1. Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder in the elderly, affecting approximately 10 million individuals globally[
1]. It is characterized by a progressive loss of dopaminergic neurons in the substantia nigra, leading to a range of motor symptoms, such as bradykinesia, rigidity, rest tremor, and postural instability. In addition, patients often experience non-motor symptoms, including sleep disturbances, orthostatic hypotension, and cognitive decline[
2]. Above all the dopaminergic treatments, Levodopa remains the cornerstone of PD treatment, effectively managing motor symptoms in the early stages of the disease [
3]. However, as PD progresses, patients frequently encounter motor fluctuations, especially wearing-off and levodopa-induced dyskinesia, that complicate disease management and affect their daily living quality of life [
4]. These motor fluctuations can occur at varying intervals weekly, daily, or even within the same day. However, PD symptoms are usually evaluated with anamnesis and clinical scales in clinical studies and daily practice, like a snapshot examination[
5]. In this examination, patients may not recall symptoms, especially the exact time of their ‘‘on’‘ and ‘‘off’‘ periods in a limited time, potentially hindering the main problems, so the optimal treatment of the disease may not be provided. Therefore, accurate and consistent self-reporting of patient data is vital in a personalized approach to tailor treatment targeting each patient’s specific needs and adjusting medication regimens more effectively [
5,
6]. Several paper-based patient diaries are specifically designed for patients with PD to help them track their motor symptoms [
7]. They are generally used in clinical practice for documenting patients’ motor complications or ‘‘on’‘ and ‘‘off’‘ periods within a specific time frame (e.g., hourly, every 30 min) for a day or a week in a structured manner. The most widely used paper diary for tracking motor symptoms was developed by Hauser et al.[
8]. Other paper diaries for PD have also been published [
9,
10,
11]. These diaries used to be a standard follow-up method, providing valuable insights into experiences and motor fluctuations throughout the day. Still, their reliability and accuracy are uncertain for a few reasons. Foremost, it is unclear if the paper diaries were completed on time, which could impact the results. In addition, issues like low adherence, multiple entries for the same time interval (data duplication), diary fatigue, filling in the retrospectively at once, and incomplete-missing-illegible diaries can undermine the quality of the data and potentially lead to suboptimal treatment decisions[
12].
Thus, digital health technologies have introduced wireless electronic diaries (e-diaries) as a contemporary approach to monitoring PD symptoms and overcoming these challenges. Available on multiple platforms, including tablets, smartphone apps, and the web, these e-diaries have several advantages over paper diaries[
13]. Among them, smartphone app e-diaries stand out as they are convenient and easy to use. In response, these digital tools improve the timeliness of data entry (with alerts prompting patients to enter answers on time) and improve the accuracy of responses[
7], which ensures data quality and helps improve patient compliance and retention[
12]. Although smartphone-based diaries hold promise for monitoring PD symptoms, only a few studies have been published to evaluate their use in monitoring PD symptoms. Since the adoption of these digital tools continues to develop, it becomes increasingly important to assess their utility.
This study aims to evaluate the effectiveness, compliance, accuracy, and patient preferences between traditional paper diaries and our new smartphone application (My Parkinson’s) in tracking motor symptoms in PD and evaluate the agreement between clinical examination notes and patient-reported data.
2. Materials and Methods
2.1. Study Design and Participants
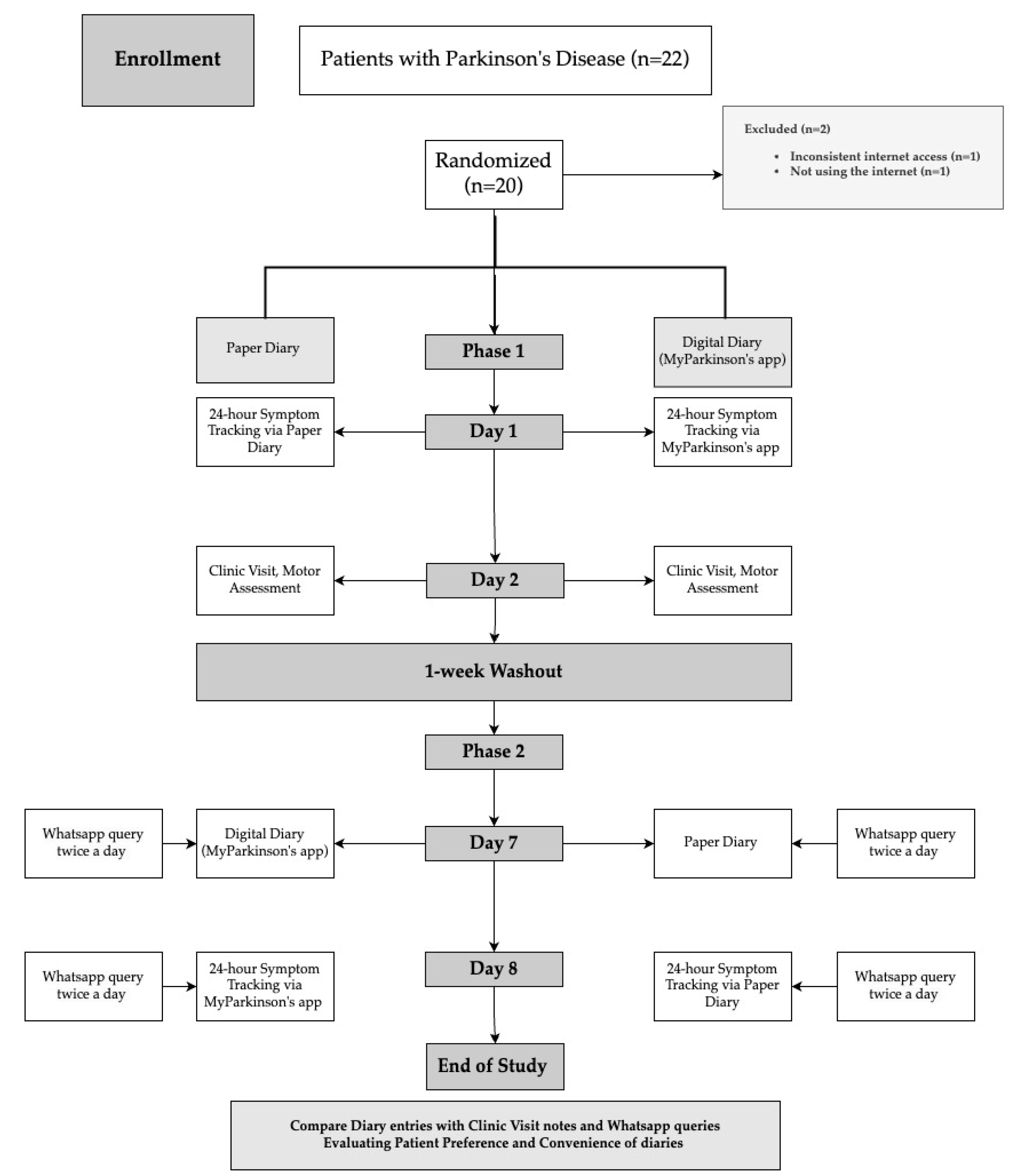
This single-center, prospective, randomized cross-over study enrolled 22 patients with advanced PD from June 2022 to January 2023. The UK Parkinson’s Disease Society Brain Bank criteria were used by one[
14], and all participants who met these diagnostic criteria and had motor fluctuations and dyskinesias were enrolled in the study.
The study adhered to the principles of the Helsinki Declaration and received approval from the local Institutional Review Board (Approval No. 23/24.05.2022). Written informed consent was obtained from all participants before their inclusion in the study. Inclusion criteria were as follows: a baseline Mini-Mental State Examination (MMSE) score of at least 25 to ensure sufficient cognitive function, which could interfere with the results[
15]. For this study, we sought at least primary school graduates to enable comprehension of the instructions and effectively use mobile applications. In addition, Internet accessibility and smartphone use in this study were prerequisites because all motor symptom assessments of the patient were digitally monitored.
Power analysis was conducted using G*Power software (Version 3.1.9.7). Based on an expected effect size of d = 0.5, an alpha level of 0.05, and a desired power of 0.80, the analysis indicated that a minimum sample size of 20 participants per group was required.
Participants had to maintain a stable antiparkinsonian medication regimen for at least one month before and during the study to remove potential confounding effects possibly related to medication changes on the study outcomes. Individuals who took medications that might have altered their parkinsonism or dyskinesias and those with major depression, psychosis, or other severe medical conditions that could affect the results were excluded from the study.
All participants’ demographic and clinical characteristics, including age, gender, education, disease duration, motor complication duration, and Levodopa equivalent daily doses (LEDD)[
16], were recorded. Motor symptoms of PD were assessed using the UPDRS Part III [
17]and staged according to the Hoehn and Yahr (H&Y) scale [
18]. At the end of the study, patients were asked to evaluate both diaries in terms of convenience and preference.
2.2. Procedures
This study uses a randomized cross-over design to investigate compliance, accuracy, and patient preferences between traditional paper diaries and the electronic diary application My Parkinson’s for tracking motor symptoms in PD.
2.3. Randomization and Group Assignment
The participants were randomly allocated into one of two groups with Random.org.[
19]
Group 1:
First Phase (Phase I):
Day 1: Participants in this group were initially given a paper diary. At home, they were instructed to record their PD-related symptoms (on-off fluctuations, dyskinesia, and tremors) by self-reporting every hour over 24 hours to capture their motor fluctuations.
Day 2: First Clinic Visit The day after the 24-hour monitoring period at home, patients attended a clinic visit. During their follow-up visit, a movement disorders specialist evaluated their motor symptoms according to anamnestic data (interview in the medical routine without access to recorded diaries). We included this step to replicate the circumstance of a typical clinic evaluation, which is based on patient recall and clinician judgment.
Second Phase (Phase II):
1 Week later (Day 7-Cross-over): Following a 1-week washout period to mediate any effects due to carry-over from Phase I, the PD patients were introduced, this time with access to My Parkinson’s digital diary. They downloaded apps on their smartphones and received standardized training in Turkish for using this system with a train-the-trainer model. They went on to track their motor symptoms every hour for 24 hours
Day 8 –Second Clinic Visit (Cross-over): On the second day, motor symptoms were re-evaluated by the movement disorders specialist blindly (NDC, author) using a similar method to the first clinic visit to analyze and compare symptom data collected by paper diary vs. digital diary computed at home as a direct comparison of last measurement results from patient diaries.
Group 2:
First Phase (Phase I): This group of participants started the study with a My Parkinson’s digital diary. They followed the exact same protocols as Group 1. They tracked their symptoms for 24 hours, followed by a clinic visit the next day.
Second Phase (Phase II): (Cross-over): Like in Group 1, these participants switched to using the paper diary for another 24-hour symptom-tracking after the one-week washout period. During a second clinic visit, their symptoms were then assessed blindly by the same movement disorders specialist (NDC, author).
All the patients were asked about their Parkinson ‘s-related symptoms via WhatsApp twice a day at randomized hours on both Day 1 and Day 7. These data were recorded and analyzed to identify discrepancies between what patients recorded in their diaries and what they reported in real-time for intra-rater reliability of each diary method
Compliance and Data Analysis Patient compliance with the diaries was evaluated by retrospectively comparing the recorded status randomly asked via WhatsApp records at documented hours at the diary keeping days. This analysis helped to identify discrepancies between what patients recorded in their diaries and what they reported in real-time, providing insight into the reliability of each diary method
End of Study Evaluation Participants were asked to rate the paper and digital diaries in preference and convenience after the study. This charting is a subjective assessment that allowed us to estimate patient satisfaction as well as ease of usability
Randomization and Group Assignment are summarized in
Figure 1.
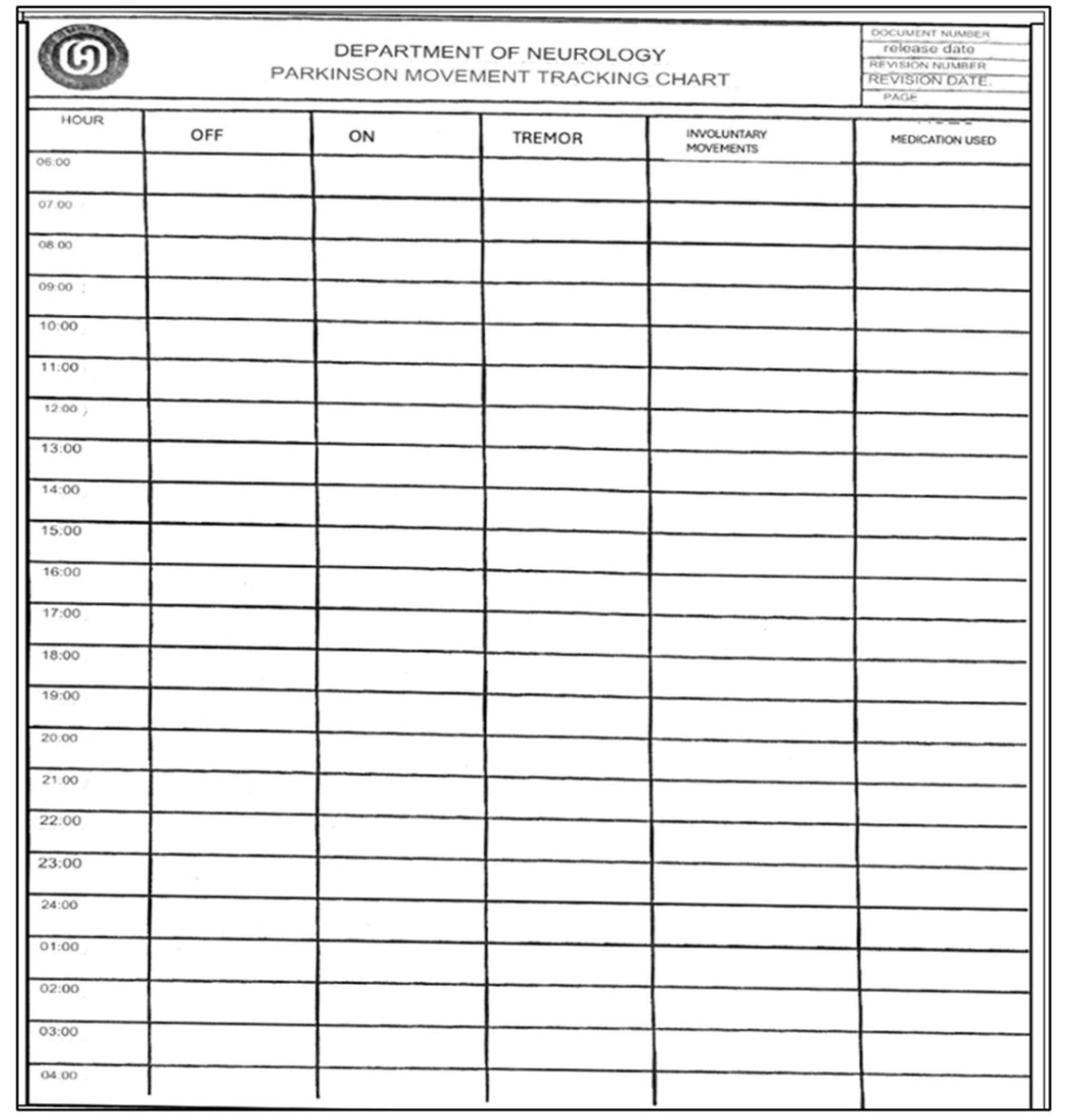
Features of the Paper Diary
This paper chart is a 24-hour diary for movement tracking designed explicitly for PD patients. With this diary, patients can systematically record and monitor their motor symptoms and medication usage (
Figure 2). The diary is divided into hourly intervals between the starting point of 06:00 am and the ending point of 04:00 am (4:00 am of the following day), allowing for continuous observation throughout the day and night whenever the patient is awake. The essential columns in the chart are “On” and “Off,” which may indicate periods of the patient’s motor state. Columns such as “Tremor” and” Involuntary Movements” can track the most common symptoms of tremors and a side effect of dyskinesia caused by medication or a symptom of the disease. Finally, the record can be made in the column “Medication Use” to write which drug was taken at each hour. This Turkish chart is a revised form of Hauser et al.’s[
8]. This diary is more detailed to provide more understandable patient fluctuations daily and optimize treatment.
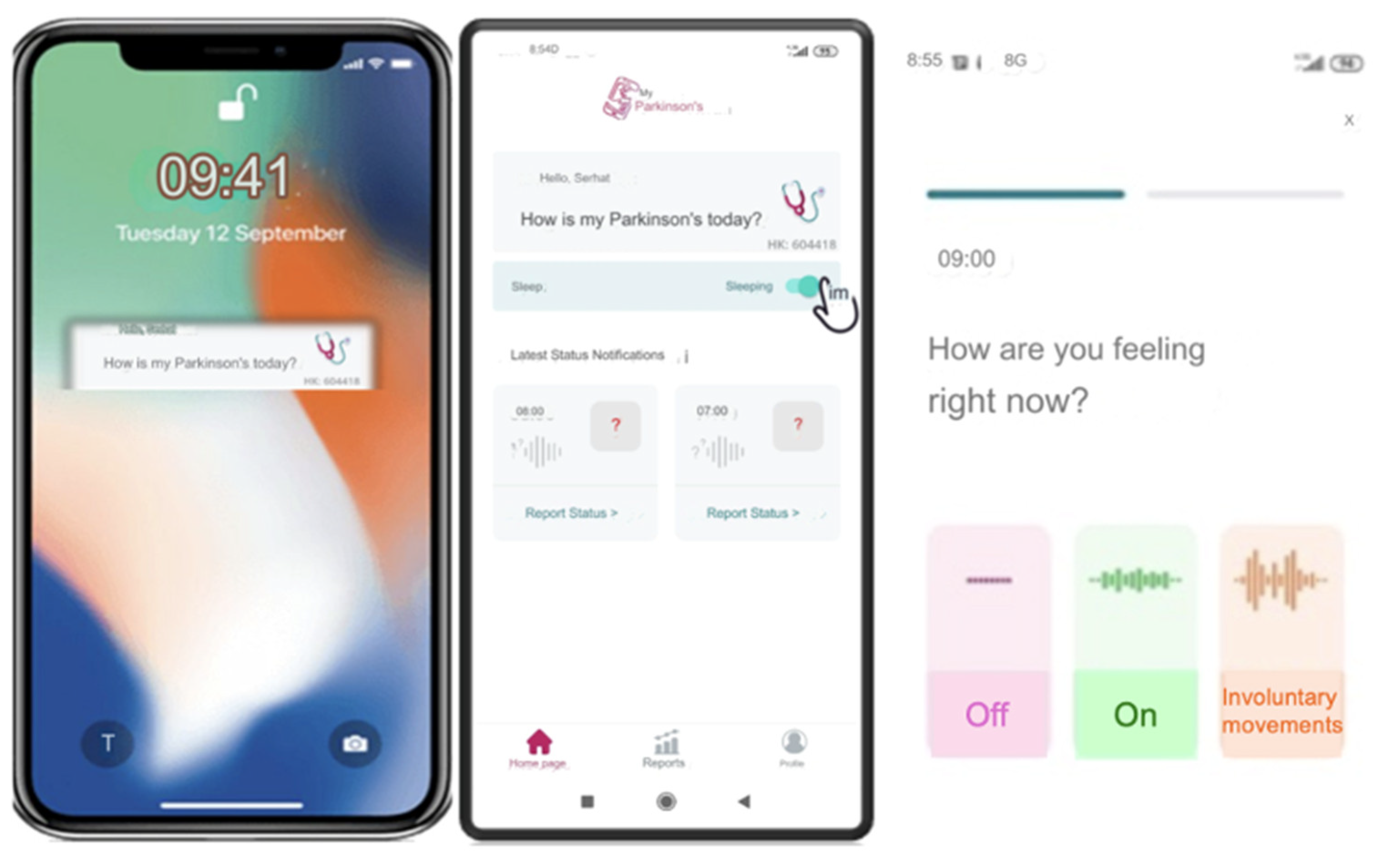
Features of the Smartphone Application Digital Diary (My Parkinson’s)
“My Parkinson’s” is a smartphone application developed by a movement disorders specialist (SO, author). The app is free to download from Google Play on Android and the app store on iOS. This novel digital motor tracking app for patients with PD provides a real-time, remote monitoring and quantification tool empowering physicians to optimize care. The app is like a diary that allows users to enter information about their motor condition, appear “On” and “Off” throughout the day, and involuntary movements such as tremors or dyskinesia typically occur in PD.
The app’s interface is designed with simplicity and accessibility, especially to make it easy for users to input their symptoms with just a few taps, considering it will be used mainly by older people. Once patients register in the app’s patient section, they will receive an ID on their screen. They should then share this ID with their clinician so the clinician can access the patient’s data in real-time using this code. The question ‘How is my Parkinson’s today?’’ pops up on the phone screen every hour or at the desired interval (from 15 min to every four hours) as a reminder. When they tap the question button, the patient is directed to the application and a page where the patient is prompted with the question, “How are you feeling now?” and is presented with three options: ‘‘off’‘, ‘‘on’‘ and ‘‘involuntary movements’‘
Figure 3 This functionality enables real-time tracking, allowing the clinician to monitor the patient’s condition continuously. The data on the server can be accessed only by using an access code (handed to a physician by his patient), and this access does not work forever, as each report has its unique one-time code. This information is immediately shared with their clinician, with whom they have previously shared a unique patient code.
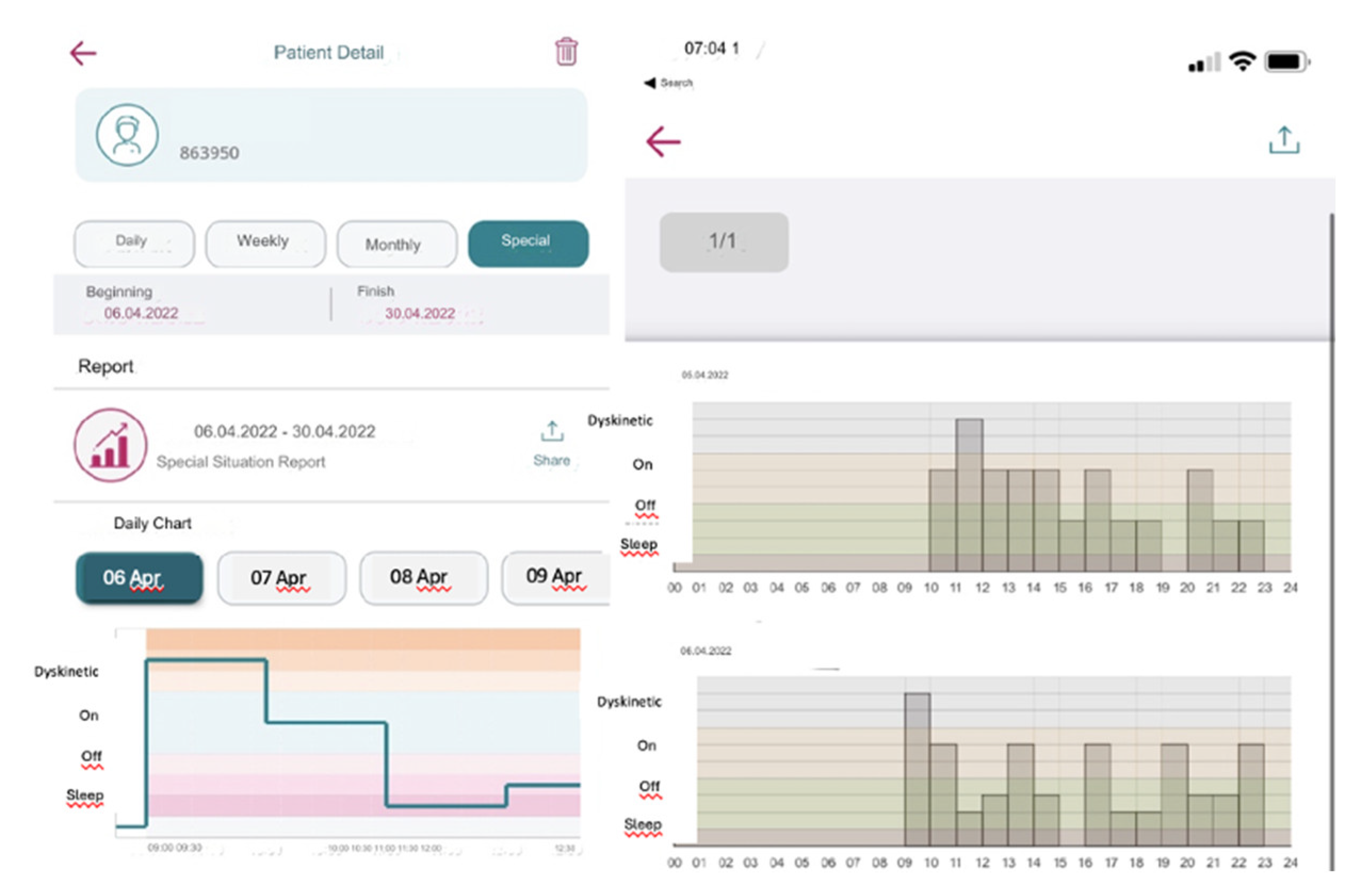
If the patient forgets to answer or cannot answer the question, they can only respond to the next question. They are not allowed to go back and answer the previous question/s to ensure the integrity of the tracking system and prevent retrospective adjustments, changes in current data, or multiple entries at once. The app collects these entries into a single timeline and visually organizes them chronologically over numerous days. It then reviews detailed graphs displaying data over various intervals and adjusts treatments as needed (
Figure 4). This feature has been added to be useful for patients and clinicians in determining when exactly symptoms are getting worse or when medication works best.
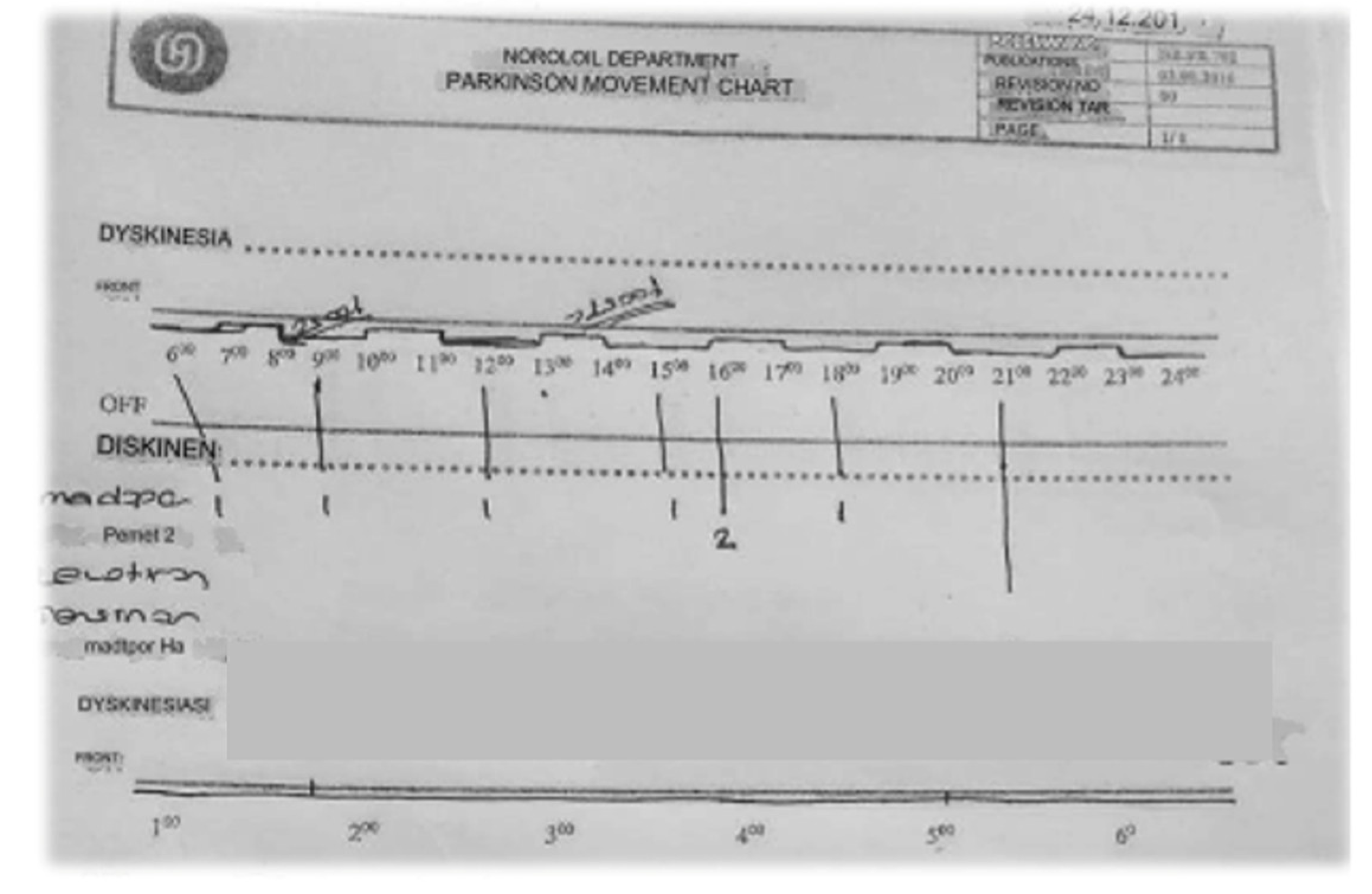
24-Hour Parkinson’s Disease Motor Symptom Monitoring Chart:
This chart is designed for clinic visits in our department. During the visit, the clinician records motor fluctuations in dyskinesias and “on” and” off” periods based on the patient’s anamnesis. The timeline at the top of the chart spans from 6:00 am to 2:00 am the following day, with annotations marking the patient’s motor status at different times. Medication names are noted along the left side, with an arrow indicating when the patient took each medication. The chart visually represents the correlation between medication administration and the patient’s motor fluctuations, providing valuable data for clinicians to adjust treatment plans. The precise identification of” on” and” off” periods, along with times of dyskinesia, allows for a more tailored and effective management of Parkinson’s Disease symptoms (
Figure 5).
2.4. Statistical Analyses
All statistical analyses were conducted using SPSS software, version 22. Descriptive statistics were presented as frequencies (n) and percentages (%) for categorical variables and as medians with corresponding minimum and maximum values for continuous variables. The weighted Kappa (κ) statistic assessed inter-rater agreement for each bias risk assessment tool domain and the overall evaluation.
4. Discussion
This study results show that the electronic diary was rated better for overall patient adherence and accuracy among patients who initiated paper diaries. Higher compliance is observed with the digital diary compared to other measures, and there is more significant agreement with clinical notes. Electronic diaries offer advantages by improving compliance through reminders and reducing recall bias, but they also present challenges, especially in extensive multicenter studies [
12].
In our study, paper diaries provided worse compliance and accuracy to real-time data than digital diaries in monitoring motor symptoms of PD. While conventional paper diaries have been widely used, they face compliance issues and recall bias. Löhle et al. assessed the validity of the Parkinson’s disease (PD) home diary (paper diary) for quantifying motor states by comparing it to direct clinical observation. The results indicate that the diary often inaccurately reflects actual motor states, with overall agreement at 59.8% (Cohen’s Kappa 0.387) and significant discrepancies in documenting “On” without dyskinesia (52.3% vs. 38.9%) and “On” with dyskinesia (21.5% vs. 34.2%), suggesting it may be an unreliable tool for assessing motor fluctuations in advanced PD [
20].
The digital world is evolving, especially after the COVID-19 pandemic; telemedicine and digital tools have become an irreplaceable part of life [
21]. Marxreiter et al. evaluated the use of digital technologies among Parkinson’s disease (PD) patients across different age groups. The study surveyed 190 Parkinson’s disease (PD) patients and found that 75% accessed disease-related information online, with digital technology use comparable to the general population. However, smartphone usage decreased in advanced PD patients due to motor impairments, suggesting that while PD patients are adopting digital tools, future healthcare technologies must account for motor difficulties in smartphone use [
22]. Habets et al. developed and validated an electronic diary that monitors Parkinson’s disease symptoms during daily life. In this study, 20 patients used the developed eDiary for 14 days. The results indicated moderate-to-strong relationships between mood, motor symptom severity, and motor performance slowness of the implemented eDiary, demonstrating the validity of psychometric properties. This study also implies the importance of carefully developing the eDiary and patient selection for successful routine application [
23]. Lakshminarayana et al. assessed the effect of using a smartphone-based Parkinson’s Tracker App (PTA) for people with PD on enhancing medication adherence and clinical consultation experience by conducting multicenter RCT in England and Scotland. The trial included 215 participants and found that the PTA more than doubled medication adherence, and patients reported quality of consultation compared to usual care.
Furthermore, 72% of participants used the app weekly for all four months, reflecting a high level of patient engagement. This was consistent with the results in that improvements were seen in non-motor symptoms and QOL but did not reach statistical significance. This study concluded that the PTA assists self-management and improves clinical care for Parkinson’s disease; however, further research is needed on the long-term outcome of using the tool [
24].
Previous studies comparing smartphone and paper diaries for chronic diseases show varying results [
25,
26,
27]. Regarding digital diaries, Lyons et al. evaluated the feasibility and compliance of electronic diaries in PD patients. Over seven days, 12 patients recorded their motor function every half hour, with the diary sending alarms for missed entries. The compliance rate was exceptionally high at 99.98% within 24 hours, with an average response time of 63 minutes during waking hours. Patients successfully operated the electronic diaries, achieving over 89% acquisition compliance, and the average response compliance was around one hour, providing valuable data on response patterns. In the questionnaire conducted after using the electronic diary in the study, half of the patients preferred the electronic diary over the paper diary, and 83% of the patients stated they would use this device to evaluate a new therapy for PD [
28]. Reimer et al.[
10] examined the utility and reliability of a CAPSIT-PD on/off diary using a four-point scale to measure fluctuations in Parkinson’s disease. Although agreement between patients and clinicians was good overall, the study showed that the CAPSIT-PD diary needed to be administered for too long a duration (> seven days) because shorter periods (one to three days) did not predict motor fluctuations over time. Our study’s relatively higher compliance with the digital diary is consistent with other up-to-date studies examining digital health tools within PD. Chuapakdee et al. developed a simplified, symptom-based, and electronic Parkinson’s disease (PD) diary as part of monitoring motor complications (MCs) in advanced PD. They compared it with traditional paper diaries regarding feasibility and critical outcomes. The electronic diary (e-PD) was created using a smartphone application with alarms and a picture response option to record in real-time ON/OFF states and dyskinesias. Seventeen non-demented PD patients with stable medication regimens used electronic and paper diaries simultaneously for at least one week. There was no significant difference in the time of ON/OFF/dyskinesia recorded by each method, although an 80.2% coefficient existed for functional state crossed between two diaries. The new method, the e-PD diary, had an accuracy of 81.1% with a sensitivity and specificity of 89.8% and 68.5%, respectively. Additionally, patients reported superior compliance and satisfaction with the e-PD diary. Conclusions: the e-PD diary is a feasible and accurate tool that could optimize motor complications management in PD[
29].
The higher data accuracy from the digital diary application used in our study is consistent with the findings by Ossig et al., who compared paper diaries to sensor-based digital monitoring systems [
11]. Interestingly, digital monitoring devices even detected subtle motor fluctuations not otherwise captured by traditional diaries[
7] On the other hand, the study by Nyholm et al. compares the effectiveness of conventional paper diaries and electronic diaries for tracking Parkinson’s disease (PD) symptoms, particularly in the presence of motor fluctuations. Twenty patients with PD, diagnosed for at least five years, were randomly assigned to use either a paper or electronic diary over eight days within a month. Patients answered questions every two hours for 12 hours. The results showed that median compliance was slightly higher with the paper diary (98%) than the electronic diary (88%). However, strict compliance linked to the scheduled times was lower in the paper diary. Age and prior computer experience did not influence the ability to use the electronic diary. The study concludes that electronic diaries effectively assess PD symptoms, offering real-time data capture and accurate compliance tracking[
30]. Like many digital diaries in the literature, we also observed high-performance variability by supporting the idea that digital diaries improve patients’ contribution by allowing patients to receive self-reports in real-time and create a better understanding of their state as said states are affected by interventions. However, there are challenges to be overcome; for example, patient adherence is shaped by technology-related barriers such as those described in Terroba-Chambi et al.’s paper [
31]. Riggare et al. mentioned despite the potential of digital health to revolutionize Parkinson’s care, existing solutions frequently fall short of addressing their specific needs [
32]. Espay et al. stated that while technology captures more detailed data on Parkinson’s disease, it hasn’t yet improved diagnosis or treatment. Challenges include incompatible platforms, the need for widespread sensor use, and the gap between large data sets and clinical applications. A task force is working to develop open-source platforms for personalized treatment, early detection, and better management of tools that may struggle to have the same transformative impact on PD without managing usability and patient education [
33]. Arora et al. highlight the need for patient education and support in implementing digital tools. Digital diaries were generally more acceptable, but acceptability depended on patients’ experience with this technology, as the study showed [
34]. In chronic disease management, patient engagement is essential as the care needs to be monitored regularly and requires the participation of patients themselves. Since the number of patients was small and the ages of the patients were quite similar, we could not analyze the effect of age in our study. Age and education level may affect the preference for using a digital app rather than keeping a written track of symptoms in patients with Parkinson’s disease (PD). Research indicates that given the proper education and familiar interface, older patients can use digital tools for symptom tracking[
35,
36]. Mobile Apps for PD have demonstrated increased usability with familiarity and support; they apply even to an aging population[
25]. People with higher levels of education are more knowledgeable about PD and accept digital health technologies with technical skills and an orientation toward using new tools [
37]
Digital tools play an increasingly important role in every aspect of Parkinson’s disease. For instance one important tool that plays a vital role in helping people improve their movements and in relieving the gait freezing (FOG) resulting from Parkinson’s Disease is using visual cues. Prolonged stride length, an early warning sign of shuffling backwards or cessation, can be eliminated especially by foot tapping lightly. Stripes across the scene effectively reduce sequence effect, a slow shortening of steps until they freeze. They’re better than mobile lasers or medication [
38]. My Parkinson’s app, which is digitized, reinforces these strategies by enabling self-management practices and keeping an eye on symptoms. These apps remind patients to record their condition, such as FOG–at this moment, such information is not yet available. They can introduce a large bias to analyze their own illness unless they have been universally observed and accepted in hospital records. Mobilephone applications can also be combined with real-time auditory cues across AR to effectively serve both sound and visual intervention in the control of PD.
The availability of “The Parkinson’s” interface in Turkish is a statement about how our study group is mainly made up of Turkish speakers. But this single-language setup will restrict those who might benefit from usage of the app to track Parkinson’s Disease-related motor symptoms. As Parkinson’s disease is distributed throughout the globe, it is essential that multilingual support will be provided. We are currently in the process of expanding the widely spoken languages such as English and Spanish
The multilingual support may extend both the reach and the ease of use of “My Parkinson’s” to diverse patient populations. Apps like this one will gradually work themselves into different and more complicated clinical environments, assisting patients better to keep their doctor’s appointments across all kinds of population groups rather than simply one. This adjustment will allow for sharing of research findings across cultures and lay the basis for a more complete understanding of how PD operates and is treated in different linguistic regions, adding the languages is also one more important step toward making sure digital health tools like “My Parkinson’s” become global medical facilities.
The results of our study bear clinical relevance, as they imply an ability for digital diaries to shape how PD is managed in the future due to their potential for integrating more precise symptomatology that occurs on a day-to-day basis. This is consistent with the observations of Dorsey et al.’s [
39], who noted the potential for digital tools to enhance clinical decision-making by reinforcing data available with greater granularity and immediacy than that within traditional methods. Moreover, incorporating digital tools in day-to-day clinic practice is a game-changer, allowing new possibilities for 24/7 monitoring and truly patient-centered proactive care[
40].
Among all the smartphone apps tracking motor symptoms of PD, ‘MyParkinson’s’ app is an excellent tool among these new apps -- with direct clinician integration and real-time symptom tracking through its ongoing symptom logs. In all, human health care will improve considerably using this unique program. One of the most distinctive features of ‘MyParkinson’s’ is real-time data sharing, which means clinicians can monitor patients’ motor symptoms as they occur and adjust treatment strategies accordingly. It is essential in dynamic clinical environments where proper intervention can make a huge difference in patient outcomes. In contrast,”mPower” or Parkinson’s mHealth are primarily concerned with data collection, and these fail to give clinicians real-time access, limiting their usefulness in responsive care settings. I MyParkinson’s provides self-management and pertinent customization according to patient or provider preference, leading to more personalized care. In contrast, mPower and Parkinson’s mHealth require strict tracking protocols that may not align with the customized nature of PD [
41]. Regarding logging of symptoms, MyParkinson currently covers motor symptoms, with non-motor to be incorporated in future releases. The primary added value of MyParkinson’s capturing motor and NM symptoms is its potential to integrate real-time feedback into clinical care for responsive symptom management, a feature that neither uMotif nor mPower currently provides[
24].
MyParkinsons is unique in that it sends reminders for symptom logging to log data at regular intervals, which minimizes recall bias and ensures the quality of registered data. Other apps e.g., mPower, and Parkinson mHealth, allow more flexible logging with possible transgressions from delayed data entry, possibly introducing bias[
7]. And, with a focus on availability, MyParkinsons is available in Turkish and will have additional language support added soon. While this is a welcome increase in diversity, it contrasts with the limited availability of migration tools like mPower, which can only be used in English. Global healthcare platforms require this option, and uMotif is outstanding with its multiple-language support[
24].
In summary, MyParkinson’s provides an all-around customizable platform facilitating the patient-physical therapy relationship by allowing real-time data sharing between the rehabilitation system and clinician, offering interactive sessions via various mediums, and providing easy-to-use patient interfaces. Despite the additional features in apps like uMotif and Roche PD Mobile Application V2, which can provide machine learning insights, MyParkinson offers a good trade-off between current care and being easy to use for patients. This aligns with the rising trend of integrated digital platforms for PD management, as shown by Vizcarra et al., highlighting the importance of e-diaries to improve clinical and research results [
7]. A systematic comparison of MyPakinson’s and other motor-tracking e-diary apps is summarized in the table in the Supplementary material (Suppllement 2)
. Limitations:
This study has several limitations that are kept in mind while proposing potential solutions. Although the study did reach the sample size as determined based on a power analysis, however, the limited number of participants may be problematic for generalizing the results from this study. Although large enough to detect the expected effect, this sample size is not necessarily sufficient to identify more subtle effects that might be present in a larger population. Moreover, small sample sizes could result the observed variability within some subgroups to be decreased and this may contribute to a reduction of the robustness of subgroup analyses. Another limitation of this study is that the patients might have experienced an influence on their behavior by using a digital diary which could potentially have been due to increased motivation derived from the “fashionable” or novel nature of the technology. The excitement of a new method on its own will have an effect, we however did not intend to control for those aspects since this was primarily examine the compliance in relation to diary smudging and accuracy between paper versus digital diaries. Our results suggest that future research should consider either including a third group or using alternative assessments to better capture the novelty effect for these diary methods over time.
Another limitation of our research might be that it relied on patient-reported evaluations, which despite the fact that they were recorded in real time and by the hour, might still have been intrinsically marred from recall bias. In PD specifically, this aspect can play a vital role--cognitive impairments may affect the memory and recall of symptoms If it was essential to try and diminish recall bias by our process, we still have not found answers as to whether this in itself is enough--with a lack of objective measurements like wearable sensors being another potential limiting factor on how precisely different symptoms will be reported in future research. Therefore, it is suggested that future studies should integrate such technology to give uninterrupted and unselective monitoring from the patient’s end; hopefully, these methods can be better verified by results based on previously applied experimentation.
Hence, it will affect the generalizability of results as we lack an adequate cohort or numbers to support robust analysis. Moreover, the need for familiarity with smartphones and internet access to participate introduces selection bias. A limitation of this study is that the short follow-up duration (24 hours) may not capture complete long-term use and compliance with diaries. Patients ‘ experience with technology was ignored; some might have had little familiarity with how to use the digital diary. As far as the treatment of Parkinson’s disease (PD) is concerned, the good management and tracking required for relief of symptoms are invaluable.When the application “My Parkinson’s” became the first to take both motor and non-motor symptoms into account, it began blazing a new trail in PD management not just for patients but also for clinicians. Sleep disturbances, tiredness and anxiety, non-motor symptoms just like these extend the current technique of care anxiety to people living with a set of issues completely different at every hour of the day. In this new framework improved treatment of motor symptoms should bring about much better quality care because it deals with all the often neglected non-motor symptoms of which drama queen patient’s lives are mostly comprised.