Submitted:
27 August 2024
Posted:
29 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Methane Measurement
2.1.1. Figaro TGS Sensors
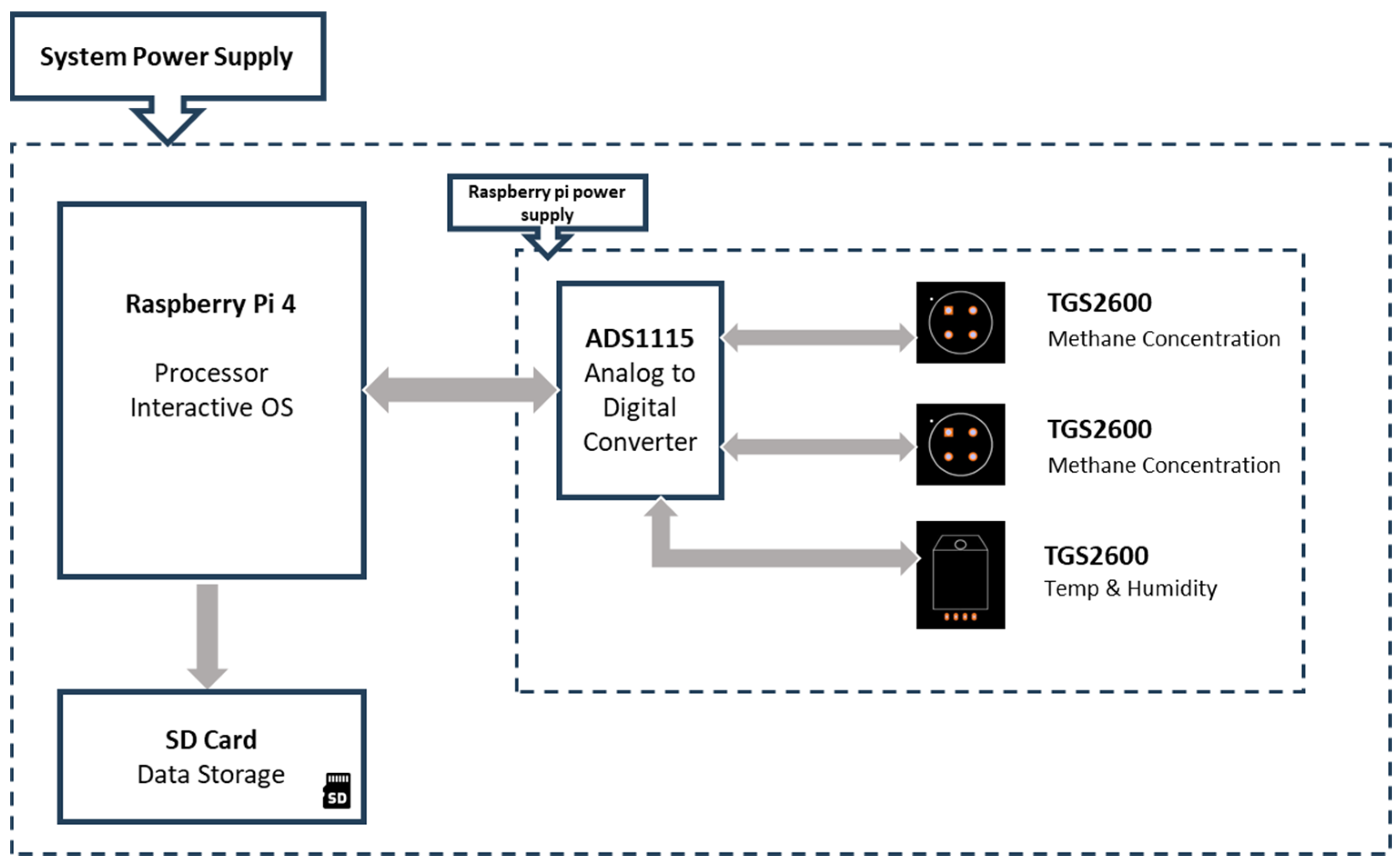
2.1.2. Reference instrument—Aeris MIRA Ultra Mobile LDS
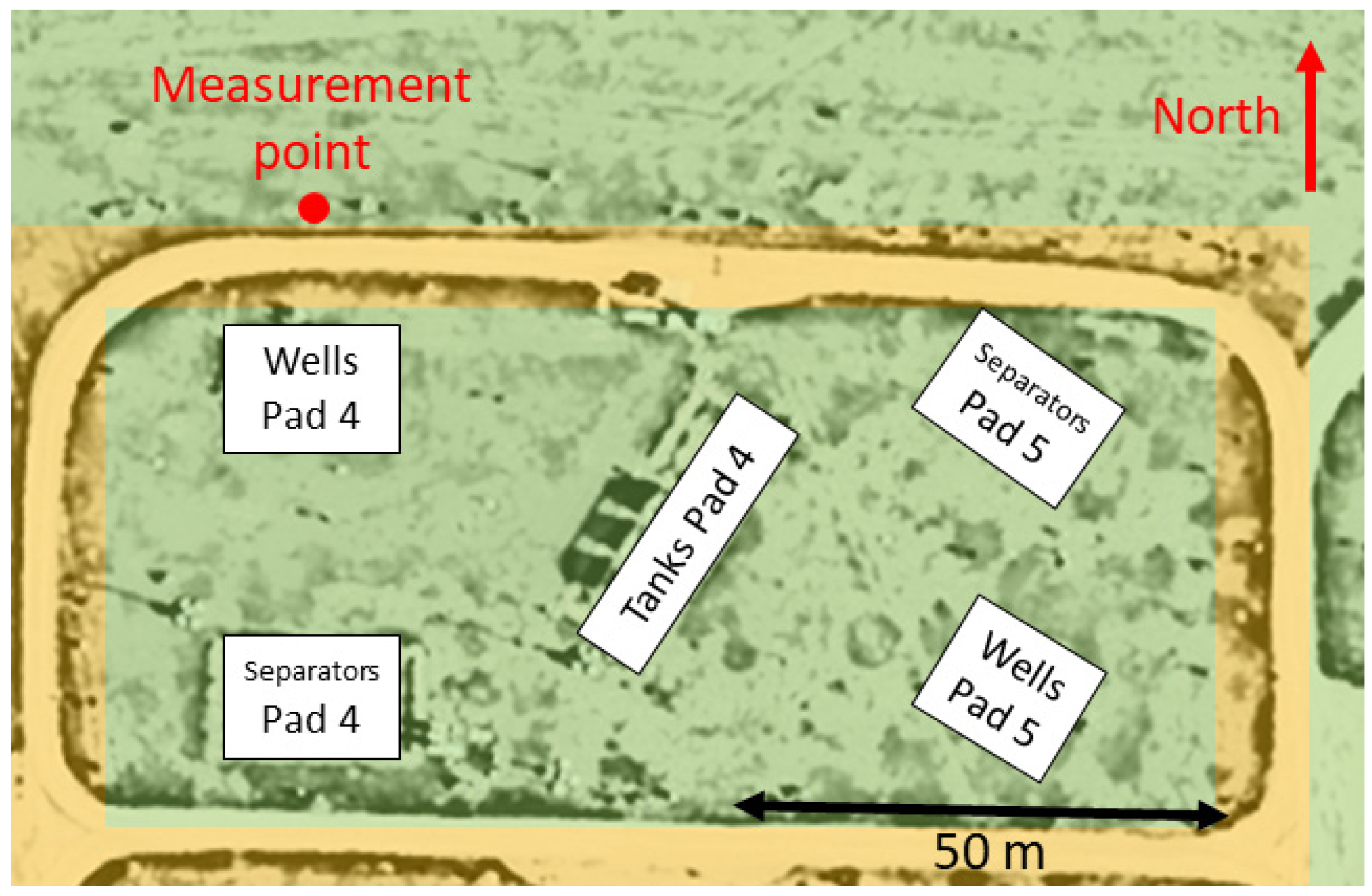
2.2. Controlled Methane Release Experiments
2.3. Eugster and Kling (2012) Metal Oxide Sensor Calibration
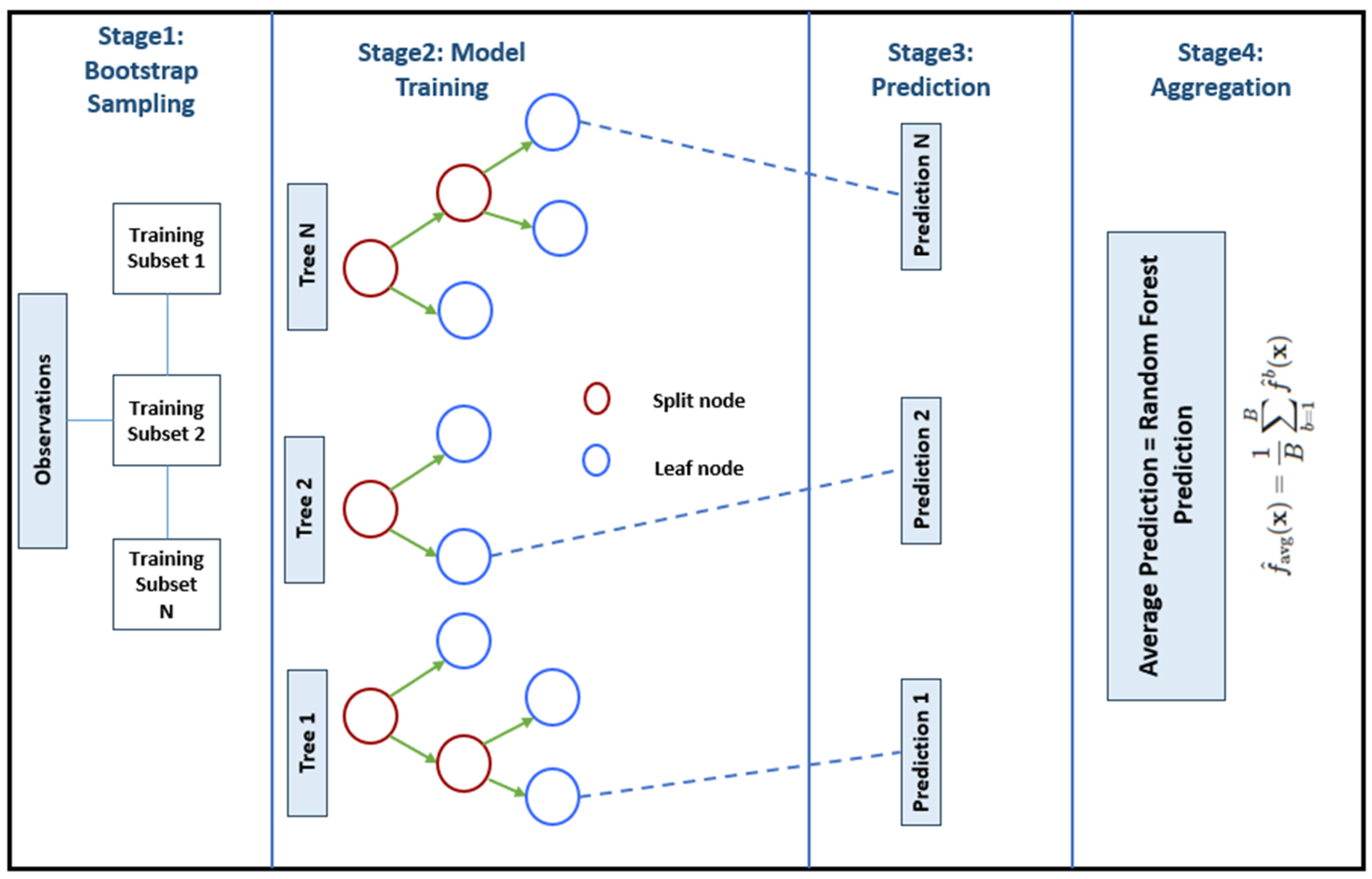
2.4. Machine Learning Calibration
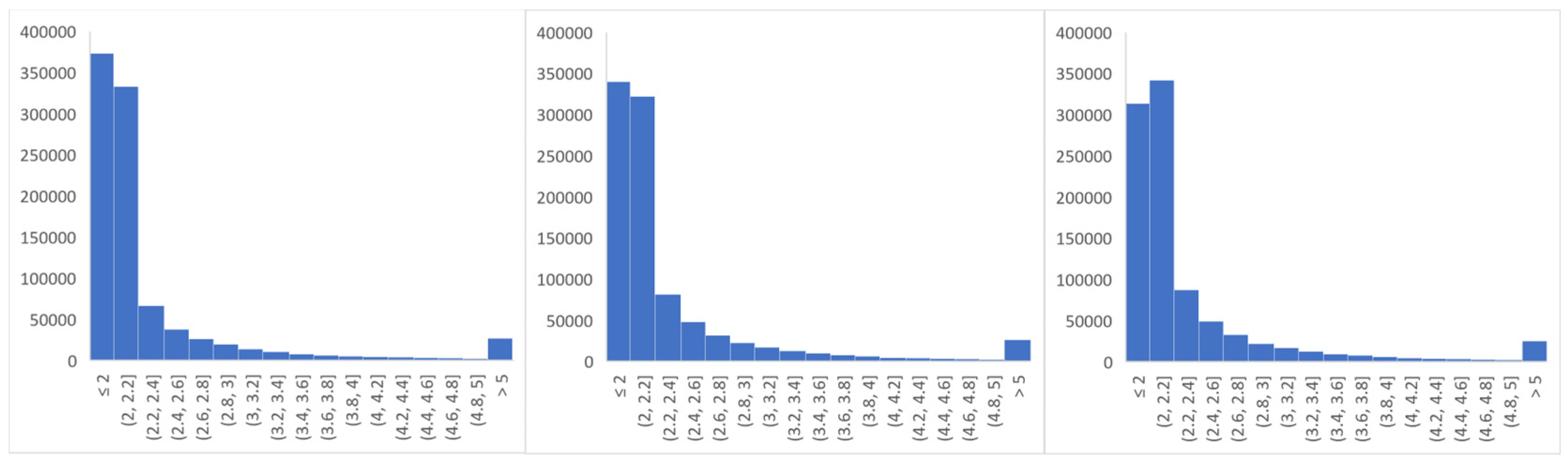
2.4.1. Data Preprocessing
2.4.2. Model Training
2.4.4. Model Evaluation Metrics
3. Results
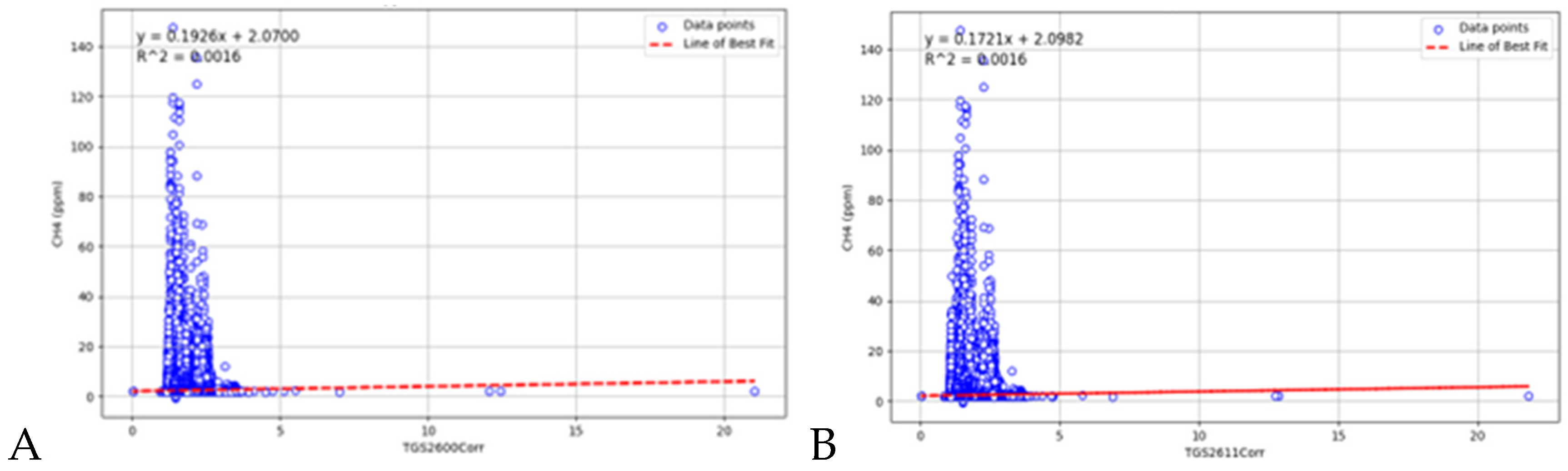
3.1. Eugster and Kling (2012) Calibration Method
3.1.1. Calibration Curves
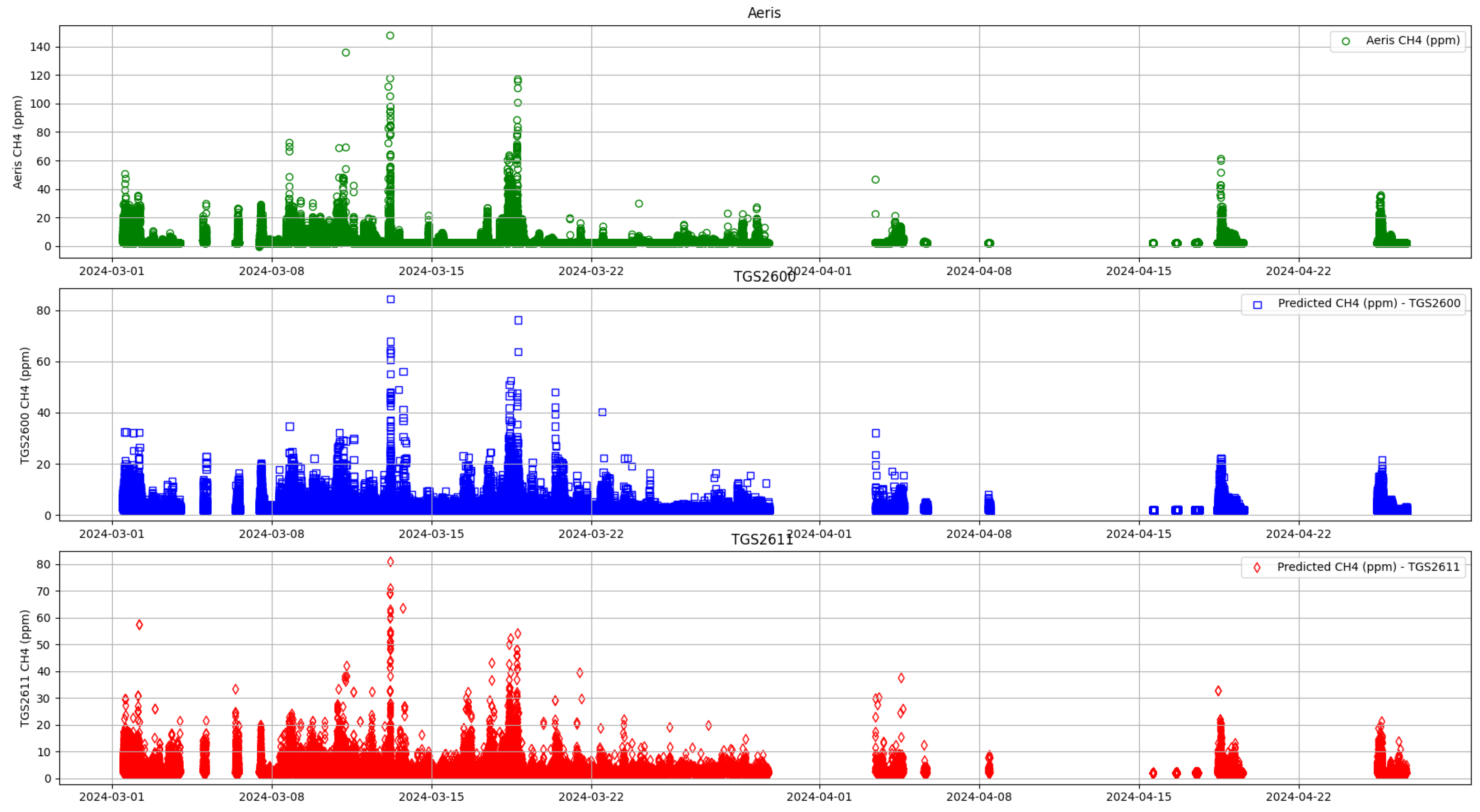
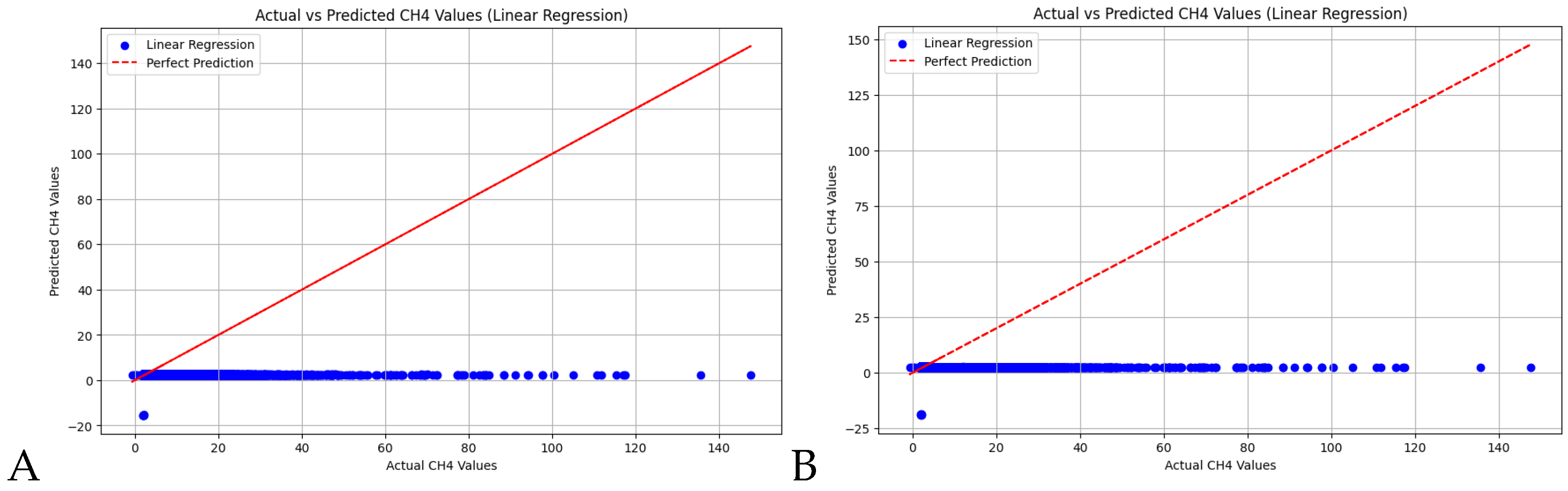
3.2. Machine Learning Methods
4. Discussion
4.1. Random Forest Calibration versus Linear Regression Calibration
4.2. Influence of Humidity & Temperature
4.3. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- IPCC Climate Change 2013 - The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, 2014. ISBN 978-1-107-41532-4.
- IPCC Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [H.-O. Pörtner, D.C. Roberts, M. Tignor, E.S. Poloczanska, K. Mintenbeck, A. Alegría, M. Craig, S. Langsdorf, S. Löschke, V. Möller, A. Okem, B. Rama (Eds.)].; Cambridge University Press.: Cambridge University Press, Cambridge, UK and New York, NY, USA, 2022. ISBN 978-1-00-932584-4.
- Global Methane Pledge Global Methane Pledge - Fast Action on Methane to Keep a 1.5°C Future within Reach. Available online: www.globalmethanepledge.org (accessed on 15 September 2022).
- UNFCCC Paris Agreement. United Nations Framework Convention on Climate Change. FCCC/CP/2015/L.9/Rev.1. Available online: https://unfccc.int/documents/9064 (accessed on 16 June 2023).
- UNEP/CCAC United Nations Environment Programme and Climate and Clean Air Coalition (2021). Global Methane Assessment: Benefits and Costs of Mitigating Methane Emissions. Nairobi: United Nations Environment Programme. Available online: https://www.ccacoalition.org/en/resources/global-methane-assessment-full-report (accessed on 25 October 2022).
- Bell, C.; Ilonze, C.; Duggan, A.; Zimmerle, D. Performance of Continuous Emission Monitoring Solutions under a Single-Blind Controlled Testing Protocol. Environ. Sci. Technol. 2023, 57, 5794–5805. [Google Scholar] [CrossRef] [PubMed]
- Caulton, D.R.; Lu, J.M.; Lane, H.M.; Buchholz, B.; Fitts, J.P.; Golston, L.M.; Guo, X.; Li, Q.; McSpiritt, J.; Pan, D.; et al. Importance of Superemitter Natural Gas Well Pads in the Marcellus Shale. Environ. Sci. Technol. 2019, 53, 4747–4754. [Google Scholar] [CrossRef] [PubMed]
- Riddick, S.N.; Cheptonui, F.; Yuan, K.; Mbua, M.; Day, R.; Vaughn, T.L.; Duggan, A.; Bennett, K.E.; Zimmerle, D.J. Estimating Regional Methane Emission Factors from Energy and Agricultural Sector Sources Using a Portable Measurement System: Case Study of the Denver–Julesburg Basin. Sensors 2022, 22, 7410. [Google Scholar] [CrossRef] [PubMed]
- Albertson, John.D.; Harvey, T.; Foderaro, G.; Zhu, P.; Zhou, X.; Ferrari, S.; Amin, M.S.; Modrak, M.; Brantley, H.; Thoma, E.D. A Mobile Sensing Approach for Regional Surveillance of Fugitive Methane Emissions in Oil and Gas Production. Environ. Sci. Technol. 2016, 50, 2487–2497. [CrossRef]
- GRI and EPA Harrison, M.R., Shires, T.M., Wessels, J.K., Cowgill, R. M. Methane Emissions from the Natural Gas Industry, Volumes 1 – 15, Final Report, GRI-94/0257 and EPA-600/R-96- 080, Gas Research Institute and US Environmental Protection Agency, June 1996. 1996.
- Campbell, L.M.; Campbell, M.V.; Epperson, D.L. Methane Emissions from the Natural Gas Industry, Volume 2: Technical Report, Final Report, GRI-94/0257.1 and EPA-600/R-96-080b. Gas Research Institute and U.S. Environmental Protection Agency. 1996.
- Riddick, S.N.; Mauzerall, D.L. Likely Substantial Underestimation of Reported Methane Emissions from United Kingdom Upstream Oil and Gas Activities. Energy Environ. Sci. 2023, 16, 295–304. [Google Scholar] [CrossRef]
- Caulton, D.R.; Li, Q.; Bou-Zeid, E.; Fitts, J.P.; Golston, L.M.; Pan, D.; Lu, J.; Lane, H.M.; Buchholz, B.; Guo, X.; et al. Quantifying Uncertainties from Mobile-Laboratory-Derived Emissions of Well Pads Using Inverse Gaussian Methods. Atmospheric Chem. Phys. 2018, 18, 15145–15168. [Google Scholar] [CrossRef]
- Peischl, J.; Eilerman, S.J.; Neuman, J.A.; Aikin, K.C.; de Gouw, J.; Gilman, J.B.; Herndon, S.C.; Nadkarni, R.; Trainer, M.; Warneke, C.; et al. Quantifying Methane and Ethane Emissions to the Atmosphere From Central and Western U.S. Oil and Natural Gas Production Regions. J. Geophys. Res. Atmospheres 2018. [CrossRef]
- Pétron, G.; Karion, A.; Sweeney, C.; Miller, B.R.; Montzka, S.A.; Frost, G.J.; Trainer, M.; Tans, P.; Andrews, A.; Kofler, J.; et al. A New Look at Methane and Nonmethane Hydrocarbon Emissions from Oil and Natural Gas Operations in the Colorado Denver-Julesburg Basin. J. Geophys. Res. Atmospheres 2014, 119, 6836–6852. [Google Scholar] [CrossRef]
- Barkley, Z.; Davis, K.; Miles, N.; Richardson, S.; Deng, A.; Hmiel, B.; Lyon, D.; Lauvaux, T. Quantification of Oil and Gas Methane Emissions in the Delaware and Marcellus Basins Using a Network of Continuous Tower-Based Measurements. Atmospheric Chem. Phys. 2023, 23, 6127–6144. [Google Scholar] [CrossRef]
- Riddick, S.N.; Ancona, R.; Cheptonui, F.; Bell, C.S.; Duggan, A.; Bennett, K.E.; Zimmerle, D.J. A Cautionary Report of Calculating Methane Emissions Using Low-Cost Fence-Line Sensors. Elem. Sci. Anthr. 2022, 10, 00021. [Google Scholar] [CrossRef]
- Cho, Y.; Smits, K.M.; Riddick, S.N.; Zimmerle, D.J. Calibration and Field Deployment of Low-Cost Sensor Network to Monitor Underground Pipeline Leakage. Sens. Actuators B Chem. 2022, 355, 131276. [Google Scholar] [CrossRef]
- Vaughn, T.L.; Bell, C.S.; Pickering, C.K.; Schwietzke, S.; Heath, G.A.; Pétron, G.; Zimmerle, D.J.; Schnell, R.C.; Nummedal, D. Temporal Variability Largely Explains Top-down/Bottom-up Difference in Methane Emission Estimates from a Natural Gas Production Region. Proc. Natl. Acad. Sci. 2018, 115, 11712–11717. [Google Scholar] [CrossRef]
- Riddick, S.N.; Mbua, M.; Santos, A.; Hartzell, W.; Zimmerle, D.J. Potential Underestimate in Reported Bottom-up Methane Emissions from Oil and Gas Operations in the Delaware Basin. Atmosphere 2024, 15, 202. [Google Scholar] [CrossRef]
- Bell, C.S.; Vaughn, T.L.; Zimmerle, D.; Herndon, S.C.; Yacovitch, T.I.; Heath, G.A.; Pétron, G.; Edie, R.; Field, R.A.; Murphy, S.M.; et al. Comparison of Methane Emission Estimates from Multiple Measurement Techniques at Natural Gas Production Pads. Elem Sci Anth 2017, 5, 79. [Google Scholar] [CrossRef]
- Bell, C.; Rutherford, J.; Brandt, A.; Sherwin, E.; Vaughn, T.; Zimmerle, D. Single-Blind Determination of Methane Detection Limits and Quantification Accuracy Using Aircraft-Based LiDAR. Elem. Sci. Anthr. 2022, 10, 00080. [Google Scholar] [CrossRef]
- Shah, A.; Laurent, O.; Lienhardt, L.; Broquet, G.; Rivera Martinez, R.; Allegrini, E.; Ciais, P. Characterising the Methane Gas and Environmental Response of the Figaro Taguchi Gas Sensor (TGS) 2611-E00. Atmospheric Meas. Tech. 2023, 16, 3391–3419. [Google Scholar] [CrossRef]
- Shah, A.; Laurent, O.; Broquet, G.; Philippon, C.; Kumar, P.; Allegrini, E.; Ciais, P. Determining Methane Mole Fraction at a Landfill Site Using the Figaro Taguchi Gas Sensor 2611-C00 and Wind Direction Measurements. Environ. Sci. Atmospheres 2024, 4, 362–386. [Google Scholar] [CrossRef]
- Eugster, W.; Kling, G.W. Performance of a Low-Cost Methane Sensor for Ambient Concentration Measurements in Preliminary Studies. Atmospheric Meas. Tech. 2012, 5, 1925–1934. [Google Scholar] [CrossRef]
- Eugster, W.; Laundre, J.; Eugster, J.; Kling, G.W. Long-Term Reliability of the Figaro TGS 2600 Solid-State Methane Sensor under Low-Arctic Conditions at Toolik Lake, Alaska. Atmospheric Meas. Tech. 2020, 13, 2681–2695. [Google Scholar] [CrossRef]
- Lin, J.J.Y.; Buehler, C.; Datta, A.; Gentner, D.R.; Koehler, K.; Zamora, M.L. Laboratory and Field Evaluation of a Low-Cost Methane Sensor and Key Environmental Factors for Sensor Calibration. Environ. Sci. Atmospheres 2023, 3, 683–694. [Google Scholar] [CrossRef]
- Nagahage, I.S.P.; Nagahage, E.A.A.D.; Fujino, T. Assessment of the Applicability of a Low-Cost Sensor–Based Methane Monitoring System for Continuous Multi-Channel Sampling. Environ. Monit. Assess. 2021, 193, 509. [Google Scholar] [CrossRef]
- Sugriwan, I.; Soesanto, O. Development of TGS2611 Methane Sensor and SHT11 Humidity and Temperature Sensor for Measuring Greenhouse Gas on Peatlands in South Kalimantan, Indonesia. J. Phys. Conf. Ser. 2017, 853, 012006. [Google Scholar] [CrossRef]
- Riddick, S.N.; Mauzerall, D.L.; Celia, M.; Allen, G.; Pitt, J.; Kang, M.; Riddick, J.C. The Calibration and Deployment of a Low-Cost Methane Sensor. Atmos. Environ. 2020, 230, 117440. [Google Scholar] [CrossRef]
- Figaro Production Information. TGS 2611 - for the Detection of Methane. Available online: Https://Www.Figarosensor.Com/Product/Docs/TGS%202611C00(1013).Pdf (accessed on 26 August 2021).
- Andrews, B.; Chakrabarti, A.; Dauphin, M.; Speck, A. Application of Machine Learning for Calibrating Gas Sensors for Methane Emissions Monitoring. Sensors 2023, 23, 9898. [Google Scholar] [CrossRef] [PubMed]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- MIRA Mobile Methane/Ethane Analyzers. Aeris Technol.
- Ellis, C. Hyperparameter Tuning in Random Forests. Available online: https://crunchingthedata.com/hyperparameter-tuning-in-random-forests/ (accessed on 13 August 2024).
- Furuta, D.; Sayahi, T.; Li, J.; Wilson, B.; Presto, A.A.; Li, J. Characterization of Inexpensive Metal Oxide Sensor Performance for Trace Methane Detection. Atmospheric Meas. Tech. 2022, 15, 5117–5128. [Google Scholar] [CrossRef]
- Collier-Oxandale, A.; Casey, J.G.; Piedrahita, R.; Ortega, J.; Halliday, H.; Johnston, J.; Hannigan, M.P. Assessing a Low-Cost Methane Sensor Quantification System for Use in Complex Rural and Urban Environments. Atmospheric Meas. Tech. 2018, 11, 3569–3594. [Google Scholar] [CrossRef]
| Metric | TGS2600-RF | TGS2611-RF |
|---|---|---|
| R-squared (R²) | 0.28 | 0.25 |
| Mean Absolute Error (MAE) | 0.33 | 0.36 |
| Mean Squared Error (MSE) | 2.19 | 2.28 |
| Root Mean Squared Error (RMSE) | 1.48 | 1.51 |
| Mean Absolute Percentage Error (MAPE) | 9.66 | 10.76 |
| Explained Variance Score | 0.28 | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





