the basal ganglia (comprising the nucleus accumbens, caudate nucleus and putamen), they form the frontostriatal circuit. Neuroimaging studies show structural and functional abnormalities in all of these structures in patients with ADHD, extending into the amygdala and cerebellum. c | Neurotransmitter circuits in the brain are involved in ADHD. The dopamine system plays an important part in planning and initiation of motor responses, activation, switching, reaction to novelty and processing of reward. The noradrenergic system influences arousal modulation, signal-to-noise ratios in cortical areas, state-dependent cognitive processes and cognitive preparation of urgent stimuli. d | Executive control networks are affected in patients with ADHD. The executive control and corticocerebellar networks coordinate executive functioning, that is, planning, goal-directed behaviour, inhibition, working memory and the flexible adaptation to context. These networks are underactivated and have lower internal functional connectivity in individuals with ADHD compared with individuals without the disorder. e | ADHD involves the reward network. The ventromedial prefrontal cortex, orbitofrontal cortex and ventral striatum are at the centre of the brain network that responds to anticipation and receipt of reward. Other structures involved are the thalamus, the amygdala and the cell bodies of dopaminergic neurons in the substantia nigra, which, as indicated by the arrows, interact in a complex manner. Behavioural and neural responses to reward are abnormal in ADHD. f | The alerting network is impaired in ADHD. The frontal and parietal cortical areas and the thalamus intensively interact in the alerting network (indicated by the arrows), which supports attentional functioning and is weaker in individuals with ADHD than in controls. g | ADHD involves the default-mode network (DMN). The DMN consists of the medial prefrontal cortex and the posterior cingulate cortex (medial view) as well as the lateral parietal cortex and the medial temporal lobe (lateral view). DMN fluctuations are 180 degrees out of phase with fluctuations in networks that become activated during externally oriented tasks, presumably reflecting competition between opposing processes for processing resources. Negative correlations between the DMN and the frontoparietal control network are weaker in patients with ADHD than in people who do not have the disorder. Source: Attention-deficit/hyperactivity disorder, Faraone et al, Nature Reviews, 2015.
Image 3.
Brain mechanisms in attention-deficit/hyperactivity disorder. a | The cortical regions (lateral view) of the. brain have a role in attention-deficit/hyperactivity disorder (ADHD). The dorsolateral prefrontal cortex is linked to working memory, the ventromedial prefrontal cortex to complex decision making and strategic planning, and the parietal cortex to orientation of attention. b | ADHD involves the subcortical structures (medial view) of the brain. The ventral anterior cingulate cortex and the dorsal anterior cingulate cortex subserve affective and cognitive components of executive control. Together with the basal ganglia (comprising the nucleus accumbens, caudate nucleus and putamen), they form the frontostriatal circuit. Neuroimaging studies show structural and functional abnormalities in all of these structures in patients with ADHD, extending into the amygdala and cerebellum. c | Neurotransmitter circuits in the brain are involved in ADHD. The dopamine system plays an important part in planning and initiation of motor responses, activation, switching, reaction to novelty and processing of reward. The noradrenergic system influences arousal modulation, signal-to-noise ratios in cortical areas, state-dependent cognitive processes and cognitive preparation of urgent stimuli. d | Executive control networks are affected in patients with ADHD. The executive control and corticocerebellar networks coordinate executive functioning, that is, planning, goal-directed behaviour, inhibition, working memory and the flexible adaptation to context. These networks are underactivated and have lower internal functional connectivity in individuals with ADHD compared with individuals without the disorder. e | ADHD involves the reward network. The ventromedial prefrontal cortex, orbitofrontal cortex and ventral striatum are at the centre of the brain network that responds to anticipation and receipt of reward. Other structures involved are the thalamus, the amygdala and the cell bodies of dopaminergic neurons in the substantia nigra, which, as indicated by the arrows, interact in a complex manner. Behavioural and neural responses to reward are abnormal in ADHD. f | The alerting network is impaired in ADHD. The frontal and parietal cortical areas and the thalamus intensively interact in the alerting network (indicated by the arrows), which supports attentional functioning and is weaker in individuals with ADHD than in controls. g | ADHD involves the default-mode network (DMN). The DMN consists of the medial prefrontal cortex and the posterior cingulate cortex (medial view) as well as the lateral parietal cortex and the medial temporal lobe (lateral view). DMN fluctuations are 180 degrees out of phase with fluctuations in networks that become activated during externally oriented tasks, presumably reflecting competition between opposing processes for processing resources. Negative correlations between the DMN and the frontoparietal control network are weaker in patients with ADHD than in people who do not have the disorder. Source: Attention-deficit/hyperactivity disorder, Faraone et al, Nature Reviews, 2015.
Default Mode Network (DMN): The DMN is a large-scale brain network involved in self-referential processing, mind-wandering, and task-independent thought. Altered DMN activity and connectivity have been consistently reported in individuals with ADHD, with the most significant differences observed in the posterior cingulate cortex (PCC), medial PFC (mPFC), and angular gyrus (Castellanos & Proal, 2016; Cortese, 2012).
The DMN interference hypothesis proposes that excessive DMN activity and
impaired DMN suppression during cognitive tasks contribute to the attentional and cognitive deficits observed in individuals with ADHD (Sonuga-Barke &
Castellanos, 2007; Castellanos & Proal, 2016).
The FPN (Fronto-Parietal Node is a large-scale brain network involved in executive functions, such as attention, working memory, and cognitive control. Altered FPN activity and connectivity have been reported in individuals with ADHD, with the most significant differences observed in the DL-PFC, inferior parietal lobule (IPL), and intraparietal sulcus (IPS) (Castellanos & Proal, 2016; Cortese, 2012).
FPN Dysfunction Hypothesis: The FPN dysfunction hypothesis proposes that impaired FPN activity and connectivity contribute to the executive function deficits observed in individuals with ADHD (Castellanos & Proal, 2016; Cortese, 2012).
Ventral Attention Network is a large-scale brain network involved in detecting and orienting to salient stimuli in the environment. Altered VAN activity and connectivity have been reported in individuals with ADHD, with the most significant differences observed in the temporoparietal junction (TPJ), ventral frontal cortex (VFC), and anterior insula (AI) (Castellanos & Proal, 2016; Cortese, 2012).
The VAN dysfunction hypothesis proposes that impaired VAN activity and connectivity contribute to the attentional and emotional dysregulation observed in individuals with ADHD (Castellanos & Proal, 2016; Cortese, 2012).
The dual pathway model integrates the DMN interference and FPN dysfunction hypotheses, proposing that ADHD symptoms arise from an imbalance between the DMN and FPN (Sonuga-Barke & Castellanos, 2007; Castellanos & Proal, 2016). According to this model, excessive DMN activity and impaired DMN suppression interfere with FPN function, leading to attentional and cognitive deficits. Conversely, impaired FPN function fails to regulate DMN activity, resulting in a vicious cycle that exacerbates ADHD symptoms.
Section 2.6 Clinical Implications and Future Directions
The neuroanatomical and brain wave characteristics of ADHD have significant clinical implications for the diagnosis, treatment, and management of this condition.
Diagnostic Biomarkers: The identification of reliable and objective biomarkers for ADHD is a critical goal in the field. Neuroimaging and electrophysiological measures, such as the TBR, P300, and DMN connectivity, hold promise as potential biomarkers for ADHD (Arns et al., 2008; Snyder & Hall, 2006; Castellanos & Proal, 2016). However, further research is needed to validate some of these measures and establish their clinical utility.
Treatment Selection and Optimization: Neuroimaging and electrophysiological measures may also be useful in predicting treatment response and optimizing treatment selection for individuals with ADHD. For example, the TBR has been proposed as a potential biomarker for predicting response to stimulant medication (Arns et al., 2008; Snyder & Hall, 2006). Additionally, neurofeedback and other neuromodulatory interventions targeting specific brain wave
characteristics or neural networks may offer promising alternatives or adjuncts to traditional pharmacological treatments (Arns et al., 2009; Cortese et al., 2016).
Longitudinal studies are essential for understanding the developmental trajectories of ADHD and the neural mechanisms underlying symptom persistence, remission, and late-onset cases. Such studies can provide valuable
insights into the dynamic interplay between neuroanatomical, brain wave, and clinical characteristics over time (Shaw et al., 2013; Franke et al., 2018).
Integrating multimodal neuroimaging and electrophysiological data is crucial for obtaining a comprehensive understanding of ADHD neurobiology.
Combining structural, functional, and connectivity measures from different modalities can provide complementary information and help elucidate the complex neural networks and dynamics underlying ADHD symptoms (Castellanos & Proal, 2016; Cortese, 2012).
Neurostimulants as Lisdexanfetamine and Metilfenidateand have been highly effective in the treatment of ADHD, especially in adults, despite its heterogeneous spectrum. Several adjuvant drugs are available, as atomoxetine, , venlafaxine and clonidine, which form a powerful therapeutical arsenal against the disease, something atypical for a neuropsychiatric disease, usually with lower rates of satisfactory resolution.
The ultimate goal of ADHD research is to develop personalized medicine approaches that tailor treatments to the unique needs and characteristics of each individual. Achieving this goal will require a deep understanding of the neuroanatomical, brain wave, and clinical heterogeneity of ADHD, as well as the development of sophisticated analytical tools and algorithms for integrating and interpreting multimodal data (Castellanos & Proal, 2016; Cortese, 2012).