Submitted:
27 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Dietary Nutrients and NCDs
3. Strategies to Increase Tomato Properties as FF
3.1. The Strategy to Improve Tomatoes as FF Using the Whole Fruit
3.2. Properties of WTFS
- a)
- b)
- c)
- d)
- e)
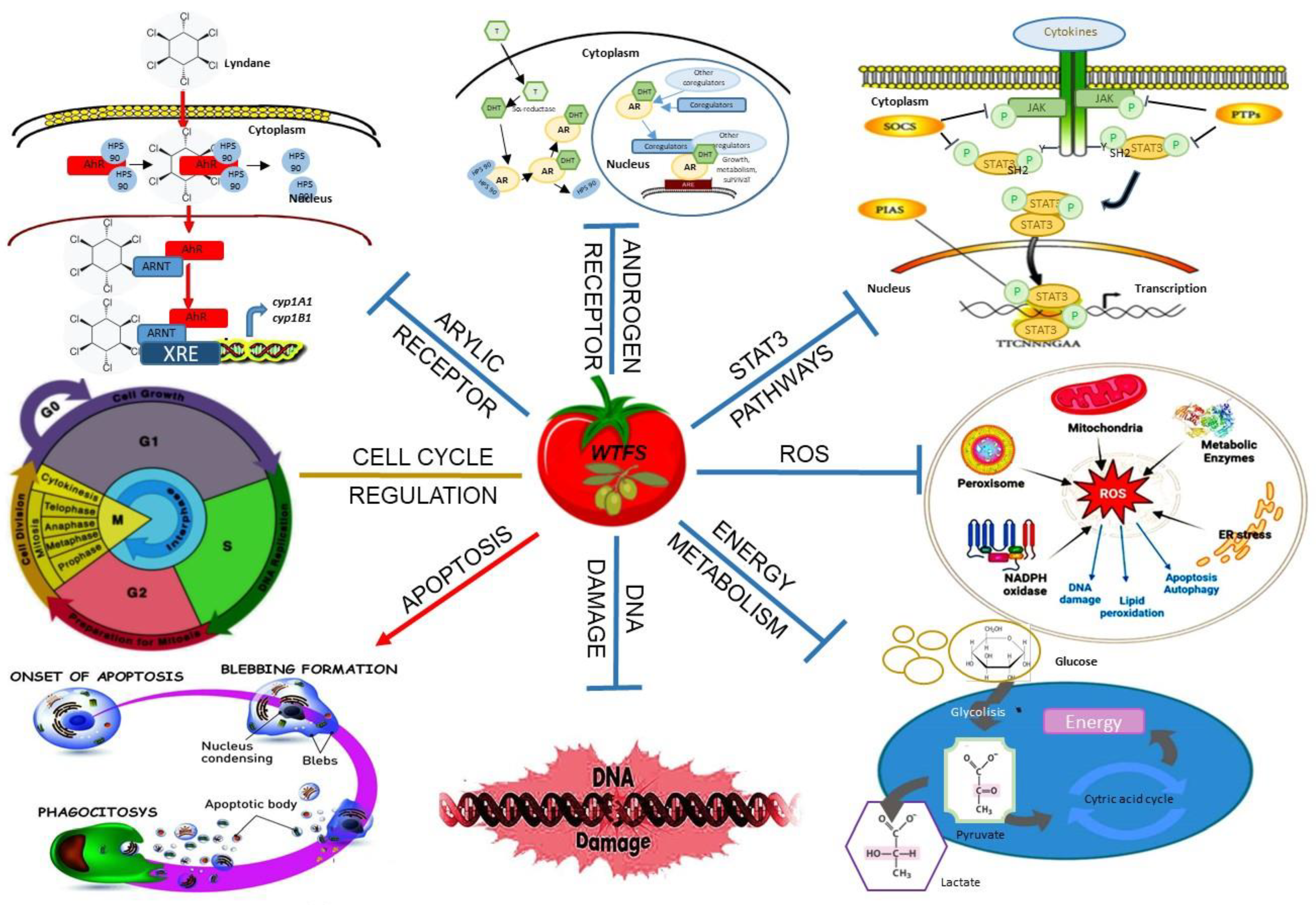
- it contains olive polyphenols, endowed with converging biologic activities with lycopene in increasing apoptosis, preventing DNA damage, oxidative stress, receptors modulation, and activation of signal transducer and activator of transcription-3 (STAT-3) [99], a key modulator of the expression of a wide range of oncogenic [102] and inflammation-related genes [103], and of tumor cell energy metabolism [104] (Figure 1);
- f)
- its in vitro antioxidant activity is comparable to N-acetyl-cysteine [105];
- g)
- it has a translational potential in clinical settings. This property has been explored in human prostate benign hypertrophy (PBH), a frequent age-dependent disease sustained by chronic inflammation [106]. PBH has been proven to benefit in a dose-dependent manner from lycopene supplementation due to the property of the carotenoid [56] and its metabolite to concentrate in the gland. In the phase II prospective, randomized double-blinded, placebo-controlled study, the WTFS consumption significantly improved the patient’s urinary tract symptoms and quality of life. Prostate-specific antigen (PSA) levels, when elevated prior to WTFS uptake, decreased, with unchanged free/bound fractions [108]. This improvement has been also documented in patients bearing low-grade chronic inflammation, i.e. HIV-infected individuals with PBH [109]. Differently from tomato based culinary preparations [110,111], at the max daily dosage used in clinical trials [108,109], no side effects were documented in tomato allergy-free individuals. Whether the prolonged uptake of WTFS is side-effect-free remains to be explored;
- h)
- it retains the sensory properties, i.e. aroma, taste, and color of red tomatoes. It can easily form a “granular suspension” in any liquid;
- i)
- further heating for culinary use does not impair its biological activity [112];
- l)
- it has an average nutritional value of 3,34 Kcal/g, thus acceptable under calories restricted diets.
4. WTFS potential in Improving Healthy Dietary Regimens
- a)
- b)
- contrasting aging-related carotenoid deficiency [21,142,143]. Indeed, the consumption of whole heat-processed tomatoes ameliorates the carotenoid status of healthy subjects and prevent their depletion in antiviral-treated patients, resulting in improved oxidative status [96] and associated NCDs [21];
- c)
- buffering the unhealthy effects of the spreading Western diet [144].
4.1. WTFS Use According to Whole Tomato Health Claims
4.2. Exploratory/Potential Use of WTFS
5. Conclusions
- a)
- b)
- c)
- increasing the olive mill wastewater content [195];
- d)
- exploring the possibility of developing more focused healthy properties by increasing the concentration of some of its components, i.e. lutein [196], or combining with other healthy micronutrients which may further improve the biological activity of those present in WTFS.
Patent
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Center for Disease Control and Prevention. Global Health Protection and Security. Available online: https://www.cdc.gov/globalhealth/healthprotection/ncd/global-ncd-overview.html (accessed on 6 may 2024).
- Permanyer, I.; Trias-Llimós, S.; Spijker, J.J.A. Best-practice healthy life expectancy vs. life expectancy: catching up or lagging behind? Proc. Natl. Acad. Sci. U S A 2021, 118, e2115273118:1-e2115273118:3. [CrossRef]
- World Health Organization. Global status report on noncommunicable diseases, 2014. Available online: https://www.who.int/publications/i/item/9789241564854 (accessed on 6 may 2024).
- International Federation of Medical Students’ Associations. Noncommunicable Diseases and the 4 most common shared risk factors. Available online: https://ifmsa.org/wp-content/uploads/2018/03/Noncommunicable-Diseases.pdf (accessed on 6 may 2024).
- Penman-Aguilar, A.; Talih, M.; Huang, D.; Moonesinghe, R.; Bouye, K.; Beckles, G. Measurement of health disparities, health inequities, and social determinants of health to support the advancement of health equity. J. Public Health Manag. Pract. 2016, 22, S33-S42. [CrossRef]
- Garry, S.; Checchi, F. Armed conflict and public health: into the 21st century. J. Public Health 2020, 42, e287-e298. [CrossRef]
- Akombi-Inyang, B.; Huda, M.N.; Schutte, A.E.; Macniven, R.; Lin, S.; Rawstorne, P.; Xu, X.; Renzaho, A. The association between post-migration nutrition and lifestyle transition and the risk of developing chronic diseases among Sub-Saharan African migrants: a mixed method systematic review protocol. Int. J. Environ. Res. Public Health 2021, 18, 4706:1-4706:7. [CrossRef]
- Patil, R.R. Urbanization as a determinant of health: a socioepidemiological perspective. Soc. Work Public Health 2014, 29, 335-341. [CrossRef]
- Mialon, M.; Ho, M.; Carriedo, A.; Ruskin, G.; Crosbie, E. Beyond nutrition and physical activity: food industry shaping of the very principles of scientific integrity. Global Health 2021, 17, 37:1-37:13. [CrossRef]
- Furlow, B. Cancer misinformation puts patients in harm’s way. Lancet Oncol. 2024, 25, 165-166. [CrossRef]
- World Health Organization. Health literacy development for the prevention and control of noncommunicable diseases: Volume 1. Overview. Available online: https://www.who.int/publications/i/item/9789240055339(accessed on 6 may 2024).
- Myers, S.S. Planetary health: protecting human health on a rapidly changing planet. Lancet 2017, 390, 2860-2868. [CrossRef]
- Raffetti, E.; Ahrne, M.; Doring, S.; Hagstron, A.; Mazzoleni, M.; Messori, G.; Rusca, M.; Zarantonelllo L. Sustainable transformations for healthcare systems in a changing climate. Cell Reports Sustainability 2024, 1, 100054:1-100054:4. [CrossRef]
- Parthasarathy, S. Innovation as a force for equity. Sci. Technol. 2022, 38, 30-36. https://issues.org/health-innovation-system-force-equity-shobita-parthasarathy/.
- Sousa, L.P.; Alessandri, A.L.; Pinho, V.; Teixeira, M.M. Pharmacological strategies to resolve acute inflammation. Curr. Opin. Pharmacol. 2013, 13, 625-631. [CrossRef]
- Panezai, J.; Van Dyke, T.E. Resolution of inflammation: intervention strategies and future applications. Toxicol. Appl. Pharmacol. 2022, 449, 116089:1-116089:15. [CrossRef]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [CrossRef]
- Candore, G.; Caruso, C.; Jirillo, E.; Magrone, T.; Vasto, S. Low grade inflammation as a common pathogenetic denominator in age-related diseases: novel drug targets for anti-ageing strategies and successful ageing achievement. Curr. Pharm. Des. 2010, 16, 584-596. [CrossRef]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Forum on Drug Discovery, Development, and Translation. Innovation in Drug Research and Development for Prevalent Chronic Diseases: Proceedings of a Workshop. The National Academies Press: Washington, DC 20001, 2021. [CrossRef]
- Rönnbäck, C.; Hansson, E. The importance and control of low-grade inflammation due to damage of cellular barrier systems that may lead to systemic inflammation. Front. Neurol. 2019, 10, 533:1-533:8. [CrossRef]
- Chaudhary, M.R.; Chaudhary, S.; Sharma, Y.; Singh T.A.; Mishra, A.K.; Sharma, S.; Mehdi. M.M. Aging, oxidative stress and degenerative diseases: mechanisms, complications and emerging therapeutic strategies. Biogerontology 2023, 24, 609-662. [CrossRef]
- Riboli, E.; Hunt, K.J.; Slimani, N.; Ferrari, P.; Norat, T.; Fahey, M.; Charrondière, U.R.; Hémon, B.; Casagrande, C.; Vignat, J.; Overvad, K.; Tjønneland, A.; Clavel-Chapelon, F.; Thiébaut, A.; Wahrendorf, J.; Boeing, H.; Trichopoulos, D.; Trichopoulou, A.; Vineis, P.; Palli, D.; Bueno-De-Mesquita, H.B.; Peeters, P.H.; Lund, E.; Engeset, D.; González, C.A.; Barricarte, A.; Berglund, G.; Hallmans, G.; Day, N.E.; Key, T.J.; Kaaks, R.; Saracci, R. European prospective investigation into cancer and nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002, 5, 1113-1124. [CrossRef]
- Magni, P.; Bier, D.M.; Pecorelli, S.; Agostoni, C.; Astrup, A.; Brighenti, F.; Cook, R.; Folco, E.; Fontana, L.; Gibson, R.A.; Guerra, R.; Guyatt, G.H.; Ioannidis, J.P.; Jackson, A.S.; Klurfeld, D.M.; Makrides, M.; Mathioudakis, B.; Monaco, A.; Patel, C.J.; Racagni, G.; Schünemann, H.J.; Shamir, R.; Zmora, N.; Peracino, A. Perspective: improving nutritional guidelines for sustainable health policies: current status and perspectives. Adv. Nutr. 2017, 8, 532-545. [CrossRef]
- EAT–Lancet 2.0 Commissioners and contributing authors. Electronic address: fabrice@eatforum.org. EAT-Lancet Commission 2.0: securing a just transition to healthy, environmentally sustainable diets for all. Lancet, 2023, 402, 352-354. [CrossRef]
- Schwingshackl, L.; Morze, J.; Hoffmann, G. Mediterranean diet and health status: active ingredients and pharmacological mechanisms. Br. J. Pharmacol. 2020, 177, 1241-1257. [CrossRef]
- Mozaffarian, D. Mediterranean diet for primary prevention of cardiovascular disease. N. Engl. J. Med. 2013, 369, 673-674. [CrossRef]
- Kiani, A.K.; Medori, M.C.; Bonetti, G.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A.; Stuppia, L.; Connelly, S.T.; Herbst, K.L.; Bertelli, M. Modern vision of the mediterranean diet. J. Prev. Med. Hyg. 2022, 63, E36-E43. [CrossRef]
- Martínez-González, MÁ.; Hershey, M.S.; Zazpe, I.; Trichopoulou. A. Transferability of the mediterranean diet to non-mediterranean countries. What is and what is not the mediterranean diet. Nutrients 2017, 9, 1226:1-1226:14. [CrossRef]
- Aronson, J.K. Defining ‘nutraceuticals’: neither nutritious nor pharmaceutical. Br. J. Clin. Pharmacol. 2017, 83, 8-19. [CrossRef]
- Aghajanpour, M.; Nazer, M.R.; Obeidavi, Z.; Akbari, M.; Ezati, P.; Kor, N.M. Functional foods and their role in cancer prevention and health promotion: a comprehensive review. Am. J. Cancer Res. 2017, 7, 740-769.
- Alongi, M.; Anese, M. Re-thinking functional food development through a holistic approach. J. Funct. Foods 2021, 81, 104466: 1-104466:13. [CrossRef]
- Maillot, M.; Vieux, F.; Delaere, F.; Lluch, A.; Darmon, N. Dietary changes needed to reach nutritional adequacy without increasing diet cost according to income: an analysis among French adults. PLoS One 2017, 12, e0174679:1-e0174679:20. [CrossRef]
- Vats, S.; Bansal, R.; Rana, N.; Kumawat, S.; Bhatt, V.; Jadhav, P.; Kale, V.; Sathe, A.; Sonah, H.; Jugdaohsingh, R.; Sharma, T.R.; Deshmukh, R. Unexplored nutritive potential of tomato to combat global malnutrition. Crit. Rev. Food Sci. Nutr. 2022, 62, 1003-1034. [CrossRef]
- Naureen, Z.; Dhuli, K.; Donato, K.; Aquilanti, B.; Velluti, V.; Matera, G.; Iaconelli, A. Bertelli, M. Foods of the mediterranean diet: tomato, olives, chili pepper, wheat flour and wheat germ. J. Prev. Med. Hyg. 2022, 63, E4-E11. [CrossRef]
- Ritchie, H.; Rosado, P.; Roser, M. Agricultural production. Available online: https://ourworldindata.org/agricultural-production (accessed on 6 may 2024).
- Branthôme, F.-X. Worldwide consumption of tomato Products, 2018/2019 (Part 1). 2020 WPTC Congress. Available online: https://www.tomatonews.com/en/worldwide-consumption-of-tomato-products-20182019-part-1_2_994.html (accessed on 6 may 2024).
- Mordor Intelligence. Tomato market size & share analysis - Growth trends & forecasts (2024 - 2029). Available online: https://www.mordorintelligence.com/industry-reports/tomato-market (accessed on 6 may 2024).
- Hanson, C. All recipes. Available on line: http://www.allrecipes.com/gallery/world-recipes-for-fresh-tomatoes/ (accessed on 6 may 2024).
- Trombino, S.; Cassano, R.; Procopio, D.; Di Gioia, M.L.; Barone E. Valorization of tomato waste as a source of carotenoids. Molecules 2021, 26, 5062:1-5062:19. [CrossRef]
- Madia, V.N.; De Vita, D.; Ialongo, D.; Tudino, V.; De Leo, A.; Scipione, L.; Di Santo, R.; Costi, R.; Messore, A. Recent advances in recovery of lycopene from tomato waste: a potent antioxidant with endless benefits. Molecules 2021, 26, 4495:1-4495:18. [CrossRef]
- Li, Y.; Wang, H.; Zhang, Y.; Martin, C. Can the world’s favorite fruit, tomato, provide an effective biosynthetic chassis for high-value metabolites? Plant Cell Rep. 2018, 37, 1443-1450. [CrossRef]
- Bhattarai, K.; Sharma, S.; Panthee, D.R. Diversity among modern tomato genotypes at different levels in fresh-market breeding. Int. J. Agron. 2018, 2018, 4170432:1-4170432:16. [CrossRef]
- Frusciante, L.; Carli, P.; Ercolano, M.R.; Pernice, R.; Di Matteo, A.; Fogliano, V.; Pellegrini, N. Antioxidant nutritional quality of tomato. Mol. Nutr. Food Res. 2007, 51, 609-617. [CrossRef]
- Erika, C.; Ulrich, D.; Naumann, M.; Smit, I.; Horneburg, B.; Pawelzik, E. Flavor and other quality traits of tomato cultivars bred for diverse production systems as revealed in organic low-input management. Front. Nutr. 2022, 9, 916642:1-916642:19. [CrossRef]
- Sainju, U.M.; Singh, B.P.; Rahman, S.; Reddy, V.R. Tillage, cover cropping, and nitrogen fertilization influence tomato yield and nitrogen uptake. HortSci. 2000, 35, 217–221. [CrossRef]
- Salem, N.M.; Albanna, L.S.; Awwad, A.M. Toxic heavy metals accumulation in tomato plant (Solanum lycopersicum). ARPN J. Agric. Biol. Sci. 2016, 11, 399–404.
- Ilić, Z.S.; Kapoulas, N.; Šunić, L.; Beković, D.; Mirecki, N. Heavy metals and nitrate content in tomato fruit grown in organic and conventional production systems. Pol. J. Environ. Stud. 2014, 23, 2027-2032. [CrossRef]
- Abou-Arab, A.A.K. Behavior of pesticides in tomatoes during commercial and home preparation. Food Chem. 1999, 4, 509–514. [CrossRef]
- Hedayati, N.; Naeini, M.B.; Nezami, A.; Hosseinzadeh, H.; Wallace Hayes, A.; Hosseini, S.; Imenshahidi, M.; Karimi, G. Protective effect of lycopene against chemical and natural toxins: a review. Biofactors 2019, 45, 5–23. [CrossRef]
- Arazuri, S.; Jaren, C.; Arana, I.; Pérez de Ciriza, J.J. Influence of mechanical harvest on the physical properties of processing tomato (Lycopersicon esculentum Mill.). J. Food Eng. 2007, 80, 190-198. [CrossRef]
- Medland, L. ‘There is no time’: Agri-food internal migrant workers in Morocco’s tomato industry. J. Rural Stud. 2021, 88, 482-490. [CrossRef]
- Cannon G.; Leitzmann C. Food and nutrition science: the new paradigm. Asia Pac. J. Clin. Nutr. 2022, 31, 1-15. [CrossRef]
- European Food Safety Authority. Assesses safety of lycopene in foods. 2008. Available on line: https://www.efsa.europa.eu/en/news/efsa-assesses-safety-lycopene-foods (accessed on 6 may 2024).
- U.S. Food & Drug Administration. Generally Recognized as Safe (GRAS). Available on line: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 6 may 2024).
- Subhash, K.; Bose, C.; Agrawal, B.K. Effect of short term supplementation of tomatoes on antioxidant enzymes and lipid peroxidation in type-II diabetes. Indian. J. Clin. Biochem. 2007, 22, 95-98. [CrossRef]
- Boileau, T.W.; Boileau, A.C.; Erdman, J.W.Jr. Bioavailability of all-trans and cis-isomers of lycopene. Exp. Biol. Med. 2002, 227, 914–919. [CrossRef]
- Bohn, T.; Desmarchelier, C.; Dragsted, L.O.; Nielsen, C.S.; Stahl, W.; Rühl, R.; Keijer, J.; Borel, P. Host-related factors explaining interindividual variability of carotenoid bioavailability and tissue concentrations in humans. Mol. Nutr. Food Res. 2017, 61, 1600685:1-1600685:37. [CrossRef]
- Amorim, A.D.G.N.; Vasconcelos, A.G.; Souza, J.; Oliveira, A.; Gullón B.; de Souza de Almeida Leite J.R.; Pintado M. Bio-availability, anticancer potential, and chemical data of lycopene: an overview and technological prospecting. Antioxidants 2022, 11, 360:1-360:22. [CrossRef]
- Vallverdú-Queralt, A.; Regueiro, J.; de Alvarenga, J.F.; Torrado, X.; Lamuela-Raventos, R.M. Carotenoid profile of tomato sauces: effect of cooking time and content of extra virgin olive oil. Int. J. Mol. Sci. 2015, 16, 9588-9599. [CrossRef]
- Magne, T.M.; da Silva de Barros, A.O.; de Almeida Fechine, P.B; Rebelo Alencar, L.M.; Ricci-Junior, E.; Santos-Oliveira, R. Lycopene as a multifunctional platform for the treatment of cancer and inflammation. Rev. Bras. Farmacogn. 2022, 32, 321–330. [CrossRef]
- Mein, J.R.; Lian, F.; Wang, X.D. Biological activity of lycopene metabolites: implications for cancer prevention. Nutr. Rev. 2008, 66, 667–683. [CrossRef]
- Caseiro, M.; Ascenso, A.; Costa, A.; Creagh-Flynn, J.; Johnson, M.; Simoes, S. Lycopene in human health. LWT 2020, 127, 109323:1-109323:16. [CrossRef]
- Marquez, C.S.; Reis Lima, M.J.; Oliveira, J.; Teixeira-Lemos, E. Tomato lycopene: functional proprieties and health benefits. [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [CrossRef]
- Ross, A.B.; Vuong leT.; Ruckle, J.; Synal, H.A.; Schulze-König, T.; Wertz, K.; Rümbeli, R.; Liberman, R.G.; Skipper, P.L.; Tannenbaum, S.R.; Bourgeois, A.; Guy, P.A.; Enslen, M.; Nielsen, I.L.; Kochhar, S.; Richelle, M.; Fay, L.B.; Williamson, G. Lycopene bioavailability and metabolism in humans: an accelerator mass spectrometry study. Am. J. Clin. Nutr. 2011, 93, 1263-1273. [CrossRef]
- Paetau, I.; Khachik, F.; Brown, E.D.; Beecher, G.R.; Kramer, T.R.; Chittams, J.; Clevidence B.A. Chronic ingestion of lycopene-rich tomato juice or lycopene supplements significantly increases plasma concentrations of lycopene and related tomato carotenoids in humans. Am. J. Clin. Nutr. 1998, 68, 1187-1195. [CrossRef]
- van Steenwijk, H.P.; Bast, A.; de Boer, A. The role of circulating lycopene in low-grade chronic inflammation: a systematic review of the literature. Molecules, 2020, 25, 4378:1-4378:22. [CrossRef]
- Nandi, P. Lycopene Market Research Report Information by Source. 2024 Available on line: https://www.marketresearchfuture.com/reports/lycopene-market-20296 (accessed on 6 may 2024).
- Liang, X.; Ma, C.; Yan, X.; Liu, X.; Liu, F. Advances in research on bioactivity, metabolism, stability and delivery systems of lycopene. Trends Food Sci. Technol. 2019, 93, 185-196. [CrossRef]
- Lambelet, P.; Richelle, M.; Bortlik, K.; Franceschi, F.; Giori, A.M. Improving the stability of lycopene Z-isomers in isomerised tomato extracts. Food Chem. 2009, 112, 156-161. [CrossRef]
- Li, M.; Xia, Q.; Zhang, H.; Zhang, R.; Yang, J. Metabolic engineering of different microbial hosts for lycopene production. J. Agric. Food Chem. 2020, 68, 14104-14122. [CrossRef]
- Canene-Adams, K.; Lindshield, B.L.; Wang, S.; Jeffery, E.H.; Clinton, S.K.; Erdman, J.W.Jr. Combinations of tomato and broccoli enhance antitumor activity in dunning r3327-h prostate adenocarcinomas. Cancer Res. 2007, 67, 836–843. [CrossRef]
- Rowles, J.L. 3rd; Erdman, J.W.Jr. Carotenoids and their role in cancer prevention. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2020, 1865, 158613:1-158613:9. [CrossRef]
- Tamanna, N.; Mahmood, N. Food processing and Maillard reaction products: effect on human health and nutrition. Int. J. Food Sci. 2015, 2015, 526762:1-526762:6. [CrossRef]
- Canene-Adams, K.; Campbell, J.K.; Zaripheh, S.; Jeffery, E.H.; Erdman, J.W.Jr. The tomato as a functional food. J. Nutr. 2005, 135, 1226–1230. [CrossRef]
- Linnewiel-Hermoni, K.; Khanin, M.; Danilenko, M.; Zango, G.; Amo, Y.; Levy, J.; Sharoni, Y. The anti-cancer effects of carotenoids and other phytonutrients resides in their combined activity. Arch. Biochem. Biophys. 2015, 572, 28–35. [CrossRef]
- Mohri, S.; Takahashi, H.; Sakai, M.; Takahashi, S.; Waki, N.; Aizawa, K.; Suganuma, H.; Ara, T.; Matsumura, Y.; Shibata, D.; Goto, T.; Kawada, T. Wide-range screening of anti-inflammatory compounds in tomato using LC-MS and elucidating the mechanism of their functions. PLoS ONE 2018, 13, e0191203:1-e0191203:21. [CrossRef]
- Mazidi, M.; Katsiki, N.; George, E.S.; Banach, M. Tomato and lycopene consumption is inversely associated with total and cause-specific mortality: a population-based cohort study, on behalf of the International Lipid Expert Panel (ILEP). Br. J. Nutr. 2020, 124, 1303-1310. [CrossRef]
- Mazidi, M.; Ferns, G.A.; Banach, M. A high consumption of tomato and lycopene is associated with a lower risk of cancer mortality: results from a multi-ethnic cohort. Public Health Nutr. 2020, 23, 1569-1575. [CrossRef]
- Landrier, J.F.; Breniere, T.; Sani, L.; Desmarchelier, C.; Mounien, L.; Borel, P. Effect of tomato, tomato-derived products and lycopene on metabolic inflammation: from epidemiological data to molecular mechanisms. Nutr. Res. Rev. 2023, 1-17. [CrossRef]
- Collins, E. J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: an extensive review of the associated health impacts of tomatoes and factors that can affect their cultivation. Biology 2022, 11, 239:1-239:44. [CrossRef]
- Unlu, N.Z.; Bohn, T.; Francis, D.M.; Nagaraja, H.N.; Clinton. S.K.; Schwartz, S.J. Lycopene from heat-induced cis-isomer-rich tomato sauce is more bioavailable than from all-trans-rich tomato sauce in human subjects. Br. J. Nutr. 2007, 98, 140-146. [CrossRef]
- Pannellini, T.; Iezzi, M.; Liberatore, M.; Sabatini, F.; Iacobelli, S.; Rossi, C.; Alberti, S.; Di Ilio, C.; Vitaglione, P.; Fogliano, V.; Piantelli, M. A dietary tomato supplement prevents prostate cancer in TRAMP mice. Cancer Prev. Res. 2010, 3, 1284–1291. [CrossRef]
- Applegate, C.; Rowles, J. 3rd; Miller, R.; Wallig, M.; Clinton, S.; O’Brien, W.; Erdman, J.Jr. Dietary tomato, but not lycopene supplementation, impacts molecular outcomes of castration-resistant prostate cancer in the TRAMP model (P05-015-19). Curr. Dev. Nutr. 2019, 3, 438. [CrossRef]
- Conlon, L.E.; Wallig, M.A.; Erdman, J.W.Jr. Low-lycopene containing tomato powder diet does not protect against prostate cancer in TRAMP mice. Nutr. Res. 2015, 35, 882-890. [CrossRef]
- Applegate, C.C.; Lowerison, M.R.; Hambley, E.; Song, P.; Wallig, M.A.; Erdman, J.W.Jr. Dietary tomato inhibits angiogenesis in TRAMP prostate cancer but is not protective with a Western-style diet in this pilot study. Sci. Rep. 2021, 11, 18548:1-18548:13. [CrossRef]
- Fogliano, V.; Iacobelli, S.; Piantelli, M. Euro Patent 3 052 113 B1, Italian Health Ministry (registration n. 68843, 2018–2019) Available online: https://worldwide.espacenet.com/patent/search/family/049226079/publication/EP3052113A1?q=3052113 (accessed on 6 may 2024).
- Piroddi, M.; Albini, A.; Fabiani, R.; Giovannelli, L.; Luceri, C.; Natella, F.; Rosignoli, P.; Rossi, T.; Taticchi, A.; Servili, M.; Galli, F. Nutrigenomics of extra-virgin olive oil: a review. Biofactors 2017, 43, 17–41. [CrossRef]
- Luo, C.; Li, Y.; Wang, H.; Cui, Y.; Feng, Z.; Li, H.; Li, Y.; Wang, Y.; Wurtz, K.; Weber, P.; Long, J.; Liu, J. Hydroxytyrosol promotes superoxide production and defects in autophagy leading to anti-proliferation and apoptosis on human prostate cancer cells. Curr. Cancer Drug Targets 2013, 13, 625–639. [CrossRef]
- Zubair, H.; Bhardwaj, A.; Ahmad, A.; Srivastava, S.K.; Khan, M.A.; Patel, G.K.; Singh, S.; Singh, A.P. Hydroxytyrosol induces apoptosis and cell cycle arrest and suppresses multiple oncogenic signaling pathways in prostate cancer cells. Nutr. Cancer 2017, 69, 932–942. [CrossRef]
- Albini, A.; Indraccolo, S.; Noonan, D.M.; Pfeffer, U. Functional genomics of endothelial cells treated with anti-angiogenic or angiopreventive drugs. Clin. Exp. Metastasis 2010, 27, 419–439. [CrossRef]
- Pounis, G.; Bonaccio, M.; Di Castelnuovo, A.; Costanzo, S.; de Curtis, A.; Persichillo, M.; Sieri, S.; Donati, M.B.; Cerletti, C.; de Gaetano, G.; Iacoviello, L. Polyphenol intake is associated with low-grade inflammation, using a novel data analysis from the Moli-sani Study. Thromb. Haemost. 2016, 115, 344-352. [CrossRef]
- Peroulis, N.; Androutsopoulos, V. P.; Notas, G.; Koinaki, S.; Giakoumaki, E.; Spyros, A.; Manolopoulou, Ε.; Kargaki, S.; Tzardi, M.; Moustou, E.; Stephanou, E. G.; Bakogeorgou, E.; Malliaraki, N.; Niniraki, M.; Lionis, C.; Castanas, E.; Kampa, M. Significant metabolic improvement by a water extract of olives: animal and human evidence. Eur. J. Nutr. 2019, 58, 2545-2560. [CrossRef]
- Toma, R.B.; Frank, G.C.; Nakayama, K.; Tawfik, E. Lycopene content in raw tomato varieties and tomato products. J. Foodserv. 2008, 19, 127-132. [CrossRef]
- Sidhu, G.K.; Singh, M.; Kaur, P. Effect of operational parameters on physicochemical quality and recovery of spray-dried tomato powder. J. Food Process Preserv. 2019, 43, e14120:1-e14120:9. [CrossRef]
- Vitaglione, P.; Fogliano, V.; Stingo, S.; Scalfi, L.; Caporaso, N.; Morisco, F. Development of a tomato-based food for special medical purposes as therapy adjuvant for patients with HCV infection. Eur. J. Clin. Nutr. 2007, 61, 906-915. [CrossRef]
- Soares, N.D.C.P.; Elias, M.B.; Lima Machado, C.; Trindade, B.B.; Borojevic, R.; Teodoro, A.J. Comparative analysis of lycopene content from different tomato-based food products on the cellular activity of prostate cancer cell lines. Foods 2019, 8, 201:1-201:14. [CrossRef]
- Li, J.; Yang, Z.; Zhang, Y.; Gao, B.; Niu, Y.; Lucy Yu, L. The structural and functional characteristics of soluble dietary fibers modified from tomato pomace with increased content of lycopene. Food Chem. 2022, 382, 132333:1-132333:7. [CrossRef]
- Rubini, E.; Minacori, M.; Paglia, G.; Macone, A.; Chichiarelli, S.; Altieri, F.; Eufemi, M. Tomato and olive bioactive compounds: A natural shield against the cellular effects induced by β-hexachlorocyclohexane-activated signaling pathways. Molecules 2021, 26, 7135:1-7135:23. [CrossRef]
- Johnson, E. J.; Qin, J.; Krinsky, N. I.; Russell, R. M. Ingestion by men of a combined dose of beta-carotene and lycopene does not affect the absorption of beta-carotene but improves that of lycopene. J. Nutr. 1997, 127, 1833-1837. [CrossRef]
- Yang, C.; Zhang, S.; Shi, R.; Yu, J.; Li, S.; Tao, G.; Tsao, R.; Zhang, J.; Zhang, L. LC-MS/MS for simultaneous detection and quantification of Amadori compounds in tomato products and dry foods and factors affecting the formation and antioxidant activities. J Food Sci. 2020, 85, 1007-1017. [CrossRef]
- Tesoriere, A.; Dinarello, A.; Argenton, F. The roles of post-translational modifications in STAT3 biological activities and functions. Biomedicines 2021, 9, 956:1-956:20. [CrossRef]
- Matsuda, T. The physiological and pathophysiological role of IL-6/STAT3-mediated signal transduction and STAT3 binding partners in therapeutic applications. Biol. Pharm. Bull. 2023, 46, 364-378. [CrossRef]
- Marrocco, I.; Altieri, F.; Rubini, E.; Paglia, G.; Chichiarelli, S.; Giamogante, F.; Macone, A.; Perugia, G.; Magliocca, F. M.; Gurtner, A.; Maras, B.; Ragno, R.; Patsilinakos, A.; Manganaro, R.; Eufemi, M. Shmt2: a Stat3 signaling new player in prostate cancer energy metabolism. Cells 2019, 8, 1048:1-1048:20. [CrossRef]
- Natali, P. G.; Piantelli, M.; Minacori, M.; Eufemi, M.; Imberti, L. Improving whole tomato transformation for prostate health: benign prostate hypertrophy as an exploratory model. Int. J. Mol. Sci. 2023, 24, 5795:1-5795:15. [CrossRef]
- Krušlin, B.; Tomas, D.; Džombeta, T.; Milković-Periša, M.; Ulamec, M. Inflammation in prostatic hyperplasia and carcinoma-basic scientific approach. Front. Oncol. 2017, 7, 77:1-77:7. [CrossRef]
- Grainger, E.M.; Moran, N.E.; Francis, D.M.; Schwartz, S.J.; Wan, L.; Thomas-Ahner, J.; Kopec, R.E.; Riedl, K.M.; Young, G.S.; Abaza, R.; Bahnson, R.R.; Clinton, S.K. A novel tomato-soy juice induces a dose-response increase in urinary and plasma phytochemical biomarkers in men with prostate cancer. J. Nutr. 2019, 149, 26-35. [CrossRef]
- Cormio, L.; Calò, B.; Falagario, U.; Iezzi, M.; Lamolinara, A.; Vitaglione, P.; Silecchia, G.; Carrieri, G.; Fogliano, V.; Iacobelli, S.; Natali, P.G.; Piantelli, M. Improvement of urinary tract symptoms and quality of life in benign prostate hyperplasia patients associated with consumption of a newly developed whole tomato-based food supplement: a phase II prospective, randomized double-blinded, placebo-controlled study. J. Transl. Med. 2021, 19, 24:1-24:8. [CrossRef]
- Quiros-Roldan, E.; Carriero, C.; Paghera, S.; Degli Antoni, M.; Fiorini, C.; Quaresima, V.; Castelli, F.; Imberti, L. Symptoms and quality of life in HIV-infected patients with benign prostatic hyperplasia are improved by the consumption of a newly developed whole tomato-based food supplement. A phase II prospective, randomized double-blinded, placebo-controlled study. J. Funct. Foods 2021, 82, 104495:1-104495:8. [CrossRef]
- Włodarczyk, K.; Smolińska, B.; Majak, I. Tomato allergy: the characterization of the selected allergens and antioxidants of tomato (Solanum lycopersicum)-A review. Antioxidants 2022, 11, 644:1-644:20. [CrossRef]
- Salehi, B.; Sharifi-Rad, R.; Sharopov, F.; Namiesnik, J.; Roointan, A.; Kamle, M.; Kumar, P.; Martins, N.; Sharifi-Rad, J. Beneficial effects and potential risks of tomato consumption for human health: an overview. Nutrition 2019, 62, 201-208. [CrossRef]
- Graziani, G.; Pernice, R.; Lanzuise, S.; Vitaglione, P.; Anese M.; Fogliano, V. Effect of peeling and heating on carotenoid content and antioxidant activity of tomato and tomato-virgin olive oil systems. Eur. Food Res. Technol. 2003, 216, 116–121. [CrossRef]
- Lichtenstein, A.H.; Russell, R.M. Essential nutrients: food or supplements? Where should the emphasis be? JAMA 2005, 294, 351-358. [CrossRef]
- National Academy of Sciences. The Challenge of Feeding the World Sustainably: Summary of the US-UK Scientific Forum on Sustainable Agriculture, National Academy Press: Washington, DC, 20001, 2021. [CrossRef]
- Hoffman, R.; Gerber, M. Food processing and the mediterranean diet. Nutrients 2015, 7, 7925-7964. [CrossRef]
- Clark, J. S.; Dyer, K. A.; Davis, C. R.; Shivappa, N.; Hébert, J. R.; Woodman, R.; Hodgson, J. M.; Murphy, K. J. Adherence to a mediterranean diet for 6 months improves the dietary inflammatory index in a western population: results from the MedLey Study. Nutrients 2023, 15, 366:1-366:14. [CrossRef]
- Barbaresko, J.; Koch, M.; Schulze, M.B.; Nöthlings, U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr. Rev. 2013, 71, 511-527. [CrossRef]
- Bonaccio, M.; Costanzo, S.; Di Castelnuovo, A.; Gialluisi, A.; Ruggiero, E.; De Curtis, A.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello. L. Increased adherence to a mediterranean diet is associated with reduced low-grade inflammation after a 12.7-year period: results from the Moli-sani Study. J. Acad. Nutr. Diet 2023, 123, 783-795.e7. [CrossRef]
- Hall, K.D.; Ayuketah, A.; Brychta, R.; Cai, H.; Cassimatis, T.; Chen, K.Y.; Chung, S.T.; Costa, E.; Courville, A.; Darcey, V.; Fletcher, L.A.; Forde, C.G.; Gharib, A.M.; Guo, J.; Howard, R.; Joseph, P.V.; McGehee, S.; Ouwerkerk, R.; Raisinger, K.; Rozga, I.; Stagliano, M.; Walter, M.; Walter, P.J.; Yang, S.; Zhou, M. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab. 2020, 32, 690. [CrossRef]
- Bechthold, A.; Boeing, H.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Iqbal, K.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Schlesinger, S.; Schwingshackl, L. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 1071-1090. [CrossRef]
- Schwingshackl, L.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Schwedhelm, C.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 363-375. [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Lampousi, A.M.; Knüppel, S.; Iqbal, K.; Bechthold, A.; Schlesinger, S.; Boeing, H. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am. J. Clin. Nutr. 2017, 105, 1462-1473. [CrossRef]
- Schwingshackl, L.; Schwedhelm, C.; Hoffmann, G.; Knüppel, S.; Laure Preterre A.; Iqbal, K.; Bechthold, A.; De Henauw, S.; Michels, N.; Devleesschauwer, B.; Boeing, H.; Schlesinger, S. Food groups and risk of colorectal cancer. Int. J. Cancer 2018, 142, 1748-1758. [CrossRef]
- Schlesinger, S.; Neuenschwander, M.; Schwedhelm, C.; Hoffmann, G.; Bechthold, A.; Boeing, H.; Schwingshackl, L. Food groups and risk of overweight, obesity, and weight gain: a systematic review and dose-response meta-analysis of prospective studies. Adv. Nutr. 2019, 10, 205-218. [CrossRef]
- Xu, X.; Li, S.; Zhu, Y. Dietary intake of tomato and lycopene and risk of all-cause and cause-specific mortality: results from a prospective study. Front. Nutr. 2021, 8, 684859:1-684859:9. [CrossRef]
- Guasch-Ferré, M.; Willett, W.C. The Mediterranean diet and health: a comprehensive overview. J. Intern. Med. 2021, 290, 549-566. [CrossRef]
- de Lorgeril, M.; Salen, P.; Martin, J. L.; Monjaud, I.; Delaye, J.; Mamelle, N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation 1999, 99, 779-785. [CrossRef]
- Corella, D.; Coltell, O.; Macian, F.; Ordovás, J. M. Advances in understanding the molecular basis of the mediterranean diet effect. Annu. Rev. Food Sci. Technol. 2018, 9, 227-249. [CrossRef]
- Bonaccio, M.; Di Castelnuovo, A.; Pounis, G.; Costanzo, S.; Persichillo, M.; Cerletti, C.; Donati, M.B.; de Gaetano, G.; Iacoviello, L; Moli-sani Study Investigators. High adherence to the Mediterranean diet is associated with cardiovascular protection in higher but not in lower socioeconomic groups: prospective findings from the Moli-sani study. Int. J. Epidemiol. 2017, 46, 1478-1487. [CrossRef]
- Tong, T.Y.N.; Imamura, F.; Monsivais, P.; Brage, S.; Griffin, S.J.; Wareham, N.J.; Forouhi, N.G. Dietary cost associated with adherence to the mediterranean diet, and its variation by socio-economic factors in the UK Fenland Study. Br. J. Nutr. 2018, 119, 685-694. [CrossRef]
- Caparello, G.; Galluccio, A.; Giordano, C.; Lofaro, D.; Barone, I.; Morelli, C.; Sisci, D.; Catalano, S.; Andò, S.; Bonofiglio, D. Adherence to the mediterranean diet pattern among university staff: a cross-sectional web-based epidemiological study in Southern Italy. Int. J. Food Sci. Nutr. 2020, 71, 581-592. [CrossRef]
- Maroto-Rodriguez, J.; Delgado-Velandia, M.; Ortolá, R.; Perez-Cornago, A.; Kales, S. N.; Rodríguez-Artalejo, F.; Sotos-Prieto, M. Association of a mediterranean lifestyle with all-cause and cause-specific mortality: a prospective study from the UK biobank. Mayo Clin. Proc. 2024, 99, 551-563. [CrossRef]
- Buscemi, S. What are the determinants of adherence to the mediterranean diet? Int. J. Food Sci. Nutr. 2021, 72, 143-144. [CrossRef]
- Mattavelli, E.; Olmastroni, E.; Bonofiglio, D.; Catapano, A. L.; Baragetti, A.; Magni, P. Adherence to the mediterranean diet: impact of geographical location of the observations. Nutrients 2022, 14, 2040:1-2040:11. [CrossRef]
- Colao, A.; Vetrani, C.; Muscogiuri, G.; Barrea, L.; Tricopoulou, A.; Soldati, L.; Piscitelli, P.; UNESCO Chair on Health Education and Sustainable Development. “Planeterranean” diet: extending worldwide the health benefits of mediterranean diet based on nutritional properties of locally available foods. J. Transl. Med. 2022, 20, 232:1-232:3. [CrossRef]
- Toydemir, G.; Gultekin Subasi, B.; Hall, R. D.; Beekwilder, J.; Boyacioglu, D.; Capanoglu, E. Effect of food processing on antioxidants, their bioavailability and potential relevance to human health. Food Chem. X. 2022, 14, 100334:1-100334:15. [CrossRef]
- Vitucci, D.; Amoresano, A.; Nunziato, M.; Muoio, S.; Alfieri, A.; Oriani, G.; Scalfi, L.; Frusciante, L.; Rigano, M. M.; Pucci, P.; Fontana, L.; Buono, P.; Salvatore, F. Nutritional controlled preparation and administration of different tomato purées indicate increase of β-carotene and lycopene isoforms, and of antioxidant potential in human blood bioavailability: a pilot study. Nutrients 2021, 13, 1336:1-1336:14. [CrossRef]
- Burton-Freeman B.; Sesso H.D. Whole food versus supplement: comparing the clinical evidence of tomato intake and lycopene supplementation on cardiovascular risk factors. Adv. Nutr. 2014, 5, 457-485. [CrossRef]
- Basu A.; Imrhan V. Tomatoes versus lycopene in oxidative stress and carcinogenesis: conclusions from clinical trials. Eur. J. Clin. Nutr. 2007, 61, 295-303. [CrossRef]
- Gitenay, D.; Lyan, B.; Rambeau, M.; Mazur, A.; Rock, E. Comparison of lycopene and tomato effects on biomarkers of oxidative stress in vitamin E deficient rats. Eur. J. Nutr. 2007, 46, 468-475. [CrossRef]
- Rishor-Olney, C.R.; Hinson, M.R. Mediterranean Diet. In StatPearls; Treasure Island (FL): StatPearls Publishing, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK557733/ (accessed on 6 may 2024).
- Petyaev, I.M. Lycopene deficiency in ageing and cardiovascular disease. Oxid. Med. Cell. Longev. 2016, 2016, 3218605:1-3218605:6. [CrossRef]
- Khan, U.M.; Sevindik, M.; Zarrabi, A.; Nami, M.; Ozdemir, B.; Kaplan, D.N.; Selamoglu, Z.; Hasan, M.; Kumar, M.; Alshehri, M.M.; Sharifi-Rad, J. Lycopene: food sources, biological activities, and human health benefits. Oxid. Med. Cell. Longev. 2021, 2021, 2713511:1-2713511:10. [CrossRef]
- Clemente-Suárez, V. J.; Beltrán-Velasco, A. I.; Redondo-Flórez, L.; Martín-Rodríguez, A.; Tornero-Aguilera, J. F. Global impacts of western diet and its effects on metabolism and health: a narrative review. Nutrients 2023, 15, 2749:1-2749:43. [CrossRef]
- Sun, J.; Wu, K.; Wang, P.; Wang, Y.; Wang, D.; Zhao, W.; Zhao, Y.; Zhang, C.; Zhao, X. Dietary Tomato Pectin Attenuates Hepatic Insulin Resistance and Inflammation in High-Fat-Diet Mice by Regulating the PI3K/AKT Pathway. Foods 2024, 13, 444:1-444:11. [CrossRef]
- Zeng, Z.; He, W.; Jia, Z.; Hao, S. Lycopene improves insulin sensitivity through inhibition of STAT3/Srebp-1c-mediated lipid accumulation and inflammation in mice fed a high-fat diet. Exp. Clin. Endocrinol. Diabetes 2017, 125, 610-617. [CrossRef]
- Figueiredo, I. D.; Lima, T. F. O.; Inácio, M. D.; Costa, M. C.; Assis, R. P.; Brunetti, I. L.; Baviera, A. M. Lycopene improves the metformin effects on glycemic control and decreases biomarkers of glycoxidative stress in diabetic rats. Diabetes Metab. Syndr. Obes. 2020, 13, 3117-3135. [CrossRef]
- Leh, H. E.; Lee, L. K. Lycopene: a potent antioxidant for the amelioration of type II diabetes mellitus. Molecules 2022, 27, 2335:1-2335:20. [CrossRef]
- Egbuna, C.; Awuchi, C.G.; Kushwaha, G.; Rudrapal, M.; Patrick-Iwuanyanwu, K.C.; Singh, O.; Odoh, U.E.; Khan, J.; Jeevanandam, J.; Kumarasamy, S.; Chukwube, V.O.; Narayanan, M.; Palai, S.; Găman, M.A.; Uche, C.Z.; Ogaji, D.S.; Ezeofor, N.J.; Mtewa A.G.; Patrick-Iwuanyanwu, C.C.; Kesh, S.S.; Shivamallu, C.; Saravanan, K.; Tijjani, H.; Akram, M.; Ifemeje, J.C.; Olisah, M.C.; Chikwendu, C.J. Bioactive compounds effective against type 2 diabetes mellitus: a systematic review. Curr. Top. Med. Chem. 2021, 21, 1067-1095. [CrossRef]
- Council of Europe. Homeostasis, a model to distinguish between foods (including food supplements) and medical products. Available on line: https://www.dgav.pt/wp-content/uploads/2021/04/LINK-10-Homeostasis.pdf (accessed on 6 may 2024).
- Ministero della Salute. Disciplina dell’impiego negli integratori alimentari di Sostanze e preparati vegetali. Available on line: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2019&codLeg=70165&parte=2&serie= (accessed on 6 may 2024).
- Ma, S.; Li, R.; Gong, X.; Shi, W.; Zhong, X. Lycopene reduces in utero bisphenol A exposure-induced mortality, benefits hormones, and development of reproductive organs in offspring mice. Environ. Sci. Pollut. Res. Int. 2018, 25, 24041-24051. [CrossRef]
- Calniquer, G.; Khanin, M.; Ovadia, H.; Linnewiel-Hermoni, K.; Stepensky, D.; Trachtenberg, A.; Sedlov, T.; Braverman, O.; Levy, J.; Sharoni, Y. Combined effects of carotenoids and polyphenols in balancing the response of skin cells to UV irradiation. Molecules 2021, 26, 1931:1-1931:16. [CrossRef]
- Zhang, X.; Zhou, Q.; Qi, Y.; Chen, X.; Deng, J.; Zhang, Y.; Li, R.; Fan, J. The effect of tomato and lycopene on clinical characteristics and molecular markers of UV-induced skin deterioration: a systematic review and meta-analysis of intervention trials. Crit. Rev. Food Sci. Nutr. 2023, 1-20. [CrossRef]
- The Lancet Oncology. Climate change and skin cancer: urgent call for action. Lancet Oncol. 2023, 24, 823. [CrossRef]
- Liu, D.; Shoag, J. E.; Poliak, D.; Goueli, R. S.; Ravikumar, V.; Redmond, D.; Vosoughi, A.; Fontugne, J.; Pan, H.; Lee, D.; Thomas, D.; Salari, K.; Wang, Z.; Romanel, A.; Te, A.; Lee, R.; Chughtai, B.; Olumi, A. F.; Mosquera, J. M.; Demichelis, F.; Elemento, O.; Rubin, M.A.; Sboner, A.; Barbieri, C.E. Integrative multiplatform molecular profiling of benign prostatic hyperplasia identifies distinct subtypes. Nat. Commun. 2020, 11, 1987:1-1987:9. [CrossRef]
- Er, V.; Lane, J. A.; Martin, R. M.; Emmett, P.; Gilbert, R.; Avery, K. N.; Walsh, E.; Donovan, J. L.; Neal, D. E.; Hamdy, F. C.; Jeffreys, M. Adherence to dietary and lifestyle recommendations and prostate cancer risk in the prostate testing for cancer and treatment (ProtecT) trial. Cancer Epidemiol. Biomarkers Prev. 2014, 23, 2066-2077. [CrossRef]
- Loeb, S.; Fu, B.C.; Bauer, S.R.; Pernar, C.H.; Chan, J.M.; Van Blarigan, E.L.; Giovannucci, E.L.; Kenfield, S.A.; Mucci, L.A. Association of plant-based diet index with prostate cancer risk. Am. J. Clin. Nutr. 2022, 115, 662-670. [CrossRef]
- Beebe-Dimmer, J. L.; Kapron, A. L.; Fraser, A. M.; Smith, K. R.; Cooney, K. A. Risk of prostate cancer associated with familial and hereditary cancer syndromes. J. Clin. Oncol. 2020, 38, 1807-1813. [CrossRef]
- Han, G. M.; Meza, J. L.; Soliman, G. A.; Islam, K. M.; Watanabe-Galloway, S. Higher levels of serum lycopene are associated with reduced mortality in individuals with metabolic syndrome. Nutr. Res. 2016, 36, 402–407. [CrossRef]
- Moran, N.E.; Thomas-Ahner, J.M.; Wan, L.; Zuniga, K.E.; Erdman, J.W.; Clinton, S.K. Tomatoes, lycopene, and prostate cancer: what have we learned from experimental models? J. Nutr. 2022, 152, 1381-1403. [CrossRef]
- O’Kennedy, N.; Crosbie, L.; Song, H. J.; Zhang, X.; Horgan, G.; Duttaroy, A. K. A randomised controlled trial comparing a dietary antiplatelet, the water-soluble tomato extract Fruitflow, with 75 mg aspirin in healthy subjects. Eur. J. Clin. Nutr. 2017, 71, 723-730. [CrossRef]
- Hsiao, G.; Wang, Y.; Tzu, N. H.; Fong, T. H.; Shen, M. Y.; Lin, K. H.; Chou, D. S.; Sheu, J. R. Inhibitory effects of lycopene on in vitro platelet activation and in vivo prevention of thrombus formation. J. Lab. Clin. Med. 2005, 146, 216-226. [CrossRef]
- Dell’Agli, M.; Maschi, O.; Galli, G. V.; Fagnani, R.; Dal Cero, E.; Caruso, D.; Bosisio, E. Inhibition of platelet aggregation by olive oil phenols via cAMP-phosphodiesterase. Br. J. Nutr. 2008, 99, 945-951. [CrossRef]
- Fuentes, E.; Forero-Doria, O.; Carrasco, G.; Maricán, A.; Santos, L. S.; Alarcón, M.; Palomo, I. Effect of tomato industrial processing on phenolic profile and antiplatelet activity. Molecules 2013, 18, 11526-11536. [CrossRef]
- Concha-Meyer, A.; Palomo, I.; Plaza, A.; Gadioli Tarone, A.; Maróstica Junior, M. R.; Sáyago-Ayerdi, S. G.; Fuentes, E. Platelet anti-aggregant activity and bioactive compounds of ultrasound-assisted extracts from whole and seedless tomato pomace. Foods 2020, 9, 1564:1-1564:14. [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Shaheen, S.; El Haouari, M.; Azzini, E.; Butnariu, M.; Sarac, I.; Pentea, M.; Ramírez-Alarcón, K.; Martorell, M.; Kumar, M.; Docea, A. O.; Cruz-Martins, N.; Calina, D. Flavonoids as potential anti-platelet aggregation agents: from biochemistry to health promoting abilities. Crit. Rev. Food Sci. Nutr. 2022, 62, 8045-8058. [CrossRef]
- Rodríguez-Azúa, R.; Treuer, A.; Moore-Carrasco, R.; Cortacáns, D.; Gutiérrez, M.; Astudillo, L.; Fuentes, E.; Palomo, I. Effect of tomato industrial processing (different hybrids, paste, and pomace) on inhibition of platelet function in vitro, ex vivo, and in vivo. J. Med. Food 2014, 17, 505-511. [CrossRef]
- Krasinska, B.; Osińska, A.; Osinski, M.; Krasinska, A.; Rzymski, P.; Tykarski, A.; Krasiński, Z. Standardised tomato extract as an alternative to acetylsalicylic acid in patients with primary hypertension and high cardiovascular risk - a randomised, controlled trial. Arch. Med. Sci. 2018, 14, 773-780. [CrossRef]
- Pulcinelli, F.; Curreli, M.; Natali, P. G.; Quaresima, V.; Imberti, L.; Piantelli, M. Development of the whole tomato and olive-based food supplement enriched with anti-platelet aggregating nutrients. Nutr. Health 2023, 29, 193-197. [CrossRef]
- Drachman, J. G.; Rojnuckarin, P.; Kaushansky, K. Thrombopoietin signal transduction: studies from cell lines and primary cells. Methods 1999, 17, 238-249. [CrossRef]
- Zhou, Z.; Gushiken, F. C.; Bolgiano, D.; Salsbery, B. J.; Aghakasiri, N.; Jing, N.; Wu, X.; Vijayan, K. V.; Rumbaut, R. E.; Adachi, R.; Lopez, J. A.; Dong, J. F. Signal transducer and activator of transcription 3 (STAT3) regulates collagen-induced platelet aggregation independently of its transcription factor activity. Circulation 2013, 127, 476-485. [CrossRef]
- Lu, W. J.; Lin, K. C.; Huang, S. Y.; Thomas, P. A.; Wu, Y. H.; Wu, H. C.; Lin, K. H.; Sheu, J. R. Role of a Janus kinase 2-dependent signaling pathway in platelet activation. Thromb. Res. 2014, 133, 1088-1096. [CrossRef]
- Guo, W.; Huang, D.; Li, S. Lycopene alleviates oxidative stress-induced cell injury in human vascular endothelial cells by encouraging the SIRT1/Nrf2/HO-1 pathway. Clin. Exp. Hypertens. 2023, 45, 2205051:1-2205051:11. [CrossRef]
- Mozos, I.; Stoian, D.; Caraba, A.; Malainer, C.; Horbańczuk, J. O.; Atanasov, A. G. Lycopene and vascular health. Front. Pharmacol. 2018, 9, 521:1-521:16. [CrossRef]
- Cloud, G. C.; Williamson, J. D.; Thao, L. T. P.; Tran, C.; Eaton, C. B.; Wolfe, R.; Nelson, M. R.; Reid, C. M.; Newman, A. B.; Lockery, J.; Fitzgerald, S. M.; Murray, A. M.; Shah, R. C.; Woods, R. L.; Donnan, G. A.; McNeil, J. J. Low-dose aspirin and the risk of stroke and intracerebral bleeding in healthy older people: secondary analysis of a randomized clinical trial. JAMA Netw. Open 2023, 6, e2325803:1-e2325803:12. [CrossRef]
- Abir, M. H.; Mahamud, A. G. M. S. U.; Tonny, S. H.; Anu, M. S.; Hossain, K. H. S.; Protic, I. A.; Khan, M. S. U.; Baroi, A.; Moni, A.; Uddin, M. J. Pharmacological potentials of lycopene against aging and aging-related disorders: a review. Food Sci. Nutr. 2023, 11, 5701-5735. [CrossRef]
- Zhao, Y.; Ma, D. X.; Wang, H. G.; Li, M. Z.; Talukder, M.; Wang, H. R.; Li, J. L. Lycopene prevents DEHP-induced liver lipid metabolism disorder by inhibiting the HIF-1α-induced PPARα/PPARγ/FXR/LXR system. J. Agric. Food Chem. 2020, 68, 11468-11479. [CrossRef]
- Tripathi, V.; Edrisi, S. A.; Chaurasia, R.; Pandey, K. K.; Dinesh, D.; Srivastava, R.; Srivastava, P.; Abhilash, P. C. Restoring HCHs polluted land as one of the priority activities during the UN-international decade on ecosystem restoration (2021-2030): a call for global action. Sci. Total Environ. 2019, 689, 1304-1315. [CrossRef]
- Vijgen, J.; Aliyeva, G.; Weber, R. The Forum of the International HCH and pesticides association--a platform for international cooperation. Environ. Sci. Pollut. Res. Int. 2013, 20, 2081-2086. [CrossRef]
- U.S. Environmental Protection Agency. Lindane (Gamma-Hexachlorocyclohexane). Available on line: https://www.epa.gov/sites/default/files/2016-09/documents/lindane.pdf (accessed on 6 may 2024).
- Fernández-Bedmar, Z.; Anter, J.; Alonso Moraga, Á. Anti/genotoxic, longevity inductive, cytotoxic, and clastogenic-related bioactivities of tomato and lycopene. Environ. Mol. Mutagen. 2018, 59, 427-437. [CrossRef]
- Elsayed, A.; Elkomy, A.; Alkafafy, M.; Elkammar, R.; El-Shafey, A.; Soliman, A.; Aboubakr, M. Testicular toxicity of cisplatin in rats: ameliorative effect of lycopene and N-acetylcysteine. Environ. Sci. Pollut. Res. Int. 2022, 29, 24077-24084. [CrossRef]
- Perrone, P.; Lettieri, G.; Marinaro, C.; Longo, V.; Capone, S.; Forleo, A.; Pappalardo, S.; Montano, L.; Piscopo, M. Molecular alterations and severe abnormalities in spermatozoa of young men living in the “Valley of Sacco river” (Latium, Italy): a preliminary study. Int. J. Environ. Res. Public Health 2022, 19, 11023:1-11023:18. [CrossRef]
- Montano, L.; Ceretti, E.; Donato, F.; Bergamo, P.; Zani, C.; Viola, G. C. V.; Notari, T.; Pappalardo, S.; Zani, D.; Ubaldi, S.; Bollati, V.; Consales, C.; Leter, G.; Trifuoggi, M.; Amoresano, A.; Lorenzetti, S.; FASt study group. Effects of a lifestyle change intervention on semen quality in healthy young men living in highly polluted areas in Italy: the FASt Randomized Controlled Trial. Eur. Urol. Focus 2022, 8, 351-359. [CrossRef]
- Montano, L.; Maugeri, A.; Volpe, M. G.; Micali, S.; Mirone, V.; Mantovani, A.; Navarra, M.; Piscopo, M. Mediterranean diet as a shield against male infertility and cancer risk induced by environmental pollutants: a focus on flavonoids. Int. J. Mol. Sci. 2022, 23, 1568:1-1568:24. [CrossRef]
- Pan American Health Organization. Economics of NCDs. Available on line: https://www.paho.org/en/topics/economics-ncds (accessed on 6 may 2024).
- World Cancer Research Fund International. The link between food, nutrition, diet and non-communicable disease. Available on line: https://www.wcrf.org/wp-content/uploads/2021/07/WCRF-NCD-A4-WEB.pdf (accessed on 6 may 2024).
- Afshin, A.; Micha, R.; Webb, M.; Capewell, S.; Whitsel, L.; Rubinstein, A.; Prabhakaran, D.; Suhrcke, M.; Mozaffarian, D. Effectiveness of dietary policies to reduce noncommunicable diseases. In Cardiovascular, Respiratory, and Related Disorders, 3rd ed.; Prabhakaran, D., Anand, S., Gaziano, T.A., et al., Eds.; The International Bank for Reconstruction and Development/The World Bank: Washington (DC), U.S.A., 2017; Chapter 6, Available online: https://www.ncbi.nlm.nih.gov/books/NBK525147/. [CrossRef]
- Afshin, A.; Penalvo, J.; Del Gobbo, L.; Kashaf, M.; Micha, R.; Morrish, K.; Pearson-Stuttard, J.; Rehm, C.; Shangguan, S.; Smith, J. D.; Mozaffarian, D. CVD prevention through policy: a review of mass media, food/menu labeling, taxation/subsidies, built environment, school procurement, worksite wellness, and marketing standards to improve diet. Curr. Cardiol. Rep. 2015, 17, 98:1-98:12. [CrossRef]
- Gholami, F.; Antonio, J.; Evans, C.; Cheraghi, K.; Rahmani, L.; Amirnezhad, F. Tomato powder is more effective than lycopene to alleviate exercise-induced lipid peroxidation in well-trained male athletes: randomized, double-blinded cross-over study. J. Int. Soc. Sports Nutr. 2021, 18, 17:1-17:7. [CrossRef]
- Ziaee, A.; Albadarin, A. B.; Padrela, L.; Femmer, T.; O’Reilly, E.; Walker, G. Spray drying of pharmaceuticals and biopharmaceuticals: critical parameters and experimental process optimization approaches. Eur. J. Pharm. Sci. 2019, 127, 300-318. [CrossRef]
- Nishimura, M.; Tominaga, N.; Ishikawa-Takano, Y.; Maeda-Yamamoto, M.; Nishihira, J. Effect of 12-week daily intake of the high-lycopene tomato (Solanum Lycopersicum), a variety named “PR-7”, on lipid metabolism: a randomized, double-blind, placebo-controlled, parallel-group study. Nutrients 2019, 11, 1177:1-1177:13. [CrossRef]
- Mossine, V. V.; Chopra, P.; Mawhinney, T. P. Interaction of tomato lycopene and ketosamine against rat prostate tumorigenesis. Cancer Res. 2008, 68, 4384-4391. [CrossRef]
- Bulotta, S.; Celano, M.; Lepore, S. M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219:1-219:9. [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and their roles in age-related macular degeneration-neurodegenerative disease. Nutrients 2022, 14, 827:1-827:14. [CrossRef]
- Szabo, K.; Cătoi, A. F.; Vodnar, D. C. Bioactive compounds extracted from tomato processing by-products as a source of valuable nutrients. Plant. Foods Hum. Nutr. 2018, 73, 268-277. [CrossRef]
- Plants of the World Online. Available on line: http://www.plantsoftheworldonline.org (accessed on 6 may 2024).
- Islam, Z.; Islam, S. M. R.; Hossen, F.; Mahtab-Ul-Islam, K.; Hasan, M. R.; Karim, R. Moringa oleifera is a prominent source of nutrients with potential health benefits. Int. J. Food Sci. 2021, 2021, 6627265:1-6627265:11. [CrossRef]
- Facts and Factors. Global moringa products market anticipates to reach USD 8,400 million by 2026. Available on line: https://www.fnfresearch.com/news/global-moringa-products-market-anticipates-to-reach-(accessed on 6 may 2024).
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; Wan, Q.; Zhou, Y. Tomato and lycopene and multiple health outcomes: umbrella review. Food Chem. 2021, 343, 128396:1-128396:8. [CrossRef]
| Refs. | |||
|---|---|---|---|
| Refs. | |||
| Worldwide second high yield crop | [35] | High biodiversity | [42] |
| High consumption rate | [36] | High chemodiversity | [43] |
| Expected 5% increasing market in the near future | [37] | High nutrition yield | [44] |
| Unique culinary versatility with wide acceptance in different dietary regimens | [38] | Cultivation requires timely controlled irrigation and moderate soil tillage | [45] |
| High recyclability of industrial processing waste and packaging | [39] | Growth Not sensitive to increased CO2 environmental concentrations | [45] |
| Facilitator of circular economy | [37,40] | It is an “excluder plant” when referred to soil contaminants | [46] |
| It could become a scaffold for the development of a variety of dietary supplements of more targeted health claims | [41] | Organic and conventional cultivations have not no significant difference in heavy metal content Residues of pesticides efficiently removed by washing and cooking |
[47] [48,49] |
| Tomato powder | WTFS | |||
|---|---|---|---|---|
| Tomato (98%) | Olive waste water (2%) | |||
| Carbohydrates | 66 g | 63.5 g | Oleuropeinaglycon | 6 g |
| Proteins | 10.2 g | 16.5 g | Ligtrosideaglycan | 2 g |
| Lipids | 1.6 g | 3.4 g | Oleuropeindialdehydeaglycane | 16 g |
| Total carotenoids | 142.2 mg | 500 mg | 7 g | |
| All-trans lycopene | 109.2 mg | 250 mg | Verbascoside | 6 g |
| 5-cis lycopene | 7.4 mg | 35 mg | Pinoresinol and deacetoxy-pinoresinol | 5 g |
| Lycopene isomers | 15.7 mg | 190 mg | Thyrosol | 3 g |
| β-carotene | 8.7 mg | 22 mg | Hydroxy-thyrosol | 10 g |
| Lutein | 1.2 mg | 3 mg | Unindefined polyphenols | 8 g |
| α-tocopherol | 1.9 mg | 2.3 mg | Polysaccharides | 33 g |
| Total flavonoids | 15.3 mg | 200 mg | Humidity | < 4 g |
| Quercetin derivates | 1.1 mg | 140 mg | ||
| Naringenin derivates | 4.2 mg | 60 mg | ||
| Ketosamines | - | 8 mg | ||
| Fru-His | - | 0.06 mg | ||
| Fibers | ND | 15.9 mg | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





