Submitted:
29 August 2024
Posted:
30 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
2.1. In Silico Studies
2.1.1. ADMET Properties
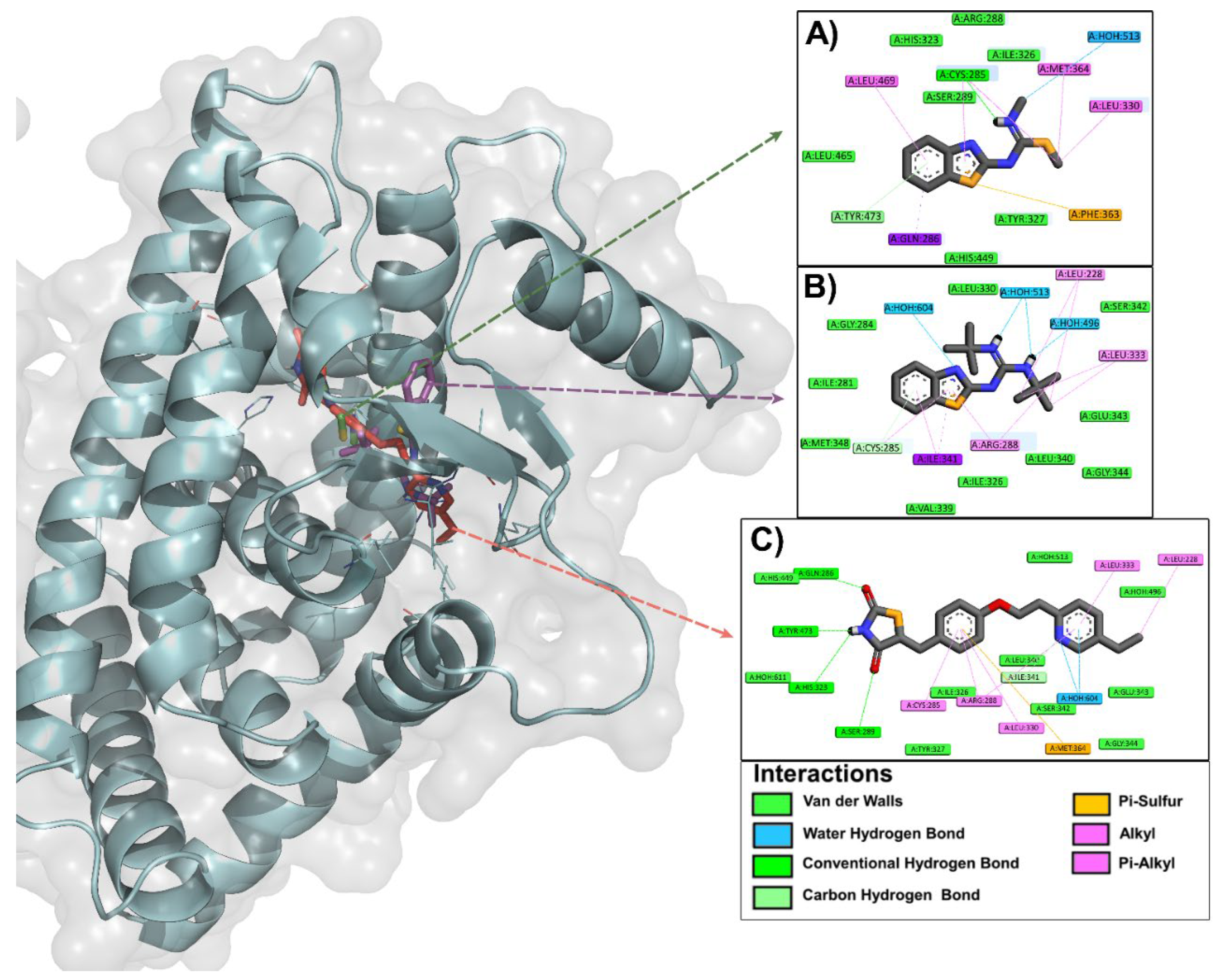
2.1.2. Molecular Docking
2.2. In Vivo Studies
2.2.1. Acute Oral Toxicity Test (AOT) of Compounds 3b and 4y
2.2.2. Acute and Subchronic Effect of Compounds 3b and 4y in the Rat Model with T2D
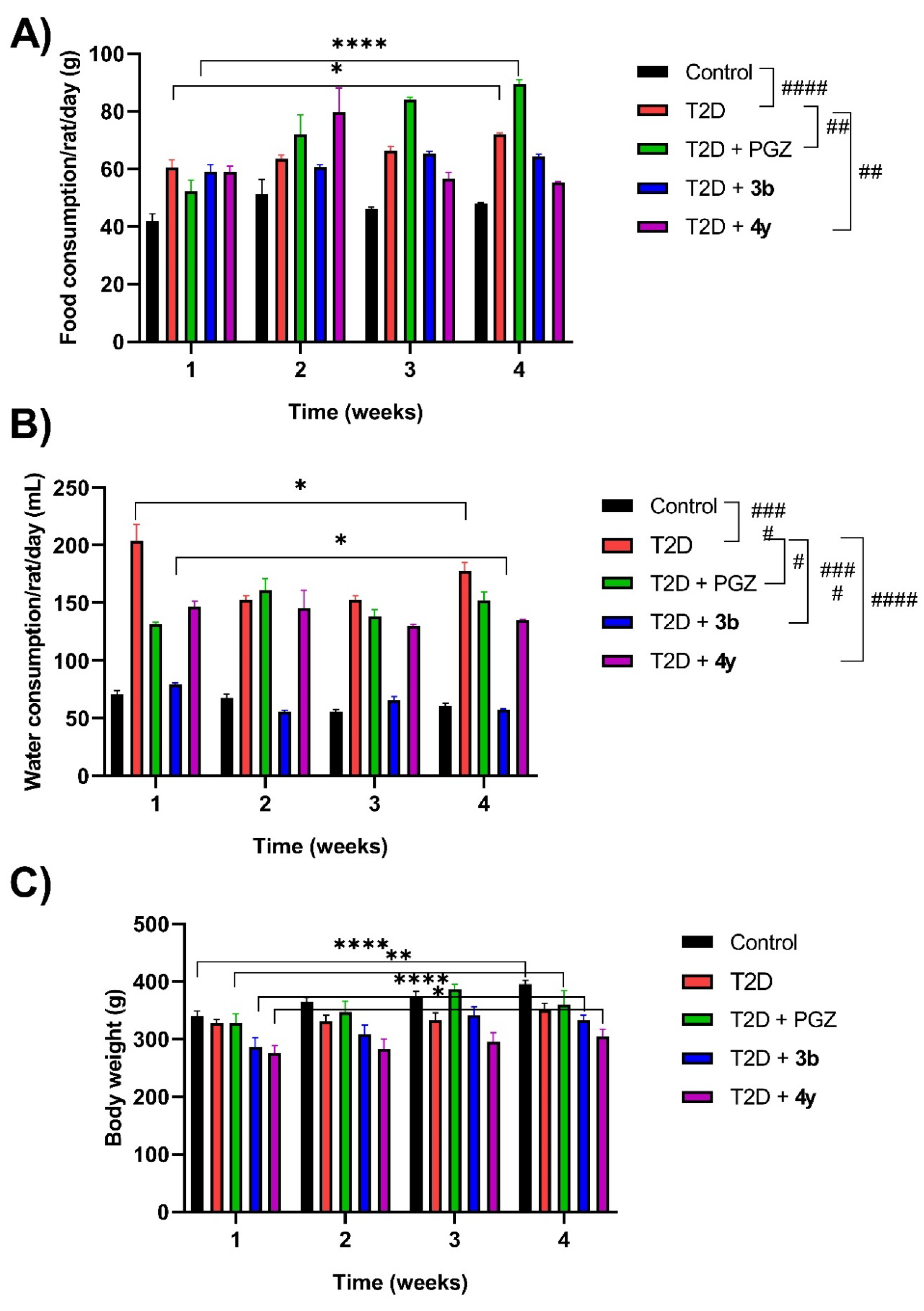
2.3.2.1. Food and Water Consumption and Body Weight
3. Materials and Methods
3.1. Chemicals
3.2. In Silico Studies
3.2.1. ADMET Properties Prediction
3.2.2. Analysis of the Binding Mode and Ligand-Protein Interactions by Molecular Docking
3.3. In Vivo Studies
3.3.1. Animals
3.3.2. Experimental Design
3.3.3. Acute Oral Toxicity (AOT) Evaluation
3.3.4. Acute and Subchronic Assessment in the T2D Model
3.3.5. Record of Food and Water Consumption and Body Weight
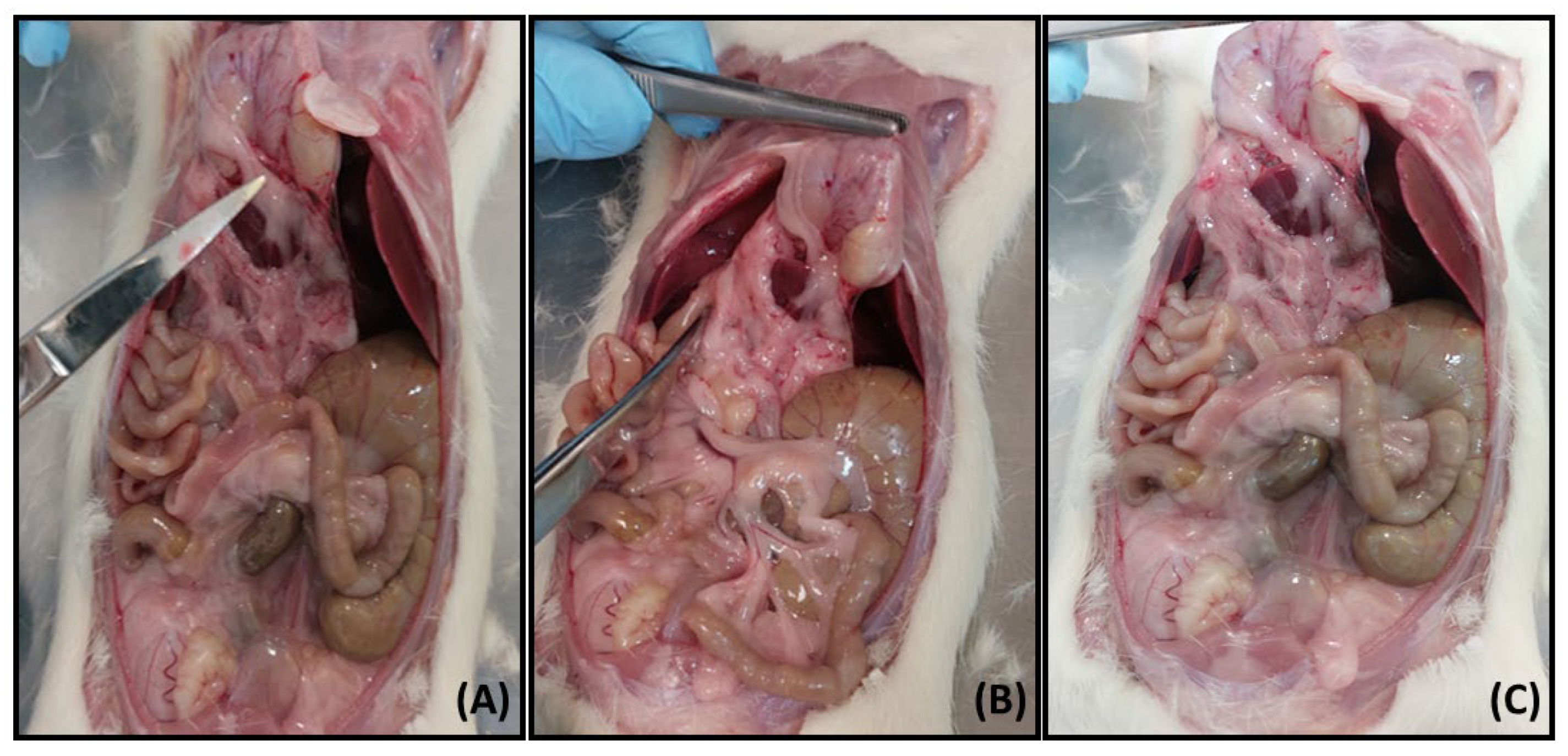
3.4. Ex Vivo Studies
3.4.1. Sample Collection and Processing
3.5. Statistic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magliano, D.J.; Boyko, E.J.; Balkau, B.; Barengo, N.; Barr, E.; Basit, A.; Bhata, D.; Bommer, C.; Booth, G.; Cariou, B.; et al. International Diabetes Federation IDF Diabetes Atlas, 10th ed.; Berkeley Communications: Reading, UK, 2021; Volume 102, ISBN 9782930229980. [Google Scholar]
- Al-Muzafar, H.M.; Alshehri, F.S.; Amin, K.A. The role of pioglitazone in antioxidant, anti-inflammatory, and insulin sensitivity in a high fat-carbohydrate diet-induced rat model of insulin resistance. Braz. J. Med. Biol. Res. 2021, 54, e10782. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, S.A.; Elkhalifa, A.E.O.; Mehmood, K.; Adnan, M.; Khan, M.A.; Eltoum, N.E.; Krishnan, A.; Baig, M.S. Multi-targeted molecular docking, pharmacokinetics, and drug-likeness evaluation of okra-derived ligand abscisic acid targeting signaling proteins involved in the development of diabetes. Molecules 2021, 26, 5957. [Google Scholar] [CrossRef] [PubMed]
- Dowarah, J.; Singh, V.P. Anti-diabetic drugs recent approaches and advancements. Bioorg. Med. Chem. 2020, 28, 115263. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global trends in diabetes complications: A review of current evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef]
- Kousaxidis, A.; Petrou, A.; Lavrentak, i.V.; Fesatidou, M.; Nicolaou, I.; Geronikaki, A. Aldose reductase and protein tyrosine phosphatase 1B inhibitors as a promising therapeutic approach for diabetes mellitus. Eur. J. Med. Chem. 2020, 207, 112742. [Google Scholar] [CrossRef]
- Shiming, Z.; Mak, K.-K.; Balijepalli, M.K.; Chakravarthi, S.; Pichika, M.R. Swietenine potentiates the antihyperglycemic and antioxidant activity of metformin in streptozotocin induced diabetic rats. Biomed. Pharmacother. 2021, 139, 111576. [Google Scholar] [CrossRef]
- Álvarez-Almazán, S.; Solís-Domínguez, L.C.; Duperou-Luna, P.; Fuerte-Gómez, T.; González-Andrade, M.; Aranda-Barradas, M.E.; Palacios-Espinosa, J.F.; Pérez-Villanueva, J.; Matadamas-Martínez, F.; Miranda-Castro, S.P.; et al. Anti-diabetic activity of glycyrrhetinic acid derivatives FC-114 and FC-122: scale-up, in silico, in vitro, and in vivo studies. Int. J. Mol. Sci. 2023, 24, 12812. [Google Scholar] [CrossRef]
- Tomic, D.; Shaw, J.E.; Magliano, D.J. The burden and risks of emerging complications of diabetes mellitus. Nat. Rev. Endocrinol. 2022, 18, 525–539. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- University of Oxford Our World in Data. Diabetes Prevalence. 2021. Available online: https://ourworldindata.org/search?q=diabetes (accessed on 10 October 2023).
- Bello-Chavolla, O.Y.; Antonio-Villa, N.E.; Fermín-Martínez, C.A.; Fernández-Chirino, L.; Vargas-Vázquez, A.; Ramírez-García, D.; Basile-Alvarez, M.R.; Hoyos-Lázaro, A.E.; Carrillo-Larco, R.M.; Wexler, D.J.; et al. Diabetes-related excess mortality in Mexico: a comparative analysis of national death registries between 2017–2019 and 2020. Diabetes Care 2022, 45, 2957–2966. [Google Scholar] [CrossRef]
- U.S. Department of Health & Human Services. What is diabetes? Available online: https://www.cdc.gov/diabetes/basics/diabetes.html (accessed on 10 October 2023).
- Almeida, C.; Monteiro, C.; Silvestre, S. Inhibitors of 11β-hydroxysteroid dehydrogenase type 1 as potential drugs for type 2 diabetes mellitus—a systematic review of clinical and in vivo preclinical studies. Sci. Pharm. 2021, 89, 5. [Google Scholar] [CrossRef]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Simos, Y.V.; Spyrou, K.; Patila, M.; Karouta, N.; Stamatis, H.; Gournis, D.; Dounousi, E.; Peschos, D. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J. Pharm. Sci. 2021, 16, 62–76. [Google Scholar] [CrossRef]
- Yadav, R.K.; Kumar, R.; Singh, H.; Mazumdar, A.; Salahuddin; Chauhan, B.; Abdullah, M.M. Recent insights on synthetic methods and pharmacological potential in relation with structure of benzothiazoles. Med. Chem. 2023, 19, 325–360. [Google Scholar] [CrossRef] [PubMed]
- Dahlén, A.D.; Dashi, G.; Maslov, I.; Attwood, M.M.; Jonsson, J.; Trukhan, V.; Schiöth, H.B. Trends in antidiabetic drug discovery: FDA approved drugs, new drugs in clinical trials and global sales. Front. Pharmacol. 2022, 12, 807548. [Google Scholar] [CrossRef] [PubMed]
- Konkwo, C.; Perry, R.J. Imeglimin: Current development and future potential in type 2 diabetes. Drugs 2021, 81, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Artasensi, A.; Pedretti, A.; Vistoli, G.; Fumagalli, L. Type 2 diabetes mellitus: A review of multi-target drugs. Molecules 2020, 25, 1987. [Google Scholar] [CrossRef]
- Seth, S. A comprehensive review on recent advances in synthesis & pharmacotherapeutic potential of benzothiazoles. Antiinflamm. Antiallergy Agents Med. Chem. 2015, 14, 98–112. [Google Scholar]
- Sumit; Kumar, A.; Mishra, A.K. Advancement in pharmacological activities of benzothiazole and its derivatives: An up to date review. Mini Rev. Med. Chem. 2021, 21, 314–335. [Google Scholar] [CrossRef]
- Bhutani, R.; Pathak, D.P.; Kapoor, G.; Husain, A.; Iqbal, M.A. Novel hybrids of benzothiazole-1,3,4-oxadiazole-4-thiazolidinone: Synthesis, in silico ADME study, molecular docking and in vivo anti-diabetic assessment. Bioorg. Chem. 2019, 83, 6–19. [Google Scholar] [CrossRef]
- Gupta, K.; Sirbaiya, A.K.; Kumar, V.; Rahman, M.A. Current perspective of synthesis of medicinally relevant benzothiazole based molecules: Potential for antimicrobial and anti-inflammatory activities. Mini Rev. Med. Chem. 2022, 22, 1895–1935. [Google Scholar] [CrossRef]
- Haroun, M. Review on the developments of benzothiazole-containing antimicrobial agents. Curr. Top Med. Chem. 2022, 22, 2630–2659. [Google Scholar] [CrossRef]
- Keri, R.S.; Patil, M.R.; Patil, S.A.; Budagumpi, S. A comprehensive review in current developments of benzothiazole-based molecules in medicinal chemistry. Eur. J. Med. Chem. 2015, 89, 207–251. [Google Scholar] [CrossRef] [PubMed]
- Rouf, A.; Tanyeli, C. Bioactive thiazole and benzothiazole derivatives. Eur. J. Med. Chem. 2015, 97, 911–927. [Google Scholar] [CrossRef]
- Ciocci Pardo, A.; González Arbeláez, L.F.; Fantinelli, J.C.; Álvarez, B.V.; Mosca, S.M.; Swenson, E.R. Myocardial and mitochondrial effects of the anhydrase carbonic inhibitor ethoxzolamide in ischemia-reperfusion. Physiol. Rep. 2021, 9, e15093. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, M.J.; Tabary, N.; Martel, B.; Cazaux, F.; Oliva, A.; Taboada, P.; Concheiro, A.; Alvarez-Lorenzo, C. Poly-(cyclo)dextrins as ethoxzolamide carriers in ophthalmic solutions and in contact lenses. Carbohydr. Polym. 2013, 98, 1343–1352. [Google Scholar] [CrossRef]
- Modak, J.K.; Tikhomirova, A.; Gorrell, R.J.; Rahman, M.M.; Kotsanas, D.; Korman, T.M.; Garcia-Bustos, J.; Kwok, T.; Ferrero, R.L.; Supuran, C.T.; Roujeinikova, A. Anti-Helicobacter pylori activity of ethoxzolamide. J. Enzyme Inhib. Med. Chem. 2019, 34, 1660–1667. [Google Scholar] [CrossRef]
- Rahman, M.M.; Tikhomirova, A.; Modak, J.K.; Hutton, M.L.; Supuran, C.T.; Roujeinikova, A. Antibacterial activity of ethoxzolamide against Helicobacter pylori strains SS1 and 26695. Gut Pathog. 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Chiò, A.; Mazzini, L.; Mora, G. Disease-modifying therapies in amyotrophic lateral sclerosis. Neuropharmacology 2020, 167, 107986. [Google Scholar] [CrossRef]
- Jaiswal, M.K. Riluzole and edaravone: A tale of two amyotrophic lateral sclerosis drugs. Med. Res. Rev. 2019, 39, 733–748. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Hartley, L.W.; Schmidtke, J.R. The immunomodulatory action of frentizole, a novel immunosuppressive agent. Immunopharmacology 1982, 5, 169–179. [Google Scholar] [CrossRef]
- Aitken, L.; Benek, O.; McKelvie, B.E.; Hughes, R.E.; Hroch, L.; Schmidt, M.; Major, L.L.; Vinklarova, L.; Kuca, K.; Smith, T.K.; Musilek, K.; Gunn-Moore, F.J. Novel benzothiazole-based ureas as 17β-HSD10 inhibitors, a potential Alzheimer’s disease treatment. Molecules 2019, 24, 2757. [Google Scholar] [CrossRef] [PubMed]
- Fišar, Z.; Musílek, K.; Benek, O.; Hroch, L.; Vinklářová, L.; Schmidt, M.; Hroudová, J.; Raboch, J. Effects of novel 17β-hydroxysteroid dehydrogenase type 10 inhibitors on mitochondrial respiration. Toxicol. Lett. 2021, 339, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Hroch, L.; Guest, P.; Benek, O.; Soukup, O.; Janockova, J.; Dolezal, R.; Kuca, K.; Aitken, L.; Smith, T.K.; Gunn-Moore, F.; Zala, D.; Ramsay, R.R.; Musilek, K. Synthesis and evaluation of frentizole-based indolyl thiourea analogues as MAO/ABAD inhibitors for Alzheimer’s disease treatment. Bioorg. Med. Chem. 2017, 25, 1143–1152. [Google Scholar] [CrossRef]
- Badawy, D.; El-Bassossy, H.M.; Fahmy, A.; Azhar, A. Aldose reductase inhibitors zopolrestat and ferulic acid alleviate hypertension associated with diabetes: effect on vascular reactivity. Can. J. Physiol. Pharmacol. 2013, 91, 101–107. [Google Scholar] [CrossRef]
- Bhutani, R.; Pathak, D.P.; Kapoor, G.; Husain, A.; Kant, R.; Iqbal, M.A. Synthesis, molecular modelling studies and ADME prediction of benzothiazole clubbed oxadiazole-Mannich bases, and evaluation of their anti-diabetic activity through in vivo model. Bioorg. Chem. 2018, 77, 6–15. [Google Scholar] [CrossRef]
- Gim, H.J.; Cheon, Y.J.; Ryu, J.H.; Jeon, R. Design and synthesis of benzoxazole containing indole analogs as peroxisome proliferator-activated receptor-γ/δ dual agonists. Bioorg. Med. Chem. Lett. 2011, 21, 3057–3061. [Google Scholar] [CrossRef] [PubMed]
- Haroun, M. Novel Hybrids of pyrazolidinedione and benzothiazole as TZD analogues. rationale design, synthesis and in vivo anti-diabetic evaluation. Med. Chem. 2019, 15, 624–633. [Google Scholar] [CrossRef]
- Haroun, M. In silico design, synthesis and evaluation of novel series of benzothiazole- based pyrazolidinediones as potent hypoglycemic agents. Med. Chem. 2020, 16, 812–825. [Google Scholar] [CrossRef]
- Kharbanda, C.; Alam, M.S.; Hamid, H.; Javed, K.; Bano, S.; Ali, Y.; Dhulap, A.; Alam, P.; Pasha, M.A. Novel piperine derivatives with antidiabetic effect as PPAR-γ agonists. Chem. Biol. Drug Des. 2016, 88, 354–362. [Google Scholar] [CrossRef]
- Cruz, A.; Padilla-Martínez, I.I.; García-Báez, E.V. A synthetic method to access symmetric and non-symmetric 2-(N,N’-disubstituted)guanidinebenzothiazoles. Molecules 2012, 17, 10178–10191. [Google Scholar] [CrossRef] [PubMed]
- Mendieta-Wejebe, J.E.; Rosales-Hernández, M.C.; Padilla-Martínez, I.I.; García-Báez, E.V.; Cruz, A. Design, synthesis and biological activities of (thio)urea benzothiazole derivatives. Int. J. Mol. Sci. 2023, 24, 9488. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Martínez, I.I.; González-Encarnación, J.M.; García-Báez, E.V.; Cruz, A.; Ramos-Organillo, Á.A. Isothioureas, ureas, and their N-methyl amides from 2-aminobenzothiazole and chiral amino acids. Molecules 2019, 24, 3391. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Hernández, M.C.; Mendieta-Wejebe, J.E.; Padilla-Martínez, I.I.; García-Báez, E.V.; Cruz, A. Synthesis and biological importance of 2-(thio)ureabenzothiazoles. Molecules 2022, 27, 6104. [Google Scholar] [CrossRef]
- Rosales-Hernández, M.C.; Mendieta-Wejebe, J.E.; Tamay-Cach, F.; Cruz, A. Synthetic procedures to access 2-guanidinobenzazoles of biological interest. Curr. Org. Synth. 2023, 20, 504–522. [Google Scholar]
- Rosales-Hernández, M.C.; Cruz, A.; Mendieta-Wejebe, J.E.; Tamay-Cach, F. 2-Guanidinobenzazoles as building blocks to afford biologically active derivatives. Curr. Org. Chem. 2023, 27, 38–54. [Google Scholar] [CrossRef]
- Lipinski, C.A. Rule of five in 2015 and beyond: Target and ligand structural limitations, ligand chemistry structure and drug discovery project decisions. Adv. Drug Deliv. Rev. 2016, 101, 34–41. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Cornelissen, F.M.G.; Market, G.; Deutsch, G.; Antonara, M.; Faaij, N.; Bartelink, I.; Noske, D.; Vandertop, W.P.; Bender, A.; Westerman, B.A. Explaining blood-brain barrier permeability of small molecules by integrated analysis of different transport mechanisms. J. Med. Chem. 2023, 66, 7253–7267. [Google Scholar] [CrossRef]
- Daina, A.; Zoete, V. A BOILED-egg to predict gastrointestinal absorption and brain penetration of small molecules. ChemMedChem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.; Doerksen, R.J. Topological polar surface area: a useful descriptor in 2D-QSAR. Curr. Med. Chem. 2009, 16, 21–41. [Google Scholar] [CrossRef]
- United Nations Economic Commission for Europe. Sustainable Development Goals. About the GHS. Available online: https://unece.org/about-ghs (accessed on 30 January 2023).
- Arana, M.R.; Altenberg, G.A. ATP-binding Cassette Exporters: Structure and Mechanism with a Focus on P-glycoprotein and MRP1. Curr. Med. Chem. 2019, 26, 1062–1078. [Google Scholar] [PubMed]
- Beis, K. Structural basis for the mechanism of ABC transporters. Biochem. Soc. Trans. 2015, 43, 889–893. [Google Scholar] [CrossRef] [PubMed]
- Di, L. The role of drug metabolizing enzymes in clearance. Expert Opin. Drug Metab. Toxicol. 2014, 10, 379–393. [Google Scholar]
- Chinnam, P.; Mohsin, M.; Shafee, L.M. Evaluation of acute toxicity of pioglitazone in mice. Toxicol. Int. 2012, 19, 250–254. [Google Scholar] [CrossRef]
- Muralikumar, S.; Vetrivel, U.; Narayanasamy, A.; Das, U.N. Probing the intermolecular interactions of PPARγ-LBD with polyunsaturated fatty acids and their anti-inflammatory metabolites to infer most potential binding moieties. Lipids Health Dis. 2017, 16, 17. [Google Scholar]
- Álvarez-Almazán, S.; Bello, M.; Tamay-Cach, F.; Martínez-Archundia, M.; Alemán-González-Duhart, D.; Correa-Basurto, J.; Mendieta-Wejebe, J.E. Study of new interactions of glitazone’s stereoisomers and the endogenous ligand 15d-PGJ2 on six different PPAR gamma proteins. Biochem. Pharmacol. 2017, 142, 168–193. [Google Scholar]
- Álvarez-Almazán, S.; Navarrete-Vázquez, G.; Padilla-Martínez, I.I.; Correa-Basurto, J.; Alemán-González-Duhart, D.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. A new symmetrical thiazolidinedione derivative: in silico design, synthesis, and in vivo evaluation on a streptozotocin-induced rat model of diabetes. Processes 2021, 9, 1294. [Google Scholar] [CrossRef]
- Kroker, A.J.; Bruning, J.B. Review of the structural and dynamic mechanisms of PPARγ partial agonism. PPAR Res. 2015, 2015, 816856. [Google Scholar] [CrossRef]
- Organisation for Economic Co-operation and Development (OECD). OECD Guidelines for the Testing of Chemicals. Test Guideline No. 425. Acute Oral Toxicity: Up-and-Down-Procedure (UDP). Paris, France, 2008, p.p. 1–28. Available online: https://www.oecd.org/env/test-no-425-acute-oral-toxicity-up-and-down-procedure-9789264071049-en.htm (accessed on 16 January 2023).
- Alemán-González-Duhart, D.; Tamay-Cach, F.; Correa-Basurto, J.; Padilla-Martínez, I.I.; Álvarez-Almazán, S.; Mendieta-Wejebe, J.E. In silico design, chemical synthesis and toxicological evaluation of 1,3-thiazolidine-2,4-dione derivatives as PPARγ agonists. Regul. Toxicol. Pharmacol. 2017, 86, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Alemán-González-Duhart, D.; Álvarez-Almazán, S.; Valdes, M.; Tamay-Cach, F.; Mendieta-Wejebe, J.E. In vivo and ex vivo evaluation of 1,3-thiazolidine-2,4-dione derivatives as euglycemic agents. PPAR Res. 2021, 2021, 5100531. [Google Scholar] [PubMed]
- Ahmed, Y.M.; Abdelgawad, M.A.; Shalaby, K.; Ghoneim, M.M.; AboulMagd, A.M.; Abdelwahab, N.S.; Hassan, H.M.; Othman, A.M. Pioglitazone synthetic analogue ameliorates streptozotocin-induced diabetes mellitus through modulation of ACE 2/angiotensin 1-7 via PI3K/AKT/mTOR signaling pathway. Pharmaceuticals 2022, 15, 341. [Google Scholar] [CrossRef] [PubMed]
- Frederico, M.J.S.; Castro, A.J.G.; Menegaz, D.; Murat, C.B.; Mendes, C.P.; Mascarello, A.; Nunes, R.J.; Silva, F.R.M.B. Mechanism of action of novel glibenclamide derivatives on potassium and calcium channels for insulin secretion. Curr. Drug Targets 2017, 18, 641–650. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef]
- Ghasemi, A.; Jeddi, S. Streptozotocin as a tool for induction of rat models of diabetes: a practical guide. EXCLI J. 2023, 22, 274–294. [Google Scholar]
- Kaur, R.; Sodhi, R.K.; Aggarwal, N.; Kaur, J.; Jain, U.K. Renoprotective effect of lansoprazole in streptozotocin-induced diabetic nephropathy in Wistar rats. Naunyn Schmiedebergs Arch. Pharmacol. 2016, 389, 73–85. [Google Scholar]
- Valdés, M.; Calzada, F.; Mendieta-Wejebe, J.E.; Merlín-Lucas, V.; Velázquez, C.; Barbosa, E. Antihyperglycemic effects of Annona diversifolia Safford and its acyclic terpenoids: α-glucosidase and selective SGLT1 inhibitors. Molecules 2020, 25, 3361. [Google Scholar] [CrossRef]
- Madhuri, K.; Naik, P.R. Modulatory effect of garcinol in streptozotocin-induced diabetic Wistar rats. Arch. Physiol. Biochem. 2017, 123, 322–329. [Google Scholar] [CrossRef]
- Abo-elmatty, D.M.; Essawy, S.S.; Badr, J.M.; Sterner, O. Antioxidant and anti-inflammatory effects of Urtica pilulifera extracts in type 2 diabetic rats. J. Ethnopharmacol. 2013, 145, 269–277. [Google Scholar]
- Lasram, M.M.; Bouzid, K.; Douib, I.B.; Annabi, A.; El Elj, N.; El Fazaa, S.; Abdelmoula, J.; Gharbi, N. Lipid metabolism disturbances contribute to insulin resistance and decrease insulin sensitivity by malathion exposure in Wistar rat. Drug Chem. Toxicol. 2015, 38, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Molinspiration Cheminformatics. Available online: https://www.molinspiration.com (accessed on 21 September 2022).
- Openmolecules.org. DataWarrior. Available online: https://openmolecules.org/datawarrior/ (accessed on 25 September 2022).
- ProTox 3.0. Prediction of Toxicity of Chemicals. Tox-Prediction. Available online: https://tox-new.charite.de/protox_II/ (accessed on 5 October 2022).
- SwissADME. SwissDrugDesign. Available online: http://www.swissadme.ch/ (accessed on 12 October 2022).
- Nolte, R.T.; Wisely, G.B.; Westin, S.; Cobb, J.E.; Lambert, M.H.; Kurokawa, R.; Rosenfeld, M.G.; Willson, T.M.; Glass, C.K.; Milburn, M.V. Ligand binding and co-activator assembly of the peroxisome proliferator-activated receptor-gamma. Nature 1998, 395, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Vangone, A.; Schaarschmidt, J.; Koukos, P.; Geng, C.; Citro, N.; Trellet, M.E.; Xue, L.C.; Bonvin, A.M.J.J. Large-scale prediction of binding affinity in protein-small ligand complexes: the PRODIGY-LIG web server. Bioinformatics 2019, 35, 1585–1587. [Google Scholar]
- Ferdowsian, H.R.; Beck, N. Ethical and scientific considerations regarding animal testing and research. PLoS One 2011, 6, e24059. [Google Scholar]
- Morton, D.B. Humane endpoints in animal experimentation for biomedical research: ethical, legal and practical aspects. 1998; pp:5-12.
- The ARRIVE guidelines 2.0. ARRIVE Essential 10. Available online: https://arriveguidelines.org/arrive-guidelines (accessed on 5 January 2023).
- Maheshwari, R.A.; Parmar, G.R.; Hinsu, D.; Seth, A.K.; Balaraman, R. Novel therapeutic intervention of coenzyme Q10 and its combination with pioglitazone on the mRNA expression level of adipocytokines in diabetic rats. Life Sci. 2020, 258, 118155. [Google Scholar]
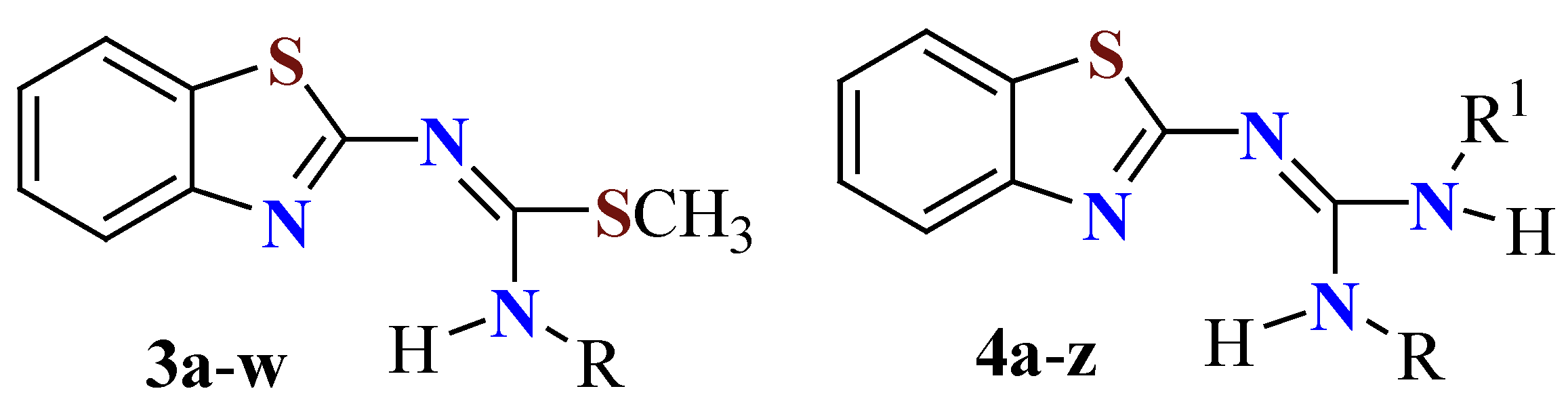
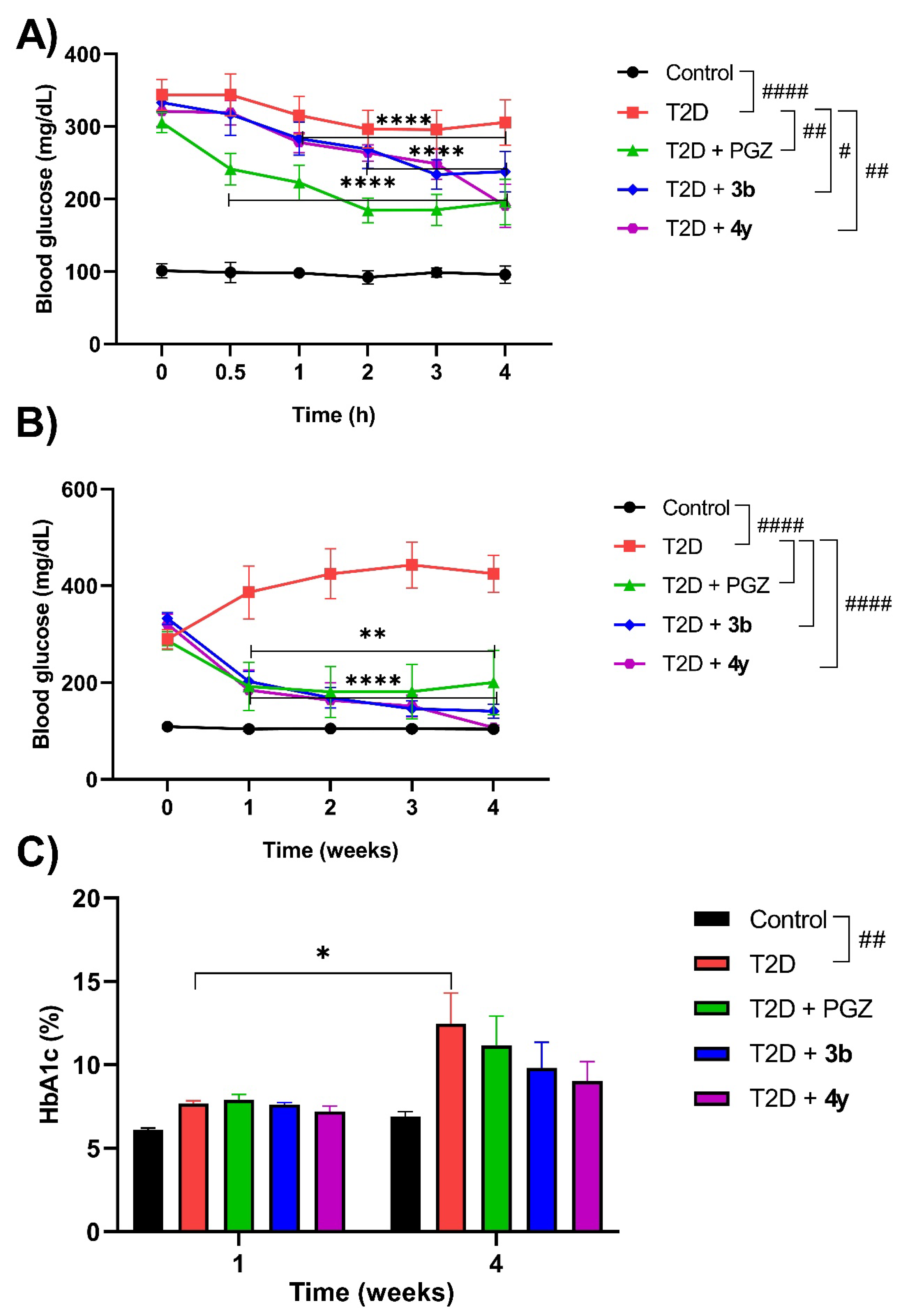
| Compound | Chemical structure |
|---|---|
| 3a | 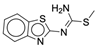 |
| 3b | 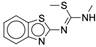 |
| 4a | 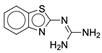 |
| 4b | 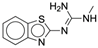 |
| 4c | 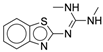 |
| 4r | 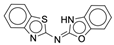 |
| 4s | 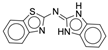 |
| 4x | 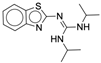 |
| 4y | 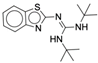 |
| Compound | Physicochemical properties | Toxicity (DW) | ODLS | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MW (g/mol) |
cLogP | nON | nOHNH | nrotb | TPSA (Å2) |
M | T | IE | RE | ||
| 3a | 223.33 | 2.41 | 3 | 2 | 2 | 51.28 | Χ | Χ | Χ | Χ | 1.0 |
| 3b | 237.35 | 2.79 | 3 | 1 | 3 | 37.28 | Χ | Χ | Χ | Χ | 1.0 |
| 4a | 192.25 | 2.29 | 4 | 4 | 1 | 77.30 | Χ | Χ | Χ | Χ | 1.0 |
| 4b | 206.27 | 2.67 | 4 | 3 | 2 | 63.31 | Χ | Χ | Χ | Χ | 1.0 |
| 4c | 220.30 | 3.04 | 4 | 2 | 3 | 49.31 | Χ | Χ | Χ | Χ | 1.0 |
| 4r | 267.31 | 4.14 | 4 | 1 | 1 | 54.19 | Χ | Χ | Χ | Χ | 1.0 |
| 4s | 266.33 | 4.04 | 4 | 2 | 1 | 56.84 | Χ | Χ | Χ | Χ | 1.0 |
| 4x | 276.41 | 4.38 | 4 | 2 | 5 | 49.31 | Χ | Χ | Χ | Χ | 1.0 |
| 4y | 304.46 | 5.41 | 4 | 2 | 5 | 49.31 | Χ | Χ | Χ | Χ | 0.92 |
| PGZ | 356.45 | 3.07 | 5 | 1 | 7 | 68.30 | Χ | Χ | Χ | Χ | 1.0 |
| Compound | LD50 (mg/kg) |
Class | Hepatotoxicity | Inmunotoxicity | Citotoxicity |
|---|---|---|---|---|---|
| 3a | 1190 | IV | X | X | X |
| 3b | 1000 | IV | X | X | X |
| 4a | 1190 | IV | X | X | X |
| 4b | 1190 | IV | X | X | X |
| 4c | 1190 | IV | X | X | X |
| 4r | 1190 | IV | X | X | X |
| 4s | 1190 | IV | X | X | X |
| 4x | 1190 | IV | X | X | X |
| 4y | 1190 | IV | X | X | X |
| PGZ | 1000 | IV | X | X | X |
| Compound | Aqueous solubility | Pharmacokinetics | ODLS | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| logS | Solubility (mg/mL) |
Class | GI | BBB | P-gp | CYP450 inhibitor | BD | ||||||||||
| 1A2 | 2C19 | 2C9 | 2D6 | 3A4 | |||||||||||||
| 3a | -3.00 | 2.24e-01 | III | √ | X | X | √ | √ | X | X | X | 0.55 | 0.84 | ||||
| 3b | -3.24 | 1.37e-01 | III | √ | X | X | √ | √ | √ | X | X | 0.55 | 0.80 | ||||
| 4a | -1.95 | 2.15e+00 | IV | √ | X | X | √ | X | X | X | X | 0.55 | 0.92 | ||||
| 4b | -2.19 | 1.34e+00 | III | √ | X | X | √ | X | X | X | X | 0.55 | 0.88 | ||||
| 4c | -2.43 | 8.22e-01 | III | √ | X | X | √ | X | X | X | X | 0.55 | 0.88 | ||||
| 4r | -4.51 | 8.31e-03 | II | √ | X | X | √ | X | X | X | X | 0.55 | 0.84 | ||||
| 4s | -4.14 | 1.95e-02 | II | √ | X | X | √ | X | X | X | √ | 0.55 | 0.80 | ||||
| 4x | -3.56 | 7.64e-02 | III | √ | √ | X | √ | √ | √ | X | X | 0.55 | 0.80 | ||||
| 4y | -3.93 | 3.56e-02 | III | √ | X | X | √ | √ | √ | X | X | 0.55 | 0.80 | ||||
| PGZ | -4.31 | 1.76e-02 | II | √ | X | X | √ | √ | √ | √ | √ | 0.55 | 0.68 | ||||
| Compound | Drug-likeness | Medicinal chemistsry | |||||||||||||||
| Lipinski | Ghose | Veber | Egan | Muegge | PAINS | Brenk | Synthesis accesibility | ||||||||||
| 3a | √ | √ | √ | √ | √ | 0 | 2 | 2.83 | |||||||||
| 3b | √ | √ | √ | √ | √ | 0 | 2 | 3.03 | |||||||||
| 4a | √ | √ | √ | √ | X | 0 | 2 | 2.50 | |||||||||
| 4b | √ | √ | √ | √ | √ | 0 | 2 | 2.69 | |||||||||
| 4c | √ | √ | √ | √ | √ | 0 | 2 | 2.77 | |||||||||
| 4r | √ | √ | √ | √ | √ | 0 | 0 | 3.22 | |||||||||
| 4s | √ | √ | √ | √ | √ | 0 | 0 | 2.86 | |||||||||
| 4x | √ | √ | √ | √ | √ | 0 | 2 | 3.16 | |||||||||
| 4y | √ | √ | √ | √ | √ | 0 | 2 | 3.38 | |||||||||
| PGZ | √ | √ | √ | √ | √ | 0 | 1 | 3.46 | |||||||||
| Compound 3b | |||
|
Doses (mg/kg) |
Mortality rate (%) |
LD50 (mg/kg) |
GHS category |
| 175 | 0 | > 1750 | Clase IV |
| 550 | 0 | ||
| 1750 | 0 | ||
| Compound 4y | |||
|
Doses (mg/kg) |
Mortality rate (%) |
LD50 (mg/kg) |
GHS category |
| 175 | 0 | > 1750 | Clase IV |
| 550 | 0 | ||
| 1750 | 0 | ||
| Compound | Organ weight (g) |
||||
|---|---|---|---|---|---|
| Spleen | Stomach | Liver | Intestine | Kidney | |
| Vehículo | 0.90 | 3.41 | 14.08 | 26.23 | 2.85 |
| 3b* | 0.82 ± 0.05 | 2.93 ± 0.49 | 13.71 ± 2.11 | 25.04 ± 1.04 | 2.50 ± 0.04 |
| 4y* | 0.84 ± 0.04 | 2.37 ± 0.40 | 14.71 ± 2.11 | 24.04 ± 0.94 | 2.95 ± 0.05 |
| Group | Parameter | |||
|---|---|---|---|---|
| TG (mg/dL) |
T-Cho (mg/dL) |
HDL-C (mg/dL) |
LDL-C (mg/dL) |
|
| Control | 107 ± 8 | 85 ± 3 | 10 ± 0 | 83 ± 3 |
| T2D | 128 ± 29 | 95 ± 11 | 12 ± 1 | 92 ± 10 |
| T2D + PGZ | 65 ± 14 | 79 ± 4 | 14 ± 2 | 76 ± 4 |
| T2D + 3b | 91 ± 12 | 79 ± 3 | 13 ± 1 | 76 ± 2 |
| T2D + 4y | 71 ± 15 | 74 ± 3 | 14 ± 2 | 71 ± 3 |
| Grupo | Parameter | ||
|---|---|---|---|
| ALT/GPT (U/L) |
AST/GOT (U/L) |
GGT (U/L) |
|
| Control | 53 ± 7 | 263 ± 26 | 10 ± 0 |
| T2D | 57 ± 12 | 194 ± 19 | 11 ± 2 |
| T2D + PGZ | 54 ± 11 | 285 ± 11 | 12 ± 1 |
| T2D + 3b | 31 ± 2 | 211 ± 14 | 10 ± 0 |
| T2D + 4y | 49 ± 9 | 236 ± 28 | 10 ± 0 |
| EXPERIMENTAL GROUPS | |||
|---|---|---|---|
| HEALTHY | WITH T2D | ||
| Name | Treatment | Name | Treatment |
| Healthy without treatment (n = 6) |
T2D without treatment (n = 6) |
STZ, 45 mg/kg *Note 1 |
|
| Healthy + Vehículo (n = 6) |
Vehicle, 1 mL *Note 2 |
T2D + Vehicle (n = 6) |
STZ, 45 mg/kg + Vehicle, 1 mL |
| Healthy + PGZ (n = 6) |
PGZ, 15 mg/kg | T2D + PGZ (n = 6) |
STZ, 45 mg/kg + PGZ, 15 mg/kg |
| Healthy + 3b or 4y (n = 6) |
Compound 3b or 4y *Note 3 |
T2D + 3b or 4y (n = 6) |
STZ, 45 mg/kg + Compound 3b or 4y |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





