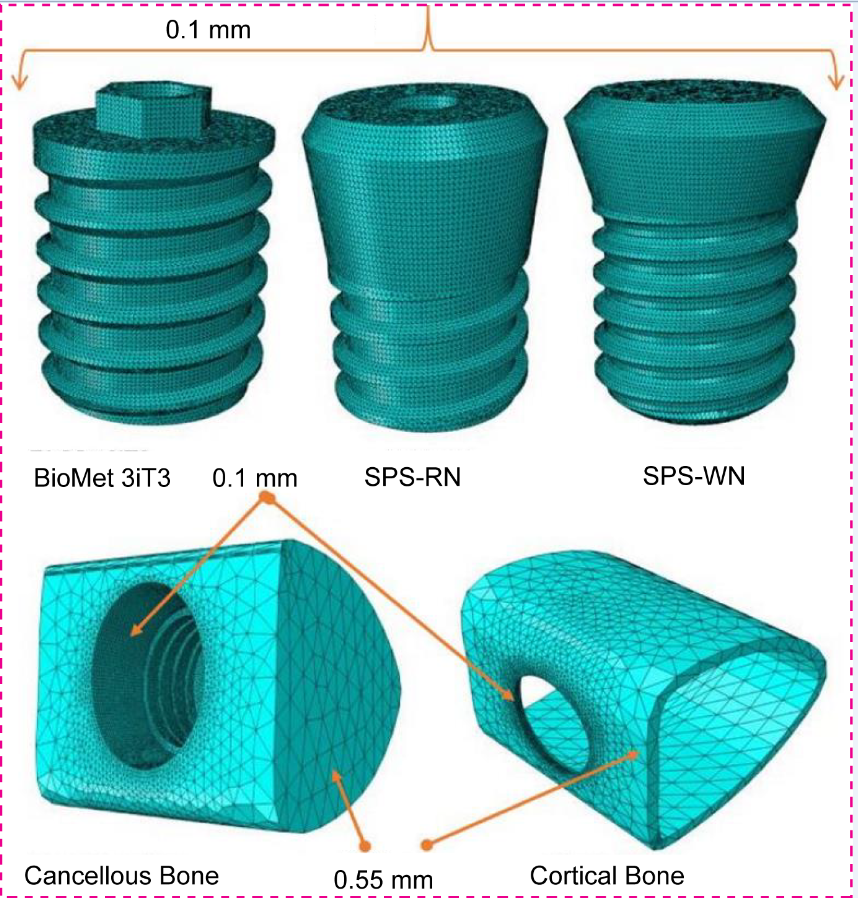
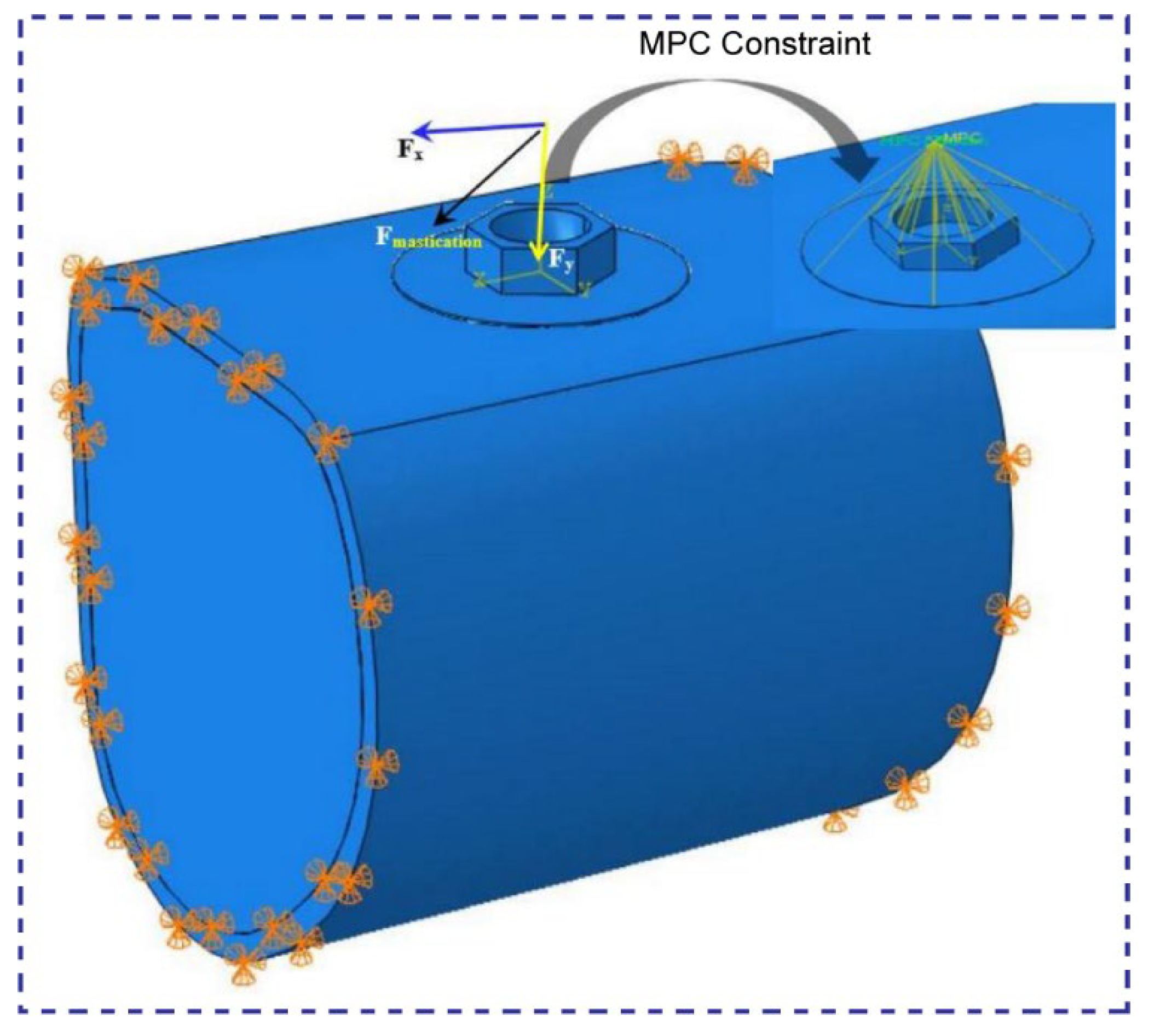
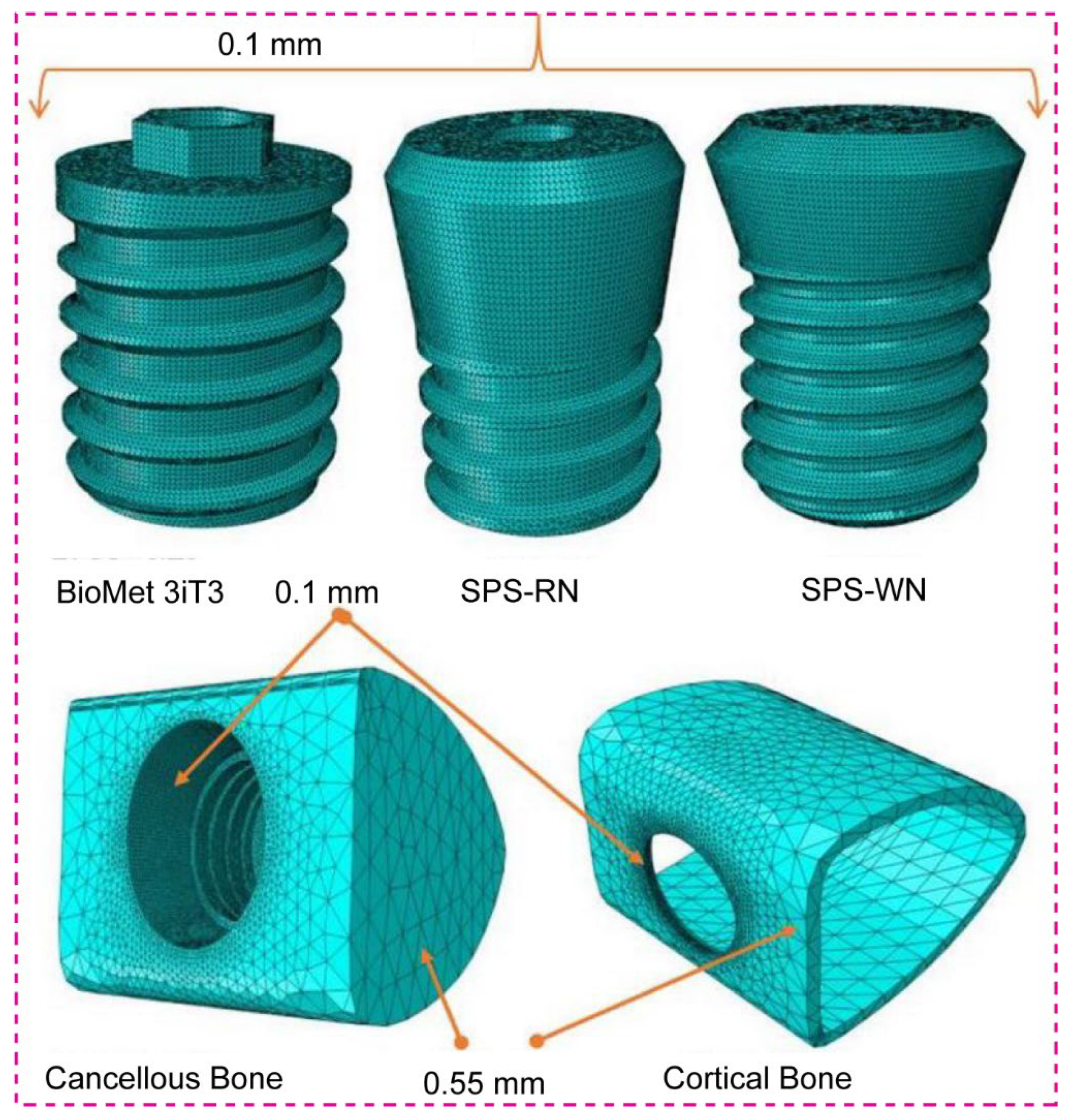
1. Introduction
Dental implants have transformed the sector by offering a long-lasting, functionally efficient, and aesthetically beautiful alternative to missing teeth [
1,
2]. Dental implants aim to integrate directly with the jawbone, a process known as osseointegration, unlike typical dental prostheses like dentures or bridges[
3,
4,
5]. This integration is critical to the implant’s long-term durability and success because it enables it to operate like a natural tooth root, firmly supporting the overlying prosthesis[
6,
7,
8]. Short dental implants have become a more prevalent dental restorative treatment, mainly for individuals with poor bone health[
9,
10,
11]. These implants are a stable and durable strategy for restoring lost teeth, with significant benefits over conventional dentures or bridges. Unlike standard dental artificial teeth, short dental implants directly connect into the jawbone through a process known as osseointegration[
12,
13]. This bonding is important for recovering teeth’s function in addition to their original physique because it enables the implant to act analogously to an actual tooth root, firmly holding the prosthesis.
However, the recipient’s bone qualities, along with the surgical approach and implant quality, heavily influence the success of dental implants[
14,
15,
16]. In recipients with an appropriate quantity of bone and density, osseointegration typically occurs readily, resulting in an anchored and safe implant. However, a considerable minority of patients, notably those with low bone density or height, have major difficulties in attaining effective osseointegration[
12,
17,
18,
19]. The efficacy of dental implants is intimately related to osseointegration, in which bone forms around and binds with the implant surface, forming a firm base[
20,
21,
22]. These properties are particularly prevalent in Bone Types III and IV, where the bone is either less thick or exceedingly porous, thereby increasing the likelihood of malfunctioning implants[
23,
24,
25,
26].
Short dental implants have emerged as a potential solution for individuals with poor bone health in the context of dental implantology[
27,
28,
29]. Traditionally, invasive operations like bone grafting or sinus lifts treated patients with insufficient bone height or density, augmenting the bone and providing a solid foundation for standard-length implants[
30]. While successful, these techniques are linked to greater surgical complexity, longer recovery periods, and higher expenses[
31,
32,
33]. Short implants, typically less than 7 mm in length, produce adequate results in situations where regular implants may be prohibited[
34,
35,
36,
37]. Short dental implants have grown in popularity because of their potential to offer excellent results in situations when regular implants would be undesirable. Small implants, when implanted in the accessible bone, may eliminate the need for extensive grafting operations in patients with limited bone height, particularly in the posterior maxilla[
38,
39,
40]. Also, when a patient has low bone density, short implants put less stress on the bone around them. This may help the distribution of occlusal force and lower the risk of overworking the bone-implant contact[
34,
41,
42].
The practical use of short dental implants has benefited from advancements in implant design and surface technology, as they have proven to enhance osseointegration, even in challenging bone conditions[
13,
43,
44]. Despite these advances, research into the biomechanical behavior of short implants with varied degrees of osseointegration is still underway. Understanding how varied degrees of osseointegration influence stress distribution and strain at the bone-implant interface is critical for optimizing implant designs and improving patient outcomes, especially in individuals with low bone density [
45,
46,
47].
A variety of industries, including automobile engineering[
48,
49] , dentistry [
50,
51], and heat transfer studies [
52], use the Finite Element Method (FEM), an advanced and adaptable computational method. In the field of dental implantology, FEM is critical for biomechanical characterization of dental implants and surrounding bone structures[
50,
51]. This technology enables researchers and physicians to anticipate how implants will interact with bone in various settings by simulating and evaluating complex mechanical characteristics in detail [
53]. Researchers have widely used FEM to estimate stress distribution inside dental implants, as well as in compact and spongy bone, under various loading conditions and degrees of osseointegration [
24].
This research study significant biomechanical effects of different osseointegration percentages in three different short dental implant models: BioMet 3iT3, SPS-RN, and SPS-WN. The main goal is to find the best short dental implant among these options for replacing natural teeth in people with low bone density and not enough bone height, specifically less than 7 mm. Traditional approaches to treating these difficult illnesses sometimes include intrusive operations such as bone grafting, which are associated with greater risks, longer recovery periods, and higher costs. Short dental implants, on the other hand, are a less intrusive option that may eliminate the need for bone augmentation while still giving effective and long-lasting results. To do this, we used nonlinear three-dimensional finite element analysis (FEA) to model how the bone and implant interact when the bone density and osseointegration are different. The study looked at a lot of important things, like the highest stress at the bone-implant contact (BIC), especially at the implant neck and bone hole locations, as well as the highest and lowest primary strains at the BIC. Furthermore, we investigated the distribution of maximal von Mises stresses across each of the three short dental implants. These biomechanical factors are critical for determining the implants’ capacity to efficiently distribute mechanical loads, reduce localized stress concentrations, and improve overall stability and durability. This study aims to shed light on the comparative performance of these short implants in clinical settings where bone grafting is not feasible. The goal of this research is to help doctors choose the optimal implant for patients with low bone density and restricted bone height by determining the implant type that provides the highest biomechanical performance under compromised bone circumstances. Finally, this study not only adds to the current body of evidence, but it also has the potential to impact medical decisions, resulting in better patient outcomes and defining a new standard for dental implantology in difficult situations.
3. Results
3.1. Maximum Stress and Maximum Strain Bone
3.1.1. BioMet 3iT3 Short Implant
Table 4 shows that the mechanical response of cortical and cancellous bones varies significantly with osseointegration level. The findings demonstrate that when the osseointegration percentage in cortical bone increases from 25% to 75%, the maximum stress rises significantly from 158.4 MPa to 174.3 MPa. This implies that an improvement in bone-implant contact enhances the transmission of load to the cortical bone, leading to a rise in stress levels. Despite the increased stress, the strain inside the cortical bone reduces substantially, from 0.02559 at 25% osseointegration to 0.009997 at 75%. This decrease in strain suggests that the cortical bone is better able to bear applied stresses as osseointegration advances, most likely due to a more solid and durable bone-implant contact.
Table 3 shows a somewhat different trend in cancellous bone. The maximum stress falls significantly as osseointegration progresses, decreasing from 19.23 MPa at 25% to 17.79 MPa at 75%. This means that the cancellous bone is under less stress when the implant integrates with the surrounding bone tissue. Similarly, strain in cancellous bone drops considerably with improved osseointegration, from 0.106 at 25% to 0.03264 at 75%. This decrease in strain demonstrates the beneficial effect of improved osseointegration on the mechanical stability of the implant, since it minimizes the risk of excessive deformation in the surrounding bone.
3.1.2. Standard Plus Short (SPS) Implants with Regular Neck (SRN)
The results in
Table 5 show that the Standard Regular Neck SRN-Straumann
® Standard Plus Short (SPS) Implants put different amounts of stress and strain on the cortical and cancellous bones at different osseointegration percentages. The findings reveal significant variations in how bone types III and IV respond to patient bite loads. When the osseointegration percentage for Bone Type III goes from 25% to 100%, the highest stress in the cortical bone changes. It goes from 137 MPa at 25% osseointegration to 136.6 MPa at 100% osseointegration. However, the maximum strain in the cortical bone drops dramatically, from 0.04126 at 25% osseointegration to 0.009295 at 100%. This suggests that with increased osseointegration, the cortical bone deforms less under strain, implying a more stable bone-implant contact. The maximum stress in type III cancellous bone decreases from 38.48 MPa at 25% osseointegration to 33.32 MPa at 100%. Similarly, the maximum strain in cancellous bone decreases significantly, from 0.1099 at 25% osseointegration to 0.02463 at 100%. These findings show that increased osseointegration not only lowers stress levels but also significantly decreases strain in cancellous bone, improving the implant’s durability. For Bone Type IV, the consequences are more apparent. The maximum stress in the cortical bone is much greater, beginning at 330.1 MPa at 25% osseointegration and dropping to 300.1 MPa at 100%. The strain in the cortical bone drops significantly, from 0.08977 at 25% osseointegration to 0.02171 at 100%. These findings imply that since bone type IV is less dense, it endures more stress at first, but as osseointegration progresses, the bone’s capacity to withstand deformation under load increases.
The maximum stress in type IV cancellous bone decreases significantly, from 26.12 MPa at 25% osseointegration to 25.45 MPa at 100%. The strain shows a similar trend, falling from 0.4199 at 25% osseointegration to 0.1027 at 100%. These results show that even though stress levels in cancellous bone are usually lower than those in cortical bone, the decrease in strain that comes with better osseointegration is very important for keeping the structure of the implant, especially in bone that isn’t very dense.
3.1.3. Standard Plus Short (SPS) Implants with Wide Neck (SWN)
The data in
Table 6 shows that the Standard Wide Neck SWN-Straumann
® Standard Plus Short (SPS) Implants, which are 4 mm long and 4.8 mm wide, cause different levels of stress and strain in the cortical and cancellous bones when the patient bites down on them. This happens at different levels of osseointegration. For bone type III, the findings reveal that when the osseointegration percentage grows from 25% to 100%, the maximum stress in the cortical bone drops from 135.5 MPa to 114.3 MPa. This tendency shows that as osseointegration develops, the implant becomes more closely integrated with the bone, prompting the cortical bone to endure less stress. As a result, the strain in the cortical bone drops substantially, from 0.04750 at 25% osseointegration to 0.009631 at 100%. This reduction in strain suggests that the bone deforms less under stress as osteointegration proceeds, which improves implant stability. The maximum stress in Type III cancellous bone decreases slightly, from 14.78 MPa at 25% osseointegration to 13.34 MPa at 100%. Similarly, the strain in cancellous bone decreases from 0.06905 at 25% to 0.01645 with 100% osseointegration. Although cancellous bone typically experiences lower stress levels than cortical bone, it also benefits from enhanced osseointegration, creating a more stable and supportive environment for the implant.
The findings for bone type IV demonstrate a more significant trend. At 25% osseointegration, the maximum stress in the cortical bone is 399.1 MPa, which decreases to 322.7 MPa at 100%. The equivalent strain in cortical bone drops significantly, from 0.09706 at 25% osseointegration to 0.02004 at 100%. This suggests that increased osseointegration significantly reduces both stress and strain in less dense Bone Type IV, which is critical for lowering the likelihood of implant failure due to excessive loading. The maximum stress in Type IV cancellous bone is relatively constant, ranging from 15.73 MPa at 25% osseointegration to 15.68 MPa at 100%. However, the strain drops significantly, from 0.2803 at 25% to 0.06946 with 100% osseointegration. This large decrease in strain indicates that with increased osseointegration, the cancellous bone is better able to distribute the applied stresses, lowering the chance of deformation and improving overall implant stability.
Table 6.
Maximum stress and maximum strain for Standard Wide Neck SWN- Straumann® Standard Plus Short (SPS) Implants, at 4 mm long, 4.8 mm diameter produced in bones due to applied patient biting load.
Table 6.
Maximum stress and maximum strain for Standard Wide Neck SWN- Straumann® Standard Plus Short (SPS) Implants, at 4 mm long, 4.8 mm diameter produced in bones due to applied patient biting load.
| Bone type |
Osseointegration (%) |
Highest Stress |
Highest Strain |
| Cortical (MPa) |
Cancellous (MPa) |
Cortical |
Cancellous |
| III |
25 |
135.5 |
14.78 |
0.04750 |
0.06905 |
| 50 |
127.1 |
14.27 |
0.02200 |
0.03396 |
| 75 |
120.2 |
13.79 |
0.01368 |
0.02228 |
| 100 |
114.3 |
13.34 |
0.009631 |
0.01645 |
| IV |
25 |
399.1 |
15.73 |
0.09706 |
0.2803 |
| 50 |
369.0 |
15.72 |
0.04523 |
0.1398 |
| 75 |
344.0 |
15.70 |
0.02831 |
0.09292 |
| 100 |
322.7 |
15.68 |
0.02004 |
0.06946 |
3.2. Maximum Shear Stresses along the Three-Plane
According to
Table 7, the highest shear stresses in three planes (Sxy, Sxz, and Syz) for different implant types under a static mastication load vary significantly in cancellous and cortical bones at different stages of osseointegration.
As osseointegration grows from 25% to 100% in the BioMet 3iT3 implant model, shear stresses in cancellous bone vary to some extent. Specifically, the shear stress in the XY plane (Sxy) begins at 0.9718 MPa at 25% osseointegration and increases gradually to 1.581 MPa at 100%. The XZ and YZ planes exhibit similar tendencies, with stress levels typically increasing as osteointegration improves. Cortical bone, on the other hand, experiences more significant variations in shear stresses. For example, the shear stress in the XY plane (Sxy) rises from 97.53 MPa at 25% osseointegration to 102.8 MPa at 100%, but the XZ plane (Sxz) increases significantly from 55.37 MPa to 67.93 MPa over the same range. This suggests that when osseointegration advances, the cortical bone experiences increased shear stresses, perhaps indicating improved load transmission and stability. The shear stresses in cancellous bone in the SPS-RN (Standard Plus Regular Neck) implant model are rather consistent across varying osseointegration levels. The Sxy stress in the XY plane, for example, maintains at 1.55 MPa for all osseointegration levels. Shear stresses in cortical bone, on the other hand, fall in a clear pattern. The XY plane stress (Sxy) drops from 78.34 MPa at 25% osseointegration to 75.28 MPa at 100%. This reduction implies that when bone-implant contact improves, the distribution of shear loads becomes more uniform, possibly lowering the likelihood of localized stress concentrations. It was found that shear stresses in cancellous bone are usually lower in the SPS-WN (Standard Plus Wide Neck) implant model than in the others. The Sxy stress in the XY plane drops from 1.105 MPa at 25% osseointegration to 1.095 MPa at 100%. The cortical bone experiences a greater decrease in shear forces as osteointegration advances. For instance, the Sxy stress in the XY plane decreases from 62.77 MPa at 25% osseointegration to 57.96 MPa at 100%, with comparable trends observed in the other planes. This decrease in shear stress, especially in cortical bone, shows that the implant’s stability increases with improved osseointegration, as the bone becomes more capable of handling the imposed stresses without excessive shear.
3.3. Maximum von Mises Stress at the Bone-Implant Contact (BIC)
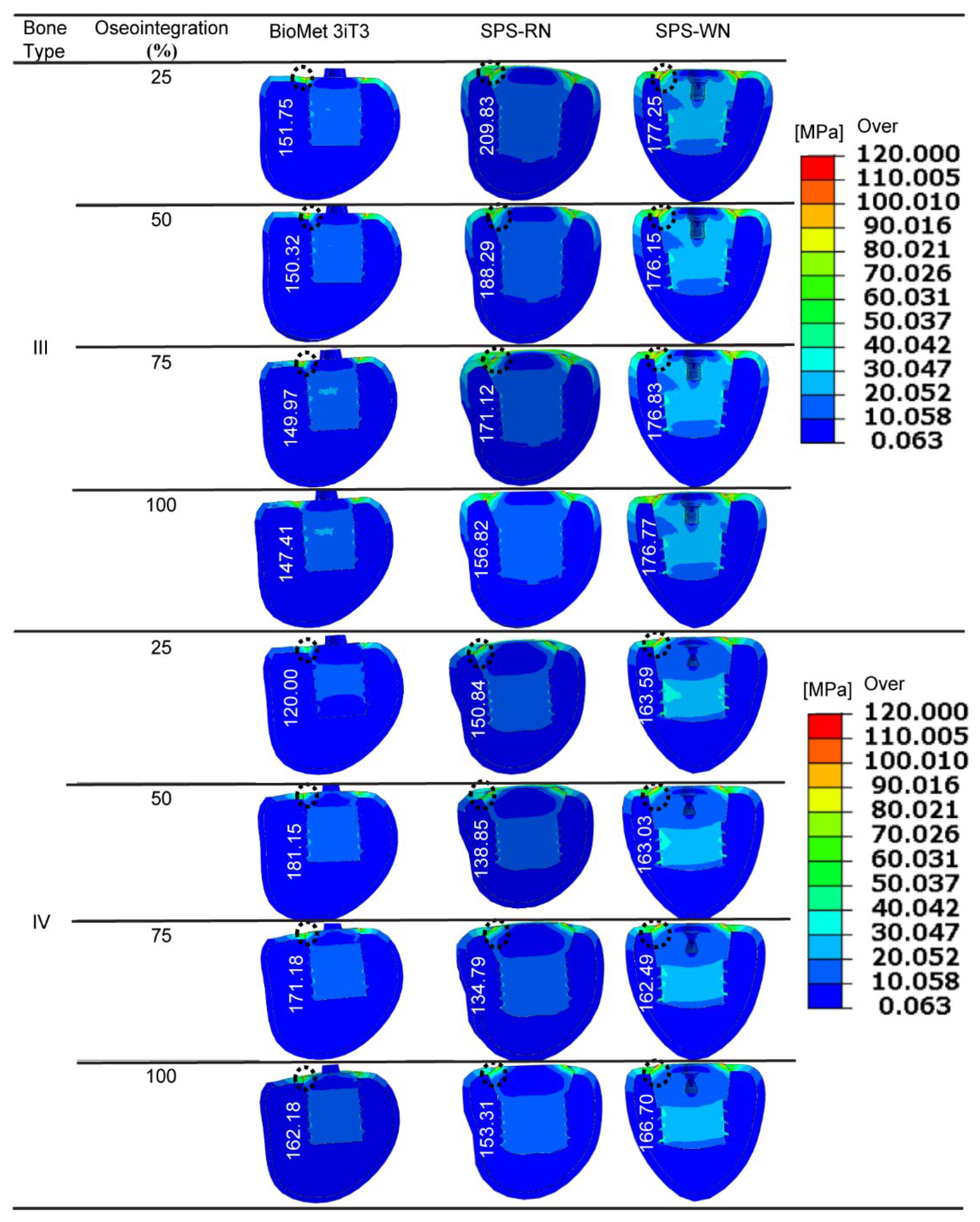
For three types of short implants—BioMet 3iT3, SPS-WN, and SPS-RN—at different stages of osseointegration,
Figure 4 below shows the highest von Mises stress at the bone-implant contact (BIC) area. This is the area where the bone hole meets the implant’s angled neck. The black dotted circles in the illustrations represent the highest stress position in the associated contour plots.
For all implant types, including Bone Type III, the maximum von Mises stress values decrease as osseointegration increases. For example, the BioMet 3iT3 implant experiences a stress drop from 151.75 MPa at 25% osseointegration to 147.41 MPa at 100% osseointegration. The SPS-WN implant exhibits a greater reduction in stress, from 209.83 MPa at 25% osseointegration to 156.82 MPa at 100%. Similarly, the SPS-RN implant experiences a slight drop from 177.25 MPa to 176.77 MPa across the same range. These findings show that when osteointegration improves, the load distribution at the bone-implant interface becomes more uniform, resulting in reduced stress concentrations and perhaps increasing implant stability. In bone type IV, the pattern is more complicated. The BioMet 3iT3 implant’s von Mises stress first increases from 120 MPa at 25% osseointegration to 181.15 MPa at 50%, then gradually decreases to 162.18 MPa at 100% osseointegration. The stress on the SPS-WN implant, on the other hand, drops from 183.85 MPa at 25% osseointegration to 135.31 MPa at 100%. This shows that better osseointegration lowers stress at the interface, especially in bone that isn’t as dense. The SPS-RN implant had generally steady stress values of 162.49 MPa throughout various osseointegration phases, with a little rise to 166.7 MPa at 100% osseointegration. These findings, as seen in the following figure, emphasize the necessity of attaining perfect osseointegration to reduce stress concentrations at the bone-implant interface, possibly lowering the likelihood of implant failure and improving long-term stability. Variations in stress between implant types and bone conditions further indicate that implant design and bone quality have a considerable impact on the mechanical environment at the BIC, which is crucial for dental implant success. maximum von Mises stress
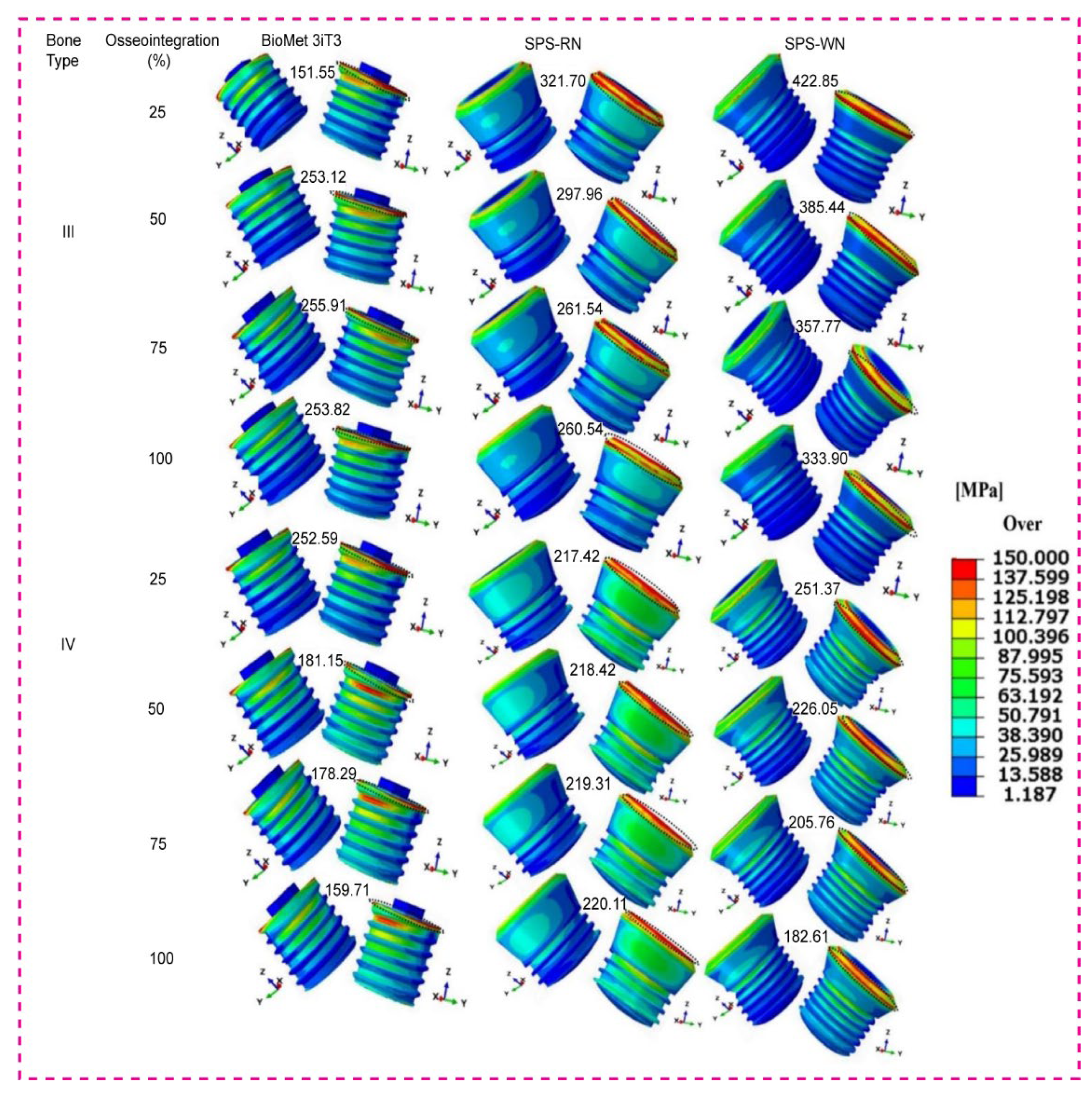
3.4. Maximum von Mises Stress in Three Types of Short Dental Implants
Contour plots of the maximum von Mises stress, representing the distribution of mechanical stress during loading, three types of short dental implants depicted in
Figure 5 below.
As osseointegration advances, Bone Type III, the BioMet 3iT3 implant, exhibits a significant rise in von Mises stress, beginning at 151.55 MPa at 25% and culminating at 255.91 MPa at 75%. Interestingly, with 100% osseointegration, the stress drops significantly to 253.82 MPa. The SPS-RN implant follows a similar trajectory, with a high starting stress of 321.7 MPa at 25% osseointegration and gradually decreasing to 260.51 MPa at 100% osseointegration. However, the SPS-WN implant suffers the greatest stress values of any implant type, beginning at 422.85 MPa at 25% osseointegration and decreasing to 333.9 MPa at 100%. These findings suggest that the SPS-WN implant puts more stress on the bone around it in Bone Type III, even when osseointegration improves. This raises the possibility of implant-bone interface stress concentration. Bone Type IV: The BioMet 3iT3 implant has a high initial von Mises stress of 252.59 MPa at 25% osseointegration, which decreases significantly to 179.51 MPa at 100%. The SPS-RN implant has a more constant stress level, beginning at 217.42 MPa at 25% osseointegration and rising slightly to 220.11 MPa at 100%. The von Mises stress on the SPS-WN implant slowly goes down, from 251.37 MPa at 25% osseointegration to 182.61 MPa at 100%. This shows that this implant design benefits the most from better osseointegration in Bone Type IV, which is less dense. Overall, the von Mises stress contours show that osseointegration is crucial for lowering stress at the bone-implant contact. When osseointegration is better, implants with higher starting stress values (SPS-WN) show bigger reductions, especially in bones that aren’t very thick (Type IV). These results, as shown in the contour plots, highlight the need for attaining optimum osseointegration to reduce mechanical stress and improve implant stability.
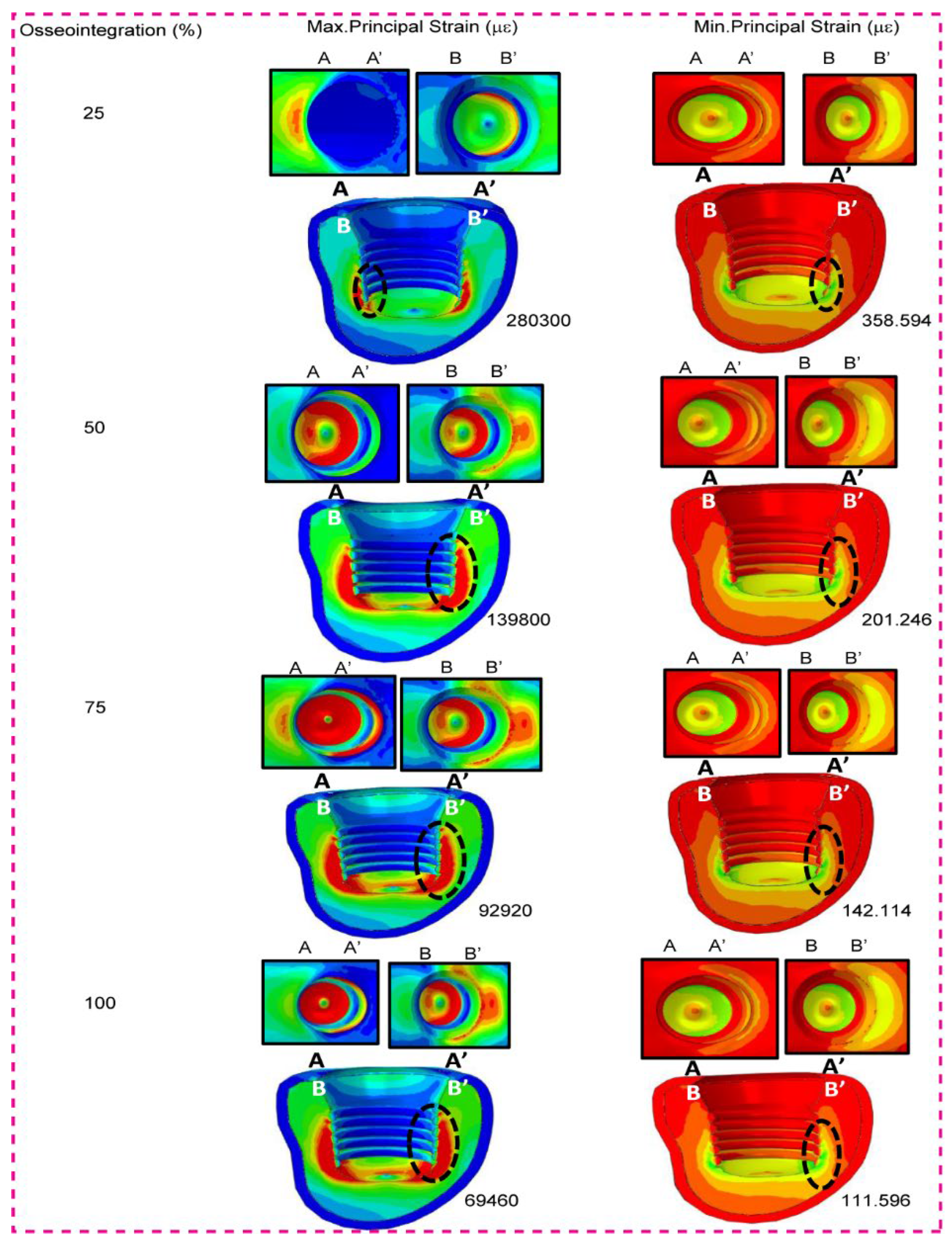
When the SPS-WN implant model was used, the table shows the highest and lowest primary stresses in micrometers (µm) for low-density cancellous bone (Bone Type IV) at different levels of osseointegration (25%, 50%, 75%, and 100%). We particularly measure the strain values at the bone-implant contact interface, and the contour plots display the locations of these strains as black dotted circles. As shown in the table and contour graphs, the maximum principal strain decreases significantly as osseointegration progresses. At 25% osseointegration, the maximum strain is 280300 µε, which gradually decreases to 69460 µε at 100% osseointegration. This decrease in maximum strain suggests that as the bone-implant contact becomes more integrated, the bone can better distribute the load, resulting in less localized deformation. With osseointegration, the minimum principal strain decreases from 358.594 at 25% to 111.596 at 100%. This pattern demonstrates a constant increase in the implant’s mechanical stability as osseointegration continues, with the bone experiencing reduced strain levels under the same loading conditions. These decreases in both maximum and lowest primary stresses indicate that establishing better osseointegration is critical for minimizing mechanical strain on the bone-implant interface, thereby improving implant stability and lifespan.
3.5. Low Density Cancellous Bone Maximum and Minimum Principal Strain
Figure 6 shows that when osseointegration happens, both the maximum and minimum principal strains for the SPS-WN implant model in low-density cancellous bone (Bone Type IV) go down by a large amount. The highest principle strain falls from 280300 at 25% osseointegration to 69460 at 100%, while the lowest principle strain drops from 358.594 to 111.596 across the same range. These decreases show that as the bone-implant contact becomes more integrated, the bone efficiently distributes mechanical stresses, reducing localized deformation and improving implant stability. Black dotted circles mark the positions of the highest and lowest principal strains at the implant-cancellous bone contact, illustrating key stress concentration zones.
3.6. High Density Cancellous Bone Maximum and Minimum Principal Strain
As osseointegration advances,
Figure 7 presented findings for the SPS-WN implant model in high-density cancellous bone (Bone Type III) demonstrate a significant reduction in both maximum and lowest principal strains. The highest main strain falls from 69050 at 25% osseointegration to 16450 at 100%, whereas the lowest principal strain drops from 157.457 to 64.1361 within the same range. These lower strains mean that as the bone-implant contact gets stronger, the bone is better able to spread mechanical stresses, which means that the implant is less likely to deform in one place and is more stable overall. Black dotted circles at the implant-cancellous bone contact depict the highest and lowest principal strains, highlighting key stress concentration locations. The contour plots further demonstrate this tendency, showing a more equal strain distribution throughout the top surface of the bone holes (A-A’ and B-B’) as osseointegration improves, which is critical for the implant’s durability.
3.7. Low- and High-Density Cancellous Bone Maximum and Minimum Principal Strain in Three Types of Short Dental Implants
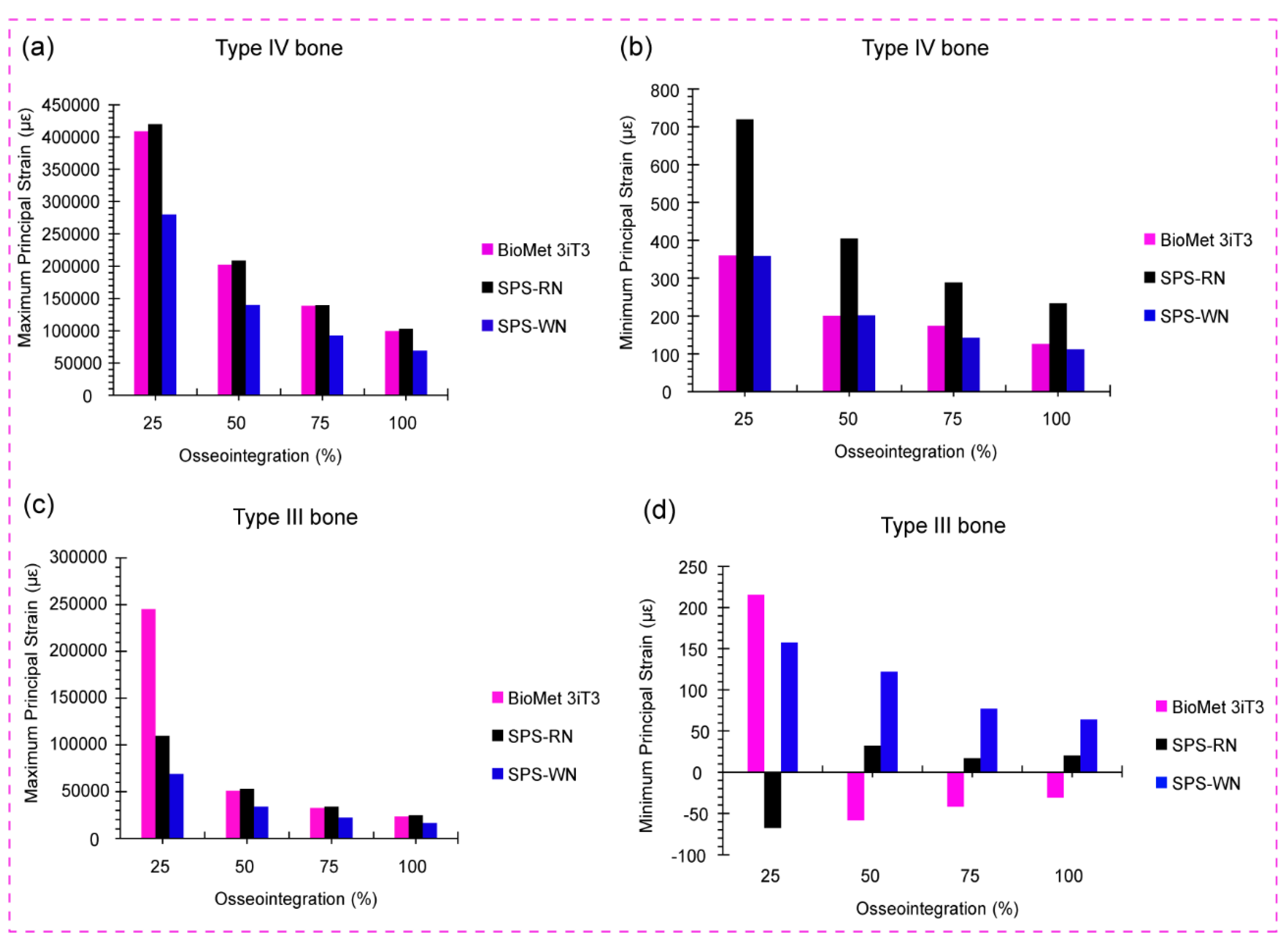
Figure 8a shows the bar chart for maximum principal strain in low-density cancellous bone (Bone Type IV) for the BioMet 3iT3, SPS-RN, and SPS-WN implant types at four different levels of osseointegration: 25%, 50%, 75%, and 100%.We can see the highest principal strain values for low-density cancellous bone (Bone Type IV) at different osseointegration levels (25%, 50%, 75%, and 100%) for three implant models below. These are BioMet 3iT3, SPS-RN, and SPS-WN. The bar chart, which originally depicted these comparisons, generated these figures.
At 25% osseointegration, the SPS-RN implant model has the greatest maximum principal strain (419862 µε), followed closely by the BioMet 3iT3 model (408801 µε). At this early stage of osseointegration, the SPS-WN implant had a significantly reduced strain of 280000 µε. As osteointegration progresses to 50%, 75%, and 100%, all three implant models show a significant decrease in maximum principal strain, with the SPS-WN model consistently having the lowest strain values at each stage. At 100% osseointegration, the SPS-WN model has the lowest maximum principal strain of 69000 µε, suggesting a significant increase in mechanical stability when the implant completely integrates with the bone. The results, shown in the bar chart, show that the SPS-WN model is better at reducing strain at higher levels of osseointegration, especially when there is 100% bone-implant contact. The SPS-RN model experiences the most strain when osseointegration is low. This decrease in stress as osseointegration progresses underscores the need for complete integration for the implant’s long-term stability and success.
The data in
Figure 8b show a considerable decrease in minimum principal strain in low-density cancellous bone (Bone Type IV) across the BioMet 3iT3, SPS-RN, and SPS-WN implant models as osseointegration advances from 25% to 100%. The SPS-WN model consistently has the lowest minimum principal strain, ranging from 280000 at 25% osseointegration to 69000 at 100%, indicating improved mechanical stability. While the BioMet 3iT3 and SPS-RN models have higher strain values throughout the osseointegration process, the highest strains were seen at the lowest levels of osseointegration. This shows how important it is to achieve full osseointegration to lower mechanical stress and improve the long-term success of the implant.
There are four levels of osseointegration shown in
Figure 8c. These levels are 25%, 50%, 75%, and 100%. The graph shows the maximum principal strain in high-density cancellous bone (Bone Type III) for the BioMet 3iT3, SPS-RN, and SPS-WN implant types. At 25% osseointegration, the BioMet 3iT3 implant model had the largest maximum principal strain, reaching 245281 µε. This is much larger than the strains reported in the SPS-RN and SPS-WN models, which were 109945 µε and 69000 µε, respectively. As osseointegration progresses, all three implant types exhibit a significant decrease in maximum principal strain. At 100% osseointegration, the BioMet 3iT3 model strain drops to 23581.7 µε, whereas the SPS-RN and SPS-WN models have even lower strain values of 24633.1 µε and 16446.6 µε, respectively. Notably, the SPS-WN model always has the lowest maximum principal strain across all levels of osteointegration. This shows that it works better at reducing strain at the bone-implant interface as osteointegration progresses.
4. Discussion
In the setting of dental implants, the results in
Table 4 show how important osseointegration is for the mechanical behavior of both cortical and cancellous bones. As osseointegration levels in cortical bone grow from 25% to 75%, maximum stress rises significantly from 158.4 MPa to 174.3 MPa. This increase in stress shows that improved bone-implant contact increases the efficiency of load transmission to the cortical bone, resulting in greater stress levels. However, the concurrent decrease in strain, from 0.02559 to 0.009997, suggests that the cortical bone becomes better capable of withstanding these loads without substantial deformation. This study implies that increased osseointegration not only strengthens the bone-implant interface, but also increases cortical bone load-bearing capability, most likely due to the formation of a more robust and lasting contact surface.
Table 3 depicts the mechanical reaction of cancellous bone, which differs from that of other bone types. As osseointegration improves, the maximum stress decreases, from 19.23 MPa at 25% to 17.79 MPa at 75%. This decrease in stress means that as the implant becomes more integrated with the surrounding cancellous bone, the bone experiences reduced stress levels, potentially lowering the risk of stress-induced bone resorption. Furthermore, the considerable reduction in strain from 0.106 to 0.03264 suggests that better osseointegration improves the mechanical stability of the cancellous bone by reducing the danger of excessive deformation.
These results have profound implications for the use of dental implants. Increasing osseointegration leads to more even load distribution and less strain in both the cortical and cancellous bones. This means that achieving high levels of osseointegration is essential for dental implants to last and work well in the long term. In clinical practice, this underlines the significance of surgical methods and implant surface treatments that promote rapid and successful osseointegration. By maximizing bone-implant contact, physicians may guarantee that implants can resist functional stresses, lower the risk of implant failure, and improve patient outcomes in restorative dentistry.
The results in
Table 5 show how important osseointegration is for controlling how the cortical and cancellous bones react to the stresses put on them by Standard Regular Neck SRN-Straumann
® Standard Plus Short (SPS) Implants. As osseointegration increases from 25% to 100% in Bone Type III, the maximum stress in the cortical bone stays generally consistent, slightly dropping from 137 MPa to 136.6 MPa. However, there is a considerable decrease in strain, from 0.04126 to 0.009295, demonstrating that with improved osseointegration, the cortical bone deforms considerably less under stress. This demonstrates that improved bone-implant contact not only stabilizes the interface, but also enables the cortical bone to withstand applied stresses more efficiently without significant deformation. As osseointegration improves, the maximum stress in cancellous bone drops from 38.48 MPa to 33.32 MPa, resulting in a considerable decrease in strain from 0.1099 to 0.02463. This trend shows that enhanced osseointegration not only decreases mechanical stress on cancellous bone, but it also improves structural stability by reducing deformation.
The benefits are much more obvious for Bone Type IV, which is less dense and exposed to increased stress levels from the start. The maximum stress in cortical bone reduces substantially, from 330.1 MPa at 25% osseointegration to 300.1 MPa at 100%. Meanwhile, the strain in cortical bone decreases from 0.08977 to 0.02171, indicating that the bone’s capacity to resist deformation improves with osseointegration. In cancellous bone, the maximum stress reduces at least from 26.12 MPa to 25.45 MPa, whereas strain decreases significantly from 0.4199 to 0.1027. These results show that even though cancellous bone is under less stress than cortical bone, the decrease in stress that comes with better osseointegration is very important for keeping the implant’s structure, especially in bone types that aren’t very dense.
These findings highlight the necessity of establishing optimum osseointegration in dental implant treatments, especially when working with less dense bone types such as Bone Type IV. The observed decrease in strain and stress levels with enhanced osseointegration indicate that improving bone-implant contact is critical for long-term implant stability and longevity. Clinically, this emphasizes the need for precise surgical methods and implant surfaces that promote quick and successful osseointegration. By providing strong and stable bone-implant interfaces, clinicians may lower the chance of implant failure, especially in difficult instances when bone density is limited, hence enhancing patient outcomes and implant life.
The stress and strain responses of the cortical and cancellous bones change a lot at different levels of osseointegration for the Standard Wide Neck SWN-Straumann
® Standard Plus Short (SPS) Implants, as shown in
Table 6. For Bone Type III, the highest stress in the cortical bone drops from 135.5 MPa to 114.3 MPa as osseointegration goes from 25% to 100%. This shows that better bone-implant contact lowers stress levels in the cortical bone. In addition, there is a significant decrease in strain from 0.04750 to 0.009631, indicating that as osseointegration advances, the cortical bone deforms less, improving implant stability. The maximum stress in cancellous bone drops at least from 14.78 MPa to 13.34 MPa, while strain also decreases from 0.06905 to 0.01645. Although cancellous bone experiences less stress than cortical bone, the decreased strain caused by enhanced osseointegration implies a more stable and supportive environment for the implant.
In Bone Type IV, the pattern is more noticeable. At 25% osseointegration, the maximum stress in cortical bone begins at 399.1 MPa and reduces to 322.7 MPa at 100% osseointegration. The related strain likewise drops substantially, from 0.09706 to 0.02004. These results emphasize the importance of osseointegration in reducing stress and strain in less dense Bone Type IV, which is critical for minimizing the likelihood of implant failure due to overloading. The maximum stress in cancellous bone stays rather constant, ranging from 15.73 MPa to 15.68 MPa, whereas strain decreases significantly from 0.2803 to 0.06946 as osseointegration improves. This large reduction in strain highlights cancellous bone’s superior capacity to disperse applied stresses, reducing deformation and contributing to implant durability.
These findings highlight the importance of obtaining excellent osseointegration in maintaining mechanical stability and success with dental implants. For denser bone types, such as Type III, enhanced osseointegration decreases both stress and strain, resulting in better load distribution and less bone deformation. In less dense bone types, such as Type IV, where higher initial stress and strain values increase the likelihood of implant failure, enhanced osseointegration is even more important. In terms of clinical practice, this emphasizes how crucial it is to choose implant configurations and surgical methods that facilitate efficient osseointegration, especially in patients with reduced bone density. By achieving these conditions, we can significantly reduce the stress on the surrounding bone, thereby enhancing implant longevity and enhancing patient outcomes in restorative dentistry.
When osseointegration goes from 25% to 100%,
Table 7 shows the changes in shear stress in three planes (Sxy, Sxz, and Syz) for different types of implants in cancellous and cortical bones. The stresses are caused by static mastication. In the BioMet 3iT3 implant model, the cancellous bone shear stresses rise over time. This is especially true in the XY plane (Sxy), where the stresses rise from 0.9718 MPa at 25% osseointegration to 1.581 MPa at 100%. The XZ and YZ planes replicate this pattern, showing that an improvement in osseointegration leads to slightly higher shear stresses in the cancellous bone, possibly because of enhanced load transfer through the implant. Cortical bone in the BioMet 3iT3 model exhibits more noticeable shear stress changes, with considerable increases seen, particularly in the XZ plane (Sxz), where stress rises from 55.37 MPa to 67.93 MPa. These results suggest that better osseointegration leads to better load distribution in the cortical bone. This creates more shear stress, which could mean that the bone-implant contact is more stable and effective. In the SPS-RN (Standard Plus Regular Neck) implant model, shear stresses in cancellous bone stay about the same at all osseointegration levels. The Sxy stress in the XY plane stays at about 1.55 MPa. But as osseointegration goes up, shear stress in cortical bone goes down a lot. This is especially true in the XY plane, where stress drops from 78.34 MPa at 25% osseointegration to 75.28 MPa at 100%. This means that as osseointegration gets better, the shear stresses in the cortical bone become more evenly distributed. This makes it less likely that there will be localized stress concentrations that could weaken the implant’s durability. In the SPS-WN (Standard Plus Wide Neck) implant model, shear stresses in cancellous bone are often lower than in the other models. As osseointegration progresses, only small decreases are seen. The Sxy stress in the XY plane drops slightly, from 1.105 MPa at 25% osseointegration to 1.095 MPa at 100%. The cortical bone experiences the most significant changes as osseointegration progresses, particularly in the XY plane, where the Sxy stress decreases from 62.77 MPa to 57.96 MPa. This lower level of shear stress in cortical bone suggests that as osseointegration gets better, the bone gets better at handling stresses. This makes implants more stable by lowering the risk of bone damage caused by shear.
These findings highlight the need for enhancing osseointegration to increase the mechanical stability of dental implants. The increase in shear stress in cortical bone found in the BioMet 3iT3 model promotes enhanced load distribution and implant stability, especially when osseointegration improves the bone-implant interaction. In contrast, the steady or decreasing shear stresses found in the SPS-RN and SPS-WN models suggest that enhanced osseointegration results in a more uniform distribution of loads, lowering the risk of localized shear stress concentrations that might possibly compromise the bone or implant. Clinically, our results highlight the need for implant designs and surface treatments that promote quick and robust osseointegration, especially for individuals with varied bone densities, to assure long-term implant success and durability.
Figure 4 shows how important osseointegration is for changing the biomechanical environment at the bone-implant contact (BIC) region, especially when von Mises stress is present. As osseointegration gets better across all implant types and bone conditions, the highest von Mises stress at the BIC goes down. This means that the load is spread out more evenly and there are fewer stress concentrations. This is especially visible in the SPS-WN implant, which shows a considerable decrease in stress from 209.83 MPa at 25% osseointegration to 156.82 MPa at 100%. Such a reduction in stress concentration is critical for improving the implant’s mechanical stability because it reduces the danger of localized bone resorption and implant failure caused by excessive stress. The findings are more complicated for bone type IV, which is less dense and more prone to mechanical failure. The first rise in von Mises stress in the BioMet 3iT3 implant model at 50% osseointegration and then the subsequent fall shows that the bone-implant contact changes as osseointegration progresses. This adaptability is significant in less dense bones because stress distribution may be unequal, making excellent osseointegration even more important for implant life. The fact that the SPS-RN implant’s stress levels stayed the same during different stages of osteointegration shows how important implant design is for maintaining consistent biomechanical function. This stability may lead to decreased mechanical problems over time, especially in clinical settings where bone quality fluctuates.
The results underline the importance of obtaining optimum osseointegration for the mechanical success of dental implants. Improved osseointegration lowers stress concentrations at the BIC, increasing implant durability and decreasing the likelihood of failure. This stresses the necessity of choosing implants with designs that facilitate consistent stress distribution, especially in individuals with reduced bone density (e.g., Type IV bone). Furthermore, the findings suggest that we can create personalized implant designs to enhance stress distribution for different bone types, thereby improving dental implantology outcomes. These findings underscore the need for patient-specific techniques in implant dentistry, where the unique biomechanical environment of each patient’s bone structure guides implant selection and placement procedures, within the broader context of biomechanics.
Figure 5 displays contour plots that demonstrate the biomechanical value of osseointegration in dental implants, particularly in lowering von Mises stress at the bone-implant contact. For Bone Type III, both the BioMet 3iT3 and SPS-RN implants show less stress as osseointegration improves. This means that the load is better distributed, and there is a lower chance of localized bone fractures. However, despite a considerable decrease, the SPS-WN implant still imposes higher stress levels, suggesting potential stress concentration hazards at the bone-implant interface, especially in denser bone types. In Bone Type IV, which is less dense, all implant types show a big drop in von Mises stress as osseointegration happens. The SPS-WN implant benefits the most from better osseointegration. This shows that adequate osseointegration is critical for improving the biomechanical stability of implants, particularly in less dense bones where implant failure is more likely. Also, the fact that both maximum and minimum principal strains go down as osseointegration gets better makes it even more important to make sure that the bone and implant have a strong contact. As strain decreases, the bone’s capacity to uniformly distribute stresses increases, reducing localized deformation and increasing implant lifetime. These findings highlight the need for adequate osseointegration in clinical applications to increase mechanical stability and the long-term success of dental implants, particularly in unfavorable bone scenarios.
The findings in
Figure 6 show a considerable decrease in both maximum and minimum principal strains in low-density cancellous bone (Bone Type IV) as osseointegration improves in the SPS-WN implant model. This significant reduction in strain suggests that improved osseointegration causes more efficient load distribution at the bone-implant interface, minimizing localized deformation and enhancing overall implant stability. The black dotted dots showing the significant stress concentration zones underline the essential places where osseointegration plays an important role in reducing mechanical strain, which is necessary for the lifetime and effectiveness of dental implants in biomechanical applications. This highlights the significance of obtaining optimum osseointegration, especially in low-density bone, in improving the mechanical performance and longevity of dental implants.
Figure 7 shows that the maximum and lowest principal strains in the SPS-WN implant model for high-density cancellous bone (Bone Type III) decrease significantly as osseointegration develops. This decrease in strain indicates the bone’s enhanced ability to distribute mechanical stresses efficiently, lowering the risk of localized deformation and improving the implant’s overall stability. The black dotted dots represent the greatest and lowest main stresses at the implant-cancellous bone contact, highlighting significant stress concentration locations. The contour plots provide evidence of a more uniform strain distribution, which strengthens the biomechanical benefit of greater osseointegration. This is important because long-term durability and clinical implant success depend on improved osseointegration.
The findings in
Figure 8a demonstrate the importance of osseointegration in lowering the maximum principal strain in low-density cancellous bone (Bone Type IV) across three implant models: BioMet 3iT3, SPS-RN, and SPS-WN. When it came to 25% osseointegration, the SPS-RN implant had the most strain, which means it had a higher chance of deforming locally and failing biomechanically in the early stages of integration. The SPS-WN model regularly demonstrates the lowest strain values, especially at 100% osseointegration, when the strain lowers significantly to 69000 µε. This shows that as osseointegration improves, the SPS-WN implant design becomes more successful at reducing strain and increasing mechanical stability, making it ideal for long-term implant success. The fact that strain went down as osseointegration went up across all models shows how important it is to get total bone-implant integration to improve load distribution, reduce mechanical stress, and make implants last longer and work better in clinical settings.
The results in
Figure 8b emphasize the critical need to achieve complete osseointegration in low-density cancellous bone (Bone Type IV) to improve implant stability. The SPS-WN implant model has a much lower minimum principal strain, especially as osseointegration increases from 25% to 100%. This shows that it can spread mechanical loads more evenly, which lowers localized stress and the chance of bone resorption. The BioMet 3iT3 and SPS-RN models, on the other hand, had higher strain values, especially at lower levels of osseointegration. This showed how biomechanically weak implants can be when they don’t integrate properly. These findings suggest that maximizing osseointegration is critical for reducing mechanical strain at the bone-implant interface, which is critical for the long-term durability and effectiveness of dental implants in clinical settings.
Figure 8c shows that the SPS-WN implant model is better for biomechanics in high-density cancellous bone (Bone Type III), since it always shows the lowest maximum principal strain at all levels of osseointegration. This shows better load distribution and lower mechanical stress at the bone-implant contact, which is important for reducing the risk of localized bone damage and improving implant stability. The considerable decrease in strain reported across all implant types as osseointegration builds demonstrates the need for complete integration to maintain excellent biomechanical function. The BioMet 3iT3 model, despite initially displaying the greatest strain, gains significantly from increased osseointegration; nonetheless, the SPS-WN model’s continuously lower strain shows that its design is more successful in reducing stress and increasing long-term implant success in clinical applications.
The results shown in
Figure 8d demonstrate the importance of osseointegration in reducing the minimum principal strain in high-density cancellous bone (Bone Type III) across various implant types. The BioMet 3iT3 model initially shows the greatest strain at 25% osseointegration, suggesting a higher risk of localized bone stress. However, as osseointegration advances, all models show a notable drop in strain. The SPS-WN model has the most dramatic reduction, particularly at 75% osseointegration, when it records the lowest strain value of 16.985 µε. This demonstrates that the SPS-WN implant is highly effective at lowering mechanical stress at the bone-implant interface, which improves implant stability and reduces the risk of bone destruction. The continued decline in strain with increasing osseointegration highlights the necessity of complete integration for the long-term biomechanical success of dental implants, especially in high-density bone.