1. Introduction
The number of dengue cases in the Americas increased to 3.578.414 in 2024, with 2.888 severe dengue cases and 1039 deaths reported [
1]. Due to the lack of effective vaccines providing immunization against four serotypes of viruses and the limited development of pharmaceutical products, vector control is crucial in the fight to prevent disease outbreaks in these countries. Despite efforts to control the
Aedes aegypti mosquito, which carries the dengue virus, the measures have only provided short-term results, leading to increased new generations of mosquitoes and correlated dengue cases in some local neighborhoods. Abundant populations of
Ae aegypti facilitated the emerging and dispersion of new arboviruses from 2013 to 2015 in the Americas region, such as Chikungunya and Zika [
2], and, to a lower extent, the invasive
Aedes albopictus [
3]. On the other hand, most government vector control strategies, namely chemical, biological, and educational, focus on the adult, pupa, and larvae stages. Control of the egg stage is almost nonexistent; its tiny size, black color, and attachment to the inner sides of artificial containers protect it from the field technicians' sight and removal. During the egg-laying, the female places them individually in a humid film above the water surface and is made by capillarity. It takes about 72-h for whole embryo maturation and egg melanization. If water evaporation occurs in the following days, this embryo can survive up to 6 months to two years. Despite its importance during arbovirus transmission, egg populations in
Aedes mosquitoes have been poorly studied. Nature provided a sticky substance produced by the female accessory gland containing hyaluronic acid to glue her eggs onto the larval site walls [
4]. This trait accelerated the vector geographical dispersion mainly through glued eggs in the discarded tires [
5]. Similarly, Lozano-Denis
et al. (2019) [
6] reported that around 5% of the Dengue virus in eggs was collected in 20,000 ovitraps in four states of southern Mexico. This high rate of vector transovarial transmission is strong evidence of the epidemiological importance of explaining the outbreaks beginning in endemic places. The question of how these eggs may tolerate desiccation for such a prolonged period relies on a physiological stage defined as diapause. For insects in the tropics, specific types of diapause occur. Diapause and quiescence are two forms of dormancy found in insects. Diapause is characterized as a dormant state that is hormonally programmed in advance and is not immediately ended when favorable conditions are present. On the other hand, quiescence is a dormancy state that occurs in response to unfavorable environmental conditions and ends immediately when favorable conditions return. For instance, the inability of
Ae. aegypti eggs laid above the water line to hatch are considered quiescent because development can promptly resume upon immersion in water. [
7].
This study aimed to generate new data on the populations of dormant Ae. aegypti and Ae. albopictus eggs during the dry season or inter-epidemic period. The study focused on three objectives: 1) using ovitraps to identify the species of female mosquitoes laying eggs, 2) assessing the populations of desiccated eggs and their backyard containers, and 3) evaluating the killing effectiveness of liquid Temephos 5% dissolved in mineral oil as potential ovicide of quiescent eggs.
2. Materials and Methods
2.1. Study Area
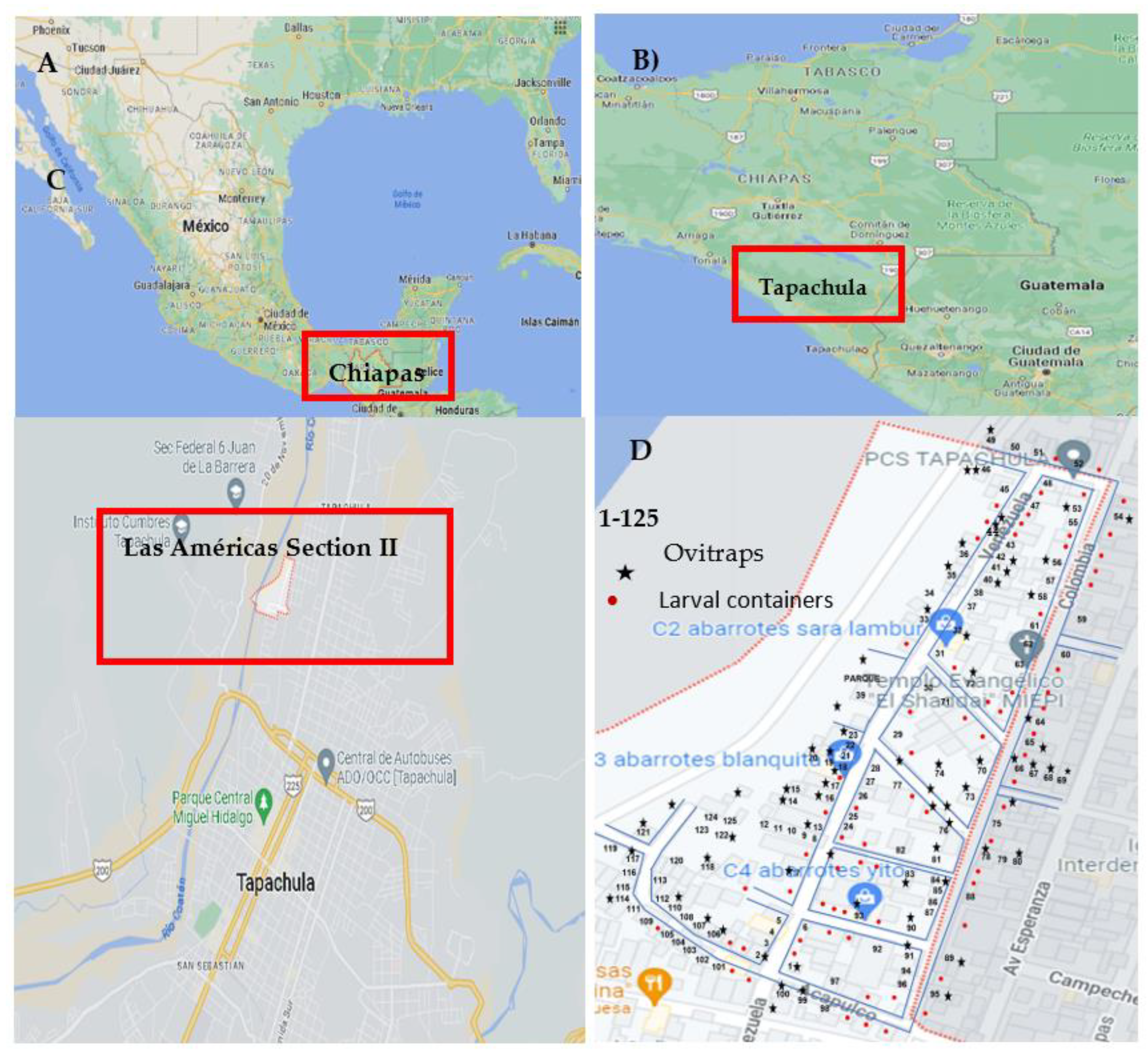
The research was conducted in the Las Americas section II neighborhood in Tapachula, Chiapas, Mexico. This neighborhood is located 5.13 km east of Tapachula's geographic center. It is also 3.69 km north of Tapachula's urban center (refer to
Figure 1). The neighborhood covers an area of 11 hectares and includes infrastructure for 309 homes. The population density is 1,042 people per square kilometer, with residents of various nationalities. The average age of the residents is 30 years, and they have an average of 8 years of schooling [
8].
The study area is experiencing disorganized urbanization. It is located next to the Coatán River, which creates ideal shade, humidity, and temperature conditions for Aedes mosquitoes. Despite having operational electricity, drainage, and drinking water services, water supply outages occur throughout the year, leading households to use various containers to store water.
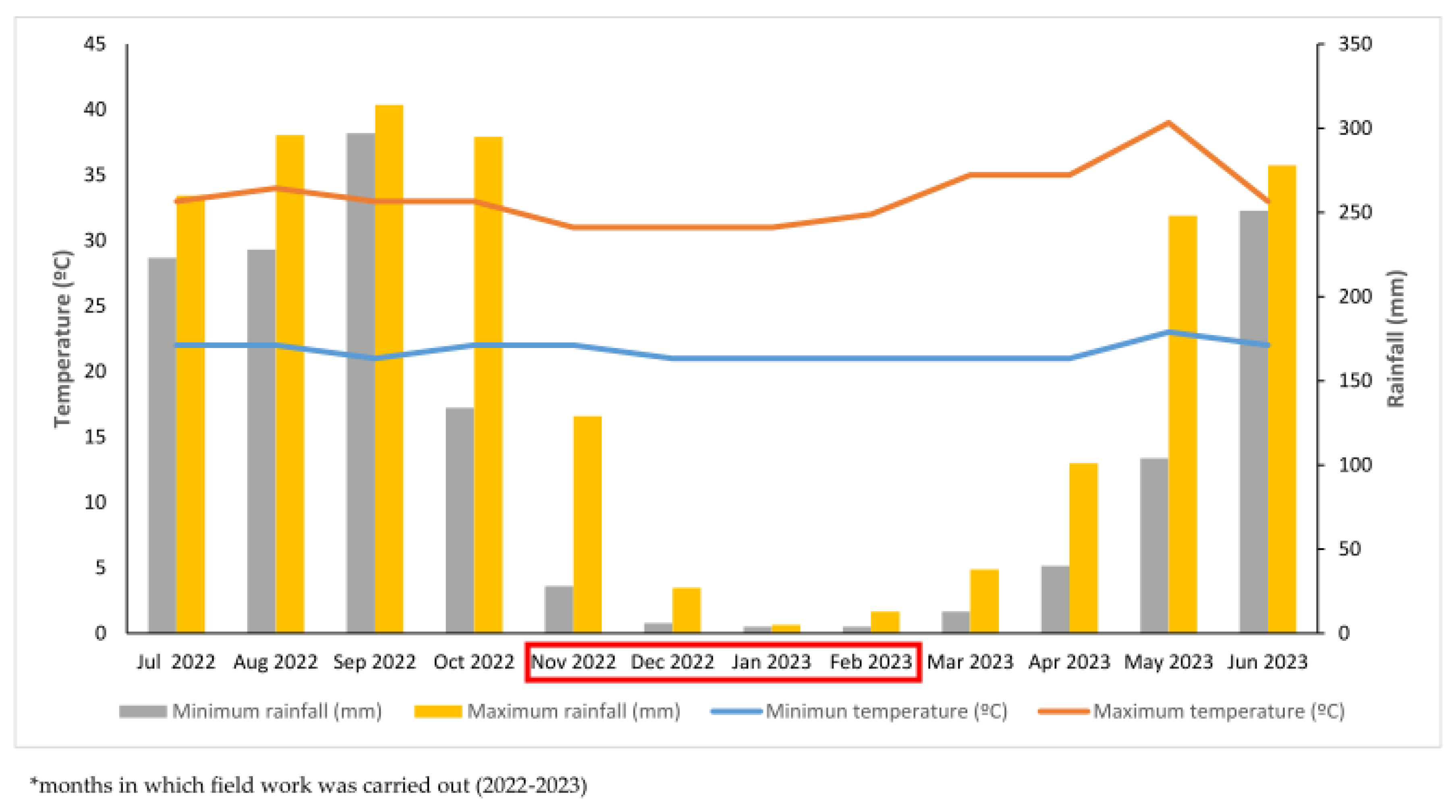
Regarding the local weather patterns, a six-month rainy season is present from June to November, whereas a dry season continues from December to late May in the following year. Annual accumulated water in mm ranges from 1,200 to 4,000 mm, including coastal areas and a mountain range of 1,500 m elevation [
9] (
Figure 2). Dengue cases are endemic yearly, with a higher monthly incidence during the peak of rainy season months [
10].
2.2. Identifying In Situ Female Egg-Laying Mosquito Species in Houses Indoors and Outdoors Using Ovitraps
Before beginning home visits, a meeting was held with neighborhood leaders to obtain appropriate authorization. Permission was also requested from the heads of the families to enter the homes, and individual talks were held to explain the project's objectives and process. Once authorized by the community leader, we presented ourselves duly identified at each home, where we offered a brief presentation about our work and its objectives and answered the residents' questions.
Monitoring took place from November to December 2022. Out of 309 houses in the area, 125 (40.45%) agreed to take part (see
Figure 1D). Each home was georeferenced, and an intra- and peri-home inspection was conducted to identify larval containers. The inspection also checked if any water containers were treated with larval control methods for
Ae. aegypti (such as Temephos-abate® or Spinosad®) authorized by the state Secretary of Health. Additionally, the residents were interviewed about the latest visit from the Health Secretary's staff and whether they had used any
Ae—aegypti control methods.
Two ovitraps were installed in each home, consisting of a 0.5 L black plastic container filled with 300 ml of tap water and an 8 x 15 cm strip of filter paper. One ovitrap was placed in the bedroom, and the other was near water storage sources. They remained there for four days (96 hours). On the fifth day, the homes were again visited to collect the egg-positive ovitraps labeled and stored in plastic bags. These samples were taken to the insectary of the Regional Public Health Center of Mexico (CRISP-INSP) in Tapachula. As a first step to stimulate egg hatching, the filter paper stripes of each ovitrap were placed in a 1-L plastic beaker containing 750 ml of tap water and larval food. After 24 h and once hatched, the larvae were transferred to individuals 22 x 35 x 5 cm trays containing 1.2 L of water and 1.5 cm deep. The first and second instar larvae were fed during the first three days with 0.4 g of a larval diet (mixture of proteins, fats, fiber, and minerals, previously ground, sieved, and sterilized; 5001 Laboratory Rodent Diet (Lab Diet, CA, USA). The third and fourth instar larvae were fed with 0.8 g of this same diet until the sixth day. Similarly, pupae were transferred to 20 cm diameter trays and covered with a tricot mesh with rubber bands to confine the adults after their emergence. Adult mosquitoes were counted, sexed, and taxonomically identified [
11]. On the other hand, the negative ovitraps were washed with tap water, the filter paper strips were replaced, and 300 ml of tap water was added. These ovitraps were placed back at the monitoring site for four additional days, repeating the abovementioned process.
2.3. Sampling of Aedes quiescent Eggs and Household Wet and Dry Larval Breeding Sites
Monitoring took place from January to February 2023. Of the 125 homes that participated in the ovitrap study to detect the presence of
Aedes mosquitoes, all were authorized to continue with the project’s next phase. As a first step, an inspection was carried out both inside and around the homes to identify possible mosquito breeding sites. All containers filled full or partly with water were examined and visually inspected for eggs and larvae. In contrast, dry or water-emptied containers with favorable conditions to become breeding sites were identified, where only eggs were sought (
Figure 3).
All possible dry-season 83 breeding sites identified were correctly labeled and geo-referenced. The family heads authorized the physical collection of the breeding sites, which were packed with plastic and taken to the CRISP-INSP laboratory. There, tap water and larval food were added to all sampled containers to stimulate the hatching of the eggs. These first instar larvae obtained from each dry season breeding site and larvae transported from homes were transferred to 22 x 35 x 5 cm plastic trays containing 1.2 L of water and 1.5 cm deep—the The same larval diet and handling until adult emergence were similarly managed like the ovitrap eggs.
In cases where permission was not obtained to remove breeding sites from households, we collected larvae and added tap water and food as needed. After 48 hours, we returned to collect the larvae, which were transported to the CRISP-INSP laboratory for analysis in 4 1⁄2 x 9” Whirl-Pak® sample bags.
2.4. Temephos 5% Dissolved in Odorless Mineral Spirit Bioassay as Potential Operational Control for Quiescent Egg Populations
Temephos 5% was selected to run bioassays considering the following reasoning: a) Temephos 1% sand granules are a close organophosphate formulation and well-known larvicide for field operators elsewhere, b) extreme conditions of elevated temperatures and poor relative humidity during the dry season demand a more challenging product such as the mineral oil with a boiling point of 360 °C along with carrying a higher concentration of 5% of Temephos. Although frequent reports of insecticide resistance are available, the World Health Organization experts keep their recommendation of use [
13]. In this experiment, gravid female mosquitoes raised in a lab from eggs collected in the study area were released into 30 x 30 cm metal cages. Inside each cage, a plastic container with a damp Whatman #2 filter paper was provided for the females to lay their eggs. The eggs on the filter paper were allowed to embryonate for 72 hours. Afterward, the developed eggs were counted and randomly distributed into 24 plastic containers, each capable of holding 500 mL, with each container containing between 12 and 65 eggs. Subsequently, the containers were randomly selected, and three different treatments were applied: a) 3 mL of a 5% Temephos solution (Alabaster 500 EC® + mineral oil), b) 3 mL of mineral oil, and c) control treatment where nothing was applied to the eggs. These treatments were applied using a 300 mL capacity hand-held plastic sprayer. Two days after the treatments were applied, 400 mL of clean water and fish food were added to encourage hatching. Monitoring continued for 72 hours, and the percentage of eggs hatched in each container was recorded using the following formula: (number of hatched larvae / total number of eggs) x 100. Eight observations were made per treatment, and the bioassay was repeated three times.
2.5. Data Analysis
Descriptive statistical and plot distribution analyses were conducted on mosquito
Aedes hatching, emergence percentages, and proportions
. aegypti and
Ae. albopictus from ovitraps. Likewise, the frequency distribution of the desiccated or quiescent egg types of containers found in the locality was assessed. The independence test of the Mann-Whitney U test for nonparametric data was calculated to compare species hatching rates [
14]. The effectiveness of Temephos, 5% as a killing compound on desiccated eggs, was measured by comparing the hatching percentage of each treatment using the Kruskal-Wallis test and the Dwass-Steel-Critchlow-Fligner pairwise comparison.
3. Results
3.1. Species of Aedes Identified after Rearing Adult Stages from Eggs Collected in the Household Ovitraps
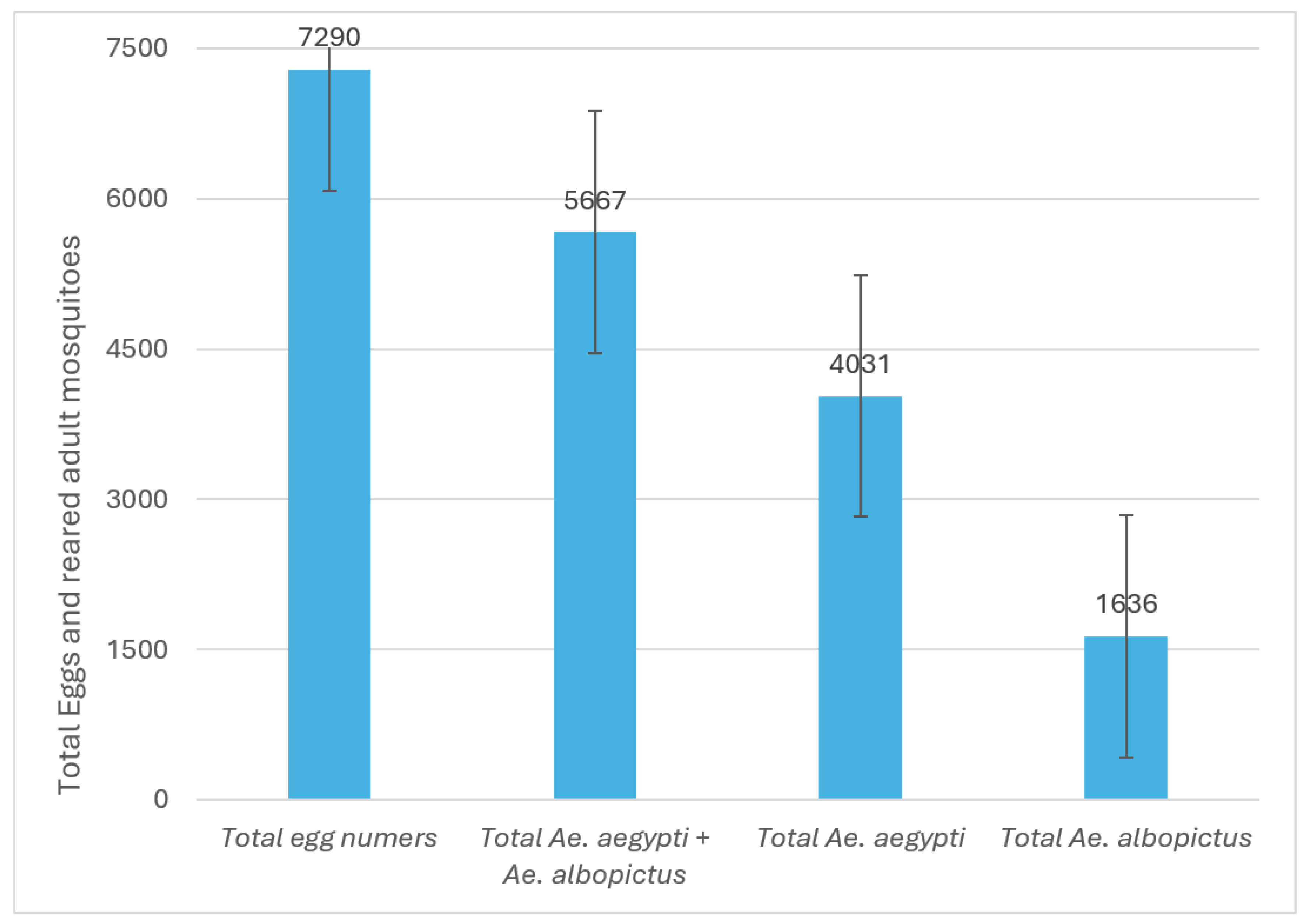
Despite the rainy season ending nearly a month prior at the study site, a high level of egg-laying activity was observed. Out of the 250 ovitraps that were placed across 125 participating houses, 211 (84.4%) were found to be positive for
Aedes eggs, while only 39 (15.6%) tested negative (see
Figure 1D). Seven thousand two hundred ninety eggs were recorded, with a pooled hatching rate of 78.5 ± 15.6% (5,667 eggs developed into adult stages). Among the adults identified, the majority, accounting for 4,031 individuals (71.1%), were
Ae. aegypti, while 1,636 (28.8%) were
Ae. albopictus (
Figure 4). The total count of mosquitoes per species and sex was
Ae. aegypti 1,861 (32.8%) females and 2,170 (38.3%) males, and
Ae. albopictus 767 (13.5%) females and 869 (15.3%) males were found, respectively. Interestingly, the hatching rates per mosquito species were significantly higher, 55.5% for
Ae. aegypti and lower 22.4% for
Ae. albopictus.(Mann-Whitney
U = 16878,
P ≤ 0.005) Eggs of the single species
Ae. aegypti, were found in 209 (83.6%) ovitraps, while only 114 (45.4% for
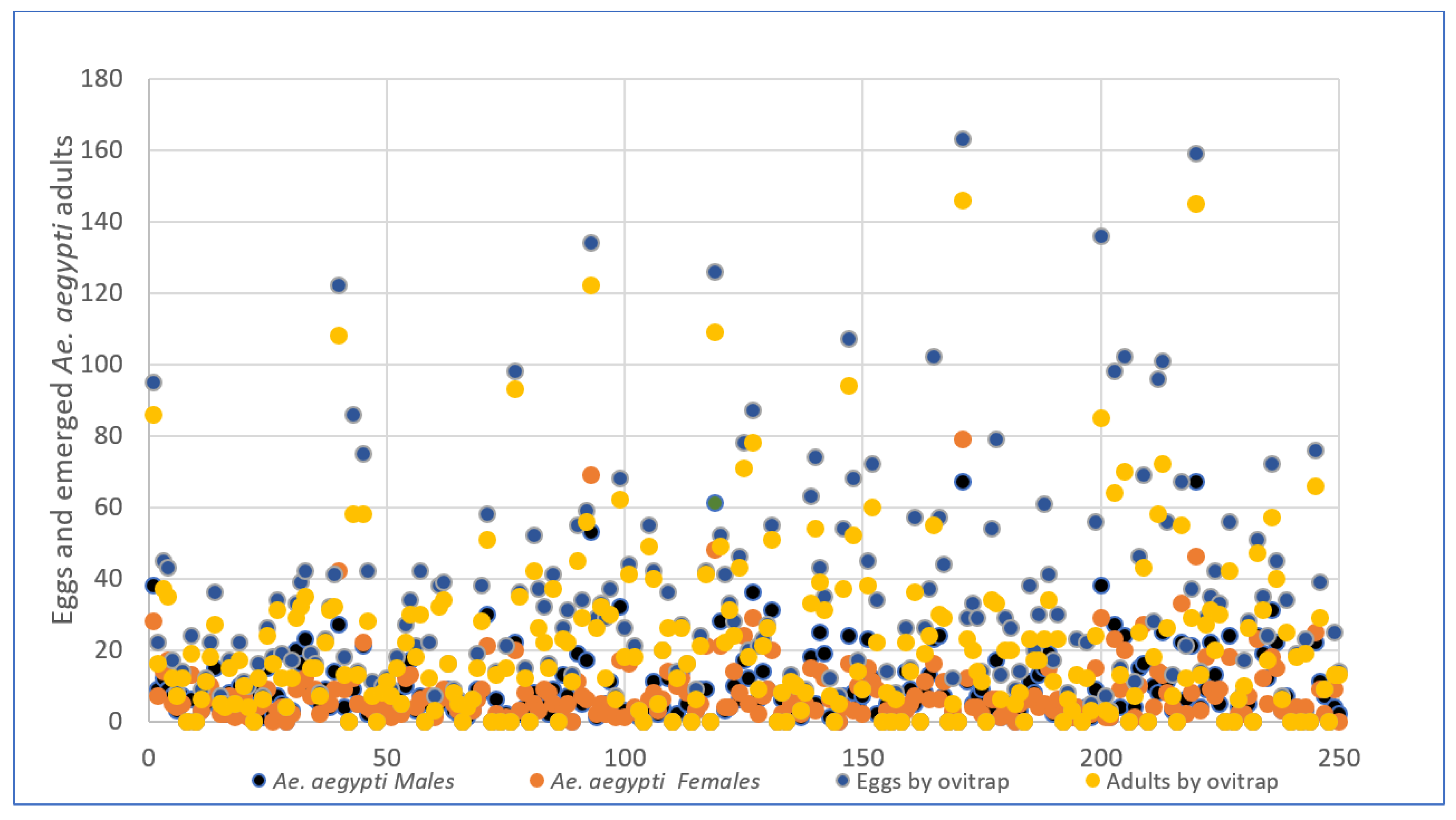
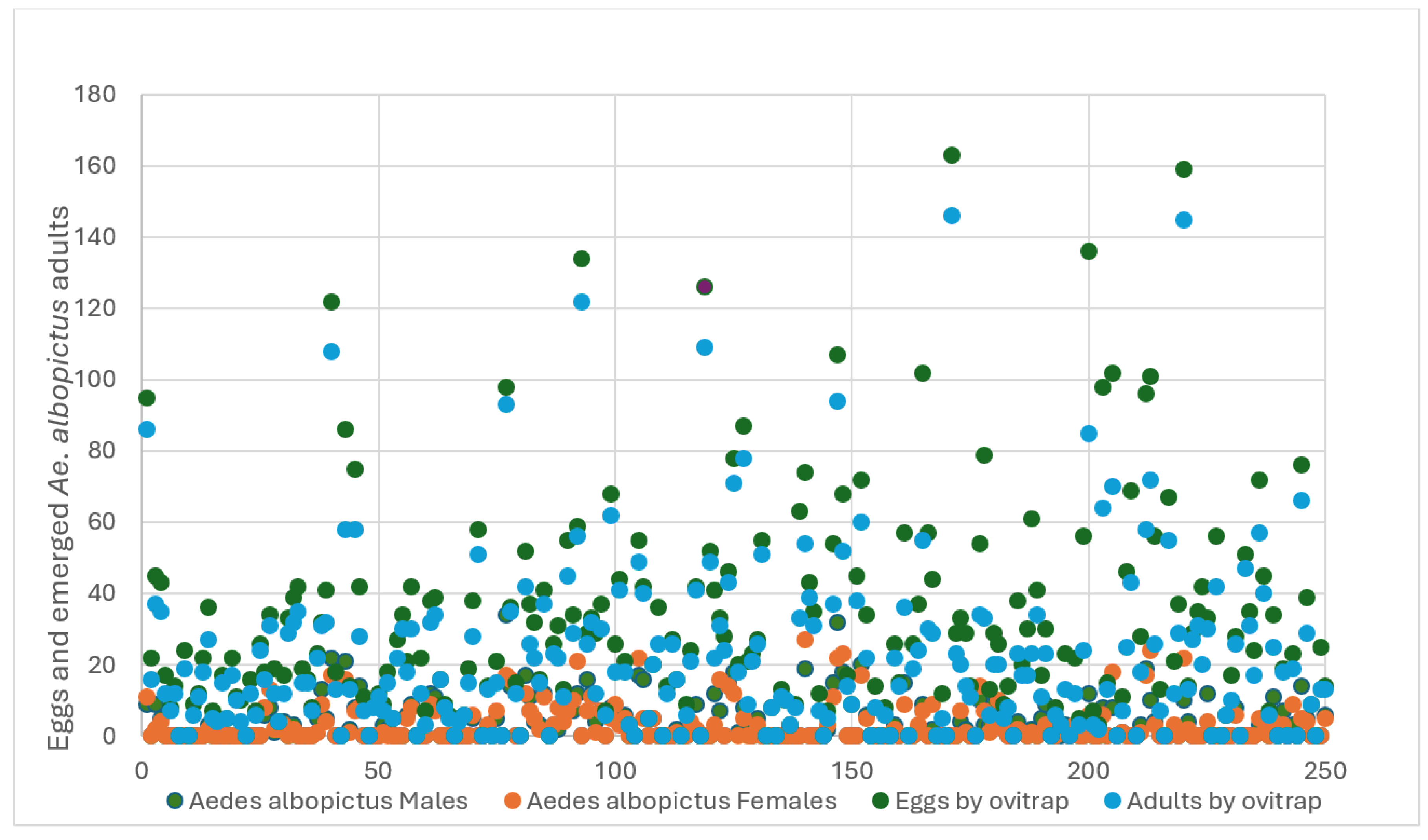
Ae. albopictus, respectively. Both culicine mosquitoes were overlapped in 108 ovitraps (43.2%), indicating species niche competition. On the other hand, 14% of the ovitraps did not attract egg-laying females of the two species. Most of the sampled egg numbers in ovitraps were ≤20 and had a similar distribution pattern in both species. However, some ovitraps recorded ≥100 eggs (
Figure 5 and
Figure 6).
3.2. Sampling and Species Identification of Aedes quiescent Eggs and Household Wet and Dry Larval Breeding Sites
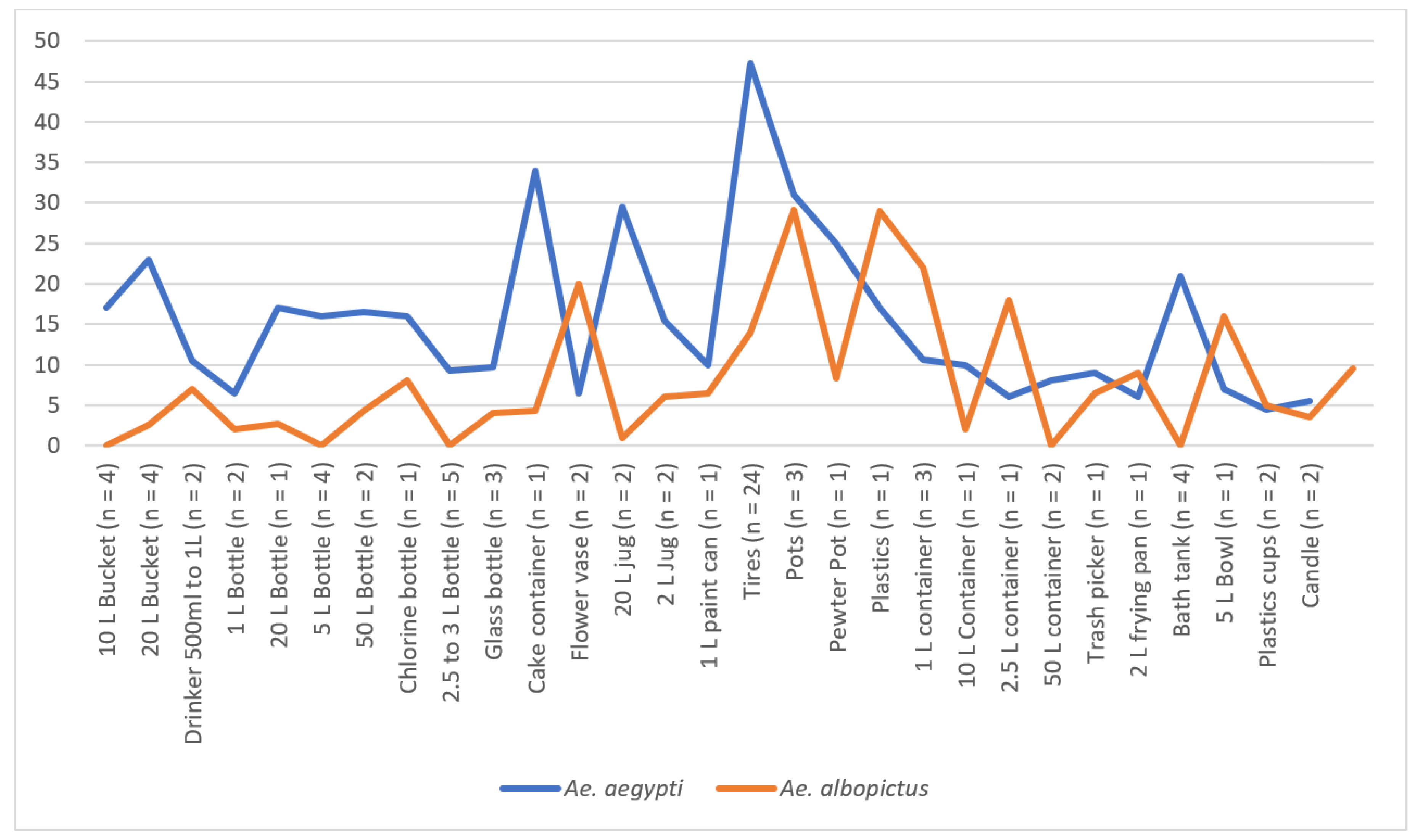
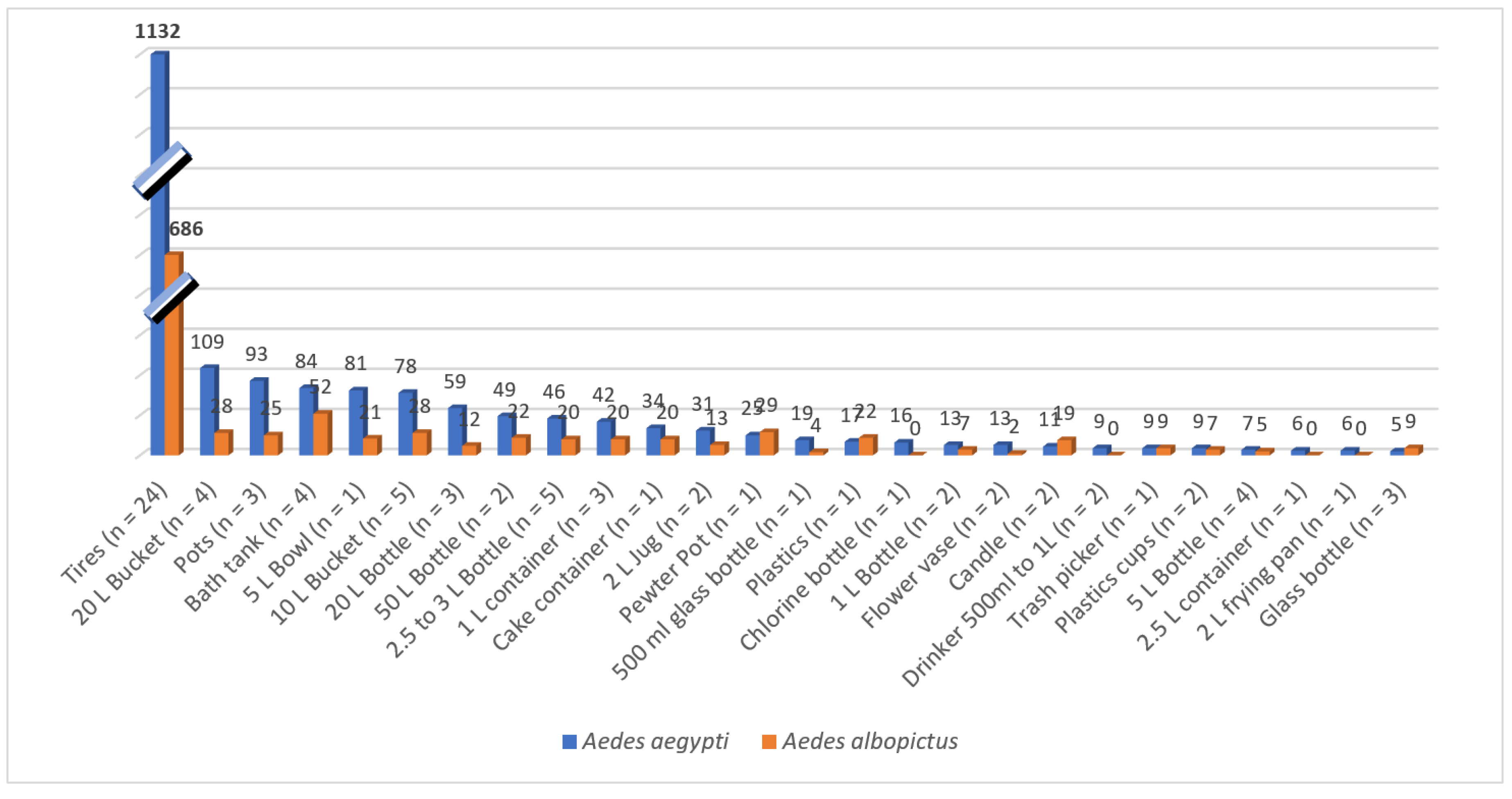
The identification of 500 potential breeding sites for developing
Aedes aegypti and
Aedes albopictus mosquitoes was conducted across 125 houses in the Las Americas neighborhood. From the thousands of artificial containers, only those that could be handled and transported with authorization from the heads of the family were selected. These containers were classified based on their water storage capacity (tires, less than 10 L, and greater than 10 L). In our sampling, water storage tanks larger than 500 L were not considered since they had been previously treated with the larvicide Spinosad® as part of the Ministry of Health's control methods. Dry season larval sites such as tires, 20 L buckets, 3 L vases, 3 L bottles, toilet bowls, 1 L plastic containers, 2 L drinkers, flowerpots, 5 L pots, etc., were collected. In total, 83 potential breeding sites were transported to further monitor the development of larvae and adults. Among the 83 containers examined, 28.9% were discarded tires, while the remaining 71.1% held various types of containers, with 56.6% being smaller than 10 liters and 14.5% larger than 10 liters. Within the 77 containers in the community homes collected with water, mosquito eggs were monitored until their adult emergence in the laboratory (
Figure 7). A total of 3,063 mosquitoes emerged, with 1,818 (59.3%) found in tires, 919 (30.0%) in containers with a capacity of less than 20 liters, and 326 (10.6%) in containers with a capacity of 20 to 50 liters. Of these, 2,108 (65.1%) were identified as
Ae. aegypti, comprising 52.8% females and 47.2% males. Additionally, 1,062 (34.5%) were identified as
Ae. albopictus, with 58.5% male and 41.5% female.
Our study found that a specific set of containers, including three discarded tires, one unused toilet tank, one 2-L cooking pan, and one 1-L plastic bottle, effectively showcased mosquitoes' quiescent eggs. These containers were collected from household backyards and brought to the research institute. After adding water and larval food to stimulate hatching, we observed the emergence of 188
Aedes mosquitoes within 24-48 hours under insectary conditions. Of these, 113 (60.1%) were
Ae. aegypti and 75 (39.9%)
Ae. albopictus (
Table 1). Frequency distribution of the average eggs and adult production showed discarded tires as the highest larval growing site, even with both species overlapping in southern Mexico (
Figure 4). However, more than 50% of available wet and dry containers were also occupied by
Ae. albopictus, an invasive mosquito with remarkable physiological plasticity (
Figure 7).
Figure 8.
Average number of Aedes aegypti and albopictus mosquitoes emerging from the collected in 83 containers in Tapachula, Chiapas, Southern Mexico.
Figure 8.
Average number of Aedes aegypti and albopictus mosquitoes emerging from the collected in 83 containers in Tapachula, Chiapas, Southern Mexico.
3.3. Effectiveness of Temephos 5% Bioassays on Dried Ae. aegypti Eggs
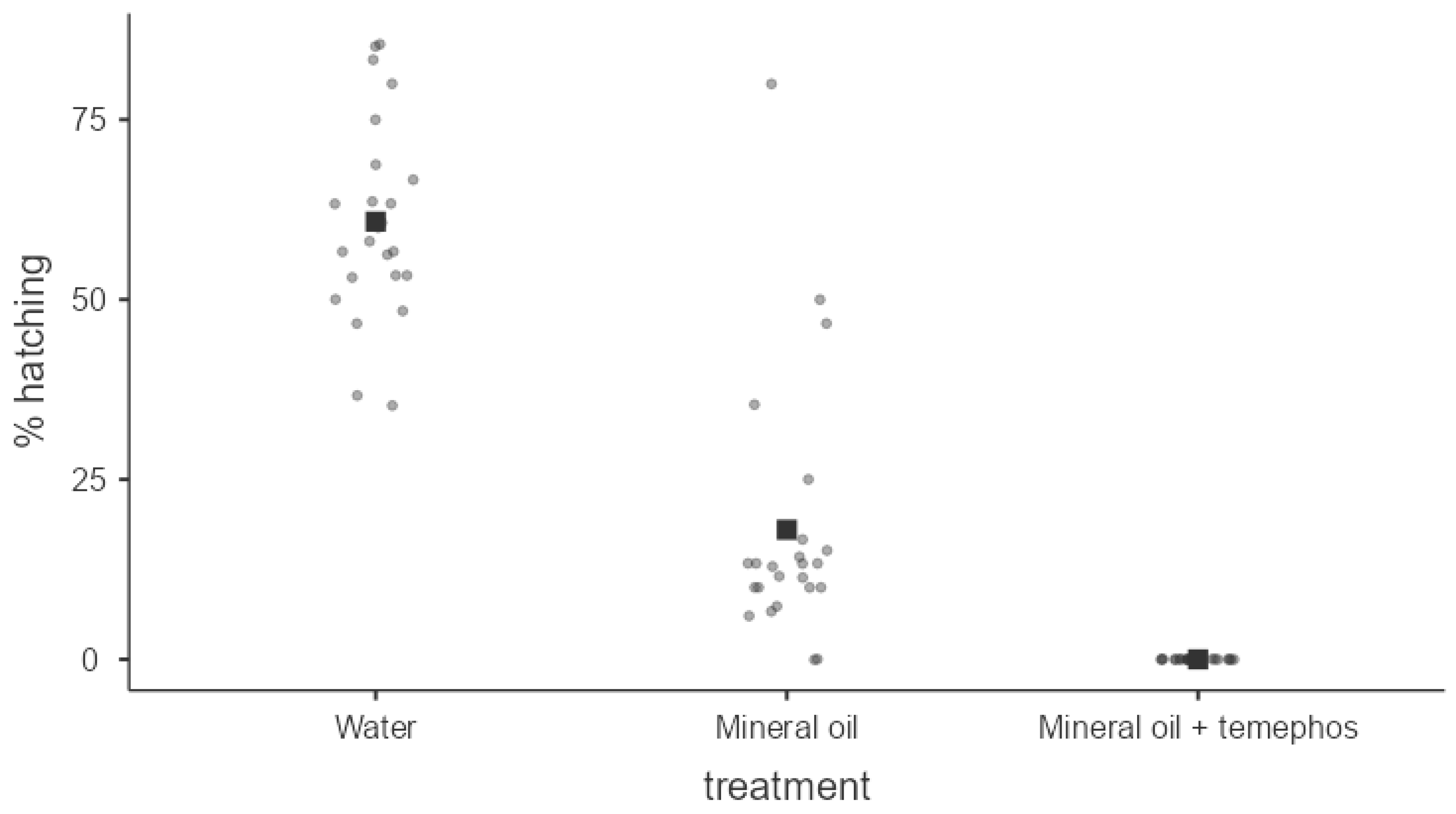
The hatching of dry eggs was fully inhibited by the formulation of 5% Temephos dissolved in mineral spirit oil. This consistency of 100% ovicidal activity was repeated during the three essays, each with eight repetitions (Kruskal-Wallis test
X2= 60.4; fd = 2;
P < 0.001, and differences in all Dwass-Steel-Critchlow-Fligner pairwise comparisons (
P < 0.001). The treatment using only mineral oil resulted in an average of 18% blockage of egg hatching across multiple bioassays and repetitions. The untreated control group had an average hatching rate of 60.8% (
Figure 9). Our preliminary results are evidence of the poorly documented ovicidal activity of Temephos for Dengue vectors elsewhere. Results also show a potential strategy to apply control measures during the dry inter-epidemic season on small-sized breeding sites carrying quiescent eggs in house backyards and most related sites.
4. Discussion
In most dengue-endemic countries in Latin America, routine vector control operations are conducted following the WHO Guidelines (2009) [
12]. However, despite experts' recommendations, Integrated Vector Control and Management strategies are only partially accomplished. Adult mosquitoes are intensively attacked using either vehicle-mounted or hand-held space-spraying chemicals. Concurrently, larvae populations are intoxicated in their artificial habitats, mostly with Temephos 1%, Pyriproxyfen, or Spinosad. Although WHO guidelines prioritize environmental management to eliminate larval household containers, which is a very effective action in the field, the truth is that house owners remove only a few larger containers, such as old mattresses and discarded tires. Regarding removing smaller containers, they volunteer to participate in community cleaning campaigns headed by the city authorities. Very often, this task has no supervision, and under dwellers' judgment, many containers will be beneficial for the future. In other words, only more significant≥20 L water deposits are treated by government programs, and consequently, thousands of small artificial containers remain in the houses' backyards. An all-stages
Aedes control strategy is not being implemented in practice. Whereas the adult, pupae, and larvae stages have some recommended treatments, the lack of mosquito eggs-specific control methods is remarkable. Some reasons for the overlooking situation are attributed to the small size of mosquito eggs, ≤1 mm, very difficult to see by the busy program technicians; additionally, eggs are glued to the container's inner walls with a sticky hyaluronic acid produced by the female accessory gland. Amazingly, after embryogenesis and melanization, the resilient egg populations may resist desiccation for up to 6 months or one year [
4].
4.1. Aedes Species Laying an Egg in Ovitrap in Early Dry Season
This study, conducted in the early months of the local dry season and linked to the tropical diapause period, found significant quiescent eggs. Of 250 ovitraps settled in 125 participating households, 84.4% showed positive filter paper stripes. These eggs developed adults reared and identified as
Ae. aegypti and
Ae. albopictus. We observed that both species shared 108 ovitraps (43.2%) which might represent some extent of niche competition of
Ae. albopictus, an invasive species in Mexico since 1997 [
3]. However, the hatching rates of
Ae. albopictus were significantly lower than
Ae. aegypti, 55.5% and 22.4%, respectively. Based on the hatching rates, the egg populations of
Ae. aegypti in southern Mexico show a form of quiescent dormancy. This is an adaptive response to unfavorable conditions. However, this dormancy can be reversed when dormant eggs are exposed to external stimuli, such as the rainy season.[
7]. Conversely, the low result for
Ae. albopictus may define the need to extend a longer field period associated with Delayed hatch (DH) overwintering species, a genetically programmed diapause such as in temperate countries [
15]. On the other hand, besides the egg stage, the results of significant surviving egg-laying females in the dry period must also be addressed by the control MoH programs. In addition to sporadic scarce and short rains filling temporary larval containers to help females’ survivorship, it is reported that delayed hatching (DH) egg-laying behavior for some
Aedes and other mosquito species [
14]. Whereas Rapid Hatching species (RH) are used to lay eggs after a 2–3-day embryonic period, DH females may take months or years. The eggs of DH usually hatch at different times, even if they are from the same female. This staggered hatching helps prevent all the larvae from emerging at once in a small space where there isn't enough food. A mixed RH and DH behavior is also reported for some species and geographical locations. Both
Aedes species in southern Mexico probably showed a similar pattern.
4.2. Egg-Laying Activity and Egg of at Early Dry Season and Findings of Desiccated Eggs in Preferred Dry and Wet Containers
As seen in
Figure 7, the two groups of larval containers searched for quiescent eggs produced adult culicines of the two
Aedes species. The first group consisted of 77 containers, which still maintained some rainwater left from the late rainy and sporadic months during the ongoing dry season. The second group comprised only six containers without rainwater or thoroughly dried and were screened to look for quiescent and desiccated eggs. Quiescent eggs-producing adults of the two vector species were consistently identified in these 77 temporary containers, i.e.,
Ae. aegypti in 100% and
Ae. albopictus only colonizing 72.0%, respectively. Males and females were almost evenly sampled over ≥ 95% of 29 assorted categories of larval containers (
Figure 8). A total of 3,063 males and females of both
Aedes emerged and were identified, corresponding to 65.4%
Ae. aegypti, and 34.6%
Ae. albopictus, respectively. Discarded tires from the backyards accounted for the most significant adult production after pooling the two species, recording 59.3%. The remaining 2-20 L containers accounted for around 25% more mosquito productivity. Regarding a preferred egg-laying container, both
Aedes species were found in similar artificial containers in southern Mexico. We assumed that the average higher adult productivity might be attributed to ≥ 5-L containers sustaining the early dry season vector production due to prolonged water evaporation (
Figure 7). From the vector control point of view, we call attention to the “trash-like” representing the rest of the house's backyard containers found in our study. According to Day's (2014) [
15] definition, quiescent eggs are dormant but quickly respond to environmental factors such as rainfall; in our case, water and larval food were added, and successful hatching rates were recorded. Therefore, we report field evidence here that they are genuinely quiescent eggs being carried in backyard-specific containers. This finding supports the importance of applying effective measures and not only treating these containers as domestic trash. This result consistently happens since larvicidal activity by government programs is targeted at only treating meant larger containers ≥ 5, 10, or 20 liters: 55-Ga barrels, 200-L cement tanks, cisterns, etc. The group of six fully dry containers shown in
Table 1 is our most substantial finding describing quiescent eggs. Both species were found, accounting for 188 males and females. Similarly, the higher mosquito habitats were discarded tires, a 2-L cooking pan, and an abandoned toilet tank.
Over the years, we have observed in several cities in Mexico that Dengue fever outbreaks are repeatedly reported yearly in the same city spots. Even when the documented log of local program vector control measures describes intense ULV space-spraying, larviciding activities, and community-based cleaning campaigns with significant clinical case reduction, the rest of Latin American countries also know this
in-situ epidemic pattern. A likely explanation for this vector population reinfestations when the transmission season begins, causing outbreaks in the same spots, is correlated to the neglected quiescent egg populations. We should recall that most insect pests follow the “R” reproductive strategy; they have a higher intrinsic reproductive rate and tend to produce large amounts of eggs and offspring [
16]. On the other hand, the impact of control activities between life cycle and disease is widely documented for adults, pupal, and larvae populations. The absence or lack of research on the role of the egg stage is always neglected. The paramount epidemiological importance of vertical virus transmission is firmly documented in many countries. In Mexico, Danis-Lozano (2019)
et al. [
6] report up to 7.8% of adults collected eggs in ovitraps in 9 cities of southern Mexico. In Mexico, Danis-Lozano (2019)
et al. [
5] report up to 7.8% of adults collected eggs in ovitraps in 9 cities of southern Mexico. Transovarial transmission in infected males is relevant from an entomo-epidemiological point of view since it shortens the transmission cycles in dengue-endemic areas. In this study, a high proportion of males emerging from ovitraps can be considered a risk factor since the probability of infecting wild females is high. Quiescent or uncontrolled
Aedes eggs can potentially play a role in pathogen maintenance during inter-epidemic periods. Secondly, the ability of the female to glue the eggs in the container's inner wall using a sticky hyaluronic acid substance has expanded both vector's geographical distribution. The commercial trade of recycled truck tires in regions and countries better exemplifies this phenomenon [
17]. Third, adaptation uses two egg-hatching strategies by
Ae. aegypti and
Ae. albopictus, such as quiescent eggs that promptly are stimulated if rain is present, Rapid Hatching (RH), or keep as a desiccated egg through a Delayed Hatching (DH) patiently waiting for the rainy and outbreaks season [
1].
4.3. Bioassay Temephos 5% as Ovicide
Regarding
Aedes vector control by government programs, measures are drastically reduced during the dry season. However, many untreated, desiccated, quiescent eggs remain hidden and glued to thousands of small, trash-like containers in residential and non-residential larval habitats [
18]. Our results using Temephos 5% dissolved in mineral oil resulted in 100% blocking
Ae. aegypti egg hatching. We cannot define Temephos as ovicide; perhaps some eggs hatched, and Temephos's active ingredient intoxicated the first instar stage, acting as a larvicide. However, for practical purposes, it may be a potential tool to eliminate the latent embryo population living in the dry season egg populations. On the other hand, mineral oil, being a high ambient temperature solvent, will keep the active ingredient for 4-6 dry season months after being sprayed in many small backyard containers, discarded tires, etc. Modern Integrated Vector Control programs must include a dry season component against quiescent egg populations [
19].
5. Conclusions
Although WHO guidelines for vector control during Dengue fever outbreaks indicate integrated control measures, the focus is on targeting adult, pupal, and larval stages, with little attention given to controlling the eggs of the Aedes species responsible. Frequent observations of city spot epidemics in year-to-year cycles call attention to the possibility of mosquito reinfestations in situ by neglected and uncontrolled dormant eggs. Our study was conducted in Tapachula, a dengue-endemic city in southern Mexico, aiming to generate new insights into the role of eggs in improving vector control in the future. During the dry season months, a group of 250 ovitraps were placed in 125 houses backyards, recording 211(84.4%) positive ovitraps. Adults who emerged from these eggs were identified as Ae. aegypti and Ae. albopictus, the two more critical vector species in Mexico. We demonstrated the definition of the quiescent diapause stage because desiccated field-collected eggs responded to external stimuli such as added water and larval food to estimate hatching. Assorted artificial containers with 1-20 L, “trash-like,” were recorded as dry season larval containers. This is important because only larger water containers are treated with chemical larvicides in Mexico, whereas community-based cleaning campaigns are supposed to eliminate these “trash” containers. However, the final destiny of trash containers is poorly documented after they are collected and transported by the city waste disposal trucks. It is not difficult to suspect that they are not treated with mosquito egg ovicide actions despite the resilient desiccated eggs glued to their walls. Additionally, mosquitoes, like other insects, have an R reproductive strategy, which means the egg-laying of overwhelming offspring to survive harsh conditions. We argue that quiescent egg populations may reinfest the same areas in the following year when favorable factors such as consistent rains return. Besides increasing reports of Dengue vertical transmission elsewhere, the ability to quiescent eggs to either a Rapid (RH) or Delayed egg hatching (DH) is an evolutionary trait allowing great colonizing success of both Ae. aegypti and Ae. albopictus.
Specific treatments must be targeted in the field to control dry-season egg populations. We have shown in this study that spraying dry eggs with Temephos 5% dissolved in mineral oil may be an interesting measure applied during the dry season months to prevent future rainy season outbreaks. Additional studies on the role of egg populations of Aedes vectors are urgently needed, considering large outbreaks of Dengue, Chikungunya, and Zika continue to be reported in Mexico and Latin America, and vaccines and antiviral drugs will take longer.
Author Contributions
Conceptualization, B.L.-M., and I.F.-S.; Data curation, K.E.P.-M., B.L.-M., and P.C.M.-A.; Formal analysis, L.A.C.V., K.E.P.-M., R.D.-L., and R.M.S.-C.; Funding acquisition, I.F.-S.; Investigation, B.L.-M., and M.A.D.G.; Methodology, L.A.C.V., J.D.-B. and M.A.D.G.; Project administration, R.M.S.-C.; Resources, R.D.-L., M.A.D.G. and P.C.M.-A.; Software, R.M.S.-C.; Validation, K.E.P.-M., and J.D.-B.; Visualization, P.C.M.-A., and I.F.-S.; Writing – original draft, J.I.N.-K. and J.D.-B.; Writing – review & editing, J.I.N.-K., L.A.C.V., R.D.-L., R.M.S.-C. and I.F.-S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Programa de Apoyo a la Ciencia, Tecnología e Innovación Científica ProACTI de la UANL, Proyecto 43-BQ-2023 y Proyecto 14-BQ-2023
Acknowledgments
We would like to thank the field technicians Crescencio Díaz and Sandra Robles (CRISP/INSP) for their support in establishing and maintaining the mosquito colonies and for their work in the semi-field and field tests
Conflicts of Interest
The authors declare no conflicts of interest
References
- Pan American Health Organization (OPS). Handbook for Integrated Vector Management in the Americas. 2024. https://iris.paho.org/handle/10665.2/51759 (accessed on 1 January 2024).
- Fernández-Salas I, EE Díaz-González, H López-Gatell, C Alpuche-Aranda. 2016. Chikungunya and Zika virus dissemination in the Americas: different arboviruses reflecting the same spreading routes and poor vector-control policies. Current opinion in infectious diseases 29 (5), 467-475.
- Ibañez-Bernal, S., Briseño, B., Mutebi, J. P., Argot, E., Rodriguez, G., Martinez-Campos, C., Paz, R., La Fuente-San Roman, P. D., Tapia-Conyer, R., & Flisser, A. (1997). The first record in America was of Aedes albopictus naturally infected with dengue virus during the 1995 outbreak in Reynosa, Mexico. Medical and Veterinary Entomology, 11(4), 305-309. https://doi.org/10.1111/j.1365-2915.1997.tb00413.x.
- Clements, A. N. (2011). The biology of mosquitoes: viral, arboviral and bacterial pathogens (Vol. 3). Cabi 2011.
- Alvarado-Moreno MS, M Laguna-Aguilar, OS Sánchez Rodríguez, RM Sánchez-Casas, R Ramírez-Jiménez, EA Zarate-Nahón, N Achee, JP Grieco, I Fernández-Salas. 2013 Potential community-based control by use of plastic film to block Aedes aegypti (L.) egg adhesion. Southwestern Entomologist 38 (4), 605-614.
- Danis-Lozano, R., Díaz-González, E. E., Malo-García, I. R., Rodriguez, M. H., Ramos-Castañeda, J., Juárez-Palma, L., ... & Fernández-Salas, I. (2019). Vertical transmission of dengue virus in Aedes aegypti and its role in the epidemiological persistence of dengue in Central and Southern Mexico. Tropical Medicine & International Health, 24(11), 1311-1319.
- Denlinger, D. L.; Armbruster, P. A. Molecular physiology of mosquito diapause. In Advances in insect physiology. Academic Press. 2016. Vol. 51, pp. 329-361.
- 8. MarketDataMexico.2023. https://www.marketdatamexico.com/es/article/Colonia-Las-Americas-Secc-I-Tapachula-Chiapas.
- 9. INEGI. Instituto Nacional de Estadística y Geografía. Información por entidad. Chiapas. Territorio. Clima. 2024. Available online https://cuentame.inegi.org.mx/monografias/informacion/chis/territorio/clima.aspx?tema=me&e=07#:~:text=M%C3%A1s%20de%20la%20mitad%20de,C%20en%20la%20Llanura%20Coste%C3%B1a (accessed on 1 July 2024).
- Boletin de Epidemiologia (2023). Sistema Nacional de Vigilancia Epidemiológica. Secrertaría de Salud, Mexico. https://www.gob.mx/cms/uploads/attachment/file/879365/sem52.pdf (accceded on August 26th 2024).
- Clark-Gil, S., y Darsie, RF (1983). Los mosquitos de Guatemala. Mosq Syst, 15 (3), 151-294.
- World Health Organization (2009). Guidelines for Diagnosis, treatment, prevention, and control. Geneva, Switzerland ISBN 978 92 4 154787 1.
- World Health Organization (WHO). WHO specifications and evaluations for public health pesticides. 2008. Available online: https://extranet.who.int/prequal/sites/default/files/vcp-documents/WHOVC-SP_Temephos_2010.pdf (accessed on 1 July 2024).
- Zar, J. Statistical Analysis. Prentice Hall. 2008. Available online: https://bayesmath.com/wp-content/uploads/2021/05/Jerrold-H.-Zar-Biostatistical-Analysis-5th-Edition-Prentice-Hall-2009.pdf (accessed on 1 July 2024).
- Day, J.F. Mosquito Oviposition Behavior and Vector Control. Insects 2016, 7, 65. https://doi.org/10.3390/insects7040065.
- Singh, A.; Vonk, J.; Shackelford, T. r-Reproductive Strategy. Encyclopedia of Animal Cognition and Behavior 1 6. Springer International Publishing. 2019. Available online: https://www.researchgate.net/publication/354149717_r_r-Reproductive_Strategy#fullTextFileContent (accessed on 1 July 2024).
- Bennett, K.L., Gómez Martínez, C., Almanza, A. et al. High infestation of invasive Aedes mosquitoes in used tires along the local transport network of Panama. Parasites Vectors 12, 264 (2019). https://doi.org/10.1186/s13071-019-3522-8.
- Morrison, A.C.; Sihuincha, M.; Stancil, J.D.; Zamora, E.; Astete, H.; Olson, J.G.; … Scott, T.W. (2006). Producción de Aedes aegypti (Diptera: Culicidae) en sitios no residenciales de la ciudad amazónica de Iquitos, Perú. Ann Trop Med Parasitol. 2006. 100:1, 73–86. https://doi.org/10.1179/136485906X105534.
- World Health Organization (2017). Global Vector Control Response 2017-20130. : CC BY-NC-SA 3.0 IGO. http://apps.who.int/iris.
Figure 1.
A) Geographic location of the state of Chiapas in the Mexican national territory. B) LocaTable 14. 9277, Longitude: -92.2602). D) Geographic limitations of the Las Americas section II and its border with the Coatan River (Google Maps, @ INEGI 2023).
Figure 1.
A) Geographic location of the state of Chiapas in the Mexican national territory. B) LocaTable 14. 9277, Longitude: -92.2602). D) Geographic limitations of the Las Americas section II and its border with the Coatan River (Google Maps, @ INEGI 2023).
Figure 2.
Distribution of rainfall (mm) and temperatures (°C) in Tapachula, Chiapas, Mexico, from July 2022 to July 2023.
Figure 2.
Distribution of rainfall (mm) and temperatures (°C) in Tapachula, Chiapas, Mexico, from July 2022 to July 2023.
Figure 3.
Examples of dry season breeding sites (wet and dry) identified and monitored for the development of Aedes mosquitoes in the Las Americas section II in Tapachula, Chiapas, Mexico. A) 20 L plastic container with water, B) tire with water, C) 5 L plastic container with water, D) 2 L plastic bottle with water, E) plastic vase with plants, F) plastic container 3 L with water, G) accumulated plastic containers, H) plastic container with water.
Figure 3.
Examples of dry season breeding sites (wet and dry) identified and monitored for the development of Aedes mosquitoes in the Las Americas section II in Tapachula, Chiapas, Mexico. A) 20 L plastic container with water, B) tire with water, C) 5 L plastic container with water, D) 2 L plastic bottle with water, E) plastic vase with plants, F) plastic container 3 L with water, G) accumulated plastic containers, H) plastic container with water.
Figure 4.
Total Aedes aegypti and Aedes albopictus egg numbers laid in 250 ovitraps and reared adults. Sampling was conducted in 125 backyard households during the dry season in the locality of the Americas in Tapachula, southern Mexico.
Figure 4.
Total Aedes aegypti and Aedes albopictus egg numbers laid in 250 ovitraps and reared adults. Sampling was conducted in 125 backyard households during the dry season in the locality of the Americas in Tapachula, southern Mexico.
Figure 5.
shows the dispersion of the 7,290 eggs collected in 125 houses. Here, we can see the distribution of the 5,667 eggs from which adults Aedes aegypti (4,031) are female and male.
Figure 5.
shows the dispersion of the 7,290 eggs collected in 125 houses. Here, we can see the distribution of the 5,667 eggs from which adults Aedes aegypti (4,031) are female and male.
Figure 6.
shows the dispersion of the 7,290 eggs collected in 125 houses. It also shows the distribution of the 5,667 eggs from which adults Aedes albopictus (1636) hatched.
Figure 6.
shows the dispersion of the 7,290 eggs collected in 125 houses. It also shows the distribution of the 5,667 eggs from which adults Aedes albopictus (1636) hatched.
Figure 7.
Adults Aedes aegypti and Aedes albopictus emerging from dry and glued eggs collected above the water line of assorted backyard containers in Tapachula, Chiapas, Southern Mexico.
Figure 7.
Adults Aedes aegypti and Aedes albopictus emerging from dry and glued eggs collected above the water line of assorted backyard containers in Tapachula, Chiapas, Southern Mexico.
Figure 9.
Average percentage (darkest point) of hatching of Ae. aegypti eggs exposed to different treatments. Kruskal-Wallis test X2=60.4; fd=2; p<0.001. Differences in all Dwass-Steel-Critchlow-Fligner pairwise comparisons (p<0.001).
Figure 9.
Average percentage (darkest point) of hatching of Ae. aegypti eggs exposed to different treatments. Kruskal-Wallis test X2=60.4; fd=2; p<0.001. Differences in all Dwass-Steel-Critchlow-Fligner pairwise comparisons (p<0.001).
Table 1.
Adult mosquitoes Ae. aegypti and Ae. albopictus were reared from quiescent eggs collected during the dry season from inside empty household containers in Tapachula Chiapas, Mexico, 2023.
Table 1.
Adult mosquitoes Ae. aegypti and Ae. albopictus were reared from quiescent eggs collected during the dry season from inside empty household containers in Tapachula Chiapas, Mexico, 2023.
| House # |
Dry Egg-Carrying Container |
Sample Size |
Ae. Aegypti |
Ae. Albopictus |
Total Adults |
| Male |
Female |
Male |
Female |
| 7 |
Toilet tank |
1 |
4 |
2 |
13 |
9 |
28 |
| 15 |
1 L bottle |
1 |
2 |
4 |
4 |
3 |
13 |
| 43 |
Discarded tire |
1 |
34 |
39 |
24 |
10 |
107 |
| 64 |
Discarded tire |
1 |
4 |
7 |
7 |
2 |
20 |
| 100 |
Discarded tire |
1 |
3 |
8 |
3 |
0 |
14 |
| 122 |
2 L pan |
1 |
3 |
3 |
0 |
0 |
6 |
| |
TOTAL |
6 |
50 |
63 |
51 |
24 |
188 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).