1. Introduction
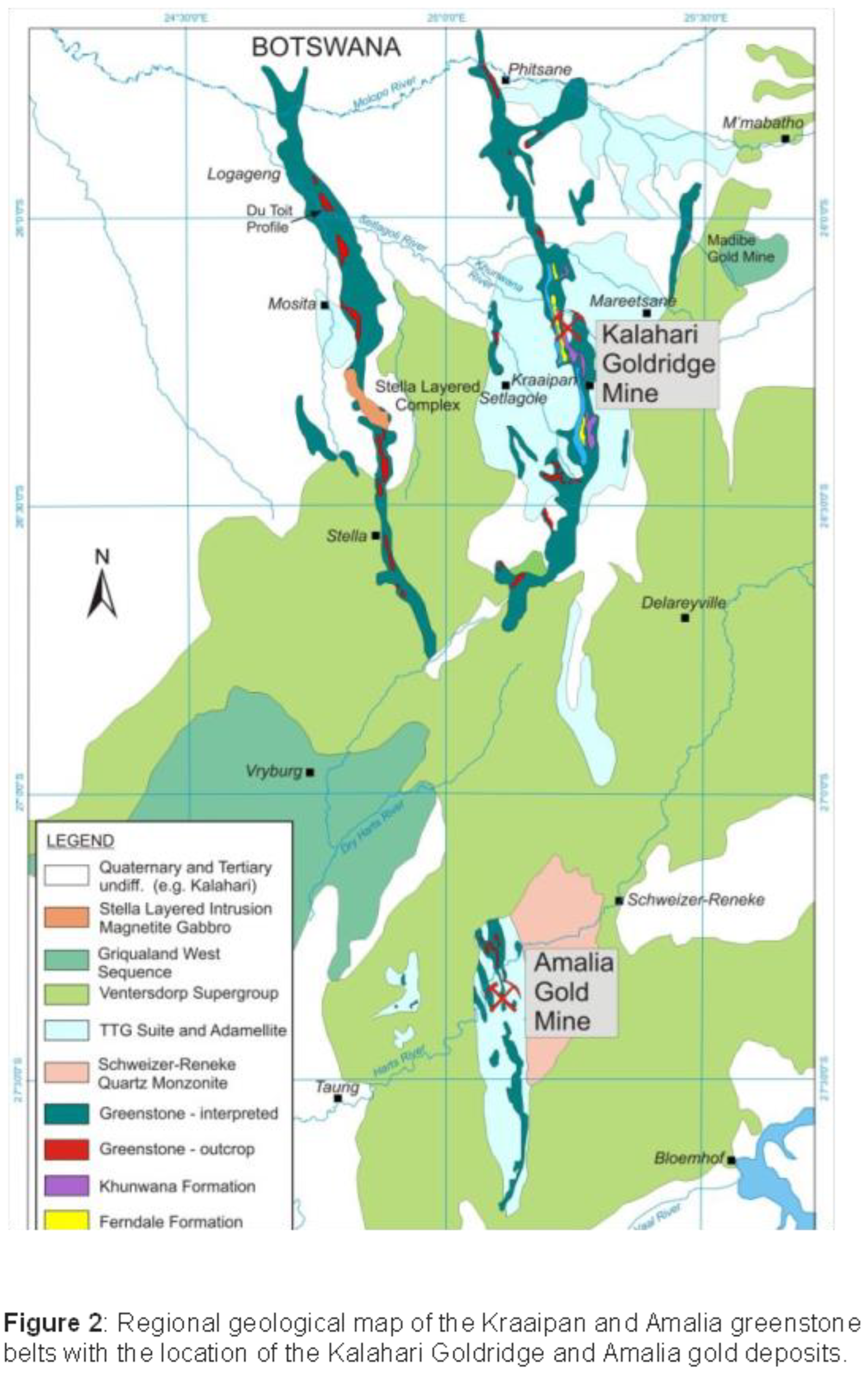
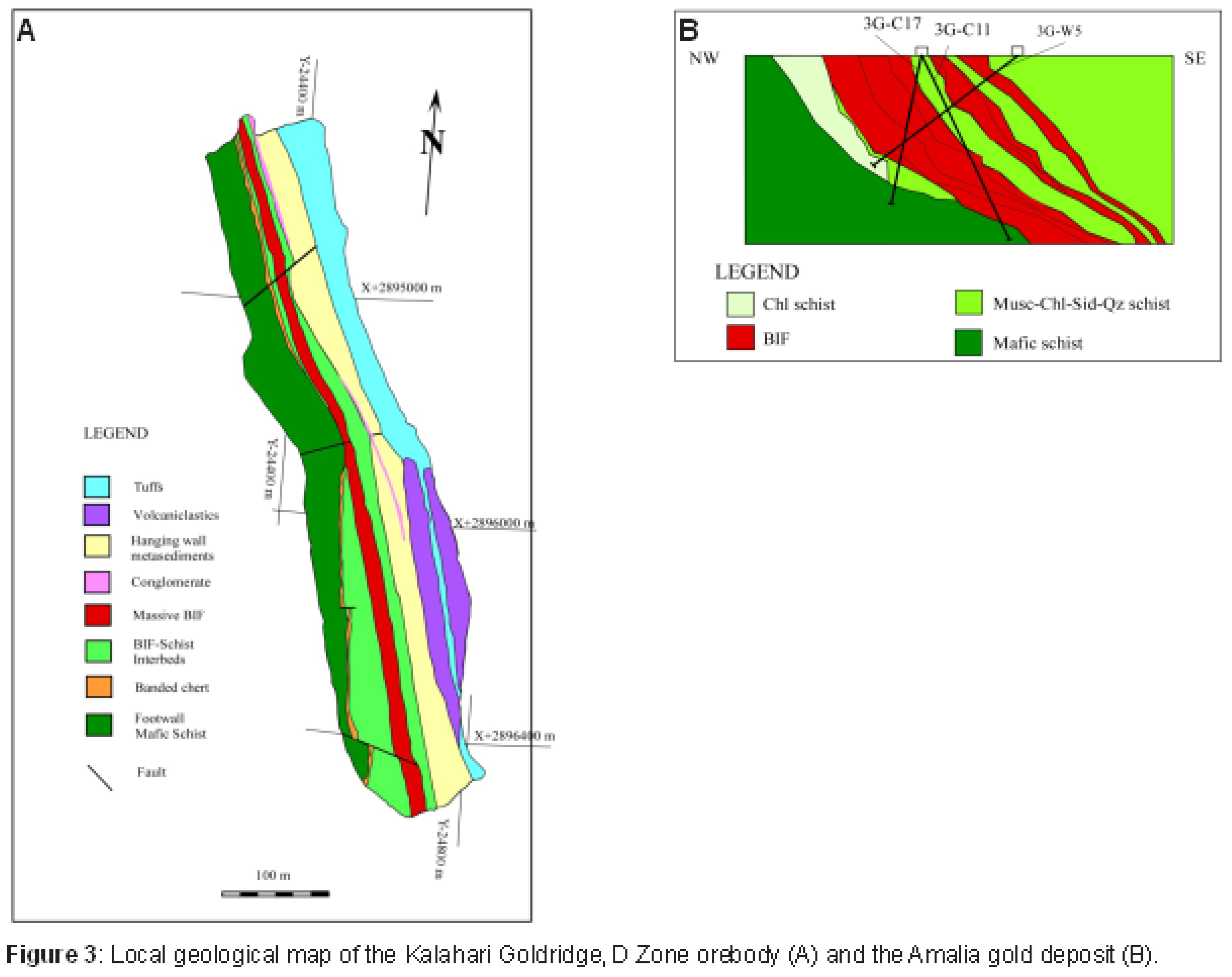
Several studies on Archaean orogenic gold deposits (e.g., Kerrick 1989; Ho et al., 1992; Groves et al. 1998; Goldfarb and Groves, 2015; Groves et al., 2020) are documented to have been formed from ore-forming fluids that originated from deep within the crust, along crustal scale faults or fissures through which these fluids have migrated to depositional sites of ore metals and associated minerals. The stable isotope data associated with most of these studies have been unable to clearly define the origin of ore-forming fluids associated with such deposits due to overlap of isotopic signatures among fluid sources. The application of radiogenic isotopes, particularly strontium (Sr) in recent years, have proven to be a useful tool in understanding the source reservoir and flow pathways of these fluids (e.g., Glodny and Grauert, 2009; Toki et al. 2022; Kim et al., 2004). The flow pathways can provide significant insight to the nature of water-rock interaction between the fluid and the rocks encountered during fluid migration; thus, providing important information on the nature and evolution of the hydrothermal fluid in ore deposit systems. The Kraaipan and Amalia greenstone belts in South Africa is a N-S trending, laterally discontinuous structure that are spatially associated with granitoids of similar petrological characteristics and age (Figure 1). The basement rocks of these greenstone belts are Archaean TTG gneisses (Anhaeusser and Walraven 1999). Epigenetic banded iron-formation (BIF)-hosted gold deposits within the Kraaipan-Amalia terrane include the Kalahari Goldridge deposit in the Kraaipan greenstone belt (to the north) and the Amalia deposit in the Amalia greenstone belt (to the south) (Figure 1), and are separated about 90 km from each other. The hanging wall units in both deposits comprises metasedimentary rocks of shales and schists, and footwall comprises mafic volcanic schists (Figure 2 and Figure 3). However, local lithological variations occur between the BIF and footwall at the Kalahari Goldridge deposit (Hammond and Moore 2006).
Despite similar geological settings, the ambient conditions associated with the mineralization in these deposits exhibit some geochemical variations (Hammond et al. 2007, Adomako-Ansah et al. 2017). It is therefore not clear if the mineralizing events in these deposits were spatially linked to a unique fluid source that were modified along different flow pathways to the depositional’ sites or they were discrete mineralizing events with distinct sources. A combination of Sr-C-O isotopic ratios from previous studies on the Kalahari Goldridge (Hammond et al. 2007, Hammond and Morishita 2009), C-O isotope on Amalia (Adomako-Ansah et al. 2017) and new Sr isotope data for the Amalia deposit, this paper attempts to unravel and evaluate the nature and evolution of the fluids associated with gold mineralization in the Kraaipan-Amalia terrane.
2. Geological Setting
The evolution history of the Kaapvaal craton includes the amalgamation of the ~3.7-Ga-old Witwatersrand block (to the east) and 3.2-Ga-old Kimberly block (to the west) along the Colesberg Lineament (CL) suture (Figure 1) by subduction-accretion and continent-continent collision processes that occurred at about 3.0-2.9 Ga ago (Corner et al. 1990, de Wit et al. 1992, McCourt 1995, Richardson et al. 2001, Schmitz et al. 2004, Errickson et al. 2009). The Kraaipan-Amalia greenstone belts are located in the Kimberly block of the Kaapvaal craton and aligned parallel to the CL. The thermo-tectonic processes is believed to have resulted in the formation of highly deformed, north-south-trending subvertical volcano-sedimentary rock units in the Kraaipan-Amalia greenstone belts that are generally fault-bounded and partially engulfed by abundant intrusive syn- to post-deformational granitoids (de Wit et al. 1992, Schmitz et al. 2004). The Kraaipan-Amalia greenstone belt is flanked to west by 3.08-2.93 Ga-old reworked TTGs and, to the east by 2.93-2.85 Ga-old Kraaipan group of post-collisional and intrusive granodioritic and magnetite-bearing quartz monzonitic plutons (Figure 1; Anhaeusser and Walraven 1999, Poujol et al. 2002, Robb and Meyer 1995). Spatial association between the Kraaipan-Amalia greenstone belts and the granodioritic-monzonitic rocks aroused speculations on a genetic link between the gold deposits within the greenstone belts and felsic igneous activity (e.g., Anhaeusser and Walraven 1999, Poujol et al. 2002, Robb and Meyer 1995). The greenstone belts are poorly exposed due to limited outcrop in the region. Sparse low-ridged BIF have been reported to be associated with a suite of agglomerates and accretionary lapilli tuffs (Jones and Anhaeusser 1993, Anhaeusser and Walraven 1999). Geophysical investigation conducted across the CL by de Wit and Tinker (2004) revealed that the Kraaipan-Amalia greenstone belt terrane was obducted unto the Kaapvaal craton from the east as an allochthonous unit by accretionary tectonics (de Wit and Tinker 2004).
3. Hydrothermal Alteration and Gold Mineralization
The geological, mineralogical and geochemical characteristics are detailed in Hammond and Moore (2006), Hammond
et al. (2007), Hammond and Morishita (2009), Kiefer (2004), Adomako-Ansah
et al. (2013) and Adomako-Ansah
et al. (2017) and are summarized in
Table 1. The host rocks to mineralization in the Kalahari Goldridge and Amalia gold deposits are magnetite-rich oxide facies BIFs (Figure 3); Hammond and Moore 2006, Adomako-Ansah
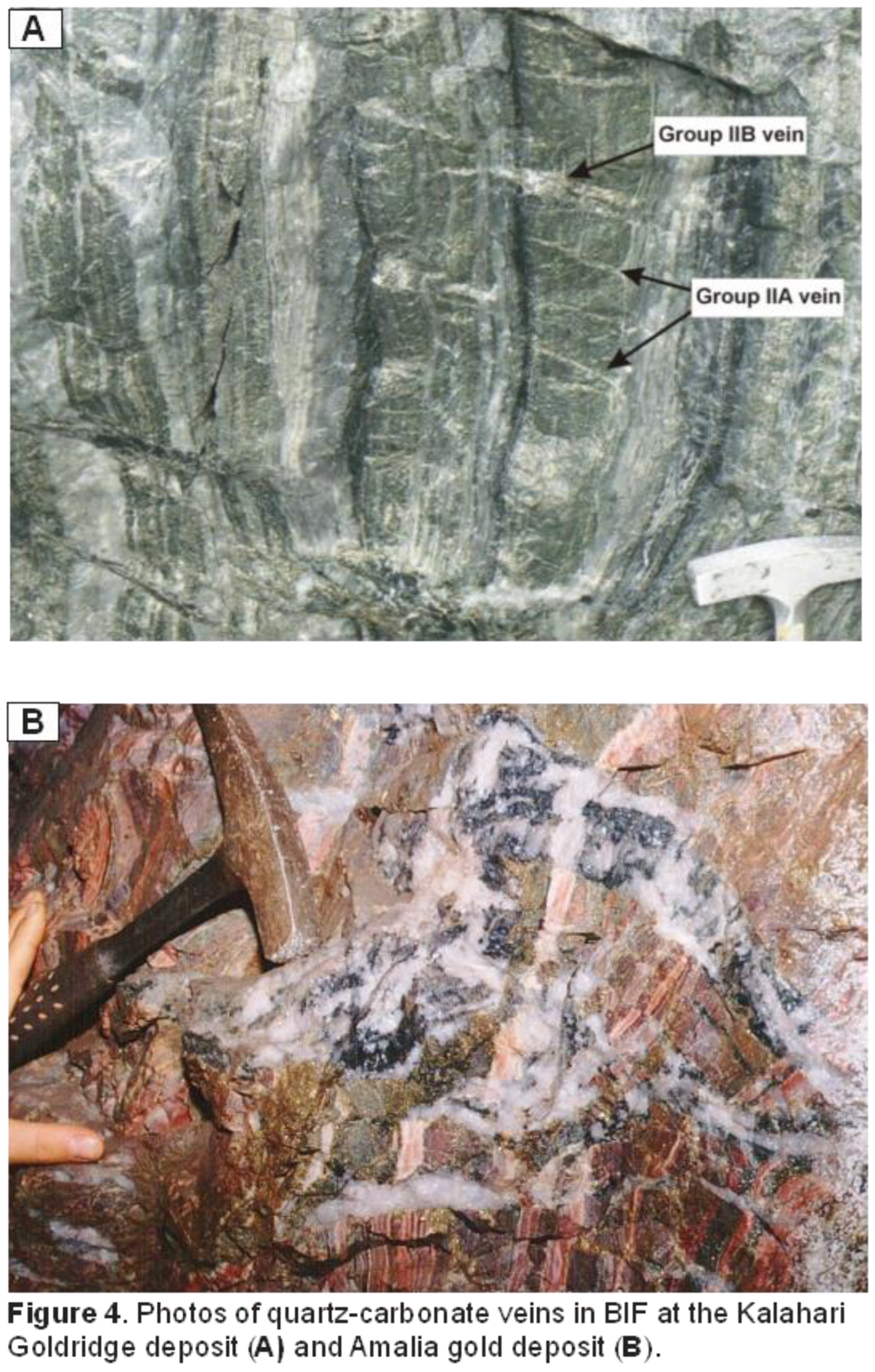
et al. 2013). Gold mineralization in both deposits is associated with quartz-carbonate veins that cut across the oxide-facies BIF layers (Figure 4). Field observation and textural evidence in both deposits indicate carbonate alteration of the BIF was pervasive and occurred alongside sulphidized alteration haloes that surrounded the contacts and selvages between the quartz-carbonate veins and the host BIF units. Hematization of magnetite was observed at Amalia, however, it was absent at Kalahari Goldridge. Geochemical evidence showed that gold mineralization in both deposits is closely associated with the altered host rocks; typically, the carbonate-altered and highly sulphidized BIF. Replacement of magnetite by pyrite and pyrrhotite is prominent at Kalahari Goldridge (Figure 5A-C), however, there is extensive replacement of magnetite by hematite and pyrite at the Amalia deposit with minor chalcopyrite and arsenopyrite (Figure 5D-F). These observations are clearly consistent with contrasting ambient redox conditions in the ore-forming fluids during mineralization in these deposits: a reduced fluid system at Kalahari Goldridge deposit and more oxidized conditions at Amalia.

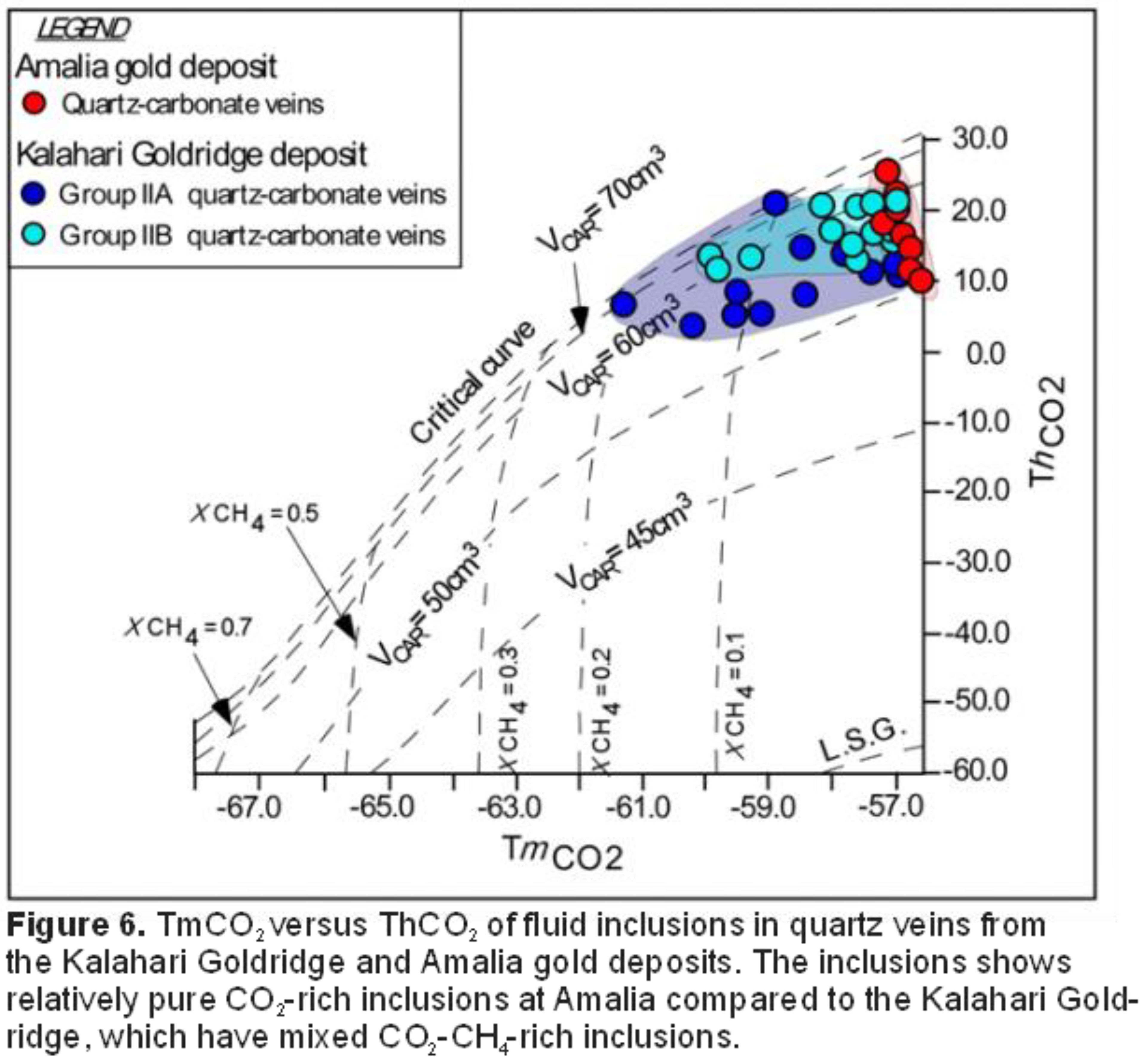
The varied redox state of these deposits is further evidenced from microthermometric and Raman analyses of fluid inclusions in quartz veins that indicated low salinity NaCl-H2O-CO2±CH4 compositions at the Kalahari Goldridge (Hammond et al. 2007) and NaCl-H2O-CO2 at Amalia Adomako-Ansah et al. 2017), Figure 6. Thus, a relatively ‘pure’ CO2-rich composition characterizes the ore-forming fluid at Amalia compared to the Kalahari Goldridge, which contains significant in CH4 in some inclusion.
4. Mineralization Temperatures Based on Chlorite Geothermometry
Chlorite is a common mineral which occurs in a wide range of geological environments such as diagenesis, low- to medium-grade metamorphism and hydrothermal alteration. The mineral displays a wide range of non-stoichiometric compositional variations depending on bulk composition of host rocks, particularly the Fe/(Fe+Mg) ratio, prevailing physico-chemical conditions such as temperatures, pressures, redox state during formation (Bryndzia and Scott, 1987; De Caritat et al., 1993; Vidal et al., 2016), and fluid chemistry in systems associated with hydrothermal deposits. The variation of chemical composition in chlorite has served as a useful tool to obtain information on the physico-chemical conditions during the evolution of mineralizing hydrothermal fluids. In particular, the chlorite geothermometry have been applied widely in the estimation of formation temperatures of chlorites in hydrothermally altered rocks in many ore deposits given its ubiquitous occurrence in such geological settings.
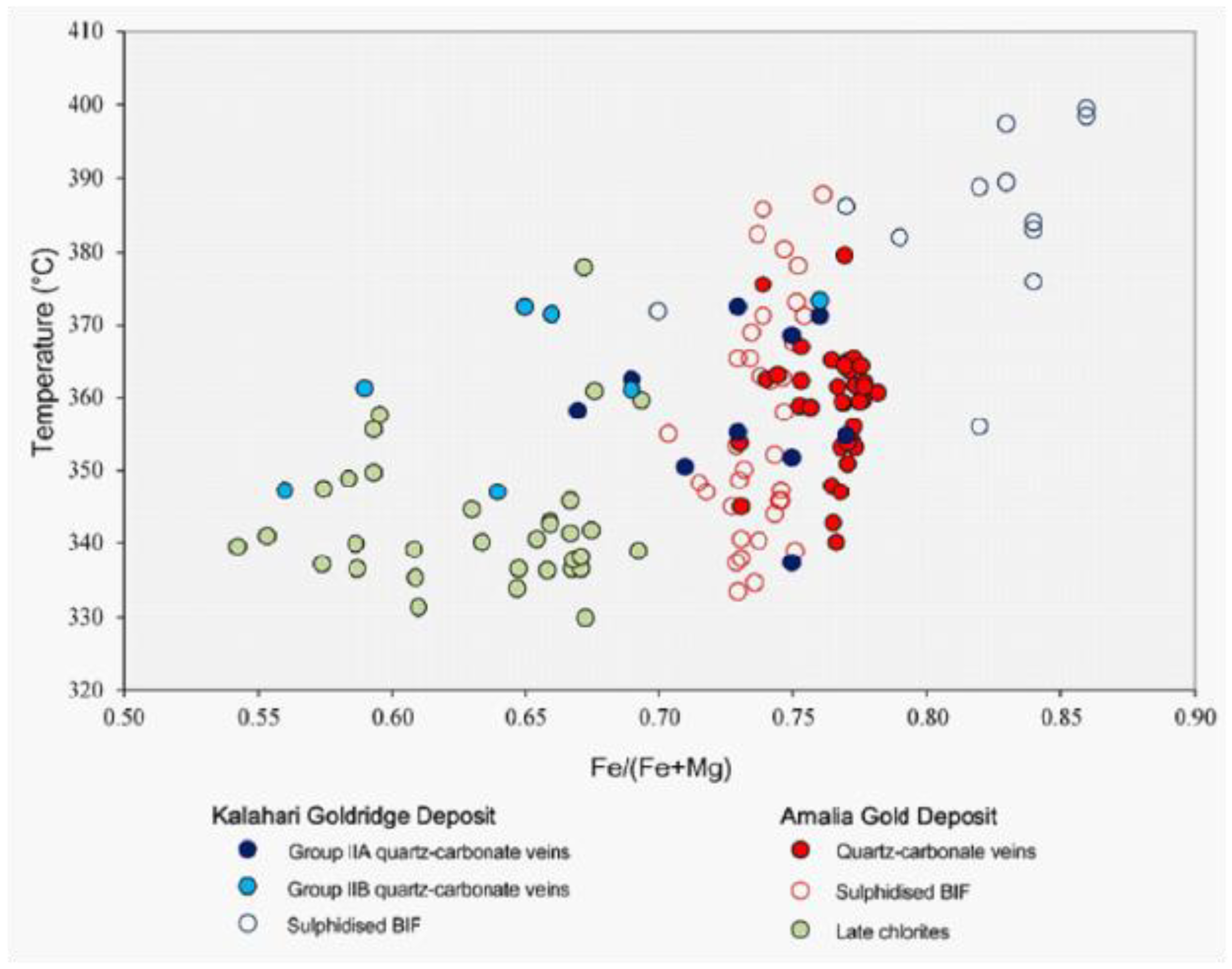
The mineralization temperatures for the Amalia and Kalahari Goldridge deposits were determined using the chlorite geothermometry data of Cathelineau and Nieva (1985) and Cathelineau (1988). The compositions of the chlorite were analysed from chlorites in mineralizing veins and hydrothermally altered BIFs. Figure 7 illustrates these ranges from 350o to 400oC at the Kalahari Goldridge deposit (Hammond et al. 2007, Hammond and Morishita, 2009), and 330o to 390oC at Amalia (Adomako-Ansah et al., 2013). However, there is an overall decrease in temperature with corresponding decreasing Fe/(Fe+Mg) ratio, with highest temperatures recorded in chlorites in altered BIF at Kalahari Goldridge and lowest temperatures associated with chlorites in late factures in Amalia BIF.
5. Isotopic (Sr, C and O) Signatures and Rb Concentrations
Rubidium-strontium, carbon and oxygen isotopic systematics in carbonates from the alteration carbonates at the Amalia and Kalahari Goldridge deposits were undertaken to deduce the nature and evolution of fluids in these deposits in an attempt to define the fluid origin in the Kraaipan-Amalia terrane. The Rb-Sr isotopic technique was based on the premise that in minerals that contain relatively low concentration of Rb (<1ppm) but high concentrations of Sr such as in carbonates arising from Sr-Ca substitution due to similar ionic radii of the two elements. Hence, the
87Sr/
86Sr ratio can remain relatively unchanged with time due to limited or no influence of radiogenic Sr from in-situ radioactive decay of Rb. The
87Sr/
86Sr ratios may therefore, represent the approximate initial compositions of the fluids at the time of carbonate crystallization. On the basis of this premise, diagnostic fluid-rock interaction trends between evolving fluid systems and crustal components can be constructed to deduce this assertion. The Rb-Sr isotope data for Kalahari Goldridge deposit reported earlier by Hammond and Morishita (2009) and compared with new strontium isotopic signatures (
87Sr/
86Sr isotopic ratios) for the Amalia gold deposit. Mineralizing quartz-carbonate veins from both deposits were crushed in a steel mortar and carbonates separated manually under binocular microscopes. The carbonates were subject to standard ion exchange technique and
87Sr/
86Sr isotopic measurements, and trace elements concentration including Sr and Rb were determined using multicollector ICP-MS, at the Department of Earth Science and Technology, Akita University in Japan for the Amalia deposits, and the Research School of Earth Science, Australian National University for the Kalahari Goldridge deposits. Six (6)
87Sr/
86Sr isotopic ratios of carbonates were determined from the Amalia veins and nine (9)
87Sr/
86Sr isotopic ratios of carbonates determined for the Kalahari Goldridge deposit (
Table 2). Analyses of the carbonate separates by MC-ICP-MS measurements following chemical separation of Rb and Sr by ion exchange chromatography yielded very radiogenic
87Sr/
86Sr ratios range from 0.703023 to 0.706643 for the carbonates from Amalia. The Sr and Rb concentrations vary from 3.4 to 157.2 ppm and 0.6 to 2.1 ppm, respectively. The
87Sr/
86Sr ratios of the vein carbonate in the Kalahari Goldridge is from 0.70354 to 0.73914, while the Sr and Rb concentrations vary from 1.1.2 to 168 ppm and 0.04 to 0.4 ppm, respectively.
The δ
13C-δ
18O data of carbonates from quartz-carbonate veins from the Amalia and Kalahari Goldridge deposits are derived from Adomako-Ansah
et al. (2017) and Hammond
et al. (2007) respectively and also summarized in
Table 2.
6. Discussion
The analytical results indicate very low Rb/Sr ratios (<1) for the vein carbonates from both deposits, suggesting that the corresponding 87Sr/86Sr ratios remained unchanged through time making the measured values a good approximation for the initial 87Sr/86Sr ratios. Consequently, the 87Sr/86Sr ratios are suitable for monitoring the nature and evolution of the ore-forming fluid(s) in the Kraaipan-Amalia terrane.
Figure 8 illustrates a binary plot of 87Sr/86Sr ratios and their corresponding Sr concentrations in vein carbonates from the Kalahari Goldridge and Amalia gold deposits, which shows that the vein carbonates from both deposits plot have a common minimum value characterized by 87Sr/86Sr isotopic ratio of 0.70354. The minimum value suggests that the ore-forming fluids for both deposits possibly originated from a common fluid reservoir source of uniform isotopic composition (87Sr/86Sr ratio = 0.70354), which is consistent with a mantle or mafic igneous signature for the ore-forming fluids. The plot also illustrates a diverging trend towards increasing radiogenic Sr values along different evolutionary flow paths for each of the quartz-carbonate veining events in the deposits. The–increasing radiogenic Sr trend can be attributed to isotopic exchange resulting from the mixing of multiple fluids of isotopically different signatures or by the water-rock interaction between ore-forming fluids and supracrustal wall rocks with higher radiogenic Sr concentrations. The fluid inclusion and stable isotope data (Hammond et al. 2007; Adomako-Ansah et al. 2017) are inconsistent with the involvement of multiple fluids in both deposits. Hence, the effect of fluid mixing is consequently ruled out. Thus, the trend to higher 87Sr/86Sr ratios illustrated in Figure 7 can be related to isotopic exchange between ore-forming fluids and the supracrustal rocks that surround the deposits. The basement rocks to the BIF-hosted Kalahari Goldridge and Amalia gold deposits are Archean TTGs of similar age and petrographic characteristics. If the ore-forming fluids interacted with only the basement TTG rocks and/or BIF units, it is expected that the deposits will exhibit similar variations or tendencies in the radiogenic Sr isotopic ratios of their respective vein carbonates. However, this is not the case. Inconsistency in the trend to higher Sr isotopic ratios between the two deposits is illustrated in Figure 7, by comparing the Sr isotopic data with the δ13C values from both deposits (e.g., Kerrich et al. 1987, Morishita and Nakano 2008). The vein carbonates from the Amalia gold deposit are characterized by relatively homogeneous and less radiogenic 87Sr/86Sr ratios. On the other hand, the 87Sr/86Sr ratios in the vein carbonates from the Kalahari Goldridge deposit are relatively heterogenous, more radiogenic and widespread: ranging from low values that are similar to that of the Amalia gold deposit to much higher radiogenic values that are not recorded in the Amalia gold deposit. Since the basement rocks are the same for both deposits, the analogy, therefore, would be that the wider spread to relatively higher radiogenic Sr isotopic values in the precipitating vein carbonates at the Kalahari Goldridge deposit resulted from the water-rock interaction between ore-forming fluids and the graphite-bearing metasedimentary rock surrounding the host BIF unit. In addition, the heterogeneous nature of the 87Sr/86Sr ratios at Kalahari Goldridge can be attributed to larger volume of fluid interaction with more crustal rocks, and relatively more intensive fluid-rock interaction. The large fluid volume is reflected by the large quartz veins (Group IIB veins) and the multiple ladder veins (Group IIA) (Hammond and Morishita 2009). Conversely, the homogeneous 87Sr/86Sr ratios in the vein carbonates from Amalia, as illustrated in Figure 7, can be attributed to interaction of ore-forming fluids with limited or restricted variety of crustal sedimentary host rocks at Amalia in comparison with Kalahari Goldridge. The observations at the Kraaipan-Amalia is consistent with several orogenic gold deposit of variable geological setting worldwide (e.g.,Goldfarb and Groves, 2015; Kerrich et al.,1987). For example, the review by Goldfarb and Groves (2015) on fluid and metal sources for orogenic gold deposits reported that strontium was derived from basement rocks below Archean greenstone belts that host these deposits or reflect a significant mantle component, with values altered along the flow path and at the site of gold deposition by host metasedimentary rock sequences. They also documented local wall rock sources for Sr or multiple strontium sources in host rocks distal to the gold deposits. Kerrich et al. (1987) noted the uniformity of initial 87Sr/86Sr ratios of mafic volcanic rocks in gold deposit-hosted terranes in the Archean Abitibi sub-province of Canada to be consistent with a homogenous upper mantle reservoir. In a similar study, the variations in 87Sr/86Sr ratios in epithermal gold deposits hosted in both the basement carbon-rich metasedimentary rocks of the Shimanto Group and the overlying andesitic volcanic rocks were investigated by Morishita and Nakano (2008) in the Kyushu region of Japan and showed relatively high 87Sr/86Sr ratios in ore-related calcite, which were inferred to indicate hydrothermal ore-forming fluid interaction with the carbon-rich metasedimentary rocks that contained much higher 87Sr/86Sr composition than the surrounding shallower volcanic rocks hosting the deposits.
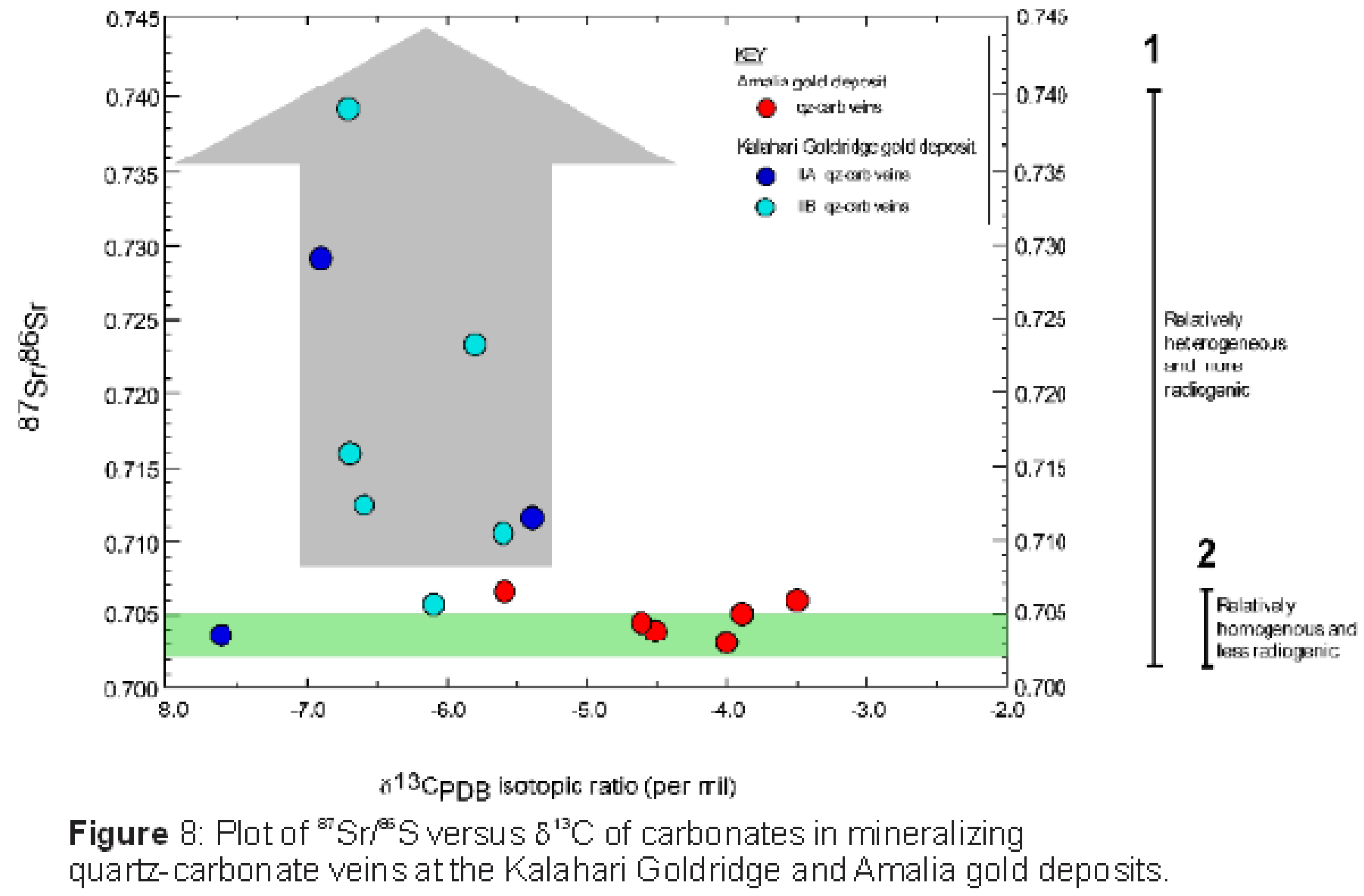
Like most Archean orogenic gold deposits, the deposits in the Kraaipan-Amalia terrane exhibit similarities in δ18Owater values in quartz from the quartz-carbonate veins; in addition to the pervasive carbonatization and sulphidation mentioned earlier (Hammond et al. 2007, Adomako-Ansah et al. 2017). However, variations exist in the δ13C values of the associated carbonates from both deposits (Figure 8). On the basis of geochemical mass balance calculations, variation in δ13C values above or below average mantle/igneous values of -5±3 ‰ (Ohmoto and Rye 1979; McCuiag and Kerrich 1998; Giuliani et al. 2014) has been attributed to basement rocks of heterogeneous TTG composition (Kerrich et al. 1987) or water-rock interactions between ore-forming fluids and carbon-rich sedimentary rocks (e.g., Morishita and Nakano 2008, Kerrich 1987). Figure 8 shows that the δ13C values of ore-related carbonates from the Kalahari Goldridge deposit (-7.6 to -5.4‰; Hammond et al. 2007) is lower than the δ13C values of the Amalia gold deposit (-5.8 to -3.5‰; Adomako-Ansah et al., 2017). In addition, the CO2-bearing ore-forming fluid at the Amalia gold deposit contains negligible CH4 concentration whereas CH4 is appreciable in the ore-forming fluid at Kalahari Goldridge deposit (Figure 6). While the presence of CH4 is interpreted to be due to interaction between the ore-forming fluid and the thick sequence of graphite-bearing metasediment that have been mapped at the Kalahari Goldridge deposit (Hammond et al. 2007), an alternative explanation that cannot be ruled out is that the ore-forming fluid possibly evolved under reduced conditions at the time of mineralization at the Kalahari Goldridge region and that the presence of the graphite-bearing metasediment buffered the reduced conditions during gold precipitation (e.g., Morishita and Nakano 2008).
As noted earlier, carbon-rich metasediments host rocks have not been observed at the Amalia gold deposit at the time of this investigation nor has it been reported by previous workers (e.g., Kiefer 2004). This reasonably explains the absence of CH4 in the Amalia ore-forming fluid. However, it is geologically unrealistic to ignore a probable occurrence of carbon-rich metasediment within Archean BIFs. Therefore, an alternative explanation for the lack of CH4 contents in the fluid inclusions at Amalia could be that the probable interaction of any sort between the fluid and (thin sequence of) carbon-rich metasediment did not alter the fluid chemistry at Amalia. Therefore, in contrast to the Amalia gold deposit, the occurrence of relatively thick sequence of carbon-rich metasedimentary rocks surrounding the Kalahari Goldridge deposit could have partly buffered the ore-forming fluid to reduced conditions, thereby resulting in lower δ13C values and the trend to higher 87Sr/86Sr ratios at the Kalahari Goldridge deposit. This could be one major explanation for the observed difference in redox conditions at the deposits.
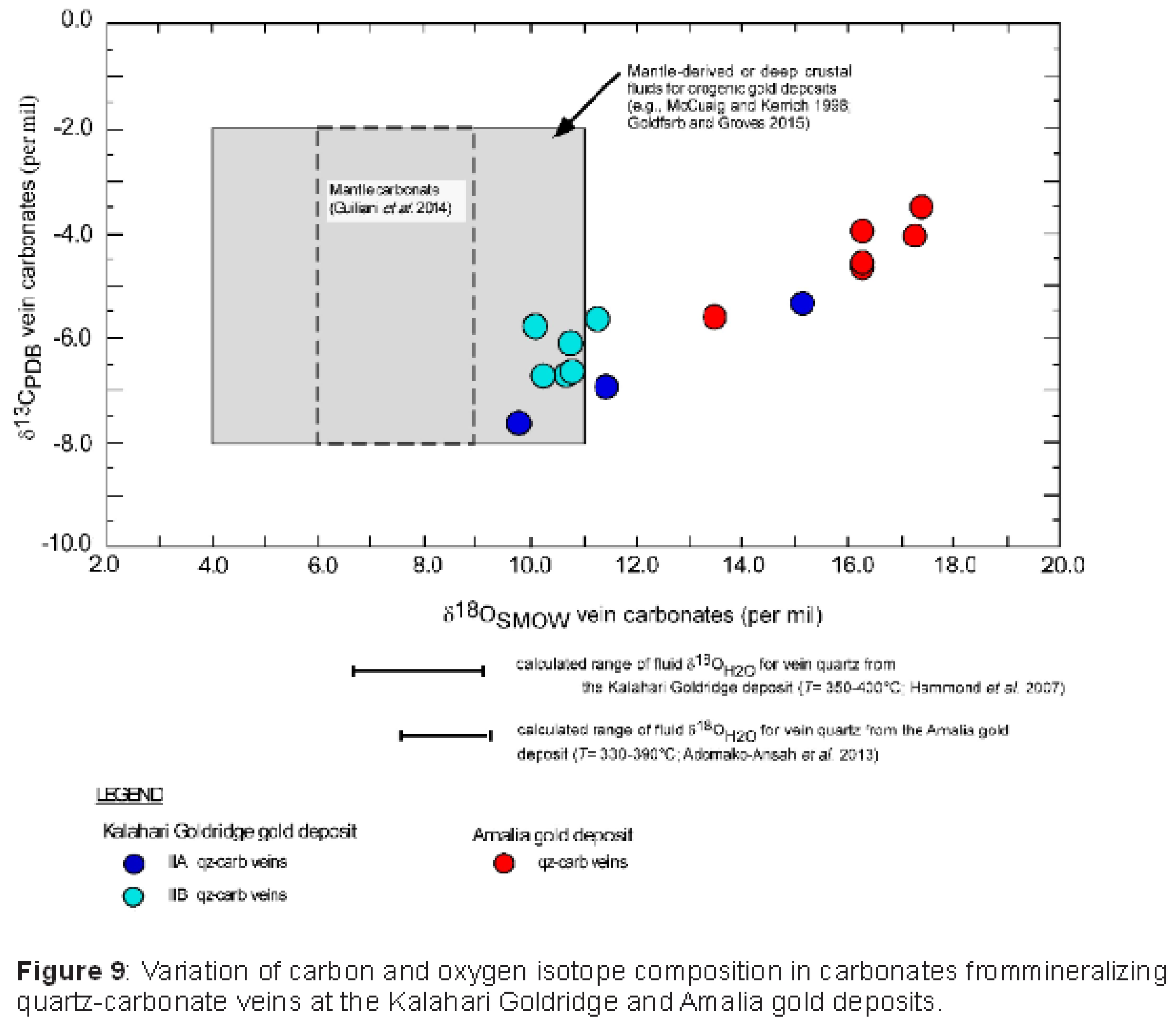
The δ13C and δ18O of vein carbonates from the Kalahari Goldridge and Amalia gold deposits show a general positive correlation (Figure 9). However, in comparison with the Kalahari Goldridge deposit, the Amalia deposit exhibits relatively higher δ13C and δ18O values between the oxygen and carbon isotope ratios of carbonate samples. This observation suggest a progressive enrichment of 18O and 13C in the fluids during fluid interaction with host rocks from Kalahari Goldridge to Amalia. Rye and Ohmoto (1974) and Ohmoto and Rye (1979) documented such positive variation in several studied individual deposits that showed increasing trend of enrichment of 18O and 13C in carbonates in the paragenetic sequence of the mineralization. Ohmoto and Rye (1979) attributed this trend to (i) decreasing temperature, (ii) increasing CO2/CH4 ratios in an evolving fluid system resulting from local fluid interaction with graphitic rocks or immiscible separation of CO2 + CH4 in the hydrothermal fluid, and/or (iii) contribution of CO2 from other sources resulting in a progressive increase in the 13C/12C and 18O/16O ratios. Therefore, the general positive variation between δ13C and δ18O in the two deposits can be attributed to increasing oxidation state in an evolving fluid system from a unique homogenous origin that is conformable with the observed 87Sr/86Sr-Sr variation illustrated in Figure 8. This can be accounted for by the variation of CH4 and CO2 concentration, as well as decreasing mineralization temperatures from the Kalahari Goldridge to the Amalia deposit as evidenced from the chlorite geothermometry (Figure 7). The differences in the degree of 18O and 13C enrichments of the carbonates in Amalia and Kalahari Goldridge may however be associated with the interaction of the ore-forming fluid with different set of host rocks during mineralization in these deposits. In particular, the vein-forming fluids interacted with sedimentary rocks having organic materials at Kalahari Goldridge in comparison with the host rocks at Amalia, which lacked or have limited organic materials. The observed trend is also consistent with the decreasing temperature trend observed from Amalia to the Kalahari Goldridge deposit (Figure 7). It is worth noting that despite the different temperatures, calculations of fluid C-O isotopic compositions (i.e. δ13CƩC and δ18OH2O) from vein carbonate and vein quartz samples overlap mantle and mantle-derived values (Figure 8); as also indicated by the Sr isotopic compositions. This suggests that upward-migrating deep crustal ore-forming fluids having mantle-like signatures were associated with the gold mineralization in the deposits. More importantly, the slight deviations in the δ13CƩC and δ18OH2O values from the mantle box plot in Figure 8 are consistent with the hypothesis that the fluids interacted with supracrustal rocks of high isotopic composition en route to the depositional sites in the Kraaipan-Amalia terrane; as also revealed by the Sr isotopic compositions.
7. Conclusion
The combination of Sr, C, and O isotopic data from the Kalahari Goldridge and Amalia BIF-hosted gold deposits have been used to evaluate the nature and evolution of the ore-forming fluids associated with gold mineralization in the Kraaipan-Amalia region of South Africa. A schematic model of the Kraaipan-Amalia gold mineralization system is illustrated in Figure 10. The two gold deposits show a common source for the ore-forming fluids on the basis of Sr isotopic data acquired on carbonates associated with the gold mineralization, irrespective of their contrasting ambient redox conditions, fluid chemistry, and temperatures of gold formation.
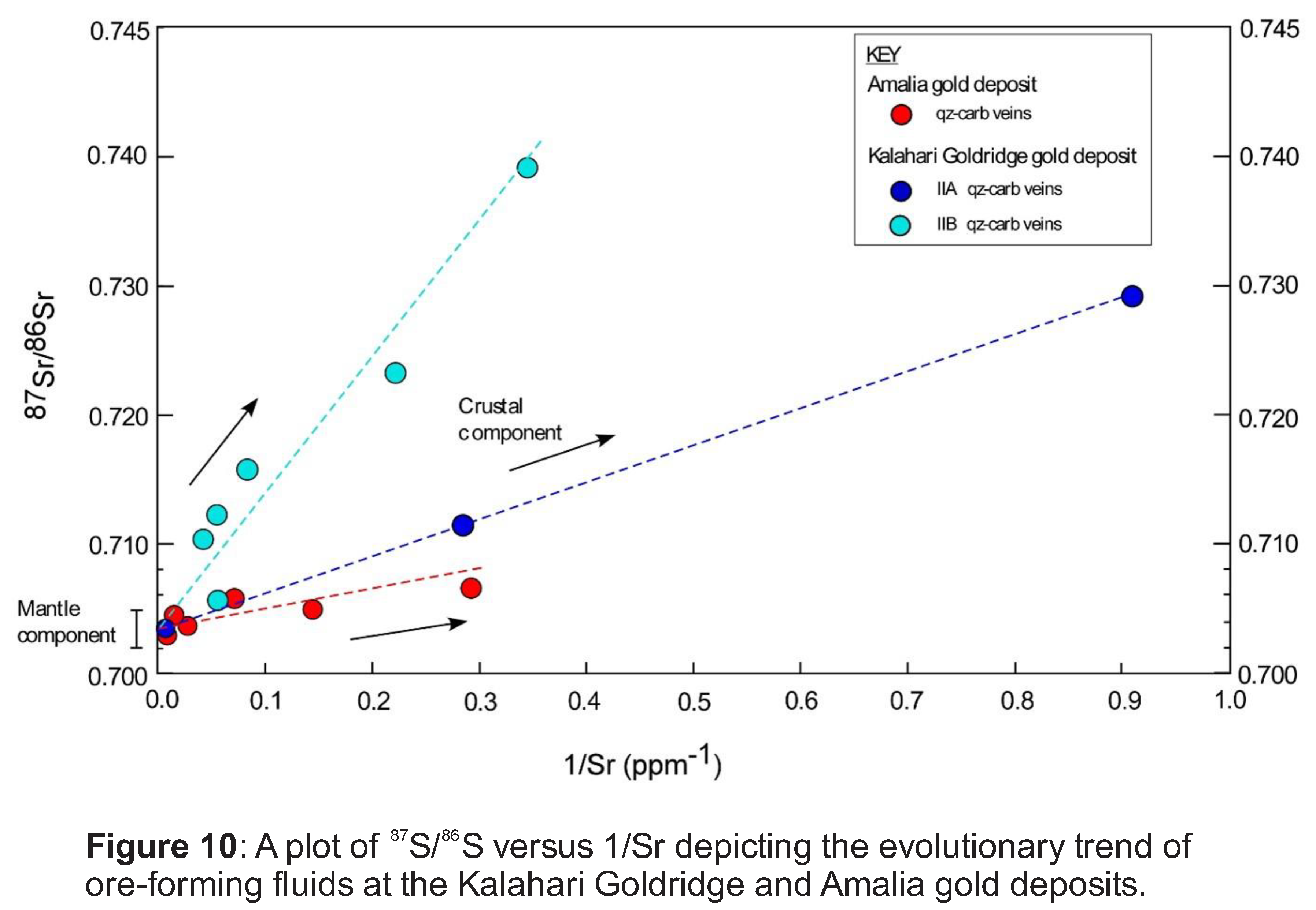
The absolute timing of the gold mineralization in these greenstone belts is not constrained, however evidence from fluid composition data and Sr-C-O isotopic ratios suggests that observed differences in redox conditions in these deposits could be attributed to different flow pathways by the evolving fluid from a common source (with minimum 87Sr/86Sr=0.70354) to the sites of gold deposition. Fluid-rock interaction between ore-forming fluid and carbonaceous meta-pelitic rock units partly resulted in reducing conditions and heterogeneity in the observed Sr-C isotopic distribution at the Kalahari Goldridge deposit. On the contrary, the oxidized character and homogeneous Sr-C isotopic distribution observed at the Amalia gold deposit is attributed to the lesser fluid-rock interaction between the ore-forming fluid and limited amount of (carbonaceous) supracrustal rocks. The results of the studies amplify the fact that although Archean orogenic gold deposits formed from fluids of similar composition in similar tectonic environments, minor differences in the deposits could be linked to variable host rock composition, redox conditions of gold formation and/or other physico-chemical parameters at individual deposits.
Acknowledgments
Prof Maruyama Yamamoto, formerly of Akita University, Japan, is specially thanked for his immense support during the Sr isotope analytical runs. We also thank Prof Toshio Mizuta for his advice during the course of this research.
References
- Adomako-Ansah, K., Mizuta, T., Hammond, N.Q., Ishiyama, D., Ogata, T., and Chiba, H., 2013. Gold mineralization in banded iron formation in the Amalia greenstone belt, South Africa: a mineralogical and sulfur isotope study: Resource Geology, v. 63, p. 119-140.
- Adomako-Ansah, K., Mizuta, T., Ishiyama, D., and Hammond, N.Q., 2017. Nature of ore-forming fluid and formation conditions of BIF-hosted gold mineralization in the Archean Amalia greenstone belt, South Africa: constraints from fluid inclusion and stable isotope studies: Ore Geology Reviews, v. 89, p. 609-626.
- Anhaeusser, C., R., and Walraven, F., 1999. Episodic granitoid emplacement in the western Kaapvaal Craton: evidence from the Archean-Kraaipan granite-greenstone terrane, South Africa: Journal of African Earth Sciences, v. 28, p. 289-309.
- Armstong, R.L., 1968. A model for the evolution of strontium and lead isotopes in a dynamic earth: Reviews of Geophysics, v. 6, 175-199.
- Bickle, M.J., 1994. The role of metamorphic decarbonation reactions in returning strontium to the silicate sediment mass: Nature, v. 367, p. 699-704.
- Bottinga, Y., 1968. Calculation of fractionation factors for carbon and oxygen isotopic exchange in the system calcite-carbon dioxide-water: Journal of Physical Chemistry, v. 72, p. 800-808.
- Corner, B., Durrheim, R.J., and Nicolaysen, L.O., 1990. Relationships between the Vredefort structure and the Witwatersrand basin within the tectonic framework of the Kaapvaal Craton as interpreted from regional gravity and aeromagnetic data: Tectonophysics, v. 171, p. 49-61.
- Deer, W.A., Howie, R.A., and Zussman, J., 1966. An Introduction to the rock forming minerals: London, Longman, 528 p.
- de Wit, M.J., Roering, C., Hart, R.J., Armstrong, R.A., de Ronde, C.E.J., Green, R.W.E., Tredoux, M., Peberdy, E., and Hart, R.A., 1992. Formation of an Archaean continent: Nature, v. 357, p. 553-562.
- de Wit, M. and Tinker, J., 2004, Crustal structures across the central Kaapvaal craton from deep-seismic reflection data: South African Journal of Geology, v. 107, p. 185-206.Donnelly, C.L., Griffin, W.L., O’Reilly, S.L, Pearson, N.J., Shee, S.R., 2011. The Kimberlites and related rocks of the Kuruman Kimberlite Province, Kaapvaal Craton, South Africa: Contributions to Mineralogy and Petrology, v. 161, p. 351-371.
- Eriksson, P.G., Banerjee, S., Nelson, D.R., Rigby, M.J., Catuneanu, O., Sarkar, S., Roberts, R.J., Ruban, D., Mtimkulu, M.N., and Raju, P.V.S., 2009. A Kaapvaal craton debate: Nucleus of an early small supercontinent or affected by an enhanced accretion event? Gondwana Research, v. 15, p. 354-372.
- Goldfarb, R.J., and Groves, D.I., 2015. Orogenic gold: common or evolving fluid and metal sources through time: Lithos, v. 233, p. 2-26.
- Groves, D.I., Goldfarb, R.J., Gebre-Mariam, M., Hagemann, S.G., and Robert, F., 1998. Orogenic gold deposits–a proposed classification in the context of their crustal distribution and relationship to other gold deposit types: Ore Geology Reviews, v. 13, p. 7-27.
- Groves, D.I., Santosh, M., Deng, J., Wang, Q., Yang, L., and Zhang, L., 2020. A holistic model for the origin of orogenic gold deposits and its implications for exploration. Mineralium Deposita, v. 55, p. 275–292.
- Giuliani, A., Phillips, D., Kamenetsky, V.S., Fiorentini, M.L., Farquhar, J., and Kendrick, M.A., 2014. Stable isotope (C, O, S) compositions of volatile-rich minerals in kimberlites: A review: Chemical Geology, v. 374-375, p. 61-63.
- Hammond, N.Q., and Moore, J.M., 2006. Archean lode gold mineralization in banded iron formation at the Kalahari Goldridge deposit, Kraaipan greenstone belt, South Africa: Mineralium Deposita, v. 41, p. 483-503.
- Hammond, N.Q., Moore, J.M., and Sheets, R.W., 2007. Physico-chemical conditions of ore-bearing fluids associated with the genesis of the Kalahari Goldridge deposit, Kraaipan greenstone belt, South Africa: Ore Geology Reviews, v. 30, p. 106-134.
- Hammond, N.Q., and Morishita, Y., 2009. Source of ore fluids at the Kalahari Goldridge deposit, Kraaipan greenstone belt, South Africa: evidence from Sr, C and O isotope signatures in carbonates: Geofluids, v. 9, p.
- Ho, S.E. Groves, D.I McNaughton, N.J. Mikucki, E.J. (1992). The source of ore fluids and solutes in Archaean lode-gold deposits of Western Australia, Journal of Volcanology and Geothermal Research, v. 50, p.
- Jacobsen, S.B., and Pimentel-Klose, M.R., 1988. Nd isotopic variations in Precambrian banded iron formations: Geophysical Research Letters, v. 15, p. 393-396.
- Jones, I.M., and Anhaeusser, C.R., 1993. Accretionary lapilli associated with Achaean banded iron formations of the Kraaipan Group, Amalia greenstone belt, South Africa: Precambrian Research, v. 61, p. 117-136.
- Kato, Y., Ohta, I., Tsunematsu, T., Watanabe, Y., Isozaki, Y., Maruyama, S., and Imai, N. (1998). Rare earth element variations in mid-Archean banded iron formations: implications for the chemistry of ocean and continent and plate tectonics: Geochimica Cosmochimica Acta, v. 62, p. 3475-3497.
- Kato, Y., Yamaguchi, K.E., and Ohmoto, H., 2006, Rare earth elements in Precambrain banded iron formations: Secular changes of Ce and Eu anomalies and evolution of atmospheric oxygen, In Kessler, S.E. and Ohmoto, H, eds., Evolution of early earth’s atmosphere, hydrosphere, and biosphere-constraints from ore deposits: Geological Society of America Memoir, v. 198, p. 269-289.
- Kerrich, R., 1987. The stable isotope geochemistry of Au-Ag vein deposits in metamorphic rocks. In Kyser, T.K., ed., Stable isotope geochemistry of low temperature processes: GAC-MAC short course v. 11, p. 318-361.
- Kerrich, R., 1989, Geochemical evidences of the sources of fluids and solutes for shear zone hosted mesothermal gold deposits. In Mineralization and Shear Zones. Edited by J.T. Bursnall. Short Course Volume 6, Geological Association of Canada.
- Kerrich, R., Fryer, B.J., King, R.W., Willmore, L.M., and van Hees, E., 1987, Crustal outgassing and LILE enrichment in major lithosphere structures, Archean Abitibi greenstone belt: evidence on the source reservoir from strontium and carbon isotope tracers: Contributions to Mineralogy and Petrology, v. 97, p. 156-168.
- Kiefer, R., 2004, Regional geology, tectonic evolution, and controls of gold mineralization in the Archean Amalia greenstone belt, South Africa [Ph.D. thesis]: Johannesburg, University of the Witwatersrand, 542 p.
- McCuaig, T.C., and Kerrich, R., 1998, P-T-t-deformation-fluid characteristics of lode gold deposits: evidence from alteration systematics. Ore Geology Reviews, v. 12, p. 381-453.
- McCourt, S., 1995. The crustal architecture of the Kaapvaal crustal block South Africa, between 3.5 and 2.0 Ga: A synopsis: Mineralium Deposita, v. 30, p. 89-97.
- McLennan, S.M., Taylor, S.R., McCulloch, M.T., and Maynard, J.B., 1990, Geochemical and Nd-Sr isotopic composition of deep-sea turbidites: crustal evolution and plate tectonics: Geochimica Cosmochimica Acta, v. 54 (7), p. 2015-2050.
- Mitchell, R.H., 1995, Kimberlites, orangeites, and related rocks. New York, Plenum Press.
- Morishita, Y., and Nakano, T., 2008, Role of basement in epithermal deposits: The Kushikino and Hishikari gold deposits, southwestern Japan: Ore Geology Reviews, v. 34, p. 597-609.
- Ohmoto, H., and Rye, R.O., 1979, Isotopes of sulphur and carbon. in Barnes, H.L., ed., Geochemistry of Hydrothermal Ore Deposits, 2nd Edition: New York, John Wiley and Sons, p. 509-562.
- Palmer, M.R, and Edmond, J.M., 1989, Strontium isotope budget of the modern ocean: Earth Planetary Science Letters, v. 92, p. 11-26.
- Peucker-Ehrenbrink, B., and Miller, M.W., 2006, Marine 87Sr/86Sr record mirrors the evolving upper continental crust: Geochimica Cosmochimica Acta, v. 70 (18), p. A487.
- Plank, T., and Langmuir, C.H., 1998. The chemical composition for subducting sediment and its consequences for the crust and mantle: Chemical Geology, v. 145, p. 325-394.
- Poujol, M., Anhaeusser, C.R., and Armstrong, R.A., 2002, Episodic granitoid emplacement in the Archean Amalia-Kraaipan terrane, South Africa: confirmation from zircon U-Pb geochronology: Journal of African Earth Sciences, v. 35, p. 147-161.
- Richardson, S.H., Shirey, S.B., Harris, J.W., and Carlson, R.W., 2001, Archean subduction recorded by Re-Os isotopes in eclogitic sulfide inclusions in Kimberley diamonds: Earth and Planetary Science Letters, v. 191, p. 257-266.
- Ridley, J., and Hagemann, S.G., 1999, Interpretation of post-entrapment fluid-inclusion re-equilibration at the Three Mile Hill, Marvel Loch and Griffins Find high temperature lode-gold deposits, Yilgarn Craton, Western Australia: Chemical Geology, v. 154, p. 257–278.
- Robb, L.J., and Meyer, F.M., 1995, The Witwatersrand Basin: Geological framework and mineralization processes: Ore Geology Reviews, v. 10, p. 67-94.
- Rye, R.O., and Ohmoto, H., 1974, Sulphur and carbon isotopes and ore genesis: a review. Economic Geology 69, 642-826.
- Sato, H., Ishiyama, D., Mizuta, T., and Ishikawa, Y., 1999, Rare earth element analysis of rock and thermal water samples by inductively coupled plasma mass spectrometry: Science and Technology Report, Faculty of Engineering and Resource Science, Akita Univiversity, 20, p. 1-8 (in Japanese with English abstract).
- Schmitz, M.D., Bowring, S.A., de Wit, M.J., and Gartz, V., 2004, Subduction and terrane collision stabilize the western Kaapvaal craton tectosphere 2.9 billion years ago: Earth and Planetary Science Letters, 222, p. 363-376.
- Shields, G.A., 2007, A normalized seawater strontium isotope curve: possible implications for Neoproterozoic-Cambrian weathering rates and the further oxygenation of the earth: eEarth, v.2, p. 35-42.
- Shields, G.A., and Veizer, J., 2002, Precambrian marine carbonate isotope database: version 1.1.: Geochemistry, Geophysics, Geosystems, v.6, p. 1-12.
- Smith, C.B., 1983, Pb, Sr and Nd isotopic evidence for sources of southern African Cretaceous kimberlites. Nature, v. 304, 51-54.
- Veizer, J., 1989, Strontium isotopes in seawater through time, Annual Review Earth Planetary Science, 17, p. 141-167.
- Yamaguchi, K.E., Bau, M. and Ohmoto, H., 2000, Constraints from REEs on the processes and environments for Precambrian banded iron formations: revaluation of the data and models. J. Goldschmidt Conference Abstract, 5 (2), p. 1110.
- Yamamoto, M., and Maruyama, T., 1996, The Sr and Nd isotopic analysis and quantitative analysis of Rb and Sr by using MAT 261: Report of the Research Institute of Natural Resources, Mining College, Akita University, v. 61, p. 7-30 (in Japanese with English abstract).
Table 1.
A. Sr isotopes data of carbonates from the Amalia gold deposit, Amalia Greenstone Belt, South Africa. B. Sr isotopes data of carbonates from the Kalahari goldridge deposit, Kraaipan Greenstone Belt (From Hammond and Morishita, 2007).
Table 1.
A. Sr isotopes data of carbonates from the Amalia gold deposit, Amalia Greenstone Belt, South Africa. B. Sr isotopes data of carbonates from the Kalahari goldridge deposit, Kraaipan Greenstone Belt (From Hammond and Morishita, 2007).
| A |
|---|
| Sample name |
δ18OSMOW (per mil) |
δ13CPDB (per mil) |
87Rb/86Sr |
Rb (ppm) |
Sr (ppm) |
Rb/Sr |
1/Sr (ppm−1) |
87Sr/86Sr |
std |
87Rb/86Sr |
| C17-20 |
17.2 |
-4.0 |
0.010 |
0.55 |
157.20 |
0.004 |
0.006 |
0.703023 |
(±7; 2σ) |
0.010 |
| C11-5A-3 |
16.3 |
-4.5 |
0.076 |
1.00 |
38.15 |
0.026 |
0.026 |
0.703747 |
(±6; 2σ) |
0.076 |
| C17-15B |
13.5 |
-5.6 |
1.23 |
1.46 |
3.43 |
0.426 |
0.292 |
0.706643 |
(±6; 2σ) |
1.23 |
| C17-23 |
16.3 |
-3.9 |
0.371 |
0.89 |
6.94 |
0.128 |
0.144 |
0.704895 |
(±6; 2σ) |
0.371 |
| N23-7A |
17.4 |
-3.5 |
0.382 |
1.88 |
14.22 |
0.132 |
0.070 |
0.705781 |
(±12; 2σ) |
0.382 |
| C17-6 |
16.3 |
-4.6 |
0.078 |
2.11 |
78.45 |
0.027 |
0.013 |
0.704318 |
(±7; 2σ) |
0.078 |
| B |
| ARC 236/11A |
11.3 |
-5.6 |
0.034 |
0.1 |
23.3 |
0.004 |
0.043 |
0.71042 |
(±1; 2σ) |
0.034 |
| ARC 236/11X |
10.1 |
-5.8 |
0.188 |
0.3 |
4.5 |
0.067 |
0.222 |
0.72325 |
(±1; 2σ) |
0.188 |
| ARC 236/16 |
15.1 |
-5.4 |
0.04 |
0.04 |
3.5 |
0.011 |
0.286 |
0.71138 |
(±1; 2σ) |
0.04 |
| MSH/W-3 |
9.8 |
-7.6 |
0.005 |
0.3 |
168 |
0.002 |
0.006 |
0.70354 |
(±1; 2σ) |
0.005 |
| GDP 531/9C |
11.5 |
-6.9 |
0.513 |
0.2 |
1.1 |
0.182 |
0.909 |
0.72907 |
(±2; 2σ) |
0.513 |
| DZ 40/1 |
10.3 |
-6.7 |
0.389 |
0.4 |
2.9 |
0.138 |
0.345 |
0.73914 |
(±2; 2σ) |
0.389 |
| DZ 40/2 |
10.6 |
-6.7 |
0.047 |
0.2 |
12.1 |
0.017 |
0.083 |
0.71583 |
(±1; 2σ) |
0.047 |
| DZ 40/3 |
10.8 |
-6.6 |
0.047 |
0.3 |
18.2 |
0.016 |
0.055 |
0.71235 |
(±1; 2σ) |
0.047 |
| GDP 531/16B |
10.8 |
-6.1 |
0.006 |
0.1 |
18.1 |
0.006 |
0.055 |
0.70564 |
(±2; 2σ) |
0.006 |
| std = standard deviation |
|
|
|
|
|
|
|
|
|
| Ank = ankerite |
|
|
|
|
|
|
|
|
|
|
| Sid = siderite |
|
|
|
|
|
|
|
|
|
|
Table 2.
A. Sr isotopes data of carbonates from the Amalia gold deposit, Amalia Greenstone Belt, South Africa. B. Sr isotopes data of carbonates from theKalahari Goldridge, Kraaipan Greenstone Belt, South Africa (Hammond & Morishita, 2009).
Table 2.
A. Sr isotopes data of carbonates from the Amalia gold deposit, Amalia Greenstone Belt, South Africa. B. Sr isotopes data of carbonates from theKalahari Goldridge, Kraaipan Greenstone Belt, South Africa (Hammond & Morishita, 2009).
| A |
|---|
| Sample name |
Sampled material |
Host rock |
Mineral |
δ18OSMOW (per mil) |
δ13CPDB (per mil) |
87Rb/86Sr |
Rb (ppm) |
Sr (ppm) |
Rb/Sr |
1/Sr (ppm−1) |
87Sr/86Sr |
|
87Rb/86Sr |
| C17-20 |
quartz-carbonate vein |
Mineralised BIF |
Ankerite |
17.2 |
-4.0 |
0.010 |
0.55 |
157.20 |
0.004 |
0.006 |
0.703023 |
(±7; 2σ) |
0.010 |
| C11-5A-3 |
quartz-carbonate vein |
Mineralised BIF |
Ankerite |
16.3 |
-4.5 |
0.076 |
1.00 |
38.15 |
0.026 |
0.026 |
0.703747 |
(±6; 2σ) |
0.076 |
| C17-15B |
quartz-carbonate vein |
Mineralised BIF |
Ankerite |
13.5 |
-5.6 |
1.23 |
1.46 |
3.43 |
0.426 |
0.292 |
0.706643 |
(±6; 2σ) |
1.23 |
| C17-23 |
quartz-carbonate vein |
Mineralised BIF |
Ankerite |
16.3 |
-3.9 |
0.371 |
0.89 |
6.94 |
0.128 |
0.144 |
0.704895 |
(±6; 2σ) |
0.371 |
| N23-7A |
quartz-carbonate vein |
Mineralised BIF |
Ankerite |
17.4 |
-3.5 |
0.382 |
1.88 |
14.22 |
0.132 |
0.070 |
0.705781 |
(±12; 2σ) |
0.382 |
| C17-6 |
quartz-carbonate vein |
H/W schist |
Ankerite |
16.3 |
-4.6 |
0.078 |
2.11 |
78.45 |
0.027 |
0.013 |
0.704318 |
(±7; 2σ) |
0.078 |
| B |
| ARC 236/11A |
quartz-carbonate vein IIB |
Mineralised BIF |
Ankerite |
11.3 |
-5.6 |
0.034 |
0.1 |
23.3 |
0.004 |
0.043 |
0.71042 |
(±1; 2σ) |
0.034 |
| ARC 236/11X |
quartz-carbonate vein IIB |
Mineralised BIF |
Siderite |
10.1 |
-5.8 |
0.188 |
0.3 |
4.5 |
0.067 |
0.222 |
0.72325 |
(±1; 2σ) |
0.188 |
| ARC 236/16 |
quartz-carbonate vein IIA |
Mineralised BIF |
Siderite |
15.1 |
-5.4 |
0.04 |
0.04 |
3.5 |
0.011 |
0.286 |
0.71138 |
(±1; 2σ) |
0.04 |
| MSH/W-3 |
quartz-carbonate vein IIA |
Mineralised BIF |
Ankerite |
9.8 |
-7.6 |
0.005 |
0.3 |
168 |
0.002 |
0.006 |
0.70354 |
(±1; 2σ) |
0.005 |
| GDP 531/9C |
quartz-carbonate vein IIA |
Mineralised BIF |
Siderite |
11.5 |
-6.9 |
0.513 |
0.2 |
1.1 |
0.182 |
0.909 |
0.72907 |
(±2; 2σ) |
0.513 |
| DZ 40/1 |
quartz-carbonate vein IIB |
Mineralised BIF |
Siderite |
10.3 |
-6.7 |
0.389 |
0.4 |
2.9 |
0.138 |
0.345 |
0.73914 |
(±2; 2σ) |
0.389 |
| DZ 40/2 |
quartz-carbonate vein IIB |
Mineralised BIF |
Siderite |
10.6 |
-6.7 |
0.047 |
0.2 |
12.1 |
0.017 |
0.083 |
0.71583 |
(±1; 2σ) |
0.047 |
| DZ 40/3 |
quartz-carbonate vein IIB |
Mineralised BIF |
Siderite |
10.8 |
-6.6 |
0.047 |
0.3 |
18.2 |
0.016 |
0.055 |
0.71235 |
(±1; 2σ) |
0.047 |
| GDP 531/16B |
quartz-carbonate vein IIB |
Mineralised BIF |
Siderite |
10.8 |
-6.1 |
0.006 |
0.1 |
18.1 |
0.006 |
0.055 |
0.70564 |
(±2; 2σ) |
0.006 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).