1. Introduction
Water is fundamental for the survival of living beings but the increasing pollution, due to the growth of industrialization and human activity, is seriously compromising the availability and healthiness of this important resource with considerable social, ecological and economic implications. Industrial wastes and intensive agricultural treatments represent the main sources of water pollution, releasing in the environment high amounts of inorganic and organic pollutants, many of which are not biodegradable and tend to accumulate in aquatic environments, threatening human and environmental health [
1,
2]
Phenolic compounds, such as dyes and pesticides, represent a hazardous class of organic contaminants produced in large amounts from industrial activities. Generally, their toxicity is associated with the presence of aromatic rings in the structure that confer to these substances mutagenic and carcinogenic properties [
3]. For these reasons, the research of innovative approaches to ensure drinking water purification but also efficient wastewater treatment is fundamental. Dyes commonly released in the environment from textile, pharmaceutical and plastic industries, are not biodegradable due to their aromatic structure and their thermal and photo stability [
4]. Thus, their persistence in the environment can cause groundwater pollution, endangering human health. For these reasons, the capability to degrade dyes from the industrial wastewater and to put back into the industrial cycle the treated water, avoiding its environmental dispersion, would be a great achievement.
Methylene blue (MB) is a typical hazardous dye with carcinogenic properties widely used in industrial plants. It is well known that a prolonged exposure to MB can cause serious damages to the nervous system and gastrointestinal apparatus. For this reason, the research of specific methodologies to eradicate the presence of this hazardous compound from water represents an important goal [
3]. Generally, biological, chemical and physical methods are widely used for dyes removal, but these technologies showed a lot of disadvantages associated with the production of hazardous by-products that cause secondary environmental pollution. In the last years a considerable increase of research of new and efficient materials able to adsorb water pollutants has been obvious. Natural materials such as clays [
5,
6], microorganisms [
7], and agricultural wastes [
8,
9,
10], were recently adopted as green sorbents for water remediation to remove organic and inorganic pollutants [
11]. However, these approaches only move the problem but do not eliminate it. Recently, the application of nanotechnology to water and wastewater treatment has gained great attention. In the last few years, a lot of studies focused on the synthesis of noble metal nanoparticles applied to water remediation have been published [
12,
13,
14]. Gold and silver nanoparticles are the most studied due to their peculiar characteristics such as large surface area, chemical reactivity, biocompatibility, catalytic and unique optical properties associated with the presence of a specific surface plasmon resonance (related with nanoparticles shape and dimension) that resulting from a typical scattering of light when noble metal nanoparticles are irradiated with specific wavelengths [
15]. For these reasons noble metal nanoparticles were widely used as nanocatalysts in water remediation [
3,
16]. Indeed, their presence catalyzes reduction and oxidation processes that convert harmful compounds into non toxic substances [
17,
18].
Generally the preparation of noble metal nanoparticles require the use of hazardous compounds and often expensive equipment, but recently a lot of research were made to exploit reducing and stabilizing agent naturally present in agricultural waste (sugars, polyphenols, tannins, anthocyanins, flavonoids etc.) used as starting materials for green metal nanoparticles preparation [
18,
19,
20,
21,
22]. Recently, Song and co-workers prepared green silver and gold nanoparticles using
Sargassum horneri extract as starting materials for dyes degradation in the presence of sodium borohydride (NaBH
4), a hazardous reducing agent [
23]. Green gold and silver nanoparticles were also obtained starting from the mangrove isolate
Pseudoalteromonas lipolytica, using peptides as stabilizing agent and employed for dyes degradation. In particular these photocatalytic systems showed an efficient degradation capacity of MB and congo red [
24]. In another work green gold nanoparticles capped by polyoxyethylene cholesteryl ether were synthesized revealing high catalytic activity in degradation of MB to Leucomethylene also in this case in the presence of NaBH
4 [
25]. However, in view of the increased global interest in the development of eco-sustainability processes for water purification, the use of hazardous compounds such as NaBH
4 should be avoided. Moreover, an easy system to remove metal nanoparticles after degradation is desirable to avoid their dispersion in the environment.
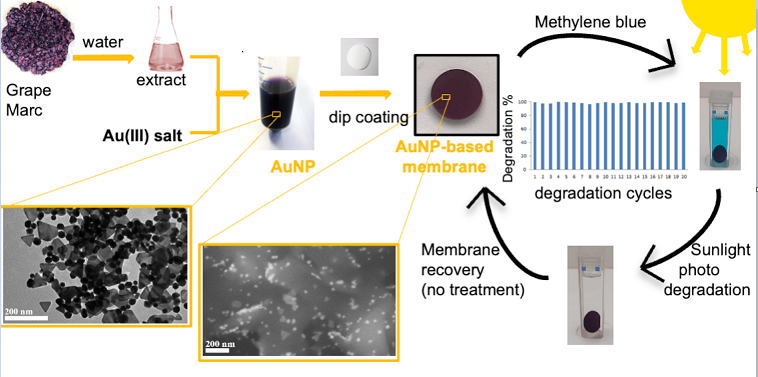
Starting from this assumption, in this work our attention was focused on the development of an efficient nanocomposite material based on green gold nanoparticles able to degrade MB present in water, without the use of other toxic substances. Firstly, green gold nanoparticles were synthesized using as starting materials an hydrothermal extract of Lambrusco winery grape marc (GM) waste, obtained from a local company, avoiding the use of hazardous compounds commonly used in metal nanoparticles preparation [
4,
18,
26]. Then, green gold nanoparticles (GM-AuNPs), after characterization, were immobilized on a commercial polyvinylidene difluoride (PVDF) membrane to obtain a stable and efficient system for MB degradation from wastewater. In this way, a combination between the high photocatalytic activity of green gold nanoparticles and the high stability of PVDF membrane support occurred, favoring an easy removal of the membrane after its use. GM-AuNPs and the modified membrane were characterized using transmission electron microscopy (TEM), scanning transmission electron microscopy (STEM) and X-Ray powder diffraction (XRD). After characterization the modified membrane was incubated with MB solutions in the dark and under sunlight radiation without the addition of other reagents and the degradation process was followed by UV-vis spectrophotometer. Fourier transform infrared spectroscopy (FT-IR) analysis was also used to demonstrate the complete degradation of MB. The same experiment was performed several times to evaluate the repeatability and the stability of the nanocomposite membrane in the time. Finally, the degradation ability of the modified membrane has been also tested on other organic pollutants such as methyl orange (MO), rhodamine B (RO) and 4-nitrophenol (4NP).
2. Materials and Methods
2.1. Materials
Gold (III) chloride trihydrate (HAuCl4⋅3H2O), MB, RO, MO, 4-NP and filter membranes Durapore ® PVDF (13 mm of diameter and 0.22 µm of pore size) were acquired from Sigma-Aldrich (Steinheim, Germany). GM wastes obtained from Lambrusco winery production (2023) of Salento area (South of Italy) were supplied from a local company (Cantina Vecchia Torre s.c.a.). Whatman 1 filters were purchased from Merck KGaA (Darmstadt, Germany). Centrifugations were conducted using a NEYA 16R High Speed refrigerated centrifuge (Giorgio Bormac s.r.l, MO, Italy) and a PK121 multispeed centrifuge (Thermo Electron Corporation, Waltham, MA, USA). All solutions were prepared using ultrapure water obtained using a water purification system (Human Corporation, Seoul, Korea).
2.2. Grape Marc Extract Preparation
GM winery wastes were sun dried for 5 days and then milled and sieved. 5 grams of the obtained powder were suspended in 100 ml of ultrapure water and heated at 65°C for 1h [
18]. After filtration with Whatman 1 filter and centrifugation at 9000 rpm for 20 minutes, the extract was aliquoted and stored at -20°C until use.
2.3. Synthesis of Green Gold Nanoparticles
50 ml of aqueous solution of HAuCl
4·3H
2O (1 mM) were heated at 80°C using a thermostatic oil bath. Then, 5 ml of GM extract were added to the solution and the mixture was vigorously stirred at 80°C for 1 hour [
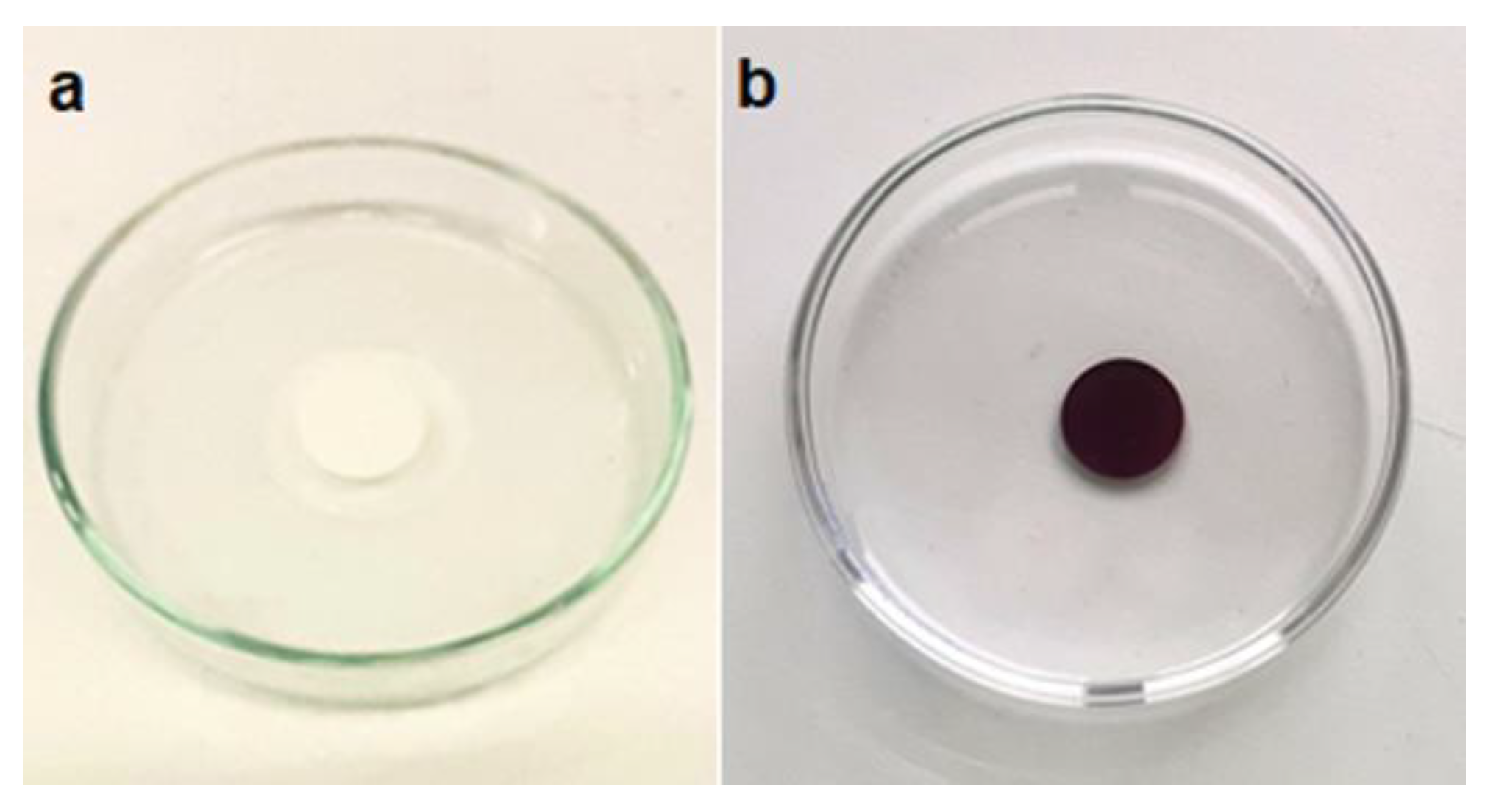
18]. After 10 minutes a color change from pale yellow to purple confirmed nanoparticles formation. The synthesis was followed by using a Jasco V-660 UV-vis spectrophotometer (Jasco, Palo Alto, CA, USA) to monitor the comparison of a Plasmon resonance peak typical of AuNPs. After 1 hour the reaction was stopped and left cooling at room temperature. The purple dispersion was stored at room temperature in the dark until use.
2.4. Modified PVDF Membrane Preparation
Commercial PVDF membrane was activated in ethanol for 3 minutes, washed and stored in ultrapure water at 4°C. After activation, the membrane was dipped in 2 ml of green AuNPs dispersion for 20 hours, then washed with ultrapure water to remove residual AuNPs and stored at 4°C in ultrapure water until further use [
27]. To evaluate the amount of GM-AuNPs deposited, PVDF membrane was weighed before deposition and then, after preparation, dried at 40°C and weighed again.
2.5. Characterization Studies
A first characterization of GM-AuNPs colloidal solution was conducted by using an Jasco V-660 UV-vis spectrophotometer. In particular, the reduction of gold ions and the comparison of plasmonic bands typical of gold nanoparticles was monitored analyzing UV-vis spectra of diluted solutions (1/10, v/v) of crude reaction in the time. UV-vis spectrophotometer was also used to follow the signal decrease in solution of MB, MO, RO and 4NP of kinetic experiments.
Electrophoretic light scattering measurements, conducted by using a Malvern Zetasizer nano ZS 90 (Worcestershire, UK), were evaluated on diluted samples to determine the zeta-potential of GM-AuNPs.
XRD analysis of synthesized GM-AuNPs and GM-AuNPs-based membrane was evaluated by using a Rigaku Ultima+ model diffractometer (Rigaku, Riga, Latvia) with a monochromatic Cu-kα radiation (λ =0.154 nm), operating at 40kV with a current of 20 mA. GM-AuNPs colloidal solution was centrifuged several times at 12000 rpm (+4°C) for 10 minutes and then drop-cast on a glass slide and dried in the oven at 30°C for 24 hours before analysis. All spectra were recorded in the 2θ range between 30 and 80°.
FT-IR analysis on GM-AuNPs-based membrane were performed using a Jasco FT-IR-660 plus spectrometer (Jasco, Palo Alto, CA, USA) applying an attenuated total reflectance (ATR) technique, with 64 scans and 2 cm-1 of resolution over the range of 3650 - 650 cm-1.
UV-vis-NIR spectra were acquired through a Shimadzu UV-3600 i Plus.
TEM analysis of GM-AuNPs aqueous dispersion was performed using JEOL JEM-1400 (JEOL, Tokyo, Japan) operating at 120kV and equipped with a CCD camera (Gatan Orius 831). A few microliters of GM-AuNPs dispersion were drop-cast onto carbon-coated Cu grids and then dried overnight at 60°C before investigation. The same sample deposited onto a copper grid was used for STEM measurements performed in a Zeiss Merlin SEM operating at 20 kV. SEM analysis of GM-AuNPs-loaded membranes were carried out in a field-gun emission SEM, Sigma 300VP, Zeiss, Germany at accelerating voltage of 15 kV, by employing a secondary electron detector under high vacuum conditions.
2.6. Photocatalytic Activity and Kinetic Studies of Modified Membrane
Photocatalytic activity of GM-AuNPs-based membrane was evaluated by incubating the modified membrane with 3ml of 5.6 mg L-1 of MB solution in a quartz cuvette 4 cm in height with 1 cm path length and kept under sunlight radiation. Reaction solution was monitored by a UV-vis spectrophotometer at regular intervals, recording the absorbance at 664 nm. The same experiment was conducted using a pristine PVDF membrane.
Degradation % was calculated using the following equation:
where A
t and A
0 were associated to the absorbance at the time t and 0 respectively.
The GM-AuNPs-based membrane was also incubated in the dark for 2 hours and the results obtained were compared.
To study kinetic performance, linearized form of a pseudo-first-order and pseudo-second-order kinetics were used as described in the following equation:
where C
0 and C
t represent the initial concentration and the concentration at the time t of dye pollutant respectively and k
1 and k
2 the pseudo-first-order and pseudo-second-order rate constants respectively [
28,
29,
30].
To evaluate the specificity of the system, the modified membrane was also incubated separately under sunlight radiation with 5.6 mg L-1 of MO, RO and 4NP solutions. All experiments were conducted in triplicate.
The Materials and Methods should be described with sufficient details to allow others to replicate and build on the published results. Please note that the publication of your manuscript implicates that you must make all materials, data, computer code, and protocols associated with the publication available to readers. Please disclose at the submission stage any restrictions on the availability of materials or information. New methods and protocols should be described in detail while well-established methods can be briefly described and appropriately cited.
2.7. Reusability of Modified Membrane in MB Degradation
The modified membrane was incubated in 3 ml of MB solution (5.6 mg L-1) in a quartz cuvette 4 cm in height with 1 cm path length and kept under sunlight radiation for 2 hours. The absorbance of the peak at 664 nm was monitored at regular intervals by UV-vis spectrophotometer. Then the modified membrane was reused for another degradation process, without any treatment. The same experiment was repeated 20 times in a period of three months.
3. Results and Discussion
3.1. Synthesis and Characterization of Green Gold Nanoparticles
Grape marc (GM) represents a high value waste obtained from the winery industry rich of relevant evaluable products such as tannins, polyphenols, pigments and unfermented sugars with important reducing and stabilizing properties [
26].The high solubility of these substances in hot water, promotes their hydrothermal extraction and they can be used as starting materials for green noble metal nanoparticles preparation instead of toxic substances generally employed [
18]. In detail, our attention was focused on the use of GM extract, obtained at 65°C, to synthesize green gold nanoparticles with a specific plasmonic band, associated to nanoparticle dimensions, that confer notable photocatalytic properties when opportunely irradiated.
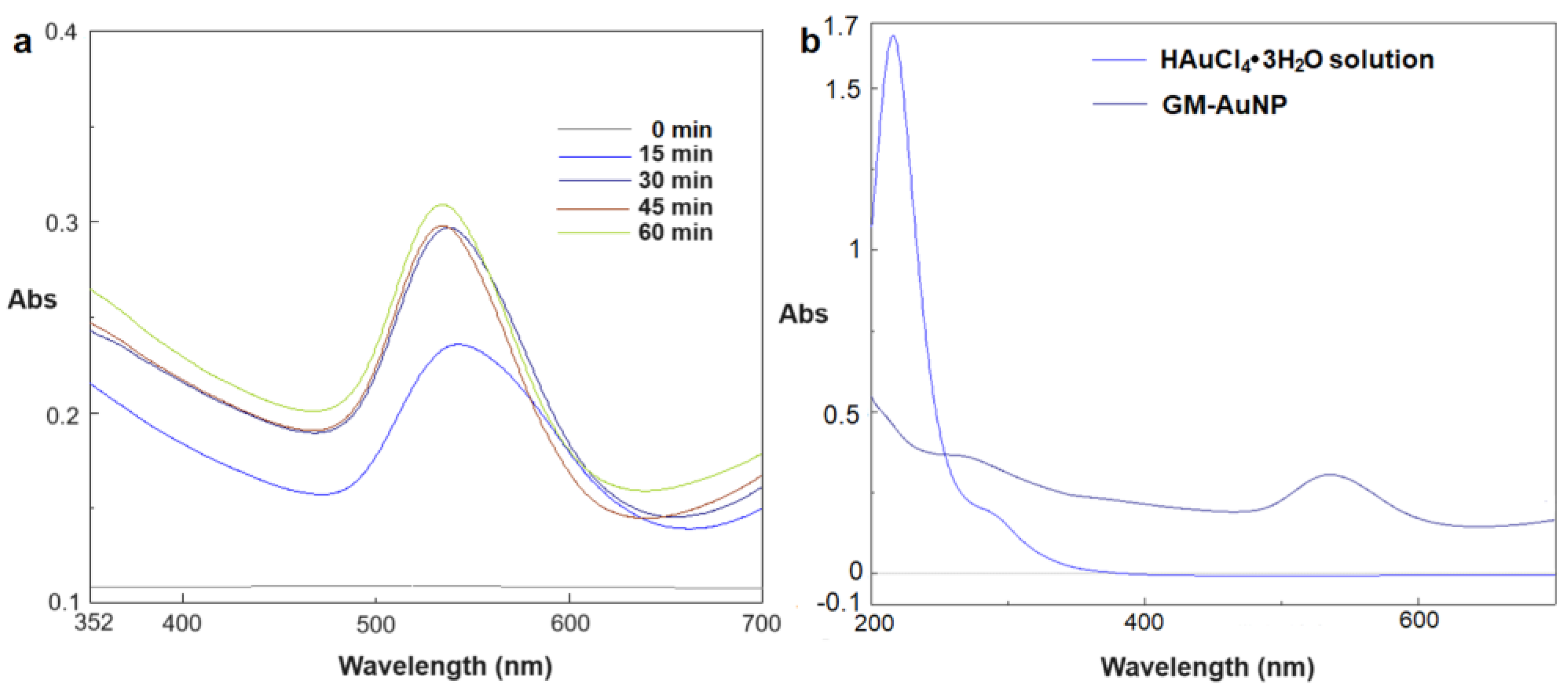
An easy and fast methodology was employed to synthesize GM-AuNPs adding the extract to an auric solution heated at 80°C. After 10 minutes of vigorous stirring the color of solution changes from pale yellow to purple, indicating the reduction of Au
3+ to Au
0. Nanoparticles formation was monitored by UV-vis spectrophotometer, analyzing diluted samples of crude reaction every 15 minutes. As it can be seen in
Figure 1a, plasmonic band at 545 nm appears after 15 minutes, shifting to 535 nm after 1 hour. The results obtained confirmed gold nanoparticles formation from GM extract through different steps. Initially, (activation step) Au
3+ ions were reduced to Au
0 and the nucleation occurred. This step was followed by the formation of larger nanoparticles (growth step) confirmed from the plasmonic band at 545 nm obtained after 15 minutes. Then, in the final step all nanoparticles take on the shape energetically favored, reducing their dimension, as confirmed from the plasmonic band shifted at 535 nm (
Figure 1a) [
31]. Overlapping the UV-vis spectra of diluted auric solution (1 mM) used for reaction and crude reaction obtained after 1 hour, the disappearance of the typical peak of gold ions (216 nm) and the appearance of a plasmonic band (535 nm) was observed, demonstrating nanoparticles formation (
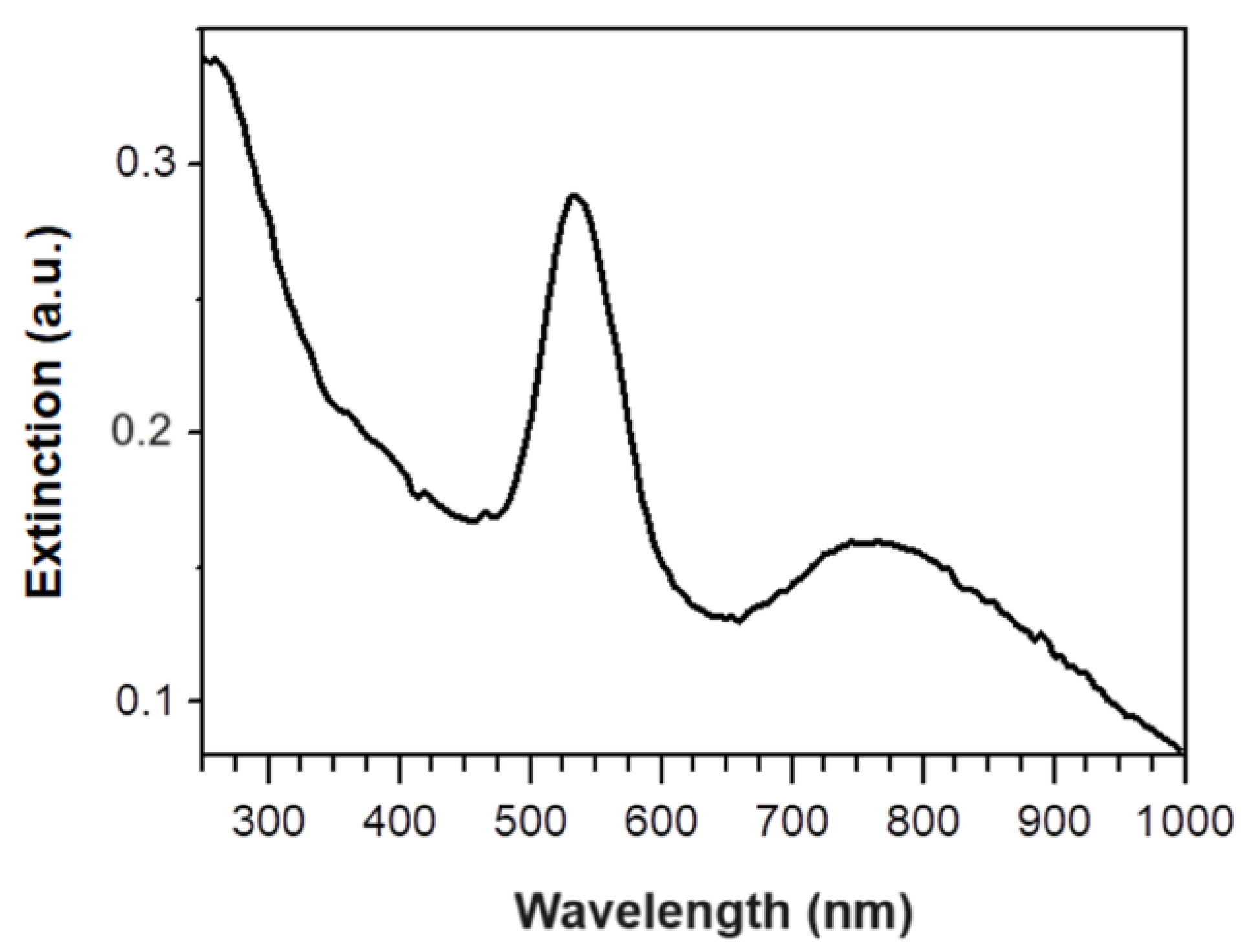
Figure 1b). Moreover, a new signal was observed over 600 nm; indeed, a band at 760 nm, probably due to in-plane dipole resonance of triangular NPs, appears in the extinction spectrum (
Figure 2).
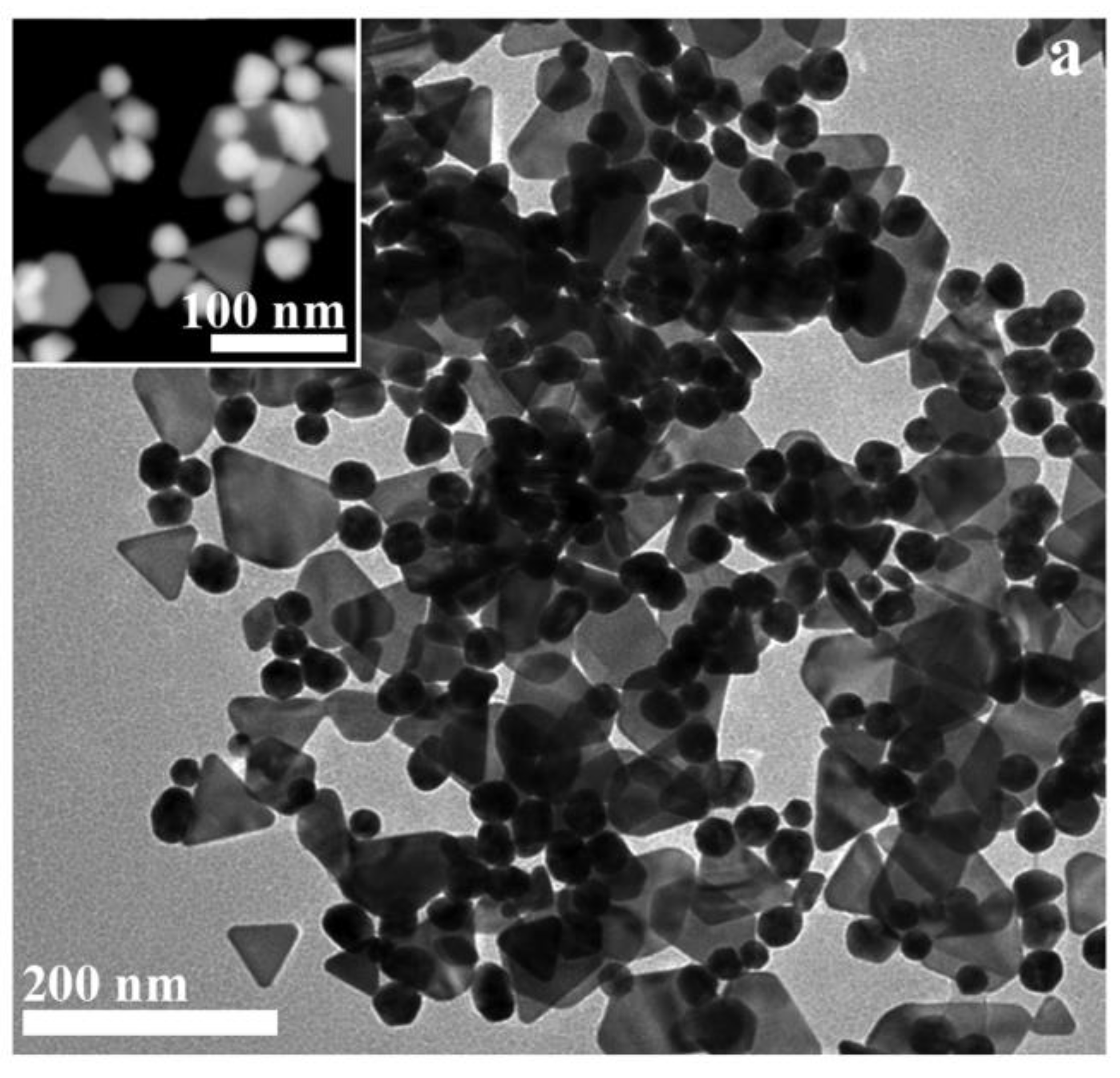
These data were confirmed from TEM analysis that revealed the evident presence of two species of crystalline colloidal NPs, respectively quasi-two-dimensional triangular platelets as large as 75-80 nm showing smoothed corners that reasonably evolve from (truncated) hexagonal profiles, as confirmed by many hexagonal shapes, and 30 nm in diameter irregularly-spherical twinned NPs (
Figure 3). STEM analysis confirmed TEM observations (see inset in
Figure 3). The GM extract contains a wide variety of bioactive compounds, particularly polyols with the assistance of other reducing and passivating agents, that are reasonably supposed to act as reducing agents throughout the synthesis and function as NP stabilizers (
Figure 3) [
32].
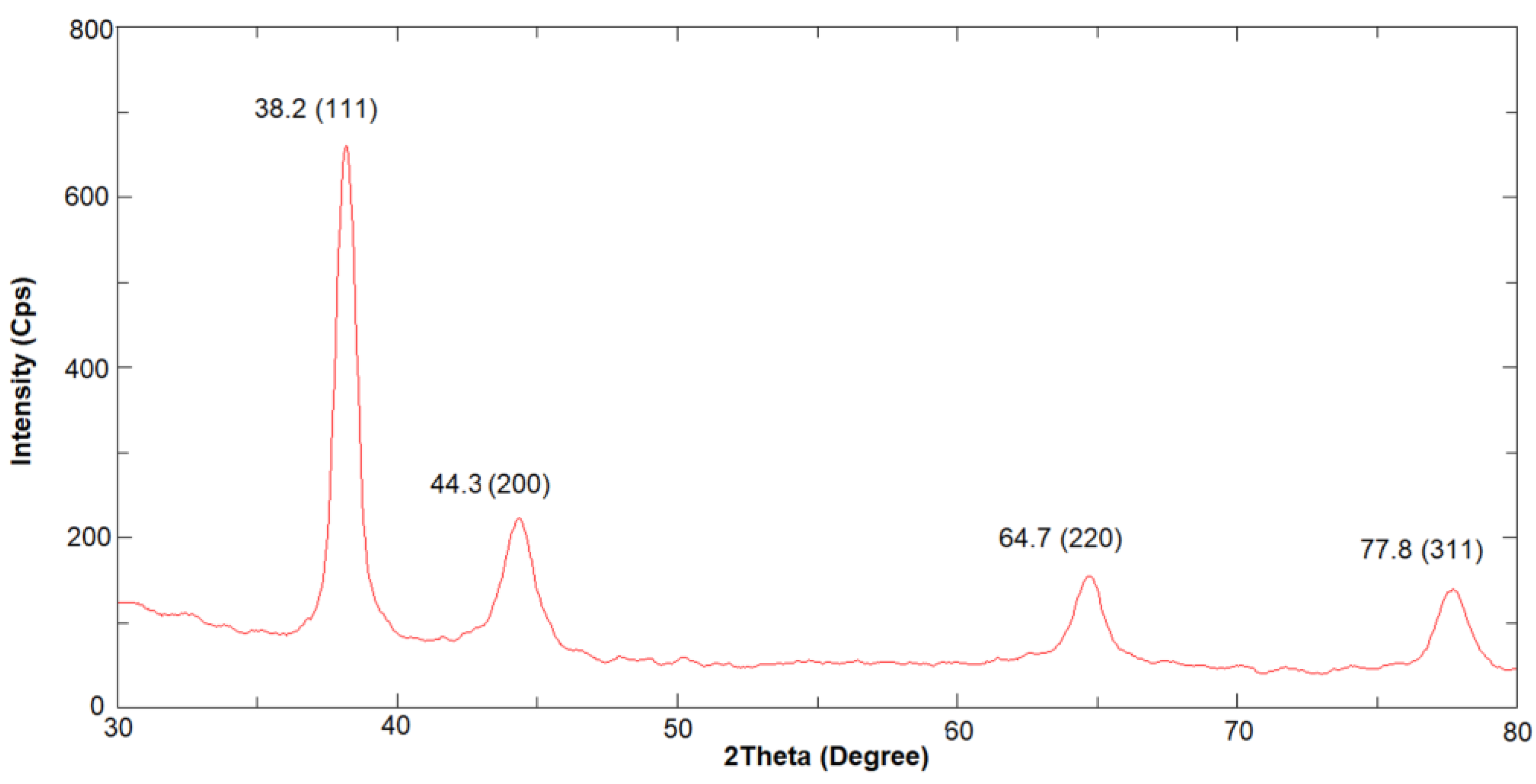
To further confirm the crystalline nature of synthesized GM-AuNPs, XRD analysis was also conducted in the range of 30-80° 2θ angle. As it can be seen in
Figure 4 four Bragg reflection peaks at 38.2°, 44.3°, 64.7° and 77.8° were obtained that correspond to Miller indexes of (111), (200), (220) and (311) respectively.
The results obtained reveal a good match with AuNPs standard diffraction pattern of face-centered cubic gold, confirming the presence of pure crystalline gold nanoparticles. The diffractogram shows the (111)-facet preferential growth of Au platelets. It can be concluded that gold nanoparticles with a crystalline structure, obtained from grape marc extract, were successfully synthesized. Moreover, zeta-potential analysis was also conducted revealing a negative charge of GM-AuNPs with a zeta-potential equal to -32.6±5.5 mV.
3.2. Dip Coating of PVDF Membrane in GM-AuNPs Dispersion
To develop a simple and fast system to test the photocatalytic activity of GM-AuNPs towards MB, gold nanoparticles were deposited by dip coating on a PVDF commercial membrane. Generally, the use of polymeric supports is desirable to avoid gold nanoparticles aggregation due to van der Waals interaction established between adjacent nanoparticles, and to facilitate an easy removal after water purification, avoiding centrifugation procedures and favoring its applicability in real systems. However, the high hydrophobicity of pristine PVDF membranes due to the fluorocarbon groups (-CF
2) restricts their use. For this reason, an easy and fast hydrophilic activation in ethanol was required before nanoparticles deposition. The infiltration of ethanol determines a temporary change from hydrophobic to hydrophilic. In this way electrostatic interactions between activated PVDF membrane and GM-AuNPs negatively charged can occur [
27]. As it can be seen in
Figure 5, an uniform layer of nanoparticles was obtained. During incubation it is very important to leave both faces of the membrane free of contact from vial surfaces in order to obtain the same deposition layer on both membrane sides. The amount of GM-AuNPs deposited was evaluated by weighting the membrane before and after dip coating, obtaining an amount of deposited GM-AuNPs equal to 158 ± 10 μg cm
-2 of membrane.
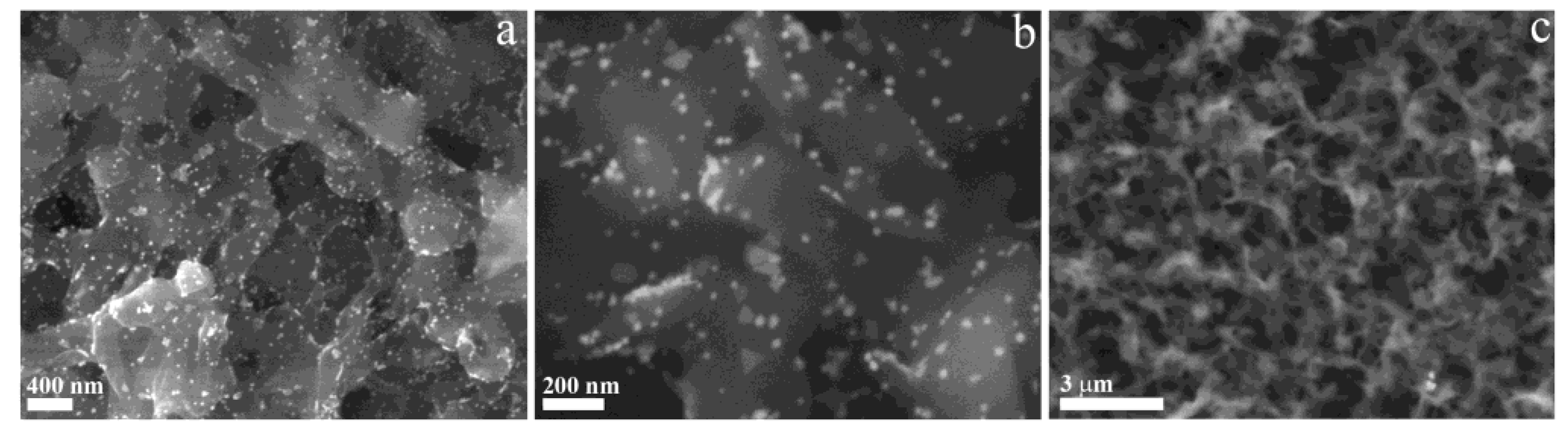
Figure 6 reports SEM micrographs of GM-AuNPs-based membrane at lower and larger magnification (see
Figure 6a-b) and of pristine membrane (
Figure 6c). GM-AuNPs are evenly dispersed (in length and depth) within the membrane on the scale of many tens of micrometers, without the formation of aggregates. The nanoparticles surface can thus be uniformly exposed to adsorbate interactions. Moreover, XRD analysis conducted on GM-AuNPs-based membrane, confirmed the presence of GM-AuNPs previously characterized. Indeed, reflection peaks at 38.3°, 44.4°, 64.9°, 77.8°, corresponding respectively to indexes of (111), (200), (220) and (311), similarly to the XRD pattern of GM-AuNPs (
Figure 4) was observed.
3.3. Photocatalytic Studies
To evaluate the photocatalytic effect of GM-AuNPs deposited on the activated membrane, different experiments were conducted under sunlight exposure, chosen to mimic real conditions. In detail, a pristine and a modified membrane were incubated with an aqueous solution of MB (5.6 mg L
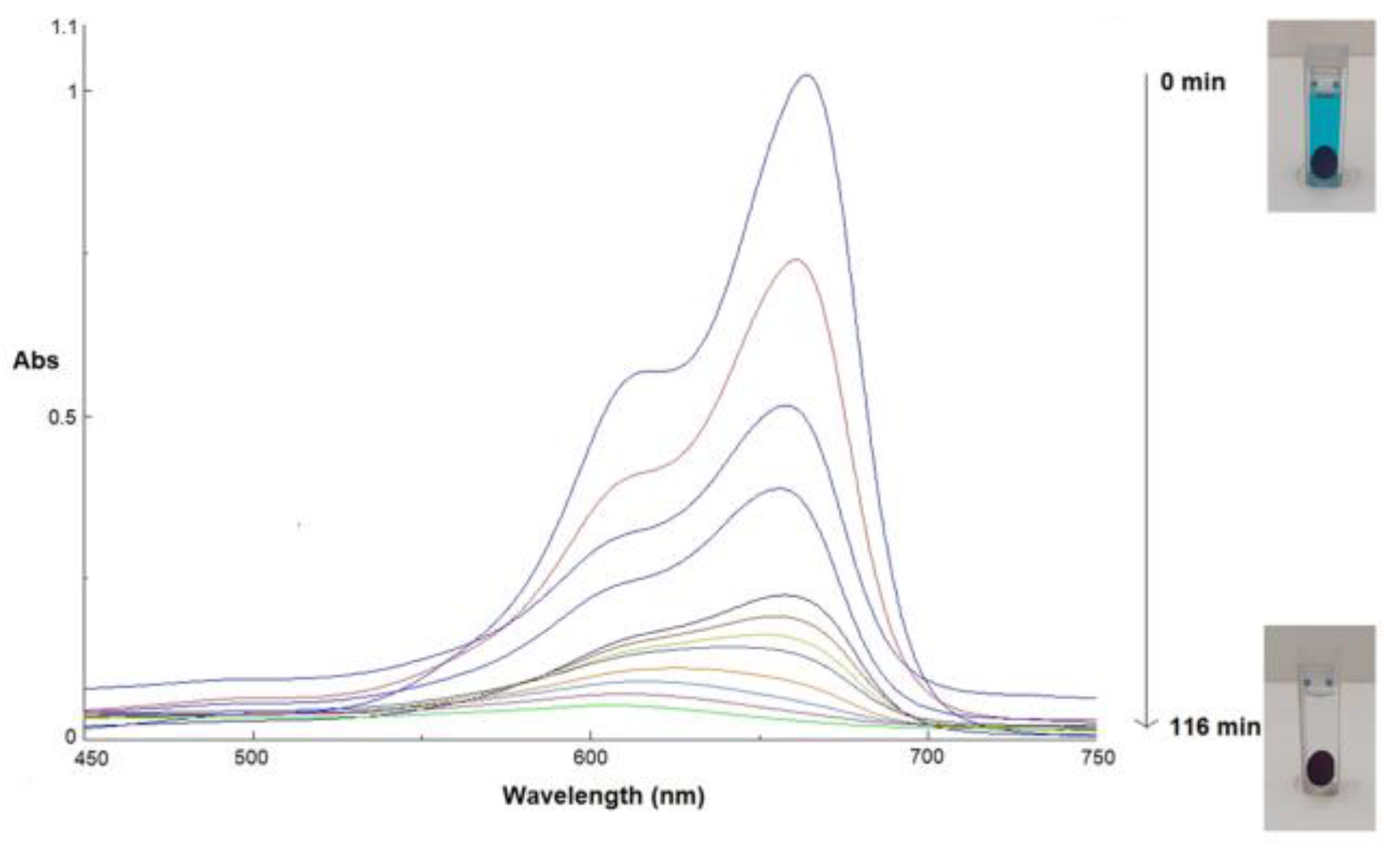
-1) and exposed to sunlight to evaluate their performance. UV-vis spectrum of MB presents two characteristic peaks at 611 and 664 nm associated with the conjugated structure of N-S heterocycle group. Under sunlight radiation of MB solution in the presence of GM-AuNPs-based membrane, a gradual reduction of adsorption peaks intensity was observed, obtaining a complete disappearance after 116 minutes (
Figure 7).
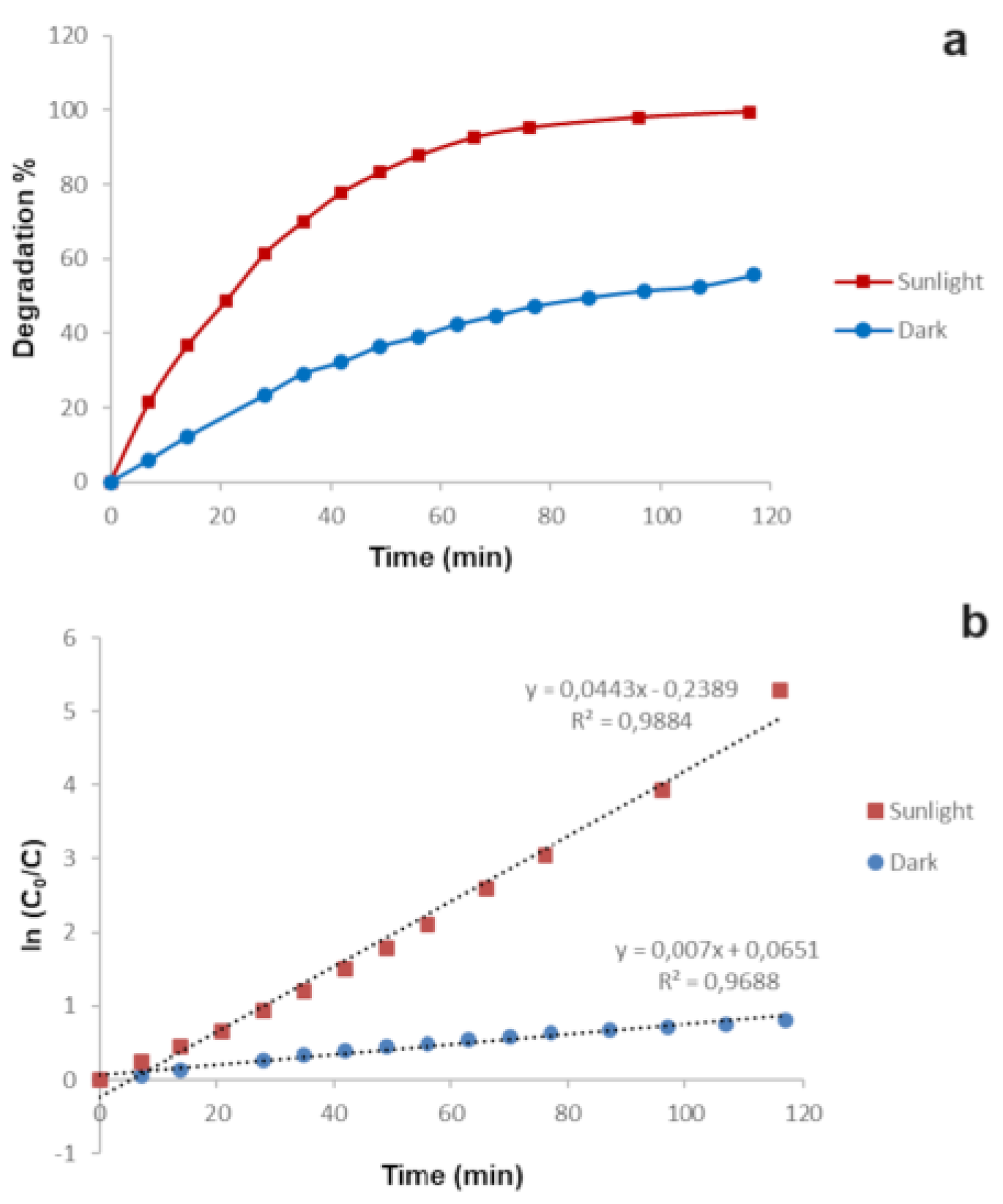
To understand the influence of sunlight radiations, the same experiment was also conducted in the dark. As it can be seen in
Figure 8a, a significant difference can be observed comparing the performance of the modified membrane in the dark and under sunlight radiation with a relevant lower removal percentage observed in the dark. Probably, only an adsorption process of the membrane occurs in the dark while the sunlight also promotes a photodegradation process mediated by GM-AuNPs that allows the complete removal of the MB. Generally, photodegradation of organic pollutants follows a pseudo-first-order kinetics [
28,
33]. As can be seen in
Figure 8b, plotting the ln(C
0/C) versus t, a linear regression was obtained with k
1 of 0.044 ± 0.011 min
-1 when the experiment was conducted under sunlight radiation, that was 5.6 times higher than the k
1 value obtained in the dark (0.007 ± 0.002 min
-1). These results confirmed the influence of sunlight radiation on the MB degradation process mediated by GM-AuNPs deposited on the membrane. Analyzing these data, it can be concluded that MB degradation occurs through a combined adsorption and photodegradation effect as found also in other works [
31].
Indeed, most probably, in a first step an adsorption of MB on the modified membrane occurs. In this way MB and GM-AuNPs are in direct contact and then the photocatalytic reaction can be started. Concerning AuNPs, depending on the wavelength excitation region, two optical contributions can be activated towards photocatalysis, namely intraband and interband excitations. In the former case the stimulation of a localized surface plasmon resonance occurs, involving electronic transitions within the sp-band. In the latter case, transitions from d- to sp-bands are associated. In the case of gold, interband transitions relate to short wavelength regions, usually below 450 nm [
34]. The two optical contributions promote two different catalytic path exciting different charge carriers; in the case of excitation of localized surface plasmon resonance high energy (hot) electrons can be created having an energy higher than the Fermi level; this could be transferred to an adsorbate or to oxygen/water molecules to produce reactive radicals. In the case of interband excitation, instead, very energy-deep holes showing low potential (below Fermi energy) can be generated; these are more available for very oxidative paths. The complete degradation of dyes suggests the formation of highly reactive superoxide and hydroxyl free radicals as most likely. In general, it is not straightforward to claim which mechanism is the more likely of the two.
Furthermore, a (photo)thermal input, a critical factor in catalysis, cannot be completely ruled out; indeed, because of characteristic shape of triangles, thermal hot-spots are created around metal apexes where an enhancement of the electromagnetic field occurs and of the electron transfer strength to adsorbed molecules [
35]. The presence in the reaction medium of these reactive species probably determined the degradation of MB initially absorbed into the membrane, causing firstly the breakage of N-CH
3 bound and after of the aromatic ring. All fragments produced are further attacked from this reactive species and finally degraded in smaller untraceable products [
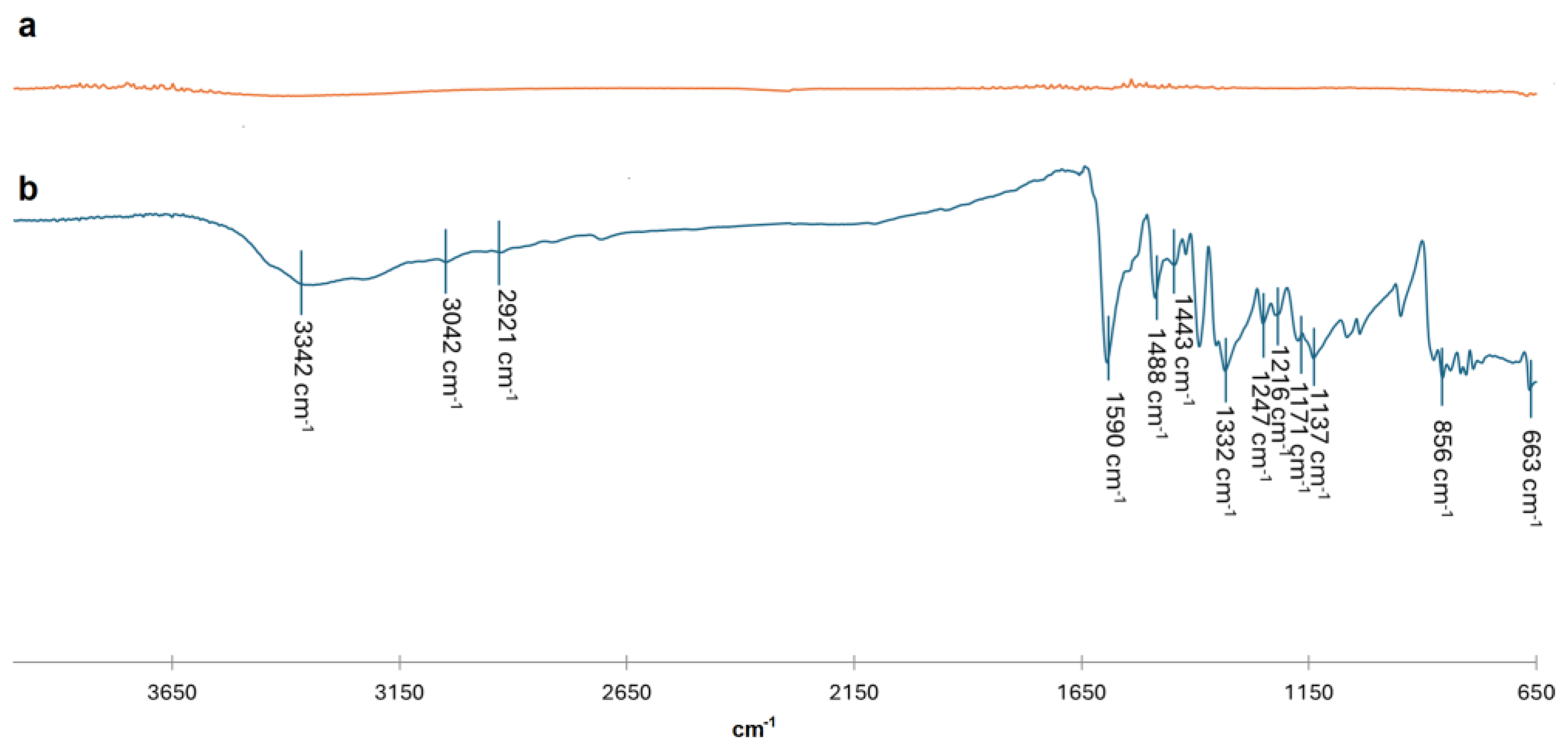
3]. To support these hypotheses, FT-IR analysis of MB solutions before and after sunlight radiation were also conducted. As it can be seen in
Figure 9b all characteristic peaks of MB functional groups can be observed. It can underline the presence of an adsorption peak at 1590 cm
-1 associated with C=C framework vibration and C=N stretching vibration of benzene ring. Moreover, the adsorption peaks at 1488 and 1443 cm
-1 were also correlated to the C=C vibration. At 1247 cm-
1 it can be seen a characteristic peak related to C=S stretching vibration. Aromatic C-H in plane bending vibration can be noted at 1216, 1171 and 1137 cm
-1, while out-plane bending vibration peaks were also found at 856 and 663 cm
-1. The broad peak at 3342 cm
-1 was associated with the O-H stretching vibration of water [
36]. After photocatalytic degradation, FT-IR spectrum of degradation products (
Figure 9a) does not show any peak associated with the presence of organic molecule. Moreover, it was interesting to note bubbles formation during the photocatalytic process suggesting the formation of gaseous species.
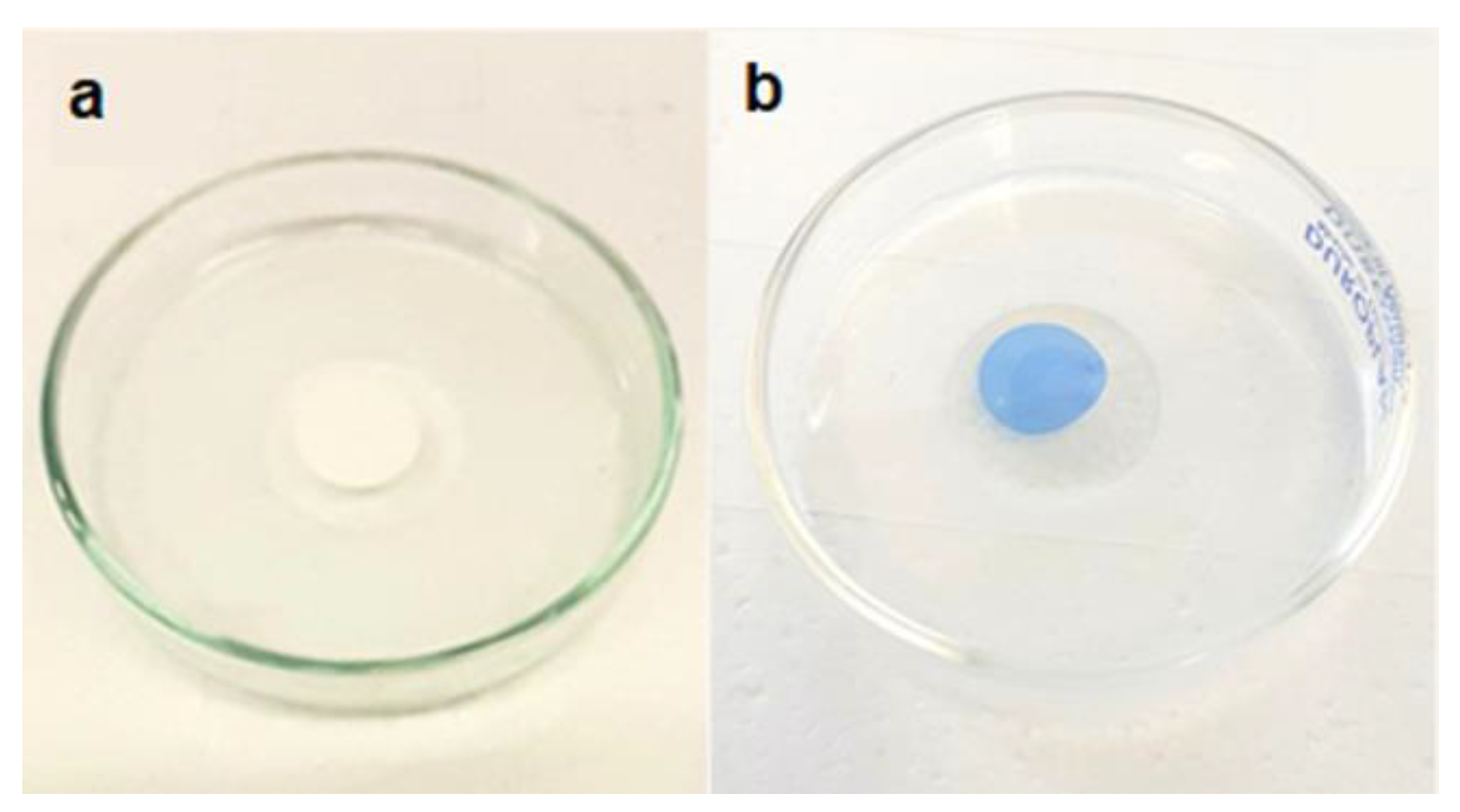
To corroborate the synergistic effect of adsorption and photodegradation process in MB degradation, the same experiment was conducted using a pristine PVDF membrane. After 2 hours of incubation with MB solution (5.6 mg L
-1), 90% of MB was removed from the solution but through an adsorption process, as it can be seen from the blue coloration of the membrane after incubation (
Figure 10).
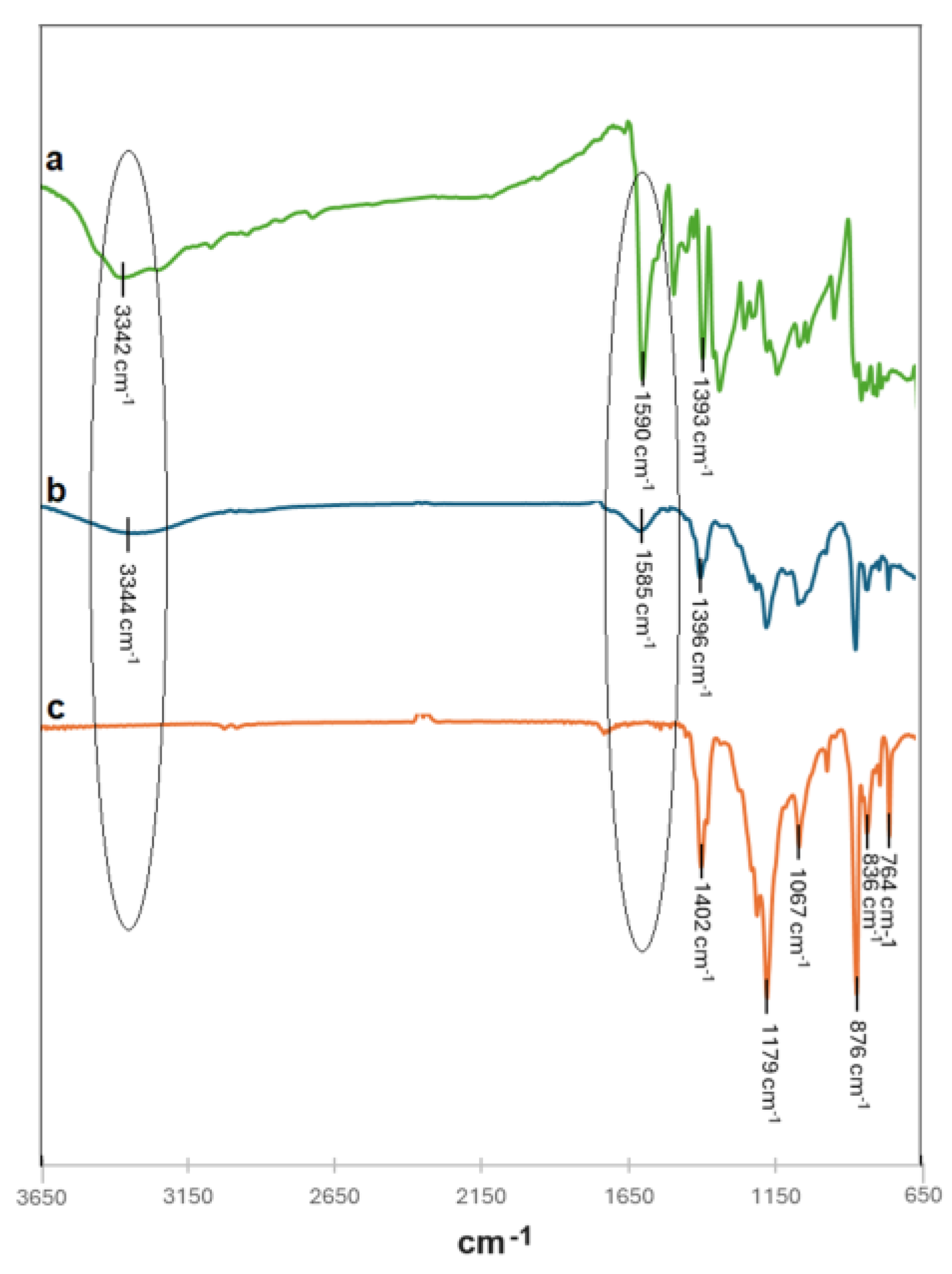
To confirm the above hypothesis, in
Figure 11, FT-IR spectra of pristine and modified membranes after sunlight radiation were reported and overlapped with MB solution spectrum.
As it can be seen, the pristine PVDF membrane after sunlight incubation showed the presence of peaks at 3344 and 1585 cm
-1 associated with the O-H stretching vibration of water and C=C framework vibration and C=N stretching vibration of benzene ring respectively (
Figure 11b) that confirmed the presence of MB adsorbed on the membrane. On the contrary, no peaks associated with MB were found in the FT-IR spectrum of GM-AuNPs-based membrane after sunlight incubation, (
Figure 11c) confirming the occurred MB degradation. Optimized conditions were also used to evaluate the performance of the modified membrane in the presence of higher MB concentrations. In
Table 1 a comparison at different MB concentrations of degradation % measured after 2 hours of sunlight radiation was reported: increasing MB concentration, the efficiency of the GM-AuNPs-based membrane decreases. The reduction of efficiency of the modified membrane in the presence of higher MB concentration was probability due to the higher MB adsorption at lower concentration that favors the direct contact with GM-AuNPs and consequently its photocatalytic degradation.
3.4. Modified Membrane Behavior with Other Organic Pollutants
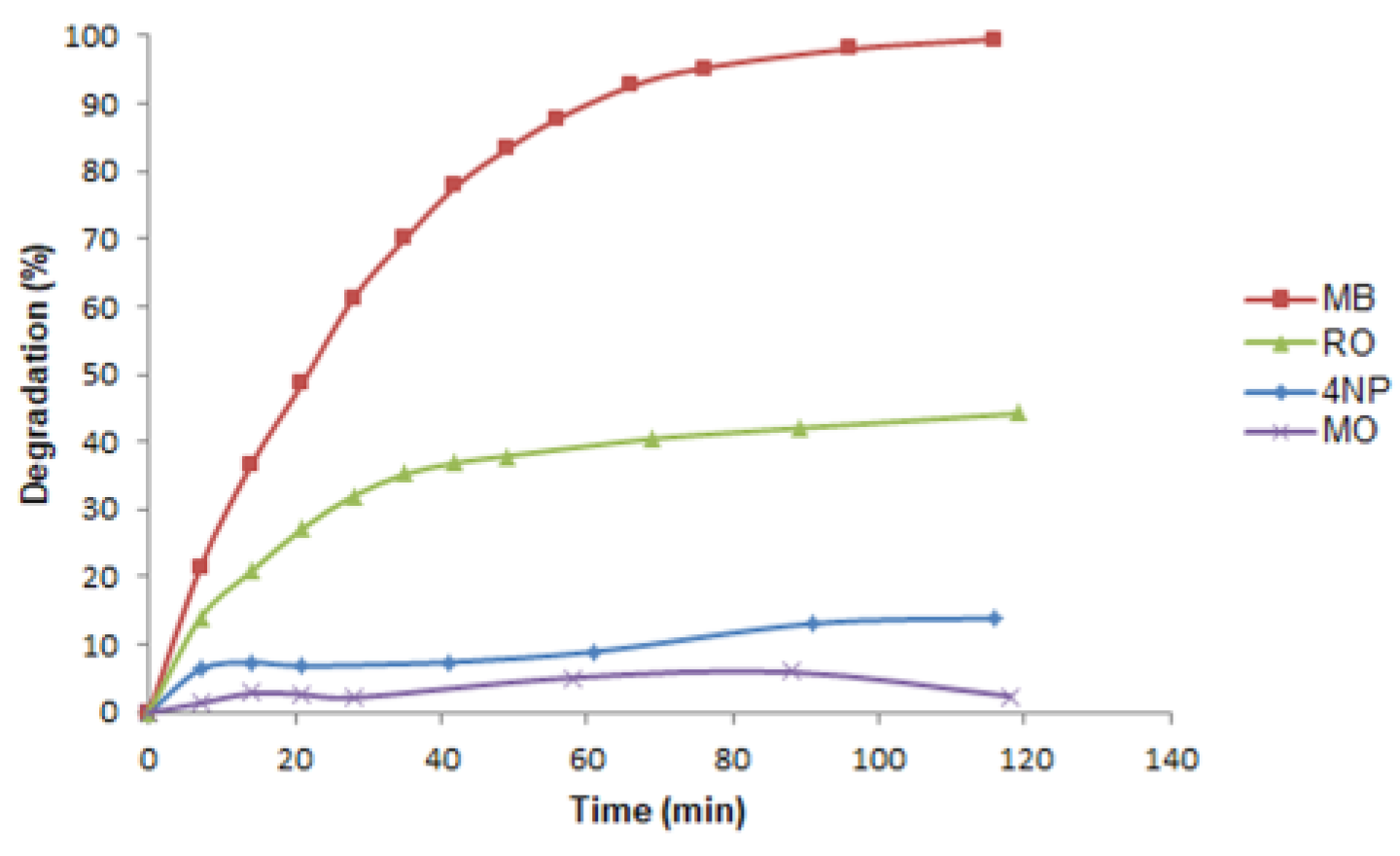
Photocatalytic performances of GM-AuNPs-based membrane were also tested using other dyes (methyl orange and rhodamine) and 4-nitrophenol, a typical pesticide widely used as a model. As it can be noted in
Figure 12, a total degradation occurred only with MB, underlining the selectivity of the system. The experiment conducted using RO as pollutant showed a decrease of the principal peak signal (554 nm) lower than 45%. However, after 2 hours of sunlight radiation, the membrane was stored in 3 ml of ultrapure water and an evident release of RO in solution (78% of the MB adsorbed), monitored by spectrophotometric analysis, was observed. Thus, it can be assumed that using RO, an adsorption process, also favored from its positive charge, occurred. Following the principal absorption peaks of MO and 4NP solution (465 and 317 nm respectively) no significant reduction after sunlight radiation was observed. Probability, the negative charge of MO produces electrostatic repulsion between MO and GM-AuNPs, negatively charged, hindering the direct contact with the nanoparticles, essential to trigger the photocatalytic reaction, as demonstrated also in other works [
31].
According to the typical adsorption peak at 317 nm, 4NP in water is present in neutral form, reducing its contact with gold nanoparticles. Kinetic data were processed using a pseudo-first-order and a pseudo-second-order kinetic models in the linear form and the results were reported in
Table 2. It is interesting to note that only using MB a linear fitting with a pseudo-first-order kinetic can be obtained (R
2= 0.9884), generally observed in photodegradation processes [
28,
33]. Instead, the incubation of GM-AuNPs-based membrane with RO, 4NP and MO showed a prevalent linearity with a pseudo-second-order kinetic in accordance with correlations coefficient obtained (
Table 2).
3.5. Reusability and Stability
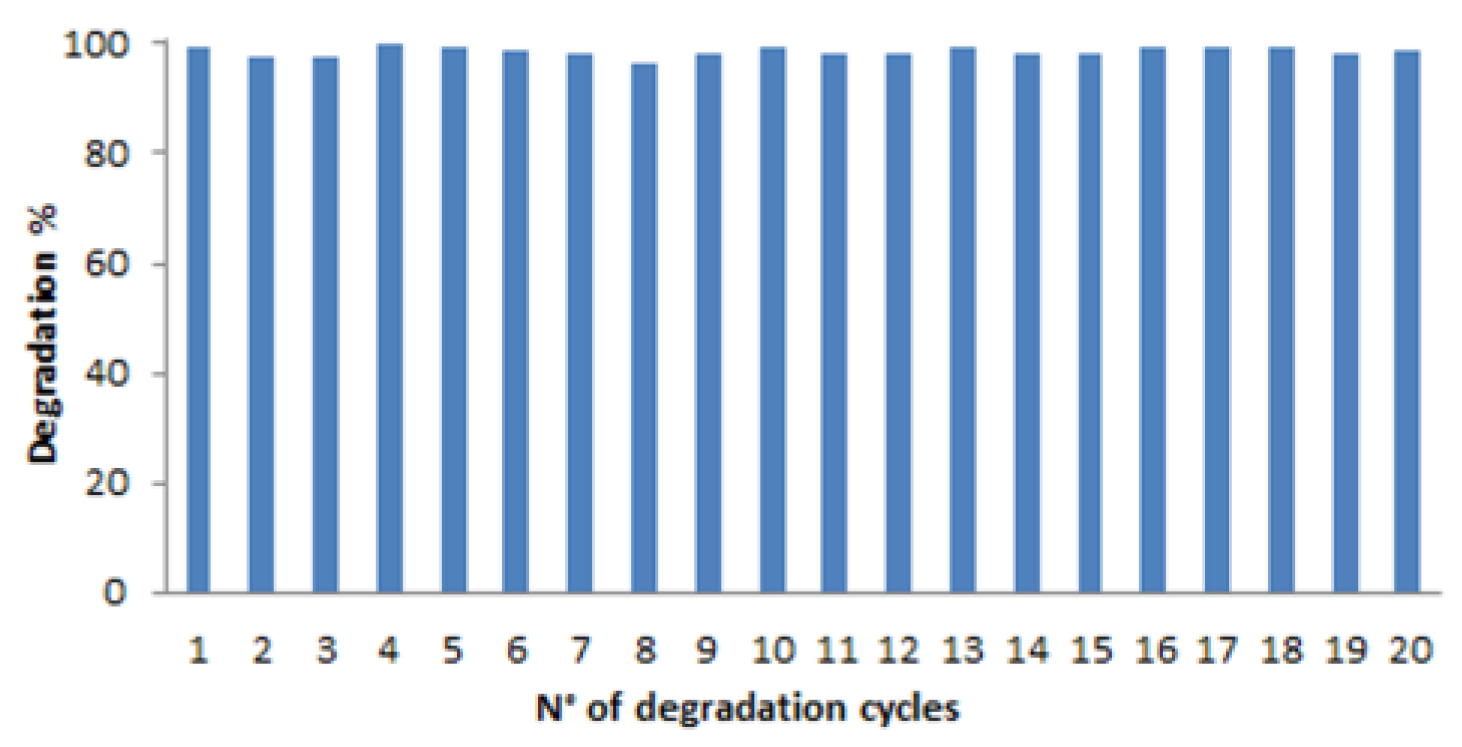
The potential reusability of the modified membrane represents the principal requirements to investigate the real applicability of the developed system in water remediation. To evaluate the performance in the time, the same modified membrane was incubated with MB solution (5.6 mg L
-1) 20 times over a period of 3 months and no loss of efficiency in MB degradation was observed, as it can be seen in
Figure 13. It is interesting to note that no washing step between each incubation was done, confirming that MB photodegradation has occurred. Indeed, if the disappearance of MB in solution was due only to an adsorption process, after few cycles, a saturation of the membrane would have happened.
Thus, an excellent nanocomposite material for MB water remediation was developed. Indeed, a high efficiency of the modified membrane was also observed in the last cycle of incubation (20th cycle) corroborates the photodegradation hypothesis previously advanced. Moreover, the high reusability determined a significant cost reduction, although gold nanoparticles were used.
5. Conclusions
In this work an easy and low cost sustainable system for MB water remediation was developed. Indeed, although an expensive gold salt was used for GM-AuNPs preparation, the absence of other reagents in their green preparation and the high reusability of the same modified membrane without losing of degradation capability, leads to an evident cost reduction. The GM-AuNPs-based membrane developed showed a very high efficiency with a power of total degradation always near to 100% during 20 cycles of incubation tested. Moreover, no sophisticated instrumentation was required, making their production and use highly sustainable. Starting from the important results obtained in this work, it would be interesting to study in the future all specific photoinduced mechanisms involved in MB photodegradation and evaluate the real possibility to exploit the peculiarity of the developed membrane in industrial wastewater treatment. Moreover, considering the excellent physical characteristics of GM-AuNPs obtained using a green route, the possible application in medicine could also be explored. Indeed, the presence of quasi-two-dimensional triangular platelets as large as 75-80 nm, in addition to irregularly-spherical twinned nanoparticles, confer to the NIR-absorbing colloidal metal nanoparticle solution, important photothermal characteristics that would make them ideal in phototherapy treatments.
Author Contributions
Conceptualization, L.M. and R.D.S.; Data curation, L.M., L.C. and R.D.S. ; Formal analysis, M.R.L., L.M., L.C. and R.D.S. ; Funding acquisition, R.D.S. and L.M. ; Investigation, L.M., L.C. and R.D.S. ; Methodology, L.M. and R.D.S.; Project administration, L.M. and R.D.S. ; Resources, R.D.S.; Supervision, R.D.S. and L.M.; Validation, L.M. and R.D.S. ; Visualization, L.M. M.R.L., L.C. and R.D.S. ; Writing – original draft, L.M., L.M. and R.D.S. ; Writing – review & editing, R.D.S. and L.M.
Funding
This research was funded by Ministry of Education, University and Research of Italy, PON “Research and Innovation” 2014-2020, Action IV.6 “Research contracts on green topics”. The authors thank the University of Salento–Department of Engineering for Innovation for funding this work (Fondi per la Ricerca di Base).
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors acknowledge Cantina Vecchia Torre s.c.a (Leverano, Lecce) for materials supplied from their winery, and the technician Donato Cannoletta for XRD analysis.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alprol, A.E.; Mansour A.T.; Abdelwahab, A.M.; Ashour, M., Advances in Green Synthesis of Metal Oxide Nanoparticles by Marine Algae for Wastewater Treatment by Adsorption and Photocatalysis Techniques. Catalysts 2023, 13, 888. [CrossRef]
- Policarpo Tonelli, F.M.; Santos Silva, C.; Silva Delgado, V.M.; Policarpo Tonelli, F.C. Algae-based green AgNPs, AuNPs, and FeNPs as potential nanoremediators. Green Process. and Synth. 2023, 12, 20230008. [CrossRef]
- Din, M.I.; Khalid, R.; Najee, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean. Prod. 2021, 298, 126567. [CrossRef]
- Omran, B.A.; Baek, K.H. Valorization of agro-industrial biowaste to green nanomaterials for wastewater treatment: Approaching green chemistry and circular economy principles. J.Environ. Manage. 2022, 311, 114806. [CrossRef]
- Del Sole, R.; Fogel, A.A.; Somin, V.A.; Vasapollo, G.; Mergola, L. Evaluation of Effective Composite Biosorbents Based on Wood Sawdust and Natural Clay for Heavy Metals Removal from Water. Materials 2023, 16, 5322. [CrossRef]
- Mamyachenkov, S.V.; Adryshev, A.K.; Seraya, N.V.; Khairullina, A.A.; Daumova, G.K. Nanostructured complex sorbent for cleaning heavy metal ions from industrial effluent. Metallurgist 2017, 61, 615–623. [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu Z.; Wang, S. Application of biochar immobilized microorganisms for pollutants removal from wastewater: A review. Sci. Total Environ. 2022, 837, 155563. http://dx.doi.org/10.1016/j.scitotenv.2022.155563.
- Del Sole, R.; Maggio, A.; Mergola, L. Green grape marc biosorbents preparation for mercury removal in aqueous media. Chem. Ind. Chem. Eng. Q. 2023, 29 (1) 1−10. [CrossRef]
- Arias Arias, F.E.; Beneduci, A.; Chidichimo, F.; Furia, E.; Straface, S. Study of the adsorption of mercury (II) on lignocellulosic materials under static and dynamic conditions. Chemosphere 2017 180, 11-23. [CrossRef]
- Mora Alvarez, N.M.; Pastrana, J.M.; Lagos, Y.; Lozada, J.J. Evaluation of mercury (Hg2+) adsorption capacity using exhausted coffee waste. Sustain. Chem. Pharm. 2018, 10, 60-70. [CrossRef]
- Husien, S.; El-taweel, R. M.; Salim A.I.; Fahim, I. S.; Said L.A.; Radwan, A.G. Review of activated carbon adsorbent material for textile dyes removal: Preparation, and modelling. Curr. Res. Green Sustain. Chem. 2022, 5, 100325. [CrossRef]
- Seoudi, R.; Al-Marhab, F.A. Synthesis, characterization and photocatalytic application of different sizes of gold nanoparticles on 4-Nitrophenol. WJNSE, 2016, 6, 120. http://dx.doi.org/10.4236/wjnse.2016.63012.
- Silva-Holguín, P.N.; Garibay-Alvarado, J.A.; Reyes-López, S.Y. Silver nanoparticles: multifunctional tool in environmental water remediation. Materials 2024, 17, 1939. [CrossRef]
- Palani,G.; Trilaksana, H.; Merlyn Sujatha, R.; Kannan, K.; Rajendran, S.; Korniejenko, K. ; Nykiel, M.; Uthayakumar, M. Silver Nanoparticles for waste water management. Molecules, 2023, 28, 3520. [CrossRef]
- Ferrari, E. Gold nanoparticle-based plasmonic biosensors. Biosensors 2023, 13, 411. [CrossRef]
- Kumari, H.; Suman, S.; Ranga, R.; Chahal, S. Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; Kumar A.; Parmar, R,. A Review on photocatalysis used for wastewater treatment: dye degradation. Water Air Soil. Pollut. 2023, 234, 349. [CrossRef]
- Huggias, S.; Serradell,M.A.; Azcárate, J.C.; Casella, M.L.; Peruzzo P.J.; Bolla, P.A. Catalytic performance in nitroarene reduction of nanocatalyst based on noble metal nanoparticles supported on polymer/s-layer protein hybrids. J. Phys. Chem. B, 2024, 128, 4809. [CrossRef]
- Mergola, L.; Carbone, L.; Stomeo, T.; Del Sole, R. Green synthesis of iridium nanoparticles from winery waste and their catalytic effectiveness in water decontamination. Materials, 2023, 16, 2060. [CrossRef]
- Lizundia, E.; Luzi, F.; Puglia, D. Organic waste valorisation towards circular and sustainable biocomposites. Green Chem. 2022, 24, 5429. [CrossRef]
- Baiocco, D.; Lavecchia, R.; Natali, S.; Zuorro, A. Production of Metal Nanoparticles by Agro-Industrial Wastes: A Green Opportunity for Nanotechnology. Chem. Eng. Trans. 2016, 47, 67. [CrossRef]
- Cui, M.; Huang,X.; Zhang, X.; Xie, Q.; Yang, D. Ultra-small iridium nanoparticles as active catalysts for the selective and efficient reduction of nitroarenes. New J.Chem. 2020, 44, 18274. [CrossRef]
- Bordiwala, R.V. Green synthesis and Applications of Metal Nanoparticles.- A Review Article. Results Chem. 2023, 5, 100832. [CrossRef]
- Song, W.C.; Kim, B.; Park, S.Y.; Park, G.; Oh, J.W. Biosynthesis of silver and gold nanoparticles using Sargassum horneri extract as catalyst for industrial dye degradation. Arab. J. Chem. 2022, 15, 104056. [CrossRef]
- Kulkarni, R.; Harip, S.; Kumar, A.R.; Deobagkar, D.; Zinjarde, S. Peptide stabilized gold and silver nanoparticles derived from the mangrove isolate Pseudoalteromonas lipolytica mediate dye decolorization. Colloids Surf. A: Physicochem. Eng. Asp. 2018, 555, 180. . [CrossRef]
- Rajput, G.; Pandya, N. Catalytic Degradation of Methylene Blue Using Gold Nanoparticles Capped by Polyoxyethylene Cholesteryl Ether. Adv. Sci. Eng. Med. 2020, 12, 1236. [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manage. 2018, 72, 99. [CrossRef]
- Sun, J.; Zhang, Z.; Liu, C.; Dai, X.; Zhou, W.; Jiang, K.; Zhang, T.; Yin, J.; Gao, J.; Yin H.; Li, H. Continuous in situ portable SERS analysis of pollutants in water and air by a highly sensitive gold nanoparticle-decorated PVDF substrate. Anal. Bioanal. Chem. 2021, 413, 5469. [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: a mini-review and new approach. RSC Advances 2023, 13, 16915. [CrossRef]
- Iqbal, A.; Ibrahim, N.H.; Rahman, N.R.A.; Saharudin, K.A.; Adam, F.; Sreekantan, S.; Yusop, R.M.; Jaafar, N.F.; Wilson, L. D. ZnO Surface Doping to Enhance the Photocatalytic Activity of Lithium Titanate/TiO2 for Methylene Blue Photodegradation under Visible Light Irradiation. Surfaces 2020,3, 301. [CrossRef]
- Basak, S.; Ali, S.; Das, D.; Sikdar, S.; Roy, M.N. Synthesis, Characterization and Visible Light Induced Photo-Degradation of Acid Orange II Dye in Aqueous Medium using a Novel Synthesized Al2MoZnO7 Nanocomposite. J. Adv. Chem. Sci. 2020, 6(2), 676. [CrossRef]
- Singh, R.K.; Behera, S.S.; Singh, K.R.; Mishra, S.; Panigrahi, B.; Sahoo, T.R.; Parhia, P.K.; Mandala, D. Biosynthesized gold nanoparticles as photocatalysts for selective degradation of cationic dye and their antimicrobial activity. J. Photoch. Photobio. A Chem. 2020, 400, 112704. [CrossRef]
-
Fiévet, S. Ammar-Merah, R. Brayner, F. Chau, M. Giraud, F. Mammeri, J.Peron, J.-Y. Piquemal, L. Sicard and G. Viau, The polyol process: a unique method for easy access to metal nanoparticles with tailored sizes, shapes and compositions. Chem. Soc. Rev. 2018, 47, 5187–5233. [CrossRef]
- Rosas-García, V.M.; Rodríguez-Nava, O.; Cuenca-Álvarez, R.; Garrido-Hernandez, A.; García-Hernández, M.; Morales-Ramírez, A.J. Photocatalytic Degradation of Methylene Blue (MB) and Methyl Orange (MO) by the Highly Oxidative Properties of SnO2 Sb2O3 Particles. Mater. Trans. 2022, 63(8), 1188. [CrossRef]
- Lyu, P.; Espinoza, R.; Nguyen, S.C. Photocatalysis of Metallic Nanoparticles: Interband vs Intraband Induced Mechanisms. J. Phys. Chem. C 2023, 127, 15685−15698. [CrossRef]
- Baumberg, J.J. Hot electron science in plasmonics and catalysis: what we argue about. Faraday Discuss. 2019, 214, 501–511. [CrossRef]
- Wang, X.Q.; Han, S.F.; Zhang, Q.W.; Zhang, N.; Zhao, D.D. Photocatalytic oxidation degradation mechanism study of methylene blue dye waste water with GR/iTO2. MATEC Web Conf. 2018, 238, 03006. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).