1. Introduction
The COVID-19 pandemic caused by SARS-CoV-2 has led to more than 700 million cases and 7 million deaths worldwide. Despite the pandemic being declared over, new cases of COVID-19 continue to be reported [
1]. Healthcare workers (HCW) working in primary care are on the front lines of the COVID-19 response and still face a high risk of infection due to frequent and close interactions with infected patients. The safety of these workers is crucial not only for their own well-being, but also for preventing transmission to uninfected patients and avoiding healthcare system backlog caused by absenteeism [
2].
The surveillance of SARS-CoV-2 among HCW is essential for monitoring their health, understanding the dynamics of transmission, and evaluating their importance as a sentinel group for detecting virus variant shifts. Efficient monitoring of SARS-CoV-2 infections can help prevent high rates of COVID-19 and is crucial for identifying vulnerable groups and reducing the virus's spread within healthcare facilities. [
3]. Despite COVID-19 vaccination having a strong protective effect against reinfection, the risk of SARS-CoV-2 infection persists among HCW. Reinfections can occur in fully vaccinated workers, but with reduced severity and mortality [
4,
5].
Several factors have been identified as potential risk factors for SARS-CoV-2 transmission among HCW, such as job role, work environment, use of personal protective equipment, vaccination status, and concurrent community and household exposure. Studies showed substantial variability in prevalence of and risk factors for SARS-CoV-2 infection among HCW, attributed to different job roles, exposure to COVID-19 patients, and healthcare settings [
6], although the risk of SARS-CoV-2 exposure for HCW is higher in the community rather than at their workplace [
7]. HCW in the Global South may face limited resources and workforce shortages, leading to overworked staff and reduced quality of care. These challenges underscore the need for targeted support and resources to protect HCW in the Global South to ensure they can provide the highest quality of care during the pandemic. Monitoring infection and seroconversion among HCW is crucial in identifying at-risk individuals, assessing the effectiveness of protective measures, and implementing timely interventions to mitigate the spread of SARS-CoV-2.
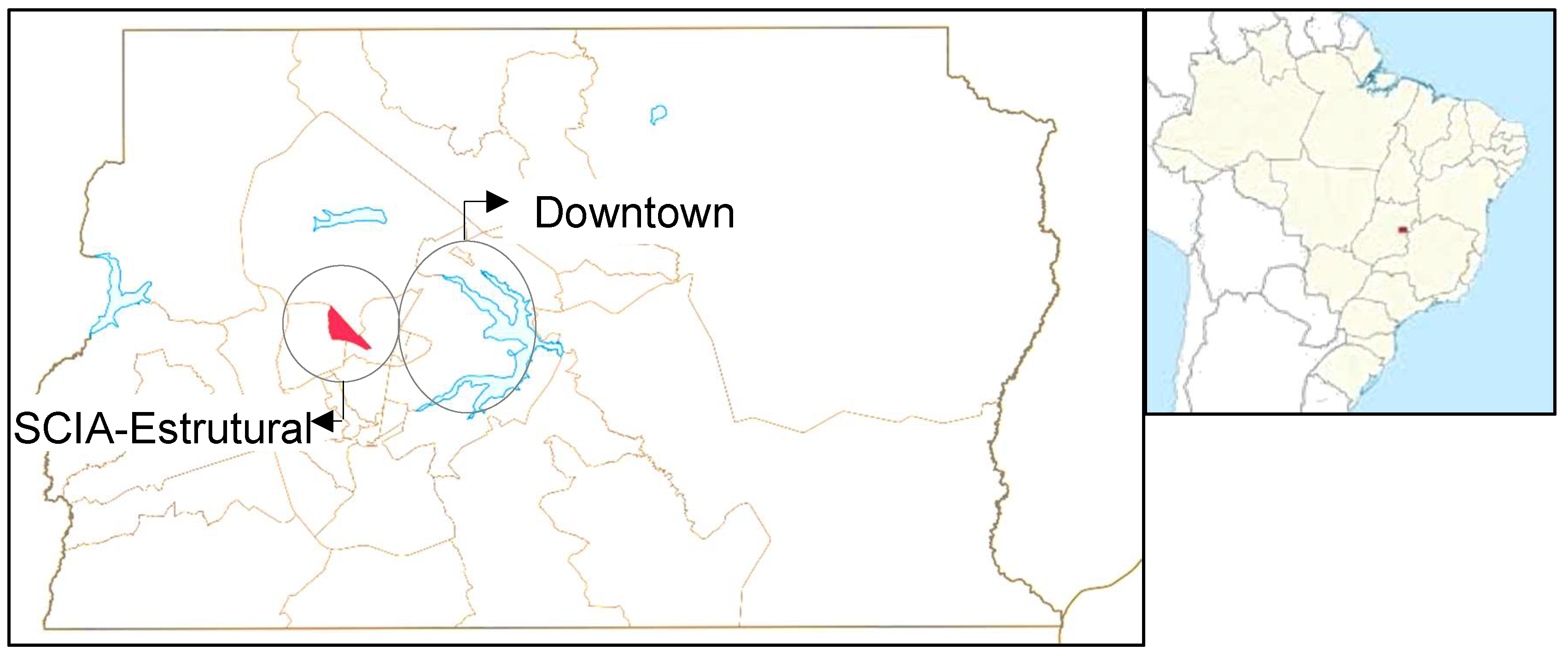
This study aims to investigate SARS-CoV-2 infection and reinfection in HCW from a primary healthcare unit serving a disenfranchised community located in Cidade Estrutural (RA XXV SCIA/Estrutural, DF, Brazil). This city, on the outskirts of the capital of Brazil, Brasília, was home to Latin America's largest untreated refuse disposal site until its decommissioning in 2018 [
8]. Our work describes the sociodemographic profile of the HCW, evaluates their frequency of infection, cases of reinfection, and post-infection IgM and IgG values in a prospective descriptive open cohort. The surveillance of SARS-CoV-2 in the healthcare workforce is a strategic approach for filling information gaps, understanding the behavior of the virus, and enabling early responses for the population.
2. Material and Methods
2.1. Study design, Settings, and Ethical Considerations
This research was a prospective analytical cohort study conducted between February and October 2021, with the staff of a primary healthcare unit (HU) at Cidade Estrutural (RA XXV SCIA/Estrutural, DF, Brazil (
Figure 1). Cidade Estrutural is characterized by considerable social challenges, where many residents face economic difficulties, inadequate housing conditions, and limited access to basic sanitation services. The city was home to the world's second-largest untreated refuse disposal site for decades, which was closed in 2018 [
8].
This research was approved by the Committees for Ethics in Research of the Faculdade de Medicina of Universidade de Brasília (CEP-FM/UnB, CAEE 39866620.4.0000.5558) and of Fundação de Ensino e Pesquisa em Ciências da Saúde (FEPECS/SES/DF, CAAE 40557020.6.3001.5553). All HCW were invited to participate and signed an informed consent to participate in this study. This work was conducted in accordance with the Ethical Principles for Medical Research in Human Subjects (Declaration of Helsinki) and Brazilian regulations (Resolution 466/12 Conep/CNS/MS).
2.2. Selection of Participants
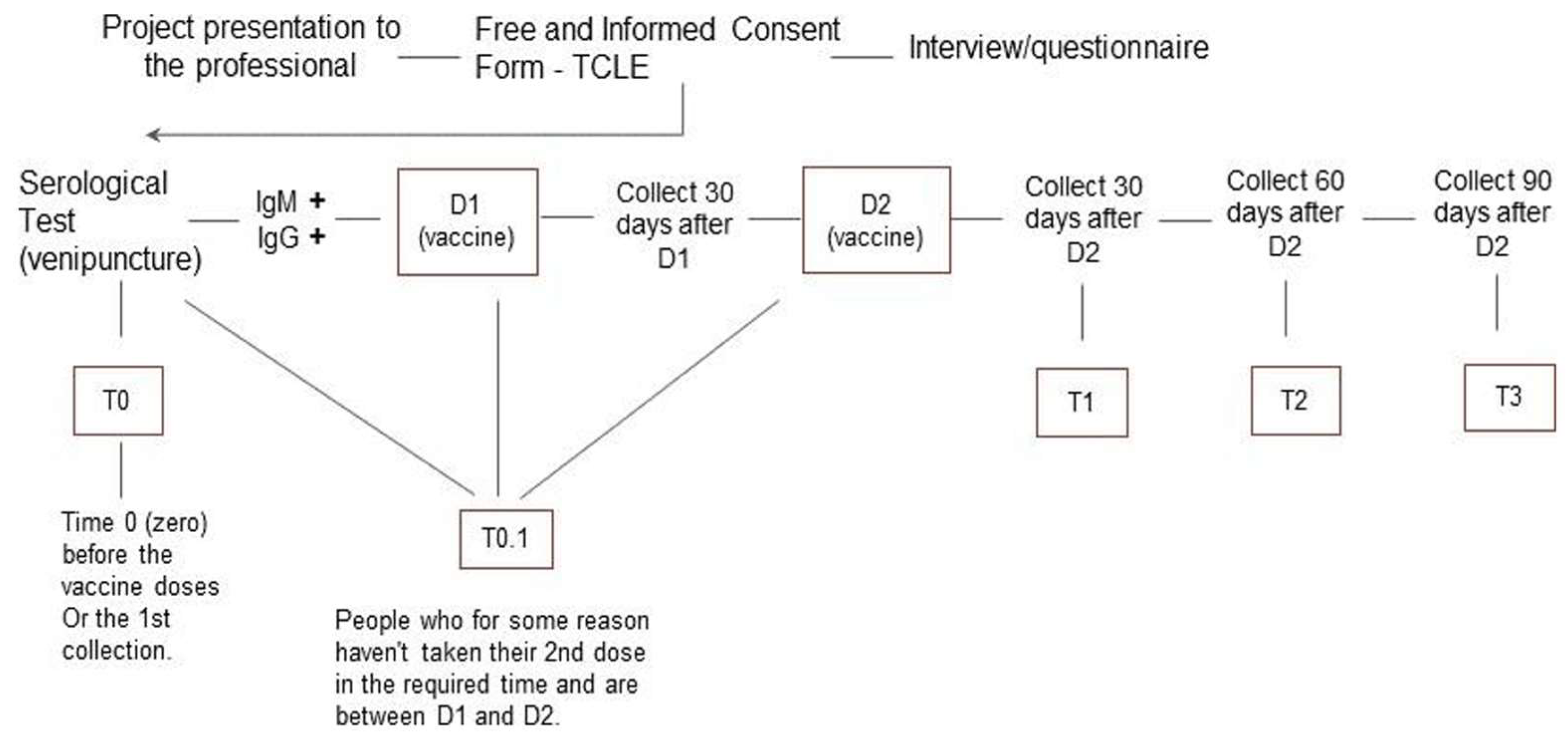
The target population was the team of 134 HCW at the HU in Cidade Estrutural. The healthcare team is responsible for providing primary care services for the population of Cidade Estrutural, including vaccinations, examinations, outpatient clinical care, schedule appointments, among other services. All HCW were invited to participate. The study included HCW who worked in the HU during the period studied that voluntarily signed the informed consent, agreed to provide biological samples (nasopharyngeal and venous blood), and answered a standardized questionnaire during the investigation. Participants were classified in three categorical groups: HCW who (i) had not yet received the first dose of the COVID-19 vaccine (Coronavac or AstraZeneca) for any reason; (ii) had received the first dose of any COVID-19 vaccine; or (iii) had received two doses of any COVID-19 vaccine. In all three cases, the biological material and primary data were collected in the period immediately preceding the scheduled vaccination date.
Participants were interviewed to obtain sociodemographic characteristics and had a venous blood sample collected every 30 ± 2 days from the initial sample collection (
Figure 2). Data on adverse effects were recorded, and serological analysis was performed at the same intervals. The follow-up period lasted eight months, during which any workers reporting flu-like symptoms were tested for COVID-19 using RT-qPCR at any time.
2.3. Serological Analysis and SARS-CoV-2 Detection
A total of 240 venous blood samples (approximately 4 mL each) was collected over a period of eight months. The samples were centrifuged, and the serum was separated and stored at -80°C until analysis. The qualitative detection of IgM and IgG antibodies against SARS-CoV-2 was performed using chemiluminescence microparticle immunoassay technology [
9]. The Abbott Architect Plus i2000SR was used to conduct tests for the detecting IgM against SARS-CoV-2 spike proteins (S) and IgG against SARS-CoV-2 nucleocapsid proteins (N). All tests were performed in accordance with the manufacturer's instructions, including calibration and daily analysis of positive/negative controls, to meet the required quality criteria.
Molecular diagnosis was conducted on demand at the Hospital Universitário de Brasília (HUB) to assess infection and reinfection from nasopharyngeal samples collected between the third and tenth day of symptoms. The samples were analyzed by RT-qPCR using the Allplex SARS-CoV-2 Assay kit (Seegene) on Quantstudio 5 (Applied Biosystems), following the manufacturer's instructions [
10]. Infection and reinfection criteria as based on RT-qPCR positive results.
2.4. Data Analysis
We assessed the frequency of infection and reinfection (positive results 90 days after an infection) among HCW according to their sociodemographic characteristics. The study analyzed the frequency of SARS-CoV-2 at nine time points, both before and after vaccination, to assess infection and reinfection. We used the Hmisc package in R 4.2.1 software, along with the RStudio 2023.03.1.446 [
11] interface.
3. Results
3.1. Characterization of the Population
From, the 134 HCW employed at the HU, 128 participated in the study. Four HCW declined to participate in the study. Three workers chose not to receive the vaccine but agreed to be monitored. During the study period, seven workers withdrew, and 12 new workers joined the study. A total of 128 workers were sampled at various stages of the study. Of these, 27 were sampled before receiving the first dose of the vaccine, 109 before the second dose, 19 immediately after the second dose, and 99 on the 30th day (± 2) after the second dose. Four workers received the AstraZeneca vaccine, while the remaining workers (124) were vaccinated with CoronaVac. Most of the workers had completed college education, earned more than six minimum wages, and worked as nursing technicians or CHW (
Table 1).
3.2. SARS-CoV-2 Infections and Reinfections
Of the 128 workers, 61 self-reported probable SARS-CoV-2 infection (47.65%; CI: 39.19-56.25) before vaccination. A total of 50 (39.06%; CI: 31.04-47.71) HCW had SARS-CoV-2 infection after vaccination, confirmed by RT-qPCR. Reinfection was identified in seven HCWs (14.00%; CI: 6.95-26.18), based on a positive RT-qPCR result after 90 days of a previous positive RT-qPCR result. The serological data from 128 HCW indicated that 68 had IgG antibodies (53.12%; CI: 44.5-61.5) and 46 had IgM antibodies (35.93%; CI: 28.14-44.54) against SARS-CoV-2 proteins.
Infections occurred in all age, race, and educational groups (
Table 1). No differences in infection were observed among the different income groups (
Table 1). However, infections were identified in HCW from different professions, mostly in community health workers (CHWs, 56%), registered nurses (50%), and licensed practice nurses (33%) (
Table 1). Following the vaccination, the percentage of infections among CHWs decreased, from 47.83% to 4.35%.
Table 2 presents the IgG and IgM values based on the time of measurement for both vaccinated and unvaccinated individuals. At the beginning of the study (T0), only 28 (22.05%) individuals had a positive IgG result before vaccination. At T1, 42 individuals who received the first dose of the vaccine tested positive for IgG (40.38%). The frequency of positive IgG gradually decreased in subsequent measurements (
Table 2). A comparable pattern was observed for IgM, although to a lesser extent.
4. Discussion
The study found that 50 (~40%) HCW in the HU located in Cidade Estrutural were infected with SARS-CoV-2 in 2021. CHW were the most infected group. The study also showed a low frequency of reinfection among these workers after vaccination. These results contribute to the understanding of SARS-CoV-2 infection dynamics among HCW of a HU serving a disenfranchised community of Brazil during the COVID-19 pandemic.
The study of HCW infection is fundamental to understanding transmission dynamics and suggesting prevention strategies. WHO [
1] recommendation of testing and isolation of asymptomatic HCW infected with SARS-CoV-2 from health care settings was critical for controlling COVID-19 transmission. This also has a direct impact on the quality of care and the mental health of HCW, minimizing additional stress [
12]. We found that approximately 40% of HCW were infected with SARS-CoV-2 in 2021. Studies reported that prior to the first dose of COVID-19 vaccination, SARS-CoV-2 infections varied between 7-58% among HCW, depending on the diagnostic method used [
13,
14,
15,
16]. It is worth noting that studies have demonstrated the effectiveness of combining serological methods with RT-qPCR to detect SARS-CoV-2, resulting in a more accurate diagnosis. In some suspected COVID-19 cases, when presenting with flu-like symptoms and having had close contact with confirmed cases, patients have tested negative twice by RT-qPCR but positive for SARS-CoV-2-specific IgM and IgG antibodies. These findings suggest a promising strategy for the prevention or rapid control of future cases [
17]. During the postvaccination, SARS-CoV-2 infections was reduced among HCW, varying between 0.5-9% [
18,
19], values slightly lower than those showed in our study. Surveillance based on accurate diagnostic methods allows for the prevention and management of COVID-19 symptoms, which can help prevent serious outcomes for HCW, including death [
13].
We detected SARS-CoV-2 infections in workers from over 18 different healthcare roles, with the majority being CHW (56%). Association between job role and SARS-CoV-2 infection was found among healthcare personnel [
6], although the risk of SARS-CoV-2 exposure for HCW was more likely to have occurred in the community and/or their households rather than at their workplace [
7]. The higher occurrence of SARS-CoV-2 infections in CHW may be linked to their direct contact with the community and visits to households, where transmission is occurring. Notably, a seroprevalence of 24% was observed in certain areas of the Federal District of Brazil during the pandemic [
20].
Our results show the dynamics of seroconversion in vaccinated individuals, indicating a positive immune response to the vaccine over time, with an initial increase at T1 and effective maintenance through T7. This analysis highlights the effectiveness of the vaccines in producing a detectable immune response in HCW, as observed in other studies [
18,
19,
21,
22]. After vaccination, participants who had a previous, self-reported SARS-CoV-2 infection had higher antibody levels than those who did not self-report a previous infection. This correlation between post-vaccination antibody levels and previous infection has been observed in other studies [
23]. The effectiveness of the COVID-19 vaccine in HCW was high, even against the Omicron variant (e.g. [
24]). Reduction in COVID-19 cases in HCW was observed in many studies [
18,
19,
21,
22]. For example, a reduction of 62% in new cases of COVID-19 among HCW seven weeks after vaccination was observed, demonstrating the effectiveness of the vaccines [
21].
COVID-19 infection among HCW is a significant public health challenge that requires the implementation of more effective protective measures. A system where hospital-based occupational health services were adapted to offer a monitoring program with daily evaluations and treatment options for HCW with SARS-CoV-2 has shown significant results. Of the 4,814 professionals enrolled, only 2% were hospitalized, and there were six deaths. The tracked professionals had lower rates of comorbidities, hospitalization, and mortality, indicating that this surveillance approach may be feasible [
25].
The study was conducted during a government-declared state of emergency, which, in conjunction with a global shortage of supplies, caused delays in the arrival of diagnostic kits. As a result, there were frequent delays in the return of results, leading some participants to seek diagnostics in the private sector. Another limitation is related to the kit used during diagnosis, as we used a qualitative method available at the beginning of the cohort. It was not possible to measure the level of antibodies after exposure. Overcoming these challenges is critical to ensure the effectiveness of the study and its effective contribution to pandemic control and the formulation of more effective and comprehensive health strategies in the future.
Surveillance offers the prospect of monitoring and follow-up, providing timely and effective health responses to health systems, populations, and governments. Incorporating routine surveillance as a public health policy could prevent future barriers to conducting research projects. COVID-19 surveillance can provide an epidemiologic perspective for controlling virus transmission. Public health surveillance by community health workers is critical, especially in disenfranchised settings. HCW play essential roles, including contact tracing and patient visits, in controlling infectious diseases such as HIV/AIDS, malaria, tuberculosis, Ebola, and COVID-19. Despite the challenges HCW face, such as unfavorable psychosocial conditions, insufficient training and resources, investment in their well-being at work and infrastructure can significantly improve their work and the quality of public health surveillance [
26,
27].
5. Conclusion
Our results demonstrate that: (i) approximately 40% of the HCWs were infected with SARS-CoV-2 after vaccination, primarily CHWs, and (ii) reinfections occurred in 14% of the HCW. The results provide valuable insights into the circulation of SARS-CoV-2 among HCWs in a primary care unit attending an unserved community of the capital of Brazil during the COVID-19 pandemic. In addition, the study indicates that some HCW were still infected even after complete immunization. This finding is consistent with previous studies that have also shown a decline in vaccine effectiveness over time, highlighting the need for revaccination.
Author Contributions
Conceptualization, W.M.R. and W.N.; methodology, A.C.P.T., R.N.B, P.P., D.C.C.A.H., A.I.P.T., C.C.G, and W.M.R.; formal analysis, A.C.P.T. and W.M.R.; investigation, A.C.P.T., R.N.B, P.P., D.C.C.A.H., A.I.P.T., C.C.G, W.N., and W.M.R; data curation, A.C.P.T., R.G-G. and W.M.R.; writing—original draft preparation, A.C.P.T., R.G-G., R.N.B and W.M.R.; writing—review and editing, R.G-G.; visualisation, A.C.P.T., R.G-G., R.N.B and W.M.R; supervision, R.G-G. and W.M.R.; project administration, W.M.R. and W.N.; funding acquisition, W.M.R. and W.N. All authors have read and agreed to the published version of the manuscript.
Financing
This work was supported by the Ministério da Educação (MEC,
http://portal.mec.gov.br / ; grant number 23106.028855/2020-74) and Fundação de Amparo à Pesquisa do Distrito Federal (FAP-DF,
http://www.fap.df.gov.br /; grant number 00193-00000495/2020-72). The funding sources were not involved in the study design, in the collection, analysis, and interpretation of the data, in the preparation of the manuscript, or in the decision to submit the manuscript to publication.
Consent for publication
All volunteers signed informed consent that the data will be disclosed to interested authorities and in reports, scientific articles and other educational dissemination.
Data availability
The data collected and analyzed for this study is available from the corresponding author upon reasonable request.
Acknowledgments
Hospital Universitário de Brasília (HUB-UnB) for assistance. Allie Melanson of Brigham Young University for English editing.
Competitive interests
The authors declare that they have no conflicting interests.
Ethical approval and consent to participate
The research was approved by the Research Ethics Committee (CEP), under protocol number: 39866620.4.0000.5558 and approved by the Research Ethics Committee of the Faculty of Medicine (CEP/FM) of the University of Brasília. We also obtained permission from the Federal District Health Department (SES/DF), with the management of the health unit, to carry out the research at the Estrutural Basic Health Unit. Authorization for the collection of data, serological samples and swabs was assessed and accepted by the UBS manager. The health professionals who agreed to take part in the research signed the Informed Consent Form (ICF).
List of abbreviations
| COVID-19 |
Coronavirus disease of 2019; |
| CHW |
Community health workers |
| HUB |
Brasília University Hospital; |
| HCW |
Health care workers |
| HU |
primary health unit |
| IEQ |
Enzyme immunoassay of chemiluminescence; |
| 95% CI |
95% confidence interval; |
| IgG |
Immunoglobulin G; |
| IgM |
Immunoglobulin M; |
| RA |
Administrative Region; |
| RT-PCR |
Reverse transcriptase reaction followed by polymerase chain reaction; |
| SARS-CoV-2 |
Severe acute respiratory syndrome coronavirus 2; |
| SCIA |
Complementary Industry and Supply Sector; |
| UnB |
University of Brasília; |
References
- WHO, 2024. COVID-19 dashboard. Available from: https://data.who.int/dashboards/covid19/cases?n=o.
- Nashwan, A.J.; Mathew, R.G.; Anil, R.; Allobaney, N.F.; Nair, S.K.; Mohamed, A.S.; Abujaber, A.A.; Balouchi, A.; Fradelos, E.C. The safety, health, and well-being of healthcare workers during COVID-19: A scoping review. AIMS Public Heal. 2023, 10, 593–609. [Google Scholar] [CrossRef]
- Padilha, D.A.; Souza, D.S.M.; Kawagoe, E.K.; Filho, V.B.; Amorim, A.N.; Barazzetti, F.H.; Schörner, M.A.; Fernandes, S.B.; Coelho, B.K.; Rovaris, D.B.; et al. Genomic Surveillance of SARS-CoV-2 in Healthcare Workers: A Critical Sentinel Group for Monitoring the SARS-CoV-2 Variant Shift. Viruses 2023, 15, 984. [Google Scholar] [CrossRef] [PubMed]
- Pizarro, A.B.; Persad, E.; Durao, S.; Nussbaumer-Streit, B.; Engela-Volker, J.S.; McElvenny, D.M.; Rhodes, S.; Martin, C.; Rhodes, S.; Martin, C.; et al. Workplace interventions to reduce the risk of SARS-CoV-2 infection outside of healthcare settings. Cochrane Database Syst. Rev. 2024, 2024, CD015112. [Google Scholar] [CrossRef]
- Cegolon, L.; Magnano, G.; Negro, C.; Filon, F.L.; on behalf of the ORCHESTRA Working Group. SARS-CoV-2 Reinfections in Health-Care Workers, 1 March 2020–31 January 2023. Viruses 2023, 15, 1551. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Trannel, A.; Heinemann, J.; Marra, A.R.; Etienne, W.; Abosi, O.J.; Holley, S.; Dains, A.; Jenn, K.E.; Meacham, H.; et al. Association between job role and coronavirus disease 2019 (COVID-19) among healthcare personnel, Iowa, 2021. Antimicrob. Steward. Heal. Epidemiology 2022, 2, e188. [Google Scholar] [CrossRef] [PubMed]
- Pouquet, M.; Decarreaux, D.; Di Domenico, L.; Sabbatini, C.E.; Prévot-Monsacre, P.; Fourié, T.; Villarroel, P.M.S.; Priet, S.; Blanché, H.; Sebaoun, J.-M.; et al. SARS-CoV-2 infection prevalence and associated factors among primary healthcare workers in France after the third COVID-19 wave. Sci. Rep. 2024, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cruvinel, V.R.N.; Marques, C.P.; Cardoso, V.; Novaes, M.R.C.G.; Araújo, W.N.; Angulo-Tuesta, A.; Escalda, P.M.F.; Galato, D.; Brito, P.; da Silva, E.N. Health conditions and occupational risks in a novel group: Waste pickers in the largest open garbage dump in Latin America. BMC Public Heal. 2019, 19, 1–15. [Google Scholar] [CrossRef]
- Lijia, S.; Lihong, S.; Huabin, W.; Xiaoping, X.; Xiaodong, L.; Yixuan, Z.; Pin, H.; Yina, X.; Xiaoyun, S.; Junqi, W. Serological chemiluminescence immunoassay for the diagnosis of SARS-CoV-2 infection. J. Clin. Lab. Anal. 2020, 34, e23466. [Google Scholar] [CrossRef]
- Teixeira, A.I.P.; de Brito, R.N.; Gontijo, C.C.; Romero, G.A.S.; Ramalho, W.M.; Haddad, R.; Noronha, E.F.; de Araújo, W.N. The role of pets in SARS-CoV-2 transmission: An exploratory analysis. Infection 2022, 51, 455–458. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, PBC: Boston, MA, 2022; RStudio 2023.03.1.446; Available online: https://posit.co/products/open-source/rstudio/ (accessed on 14 August 2023).
- Teixeira Mendes, E.; Neto, D.G.P.V.; Ferreira, G.M.; Valença, I.N.; Lima, M.P.J.S.; de Freitas, M.F.M.B.; et al. Impact of COVID-19 RT-PCR testing of asymptomatic health care workers on absenteeism and hospital transmission during the pandemic. Am J Infect Control 2023, 51, 248–254. [Google Scholar] [CrossRef]
- Caixeta, D.A.; do Carmo, M.A.V.; da Fonseca, F.G.; Nogueira, D.A.; Coelho, L.F.L.; Malaquias, L.C.C. Seroprevalence of SARS-CoV-2 in hospital workers in the southern region of Minas Gerais state in Brazil: An analysis of the pre-vaccine period. Brazilian J Microbiol 2023, 54, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Buonafine, C.P.; Paiatto, B.N.M.; Leal, F.B.; de Matos, S.F.; de Morais, C.O.; Guerra, G.G.; Martuchelli, M.V.V.; Oliveira, D.B.L.; Durigon, E.L.; Soares, C.P.; et al. High prevalence of SARS-CoV-2 infection among symptomatic healthcare workers in a large university tertiary hospital in São Paulo, Brazil. BMC Infect. Dis. 2020, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.P.; Damásio, L.C.V.D.C.; Luz, L.S.; Pereira, M.F.D.S.; Lazzerini, M. COVID-19 among health workers in Brazil: The silent wave. J. Glob. Heal. 2020, 10, 010379. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Hernández, N.; Carrillo-Ruíz, J.D.; Méndez-García, L.A.; Rizo-Téllez, S.A.; Viurcos-Sanabria, R.; Santoyo-Chávez, A.; Márquez-Franco, R.; Aguado-García, A.; Baltazar-López, N.; Tomita-Cruz, Y.; et al. High Incidence Rate of SARS-CoV-2 Infection in Health Care Workers at a Dedicated COVID-19 Hospital: Experiences of the Pandemic from a Large Mexican Hospital. Healthcare 2022, 10, 896. [Google Scholar] [CrossRef] [PubMed]
- Alamri, S.S.; Alsaieedi, A.; Khouqeer, Y.; Afeef, M.; Alharbi, S.; Algaissi, A.; Alghanmi, M.; Altorki, T.; Zawawi, A.; Alfaleh, M.A.; et al. The importance of combining serological testing with RT-PCR assays for efficient detection of COVID-19 and higher diagnostic accuracy. PeerJ 2023, 11, e15024. [Google Scholar] [CrossRef]
- Amit, S.; Beni, S.A.; Biber, A.; Grinberg, A.; Leshem, E.; Regev-Yochay, G. Postvaccination COVID-19 among Healthcare Workers, Israel. Emerg. Infect. Dis. 2021, 27, 1220–1222. [Google Scholar] [CrossRef]
- Arriola, C.S.; Soto, G.; Westercamp, M.; Bollinger, S.; Espinoza, A.; Grogl, M.; Llanos-Cuentas, A.; Matos, E.; Romero, C.; Silva, M.; et al. Effectiveness of Whole-Virus COVID-19 Vaccine among Healthcare Personnel, Lima, Peru. Emerg. Infect. Dis. 2022, 28, S238–S243. [Google Scholar] [CrossRef]
- de Brito, R.N.; Teixeira, A.I.P.; Gontijo, C.C.; Faria, R.D.S.; Ramalho, W.M.; Romero, G.A.S.; Castro, M.; Pessoa, V.; Torres, L.A.; Leite, L.P.; et al. Seroprevalence of SARS-CoV-2 and Vaccination Coverage among Residents of a Lower-Middle-Class Population in the Federal District, Brazil. Vaccines 2023, 11, 916. [Google Scholar] [CrossRef]
- Toniassoa, S.d.C.C.; Fernandesa, F.S.; Jovelevithsb, D.; Filhoc, F.F.D.; Takahasid, A.Y.; Baldina, C.P.; Pereiraa, R.M.; da Silvae, L.P.; Bruma, M.C.B. Reduction in COVID-19 prevalence in healthcare workers in a university hospital in southern Brazil after the start of vaccination. Int. J. Infect. Dis. 2021, 109, 283–285. [Google Scholar] [CrossRef]
- Goodwin, B.; Zia, H.; Lo, D.F. Assessment of early and post COVID-19 vaccination antibody response in healthcare workers: A critical review. Epidemiology Infect. 2023, 151, 1–4. [Google Scholar] [CrossRef]
- Fonseca, M.H.G.; Souza, T.d.F.G.d.; Araújo, F.M.d.C.; de Andrade, L.O.M. Dynamics of antibody response to CoronaVac vaccine. J. Med Virol. 2022, 94, 2139–2148. [Google Scholar] [CrossRef] [PubMed]
- Gaio, V.; Santos, A.J.; Amaral, P.; Viana, J.F.; Antunes, I.; Pacheco, V.; Paiva, A.; Leite, P.P.; Gonçalves, L.A.; Araújo, L.; et al. COVID-19 vaccine effectiveness among healthcare workers: A hospital-based cohort study. BMJ Open 2023, 13, e068996. [Google Scholar] [CrossRef]
- Crosby, J.C.; A Lee, R.; McGwin, G.; Heath, S.L.; A Burkholder, G.; Gravett, R.M.; Overton, E.T.; Locks, G.; E Fleece, M.; Franco, R.; et al. A COVID-19 monitoring process for healthcare workers utilizing occupational health. Occup. Med. 2023, 74, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Alhassan, J.A.K.; Alhassan, J.A.K.; Wills, O.; Wills, O. Public health surveillance through community health workers: A scoping review of evidence from 25 low-income and middle-income countries. BMJ Open 2024, 14, e079776. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.d.O.; de Faria, B.S.F.; Albuquerque, B.B.; da Silva, F.L.; Rohwedder, L.S.; de Azevedo, R.T.; Gonçalves, J.S.; Vieira, L.M.S.M.d.A.; Triches, M.I.; de Sousa, R.A.; et al. Poor Health Conditions among Brazilian Healthcare Workers: The Study Design and Baseline Characteristics of the HEROES Cohort. Healthcare 2022, 10, 2096. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).