Submitted:
28 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
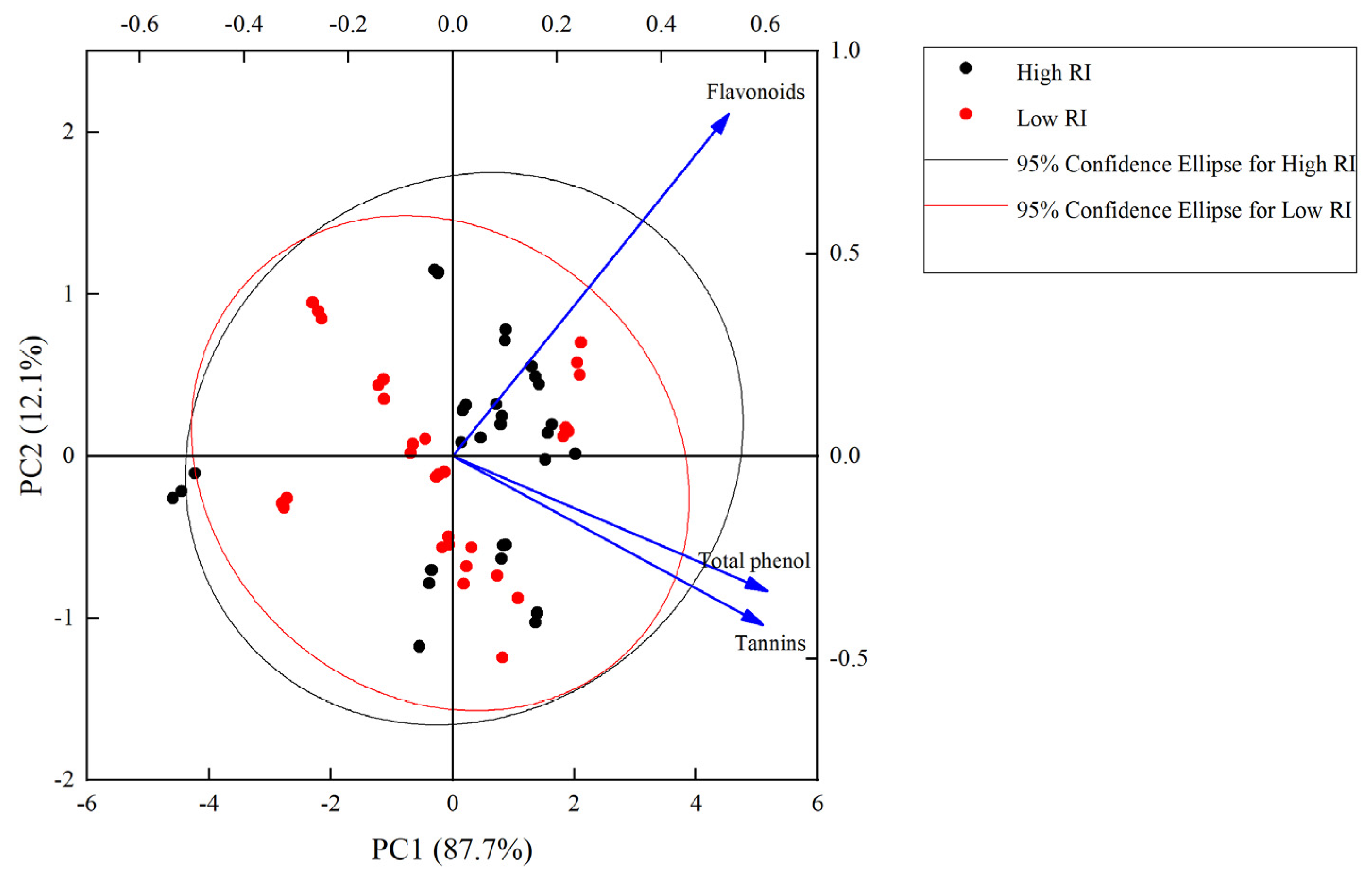
2. Results
3. Discussion
4. Materials and Methods
4.1. Selection of Species for Study
4.2. Collection of Plant Material
4.3. Preparation of Crude Extract for Phytochemical Analysis
4.4. Determination of the Total Phenolic (TPC) and Total Tannin Content (TTC)
4.5. Determination of Total Flavonoid Contents (TFC)
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velu, G.; Palanichamy, V.; Rajan, A.P. Phytochemical and Pharmacological Importance of Plant Secondary Metabolites in Modern Medicine. Bioorganic Phase in Natural Food: An Overview 2018, 135–156. [CrossRef]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A Concise Overview on the Chemistry, Occurrence, and Human Health. Phytotherapy Research 2019, 33, 2221–2243. [CrossRef]
- Hussein, R.A.; El-Anssary, A.A.; Hussein, R.A.; El-Anssary, A.A. Plants Secondary Metabolites: The Key Drivers of the Pharmacological Actions of Medicinal Plants. Herbal Medicine 2018. [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochemistry Reviews 2020, 19, 1199–1209. [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, Vol. 23, Page 762 2018, 23, 762. [CrossRef]
- Isah, T.; Isah, T. Stress and Defense Responses in Plant Secondary Metabolites Production. Biol Res 2019, 52, 39. [CrossRef]
- Pant, P.; Pandey, S.; Dall’Acqua, S. The Influence of Environmental Conditions on Secondary Metabolites in Medicinal Plants: A Literature Review. Chem Biodivers 2021, 18, e2100345. [CrossRef]
- Albuquerque, U.P.; Ramos, M.A.; Melo, J.G. New Strategies for Drug Discovery in Tropical Forests Based on Ethnobotanical and Chemical Ecological Studies. J Ethnopharmacol 2012, 140, 197–201. [CrossRef]
- Albuquerque, U.P.; de Brito, A.L.; do Nascimento, A.L.B.; de Oliveira, A.F.M.; Quixabeira, C.M.T.; de Queiroz Dias, D.; Lira, E.C.; Silva, F.S.; de Araújo Delmondes, G.; Coutinho, H.D.M. Medicinal Plants and Animals of an Important Seasonal Dry Forest in Brazil. Ethnobiology & Conservation 2020, 9.
- De Almeida, C.F.C.B.R.; De Lima E Silva, T.C.; De Amorim, E.L.C.; Maia, M.B.D.S.; De Albuquerque, U.P. Life Strategy and Chemical Composition as Predictors of the Selection of Medicinal Plants from the Caatinga (Northeast Brazil). J Arid Environ 2005, 62, 127–142. [CrossRef]
- de Sousa Araújo, T.A.; Alencar, N.L.; de Amorim, E.L.C.; de Albuquerque, U.P. A New Approach to Study Medicinal Plants with Tannins and Flavonoids Contents from the Local Knowledge. J Ethnopharmacol 2008, 120, 72–80. [CrossRef]
- Alencar, N.L.; Araújo, T.A. de S.; de Amorim, E.L.C.; de Albuquerque, U.P. Can the Apparency Hypothesis Explain the Selection of Medicinal Plants in an Area of Caatinga Vegetation? A Chemical Perspective. Acta Bot Brasilica 2009, 23, 911–911. [CrossRef]
- De Almeida, C.D.F.C.B.R.; Cavalcanti De Amorim, E.L.; De Albuquerque, U.P. Insights into the Search for New Drugs from Traditional Knowledge: An Ethnobotanical and Chemical–Ecological Perspective. http://dx‐doi.ez16.periodicos.capes.gov.br/10.3109/13880209.2010.551777 2011, 49, 864–873. [CrossRef]
- Monteiro, J.M.; Albuquerque, U.P.; Lins Neto, E.M.F.; Araújo, E.L.; Albuquerque, M.M.; Amorim, E.L.C. The Effects of Seasonal Climate Changes in the Caatinga on Tannin Levels in Myracrodruon Urundeuva (Engl.) Fr. All. and Anadenanthera Colubrina (Vell.) Brenan. Revista Brasileira de Farmacognosia 2006, 16, 338–344. [CrossRef]
- Monteiro, J.M.; Albuquerque, U.P. De; Lins-Neto, E.M.D.F.; Araújo, E.L. De; Amorim, E.L.C. De Use Patterns and Knowledge of Medicinal Species among Two Rural Communities in Brazil’s Semi-Arid Northeastern Region. J Ethnopharmacol 2006, 105, 173–186. [CrossRef]
- José, T.; Sobrinho, S.P.; Peixoto Sobrinho, T.J.S.; Castro, V.T.N.A.; Saraiva, A.M.; Almeida, D.M.; Tavares, E.A.; Amorim, E.L.C. Phenolic Content and Antioxidant Capacity of Four Cnidoscolus Species (Euphorbiaceae) Used as Ethnopharmacologicals in Caatinga, Brazil. Article in African Journal of Pharmacy and Pharmacology 2011, 5, 2310–2316. [CrossRef]
- Chaves, T.P.; Santana, C.P.; Véras, G.; Brandão, D.O.; Felismino, D.C.; Cláudia, A.; Medeiros, D.; De, D.M.; Trovão, B.M. Seasonal Variation in the Production of Secondary Metabolites and Antimicrobial Activity of Two Plant Species Used in Brazilian Traditional Medicine. Afr J Biotechnol 2013, 12, 847–853. [CrossRef]
- Amorim, E.L.C. de; Cruz, P.; Filho, J.V.; Silva, I.C.; Costa, U.; Oliveira, J.; Medeiros, M.S.; Diniz, M.; Machado, K.; Xavier, A.C.; et al. Brazilian Caatinga: Phenolic Contents, Industrial and Therapeutic Applications. 2021. [CrossRef]
- Author, A.; Bennett, B.C.; Prance, G.T. Introduced Plants in the Indigenous Pharmacopoeia of Northern South. Botany 54, 90–102.
- da Silva, A.C.O.; de Albuquerque, U.P. Woody Medicinal Plants of the Caatinga in the State of Pernambuco (Northeast Brazil). Acta Bot Brasilica 2005, 19, 17–26. [CrossRef]
- Biol Sci, B.J.; dos Santos Souza, A.; Paula Braz de Souza, A.; Farias Paiva de Lucena, R. Relative Importance of Medicinal Plants in the Semi-Arid Region of Paraíba: A Case Study in the Municipality of Congo (Paraíba, Northeast Brazil). Brazilian Journal of Biological Sciences 2016, 83–96. [CrossRef]
- de Albuquerque, U.P.; de Medeiros, P.M.; de Almeida, A.L.S.; Monteiro, J.M.; de Freitas Lins Neto, E.M.; de Melo, J.G.; dos Santos, J.P. Medicinal Plants of the Caatinga (Semi-Arid) Vegetation of NE Brazil: A Quantitative Approach. J Ethnopharmacol 2007, 114, 325–354. [CrossRef]
- Cartaxo, S.L.; de Almeida Souza, M.M.; de Albuquerque, U.P. Medicinal Plants with Bioprospecting Potential Used in Semi-Arid Northeastern Brazil. J Ethnopharmacol 2010, 131, 326–342. [CrossRef]
- Siqueira, C.F.D.Q.; Cabral, D.L.V.; Peixoto Sobrinho, T.J.D.S.; De Amorim, E.L.C.; De Melo, J.G.; Araújo, T.A.D.S.; De Albuquerque, U.P. Levels of Tannins and Flavonoids in Medicinal Plants: Evaluating Bioprospecting Strategies. Evidence-Based Complementary and Alternative Medicine 2012, 2012, 434782. [CrossRef]
- Ribeiro, D.A.; Macêdo, D.G.; Oliveira, L.G.S.; Saraiva, M.E.; Oliveira, S.F.; Souza, M.M.A.; Menezes, I.R.A. Potencial Terapêutico e Uso de Plantas Medicinais Em Uma Área de Caatinga No Estado Do Ceará, Nordeste Do Brasil. Revista Brasileira de Plantas Medicinais 2014, 16, 912–930. [CrossRef]
- Júnior, W.S.F.; Ladio, A.H.; Albuquerque, U.P. De Resilience and Adaptation in the Use of Medicinal Plants with Suspected Anti-Inflammatory Activity in the Brazilian Northeast. J Ethnopharmacol 2011, 138, 238–252. [CrossRef]
- Monteiro, J.M.; de Souza, J.S.N.; Lins Neto, E.M.F.; Scopel, K.; Trindade, E.F. Does Total Tannin Content Explain the Use Value of Spontaneous Medicinal Plants from the Brazilian Semi-Arid Region? Revista Brasileira de Farmacognosia 2014, 24, 116–123. [CrossRef]
- Campos, J.L.A.; Albuquerque, U.P. Indicators of Conservation Priorities for Medicinal Plants from Seasonal Dry Forests of Northeastern Brazil. Ecol Indic 2021, 121, 106993. [CrossRef]
- de Lucena, R.F.P.; Araújo, E. de L.; de Albuquerque, U.P. Does the Local Availability of Woody Caatinga Plants (Northeastern Brazil) Explain Their Use Value? Econ Bot 2007, 61, 347–361. [CrossRef]
- De Medeiros, P.M.; Ladio, A.H.; Albuquerque, U.P. Local Criteria for Medicinal Plant Selection. Evolutionary Ethnobiology 2015, 149–162. [CrossRef]
- Cosmo Andrade, J.; da Silva, A.R.P.; Audilene Freitas, M.; de Azevedo Ramos, B.; Sampaio Freitas, T.; de Assis G. dos Santos, F.; Leite-Andrade, M.C.; Nunes, M.; Relison Tintino, S.; da Silva, M.V.; et al. Control of Bacterial and Fungal Biofilms by Natural Products of Ziziphus Joazeiro Mart. (Rhamnaceae). Comp Immunol Microbiol Infect Dis 2019, 65, 226–233. [CrossRef]
- Andrade, J.C.; Silva, A.R.P.; Santos, A.T.L.; Freitas, M.A.; Carneiro, J.N.P.; Gonçalo, M.I.P.; de Souza, A.; Freitas, T.S.; Ribeiro, P.R.V.; Brito, E.S.; et al. UPLC-MS-ESI-QTOF Characterization and Evaluation of the Antibacterial and Modulatory Antibiotic Activity of Ziziphus Joazeiro Mart. Aqueous Extracts. South African Journal of Botany 2019, 123, 105–112. [CrossRef]
- Wink, M. Evolution of Secondary Metabolites in Legumes (Fabaceae). South African Journal of Botany 2013, 89, 164–175. [CrossRef]
- Gottlieb, O.R. Evolucao Quimica Vegetal. Cienc Cult 1987, 39, 357–360.
- Feeny, P. Chapter One PLANT APPARENCY AND CHEMICAL DEFENSE.
- Alvares, C.A.; Stape, J.L.; Sentelhas, P.C.; Leonardo, J.; Gonçalves, M.; Sparovek, G. Köppen’s Climate Classification Map for Brazil.. [CrossRef]
- Laurentino, M.; de Lima Araújo, E.; Ramos, M.A.; Cavalcanti, M.C.B.T.; Gonçalves, P.H.S.; Albuquerque, U.P. Socioeconomic and Ecological Indicators in Willingness to Accept Compensation for the Conservation of Medicinal Plants in a Tropical Dry Forest. Environ Dev Sustain 2022, 24, 4471–4489. [CrossRef]
- Rito, K.F.; Arroyo-Rodríguez, V.; Queiroz, R.T.; Leal, I.R.; Tabarelli, M. Precipitation Mediates the Effect of Human Disturbance on the Brazilian Caatinga Vegetation. Journal of Ecology 2017, 105, 828–838. [CrossRef]
- de Albuquerque, U.P. Re-Examining Hypotheses Concerning the Use and Knowledge of Medicinal Plants: A Study in the Caatinga Vegetation of NE Brazil. J Ethnobiol Ethnomed 2006, 2, 1–10. [CrossRef]
- Amorim, E.L.C. de; Castro, V.T.N. de A. de; Melo, J.G. de; Corrêa, A.J.C.; Sobrinho, T.J. da S.P.; Amorim, E.L.C. de; Castro, V.T.N. de A. de; Melo, J.G. de; Corrêa, A.J.C.; Sobrinho, T.J. da S.P. Standard Operating Procedures (SOP) for the Spectrophotometric Determination of Phenolic Compounds Contained in Plant Samples. Latest Research into Quality Control 2012. [CrossRef]
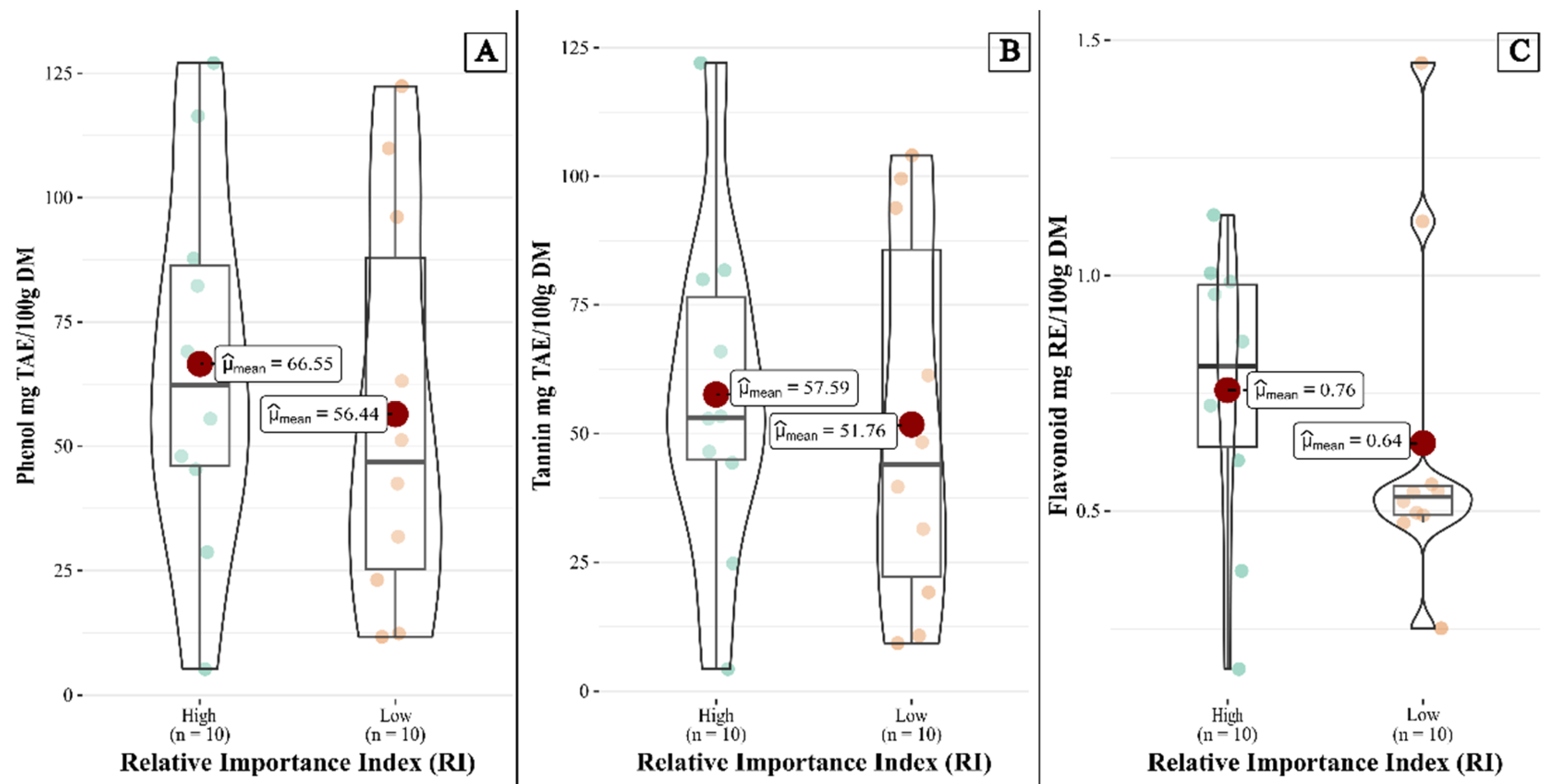
| Phenolic content | RI | Mean (± SD) | p (< 0.05) |
| Total phenols (mg TAE/100g DM) | Hight | 66.547 ± 37.955 | 0.5715 |
| Low | 56.440 ± 40.427 | ||
| Tannins (mg TAE/100g DM) | Hight | 57.586 ± 32.600 | 0.711 |
| Low | 51.760 ± 36.506 | ||
| Flavonoids (mg RE/100g DM) | Hight | 0.756 ± 0.303 | 0.457 |
| Low | 0.644 ± 0.357 |
| Species | RI | Total phenols (mg TAE/100g DM) | Tannins (mg TAE/100g DM) | Flavonoids (mg RE/100g DM) |
| Myracrodruon urundeuva | 1.94 | 116.423 ± 0.799 | 79.965 ± 1.832 | 1.005 ± 0.064 |
| Libidibia ferrea | 1.74 | 82.278 ± 0.921 | 81.741 ± 0.696 | 0.607 ± 0.018 |
| Anadenanthera colubrina | 1.69 | 127.122 ± 0.504 | 122.052 ± 0.488 | 0.756 ± 0.298 |
| Ximenia americana | 1.37 | 87.762 ± 3.393 | 65.960 ± 3.840 | 1.129 ± 0.010 |
| Handroanthus impetiginosus | 1.27 | 28.725 ± 0.475 | 24.786 ± 0.593 | 0.960 ± 0.006 |
| Anacardium occidentale | 1.26 | 69.062 ± 2.165 | 53.342 ± 2.943 | 0.860 ± 0.016 |
| Ziziphus joazeiro | 1.23 | 5.238 ± 0.199 | 4.295 ± 0.439 | 0.164 ± 0.015 |
| Hymenaea courbaril | 1.22 | 55.498 ± 0.577 | 52.884 ± 0.656 | 0.987 ± 0.211 |
| Sideroxylon obtusifolium | 1.18 | 48.000 ± 1.172 | 46.496 ± 1.395 | 0.373 ± 0.051 |
| Poincianella pyramidalis | 1.09 | 45.358 ± 1.038 | 44.340 ± 1.192 | 0.724 ± 0.047 |
| Aspidosperma pyrifolium | 0.92 | 23.151 ± 19.201 | 19.201 ± 0.626 | 0.540 ± 0.015 |
| Mimosa tenuiflora | 0.74 | 122.428 ± 3.022 | 99.549 ± 0.999 | 1.115 ± 0.023 |
| Commiphora leptophloeos | 0.72 | 31.837 ± 1.722 | 31.482 ± 1.772 | 0.542 ± 0.029 |
| Schinopsis brasiliensis | 0.62 | 109.924 ± 3.299 | 104.082 ± 3.282 | 1.452 ± 0.073 |
| Senegalia bahiensis | 0.36 | 11.727 ± 0.506 | 9.323 ± 0.601 | 0.496 ± 0.001 |
| Peltogyne pauciflora | 0.29 | 51.223 ± 1.278 | 48.343 ± 1.120 | 0.475 ± 0.014 |
| Piptadenia stipulacea | 0.21 | 12.385 ± 0.204 | 10.782 ± 0.164 | 0.251 ± 0.005 |
| Byrsonima gardneriana | 0.21 | 96.116 ± 12.011 | 93.856 ± 10.786 | 0.520 ± 0.064 |
| Erythroxylum revolutum | 0.15 | 42.498 ± 1.352 | 39.668 ± 1.281 | 0.556 ± 0.014 |
| Pityrocarpa moniliformis | 0.14 | 63.116 ± 0.517 | 61.310 ± 0.539 | 0.491 ± 0.034 |
| Family | Species | Popular name | Relative Importance Index (RI) |
| Anacardiaceae | Myracrodruon urundeuva Allemão | Aroeira | 1.94 |
| Fabaceae | Libidibia ferrea (Mart. ex Tul.) L.P.Queiroz | Pau-ferro | 1.74 |
| Fabaceae | Anadenanthera colubrina (Vell.) Brenan | Angico | 1.69 |
| Olacaceae | Ximenia americana L. | Ameixa-da-praia | 1.37 |
| Bignoniaceae | Handroanthus impetiginosus (Mart. ex DC.) Mattos | Ipê-roxo | 1.27 |
| Anacardiaceae | Anacardium occidentale L. | Cajueiro | 1.26 |
| Rhamnaceae | Ziziphus joazeiro Mart. | Juazeiro | 1.23 |
| Fabaceae | Hymenaea courbaril L. | Jatobá | 1.22 |
| Sapotaceae | Sideroxylon obtusifolium (Roem. & Schult.) T.D.Penn. | Quixaba | 1.18 |
| Fabaceae | Poincianella pyramidalis (Tul.) L.P.Queiroz | Caatingueira | 1.09 |
| Apocynaceae | Aspidosperma pyrifolium Mart. | Pereiro | 0.92 |
| Fabaceae | Mimosa tenuiflora (Willd.) Poir. | Jurema preta | 0.74 |
| Burseraceae | Commiphora leptophloeos (Mart.) J.B. Gillett | Amburana-de-cambão | 0.72 |
| Anacardiaceae | Schinopsis brasiliensis Engl. | Baraúna | 0.62 |
| Fabaceae | Senegalia bahiensis (Benth.) Seigler & Ebinger | Calumbi | 0.36 |
| Fabaceae | Peltogyne pauciflora Benth. | Pau-de morro | 0.29 |
| Fabaceae | Piptadenia stipulacea (Benth.) Ducke | Jurema branca | 0.21 |
| Malpighiaceae | Byrsonima gardneriana A.Juss. | Murici | 0.21 |
| Erythroxylaceae | Erythroxylum revolutum Mart. | Quebra-facão | 0.15 |
| Fabaceae | Pityrocarpa moniliformis (Benth.) Luckow & R.W. Jobson | Catanduva | 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





