Submitted:
27 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Isolation and Purification of Microorganisms
2.2. Macroscopic Identification
2.3. Microscopic Identification
2.4. In Vivo Fungal Activity In Vivo
2.5. In Vitro Antifungal Activity with Essential Oils
3. Results
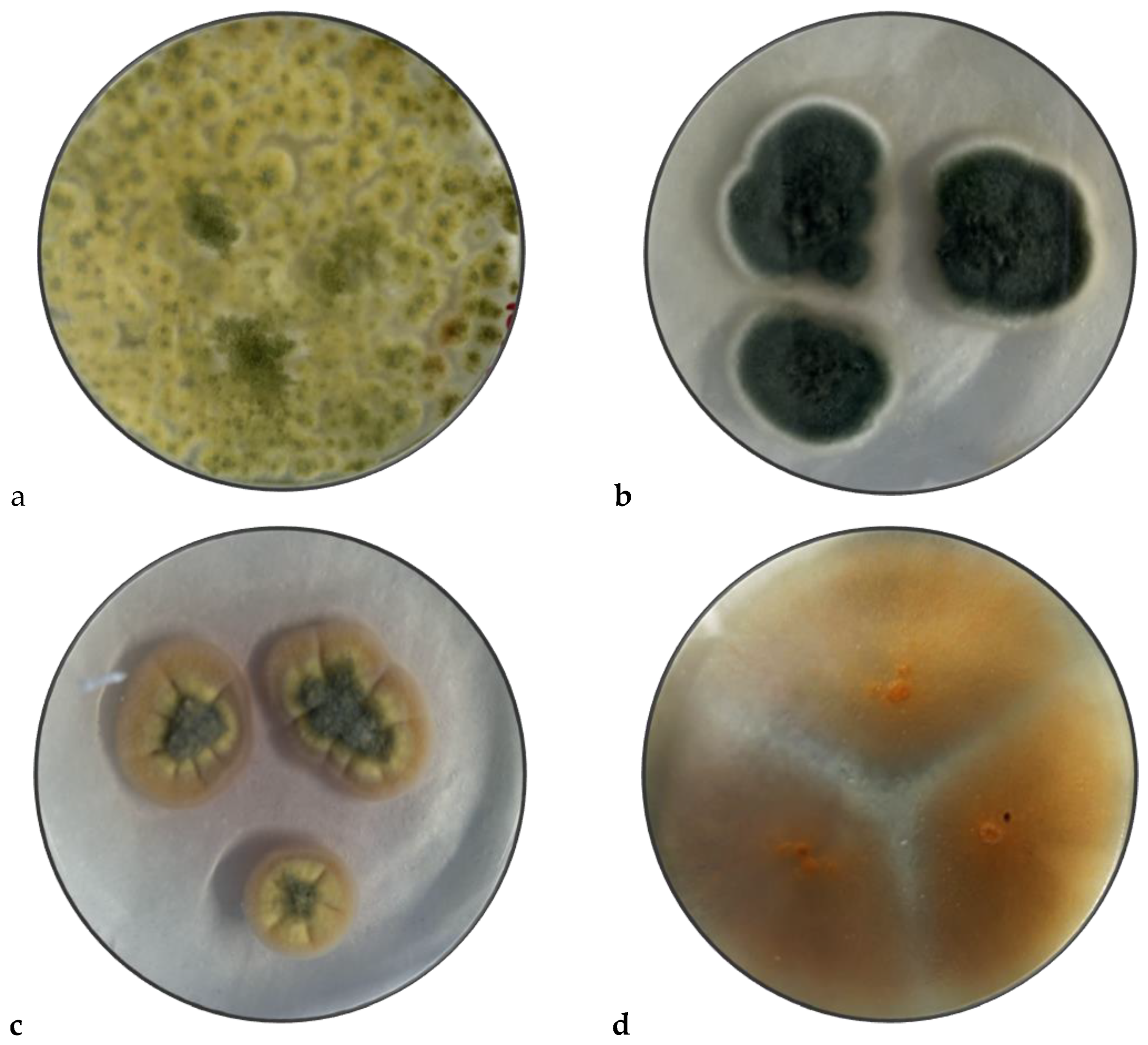
3.2. Macroscopic Identification
3.3. Microscopic identification
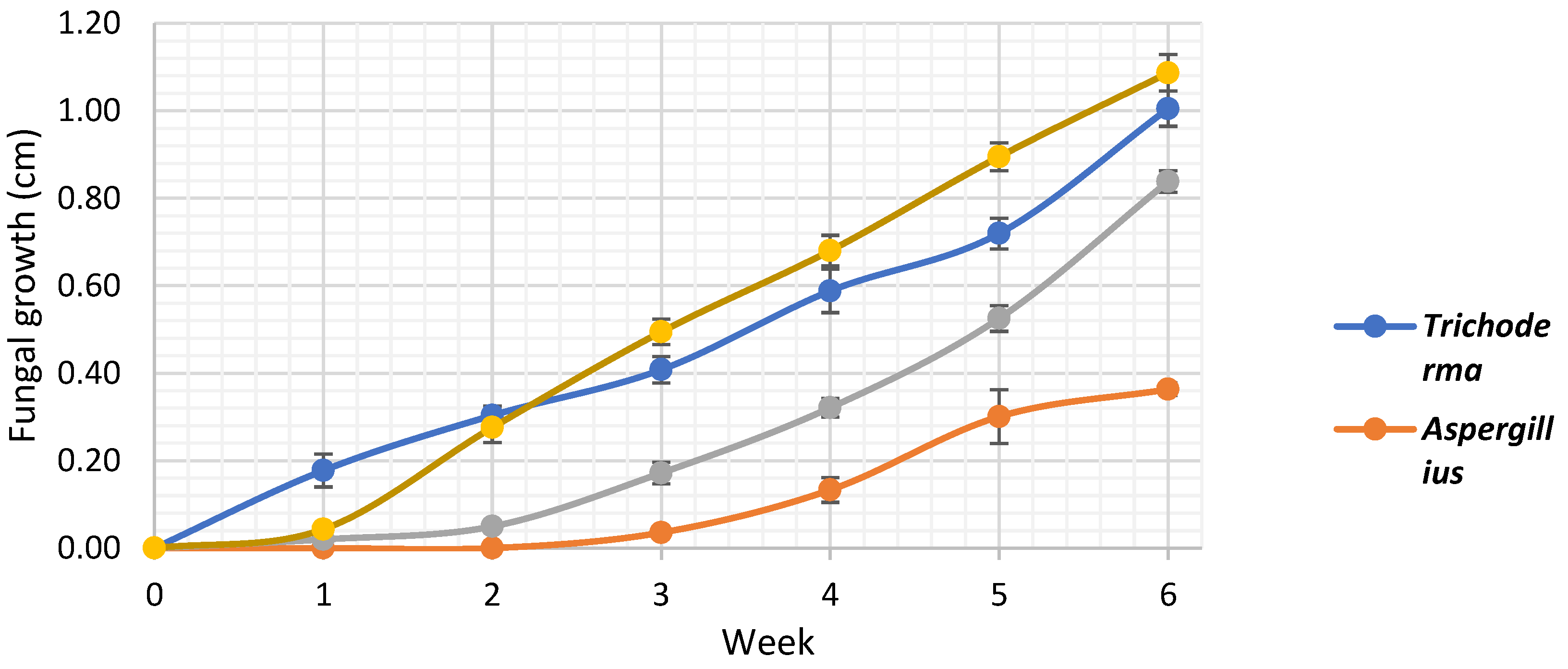
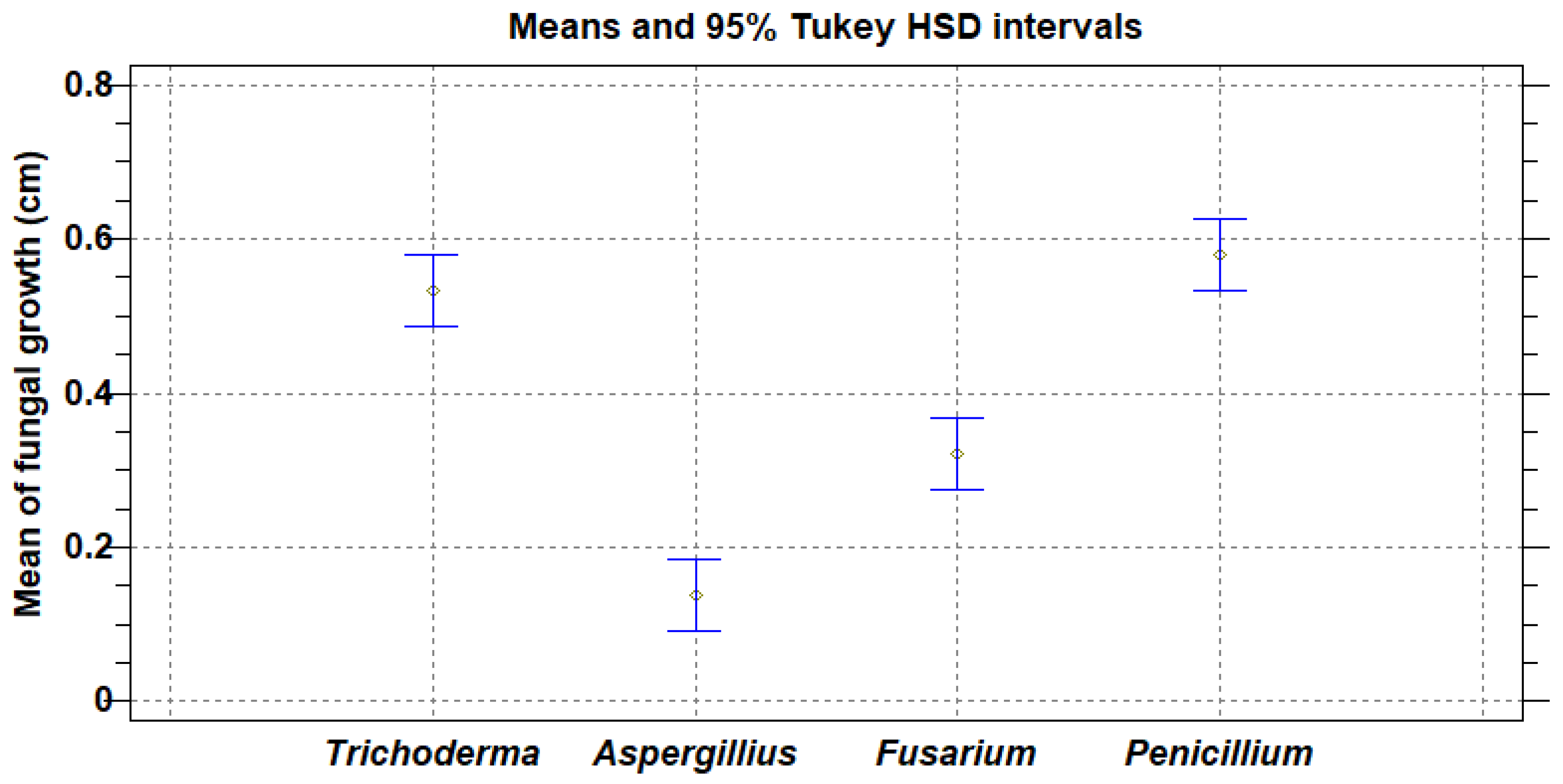
3.4. In Vivo Fungal Activity
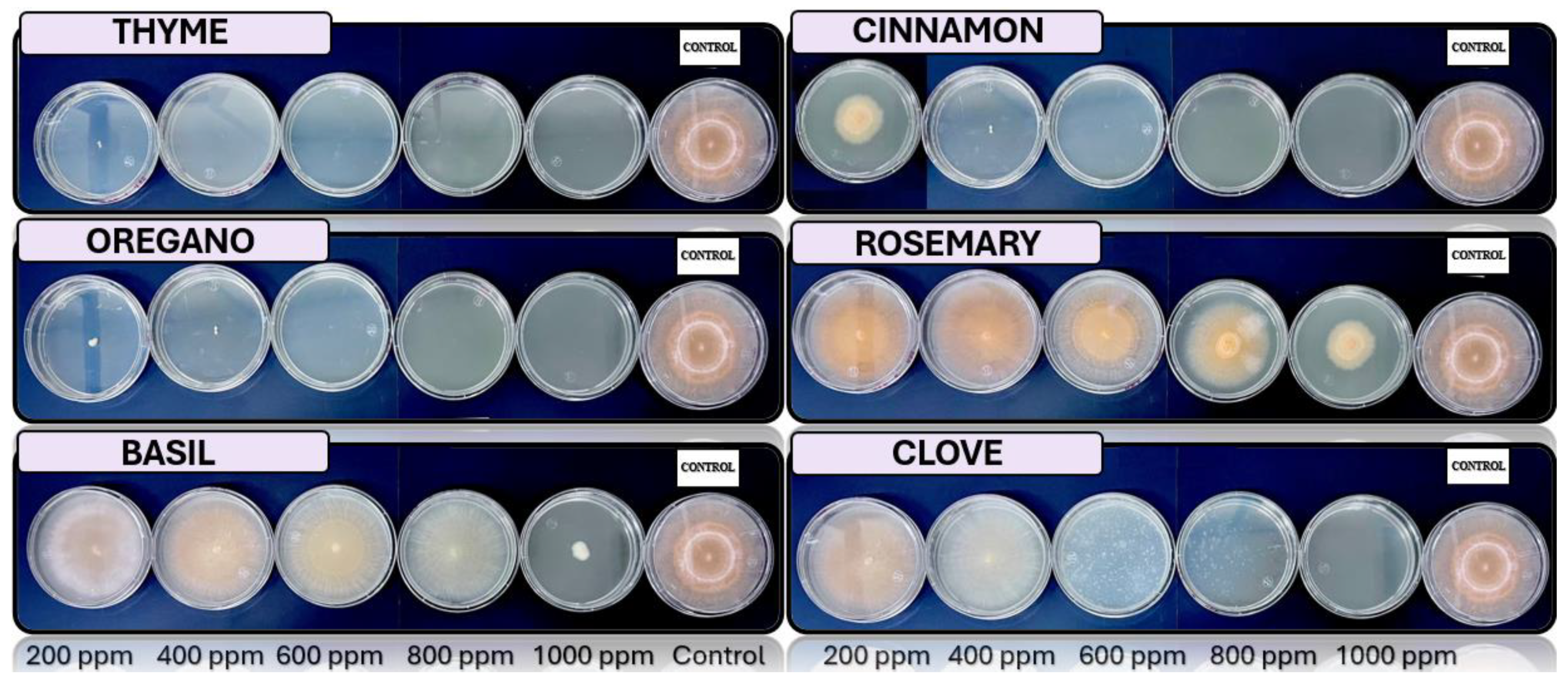
3.5. In Vitro Antifungal Activity with Essential Oils
4. Discussion
4.1. Macroscopic Identification
4.2. Microscopic Identification
4.3. In Vivo Fungal Activity
4.4. In Vitro Antifungal Activity with Essential Oils
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Galan V, Rangel A, Lopez J, Hernandez JBP, Sandoval J, Rocha HS. Propagación del banano: técnicas tradicionales, nuevas tecnologías e innovaciones. Rev Bras Frutic 2018;40. [CrossRef]
- Capa Benítez LB, Alaña Castillo TP, Benítez Narváez RM. Importancia de la producción de banano orgánico. Caso: Provincia de El Oro, Ecuador. Revista Universidad y Sociedad 2016;8:64–71.
- Ruiz Medina MD, Ruales J. Postharvest Alternatives in Banana Cultivation. 2024. [CrossRef]
- Mata Anchundia D, Suatunce Cunuhay P, Poveda Morán R. Análisis económico del banano orgánico y convencional en la provincia Los Ríos, Ecuador. Avances 2021;23:419–30.
- Aguilar-Anccota R, Arévalo-Quinde CG, Morales-Pizarro A, Galecio-Julca M, Aguilar-Anccota R, Arévalo-Quinde CG, et al. Hongos asociados a la necrosis de haces vasculares en el cultivo de banano orgánico: síntomas, aislamiento e identificación, y alternativas de manejo integrado. Scientia Agropecuaria 2021;12:249–56. [CrossRef]
- Guzmán O. El nemátodo barrenador (Radopholus similis [COBB] Thorne) del banano y plátano 2011;1.
- Castellanos D, Algecira N, Villota C. Aspectos relevantes en el almacenamiento de banano en empaques con atmósferas modificadas. 2011 2011;12:114–34.
- Barrera Necha LL, García Barrera LJ. Actividad antifúngica de aceites esenciales y sus compuestos sobre el crecimiento de Fusarium sp. aislado de papaya ( Carica papaya). Revista Científica UDO Agrícola 2008;8:33–41.
- López Luengo MT. El romero. Planta aromática con efectos antioxidantes. Offarm 2008;27:60–3.
- Flores-Villa E, Sáenz-Galindo A, Castañeda-Facio AO, Narro-Céspedes RI, Flores-Villa E, Sáenz-Galindo A, et al. Romero (Rosmarinus officinalis L.): su origen, importancia y generalidades de sus metabolitos secundarios. TIP Revista especializada en ciencias químico-biológicas 2020;23. [CrossRef]
- Ruiz M, Ávila J, Ruales J. Diseño de un recubrimiento comestible bioactivo para aplicarlo en la frutilla (Fragaria vesca) como proceso de postcosecha 2016;17:276–87.
- Características de los aceites esenciales de vegetales y las implicaciones de sus interacciones bioquímicas con los componentes de alimentos en la eficiencia de los procesos de conservación | TecnoCultura 2023.
- Mesa V a. M, Marín P, Ocampo O, Calle J, Monsalve Z, Mesa V a. M, et al. Fungicidas a partir de extractos vegetales: una alternativa en el manejo integrado de hongos fitopatógenos. RIA Revista de Investigaciones Agropecuarias 2019;45:23–30.
- Jeri R, H C. Consideraciones epidemiológicas para el manejo de la marchitez por Fusarium (Fusarium oxysporum f. sp. cubense) del banano en la región central del Perú 2012.
- Magdama F, Monserrate-Maggi L, Serrano L, García Onofre J, Jiménez-Gasco M del M. Genetic Diversity of Fusarium oxysporum f. sp. cubense, the Fusarium Wilt Pathogen of Banana, in Ecuador. Plants 2020;9:1133. [CrossRef]
- Tapia C, Amaro J. Género Fusarium. Revista Chilena de Infectología 2014;31:85–6. [CrossRef]
- Ortiz E, Riascos D, Angarita M, Castro O, Rivera C, Romero D, et al. Tópicos taxonómicos para el estudio del género Fusarium 2020;33:61–6.
- Srinivasan R, Prabhu G, Prasad M, Mishra M, Chaudhary M, Srivastava R. Penicillium. In: Amaresan N, Senthil Kumar M, Annapurna K, Kumar K, Sankaranarayanan A, editors. Beneficial Microbes in Agro-Ecology, Academic Press; 2020, p. 651–67. [CrossRef]
- Velásquez MA, Álvarez RM, Tamayo PJ, Carvalho CP. Evaluación in vitro de la actividad fungistática del aceite esencial de mandarina sobre el crecimiento de Penicillium sp. Ciencia y Tecnología Agropecuaria 2014;15:7–14. [CrossRef]
- Smith J, Henderson R. Mycotoxins and Animal Foods. 1er ed. Glasgow, Scotland: CRC Press; 1991.
- Schuster A, Schmoll M. Biology and biotechnology of Trichoderma | Applied Microbiology and Biotechnology 2010. https://link.springer.com/article/10.1007/s00253-010-2632-1 (accessed August 4, 2024). [CrossRef]
- Brotman Y, Kapuganti JG, Viterbo A. Trichoderma. Current Biology 2010;20:R390–1. [CrossRef]
- Hernández-Melchor DJ, Ferrera-Cerrato R, Alarcón A, Hernández-Melchor DJ, Ferrera-Cerrato R, Alarcón A. Trichoderma: Importancia agrícola, biotecnológica y sistemas de fermentación para producir biomasa y enzimas de interés industrial. Chilean Journal of Agricultural & Animal Sciences 2019;35:98–112. [CrossRef]
- Harman GE, Kubicek CP. Trichoderma And Gliocladium: Basic Biology, Taxonomy and Genetics. Vienna, Austria: CRC Press; 2002.
- Chang PK, Horn BW, Abe K, Gomi K. Aspergillus: Introduction. Encyclopedia of Food Microbiology: Second Edition, Elsevier Inc.; 2014, p. 77–82. [CrossRef]
- Rokas A. Aspergillus. Current Biology 2013;23:R187–8. [CrossRef]
- Vargas-Fernández JP, Wang-Wong A, Muñoz-Fonseca M, Vargas-Fernández JP, Wang-Wong A, Muñoz-Fonseca M. Microorganismos asociados a la enfermedad conocida como pudrición suave del fruto de banano (Musa sp.) y alternativas de control microbiológicas y químicas a nivel in vitro *. Agronomía Costarricense 2022;46:61–76. [CrossRef]
- Salazar E, Hernández R, Tapia A, Gómez-Alpízar L. Identificación molecular del hongo Colletotrichum spp., aislado de banano (Musa spp) de la altura en la zona de Turrialba y determinación de su sensibilidad a fungicidas poscosecha. Agronomía Costarricense 2012;36:53–68.
- Morales R, Henríquez G. Aislamiento e identificación del moho causante de antracnosis en musa paradisiaca l. (plátano) en cooperativa san carlos, el salvador y aislamiento de mohos y levaduras con capacidad antagonista. Crea Ciencia Revista Científica 2021;13:84–94. [CrossRef]
- Tortora GJ, Funke BR, Case CL. Introducción a la microbiología. Ed. Médica Panamericana; 2007.
- Vanegas J Santamaría, González N Comba, Mancilla XC Pérez. Manual de Microbiología General: Principios Básicos de Laboratorio. Editorial Tadeo Lozano; 2014.
- Suárez Contreras LY. Identificación molecular de aislamientos de Moniliophthora roreri en huertos de cacao de Norte de Santander, Colombia. Acta Agronómica 2016;65:51–7. [CrossRef]
- Aguilar Armijos JS. Identificación del hongo fitopatógeno Phoma spp. aislado a partir de plantas de uvilla (Physalis peruviana L.) en localidades de zona norte y centro-norte de la serranía ecuatoriana 2020.
- Aguilar-Anccota R, Apaza-Apaza S, Maldonado E, Calle-Cheje Y, Rafael-Rutte R, Montalvo K, et al. Control in vitro e in vivo de Thielavipsis paradoxa y Collettrichum musae cn biofungicidas en frutos de banano orgánico. Manglar 2024;21:57–63. [CrossRef]
- Aquino-Martínez JG, Vázquez-García LM, Reyes-Reyes BG. Biocontrol in vitro e in vivo de Fusarium oxysporum Schlecht. f. sp. dianthi (Prill. y Delacr.) Snyder y Hans. con Hongos Antagonistas Nativos de la Zona Florícola de Villa Guerrero, Estado de México. Revista mexicana de fitopatología 2008;26:127–37.
- Cayotopa-Torres J, Arevalo L, Pichis-García R, Olivera-Cayotopa D, Rimachi-Valle M, Kadir KJMD. New cadmium bioremediation agents: Trichodermaspecies native to the rhizosphere of cacao trees. Scientia Agropecuaria 2021;24:155–60. [CrossRef]
- Savín-Molina J, Hernández-Montiel LG, Ceiro-Catasú W, Ávila-Quezada GD, Palacios-Espinosa A, Ruiz-Espinoza FH, et al. Caracterización morfológica y potencial de biocontrol de especies de Trichoderma aisladas de suelos del semiárido. Revista mexicana de fitopatología 2021;39:435–51. [CrossRef]



| Fungus | Macroscopic characteristics | |||||||
|---|---|---|---|---|---|---|---|---|
| Upper side | Lower side | Shape | Appearance | Elevation | Shore | Surface | Color | |
| Top | Bottom | |||||||
a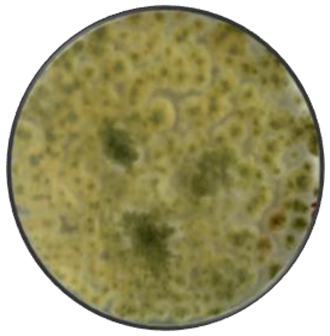
|
 |
Circulate too spherical | Cottony | Flat, slightly elevated, convex | Entire, slightly undulating | Smooth, rough and granular | White, green or yellow | Color of the fungal colony, yellow |
b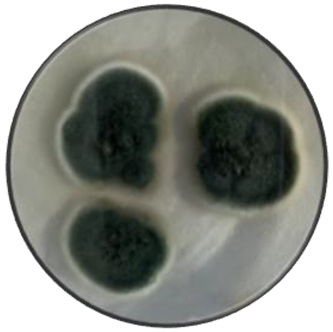
|
 |
Variable | Cottony, dusty | Convex and crateriform | Irregular and wavy | Smooth and rough | Green and white | Beige and yellow |
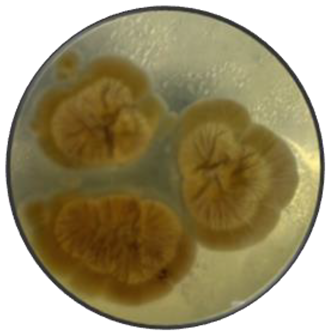
c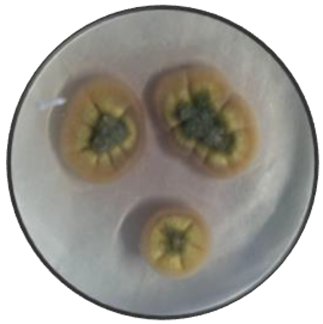
|
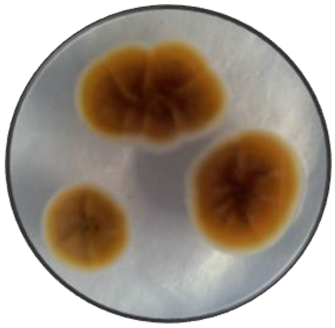 |
Variable | Velvety, Cottony and dusty | Flat, elevate and umbonate | Irregular and wavy | Smooth and rough | Green, yellow and brown | Pale yellow |
d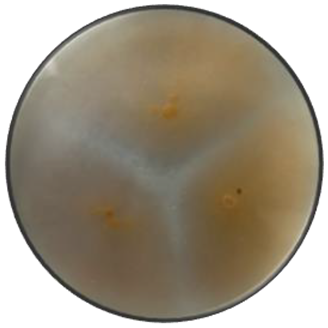
|
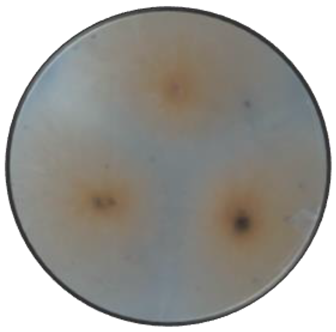 |
Cerebriform | Cottony | Flat | Irregular | Smooth | Orange | Orange |
| Fungus | Microscopic characteristics | ||||
|---|---|---|---|---|---|
| 40 X | 60 X | Hyphae | Mycelium | Spores | Appearance |
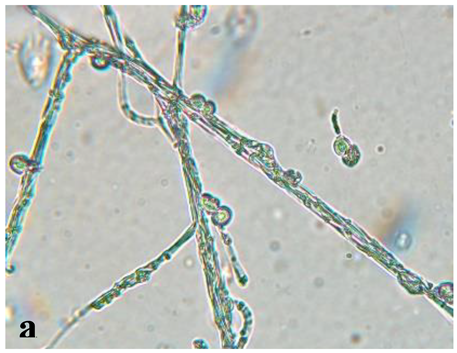 |
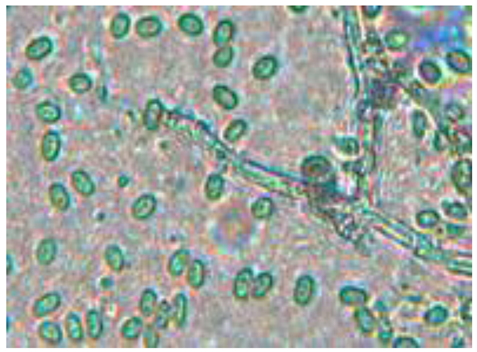 |
Septate |
Dense mycelium, often branching | Conidium (asexual), of an ellipsoid shape | Conidium scattered around of the hypha |
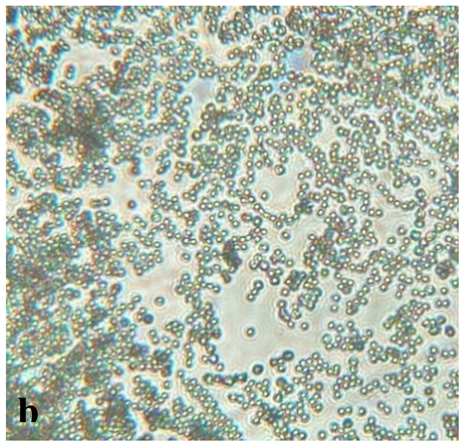 |
 |
Septate, branching |
Sporulation structures similar to a tuft | Conidium (asexual) in a circular shape and in clusters | Conidium often on a conidiophore head |
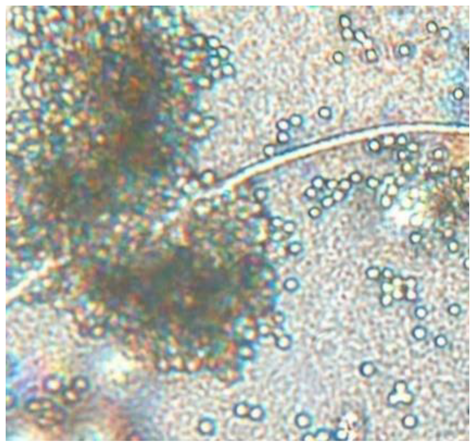
 |
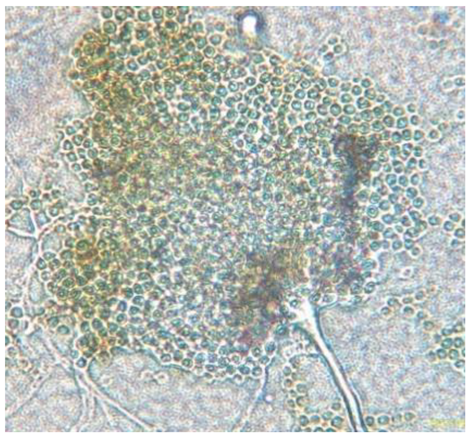 |
Septate, branching |
Mycelium profusely branching, often dense | Structure with numerous conidia (asexual) that appear like a plumose structure | Cluster of conidia with hyphae |
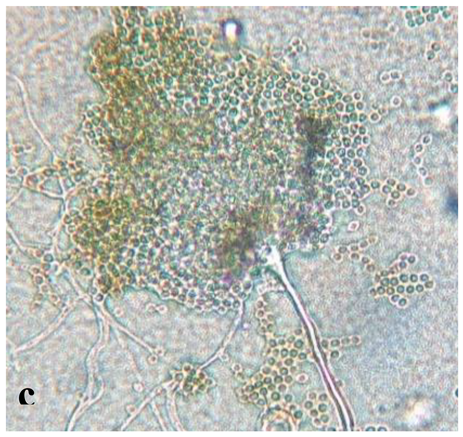
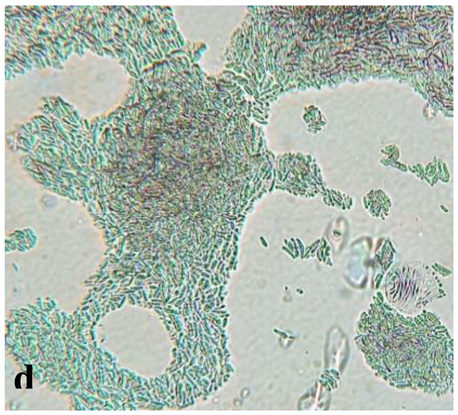 |
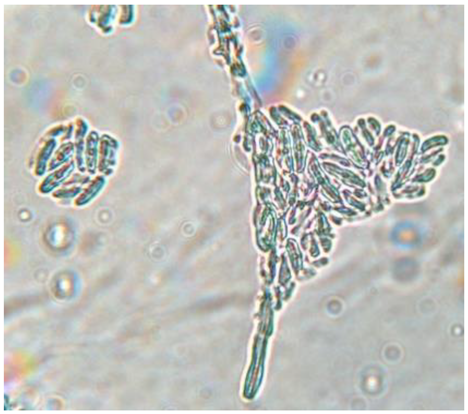 |
Septate, branching |
Form a complex and extensive network in the culture medium | Elongated and fusiform conidium forming chains | Enlarged macroconidium with septets and sections |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




