Submitted:
27 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. BIA Methods
Single Frequency-BIA (SF-BIA) and BIVA
Multiple Frequency-BIA (MF-BIA) and BIS
The Devices
Clinical Relevance of BIA in HF Patients
Clinical Relevance of BIA in CKD Patients
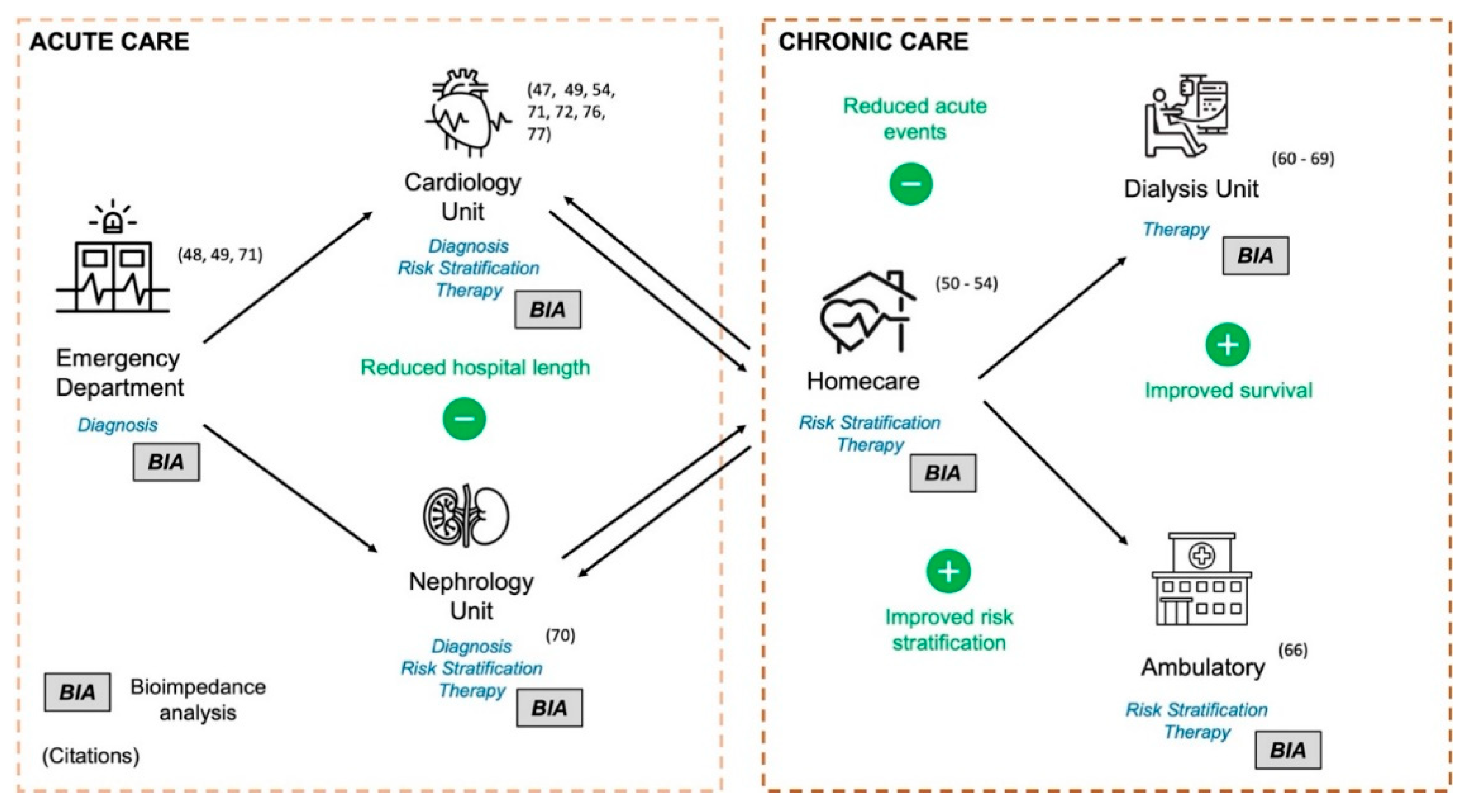
Diagnosis, Therapy, and Risk Stratification in HF and CKD
Advantages of BIA
Limitations of BIA
Future Perspectives
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bioelectrical impedance analysis in body composition measurement: National Institutes of Health Technology Assessment Conference Statement. The American journal of clinical nutrition. 1996;64(3 Suppl):524S-32S.
- Lurefkaskis HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. 1986;60(4):1327-32.
- Lukaski HC, Vega Diaz N, Talluri A, Nescolarde L. Classification of Hydration in Clinical Conditions: Indirect and Direct Approaches Using Bioimpedance. Nutrients. 2019;11(4).
- Lukaski HC, Talluri A. Phase angle as an index of physiological status: validating bioelectrical assessments of hydration and cell mass in health and disease. Rev Endocr Metab Disord. 2023;24(3):371-9.
- Baumgartner RN, Chumlea WC, Roche A. Estimation of body composition from bioelectric impedance of body segments. The American journal of clinical nutrition. 1989;50(2):221-6.
- Kotanko P, Levin NW, Zhu F. Current state of bioimpedance technologies in dialysis. Nephrology Dialysis Transplantation. 2008;23(3):808-12.
- Thomas BJ, Ward LC, Cornish BH. Bioimpedance spectrometry in the determination of body water compartments: accuracy and clinical significance. Appl Radiat Isot. 1998;49(5-6):447-55.
- Chamney PW, Krämer M, Rode C, Kleinekofort W, Wizemann V. A new technique for establishing dry weight in hemodialysis patients via whole body bioimpedance. Kidney Int. 2002;61(6):2250-8.
- Charra, B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007;11(1):21-31.
- Khalil SF, Mohktar MS, Ibrahim F. The theory and fundamentals of bioimpedance analysis in clinical status monitoring and diagnosis of diseases. Sensors. 2014;14(6):10895-928.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Gómez JM, et al. Bioelectrical impedance analysis--part I: review of principles and methods. Clin Nutr. 2004;23(5):1226-43.
- La Porta E, Lanino L, Calatroni M, Caramella E, Avella A, Quinn C, et al. Volume Balance in Chronic Kidney Disease: Evaluation Methodologies and Innovation Opportunities. Kidney & blood pressure research. 2021;46(4):396-410.
- Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. Journal of the American College of Cardiology. 2008;52(19):1527-39.
- de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, et al. Cardiovascular and noncardiovascular mortality among patients starting dialysis. Jama. 2009;302(16):1782-9.
- Deferrari G, Cipriani A, La Porta E. Renal dysfunction in cardiovascular diseases and its consequences. J Nephrol. 2021;34(1):137-53.
- Faragli A, La Porta E, Campana C, Pieske B, Kelle S, Koehler F, et al. Out-of-Hospital Care of Heart Failure Patients During and After COVID-19 Pandemic: Time for Telemedicine? 2020;2.
- Kyle UG, Bosaeus I, De Lorenzo AD, Deurenberg P, Elia M, Manuel Gómez J, et al. Bioelectrical impedance analysis—part II: utilization in clinical practice. Clinical Nutrition. 2004;23(6):1430-53.
- Hoffer EC, Meador CK, Simpson DC. Correlation of whole-body impedance with total body water volume. Journal of applied physiology. 1969;27(4):531-4.
- Lukaski, HC. Evolution of bioimpedance: a circuitous journey from estimation of physiological function to assessment of body composition and a return to clinical research. European journal of clinical nutrition. 2013;67(1):S2-S9.
- Lukaski HC, Bolonchuk WW, Hall CB, Siders WA. Validation of tetrapolar bioelectrical impedance method to assess human body composition. Journal of applied physiology. 1986;60(4):1327-32.
- Bera, TK. Bioelectrical impedance methods for noninvasive health monitoring: a review. Journal of medical engineering. 2014;2014.
- Keren H, Burkhoff D, Squara P. Evaluation of a noninvasive continuous cardiac output monitoring system based on thoracic bioreactance. 2007;293(1):H583-H9.
- Wang, L. Fundamentals of intrathoracic impedance monitoring in heart failure. The American journal of cardiology. 2007;99(10):S3-S10.
- Yang X-W, Hua W, Ding L-G, Wang J, Zheng L-H, Li C-Q, et al. OptiVol fluid index predicts acute decompensation of heart failure with a high rate of unexplained events. Journal of Geriatric Cardiology: JGC. 2013;10(3):253.
- Tang WH, Tong W. Measuring impedance in congestive heart failure: current options and clinical applications. American heart journal. 2009;157(3):402-11.
- Yu C-M, Wang L, Chau E, Chan RH-W, Kong S-L, Tang M-O, et al. Intrathoracic impedance monitoring in patients with heart failure: correlation with fluid status and feasibility of early warning preceding hospitalization. Circulation. 2005;112(6):841-8.
- Felker GM, Lee KL, Bull DA, Redfield MM, Stevenson LW, Goldsmith SR, et al. Diuretic strategies in patients with acute decompensated heart failure. The New England journal of medicine. 2011;364(9):797-805.
- Savarese G, Lund LH. Global public health burden of heart failure. Cardiac failure review. 2017;3(1):7.
- Roger, VL. Epidemiology of heart failure. Circulation research. 2013;113(6):646-59.
- Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. European journal of heart failure. 2016;18(8):891-975.
- Boorsma EM, Ter Maaten JM, Damman K, Dinh W, Gustafsson F, Goldsmith S, et al. Congestion in heart failure: a contemporary look at physiology, diagnosis and treatment. Nature reviews Cardiology. 2020;17(10):641-55.
- Ishikawa S-e. Hyponatremia associated with heart failure: pathological role of vasopressin-dependent impaired water excretion. Journal of clinical medicine. 2015;4(5):933-47.
- Palazzuoli A, Evangelista I, Nuti R. Congestion occurrence and evaluation in acute heart failure scenario: time to reconsider different pathways of volume overload. Heart failure reviews. 2020;25(1):119-31.
- Gheorghiade M, Filippatos G, De Luca L, Burnett J. Congestion in acute heart failure syndromes: an essential target of evaluation and treatment. Am J Med. 2006;119(12 Suppl 1):S3-S10.
- Pellicori P, Kaur K, Clark AL. Fluid Management in Patients with Chronic Heart Failure. Card Fail Rev. 2015;1(2):90-5.
- O’Connor CM, Stough WG, Gallup DS, Hasselblad V, Gheorghiade M. Demographics, clinical characteristics, and outcomes of patients hospitalized for decompensated heart failure: observations from the IMPACT-HF registry. J Card Fail. 2005;11(3):200-5.
- Neuberg GW, Miller AB, O’Connor CM, Belkin RN, Carson PE, Cropp AB, et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am Heart J. 2002;144(1):31-8.
- Lewin J, Ledwidge M, O’Loughlin C, McNally C, McDonald K. Clinical deterioration in established heart failure: What is the value of BNP and weight gain in aiding diagnosis? European journal of heart failure. 2005;7(6):953-7.
- Chaudhry SI, Mattera JA, Curtis JP, Spertus JA, Herrin J, Lin Z, et al. Telemonitoring in patients with heart failure. The New England journal of medicine. 2010;363(24):2301-9.
- Evangelista LS, Lee JA, Moore AA, Motie M, Ghasemzadeh H, Sarrafzadeh M, et al. Examining the effects of remote monitoring systems on activation, self-care, and quality of life in older patients with chronic heart failure. The Journal of cardiovascular nursing. 2015;30(1):51-7.
- Faragli A, Abawi D, Quinn C, Cvetkovic M, Schlabs T, Tahirovic E, et al. The role of non-invasive devices for the telemonitoring of heart failure patients. Heart Fail Rev. 2021;26(5):1063-80.
- Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, et al. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377(9766):658-66.
- Vaduganathan M, DeFilippis EM, Fonarow GC, Butler J, Mehra MR. Postmarketing Adverse Events Related to the CardioMEMS HF System. JAMA Cardiol. 2017;2(11):1277-9.
- Singh R, Varjabedian L, Kaspar G, Zughaib M. CardioMEMS in a Busy Cardiology Practice: Less than Optimal Implementation of a Valuable Tool to Reduce Heart Failure Readmissions. Cardiol Res Pract. 2018;2018:4918757.
- Koehler F, Koehler K, Deckwart O, Prescher S, Wegscheider K, Winkler S, et al. Telemedical Interventional Management in Heart Failure II (TIM-HF2), a randomised, controlled trial investigating the impact of telemedicine on unplanned cardiovascular hospitalisations and mortality in heart failure patients: study design and description of the intervention. Eur J Heart Fail. 2018;20(10):1485-93.
- Packer M, Abraham WT, Mehra MR, Yancy CW, Lawless CE, Mitchell JE, et al. Utility of impedance cardiography for the identification of short-term risk of clinical decompensation in stable patients with chronic heart failure. Journal of the American College of Cardiology. 2006;47(11):2245-52.
- Di Somma S, De Berardinis B, Bongiovanni C, Marino R, Ferri E, Alfei B. Use of BNP and bioimpedance to drive therapy in heart failure patients. Congest Heart Fail. 2010;16 Suppl 1:S56-61.
- Santarelli S, Russo V, Lalle I, De Berardinis B, Vetrone F, Magrini L, et al. Prognostic value of decreased peripheral congestion detected by Bioelectrical Impedance Vector Analysis (BIVA) in patients hospitalized for acute heart failure: BIVA prognostic value in acute heart failure. Eur Heart J Acute Cardiovasc Care. 2017;6(4):339-47.
- Anand IS, Tang WW, Greenberg BH, Chakravarthy N, Libbus I, Katra RP, et al. Design and performance of a multisensor heart failure monitoring algorithm: results from the multisensor monitoring in congestive heart failure (MUSIC) study. Journal of cardiac failure. 2012;18(4):289-95.
- Gyllensten IC, Bonomi AG, Goode KM, Reiter H, Habetha J, Amft O, et al. Early indication of decompensated heart failure in patients on home-telemonitoring: a comparison of prediction algorithms based on daily weight and noninvasive transthoracic bio-impedance. JMIR medical informatics. 2016;4(1):e4842.
- Darling CE, Dovancescu S, Saczynski JS, Riistama J, Sert Kuniyoshi F, Rock J, et al. Bioimpedance-Based Heart Failure Deterioration Prediction Using a Prototype Fluid Accumulation Vest-Mobile Phone Dyad: An Observational Study. JMIR Cardio. 2017;1(1):e1.
- Wang L, Lahtinen S, Lentz L, Rakow N, Kaszas C, Ruetz L, et al. Feasibility of using an implantable system to measure thoracic congestion in an ambulatory chronic heart failure canine model. Pacing and Clinical Electrophysiology. 2005;28(5):404-11.
- van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, et al. Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124(16):1719-26.
- Anshory M, Kuan WS, Rohman MS, Waranugraha Y, Kamila PA, Iskandar A, et al. Can non-invasive cardiac hemodynamics and fluid content system (NICaS) parameters predict Acute Heart Failure outcomes in Caucasian and Asian patients in the emergency department? Advances in medical sciences. 2024;69(1):81-9.
- McCullough K, Sharma P, Ali T, Khan I, Smith WC, MacLeod A, et al. Measuring the population burden of chronic kidney disease: a systematic literature review of the estimated prevalence of impaired kidney function. Nephrology Dialysis Transplantation. 2012;27(5):1812-21.
- McCullough PA, Chan CT, Weinhandl ED, Burkart JM, Bakris GL. Intensive Hemodialysis, Left Ventricular Hypertrophy, and Cardiovascular Disease. Am J Kidney Dis. 2016;68(5s1):S5-s14.
- Soi V, Yee J. Sodium Homeostasis in Chronic Kidney Disease. Adv Chronic Kidney Dis. 2017;24(5):325-31.
- Khan YH, Sarriff A, Adnan AS, Khan AH, Mallhi TH. Diuretics prescribing in chronic kidney disease patients: physician assessment versus bioimpedence spectroscopy. Clin Exp Nephrol. 2017;21(3):488-96.
- Davies SJ, Caskey FJ, Coyle D, Lindley E, Macdonald J, Mitra S, et al. Rationale and design of BISTRO: a randomized controlled trial to determine whether bioimpedance spectroscopy-guided fluid management maintains residual kidney function in incident haemodialysis patients. BMC Nephrol. 2017;18(1):138.
- Hur E, Usta M, Toz H, Asci G, Wabel P, Kahvecioglu S, et al. Effect of fluid management guided by bioimpedance spectroscopy on cardiovascular parameters in hemodialysis patients: a randomized controlled trial. Am J Kidney Dis. 2013;61(6):957-65.
- Onofriescu M, Hogas S, Voroneanu L, Apetrii M, Nistor I, Kanbay M, et al. Bioimpedance-guided fluid management in maintenance hemodialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2014;64(1):111-8.
- Huan-Sheng C, Yeong-Chang C, Ming-Hsing H, Fan-Lieh T, Chu-Cheng L, Tsai-Kun W, et al. Application of bioimpedance spectroscopy in Asian dialysis patients (ABISAD-III): a randomized controlled trial for clinical outcomes. Int Urol Nephrol. 2016;48(11):1897-909.
- Zoccali C, Moissl U, Chazot C, Mallamaci F, Tripepi G, Arkossy O, et al. Chronic Fluid Overload and Mortality in ESRD. J Am Soc Nephrol. 2017;28(8):2491-7.
- Dekker M, Konings C, Canaud B, Carioni P, Guinsburg A, Madero M, et al. Pre-dialysis fluid status, pre-dialysis systolic blood pressure and outcome in prevalent haemodialysis patients: results of an international cohort study on behalf of the MONDO initiative. Nephrol Dial Transplant. 2018;33(11):2027-34.
- Tabinor M, Elphick E, Dudson M, Kwok CS, Lambie M, Davies SJ. Bioimpedance-defined overhydration predicts survival in end stage kidney failure (ESKF): systematic review and subgroup meta-analysis. Scientific reports. 2018;8(1):4441.
- Horowitz L, Karadjian O, Braam B, Mavrakanas T, Weber C. Bioimpedance-Guided Monitoring of Volume Status in Patients With Kidney Disease: A Systematic Review and Meta-Analysis. Can J Kidney Health Dis. 2023;10:20543581231185433.
- Scotland G, Cruickshank M, Jacobsen E, Cooper D, Fraser C, Shimonovich M, et al. Multiple-frequency bioimpedance devices for fluid management in people with chronic kidney disease receiving dialysis: a systematic review and economic evaluation. Health Technol Assess. 2018;22(1):1-138. [CrossRef]
- Liu L, Sun Y, Chen Y, Xu J, Yuan P, Shen Y, et al. The effect of BCM guided dry weight assessment on short-term survival in Chinese hemodialysis patients : Primary results of a randomized trial - BOdy COmposition MOnitor (BOCOMO) study. BMC Nephrol. 2020;21(1):135. [CrossRef]
- Stigger K, Ribeiro LR, Cordeiro FM, Böhlke M. Incidence of hospital admissions in bioimpedance-guided fluid management among maintenance hemodialysis patients-Results of a randomized controlled trial. Hemodial Int. 2023;27(3):318-25. [CrossRef]
- Velazquez-Cecena JL, Sharma S, Nagajothi N, Khraisat A, Khosla S, Arora RR, et al. Left ventricular end diastolic pressure and serum brain natriuretic peptide levels in patients with abnormal impedance cardiography parameters. Arch Med Res. 2008;39(4):408-11.
- Parrinello G, Paterna S, Di Pasquale P, Torres D, Fatta A, Mezzero M, et al. The usefulness of bioelectrical impedance analysis in differentiating dyspnea due to decompensated heart failure. Journal of cardiac failure. 2008;14(8):676-86.
- Gastelurrutia P, Nescolarde L, Rosell-Ferrer J, Domingo M, Ribas N, Bayes-Genis A. Bioelectrical impedance vector analysis (BIVA) in stable and non-stable heart failure patients: a pilot study. Int J Cardiol. 2011;146(2):262-4.
- Zink MD, König F, Weyer S, Willmes K, Leonhardt S, Marx N, et al. Segmental bioelectrical impedance spectroscopy to monitor fluid status in heart failure. Scientific Reports. 2020;10(1):3577.
- Park JH, Jo YI, Lee JH. Clinical usefulness of bioimpedance analysis for assessing volume status in patients receiving maintenance dialysis. Korean J Intern Med. 2018;33(4):660-9.
- Maddox TM, Januzzi JL, Jr., Allen LA, Breathett K, Butler J, Davis LL, et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77(6):772-810.
- Massari F, Scicchitano P, Ciccone MM, Caldarola P, Aspromonte N, Iacoviello M, et al. Bioimpedance vector analysis predicts hospital length of stay in acute heart failure. Nutrition. 2019;61:56-60.
- Massari F, Iacoviello M, Scicchitano P, Mastropasqua F, Guida P, Riccioni G, et al. Accuracy of bioimpedance vector analysis and brain natriuretic peptide in detection of peripheral edema in acute and chronic heart failure. Heart Lung. 2016;45(4):319-26.
- Massari F, Scicchitano P, Iacoviello M, Passantino A, Guida P, Sanasi M, et al. Multiparametric approach to congestion for predicting long-term survival in heart failure. J Cardiol. 2020;75(1):47-52.
- Lindholm D, Fukaya E, Leeper NJ, Ingelsson E. Bioimpedance and new-onset heart failure: a longitudinal study of> 500 000 individuals from the general population. Journal of the American Heart Association. 2018;7(13):e008970.
- Montalibet A, Rastel D, Chaigneau C, Grenier E, McAdams E. Comparison between bioelectrical impedance spectroscopy measurements and water volume displacement of ankle oedema variations during the course of a day. Physiol Meas. 2020;41(8):085004.
- Sun SS, Chumlea WC, Heymsfield SB, Lukaski HC, Schoeller D, Friedl K, et al. Development of bioelectrical impedance analysis prediction equations for body composition with the use of a multicomponent model for use in epidemiologic surveys. The American journal of clinical nutrition. 2003;77(2):331-40.
- Chabin X, Taghli-Lamallem O, Mulliez A, Bordachar P, Jean F, Futier E, et al. Bioimpedance analysis is safe in patients with implanted cardiac electronic devices. Clin Nutr. 2019;38(2):806-11.
- Ferreira J, Pau I, Lindecrantz K, Seoane F. A Handheld and Textile-Enabled Bioimpedance System for Ubiquitous Body Composition Analysis. An Initial Functional Validation. IEEE journal of biomedical and health informatics. 2017;21(5):1224-32.
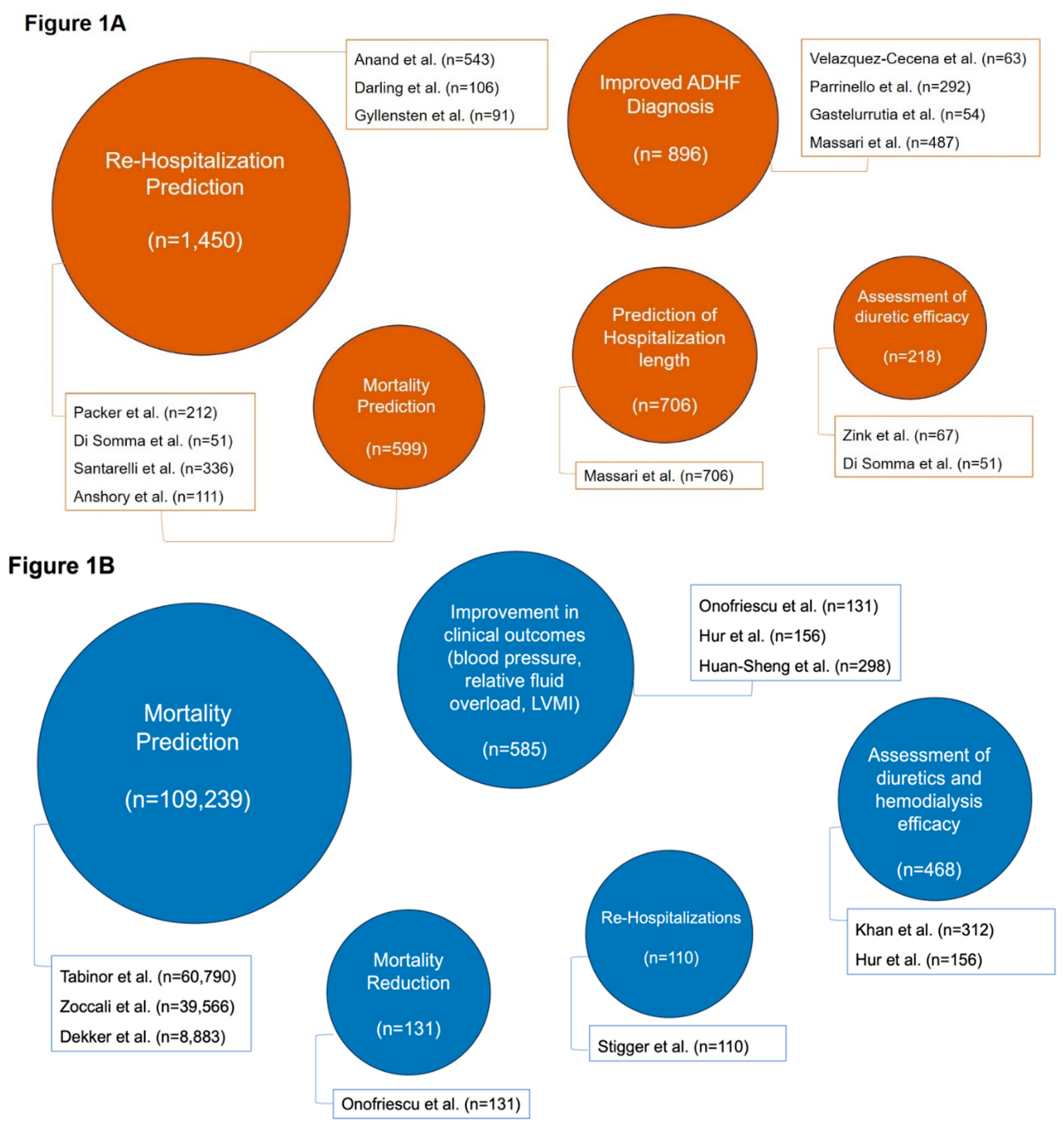
| Author | Year | Journal | Type of study | Patients | BIA Method | Endpoints | Limitations | Main Results |
|---|---|---|---|---|---|---|---|---|
| Packer - (PREDICT) |
2006 | Journal of the American College of Cardiology |
Prospective non-randomized study |
212 | Non-invasive transthoracic impedance |
All-cause mortality or HF hospitalizations in 14 days | Small sample size |
BENEFICIAL EFFECTS Clinical and ICG parameters predict HF at 14 days (p = 0.0002). High-risk ICG score presents an 8.4% event rate at 14 days (Accuracy: 41.6%) |
| Di Somma |
2010 | Congestive Heart Failure | Prospective non-randomized study |
51 | BIVA | All-cause mortality or HF hospitalizations in 90 days | Small sample size |
BENEFICIAL EFFECTS Overhydration >80.5% measured with BIVA correlated with primary endpoint at 3 months (Sensitivity: 22%; Specificity: 94.2%) |
| van Veldhuisen - (DOT-HF) | 2011 | Circulation | Randomized trial - Remote Monitoring |
335 | Intrathoracic impedance monitoring | All-cause mortality or HF hospitalizations in 15 months | Terminated prematurely due to slow enrollment |
NEUTRAL EFFECTS No difference in mortality between groups (p=0.54); number of outpatient visits was higher in the intervention group (250 versus 84; p<0.0001). |
| Anand - (MUSIC Study) |
2012 | Journal of Cardiac Failure |
Prospective non-randomized study - Remote Monitoring |
543 | Non-invasive transthoracic impedance |
ADHF event in 90 days | High exclusion rate due to device failure or withdrawal of consent |
BENEFICIAL EFFECTS Thoracic fluid content (TFC) found sensitive for predicting HF decompensation up to 9-11 days in advance. TFC increase of 7.5% from baseline was found to predict the primary endpoint (Sensitivity: 77%; Specificity: 64%). |
| Gyllensten | 2016 | JMIR Med Inform. |
Prospective non-randomized study - Remote Monitoring |
91 | Non-invasive transthoracic impedance | ADHF event in 14 days | Possibility of early intervention affecting results, small sample size |
BENEFICIAL EFFECTS Non-invasive transthoracic BIA predicts a decompensation event 2 weeks in advance (P<.001). NEUTRAL EFFECTS Non-invasive transthoracic BIA and its algorithms had a low positive predictive value for overt HF decompensation events. |
| Darling - (SENTINEL -HF) |
2017 | JMIR Cardio | Prospective non-randomized study - Remote Monitoring |
16 | Non-invasive transthoracic impedance |
ADHF event in 45 days | Small sample size, specificity was affected by false postives, homogeneous cohort |
BENEFICIAL EFFECTS An algorithm utilizing non-invasive thoracic BIA is highly predictive for the identification of recurrent HF events at 45 days (Accuracy: 72%) |
| Santarelli |
2017 | Eur Heart J – Acute Cardiovascular Care | Prospective non-randomized study |
336 | BIVA | All-cause mortality or HF hospitalizations in 90 days | Higher accuracy if BIVA is utilized with clinical signs |
BENEFICIAL EFFECTS BIVA predicts the primary endpoints at admission (area under the curve (AUC) 0.56, p<0.04) and at discharge (AUC 0.57, p<0.03). By combining BIVA with clinical signs, a high predictive value for cardiovascular events at 90 days (AUC 0.97, p<0.0001) was observed. |
| Anshory | 2024 | Advances in Medical Sciences | Prospective non-randomized study |
111 | Non-invasive transthoracic impedance + BIVA |
All-cause mortality or HF hospitalizations in 30 days | Drugs used in patient treatment and inotropic usage may have conditioned the results |
BENEFICIAL EFFECTS Hemodynamic and TBW data significantly predicted 30-day cardiovascular mortality and rehospitalization. At discharge, a value of cardiac output was a significant predictor for 30-day rehospitalization. |
| Author | Year | Journal | Type of study | Patients |
BIA Method | Endpoints | Limitations | Main Results |
|---|---|---|---|---|---|---|---|---|
| Onofriescu |
2014 | American Journal of Kidney Diseases |
Prospective randomized controlled trial - Hemodialysis |
131 | BIS |
All-cause mortality | Underpowered regarding mortality outcomes due to younger cohort and less diabetes rate |
BENEFICIAL EFFECTS BIA-based fluid management significantly reduced mortality in HD patients (HR=0.100 95% CI, 0.013-0.805). |
| Huan-Sheng – (ABISAD III) |
2016 | International Urolology and Nephrology | Prospective randomized controlled trial - Hemodialysis |
298 | BIS | All-cause hospitalizations AFO |
This study focused on FO post HD, further studies are needed to show whether other interventions besides weight adjustment play a role in this improvement. |
BENEFICIAL EFFECTS AFO incidence, cardiovascular events, or intradialytic complications were significantly reduced in the intervention group. NEUTRAL EFFECTS All-cause hospitalization rate was not different between groups. |
| Zoccali |
2017 | Journal of the American Society of Nephrology | Prospective non-randomized study - Hemodialysis |
39,566 | BIS |
All-cause mortality at 1 and 4 years | Purely observational nature of the study |
BENEFICIAL EFFECTS Baseline OHI/ECW >15% in men and >13% in women at baseline were independent predictors of mortality in HD patients (HR = 1.26, 95% CI 1.19–1.33). |
| Tabinor |
2018 | Scientfic report | Meta-analysis - Hemodialysis |
60,790 | Various BIA methods |
Mortality | Methodological heterogeneity, inadequately reported demographics and report of endpoints |
BENEFICIAL EFFECTS Baseline pre-dialysis OHI>15% is predictive for mortality in HD patients (HR = 2.28, 95% CI 1.56-3.34). |
| Dekker |
2018 | Nephrology Dialysis Transplantation. | Prospective study - Hemodialysis |
8,883 | BIS |
Mortality | No documentation of antihypertensive medications, echocardiographic results are not available and cardiac failure is likely underreported |
BENEFICIAL EFFECTS Pre-dialysis FO (>+1.1 to +2.5 L) together with pre-SBP <110 mmHg was associated with an increased mortality (HR = 1.52; 95% CI 1.06–2.17) |
| Liu - (BOCOMO Study) |
2020 | BMC Nephrology | Prospective randomized controlled trial - Hemodialysis |
445 | BIS |
All-cause mortality, myocardial infarction, cerebral infarction, cerebral hemorrhage, and peripheral vascular disease | Small cohort and limited follow up period |
NEUTRAL EFFECTS An increasing trend of survival rates in patients with BIA guided HD was observed, however no significant difference observed. (log-rank test, p= 0.07). |
| Horowitz |
2023 | Canadian Journal of Kidney Health and Disease | Systematic Review and Meta-Analysis - Hemodialysis |
2,420 | Various BIA methods |
All-cause mortality, blood pressure control, all-cause hospitalization, major adverse cardiovascular events, and change in left ventricular mass index | Heterogenity in reported endpoints/outcomes |
BENEFICIAL EFFECTS In HD patients using BIA decreases all-cause mortality and blood pressure. NEUTRAL EFFECTS No significant difference in all-cause hospitalization, major adverse cardiac event, or change in left ventricular mass index was observed. |
| Stigger |
2023 | Hemodialysis International | Prospective randomized controlled trial - Hemodialysis |
110 | BIS |
All-cause mortality, blood pressure control, all-cause hospitalization | Small sample size |
BENEFICIAL EFFECTS BIA utilization significantly reduced the incidence rate of hospital admissions in HD patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





