Submitted:
15 August 2024
Posted:
27 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
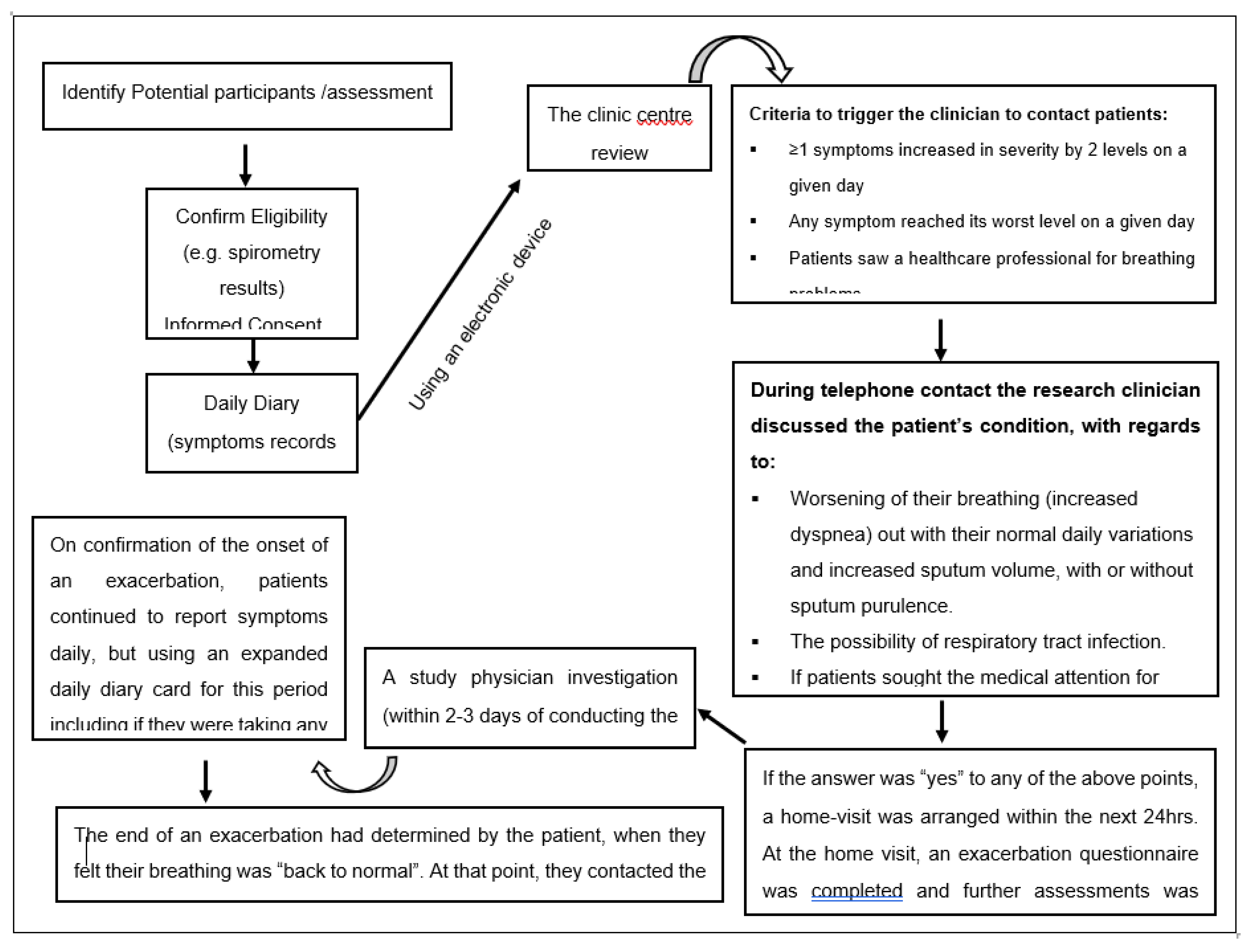
2.1. Study Design and Participants Selection Criteria
- Aged 40 years and over.
- A history of breathing problems categorized as COPD GOLD grade I to IV at the time of informed consent.
- All participants reported, at the baseline visit, being current smokers or ex-smokers (previously having consumed ten or more packs per year).
- Having less than 20% reversibility in FEV1 (forced expiratory volume in one second) and less than 200 ml increase in FEV1 following the administration of salbutamol (200 µg).
- Willingness to provide informed consent and a willingness to use the website or the telephone platform, respond to clinical team notifications, and follow professional advice. This allowed for up to six months of symptom monitoring from a distance, with follow-up assistance available by phone.
- A history of asthma.
- A history of seasonal allergic rhinitis.
- Patients with a significant co-morbidity which might prevent them from being able to withstand the study procedure.
2.2. Practitioners and Tutorial
2.3. Data Collection and Assessment of AECOPD
2.4. Follow-Up Satisfaction
- How did you like the service you received? (for patients only);
- Do you think this service is needed for every patient that enters the clinic? (for patients and practitioners);
- Was the service provided effectively? (for patients and practitioners);
- Was the time spent on the tutorial sufficient and useful? (for practitioners only).
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Characteristics of AECOPD
3.3. Patients’ and Practitioner’ Satisfaction with the Service
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- NICE Chronic Obstructive Pulmonary disease: quick reference guide, National Institute for Health and Care Excellence; National Institute for Health and Care Excellence, 2010.
- GOLD The Global Initiative for Chronic Obstructive Lung Disease (GOLD). https://goldcopd.org/2023-gold-report-2/ (1 January).
- Alsayed, A.R.; Abed, A.; Khader, H.A.; Al-Shdifat, L.M.H.; Hasoun, L.; Al-Rshaidat, M.M.D.; Alkhatib, M.; Zihlif, M. Molecular Accounting and Profiling of Human Respiratory Microbial Communities: Toward Precision Medicine by Targeting the Respiratory Microbiome for Disease Diagnosis and Treatment. Int. J. Mol. Sci. 2023, 24, 4086. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A. R.; Al-Dulaimi, A.; Alkhatib, M.; Al Maqbali, M.; Al-Najjar, M. A. A.; Al-Rshaidat, M. M. , A comprehensive clinical guide for Pneumocystis jirovecii pneumonia: A missing therapeutic target in HIV-uninfected patients. Expert Review of Respiratory Medicine 2022, 16, (11–12), 1167. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A.R.; Abed, A.; Khader, H.A.; Hasoun, L.; Al Maqbali, M.; Al Shawabkeh, M.J. The role of human rhinovirus in COPD exacerbations in Abu Dhabi: molecular epidemiology and clinical significance. Libyan J. Med. 2024, 19, 2307679. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A.R.; Abed, A.; Jarrar, Y.B.; Alshammari, F.; Alshammari, B.; Basheti, I.A.; Zihlif, M. Alteration of the Respiratory Microbiome in Hospitalized Patients with Asthma–COPD Overlap during and after an Exacerbation. J. Clin. Med. 2023, 12, 2118. [Google Scholar] [CrossRef] [PubMed]
- Alsayed, A. R.; Abed, A.; Al Shawabkeh, M. J.; Aldarawish, R. R.; Al-Shajlawi, M.; Alabbas, N. , Human Rhinovirus: Molecular and Clinical Overview. Pharmacy Practice (Granada) 2024, 22, (1), 1. [Google Scholar]
- Halpin, D.M.; Decramer, M.; Celli, B.; Kesten, S.; Liu, D.; Tashkin, D.P.; Celli, B.; Liu, D. Exacerbation frequency and course of COPD. Int. J. Chronic Obstr. Pulm. Dis. 2012, 7, 653–661. [Google Scholar] [CrossRef]
- Anzueto, A.; Leimer, I.; Kesten, S. , Impact of frequency of COPD exacerbations on pulmonary function, health status and clinical outcomes. International journal of chronic obstructive pulmonary disease 2009, 4, 245–251. [Google Scholar]
- Alsayed, A.R. Illustrating How to Use the Validated Alsayed_v1 Tools to Improve Medical Care: A Particular Reference to the Global Initiative for Asthma 2022 Recommendations. Patient Preference Adherence 2023, ume 17, 1161–1179. [Google Scholar] [CrossRef]
- Al-kilkawi, Z. M.; Basheti, I. A.; Obeidat, N. M.; Saleh, M. R.; Hamadi, S.; Abutayeh, R.; Nassar, R.; Alsayed, A. R. , Evaluation of the Association between Inhaler technique and Adherence in Asthma Control: Cross-Sectional Comparative Analysis Study between Amman and Baghdad. Pharmacy Practice 2024, 22, (1), 1–12. [Google Scholar]
- Al-Hamaden, R.A.; Abed, A.; Khader, H.; Hasoun, L.; Al-Dulaimi, A.H.; Alsayed, A. Knowledge and Practice of Healthcare Professionals in the Medical Care of Asthma Adult Patients in Jordan with a Particular Reference to Adherence to GINA Recommendations. J. Multidiscip. Heal. 2024, ume 17, 391–404. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Halloush, S.; Hasoun, L.; Alnatour, D.; Al-Dulaimi, A.; Alnajjar, M.S.; Blaibleh, A.; Al-Imam, A.; Alshammari, F.; A Khader, H. Perspectives of the community in the developing countries toward telemedicine and pharmaceutical care during the COVID-19 pandemic. Pharm. Pr. (Internet) 2022, 20, 2618–2618. [Google Scholar] [CrossRef]
- Khader, H.; Alsayed, A.; Hasoun, L. Z.; Alnatour, D.; Awajan, D.; Alhosanie, T. N.; Samara, A. , Pharmaceutical care and telemedicine during COVID-19: A cross-sectional study based on pharmacy students, pharmacists, and physicians in Jordan. Pharmacia 2022, 69, (3), 891–901. [Google Scholar] [CrossRef]
- Cruz, J.; Brooks, D.; Marques, A. Home telemonitoring effectiveness in COPD: a systematic review. Int. J. Clin. Pr. 2014, 68, 369–378. [Google Scholar] [CrossRef] [PubMed]
- McCabe, C.; McCann, M.; Brady, A.M. Computer and mobile technology interventions for self-management in chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2017, 2020, CD011425. [Google Scholar] [CrossRef]
- Alsayed, A. R.; Al-Dulaimi, A.; Alnatour, D.; Awajan, D.; Alshammari, B. , Validation of an assessment, medical problem-oriented plan, and care plan tools for demonstrating the clinical pharmacist's activities. Saudi Pharm J 2022, 30, (10), 1464–1472. [Google Scholar] [CrossRef]
- Alsayed, A.R.; Hasoun, L.; A Khader, H.; Al-Dulaimi, A.; AbuAwad, A.; Basheti, I.; Al Maqbali, M. Evaluation of the effectiveness of educational medical informatics tutorial on improving pharmacy students’ knowledge and skills about the clinical problem-solving process. Pharm. Pr. (Internet) 2022, 20, 01–08. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.W.; Hershfield, E.S.; Harding, G.K.M. Antibiotic Therapy in Exacerbations of Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N.R.; Manfreda, J.; Warren, C.P.W.; Hershfield, E.S.; Harding, G.K.M. Antibiotic Therapy in Exacerbations of Chronic Obstructive Pulmonary Disease. Ann. Intern. Med. 1987, 106, 196–204. [Google Scholar] [CrossRef]
- Al-Awaisheh, R. I.; Alsayed, A. R.; Basheti, I. A. , Assessing the Pharmacist's Role in Counseling Asthmatic Adults Using the Correct Inhaler Technique and Its Effect on Asthma Control, Adherence, and Quality of Life. Patient Prefer Adherence 2023, 17, 961–972. [Google Scholar] [CrossRef]
- Wilkinson, T.M.A.; Donaldson, G.C.; Hurst, J.R.; Seemungal, T.A.R.; Wedzicha, J.A. Early Therapy Improves Outcomes of Exacerbations of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 2004, 169, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- GOLD, Global Initiative for Chronic Obstructive Lung Disease. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease (GOLD). 2023.
- Alsayed, A.R.; Abu-Samak, M.S.; Alkhatib, M. Asthma-COPD Overlap in Clinical Practice (ACO_CP 2023): Toward Precision Medicine. J. Pers. Med. 2023, 13, 677. [Google Scholar] [CrossRef] [PubMed]
- Sund, Z.; Powell, T.; Greenwood, R.; Jarad, N. Remote daily real-time monitoring in patients with COPD – A feasibility study using a novel device. Respir. Med. 2009, 103, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Velardo, C.; Farmer, A.; Tarassenko, L. Exacerbations in Chronic Obstructive Pulmonary Disease: Identification and Prediction Using a Digital Health System. J. Med Internet Res. 2017, 19, e69. [Google Scholar] [CrossRef] [PubMed]
- Lanclus, M.; Clukers, J.; Van Holsbeke, C.; Vos, W.; Leemans, G.; Holbrechts, B.; Barboza, K.; De Backer, W.; De Backer, J. Machine Learning Algorithms Utilizing Functional Respiratory Imaging May Predict COPD Exacerbations. Acad. Radiol. 2018, 26, 1191–1199. [Google Scholar] [CrossRef]
- Cavailles, A.; Melloni, B.; Motola, S.; Dayde, F.; Laurent, M.; Le Lay, K.; Caumette, D.; Luciani, L.; Lleu, P.L.; Berthon, G.; et al. Identification of Patient Profiles with High Risk of Hospital Re-Admissions for Acute COPD Exacerbations (AECOPD) in France Using a Machine Learning Model. Int. J. Chronic Obstr. Pulm. Dis. 2020, ume 15, 949–962. [Google Scholar] [CrossRef]
- Li, X.; Xie, Y.; Zhao, H.; Zhang, H.; Yu, X.; Li, J. Telemonitoring Interventions in COPD Patients: Overview of Systematic Reviews. BioMed Res. Int. 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rubio, N.; A Parker, R.; Drost, E.M.; Pinnock, H.; Weir, C.J.; Hanley, J.; Mantoani, L.C.; MacNee, W.; McKinstry, B.; A Rabinovich, R. Home monitoring of breathing rate in people with chronic obstructive pulmonary disease: observational study of feasibility, acceptability, and change after exacerbation. Int. J. Chronic Obstr. Pulm. Dis. 2017, ume 12, 1221–1231. [Google Scholar] [CrossRef]
- Cooper, C.B.; Sirichana, W.; Arnold, M.T.; Neufeld, E.V.; Taylor, M.; Wang, X.; A Dolezal, B. Remote Patient Monitoring for the Detection of COPD Exacerbations. Int. J. Chronic Obstr. Pulm. Dis. 2020, 15, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Anthonisen, N. R.; Manfreda, J.; Warren, C. P. W. , Antiobiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987, 106, (2), 196–204. [Google Scholar] [CrossRef]
- Burge, S.; Wedzicha, J. COPD exacerbations: definitions and classifications. Eur. Respir. J. 2003, 21, 46S–53s. [Google Scholar] [CrossRef]
- So, J.Y.; Lastra, A.C.; Zhao, H.; Marchetti, N.; Criner, G.J. Daily Peak Expiratory Flow Rate and Disease Instability in Chronic Obstructive Pulmonary Disease. Chronic Obstr. Pulm. Dis. J. COPD Found. 2016, 3, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Welford, C. R. In A comprehensive computerized patient record with automated linkage to QMR, Proceedings of the Annual Symposium on Computer Application in Medical Care, 1994; American Medical Informatics Association: p 814.
- Elhanan, G.; A Socratous, S.; Cimino, J.J. In Integrating DXplain into a clinical information system using the World Wide Web, Proceedings of the AMIA Annual Fall Symposium, 1996; American Medical Informatics Association: p 348.
- Berner, E.S.; Detmer, D.E.; Simborg, D. Will the Wave Finally Break? A Brief View of the Adoption of Electronic Medical Records in the United States. J. Am. Med Informatics Assoc. 2004, 12, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Weingart, N. S.; Wilson, R. M.; Gibberd, R. W.; Harrison, B. , Epidemiology of medical error. Bmj 2000, 320, (7237), 774–777. [Google Scholar] [CrossRef]
- Burroughs, T.E.; Waterman, A.D.; Gallagher, T.H.; Waterman, B.; Adams, D.; Jeffe, D.B.; Dunagan, W.C.; Garbutt, J.; Cohen, M.M.; Cira, J.; et al. Patient Concerns about Medical Errors in Emergency Departments. Acad. Emerg. Med. 2005, 12, 57–64. [Google Scholar] [CrossRef]
- Institute of Medicine Committee on Quality of Health Care in, A., In To Err is Human: Building a Safer Health System, Kohn, L. T.; Corrigan, J. M.; Donaldson, M. S., Eds.
- Copyright 2000 by the National Academy of Sciences. All rights reserved.: Washington (DC), 2000.
- Fortescue, E.B.; Kaushal, R.; Landrigan, C.P.; McKenna, K.J.; Clapp, M.D.; Federico, F.; Goldmann, D.A.; Bates, D.W. Prioritizing Strategies for Preventing Medication Errors and Adverse Drug Events in Pediatric Inpatients. Pediatrics 2003, 111, 722–729. [Google Scholar] [CrossRef]
- Kaushal, R.; Bates, D. , Information technology and medication safety: what is the benefit? BMJ Quality & Safety 2002, 11, (3), 261–265. [Google Scholar]
| Visit | Baseline | Daily | Exacerbation | Final |
|---|---|---|---|---|
| Eligibility (inclusion/exclusion criteria) | * | |||
| Consent | * | |||
| Data collection using ASAMI database | * | * | * | |
| Symptoms (daily diary) | * | * | * | * |
| Peak Expiratory Flow Rate | * | * | * | * |
| Pulmonary Function Test (Spirometry using Spirolab®) | * | * | * | |
| Spontaneous sputum collection (optional) | * | * | ||
| Nursing assessment | * | * | * | |
| Adverse events (to study procedures) | * | * | * | |
| Concomitant medications | * | * | * |
| Daily Diary Questions | أسئلة يوميات يومية | |
|---|---|---|
| 1 | Did you cough Today? | هل سعلت اليوم؟ |
| □ No □ Mild □ Moderate □ Severe □ Very severe |
□ لا □ خفيف □ معتدل □ شديد □ شديد جدا |
|
| 2 | Did you cough up phlegm Today? | هل تسعل البلغم اليوم؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 3 | Did you have breathing problems Today? | هل عانيت من مشاكل في التنفس اليوم؟ |
| □ No □ Mild □ Moderate □ Severe □ Very severe |
□ لا □ خفيف □ معتدل □ شديد □ شديد جدا |
|
| 4 | Did breathing problems interfere with any of your regular activities (Such as working, walking, hobbies, meeting friends, shopping, or family visits) Today? | هل تعارضت مشاكل التنفس مع أي من أنشطتك المعتادة (مثل العمل ، أو المشي ، أو الهوايات ، أو مقابلة الأصدقاء ، أو التسوق ، أو الزيارات العائلية) اليوم؟ |
| □ No □ Mild □ Moderate □ Severe □ Very severe |
□ لا □ خفيف □ معتدل □ شديد □ شديد جدا |
|
| 5 | Have you had any of the symptoms of a cold or flu shown below (such as Runny/stuffy nose, change in phlegm color or stickiness, sore throat, fever, shivers, chest congestion? today? | هل عانيت من أي من أعراض البرد أو الأنفلونزا (مثل سيلان الأنف ، أو تغير في لون البلغم أو اللزوجة ، أو التهاب الحلق ، أو الحمى ، أو الرعشة ، أو احتقان الصدر؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 6 | Did you increase the frequency of using the respiratory symptoms reliever medication Today? | هل قمت بزيادة تكرار استخدام الدواء المسكن لأعراض الجهاز التنفسي اليوم؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 7 | Did your oxygen saturation become below 90% Today? | هل أصبحت قراءة التشبع بالأكسجين أقل من 90٪ اليوم؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 8 | Did you see a healthcare professional Today for breathing problems or a cold? | هل رأيت أخصائي رعاية صحية اليوم لمشاكل في التنفس أو نزلة برد؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 9 | Is there anything you want the team to contact you about? | هل هناك أي شيء تريد أن يتصل بك الفريق بشأنه؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| When exacerbation was Confirmed | عندما تم تأكيد تفاقم الحالة | |
| 1 | Did you take prednisone Today? | هل تناولت بريدنيزون اليوم؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 2 | Did you take an antibiotic Today? | هل تناولت المضاد الحيوي اليوم؟ |
| □ No □ Yes |
□ لا □ نعم |
|
| 3 | Do you think your breathing is back to normal Today? | هل تعتقد أن تنفسك وأعراضك عادت إلى طبيعته اليوم؟ |
| □ No □ Yes □ Don’t know |
□ لا □ نعم □ لا أعرف |
| Participant Descriptions | GOLD I (n = 18), II (n = 42), III (n = 24), IV (n = 3) | |
|---|---|---|
| Age, M (range), y | 59.7 (45-81) | |
| Male / Female | 57 (66) / 30 (34) | |
| Current smokers | 18 (21) | |
| Smoking, median (IQR), pack-y | 44 (40) | |
| BMI, M (SD), kg/m2 | 27.7 (5.4) | |
| Postbronchodilator FEV1% predicted at the baseline, M (SD) | 58.7 (7.5) | |
| ED visit due to breathing difficulties in the previous year | 18 (21) | |
| ≥ 1 hospitalization for breathing difficulties in the previous year | 12 (14) | |
| Participant medications at baseline | ||
| SABA | 57 (66) | |
| SAMA | 3 (3) | |
| SABA + SAMA | 18 (21) | |
| LABA | 3 (3) | |
| LAMA | 18 (21) | |
| LAMA + LABA | 45 (52) | |
| ICS + LABA | 15 (17) | |
| LAMA + ICS + LABA | 6 (7) | |
| Descriptions of AECOPD | GOLD I (n = 9), II (n = 42), III (n = 24), IV (n = 3) | |
|---|---|---|
| AECOPD no. by Anthonisen type | ||
| 1a | 81 (43) | |
| 2 | 60 (32) | |
| 3 | 48 (25) | |
| Total | 189 | |
| AECOPD per participant, No. | ||
| 0 | 3 | |
| 1 | 26 | |
| 2 | 18 | |
| 3 | 11 | |
| ≥ 4 | 8 | |
| AECOPD need any healthcare professional help | 120 (63) | |
| AECOPD required to visit a respiratory specialist | 21 (12) | |
| AECOPD required ED treatment | 18 (10) | |
| AECOPD required hospitalization | 18 (10) | |
| AECOPD lengthb, median (range), days | 9 (2-36) | |
| AECOPD with prednisone prescription, without hospitalization | 78 (41) | |
| AECOPD with an antibiotic prescription, without hospitalization | 120 (63) | |
| The absolute decrease in % predicted FEV1 postbronchodilator at AECOPD from baseline, M (SD) | 5.2 (7.3) | |
| Unresolved AECOPD (no return to normal breathing)b | 6 (3) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





