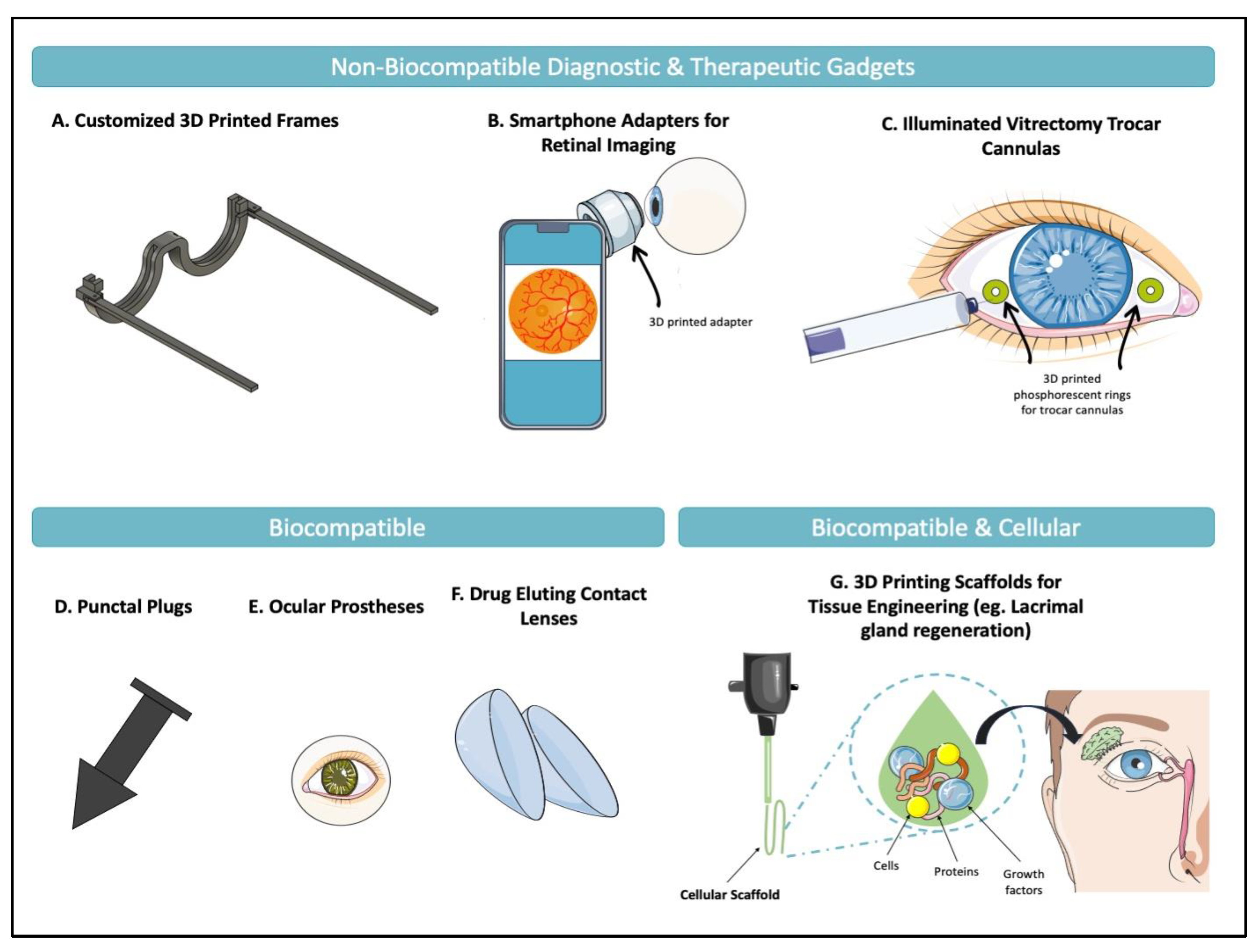
1. Introduction
In 2013, for the first time, physicians received the approval of the Food and Drug Administration (FDA) to create and place a customized, bioresorbable 3D printed tracheal splint in a newborn with life-threatening tracheobronchomalacia [
1,
2]. This revolutionary invention, approved through the emergency-use exemption, set a precedent for 3D printing in medicine. Since then, 3D printing has been increasingly gaining traction in medicine, including in ophthalmology. This traction has translated to significant developments over this past decade. For instance, recently, clinical trials of 3D-printed custom ocular prostheses have been completed (NCT05093348,
https://clinicaltrials.gov) [
3]; elsewhere the first artificial corneal tissue was bioprinted in vitro [
4].
3D printing continues to offer solutions to increasingly complex problems in ophthalmology. At the simplest level, it has allowed for the printing of non-biocompatible objects including customizable eyewear [
5,
6], adapters that enable smartphone-based retinal imaging [
7,
8], and surgical tools [
9,
10,
11]. Increasing in complexity, 3D printing uses biocompatible materials for the design of medical devices such as ocular and facial prostheses to minimize the immunogenic response following implantation. Finally, in its most advanced form to date, 3D printing involves using both cellular and acellular biomaterials to generate scaffolds that promote tissue regeneration [
12].
Figure 1 presents applications examples of 3D printing in ophthalmology that have been suggested in the literature.
These advancements have not only facilitated growth in the field but have also provided a prospect for alleviating some barriers to accessing eye care. Even in its simplest form, where 3D printing is limited to creating inanimate, non-biocompatible objects such as a smartphone fundus imaging adapter, it can create a dramatic difference to resource-stricken regions that do not have access to traditional desktop fundus cameras. The difference is stark – an open source 3D printed adapter may be manufactured for
$13, while traditional clinic fundus cameras can cost between
$10 000 to
$14 000 [
13]. Another prominent example of how 3D printing can drastically improve access to eye care is in corneal transplantation. Corneal transplants can restore sight to those living with corneal blindness from a variety of underlying etiologies including infectious, inflammatory, traumatic, or congenital causes. However, in a recent international survey of corneal transplantation and eye banking, there was a significant shortage of corneal graft tissue worldwide [
14]. Globally, the authors estimated that only 1 cornea is available for every 70 needed, leaving 12.7 million people awaiting corneal transplants [
14,
15]. Unfortunately, these shortages are most severe in the least developed countries [
16] due to a lack of eye banking infrastructure and a lack of donations possibly due to cultural attitudes or religious beliefs [
14,
15]. Corneal shortages are a complex multi-factorial problem with economic, political, cultural, and ethical barriers [
17,
18]. Regardless of the underlying causes, this shortage demands action and a multifaceted solution. In addition to encouraging donations and creating the necessary infrastructure, complementary solutions such as corneal bioengineering and bioprinting have been suggested as a potential means to alleviate the shortages [
14].
In the following sections, a diverse selection of papers is reviewed, with a focus on the newest advancements related to 3D printing in ophthalmology. Specifically, this review highlights the inventions with clinical potential and discusses how these advance the field and potentially bridge many of the disparities in access.
2. Brief Review of the Mechanisms of 3D Printing
3D printing is a method of additive manufacturing where structures are created through layer-by-layer deposition of materials. Additive manufacturing is differentiated from traditional subtractive manufacturing in that the latter takes away material from a preset block to create the desired structure [
19]. Regardless, both techniques rely on computer-aided design (CAD) to generate and guide the sequential manufacturing process.
The first step of 3D printing is to generate a digital model of the final product [
20]. This model is either designed through CAD or obtained by scanning the object. Typically, creating models through CAD is time-consuming and requires an experienced designer with knowledge of the software. Additionally, it can be difficult to replicate designs of natural anatomical structures, with their nuanced geometry. Consequently, to overcome these challenges, 3D data is now often generated by obtaining 3D scans or through other imaging modalities including CT or MRI [
21]. Through these methods, it is possible to obtain accurate data on the topography of the scanned structure; however, these scans typically require further segmentation or refinement to obtain the final model. Once the model is created, the next step is to generate a toolpath for the printer to follow. This toolpath is a point-by-point instruction guiding the printer to create the desired model. It takes into consideration many input parameters including the printing speed, the desired quality, and the printing material. Most printers have an associated software that can generate these instructions in the form of a “GCODE” file that is uploaded to the 3D printer. At this stage, the printer is set up with the appropriate material, and the print is started. Upon completion, the final product undergoes some postprocessing, which is highly dependent on the type of printer used. Many 3D printers have been applied in medicine, which have been detailed in several reviews, including the advantages and disadvantages of each [
22,
23].
3. 3D Printing in Ophthalmology
In the following sections we present the current progress and applications of 3D printing in various subspecialities and areas within ophthalmology.
3.1. Cornea
3D bioprinting is an innovative process for creating artificial organs and tissues by using additive manufacturing to provide precise and intricate arrangements of cells and tissue structures. Years of research and development have led to the current advancements of bioprinting corneal tissue. Corneal bioprinting aims to manufacture a biomimetic scaffold that supports and integrates seeded corneal cells to regenerate [
12,
24]. However, manufacturing of artificial corneas requires a deep understanding of the basic anatomy, fundamental physiological properties, and cellular components of this unique and intricate structure.
The cornea, though small, is remarkably complex. Its morphology is dome-shaped, extending 11–12 mm in diameter and having a central thickness of approximately 560 μm [
25]. The cornea is composed of cellular and acellular components. Corneal keratocytes are the most abundant of the cellular components and collagen is the most abundant acellular component, which comprises 70% of the entire corneal mass. Other constituents include glycosaminoglycans [
25]. The cornea consists of six distinct layers – epithelium, Bowman’s membrane, stroma, pre-Descemet or Duas layer [
26,
27], Descemet’s layer, and endothelium. These structural elements and layers provide the cornea with its biomechanical properties and give rise to its functional qualities. The distinct arrangement of collagen lamellae in the corneal stroma and the complex composition of the extracellular matrix (ECM) with its glycosaminoglycans, for instance, are what provide the cornea with clarity and mechanical strength. A deviation in the number of these ECM glycosaminoglycans and distortion of the collagen fibers is then the underlying pathophysiology associated with keratoconus – the condition that in itself is the leading cause of corneal transplantation [
12]. Similarly, the cornea’s symmetric curvature is fundamental to effectively transmit light and contributes to approximately two-thirds of the total eye refractive power [
28,
29].
From this simplified overview of the corneal microstructure and physiology, one can begin to appreciate the complexity of the cornea, and learn of the intricacies that arise when attempting to bioprinting corneal tissue. The creation of a biocompatible, mechanically stable, optically transparent scaffold that is capable of encapsulating corneal cells and promoting their growth, migration, proliferation, and functional expression, is undeniably difficult. Nevertheless, over the years, the field has advanced, overcoming many of these challenges; one main area of advancement is bioink development.
3.1.1. Bioink in Corneal Applications
A key component of the bioprinting process that dictates the functional and biomechanical properties of the manufactured cornea is the bioink. A bioink is a combination of biomaterial and living cells which, when deposited or crosslinked in the process of 3D bioprinting, creates the desired design [
30]. Common biomaterials, which have been utilized in corneal bioprinting include both natural and synthetic materials. The natural biomaterials collagen, gelatin, chitosan, decellularized extracellular matrix (dECM), alginate, and hyaluronic acid (HA) are the most commonly studied [
16]. These materials with their advantages and disadvantages, and relevant studies relating them to corneal bioprinting have been summarized in
Table 1.
As can be seen in
Table 1, often bioinks are formed through a combination of several complementary materials to overcome the disadvantages of one biomaterial. In a study by Wu et al., the authors bioprinted human corneal epithelial cells using a combination of collagen/gelatin/alginate incubated in a medium of sodium citrate [
35]. While such significant modifications and mixtures of materials may lead one to conclude that the design is too complex for real-life manufacturing and clinical translation, some industries have demonstrated otherwise. For instance, in the clothing industry, textiles have evolved over the past decades to accommodate a variety of functional considerations including improved antibacterial properties, durability, UV resistance, hydrophobicity, and flame-resistant properties [
51]. To achieve these functions, clothing produced globally is most often now, made of blended fabrics that combine several materials. Undoubtedly, such significant strides require time, research, and extensive trial-and-error, which, are currently underway for corneal bioprinting bioinks.
Optimizing the bioink, in the context of manufacturing artificial corneas, involves accounting for physical properties, rheological characteristics, and biological factors. In terms of the physical properties, the material ought to provide sufficient mechanical strength and optical transparency. Specifically, the structure should be capable of withstanding an intraocular pressure, at least up to 21 mmHg, and ideally have a tolerance for higher pressures. In studies, these mechanical properties are typically assessed by comparing the stress-strain curves of the material with that of the cornea [
12]. Often, with the naturally occurring materials mentioned in
Table 1, one of the common disadvantages is their weak mechanical properties. To overcome this, and thereby allow scaffolds to support themselves, researchers have identified that adding sodium alginate imparts mechanical strength when combined with other materials [
34]. When studying the optical properties, usually, the refractive index, transmittance assessments at various wavelengths, and qualitative analyses are performed [
12]. These properties have been thoroughly researched in the field, and combinations of materials have been used to optimize the transparency, including the use of GelMA to optimize the optical properties of dECM [
44]. Concerning the rheological characteristics of scaffolds, the bioink must be adjusted for gelation time and shear thinning. Both gelation and shear thinning viscosity properties are essential variables to control to ensure that the bioink is suitable for the printing process. Not only do the intrinsic properties of the material matter, but the selection of the 3D printing method and specific printer is equally important to ensure the desired outcomes. Finally, from a biological perspective, the bioink must be biocompatible, biodegradable, and have sufficient permeability to oxygen and nutrients [
16]. Biocompatibility involves ensuring that the material does not provoke an immunologic reaction and that it is nontoxic for the laden cells. Further, the material should encourage high cell viability consequently allowing the expression of proteins; often, to achieve high viability materials ought to be permeable. With every material used, biocompatibility is tested with the seedings of cells and serial assessments of cell viability.
3.1.2. Challenges and Future Prospects in Corneal Bioprinting
Clinical translation of biomimetic corneal tissue requires overcoming a few challenges and traversing through the regulatory pathways. One of the challenges encountered by researchers thus far is the bioprinting of several layers of the cornea. Most of the studies in the field have focused, unsurprisingly, on bioprinting the stromal layer, given that it is the thickest of the corneal layers. Some studies have examined epithelial bioprinting, but only a limited number of studies have assessed the potential of endothelial bioprinting [
52,
53,
54]. Unfortunately, since the endothelium remains a clinical challenge given its lack of regenerative capacity, further research in this area is essential and should be pursued. Additionally, it would be beneficial for researchers to continue examining the potential of bioprinting bilayers, such as the epithelium and stroma, which have been previously studied in a few instances [
24,
31,
55]. While current bioprinting would not meet the needs of penetrating keratoplasty (full thickness graft), having the capacity to bioprint one of the bilayers would allow us to supply tissue for DALK (Deep Anterior Lamellar Keratoplasty) which transplants all but the inner layers.
Focusing on the corneal stroma, one of the main challenges encountered in the bioprinting process is replicating the stroma’s highly ordered structure, which is crucial for its biomechanical and optical properties [
56]. Often, when cultured under standard conditions, corneal cells can lose their original phenotype and take on a fibroblastic phenotype, leading to a disorganized ECM that is similarly seen with corneal scarring. Further, when corneal cells are cultured, they often lose their ability to produce cornea-like ECM after the cell population doubles [
57]. In a study by Gouveia et al. (2019), the authors demonstrated that by regulating topographical cues in vitro, corneal stromal fibroblasts can be guided to align in a manner like their native structure [
58]. This study provides a possible path forward, and further research should focus on expanding the field’s understanding of these topographical features. Along with understanding the organization of the ECM, it is important to continue studying the incorporation of other cells and acellular components in the corneal scaffold. For instance, the cornea includes many immune cells and serves an important protective role. However, this has not yet been studied in the literature. Additional work is necessary to ensure that while replicating the cornea, all its fundamental functions are considered.
Finally, one of the main challenges to clinical translation is ensuring that the final product is compatible with regulatory pathways including appropriate sterilization techniques, storage methods, and appropriate shelf life [
12]. From a review of the literature, there is evidence to suggest that clinical testing and translation is on the horizon. This is evidenced by the copious amount of research in the field that has demonstrated significant progress in optimizing bioinks and bioprinting techniques, combined with a strong understanding of the natural cornea, and ongoing establishment of regulatory pathways [
12]. Further, some of the previously reported challenges including the printing time associated with 3D bioprinting have already been addressed, at least partially. For instance, in an experimental study, scientists developed a support scaffold that facilitates the printing of 6–12 corneas at a time, by using a combination of stereolithography printing, extrusion based printing, and micro-transfer molding techniques [
34]. The field has demonstrated clear growth over the past decade and should continue to be driven with a purpose – to alleviate the shortages of corneal transplants worldwide, and to thus, enable restoration of vision to those who are currently living with corneal blindness.
3.2. Oculoplastics
3.2.1. Ocular Prosthetics
An ocular prosthesis is an artificial substitute for an enucleated eyeball. Ocular prosthetics are an opportunity to restore function and symmetry to anophthalmic patient. The most common etiologies of enucleation and evisceration are traumatic in nature, thus, making young people often the affected demographic [
59]. Reproducing the pigmented human iris with its intricacies and manufacturing an implant that replaces the orbital volume is challenging [
60]. Historically, prostheses have been produced through two main methods: 1) ready-made stock shells or 2) custom design. Of the two methods, custom ocular prostheses are better fitting, increase the movement of the eyeball, and provide better cosmetic outcomes [
61]. Unfortunately, the fabrication of custom prostheses is time-consuming, labor-intensive, costly, and involves reliance on the experience of the ocularist [
3,
62]. These challenges undoubtedly form barriers to those living in developing countries where the health care system may already be fragile. To overcome some of these barriers, 3D printing has been implemented to create a streamlined, automated, and affordable option. An example of the success of using 3D printing for creating personalized medical aids has been its use in creating hand and arm prosthetics for patients in a rural area of Sierra Leone, a country that ranked 184 of 187 on the UN development index [
63,
64]. Through international collaboration and by harvesting the most advanced technology from around the globe, a feasibility study demonstrated that 3D printing is suitable for creating customized, 3D-printed arm prostheses in resource-stricken areas [
63]. A follow up study demonstrated an increase in the health-related quality of life with the use of these prostheses [
64].
The first documented case of a 3D-printed ocular prosthetic being designed and fitted to a patient occurred in 2016 [
65]. Since then, with the increasing emphasis on research [
60,
66,
67,
68], the field has made significant developments. First, researchers created pathways that incorporated 3D printing of prostheses while maintaining a dependence on manual painting by an ocularist [
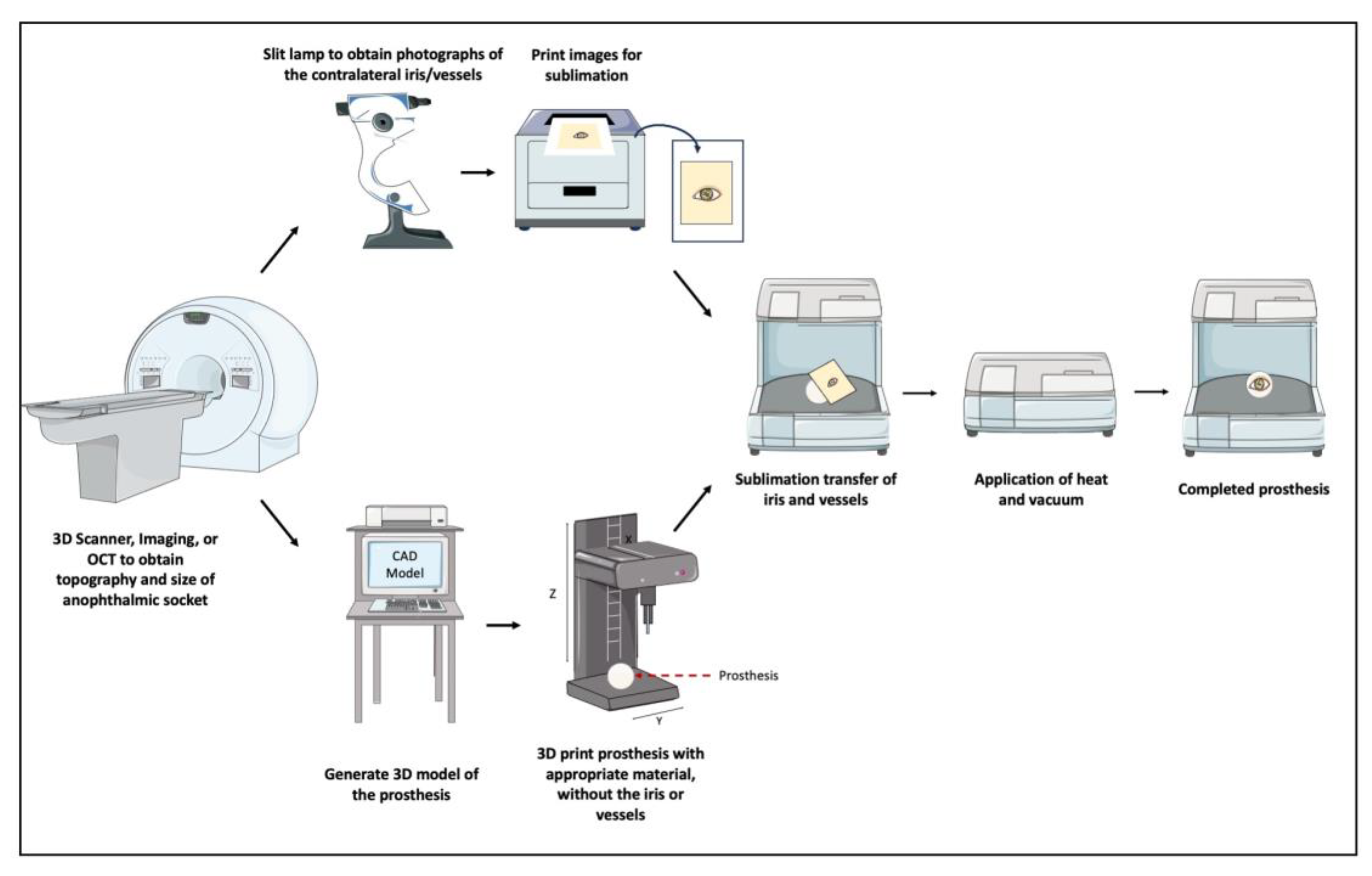
69]. Then, the process evolved to a semi-automated one, where, after obtaining an impression of the anophthalmic socket, a 3D scanner is used to obtain 3D model data of the shape of the eye, which is 3D printed without the iris or vessels. In conjunction, a slit lamp biomicroscope is used to obtain photographs of the contralateral iris/vessels, and the mirrored images were transferred onto the 3D printed model via a sublimation process [
70]. This process is demonstrated in
Figure 2. Finally, and most recently, researchers developed a fully automated digital end-to-end 3D printing pathway for creating a customized prosthesis [
3], a process that was recently studied in clinical trials (NCT05093348,
https://clinicaltrials.gov). In this process, image data from the contralateral eye is obtained by using an anterior segment optical coherence tomography device (OCT). Then, using an automatic data-driven design software, an appropriate prosthesis shape is determined, and a textured 3D model is generated. Finally, a multi-material full-color 3D printer is used to create the prosthetic [
3].
The latest 3D printed devices have demonstrated several advantages. Firstly, the material used for manufacturing these prostheses has been assessed for biocompatibility and revealed no evidence of toxicity, per the stringent criteria of ISO 10993 [
3,
71]. Secondly, 3D printing, when implemented through an automated process, can accurately replicate the color and anatomy of the adjacent eye, providing patients with the hope of restoring symmetry and promoting comfort [
3]. Thirdly, the full automation of the process minimizes labor time and is always reproducible. Given that OCT images are now being introduced into the process, it eliminates the need for impressions [
3]. This is important because impressions were needed in the traditional customized method, were time consuming, and uncomfortable for the patient. All these advantages can serve to reduce the barriers to access including cost associated with the customization and ocularist labor and the multiple visits needed to appropriately fit a prosthesis. While weighing these significant advantages, the main limitation of this design and process is the affordability [
3]. To truly make this product affordable for patients, these prostheses must be manufactured in larger quantities – a problem that is common to many industries. Scaling this product allows companies to find areas of optimization and reduce their overall costs, making such products affordable for the end user.
3.2.2. Facial & Orbital Implants
Restoring the anatomical structure of the face and orbit after a fracture is notably difficult and can unfortunately lead to problems such as diplopia and enophthalmos. While ocular prostheses are limited to mimicking the shape and function of the globe, orbital prostheses typically involve the reconstruction of the surrounding bone structure(s) and can be more complex. The application for 3D printing in orbital prosthesis has seen significant growth over the past few years, with ongoing clinical translational efforts [
72,
73,
74,
75,
76,
77]. The results have been promising thus far, but have been demonstrated predominantly in case reports [
78,
79,
80]. An overall review of the literature reveals that the use of 3D printing in orbital fracture surgeries has demonstrated several benefits, including reducing the duration of the procedure, lowering operating room costs, and achieving consistent orbital volume outcomes [
81]. Undeniably, these benefits make it possible to alleviate many barriers to care. For instance, many of the elderly with comorbidities may only have surgical interventions if operative time is reduced. However, while continuously growing, one of the areas requiring further research is the reliance on using the adjacent eye anatomy to create most of these prostheses. While it is often possible to rely on the contralateral orbit for a 3D model, this is not always possible in cases of severe trauma where both orbits may be impacted or when a systemic disease causes a need for bilateral implants. Additionally, it should be noted that facial anatomy is not perfectly symmetric, and direct mirroring can sometimes result in functional defects [
82]. As such, other approaches should continue to be researched including reconstruction software that take the contralateral eye as an input and using spline interpolation for further refinement [
83].
3.2.3. Eyelid Crutches
Another use of 3D printing in oculoplastics has been its application to manufacture eyelid crutches for patients with blepharoptosis [
84]. Blepharoptosis, or ptosis, is an abnormally low-positioned upper eyelid that can often occlude the visual axis and dramatically impact the quality of life. Often, for visually significant ptosis, operative intervention is the treatment of choice. However, in many cases such as patient refusal, high operative risk due to comorbidities, or a lack of medical and surgical infrastructure, surgical approaches may not be possible. In these cases, where certain barriers limit access, ptosis crutches may be considered [
85]. Conventional crutches have drawbacks because they are inflexible and must be tailored individually, necessitating the expertise of a specialized optician, increasing their cost to around
$80-
$100 [
84]. 3D printing offers a cost-effective alternative for producing eyelid crutches, especially with the widespread adoption of the technology. In a case report from the United States, the authors successfully printed a set of personalized eyelid crutches for a patient with recurrent ptosis, who was reluctant to pursue any further surgical intervention [
84]. The designed crutches were customized to the patient’s eyelid dimensions and their existing eyeglasses. The final printed device was produced with a biocompatible material and provided significant visual improvement to the patient. Specifically, the patient’s MRD-1 measurement, which was at -2mm prior to the crutches improved to an MRD-1 of 1mm following the application of the crutches. Further, the patient was also able to achieve adequate eye closure while wearing the crutches [
84]. This case study clearly demonstrates the application of eyelid crutches to provide the best quality care to a patient with evident barriers to eye care.
3.2.4. Dry Eye Syndrome: Lacrimal Gland Regeneration & Punctal Plugs
Keratoconjunctivitis sicca, also known as dry eye syndrome (DES), is an ocular disease that is highly prevalent, impacting more than 50% of the population in some groups [
86]. DES is characterized by inflammation and damage of the ocular surface due to tear lake hyperosmolarity secondary to a decrease in tear production or an increase in tear evaporation. Unsurprisingly, DES has significant economic burdens and impacts on quality of life [
87]. As a significant challenge in ophthalmology, researchers have leaned on 3D printing as a possible tool for addressing the disease.
Interestingly, among the solutions in development over the past two decades is regenerating the lacrimal gland [
88]. One method of 3D bioprinting that has been attempted in engineering other secretory epithelial organoids, like salivary glands, has been magnetic 3D bioprinting [
89]. In this method, biocompatible magnetic nanoparticles are employed to label cells for printing into a specific 3D arrangement [
88]. Magnetic 3D bioprinting has been successful in producing salivary gland organoids with printed cells having a 90% viability three days following differentiation [
89]. However, magnetic 3D bioprinting has not yet been trialed for the lacrimal gland. While still evidently in the research stages regarding its application for the lacrimal gland, these results are promising for the utilization of bioprinting in gland regeneration.
Another area of research addressing dry eye disease is 3D printed punctal plugs [
90,
91]. Punctal plugs are a widely used non-invasive treatment approach to alleviate symptoms of DES. By blocking the canaliculi, which usually drains the tear fluid to the nasopharynx, these plugs prevent tear drainage [
92]. Consequently, these plugs reduce the ocular tear lake hyperosmolarity, which is a major contributing factor in the pathophysiology of DES [
93]. Further, it would be advantageous to use these plugs, which can stay in the canaliculi for several weeks or months, to administer medications to patients who may be unable to adhere to frequent dosages. For instance, this can be applied to those who are elderly, or to those with limitations to their mobility. In a European experimental study, the authors developed 3D-printed punctal plugs that were loaded with dexamethasone, a commonly used corticosteroid [
90]. Their results demonstrated that punctal plugs made with 100% polyethylene glycol diacrylate exhibited prolonged drug release of more than three weeks [
90]. The application of drug-loaded punctal plugs across other subspecialties of ophthalmology is yet to be exploited. For example, drug-loaded punctal plugs is still being researched for glaucoma therapy [
94], where strict medication regimens and adherence are both necessary, but also often difficult to comply with [
95].
3.3. Drug Delivery Systems – Glaucoma, Retina, & Uveal Melanoma
Glaucoma is one of the primary causes of irreversible blindness worldwide. Due to the aging population and growing prevalence, there is an urgent need for effective treatments to alleviate the global burden of the disease. Unfortunately, glaucoma management is often demanding to patients and non compliance is prevalent [
96]. Financial hardship, older age, advanced glaucoma, and lengthier frequency of follow-up have been associated with non adherance [
96]. These factors exacerbate the necessity of an appropriate solution.
One of the management techniques for glaucoma, namely trabeculectomy, involves surgically creating a permanent fistula between the anterior chamber and the subconjunctival space, allowing the drainage of aqueous humor. While trabeculectomy is one of the suitable interventions in glaucoma treatment [
97], management with (antifibrotic) antifrotic agents like 5-fluorouracil (5-FU) is often necessary to prevent failure secondary to scarring following surgery [
98]. 5-FU requires frequent administration through subconjunctival injections, causing barriers for those with limited mobility to clinics, those at increased risks for infections, and anyone without the financial means. Consequently, sustained drug delivery systems (DDS) which can gradually release antifibrotic agents can then potentially alleviate some of these barriers to care, since they will allow patients to receive the drug at home.
In a recent international, experimental study by Ioannou et al. (2023), the authors used 3D printing to design a long-acting drug-eluting scaffold made of polycaprolactone and chitosan, infused with 5-FU [
99]. The scaffold was evaluated with cultured human conjunctival fibroblasts and demonstrated excellent biocompatibility with no significant effect on cell viability. Further, the authors evaluated the implant’s ability to suppress conjunctival fibrosis, by measuring the expression of a key fibrotic regulator, the myocardin-related transcription factor (MRTF). Their results demonstrated that the implant effectively downregulated the MRTF pathway that contributes to conjunctival fibrosis [
99]. In another international, experimental study, Mohamdeen et al. fabricated a drug-eluting contact lens that provides sustained release of timolol, a glaucoma medication [
100]. In their study, the 3D-printed contact lenses designed from biocompatible medical-grade polymers provided a sustained release of timolol over 3 days. Similar studies have evaluated the use of 3D printed contact lenses for a variety of applications [
100,
101,
102,
103,
104,
105].
When considering other applications of drug delivery systems, retinal vascular diseases are at the forefront, given the requirement for repeated intravitreal drug injections. These requirements lead to significant health and economic burdens. To address these challenges, Won et al. developed a new drug delivery system that relies on a 3D-printed drug rod to deliver two drugs—bevacizumab (BEV) and dexamethasone (DEX) from a single implant [
106]. Through this design, the rod's size and drug concentration can be optimized for drug release. The rod can be injected into the vitreous using a small-gauge needle, making the procedure less invasive. In animal models, the drug rod demonstrated superior efficacy in reducing inflammation and providing long-term suppression of neovascularization compared to traditional BEV injections, suggesting it as a promising alternative for treating retinal vascular diseases [
101].
In the realm of drug delivery, 3D printing has also been studied for brachytherapy, a type of treatment also known as internal radiation therapy [
107]. Ocular brachytherapy is often implemented in the management of uveal melanoma. In it, episcleral plaques (EPs), which are hemisphere-shaped metal objects that carry the radioactive seeds, are implanted to administer radiation. EP brackytherapy is an effective treatment, but is unfortunately associated with significant side effects due to a lack of precision in dose delivery. In a Canadian study, to improve treatment precision, Lescot et al. designed and 3D printed a tumour specific implant with a biocompatible material called Polyetheretherketone [
107]. In their study, the authors demonstrated that through the custom implant, they were able to modulate the dose profile of the radioactive material, which can be used to target the contours of cancerous tissues. Evidently, this study has implications to the future of personalized medicine, and in reducing the side effects, costs to patient and the healthcare system.
4. Future Directions
It is evident from this review that the application of 3D printing in ophthalmology is underway. While some applications, including ocular prostheses, are in clinical trials and are likely to be commercialized by companies soon, other areas still require significant research prior to clinical translation, including the use of 3D printing for drug delivery.
It is interesting to envision some of the possibilities 3D printing can have on ophthalmology. One interesting consideration is the design of custom 3D printed intraocular lens (IOL) for managing color blindness [
108]. In a study by Alam et al., the authors used a wavelength selective Atto 565 filtering dye and combined it with an in-house prepared printing resin made of highly transparent in-situ synthesized poly-2-hydroxyethyl methacrylate (pHEMA) and polyethylene glycol diacrylate (PEGDA) resin. Through this, they successfully created an IOL that successfully blocks 50% of the unwanted wavelength (565 nm) which is responsible for red-green color blindness [
108]. Similarly, it would be interesting to see the application of 3D printing to aid with other technologic advancements being studied in ophthalmology, including intraocular pressure biosensors [
109] and retinal prostheses [
110]. There is a possibility in the future to incorporate
in situ bioprinting, that is, direct printing onto the patient, when the technology is ready. The potential for this could be application in trauma settings, when, for instance, there may be a need for urgent orbital floor fracture repair. Additionally, the concept of 4D printing, where a time-based variable allowing printed materials change in response to a stimuli, may be possible.
5. Conclusions
3D printing has shown tremendous growth in ophthalmology over the past decade. It is evident from this review that the needs of patients in underserved communities can possibly be met using 3-D bioprinting technology. The technology has great implications for reducing global health disparities through improving access, lowering costs, and enabling personalized medical treatments. There are also great implications for improving patient outcomes, reducing wait times, and improving overall quality of care. Whether as demonstrated in Sierra Leone, or through the numerous examples noted here, 3D bioprinting can be used to personalize healthcare, increasing affordability and accessibility to underserved populations. It could be possible that in more stable healthcare systems around the world, such technology could be implemented to provide even more success concerning ophthalmic care access.
Author Contributions
Conceptualization, M.M. and N.G.; writing—original draft preparation, M.M. and A.G; writing—review and editing, F.M and D.G.; visualization, M.M.; supervision, N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data was created in this review.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- D. A. Zopf, S. J. Hollister, M. E. Nelson, R. G. Ohye, and G. E. Green, “Bioresorbable Airway Splint Created with a Three-Dimensional Printer,” N Engl J Med, vol. 368, no. 21, pp. 2043–2045, May 2013. [CrossRef]
- W. Huang and X. Zhang, “3D Printing: Print the Future of Ophthalmology,” Invest. Ophthalmol. Vis. Sci., vol. 55, no. 8, p. 5380, Aug. 2014. [CrossRef]
- J. Reinhard, P. Urban, S. Bell, D. Carpenter, and M. S. Sagoo, “Automatic data-driven design and 3D printing of custom ocular prostheses,” Nat Commun, vol. 15, no. 1, p. 1360, Feb. 2024. [CrossRef]
- A. Isaacson, S. Swioklo, and C. J. Connon, “3D bioprinting of a corneal stroma equivalent,” Experimental Eye Research, vol. 173, pp. 188–193, Aug. 2018. [CrossRef]
- L. Lee et al., “3-D printed spectacles: potential, challenges and the future,” Clinical and Experimental Optometry, vol. 103, no. 5, pp. 590–596, Sep. 2020. [CrossRef]
- Y. Tian, L. Li, and R. Ball, “A Qualitative Study for Parametric Designed Custom-Fit Eyewear Frames: Fit Test Evaluation and User Insights,” in Design, User Experience, and Usability, vol. 14712, A. Marcus, E. Rosenzweig, and M. M. Soares, Eds., in Lecture Notes in Computer Science, vol. 14712. , Cham: Springer Nature Switzerland, 2024, pp. 354–370. [CrossRef]
- A. A. A. Latip et al., “Development of 3D-printed universal adapter in enhancing retinal imaging accessibility,” 3D Print Med, vol. 10, no. 1, p. 23, Jul. 2024,. [CrossRef]
- G. Rubegni et al., “Design of a new 3D printed all-in-one magnetic smartphone adapter for fundus and anterior segment imaging,” European Journal of Ophthalmology, p. 11206721241246187, Apr. 2024. [CrossRef]
- A. Ruzza et al., “Preloaded donor corneal lenticules in a new validated 3D printed smart storage glide for Descemet stripping automated endothelial keratoplasty,” Br J Ophthalmol, vol. 99, no. 10, pp. 1388–1395, Oct. 2015. [CrossRef]
- P. Chandrakanth, S. Verghese, K. Chandrakanth, P. Basaiawmoit, and V. Joseph, “The Glowport – Illuminated vitrectomy trocar cannulas,” Indian Journal of Ophthalmology, Jul. 2024. [CrossRef]
- E. V. Navajas and M. Ten Hove, “Three-Dimensional Printing of a Transconjunctival Vitrectomy Trocar-Cannula System,” Ophthalmologica, vol. 237, no. 2, pp. 119–122, 2017. [CrossRef]
- H. Gómez-Fernández et al., “Comprehensive review of the state-of-the-art in corneal 3D bioprinting, including regulatory aspects,” International Journal of Pharmaceutics, vol. 662, p. 124510, Sep. 2024. [CrossRef]
- A. Hu and K. F. Damji, “New open source 3-dimensional printed smartphone fundus imaging adaptor,” Canadian Journal of Ophthalmology, vol. 54, no. 3, pp. 399–400, Jun. 2019. [CrossRef]
- P. Gain et al., “Global Survey of Corneal Transplantation and Eye Banking,” JAMA Ophthalmol, vol. 134, no. 2, p. 167, Feb. 2016. [CrossRef]
- B. H. Jeng and S. Ahmad, “In Pursuit of the Elimination of Corneal Blindness: Is Establishing Eye Banks and Training Surgeons Enough?,” Ophthalmology, vol. 128, no. 6, pp. 813–815, Jun. 2021. [CrossRef]
- L. Balters and S. Reichl, “3D bioprinting of corneal models: A review of the current state and future outlook,” J Tissue Eng, vol. 14, p. 20417314231197793, Jan. 2023. [CrossRef]
- P. M. Mathews, K. Lindsley, A. J. Aldave, and E. K. Akpek, “Etiology of Global Corneal Blindness and Current Practices of Corneal Transplantation: A Focused Review,” Cornea, vol. 37, no. 9, pp. 1198–1203, Sep. 2018. [CrossRef]
- M. Oliva, T. Schottman, and M. Gulati, “Turning the tide of corneal blindness,” Indian J Ophthalmol, vol. 60, no. 5, p. 423, 2012. [CrossRef]
- E. Moraru, G. O. Dontu, S. Cananau, and V.-A. Stanescu, “Approaches and Processing Technologies for Medical Devices: Considerations from Micro- and Macroscale Perspectives,” in International Conference on Reliable Systems Engineering (ICoRSE) - 2023, vol. 762, D. D. Cioboată, Ed., in Lecture Notes in Networks and Systems, vol. 762. , Cham: Springer Nature Switzerland, 2023, pp. 345–362. [CrossRef]
- M. H. Mobarak et al., “Recent advances of additive manufacturing in implant fabrication – A review,” Applied Surface Science Advances, vol. 18, p. 100462, Dec. 2023. [CrossRef]
- A. Haleem and M. Javaid, “Role of CT and MRI in the design and development of orthopaedic model using additive manufacturing,” Journal of Clinical Orthopaedics and Trauma, vol. 9, no. 3, pp. 213–217, Jul. 2018. [CrossRef]
- C. Dong, M. Petrovic, and I. J. Davies, “Applications of 3D printing in medicine: A review,” Annals of 3D Printed Medicine, vol. 14, p. 100149, May 2024. [CrossRef]
- N. Lin, M. Gagnon, and K. Y. Wu, “The Third Dimension of Eye Care: A Comprehensive Review of 3D Printing in Ophthalmology,” Hardware, vol. 2, no. 1, pp. 1–32, Jan. 2024. [CrossRef]
- X. Nie et al., “3D printing sequentially strengthening high-strength natural polymer hydrogel bilayer scaffold for cornea regeneration,” Regenerative Biomaterials, vol. 11, p. rbae012, Jan. 2024. [CrossRef]
- M. Sridhar, “Anatomy of cornea and ocular surface,” Indian J Ophthalmol, vol. 66, no. 2, p. 190, 2018. [CrossRef]
- H. S. Dua, L. A. Faraj, D. G. Said, T. Gray, and J. Lowe, “Human Corneal Anatomy Redefined,” Ophthalmology, vol. 120, no. 9, pp. 1778–1785, Sep. 2013. [CrossRef]
- L. E. Downie et al., “BCLA CLEAR - Anatomy and physiology of the anterior eye,” Contact Lens and Anterior Eye, vol. 44, no. 2, pp. 132–156, Apr. 2021. [CrossRef]
- J. W. Ruberti and J. D. Zieske, “Prelude to corneal tissue engineering – Gaining control of collagen organization,” Progress in Retinal and Eye Research, vol. 27, no. 5, pp. 549–577, Sep. 2008. [CrossRef]
- L. J. Muller, “The specific architecture of the anterior stroma accounts for maintenance of corneal curvature,” British Journal of Ophthalmology, vol. 85, no. 4, pp. 437–443, Apr. 2001. [CrossRef]
- P. S. Gungor-Ozkerim, I. Inci, Y. S. Zhang, A. Khademhosseini, and M. R. Dokmeci, “Bioinks for 3D bioprinting: an overview,” Biomater. Sci., vol. 6, no. 5, pp. 915–946, 2018. [CrossRef]
- A. Sorkio et al., “Human stem cell based corneal tissue mimicking structures using laser-assisted 3D bioprinting and functional bioinks,” Biomaterials, vol. 171, pp. 57–71, Jul. 2018. [CrossRef]
- D. F. Duarte Campos et al., “Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration,” Connective Tissue Research, vol. 61, no. 2, pp. 205–215, Mar. 2020. [CrossRef]
- Y. Liu, L. Ren, and Y. Wang, “Crosslinked collagen–gelatin–hyaluronic acid biomimetic film for cornea tissue engineering applications,” Materials Science and Engineering: C, vol. 33, no. 1, pp. 196–201, Jan. 2013. [CrossRef]
- S. Kutlehria, T. C. Dinh, A. Bagde, N. Patel, A. Gebeyehu, and M. Singh, “High-throughput 3D bioprinting of corneal stromal equivalents,” J Biomed Mater Res, vol. 108, no. 7, pp. 2981–2994, Oct. 2020. [CrossRef]
- Z. Wu, X. Su, Y. Xu, B. Kong, W. Sun, and S. Mi, “Bioprinting three-dimensional cell-laden tissue constructs with controllable degradation,” Sci Rep, vol. 6, no. 1, p. 24474, Apr. 2016. [CrossRef]
- M. Nikkhah, M. Akbari, A. Paul, A. Memic, A. Dolatshahi-Pirouz, and A. Khademhosseini, “Gelatin-Based Biomaterials For Tissue Engineering And Stem Cell Bioengineering,” in Biomaterials from Nature for Advanced Devices and Therapies, 1st ed., N. M. Neves and R. L. Reis, Eds., Wiley, 2016, pp. 37–62. [CrossRef]
- K. Tonsomboon and M. L. Oyen, “Composite electrospun gelatin fiber-alginate gel scaffolds for mechanically robust tissue engineered cornea,” Journal of the Mechanical Behavior of Biomedical Materials, vol. 21, pp. 185–194, May 2013. [CrossRef]
- C. Kilic Bektas and V. Hasirci, “Cell loaded 3D bioprinted GelMA hydrogels for corneal stroma engineering,” Biomater. Sci., vol. 8, no. 1, pp. 438–449, 2020. [CrossRef]
- S. S. Mahdavi, M. J. Abdekhodaie, H. Kumar, S. Mashayekhan, A. Baradaran-Rafii, and K. Kim, “Stereolithography 3D Bioprinting Method for Fabrication of Human Corneal Stroma Equivalent,” Ann Biomed Eng, vol. 48, no. 7, pp. 1955–1970, Jul. 2020. [CrossRef]
- R. Vijayaraghavan, S. Loganathan, and R. B. Valapa, “Fabrication of GelMA – Agarose Based 3D Bioprinted Photocurable Hydrogel with In Vitro Cytocompatibility and Cells Mirroring Natural Keratocytes for Corneal Stromal Regeneration,” Macromolecular Bioscience, p. 2400136, Aug. 2024. [CrossRef]
- U. Bhutani et al., “Biopolymeric corneal lenticules by digital light processing based bioprinting: a dynamic substitute for corneal transplant,” Biomed. Mater., vol. 19, no. 3, p. 035017, May 2024. [CrossRef]
- S. Ulag et al., “3D printed artificial cornea for corneal stromal transplantation,” European Polymer Journal, vol. 133, p. 109744, Jun. 2020. [CrossRef]
- H. Kim, M.-N. Park, J. Kim, J. Jang, H.-K. Kim, and D.-W. Cho, “Characterization of cornea-specific bioink: high transparency, improved in vivo safety,” J Tissue Eng, vol. 10, p. 204173141882338, Jan. 2019. [CrossRef]
- M. Zhang et al., “3D bioprinting of corneal decellularized extracellular matrix: GelMA composite hydrogel for corneal stroma engineering,” IJB, vol. 9, no. 5, p. 774, Jun. 2023. [CrossRef]
- M. Uyanıklar, G. Günal, A. Tevlek, P. Hosseinian, and H. M. Aydin, “Hybrid Cornea: Cell Laden Hydrogel Incorporated Decellularized Matrix,” ACS Biomater. Sci. Eng., vol. 6, no. 1, pp. 122–133, Jan. 2020. [CrossRef]
- A. Kostenko, S. Swioklo, and C. J. Connon, “Alginate in corneal tissue engineering,” Biomed. Mater., Jan. 2022. [CrossRef]
- B. Zhang et al., “Integrated 3D bioprinting-based geometry-control strategy for fabricating corneal substitutes,” J. Zhejiang Univ. Sci. B, vol. 20, no. 12, pp. 945–959, Dec. 2019. [CrossRef]
- M. Sun et al., “Hyaluronan Derived From the Limbus is a Key Regulator of Corneal Lymphangiogenesis,” Invest. Ophthalmol. Vis. Sci., vol. 60, no. 4, p. 1050, Mar. 2019. [CrossRef]
- A. Mörö et al., “Hyaluronic acid based next generation bioink for 3D bioprinting of human stem cell derived corneal stromal model with innervation,” Biofabrication, vol. 15, no. 1, p. 015020, Jan. 2023. [CrossRef]
- Z. Zhong et al., “Bioprinting of dual ECM scaffolds encapsulating limbal stem/progenitor cells in active and quiescent statuses,” Biofabrication, vol. 13, no. 4, p. 044101, Oct. 2021. [CrossRef]
- M. Kumar Singh, “Textiles Functionalization - A Review of Materials, Processes, and Assessment,” in Textiles for Functional Applications, B. Kumar, Ed., IntechOpen, 2021. [CrossRef]
- P. Grönroos et al., “Bioprinting of human pluripotent stem cell derived corneal endothelial cells with hydrazone crosslinked hyaluronic acid bioink,” Stem Cell Res Ther, vol. 15, no. 1, p. 81, Mar. 2024,. [CrossRef]
- K. W. Kim, S. J. Lee, S. H. Park, and J. C. Kim, “Ex Vivo Functionality of 3D Bioprinted Corneal Endothelium Engineered with Ribonuclease 5-Overexpressing Human Corneal Endothelial Cells,” Adv Healthcare Materials, vol. 7, no. 18, p. 1800398, Sep. 2018. [CrossRef]
- G. L. Duffy, H. Liang, R. L. Williams, D. A. Wellings, and K. Black, “3D reactive inkjet printing of poly-ɛ-lysine/gellan gum hydrogels for potential corneal constructs,” Materials Science and Engineering: C, vol. 131, p. 112476, Dec. 2021. [CrossRef]
- B. He et al., “3D printed biomimetic epithelium/stroma bilayer hydrogel implant for corneal regeneration,” Bioactive Materials, vol. 17, pp. 234–247, Nov. 2022. [CrossRef]
- A. L. De Araujo, “Corneal stem cells and tissue engineering: Current advances and future perspectives,” WJSC, vol. 7, no. 5, p. 806, 2015. [CrossRef]
- D. Karamichos et al., “A Role for Topographic Cues in the Organization of Collagenous Matrix by Corneal Fibroblasts and Stem Cells,” PLoS ONE, vol. 9, no. 1, p. e86260, Jan. 2014. [CrossRef]
- R. M. Gouveia, G. Lepert, S. Gupta, R. R. Mohan, C. Paterson, and C. J. Connon, “Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation,” Nat Commun, vol. 10, no. 1, p. 1496, Apr. 2019. [CrossRef]
- A. Modugno, F. Mantelli, S. Sposato, C. Moretti, A. Lambiase, and S. Bonini, “Ocular prostheses in the last century: a retrospective analysis of 8018 patients,” Eye, vol. 27, no. 7, pp. 865–870, Jul. 2013. [CrossRef]
- A. L. W. Groot, J. S. Remmers, and D. T. Hartong, “Three-Dimensional Computer-Aided Design of a Full-Color Ocular Prosthesis with Textured Iris and Sclera Manufactured in One Single Print Job,” 3D Printing and Additive Manufacturing, vol. 8, no. 6, pp. 343–348, Dec. 2021. [CrossRef]
- R. Gunaseelaraj, S. Karthikeyan, M. Kumar, T. Balamurugan, and A. Jagadeeshwaran, “Custom-made ocular prosthesis,” J Pharm Bioall Sci, vol. 4, no. 6, p. 177, 2012,. [CrossRef]
- M. C. Goiato, L. C. Bannwart, M. F. Haddad, D. M. Dos Santos, A. A. Pesqueira, and G. I. Miyahara, “Fabrication Techniques for Ocular Prostheses – An Overview,” Orbit, vol. 33, no. 3, pp. 229–233, Jun. 2014. [CrossRef]
- M. Van Der Stelt et al., “Improving Lives in Three Dimensions: The Feasibility of 3D Printing for Creating Personalized Medical Aids in a Rural Area of Sierra Leone,” The American Journal of Tropical Medicine and Hygiene, vol. 102, no. 4, pp. 905–909, Apr. 2020. [CrossRef]
- A. J. Sterkenburg et al., “Quality of life of patients with 3D-printed arm prostheses in a rural area of Sierra Leone,” Heliyon, vol. 7, no. 7, p. e07447, Jul. 2021. [CrossRef]
- S. Ruiters, Y. Sun, S. De Jong, C. Politis, and I. Mombaerts, “Computer-aided design and three-dimensional printing in the manufacturing of an ocular prosthesis,” Br J Ophthalmol, vol. 100, no. 7, pp. 879–881, Jul. 2016. [CrossRef]
- N. Puls, D. Carluccio, M. D. Batstone, and J. I. Novak, “The rise of additive manufacturing for ocular and orbital prostheses: A systematic literature review,” Annals of 3D Printed Medicine, vol. 4, p. 100036, Dec. 2021. [CrossRef]
- B. R. Kim et al., “A Pilot Clinical Study of Ocular Prosthesis Fabricated by Three-dimensional Printing and Sublimation Technique,” Korean J Ophthalmol, vol. 35, no. 1, pp. 37–43, Feb. 2021. [CrossRef]
- S.-Y. Park et al., “Custom-made artificial eyes using 3D printing for dogs: A preliminary study,” PLoS ONE, vol. 15, no. 11, p. e0242274, Nov. 2020. [CrossRef]
- Md. S. Alam, M. Sugavaneswaran, G. Arumaikkannu, and B. Mukherjee, “An innovative method of ocular prosthesis fabrication by bio-CAD and rapid 3-D printing technology: A pilot study,” Orbit, vol. 36, no. 4, pp. 223–227, Jul. 2017. [CrossRef]
- J. Ko, S. H. Kim, S. W. Baek, M. K. Chae, and J. S. Yoon, “Semi-automated fabrication of customized ocular prosthesis with three–dimensional printing and sublimation transfer printing technology,” Sci Rep, vol. 9, no. 1, p. 2968, Feb. 2019. [CrossRef]
- R. B. Kormann, R. Mörschbächer, H. Moreira, and P. Akaishi, “A three-dimensional printed photopolymer resin implant for orbital rehabilitation for evisceration,” Arquivos Brasileiros de Oftalmologia, vol. 82, no. 6, 2019. [CrossRef]
- Y. C. Kim, W. S. Jeong, T. Park, J. W. Choi, K. S. Koh, and T. S. Oh, “The accuracy of patient specific implant prebented with 3D-printed rapid prototype model for orbital wall reconstruction,” Journal of Cranio-Maxillofacial Surgery, vol. 45, no. 6, pp. 928–936, Jun. 2017,. [CrossRef]
- S. Kang et al., “Generation of customized orbital implant templates using 3-dimensional printing for orbital wall reconstruction,” Eye, vol. 32, no. 12, pp. 1864–1870, Dec. 2018. [CrossRef]
- A. Murray-Douglass, C. Snoswell, C. Winter, and R. Harris, “Three-dimensional (3D) printing for post-traumatic orbital reconstruction, a systematic review and meta-analysis,” British Journal of Oral and Maxillofacial Surgery, vol. 60, no. 9, pp. 1176–1183, Nov. 2022. [CrossRef]
- S. Mukai, T. Tsuge, S. Akaishi, R. Ogawa, and H. Kuwahara, “Utilizing 3D Printing for the Surgical Management of Orbital Floor Fractures,” Plastic and Reconstructive Surgery - Global Open, vol. 11, no. 11, p. e5433, Nov. 202. [CrossRef]
- A. B. Callahan, A. A. Campbell, C. Petris, and M. Kazim, “Low-Cost 3D Printing Orbital Implant Templates in Secondary Orbital Reconstructions,” Ophthalmic Plastic & Reconstructive Surgery, vol. 33, no. 5, pp. 376–380, Sep. 2017. [CrossRef]
- E. H. Weisson, M. Fittipaldi, C. A. Concepcion, D. Pelaez, L. Grace, and D. T. Tse, “Automated Noncontact Facial Topography Mapping, 3-Dimensional Printing, and Silicone Casting of Orbital Prosthesis,” American Journal of Ophthalmology, vol. 220, pp. 27–36, Dec. 2020. [CrossRef]
- T. S. Oh, W. S. Jeong, T. J. Chang, K. S. Koh, and J.-W. Choi, “Customized Orbital Wall Reconstruction Using Three-Dimensionally Printed Rapid Prototype Model in Patients With Orbital Wall Fracture,” Journal of Craniofacial Surgery, vol. 27, no. 8, pp. 2020–2024, Nov. 2016. [CrossRef]
- D. L. Mourits et al., “3D Orbital Reconstruction in a Patient with Microphthalmos and a Large Orbital Cyst—A Case Report,” Ophthalmic Genetics, vol. 37, no. 2, pp. 233–237, Apr. 2016. [CrossRef]
- M. Vehmeijer, M. Van Eijnatten, N. Liberton, and J. Wolff, “A Novel Method of Orbital Floor Reconstruction Using Virtual Planning, 3-Dimensional Printing, and Autologous Bone,” Journal of Oral and Maxillofacial Surgery, vol. 74, no. 8, pp. 1608–1612, Aug. 2016. [CrossRef]
- D. Amin, N. Nguyen, A. J. Manhan, J. H. Kim, S. M. Roser, and G. F. Bouloux, “Does a Point-of-Care 3-Dimensional Printer Result in a Decreased Length of Surgery for Orbital Fractures?,” Journal of Oral and Maxillofacial Surgery, p. S0278239124005974, Jul. 2024. [CrossRef]
- A. Tel et al., “Endoscopically assisted computer-guided repair of internal orbital floor fractures: an updated protocol for minimally invasive management,” Journal of Cranio-Maxillofacial Surgery, vol. 47, no. 12, pp. 1943–1951, Dec. 2019. [CrossRef]
- N. B Jamayet, Y. J Abdullah, Z. A Rajion, A. Husein, and M. K Alam, “New Approach to 3D Printing of Facial Prostheses Using Combination of Open Source Software and Conventional Techniques: A Case Report,” Bull. Tokyo Dent. Coll., vol. 58, no. 2, pp. 117–124, 2017. [CrossRef]
- M. G. Sun, D. Rojdamrongratana, M. I. Rosenblatt, V. K. Aakalu, and C. Q. Yu, “3D printing for low cost, rapid prototyping of eyelid crutches,” Orbit, vol. 38, no. 4, pp. 342–346, Jul. 2019. [CrossRef]
- O. Lapid, “Eyelid Crutches for Ptosis: A Forgotten Solution,” Plastic and Reconstructive Surgery, vol. 106, no. 5, pp. 1213–1214, Oct. 2000.
- D. Wróbel-Dudzińska, N. Osial, P. W. Stępień, A. Gorecka, and T. Żarnowski, “Prevalence of Dry Eye Symptoms and Associated Risk Factors among University Students in Poland,” IJERPH, vol. 20, no. 2, p. 1313, Jan. 2023. [CrossRef]
- C. Chan, S. Ziai, V. Myageri, J. G. Burns, and C. L. Prokopich, “Economic burden and loss of quality of life from dry eye disease in Canada,” BMJ Open Ophth, vol. 6, no. 1, p. e000709, Sep. 2021. [CrossRef]
- A. C. Lieu, M. K. Shoji, V. K. Aakalu, and C. Y. Liu, “Approaches to Restoring Lacrimal Gland Function: From stem Cells to Tissue Engineering,” Curr Ophthalmol Rep, Jul. 2024. [CrossRef]
- C. Adine, K. K. Ng, S. Rungarunlert, G. R. Souza, and J. N. Ferreira, “Engineering innervated secretory epithelial organoids by magnetic three-dimensional bioprinting for stimulating epithelial growth in salivary glands,” Biomaterials, vol. 180, pp. 52–66, Oct. 2018. [CrossRef]
- X. Xu et al., “3D Printed Punctal Plugs for Controlled Ocular Drug Delivery,” Pharmaceutics, vol. 13, no. 9, p. 1421, Sep. 2021. [CrossRef]
- T. Khanna, J. Akkara, V. Bawa, and E. Sargunam, “Designing and making an open source, 3D-printed, punctal plug with drug delivery system,” Indian J Ophthalmol, vol. 71, no. 1, p. 297, 2023. [CrossRef]
- M. M. Marcet et al., “Safety and Efficacy of Lacrimal Drainage System Plugs for Dry Eye Syndrome,” Ophthalmology, vol. 122, no. 8, pp. 1681–1687, Aug. 2015. [CrossRef]
- J. Gayton, “Etiology, prevalence, and treatment of dry eye disease,” OPTH, p. 405, Jul. 2009. [CrossRef]
- R. B. Singh, P. Ichhpujani, S. Thakur, and S. Jindal, “Promising therapeutic drug delivery systems for glaucoma: a comprehensive review,” Ophthalmol Eye Dis, vol. 12, p. 251584142090574, Jan. 2020. [CrossRef]
- L. Quaranta, A. Novella, M. Tettamanti, L. Pasina, R. N. Weinreb, and A. Nobili, “Adherence and Persistence to Medical Therapy in Glaucoma: An Overview,” Ophthalmol Ther, vol. 12, no. 5, pp. 2227–2240, Oct. 2023. [CrossRef]
- L. Tamrat, G. Gessesse, and Y. Gelaw, “Adherence to topical glaucoma medications in Ethiopian patients,” Middle East Afr J Ophthalmol, vol. 22, no. 1, p. 59, 2015. [CrossRef]
- F. M. Wagner, A. K. Schuster, K. Kianusch, J. Stingl, N. Pfeiffer, and E. M. Hoffmann, “Long-term success after trabeculectomy in open-angle glaucoma: results of a retrospective cohort study,” BMJ Open, vol. 13, no. 2, p. e068403, Feb. 2023. [CrossRef]
- S. J. Gedde, J. C. Schiffman, W. J. Feuer, L. W. Herndon, J. D. Brandt, and D. L. Budenz, “Treatment Outcomes in the Tube Versus Trabeculectomy (TVT) Study After Five Years of Follow-up,” American Journal of Ophthalmology, vol. 153, no. 5, pp. 789-803.e2, May 2012. [CrossRef]
- N. Ioannou et al., “3D-printed long-acting 5-fluorouracil implant to prevent conjunctival fibrosis in glaucoma,” Journal of Pharmacy and Pharmacology, vol. 75, no. 2, pp. 276–286, Feb. 2023. [CrossRef]
- Y. M. G. Mohamdeen et al., “Development of 3D printed drug-eluting contact lenses,” Journal of Pharmacy and Pharmacology, vol. 74, no. 10, pp. 1467–1476, Oct. 2022,. [CrossRef]
- F. Alam et al., “3D Printed Contact Lenses,” ACS Biomater. Sci. Eng., vol. 7, no. 2, pp. 794–803, Feb. 2021. [CrossRef]
- M. Hisham, A. E. Salih, and H. Butt, “3D Printing of Multimaterial Contact Lenses,” ACS Biomater. Sci. Eng., vol. 9, no. 7, pp. 4381–4391, Jul. 2023. [CrossRef]
- F. Zhao, J. Wang, L. Wang, and L. Chen, “An approach for simulating the fitting of rigid gas-permeable contact lenses using 3D printing technology,” Contact Lens and Anterior Eye, vol. 42, no. 2, pp. 165–169, Apr. 2019. [CrossRef]
- S. Hittini et al., “Fabrication of 3D-Printed Contact Lenses and Their Potential as Color Blindness Ocular Aids,” Macro Materials & Eng, vol. 308, no. 5, p. 2200601, May 2023. [CrossRef]
- F. Alam et al., “Prospects for Additive Manufacturing in Contact Lens Devices,” Adv Eng Mater, vol. 23, no. 1, p. 2000941, Jan. 2021,. [CrossRef]
- J. Y. Won et al., “3D printing of drug-loaded multi-shell rods for local delivery of bevacizumab and dexamethasone: A synergetic therapy for retinal vascular diseases,” Acta Biomaterialia, vol. 116, pp. 174–185, Oct. 2020. [CrossRef]
- T. Lescot et al., “Tumor Shape-Specific Brachytherapy Implants by 3D-Printing, Precision Radioactivity Painting, and Biomedical Imaging,” Adv Healthcare Materials, vol. 12, no. 25, p. 2300528, Oct. 2023. [CrossRef]
- F. Alam, M. Ali, M. Elsherif, A. E. Salih, N. El-Atab, and H. Butt, “3D printed intraocular lens for managing the color blindness,” Additive Manufacturing Letters, vol. 5, p. 100129, Apr. 2023,. [CrossRef]
- R. Raveendran et al., “Current Innovations in Intraocular Pressure Monitoring Biosensors for Diagnosis and Treatment of Glaucoma—Novel Strategies and Future Perspectives,” Biosensors, vol. 13, no. 6, p. 663, Jun. 2023. [CrossRef]
- K. A. Ramirez, L. E. Drew-Bear, M. Vega-Garces, H. Betancourt-Belandria, and J. F. Arevalo, “An update on visual prosthesis,” Int J Retin Vitr, vol. 9, no. 1, p. 73, Nov. 2023. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).