Submitted:
28 August 2024
Posted:
28 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
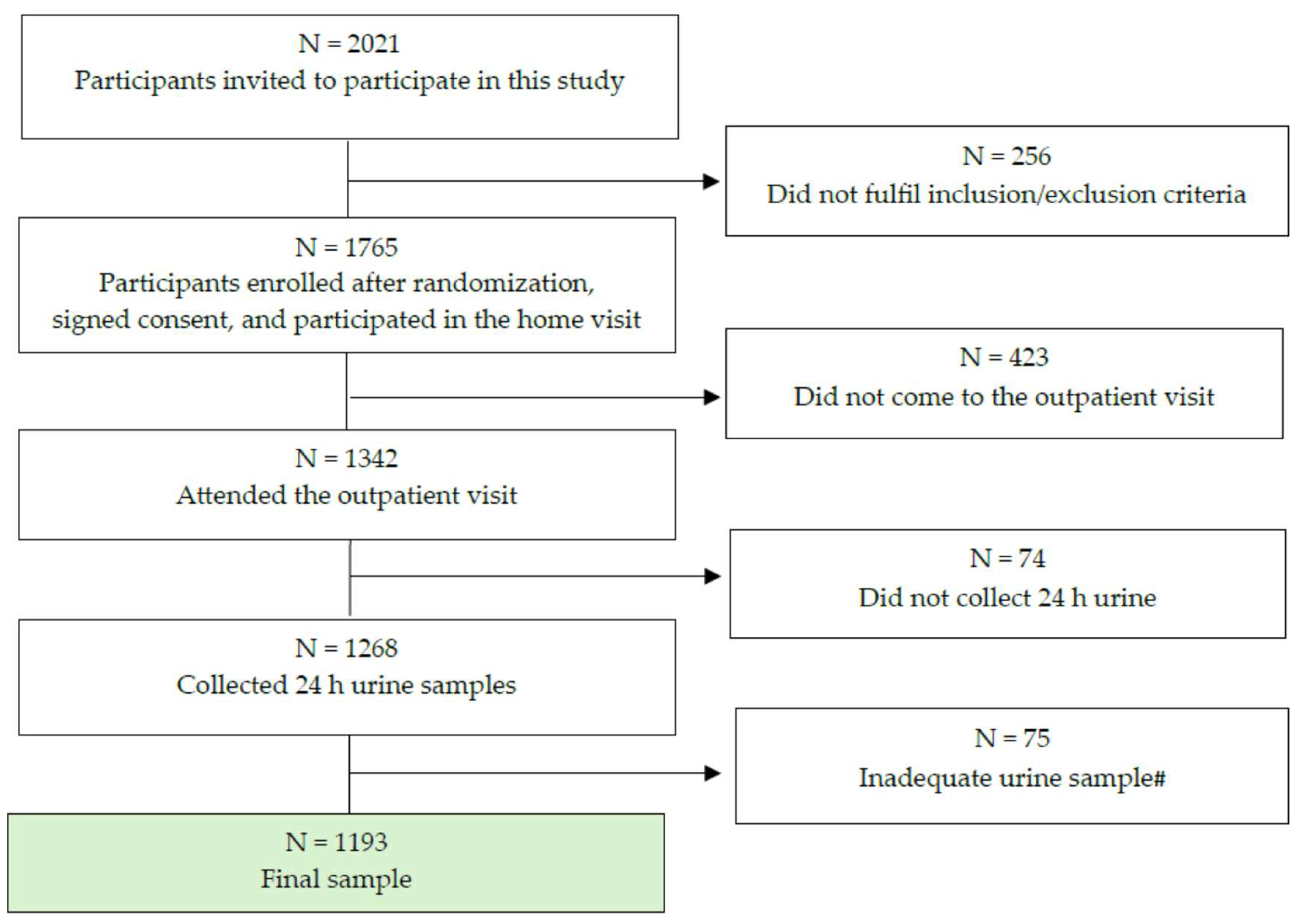
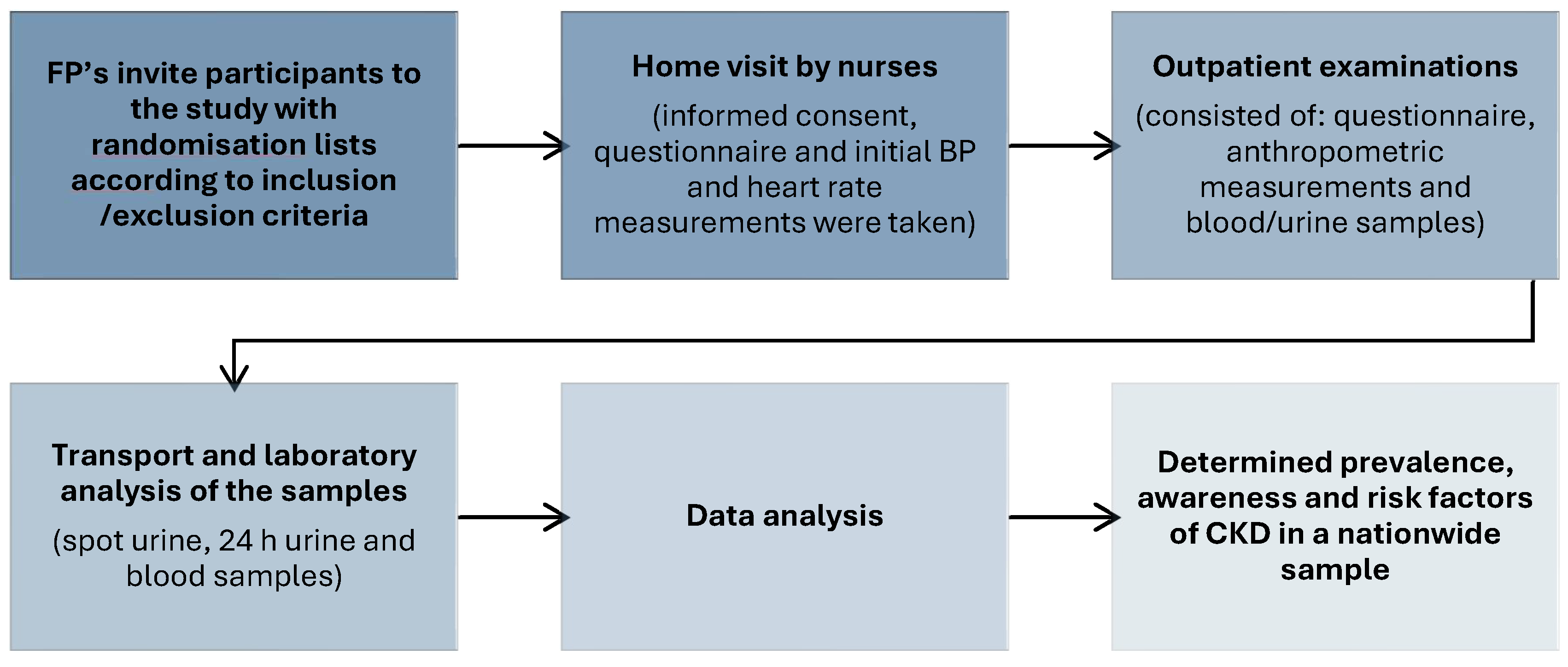
2.1. Study Design and Recruitment
2.2. Questionnaire
2.3. Anthropometry (Physical Measurements)
2.4. Laboratory Analysis
2.5. Statistical Analysis
3. Results
| Model | Coef | S.E. | Odds Ratio | 95% CI | P | Nagelkerke R2 | P for change | |
|---|---|---|---|---|---|---|---|---|
| (B) | Exp (B) | Lower | Upper | |||||
| Model 1 | 0.287 | <0.001 | ||||||
| Constant | 8.554 | 0.863 | 5186.45 | <0.001 | ||||
| Gender | 1.780 | 0.258 | 5.93 | 3.58 | 9.82 | <0.001 | ||
| Age | -0.106 | 0.013 | 0.90 | 0.87 | 0.92 | <0.001 | ||
| Model 2 | 0.304 | <0.001 | ||||||
| Constant | 8.997 | 0.936 | 8075.6 | <0.001 | ||||
| Gender | 1.837 | 0.262 | 6.280 | 3.760 | 10.48 | <0.001 | ||
| Age | -0.174 | 0.029 | 0.841 | 0.794 | 0.89 | <0.001 | ||
| ePWV | 0.359 | 0.138 | 1.432 | 1.092 | 1.878 | 0.009 | ||
| Model 3 | 0.331 | 0.557 | ||||||
| Constant | 8.407 | 1.108 | 4479.2 | <0.001 | ||||
| Gender | 1.778 | 0.265 | 5.917 | 3.517 | 9.954 | <0.001 | ||
| Age | -0.181 | 0.030 | 0.834 | 0.787 | 0.885 | <0.001 | ||
| ePWV | 0.409 | 0.141 | 1.505 | 1.141 | 1.984 | 0.004 | ||
| Diabetes | 0.995 | 0.324 | 2.705 | 1.144 | 5.105 | <0.001 | ||
| Model 4 | 0.454 | <0.001 | ||||||
| Constant | 10.198 | 1.096 | 26860.47 | <0.001 | ||||
| Gender | 1.921 | 0.299 | 6.828 | 3.8 | 12.2 | <0.001 | ||
| Age | -0.158 | 0.032 | 0.854 | 0.80 | 0.91 | <0.001 | ||
| Diabetes | 0.948 | 0.290 | 2.579 | 1.46 | 4.55 | 0.001 | ||
| ePWV | 0.408 | 0.155 | 1.504 | 1.11 | 2.03 | 0.008 | ||
| urea | -0.503 | 0.071 | 0.589 | 0.51 | 0.67 | <0.001 | ||
| Model 5 | 0.496 | 0.001 | ||||||
| Constant | 13.057 | 1.358 | 468266.30 | |||||
| Gender | 1.581 | 0.315 | 4.862 | 2.64 | 9.00 | <0.001 | ||
| Age | -0.177 | 0.034 | 0.838 | 0.78 | 0.89 | <0.001 | ||
| Diabetes | 1.009 | 0.303 | 2.743 | 1.51 | 4.96 | <0.001 | ||
| ePWV | 0.501 | 0.161 | 1.650 | 1.20 | 2.26 | 0.002 | ||
| urea | -0.442 | 0.075 | 0.643 | 0.55 | 0.74 | <0.001 | ||
| Uric acid | -0.009 | 0.002 | 0.991 | 0.98 | 0.99 | <0.001 | ||
| Model 6 | 0.518 | 0.001 | ||||||
| Constant | 17 752 | 2.091 | 51240062.3 | <0.001 | ||||
| Gender | 1.445 | 0.323 | 4.240 | 2.250 | 7.991 | <0.001 | ||
| Age | -0.187 | 0.036 | 0.829 | 0.773 | 0.890 | <0.001 | ||
| Diabetes | 1.005 | 0.315 | 2.731 | 1.472 | 5.065 | 0.001 | ||
| ePWV | 0.552 | 0.171 | 1.73 | 1.242 | 2.431 | 0.001 | ||
| urea | -0.429 | 0.80 | 0.651 | 0.557 | 0.,762 | <0.001 | ||
| Uric acid | -0.11 | 0.002 | 0.989 | 0.985 | 0.993 | <0.001 | ||
| potassium | -0.837 | 0.270 | 0.433 | 0.255 | 0.735 | 0.002 | ||
| CKD aware N = 11 |
CKD unaware N = 106 |
X2; | p | |||
|---|---|---|---|---|---|---|
| Median (IQR) | 95% CI | Median (IQR) | 95% CI | |||
| Men % | 54.5 | 23.4-83.8 | 50.0 | 40.1-59.9 | 12 653; | 0.002 |
| Age (years) | 71 (62-75) | 62.51-75.68 | 72 (65-79) | 69.2-72.9 | NS | |
| Smokers % | 18.2 | 27.3-51.8 | 11.3 | 6.0-18.9 | 12 004; | <0.017 |
| Hypertension duration (years) | 15.5 (5.5-13.2) | 54.5-56.1 | 10.0 (5.0-16.0) | 10.2-14.7 | NS | |
| Systolic blood pressure (mmHg) | 138 (126-138) | 129.3-162.6 | 137.5 (124-153) | 135.5-144.2 | NS | |
| Waist circumference % | 88.9 | 51.8-99.7 | 78.4 | 68.8-86.1 | 10 222; | 0.037 |
| Personal income < 300 Eu % | 44.3 | 37.4 -54.3 | 32.4 | 1.7-35.2 | 18 169; | 0.029 |
| Family income < 300 Eu % | 22.6 | 15.1-31.8 | 10.1 | 8.4-12.6 | 39 534; | <0.001 |
| Education < 12 years % | 50.0 | 40.1-55.9 | 54.8 | 51.8-57.7 | 20 269; | 0.009 |
| No college % | 36.8 | 27.6-46.7 | 23.5 | 21.1-26.6 | 21 771; | 0.005 |
| Atrial fibrillation % | 1.8 | 0.2-5.1 | 8.5 | 4.0-15.5 | 19 446; | 0.001 |
| Hypertension % | 63.6 | 30.8-89.1 | 72.6 | 63.1-80.9 | 5583; | 0.061 |
| Controlled hypertensive % | 23.4 | 21.0-26.9 | 21.9 | 14.4-31.0 | 12 851; | 0.045 |
| Diabetes % | 27.3 | 17.6-61.0 | 29.2 | 20.8-38.9 | 18913; | <0.001 |
| ePWV (m/s) | 11.5 (10.1-13.9) | 10.4-13.4 | 12.0 (10.6-13.4) | 11.6-12.4 | NS | |
| Urea (mmol/l) | 8.2 (3.6) | 5.7-10.6 | 8.0 (3.0) | 7.4-8.6 | NS | |
| Serum creatinine (µmol/l) | 119 (106-132) | 101.5-157.0 | 110 (91.7-118) | 106.7-121.6 | NS | |
| eGFR (ml/min/1.73m2 ) | 45.2 (38.5-58.0) | 38.4-53.6 | 55.0 (44.5-58.1) | 49.5-53.3 | NS | |
| Uric acid (µmol/l) | 350 (284-475) | 287.6-482.9 | 360.0 (304-417) | 350.5-386.4 | NS | |
4. Discussion
5. Strengths and Limitations
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020; 29;395(10225):709-733. [CrossRef]
- Mills K.T., Xu Y., Zhang W., et al. A systematic analysis of worldwide population-based data on the global burden of chronic kidney disease in 2010. Kidney Int. 2015;88:950–957. [CrossRef]
- GBD Mortality Causes of Death Collaborators Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;385:117–171. [CrossRef]
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1789–1858. [CrossRef]
- Y, Bowe B, Mokdad AH, Xian H, Yan Y, Li T, Maddukuri G, Tsai CY, Floyd T, Al-Aly Z. Analysis of the Global Burden of Disease study highlights the global, regional, and national trends of chronic kidney disease epidemiology from 1990 to 2016. Kidney Int. 2018;94(3):567-581. [CrossRef]
- Couser WG, Remuzzi G, Mendis S, Tonelli M. The contribution of chronic kidney disease to the global burden of major noncommunicable diseases. Kidney Int. 2011; 80:1258–1270. [CrossRef]
- Sarnak MJ, Levey AS, Schoolwerth AC. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association councils on kidney in cardiovascular disease, high blood pressure research, clinical cardiology, and epidemiology and prevention. Circulation. 2003; 108:2154–2169. [CrossRef]
- Matsushita K, Coresh J, Sang Y. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol. 2015; 3:514–525. [CrossRef]
- Chronic Kidney Disease Prognosis Consortium Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet. 2010; 375:2073–2081. [CrossRef]
- Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Bäck M, Benetos A, Biffi A, Boavida JM, Capodanno D, Cosyns B, Crawford C, Davos CH, Desormais I, Di Angelantonio E, Franco OH, Halvorsen S, Hobbs FDR, Hollander M, Jankowska EA, Michal M, Sacco S, Sattar N, Tokgozoglu L, Tonstad S, Tsioufis KP, van Dis I, van Gelder IC, Wanner C, Williams B; ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021 Sep 7;42(34):3227-3337. [CrossRef]
- Matsushita K, Jassal SK, Sang Y, Ballew SH, Grams ME, Surapaneni A, Arnlov J, Bansal N, Bozic M, Brenner H, Brunskill NJ, Chang AR, Chinnadurai R, Cirillo M, Correa A, Ebert N, Eckardt KU, Gansevoort RT, Gutierrez O, Hadaegh F, He J, Hwang SJ, Jafar TH, Kayama T, Kovesdy CP, Landman GW, Levey AS, Lloyd-Jones DM, Major RW, Miura K, Muntner P, Nadkarni GN, Naimark DM, Nowak C, Ohkubo T, Pena MJ, Polkinghorne KR, Sabanayagam C, Sairenchi T, Schneider MP, Shalev V, Shlipak M, Solbu MD, Stempniewicz N, Tollitt J, Valdivielso JM, van der Leeuw J, Wang AY, Wen CP, Woodward M, Yamagishi K, Yatsuya H, Zhang L, Schaeffner E, Coresh J. Incorporating kidney disease measures into cardiovascular risk prediction: Development and validation in 9 million adults from 72 datasets. EClinicalMedicine. 2020; 14; 27:100552. [CrossRef]
- Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, Goldberg R, Heidenreich PA, Hlatky MA, Jones DW, Lloyd-Jones D, Lopez-Pajares N, Ndumele CE, Orringer CE, Peralta CA, Saseen JJ, Smith SC Jr, Sperling L, Virani SS, Yeboah J. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019; 18;139(25):e1082-e1143. [CrossRef]
- Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, Cooney MT, Corrà U, Cosyns B, Deaton C, Graham I, Hall MS, Hobbs FDR, Løchen ML, Löllgen H, Marques-Vidal P, Perk J, Prescott E, Redon J, Richter DJ, Sattar N, Smulders Y, Tiberi M, van der Worp HB, van Dis I, Verschuren WMM, Binno S; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016; 1;37(29):2315-2381. [CrossRef]
- Ndumele CE, Rangaswami J, Chow SL, Neeland IJ, Tuttle KR, Khan SS, Coresh J, Mathew RO, Baker-Smith CM, Carnethon MR, Despres JP, Ho JE, Joseph JJ, Kernan WN, Khera A, Kosiborod MN, Lekavich CL, Lewis EF, Lo KB, Ozkan B, Palaniappan LP, Patel SS, Pencina MJ, Powell-Wiley TM, Sperling LS, Virani SS, Wright JT, Rajgopal Singh R, Elkind MSV; American Heart Association. Cardiovascular-Kidney-Metabolic Health: A Presidential Advisory from the American Heart Association. Circulation. 2023;14;148(20):1606-1635. [CrossRef]
- GBD. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388, 1459–1544. [CrossRef]
- Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M, Pletcher MA, Smith AE, Tang K, Yuan CW, Brown JC, Friedman J, He J, Heuton KR, Holmberg M, Patel DJ, Reidy P, Carter A, Cercy K, Chapin A, Douwes-Schultz D, Frank T, Goettsch F, Liu PY, Nandakumar V, Reitsma MB, Reuter V, Sadat N, Sorensen RJD, Srinivasan V, Updike RL, York H, Lopez AD, Lozano R, Lim SS, Mokdad AH, Vollset SE, Murray CJL. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet. 2018; 10;392(10159):2052-2090. [CrossRef]
- Radhakrishnan J, Remuzzi G, Saran R, Williams DE, Rios-Burrows N, Powe N; CDC-CKD Surveillance Team; Brück K, Wanner C, Stel VS; European CKD Burden Consortium; Venuthurupalli SK, Hoy WE, Healy HG, Salisbury A, Fassett RG; CKD.QLD group; O'Donoghue D, Roderick P, Matsuo S, Hishida A, Imai E, Iimuro S. Taming the chronic kidney disease epidemic: a global view of surveillance efforts. Kidney Int. 2014;86(2):246-50. [CrossRef]
- Carrero JJ, Hecking M, Chesnaye NC, Jager KJ. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol. 2018;14(3):151-164. [CrossRef]
- Bikbov B, Perico N, Remuzzi G; on behalf of the GBD Genitourinary Diseases Expert Group. Disparities in Chronic Kidney Disease Prevalence among Males and Females in 195 Countries: Analysis of the Global Burden of Disease 2016 Study. Nephron. 2018;139(4):313-318. [CrossRef]
- Bairey Merz CN, Dember LM, Ingelfinger JR, Vinson A, Neugarten J, Sandberg KL, Sullivan JC, Maric-Bilkan C, Rankin TL, Kimmel PL, Star RA; participants of the National Institute of Diabetes and Digestive and Kidney Diseases Workshop on “Sex and the Kidneys”. Sex and the kidneys: current understanding and research opportunities. Nat Rev Nephrol. 2019;15(12):776-783. [CrossRef]
- Silbiger SR, Neugarten J. The impact of gender on the progression of chronic renal disease. Am J Kidney Dis. 1995;25(4):515-33. [CrossRef]
- Noborisaka Y. Smoking and chronic kidney disease in healthy populations. Nephrourol Mon. 2013 Winter;5(1):655-67. [CrossRef]
- Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2021 (US Department of Health and Human Services, 2021).
- Tuot DS, Zhu Y, Velasquez A, Espinoza J, Mendez CD, Banerjee T, Hsu CY, Powe NR. Variation in Patients' Awareness of CKD according to How They Are Asked. Clin J Am Soc Nephrol. 2016; 7;11(9):1566-1573. [CrossRef]
- Ene-Iordache B, Perico N, Bikbov B, Carminati S, Remuzzi A, Perna A, Islam N, Bravo RF, Aleckovic-Halilovic M, Zou H, Zhang L, Gouda Z, Tchokhonelidze I, Abraham G, Mahdavi-Mazdeh M, Gallieni M, Codreanu I, Togtokh A, Sharma SK, Koirala P, Uprety S, Ulasi I, Remuzzi G. Chronic kidney disease and cardiovascular risk in six regions of the world (ISN-KDDC): a cross-sectional study. Lancet Glob Health. 2016;4(5):e307-19. [CrossRef]
- Florea A, Jacobs ET, Harris RB, Klimentidis YC, Thajudeen B, Kohler LN. Chronic kidney disease unawareness and determinants using 1999-2014 National Health and Nutrition Examination Survey Data. J Public Health (Oxf). 2022; 25;44(3):532-540. [CrossRef]
- Glassock RJ, Delanaye P, Rule AD. Should the definition of CKD be changed to include age adapted GFR criteria? YES. Kidney Int. 2020; 97:34-41. [CrossRef]
- Delanaye P, Jager KJ, Bökenkamp A, et al. CKD: a call for an age-adapted definition. J Am Soc Nephrol. 2019; 30:1785-1805. [CrossRef]
- Stevens PE, Levin A; Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013; 4;158(11):825-30. [CrossRef]
- Ruggenenti P, Cravedi P, Remuzzi G. Mechanisms and treatment of CKD. J Am Soc Nephrol. 2012;23(12):1917-28. [CrossRef]
- chrome-extension://.
- efaidnbmnnnibpcajpcglclefindmkaj/https://health.ec.europa.eu/system/files/2021-12/2021_chp_hr_english.pdf.
- https://www.who.int/publications/i/item/9789240015128 accessed on 26th June 2024.
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, Clement DL, Coca A, de Simone G, Dominiczak A, Kahan T, Mahfoud F, Redon J, Ruilope L, Zanchetti A, Kerins M, Kjeldsen SE, Kreutz R, Laurent S, Lip GYH, McManus R, Narkiewicz K, Ruschitzka F, Schmieder RE, Shlyakhto E, Tsioufis C, Aboyans V, Desormais I; ESC Scientific Document Group. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur Heart J. 2018; 1;39(33):3021-3104. [CrossRef]
- Vrdoljak A, Vrkić TZ, Kos J, Vitale K, Premuzić V, Laganović M, Jelaković B. Blood pressure measurement--do not sweat the small stuff and it is all small stuff?! Position paper of the Croatian national referral center for hypertension, center of excellence of the European Society of Hypertension. Lijec Vjesn. 2014;136(1-2):33-43.
- Greve SV, Blicher MK, Kruger R, Sehestedt T, Gram-Kampmann E, Rasmussen S, Vishram JK, Boutouyrie P, Laurent S, Olsen MH. Estimated carotid-femoral pulse wave velocity has similar predictive value as measured carotid-femoral pulse wave velocity. J Hypertens. 2016;34(7):1279-89. [CrossRef]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009; 5;150(9):604-12. Erratum in: Ann Intern Med. 2011; 20;155(6):408. [CrossRef]
- https://dzs.gov.hr/u-fokusu/popis-2021/88 accessed on 26th June 2024.
- Mazhar F, Sjölander A, Fu EL, Ärnlöv J, Levey AS, Coresh J, Carrero JJ. Estimating the prevalence of chronic kidney disease while accounting for nonrandom testing with inverse probability weighting. Kidney Int. 2023;103(2):416-420. [CrossRef]
- Hill NR, Fatoba ST, Oke JL, Hirst JA, O'Callaghan CA, Lasserson DS, Hobbs FD. Global Prevalence of Chronic Kidney Disease - A Systematic Review and Meta-Analysis. PLoS One. 2016; 6;11(7):e0158765. [CrossRef]
- De Nicola L, Donfrancesco C, Minutolo R, Lo Noce C, Palmieri L, De Curtis A, Iacoviello L, Zoccali C, Gesualdo L, Conte G, Vanuzzo D, Giampaoli S; ANMCO-SIN Research Group. Prevalence and cardiovascular risk profile of chronic kidney disease in Italy: results of the 2008-12 National Health Examination Survey. Nephrol Dial Transplant. 2015;30(5):806-14. [CrossRef]
- Alkerwi, A., Sauvageot, N., El Bahi, I. et al. Prevalence and related risk factors of chronic kidney disease among adults in Luxembourg: evidence from the observation of cardiovascular risk factors (ORISCAV-LUX) study. BMC Nephrol. 2017; 18, 358. [CrossRef]
- Zdrojewski Ł, Zdrojewski T, Rutkowski M, Bandosz P, Król E, Wyrzykowski B, Rutkowski B. Prevalence of chronic kidney disease in a representative sample of the Polish population: results of the NATPOL 2011 survey. Nephrol Dial Transplant. 2016;31(3):433-9. [CrossRef]
- De Nicola L, Zoccali C. Chronic kidney disease prevalence in the general population: heterogeneity and concerns. Nephrology Dialysis Transplantation. 2016; 1;31(3):331-5. [CrossRef]
- Anand S, Shivashankar R, Ali MK et al. Prevalence of chronic kidney disease in two major Indian cities and projections for associated cardiovascular disease. Kidney Int 2015; 88: 178–185. [CrossRef]
- Hooi LS, Ong LM, Ahmad G et al. A population-based study measuring the prevalence of chronic kidney disease among adults in West Malaysia. Kidney Int 2013; 84: 1034–1040. [CrossRef]
- Ponte B, Pruijm M, Marques-Vidal P et al. Determinants and burden of chronic kidney disease in the population-based CoLaus study: a crosssectional analysis. Nephrol Dial Transplant 2013; 28: 2329–2339. [CrossRef]
- White SL, Polkinghorne KR, Atkins RC et al. Comparison of the prevalence and mortality risk of CKD in Australia using the CKD Epidemiology Collaboration (CKD-EPI) and Modification of Diet in Renal Disease (MDRD) Study GFR estimating equations: the AusDiab (Australian Diabetes, Obesity and Lifestyle) Study. Am J Kidney Dis 2010; 55: 660–670. [CrossRef]
- Fraser SD, Aitken G, Taal MW et al. Exploration of chronic kidney disease prevalence estimates using new measures of kidney function in the health survey for England. PLoS One 2015; 10: e0118676. [CrossRef]
- Arora P, Vasa P, Brenner D et al. Prevalence estimates of chronic kidney disease in Canada: results of a nationally representative survey. CMAJ 2013; 185: E417–E423. [CrossRef]
- Chen F, Yang W, Weng J, Jia W, Ji L, Xiao J, Shan Z, Liu J, Tian H, Ji Q, Zhu D, Ge J, Lin L, Chen L, Guo X, Zhao Z, Li Q, Zhou Z, Shan G, Lu J; China National Diabetes and Metabolic Disorders Study Group. Albuminuria: Prevalence, associated risk factors and relationship with cardiovascular disease. J Diabetes Investig. 2014;5(4):464-71. [CrossRef]
- Zacharias, J.M., Young, T.K., Riediger, N.D. et al. Prevalence, risk factors and awareness of albuminuria on a Canadian First Nation: A community-based screening study. BMC Public Health 2012; 12, 290. [CrossRef]
- J Reed III, N Kopyt. Prevalence of Albuminuria in the U.S. Adult Population Over the age of 40: Results from the National Health and Nutrition Examination Survey (1999-2008). The Internet Journal of Nephrology. 2009 Volume 6 Number 1.
- Atkins RC, Polkinghorne KR, Briganti EM, Shaw JE, Zimmet PZ, Chadban SJ. Prevalence of albuminuria in Australia: the AusDiab kidney study. Kidney International. 2004; 1;66:S22-4. [CrossRef]
- Tanaka S, Takase H, Dohi Y, Kimura G. The prevalence and characteristics of microalbuminuria in the general population: a cross-sectional study. BMC research notes. 2013;6:1-7. [CrossRef]
- Hounkpatin HO, Harris S, Fraser SDS, Day J, Mindell JS, Taal MW, O'Donoghue D, Roderick PJ. Prevalence of chronic kidney disease in adults in England: comparison of nationally representative cross-sectional surveys from 2003 to 2016. BMJ Open. 2020; 13;10(8):e038423. [CrossRef]
- Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl (2011). 2022;12(1):7-11. [CrossRef]
- Murton M, Goff-Leggett D, Bobrowska A, Garcia Sanchez JJ, James G, Wittbrodt E, Nolan S, Sörstadius E, Pecoits-Filho R, Tuttle K. Burden of Chronic Kidney Disease by KDIGO Categories of Glomerular Filtration Rate and Albuminuria: A Systematic Review. Adv Ther. 2021;38(1):180-200. [CrossRef]
- Nishikawa K, Takahashi K, Okutani T, Yamada R, Kinaga T, Matsumoto M, Yamamoto M. Risk of chronic kidney disease in non-obese individuals with clustering of metabolic factors: a longitudinal study. Intern Med. 2015;54(4):375-82. [CrossRef]
- Rashid I, Katravath P, Tiwari P, D’Cruz S, Jaswal S, Sahu G. Hyperuricemia—a serious complication among patients with chronic kidney disease: a systematic review and meta-analysis. Explor Med. 2022;3:249. [CrossRef]
- Kieneker L, Bakker S, de Boer R, Navis G, Gansevoort R, Joosten M. Low potassium excretion but not high sodium excretion is associated with increased risk of developing chronic kidney disease Kidney International 2016; 90: 888–896;. [CrossRef]
- Winitzki D, Zacharias HU, Nadal J, Baid-Agrawal S, Schaeffner E, Schmid M, Busch M, Bergmann MM, Schultheiss U, Kotsis F, Stockmann H, Meiselbach H, Wolf G, Krane V, Sommerer C, Eckardt KU, Schneider MP, Schlieper G, Floege J, Saritas T. Educational Attainment Is Associated With Kidney and Cardiovascular Outcomes in the German CKD (GCKD) Cohort. Kidney Int Rep. 2022; 14;7(5):1004-1015. [CrossRef]
- Neugarten J, Golestaneh L. Influence of Sex on the Progression of Chronic Kidney Disease. Mayo Clin Proc. 2019;94(7):1339-1356. [CrossRef]
- Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, Manoharan A, Steigerwalt S, Wright J, Rahman M, Rosas SE, Saunders M, Sharma K, Daviglus ML, Lash JP; CRIC Investigators. Sex-Related Disparities in CKD Progression. J Am Soc Nephrol. 2019;30(1):137-146. [CrossRef]
- Hecking M, Bieber BA, Ethier J, Kautzky-Willer A, Sunder-Plassmann G, Säemann MD, Ramirez SP, Gillespie BW, Pisoni RL, Robinson BM, Port FK. Sex-specific differences in hemodialysis prevalence and practices and the male-to-female mortality rate: the Dialysis Outcomes and Practice Patterns Study (DOPPS). PLoS Med. 2014 Oct 28;11(10):e1001750. [CrossRef]
- Iseki K, Nakai S, Shinzato T, Nagura Y, Akiba T; Patient Registration Committee of the Japanese Society for Dialysis Therapy. Increasing gender difference in the incidence of chronic dialysis therapy in Japan. Ther Apher Dial. 2005 Oct;9(5):407-11. [CrossRef]
- van der Burgh AC, Rizopoulos D, Ikram MA, Hoorn EJ, Chaker L. Determinants of the Evolution of Kidney Function With Age. Kidney Int Rep. 2021; 16;6(12):3054-3063. [CrossRef]
- Gorostidi M, Sánchez-Martínez M, Ruilope LM, Graciani A, de la Cruz JJ, Santamaría R, Del Pino MD, Guallar-Castillón P, de Álvaro F, Rodríguez-Artalejo F, Banegas JR. Chronic kidney disease in Spain: Prevalence and impact of accumulation of cardiovascular risk factors. Nefrologia (Engl Ed). 2018;38(6):606-615. English, Spanish. [CrossRef]
- Liu Q, Li Z, Wang H et al. High prevalence and associated risk factors for impaired renal function and urinary abnormalities in a rural adult population from southern China. PLoS One. 2012; 7: e47100. [CrossRef]
- https://www.theisn.org/wp-content/uploads/media/ISN%20Atlas_2023%20Digital_REV_2023_10_03.pdf accessed on 21st June 2024.
- Hommos MS, Glassock RJ, Rule AD. Structural and Functional Changes in Human Kidneys with Healthy Aging. J Am Soc Nephrol. 2017;28(10):2838-2844. [CrossRef]
- De Nicola L, Minutolo R, Chiodini P, et al. The effect of increasing age on the prognosis of non-dialysis patients with chronic kidney disease receiving stable nephrology care. Kidney Int. 2012;82:482-488. [CrossRef]
- Jelaković B, Pećin I, Lang VB, Braš M, Capak K, Jelaković A, Kralj V, Miličić D, Soldo A, Bubaš M. Improving blood pressure and dyslipidemia control by increasing health literacy in Croatia-missions 70/26 & Do you know what is your number. Blood Press. 2024 Dec;33(1):2371863. [CrossRef]
| Overall CKD (any KDIGO stage) |
CKD ≥ stage 3GA A2 |
CKD < 60 ml/min/1.73 m2 |
ACR > 30 mg/g |
|||||
|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| All | 17.1 | 8.0-15.0 | 9.8 | 6.6- 9.5 | 7.9 | 6.5- 9.5 | 15.1 | 7.0-15.2 |
| Men | 19.3 | 8.3-17.3 | 11.8 | 7.6-12.8 | 10.7 | 8.0-13.3 | 17.5 | 6.9-16.0 |
| Women | 14.9 | 7.1-11.5 | 7.9 | 5.2- 8.4 | 6.5 | 4.8- 8.1 | 13.5 | 6.5-10.7 |
| Albuminuria categories | ||||||||||
| A1 | A2 | A3 | ||||||||
| ACR <30 mg/g |
ACR 30-299 mg/g |
ACR ˃300 mg/g |
Total | |||||||
| eGFR categories (ml/min/1.73 m2 | G1 | ≥ 90 | crude weighted 95% CI |
55.4 45.4 43.1-47.8 |
9.8 8.1 6.7-9.6 |
0.2 0.2 0.002-0.4 |
65.4 53.7 |
|||
| G2 | 60-89 | crude weighted 95% CI |
16.9 13.3 11.6-15.0 |
4.3 3.6 2.6-4.5 |
0.5 0.4 0.1-0.8 |
21.7 17.3 |
||||
| G3A | 45-59 | crude weighted 95% CI |
5.9 4.9 3.8-6.0 |
2.3 1.9 1.2-2.7 |
0.4 0.3 0.04-0.6 |
8.6 7.1 |
||||
| G3B | 30-44 | crude weighted 95% CI |
3.5 2.0 0.6-5.0 |
0.2 0.2 0.02-0.4 |
0 |
3.7 2.2 |
||||
| G4 | 15-29 | crude weighted 95% CI |
0.1 0.1 0,05-0.3 |
0.2 0.2 0.02-0.4 |
0.07 0.06 0.006-0.2 |
0.38 0.36 |
||||
| G5 | <15 | crude weighted 95% CI |
0.07 0.06 0.002-0.02 |
0.07 0.06 0.006-0.12 |
0.08 0.07 0.006-0.2 |
0.22 0.19 |
||||
| crude weighted |
81.87 68.37 |
16.88 14.07 |
1.25 1.04 |
100 | ||||||
| CKD risk | % | estimated number | ||||||||
| crude | weighted | of Croatian adult population | ||||||||
| Low risk | 72.3 | 58.7 | 1,846.099 | |||||||
| Moderate risk | 20.0 | 16.6 | 522.065 | |||||||
| High risk | 6.5 | 4.5 | 141.523 | |||||||
| Very high risk | 1.1 | 1.1 | 35.594 | |||||||
| CKD ≥ G3A A1 | 12.8 | 9.8 | 311.441 | |||||||
| CKD population (N = 117) |
Non-CKD population (N = 1076) |
X2; | P | |||||
|---|---|---|---|---|---|---|---|---|
| Mean (SD) | 95% CI | Mean (SD) | 95% CI | |||||
| Median (IQR)# | Median (IQR)# | |||||||
| Age # | 72 (65-75) | 68.3 - 72.3 | 58 (46-75) | 55.34-56.9 | <0.001 | |||
| Gender (men) % | 49.6 | 40.7 – 58.5 | 33.8 | 31.1- 36.6 | 57.42; | <0.001 | ||
| Hypertension duration (years)# | 10 (5-18) | 10.7 - 16.2 | 9.0 (4.0-14.0) | 9.9-11.5 | 0.005 | |||
| Systolic BP (mmHg) # | 137 (125-151) | 134.8 - 144.4 | 131 (120-144) | 143.0-134.1 | 0.001 | |||
| Diastolic BP (mmHg) | 79.7 (11.3) | 77.3 - 82.1 | 82.6 (10.3) | 82.2-83.2 | 0.011 | |||
| Hypertension (yes) | 72.1 | 63.5 – 79.6 | 61.6 | 58.6-64.2 | NS | |||
| Treated controlled (yes) | 20.7 | 15.7-30.9 | 23.4 | 21.0-25.9 | ||||
| Untreated (yes) | 28.9 | 21.2-37.6 | 20.9 | 18.6-23.4 | 7.57; | 0.057 | ||
| Heart rate (bpm) # | 75.3 (16-83.6) | 74.2 - 80.4 | 75.0 (67-83) | 74.8-76.1 | 0.168 | |||
| Height (cm) # | 166 (160-175) | 169.1 - 173.5 | 168 (162.0-175) | 168.3-169.4 | 0.198 | |||
| Weight (kg) # | 82 (63-93) | 81.6 - 88.8 | 80 (69.0-91.4) | 80.4 - 82.4 | 0.191 | |||
| Body mass index (kg/m2) # | 29.3 (26.2-32.5) | 27.9 - 29.9 | 27.9 (24.7-31.3) | 28.1 - 28.7 | 0.008 | |||
| BMI category (kg/m2) % | ||||||||
| 25-29.9 | 35.3 | 26.8 - 44.6 | 39.0 | 36.1 - 41.9 | ||||
| 30.0-34.9 | 36.1 | 27.5 – 45.4 | 23.1 | 20.6 – 25.6 | 14.52; | 0.013 | ||
| 35.0-39.9 | 9.2 | 4.7 – 15.9 | 7.3 | 5.9 – 9.0 | ||||
| Waist circumference (cm) # | 103.5 (95.0-110.2) | 100.5-105.2 | 97.0(87.0-108.0) | 96.7-98.5 | <0.001 | |||
| WC pathologic # | 80.5 | 72.2-87.2 | 64.9 | 62.0-67.7 | 11.69; | 0.003 | ||
| Body surface area (m2) # | 1.95 (1.180-1.95) | 1.95 - 2.05 | 1.93 (1.77-2.09) | 1.93 - 1.96 | 0.426 | |||
| Smokers (yes) | 10.9 | 6.1 – 17.5 | 26.4 | 23.9 – 29.0 | 15.04; | <0.001 | ||
| Daily salt intake (g/day) # | 7.2 (5.0-12.4) | 8.1 - 10.3 | 8.4 (5.6-11.4) | 8.5 - 9.0 | 0.234 | |||
| Daily salt intake > 5 grams % | 24.4 | 17.1-33.0 | 19.0 | 16.8-21.4 | NS | |||
| ePWV (m/s) # | 12.5 (10.6-13.7) | 11.4 - 12.3 | 9.5 (7.9-11.2) | 9.5 - 9.8 | <0.001 | |||
| Monthly income (< 300 Eu) % | 42.6 | 34.0-51.6 | 30.2 | 27.6-32.9 | 14.45; | 0.006 | ||
| Family monthly income (< 300 Eu) % | 22.5 | 15.6-30.7 | 8.4 | 6.9-10.2 | 34,8; | 0.001 | ||
| Education (years) % | ||||||||
| No school | 0.8 | 1.1-6.0 | 0.8 | 0.4-1.5 | ||||
| < 4 | 3.1 | 0.7-9.4 | 1.2 | 0.7-2.0 | ||||
| 4-8 | 27.9 | 10.5-27.3 | 15.6 | 14.7-19.0 | 16.44; | 0.002 | ||
| 8-12 | 45.1 | 41.4-62.9 | 55.4 | 51.7-57.4 | ||||
| ≥12 | 21.7 | 16.9 -35.8 | 26.9 | 24.1-29.1 | ||||
| Professional qualification % | ||||||||
| No college | 38.0 | 19.1-39.3 | 21.1 | 20.1-24.8 | ||||
| College | 38.8 | 34.0-55.3 | 42.7 | 39.3-45.0 | 21.043; | <0.001 | ||
| Bachelor’s degree | 12.4 | 7.9-23.4 | 17.1 | 14.8-18.9 | ||||
| Master’s degree | 9.3 | 5.5-19.5 | 17.3 | 14.9-19.2 | ||||
| Stroke ischemic % | 4.7 | 1.7 – 9.8 | 2.4 | 1.6-3.4 | 6.358; | 0.042 | ||
| Stroke hemorrhagic % | 0.8 | 0.0 – 4.2 | 0.3 | 0.1-0.9 | NS | |||
| Myocardial infarction % | 3.1 | 1.3 - 7.7 | 2.5 | 1.7 – 3.6 | NS | |||
| Heart failure % | 3.9 | 1.3 – 8.8 | 0.8 | 0.3 – 1.4 | 13.06; | 0.001 | ||
| Atrial fibrillation % | 10.1 | 5.5 – 16.6 | 2.8 | 1.9 – 3.9 | 10.25; | 0.006 | ||
| Fasting blood glucose (mmol/l) | 5.4 (4.7-6.7) | 5.6 - 6.6 | 4.9 (4.4-5.2) | 5.0 - 5.2 | <0.001 | |||
| Diabetes % | 29.5 | 21.8 -38.1 | 14.0 | 12.1-16.1 | 36.40; | <0.001 | ||
| Urea (mmol/l) # | 7.5 (6.2-9.2) | 7.6 - 9.3 | 5.2 (4.4-6.2) | 5.3 - 5.5 | <0.001 | |||
| Serum creatinine (µmol/min) # | 110 (92.0-120.5) | 119.6-138.7 | 68.0 (61.0-79.0) | 69.5-71.0 | <0.001 | |||
| eGFR (ml/min/1.73 m2) # | 54.7 (44.3-58.0) | 45.8 - 50.6 | 92.2 (81.7-101.4) | 90.0- 91.8 | <0.001 | |||
| Uric acid (µmol/l) # | 360 (304-422.1) | 368.2 -411.7 | 280 (234-338) | 285.8-294.4 | <0.001 | |||
| Total cholesterol (mmol/l) # | 4.9 (4.1-5.9) | 4.7 - 5.2 | 5.3 (1.1) | 5.3 -5.4 | 0.003 | |||
| Triglycerides (mmol/l ) # | 1.5 (1.1-2.1) | 1.5 - 2.2 | 1.3 (0.9-1.9) | 1.5 - 1.6 | 0.009 | |||
| LDL cholesterol (mmol/l)# | 2.8 (2.1-3.5) | 2.6 - 3.0 | 3.1 (2.4-3.9) | 3.1 -3.2 | <0.001 | |||
| HDL cholesterol (mmol/l) # | 1.3 (1.1-1.5) | 1.2 - 1.4 | 1.4 (1.1-1.6) | 1.4 - 1.5 | 0.022 | |||
| Serum potassium (mmol/l) # | 4.7 (4.3-5.6) | 4.6 - 4.9 | 4.5 (4.3-4.8) | 4.5 - 4.6 | 0.001 | |||
| NT pro BNP (mmol/l) # | 152.2 (76-358) | 324.0-781.1 | 73 (41.0-127.2) | 109.8-134.1 | <0.001 | |||
| Hs Troponin I (mmol/l) # | 5.0 (5.0-5.0) | 5.74 - 13.7 | 5.0 (5.0-5.0) | 5.7 - 6.5 | <0.001 | |||
| ACR (mg/g) # | 17.3 (5.6-44.1) | 26.7-291.19 | 9.4 (4.6-20.8) | 22.6-32.2 | <0.001 | |||
| ACR category (mg/g) % | ||||||||
| 30-299 | 25.0 | 17.3-34.1 | 16.4 | 14.2-18.8 | ||||
| <300 | 7.1 | 3.1-13.6 | 1.0 | 0.5-1.8 | 31.37; | <0.001 | ||
| Coef (B) | S.E. | Odds Ratio Exp (B) |
95% CI Lower Upper |
P | ||
|---|---|---|---|---|---|---|
| Age (years) | -1.00 | 0.012 | 0.90 | 0.88 | 0.92 | <0.001 |
| Gender (men) | 1.680 | 0.243 | 5.36 | 3.33 | 8.63 | <0.001 |
| Family income < 300 Eu | -1.03 | 0.31 | 0.35 | 0.66 | 1.80 | 0.004 |
| Systolic BP (mmHg) | 0.810 | 0.339 | 2.23 | 1.15 | 4.36 | 0.017 |
| Hypertension (no) | 0.481 | 0.205 | 1.61 | 1.08 | 2.42 | 0.019 |
| Hypertension duration (years) | -0.026 | 0.011 | 0.97 | 0.95 | 0.99 | 0.023 |
| Stroke ischemic (yes) | 1.114 | 0.464 | 3.04 | 1.27 | 7.56 | 0.016 |
| Heart failure (yes) | 1.757 | 0.603 | 5.79 | 1.77 | 18.87 | 0.004 |
| Atrial fibrillation (yes) | 1.158 | 0.407 | 3.18 | 1.43 | 7.07 | 0.004 |
| Body mass index (kg/m2) | -0.035 | 0.018 | 0.96 | 0.93 | 0.99 | 0.045 |
| Waist circumference (cm) | -0.021 | 0.006 | 0.97 | 0.96 | 0.99 | <0.001 |
| PWV (m/s) | -0.362 | 0.141 | 0.65 | 0.52 | 0.91 | 0.01 |
| ePWV (m/s) | -0.431 | 0.053 | 0.65 | 0.58 | 0.72 | <0.001 |
| Fasting blood glucose (mmol/l) | -0.197 | 0.044 | 0.82 | 0.75 | 0.89 | <0.001 |
| Diabetes (yes) | 1.32 | 0.233 | 3.76 | 2.38 | 5.93 | <0.001 |
| Uric acid umol/l) | -0.012 | 0.001 | 0.98 | 0.98 | 0.99 | <0.001 |
| Serum creatinine (µmol/l) | -0.372 | 0.051 | 0.68 | 0.62 | 0.76 | <0,001 |
| Urea (mmol/l) | -0.248 | 0.047 | 0.79 | 0.72 | 0.86 | <0.001 |
| Total cholesterol (mmol/l) | 0.258 | 0.085 | 1.29 | 1.09 | 1.53 | 0.002 |
| HDL cholesterol (mmol/l) | 0.818 | 0.312 | 2.26 | 1.22 | 4.17 | 0.009 |
| LDL cholesterol (mmol/l) | 0.373 | 0.114 | 1.45 | 1.16 | 1.81 | 0.001 |
| Triglycerides (mmol/l) | -0.017 | 0,059 | 0.89 | 0.79 | 0.99 | 0.046 |
| Potassium (mmol/l) | -0.727 | 0.189 | 0.48 | 0.33 | 0.81 | <0.001 |
| NT pro BNP (mmol/l) | -0.002 | 0.001 | 0.99 | 0.99 | 0.99 | <0.001 |
| hs Troponin I (mmol/l) | -0.004 | 0.01 | 0.95 | 0.93 | 0.98 | <0.001 |
| ACR | -1.0640 | 0.201 | 0.34 | 0.23 | 0.51 | <0.001 |
| ACR 30-299 (mg/g) | -0.745 | 0.273 | 0.47 | 0.27 | 0.81 | 0.006 |
| ACR > 300 (mg/g) | -2.742 | 0.507 | 0.06 | 0.02 | 0.17 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





