Preprint
Article
Safety and Efficacy of Incorporating Actellic 300CS into Soil Wall Plaster for Control of Malaria Vectors in Rural Northeastern Uganda
Altmetrics
Downloads
106
Views
47
Comments
0
supplementary.pdf (158.21KB )
This version is not peer-reviewed
Submitted:
30 August 2024
Posted:
30 August 2024
You are already at the latest version
Alerts
Abstract
Indoor residual spraying (IRS) and use of insecticide treated bed nets for malaria vector control have contributed substantially to reduction in malaria disease burden. However, these control tools have important shortcomings including being donor-dependent, expensive, and often fail because of insufficient uptake. We assessed the safety and efficacy of a user-friendly, locally tailored malaria vector control approach dubbed ‟House Decoration for Malaria Control” (HD4MC) based on incorporation of a WHO-approved insecticide, pirimiphos-methyl CS (Actellic 300CS), into a customary hut decoration practice in rural Uganda where millions of the most vulnerable and malaria-prone populations live in mud-walled huts. Three hundred and sixty households were randomly assigned to either HD4MC (120 households), IRS (120 households) or control group without any wall treatment (120 households). Entomological indices were assessed using pyrethrum spray catch, CDC Light traps and human landing catches. Organophosphate (OP) toxicity on acetyl cholinesterase activity among applicators of HD4MC was evaluated using Test-mate (Model 400) erythrocyte acetylcholinesterase (AChE) test V.2 whereas toxicity in household occupants was monitored clinically. OP level in house dust was analyzed using reversed-phase high-performance liquid chromatography (RP-HPLC). Entomological indices were compared between the 3 study arms at 1.5, 3- and 6-months post intervention. HD4MC- and IRS-treated huts had significantly reduced malaria vector density and feeding rate compared to control huts. There was no significant reduction in acetylcholinesterase activity at 1.5- and 24-hours post exposure. Organophosphate exposure did not result in any serious adverse event among the household occupants. In conclusion, HD4MC was safe and had comparable efficacy to canonical IRS.
Keywords:
Subject: Public Health and Healthcare - Public, Environmental and Occupational Health
Introduction
Malaria remains an important global development and public health challenge in Africa. An estimated 249 million malaria cases and 608,000 deaths were reported in 2022 with > 95% of these cases and deaths occurring in sub-Saharan Africa [1]. High-transmission countries like Uganda, Nigeria, Mozambique and the Democratic Republic of Congo bore the heaviest burden of malaria and together accounted for almost half of the reported global cases [1]. Control and prevention strategies targeting malaria parasites (chemotherapy) and mosquito vectors (insecticides) have been deployed in affected communities. Chemotherapeutic approaches include early treatment of malaria cases with artemisinin-based combination therapies (ACTs) and intermittent preventive therapy for prevention (IPTp) in pregnant women. The World Health Organization (WHO) recommends several chemoprophylactic strategies for national malaria control programs [2]. These strategies include seasonal malaria chemoprevention through administration of monthly doses of antimalarials in children ≥3 months old during peak transmission seasons and perennial malaria chemoprevention [2]; and mass drug administration [3]. There exists a variety of vector control methods aimed at reducing the frequency of contact between the vector and human host and reducing the vector population and capacity by killing or reducing the longevity of adult mosquitoes and larvae. These methods range from insecticidal to non-insecticidal techniques, including larval source management, entomopathogenic microorganisms, insect growth regulators, and endosymbiotic bacteria [4,5]. Mainstream adoption of some of these tools and technologies at scale in endemic populations remains problematic. Current malaria vector control is by indoor residual spraying (IRS) of WHO-approved insecticides and sleeping under insecticide-treated bed nets (ITNs). The reported reduction in malaria burden over the last decades is attributed to scale-up of both IRS and ITN in combination with chemotherapy. However, the success of chemotherapeutic and insecticide-based control and prevention approaches is undermined by the threat of drug and insecticide resistance [6]. Currently, access to the approved malaria vaccines in clinical practice remains limited [7,8,9].
The renewed call for malaria eradication and elimination emphasizes the optimization of existing interventions including vector control. However, both IRS and ITN control strategies have important shortcomings which leave vulnerable children unnecessarily exposed to malaria. Available evidence shows that the distribution, ownership, and proper use of ITNs is inefficient which undermines its effectiveness [10]. IRS is centralized, expensive (requires special equipment, trained personnel, complex logistics), donor-dependent, does not reach remote villages; and fails because of community weariness/insufficient uptake [11]. We developed a user-friendly, locally tailored malaria vector control approach ‟House Decoration for Malaria Control” (HD4MC) based on a customary hut decoration practice in rural Uganda where millions of the most vulnerable and malaria-prone populations live in mud-walled huts. Mud walls are traditionally decorated by smearing with freely available colored local materials like black or red soil mixed with cow dung or wood ash. We hypothesized that incorporating a WHO-approved insecticide, pirimiphos-methyl CS (Actellic 300CS) into soil plaster for smearing hut walls turns the customary hut decoration into a mosquito control tool because the insecticidal mud wall plaster will repel free-flying mosquitoes entering treated huts and kill mosquitoes landing on the smeared walls. Actellic 300CS is a wide spectrum organophosphate insecticide whose effectiveness against malaria mosquito vectors have been validated using IRS [12,13]. Compared against the limitations and shortcomings of IRS and ITNs, HD4MC is simple (needs little training), affordable and more sustainable (requires only water and soil), might have a higher coverage and community acceptance since it is based on a customary hut decoration practice, and could be implemented with community participation. HD4MC is also amenable to re-plastering thereby circumventing the operational difficulties associated with repeat IRS applications. Here, we report preliminary safety and efficacy data on the performance of HD4MC against the established IRS gold standard in a randomized controlled trial in a rural peasant population in northeastern Uganda.
Materials and Methods
Study Region and Population
Katakwi district is in the northeastern part of Uganda about 343km from Kampala City. The district lies at 01°54'54.0"N, 33°57'18.0"E (Latitude: 1.9150; Longitude: 33.9550) and borders Amuria, Napak, and Kumi districts in the east, north and northwest, respectively. Katakwi District has a total land area of 2,428.8 km2 (937.8 sq mi) and is made up of eight subcounties. This study took place in seven villages Abwokodia, Acurun, Ongema, Opoyongo, Oleroi, Akworo and Otujai in Abwokodia parish in Usuk sub-county. The typical rainy season in Katakwi is from March to November with marked peaks in April-May and August-October. Malaria transmission is high during the rainy season, peaking in July (72.0 cases/1000 people/month), and lower during the dry season (16.2 cases/1000 people in February) [14]. The district is predominantly inhabited by a Nilo-Hamitic indigenous population whose main occupation is peasant agriculture and small-scale animal farming. The vegetation cover is predominantly savannah grassland interspersed with seasonal and established swamps. The savanna is broken by homesteads of mud-walled, grass-thatched huts.
Baseline Entomologic Surveys
Baseline entomological survey was conducted in 4 villages (Ongema, Abwokodia, Otujai and Acurun) in August 2017 using Pyrethrum Spray Catch (PSC), Centers for Disease Prevention and Control Light Trap (CDC LT) and Human Landing Catches (HLC) following standard methods [15]. PSC: Briefly, twelve (12) houses inhabited the previous night were randomly selected in each of the 4 villages for collection of adult mosquitoes using standard PSC method [15] for seven consecutive days. Prior to PSC, all animals on the veranda, chicken and small furniture were removed from the targeted houses and white sheets were laid to completely cover the floor and all flat surfaces (under tables as well). With all windows and doors closed, knock down aerosol (Kill it) was sprayed inside and outside the house in clockwise and anti-clockwise directions by skilled entomologists. After 10 minutes the white ground sheets were removed from the houses and adult mosquitoes picked using fine forceps. CDC LT: Three houses in each of the 4 villages were randomly selected and CDC light trap (Model 512; John W. Hock Company, Gainesville, FL) was set 1 m above the floor at the occupant’s bed net from 06:00 pm and left overnight until 06:00 am. CDC LT activities were conducted for three consecutive days. HLC: HLC were carried out during the evening/night for 3 days in the villages of Otujai, Abwokodia and Acurun each village. Two teams of collectors were placed outdoors and indoors under supervision of competent entomologists. Indoor collections were carried out from 6:00pm to 6:00am, while the outdoor collections were from 6:00pm to 10:00pm, with the assumption that people turn in to sleep in their rooms by 10:00pm and thus are not at risk to outdoor biting after 10:00pm. Trained collectors/volunteers exposed the legs to the knee to serve as bait and sat as quietly as possible. Once the collector felt the mosquito landing, the flashlight was turned on to see the mosquito, which was then collected with the aspirator and placed inside a net-covered paper cup. A different paper cup was used for each hour of collection and labeled accordingly. To avoid the limitations of landing collections which includes variation in the attractiveness of human hosts/baits to mosquitoes, collectors switched sites every hour and rotated in batches between indoors and outdoors. The collectors were put on malaria prophylaxis to prevent them from being infected with malaria.
For all procedures, the mosquitoes collected in each house were stored in separate petri-dishes which were appropriately labeled (collection date and hour, village, household number). Specimens were transported to Med Biotech Laboratories field laboratory and Anopheles mosquitoes morphologically identified according to Gillies and DeMeillon keys [16].
Study Design
This was a longitudinal cohort study assessing the safety and efficacy of HD4MC in a randomized controlled trial. The study was conducted between May 2017-December 2018. Villages were randomly assigned to three intervention arms: HD4MC (Acurun and Opoyongo); IRS gold standard (Oleroi and Otujai) and non-treated control (Abwokodia, Akworo and Ongema).
Sample size calculations: We assumed that a 50 % reduction in mosquito density wasachievable based on Presidents Malaria Initiative data from Apac District-an area of comparible malaria endemicity. With a sample size of 140 huts in the HD4MC and 140 huts in the control arm (allowing for 15 % drop out), the study had a 90 % power to detect a 50 % reduction in mosquito densities by the intervention at α = 0.05 (two-tailed). We used a sample size calculator (http://www.stat.ubc.ca/~rollin/stats/ssize/n2.html) based on an assumption of a mean catch of 25 female An. gambiae s.l./trap/night in the control group and a catch of 12.5 or less in either intervention with a standard deviation (SD) of 30. The SD was calculated from the range (R) of catches/hut in Apac District using the formula SD = R/4.
Materials
Actellic 300 CS (pirimiphos-methyl) was purchased from Syngenta Crop Protection AG., Switzerland. Long sleeved coveralls, face masks, sturdy gloves and gumboots and knapsack sprayers were purchased locally. Soil and water were obtained from the villages of intervention.
Study Procedures
Household Selection and Randomization
All households within each of the seven villages were enumerated and mapped using handheld global positioning system units (Garmin e-Trex 10 GPS unit, Garmin International Inc., Olathe, KS). A household was defined as any single permanent or semi-permanent dwelling acting as the primary residence for a person or group of people that generally cook and eat together. Using a computerized number generator, random samples of households from each village were approached consecutively, and 120 households were enrolled per intervention arm (HD4MC, IRS and no-treatment). The households for the entomologic surveys and cohort studies were selected based on the following criteria i) Houses where people sleep; ii) at least one house resident 0.5–10 years of age; and ii) at least one adult resident available for providing informed consent. Exclusion criteria included i) failure or refusal to consent; ii) households with pregnant occupiers; iii) households having residents with preexisting allergies. Participants in the IRS and HD4MC arms and smearers were trained to recognize signs of organophosphate toxicity and advised to contact the study physician as soon as possible in case of suspected toxicity.
Hut Wall Treatment by HD4MC
The HD4MC intervention was carried out in the villages of Acurun and Opoyongo. The HD4MC innovation comprised of the following components: i) soil from home-owner’s garden; (ii) an insecticide (Actellic 300CS); and (iii) water (Supplementary Figure S1, left & right upper panels respectively). The best soil- which dries without leaving cracks or fissures-was chosen after several dry runs. The components were mixed to achieve a plaster mix of 2 grams (g) of insecticide per square meter of wall surface. Actellic 300 CS is supplied at a concentration of 300 g/liter in 833 ml per bottle. The WHO recommends use of 1 gram of Actellic 300 CS per square meter of sprayable surface at a nozzle/control flow valve speed of 550 ml per minute and 1.5 bar pressure with the nozzle 45 cm from the surface being sprayed for IRS [17].
For hand smearing application, we used a final concentration of 2 g of insecticide per square meter of wall surface. The surface area of the wall was calculated using the formula 2 x π x R x H where π is 3.143, R is the radius of the circular hut in meters, and H is the height in meters (invariably all village huts were circular, Supplementary Figure S1, bottom panel). A typical village hut of diameter 9 meters with a wall height of 1.5 meters has a surface area of 2 x 3.143 x 9/2 x 1.5 = 42.4 square meters. To achieve 2 g of insecticide/square meter of the smeared surface, 85 g of insecticide is required for the hut. About 283ml (an equivalent of 85g) of the insecticide was measured out using a measuring cylinder into a basin. The measuring cylinder was rinsed three times with 500 ml water (1,500 ml total) and the rinses added to the insecticide mix in the basin (Figure 2, top left panel). After mixing to ensure that the milky solution was well dispersed, more water was added to bring the mix to 10,000 ml (Supplementary Figure S2, top right panel). Four 2-liter plastic buckets (8-liter capacity) of loam soil from the garden or grounds near the home was measured into a wide plastic basin (Supplementary Figure S2, bottom left panel) and mixed with the insecticide (Figure 2, bottom right panel). The insecticide mix was stirred gently with a gloved hand into the soil until a plaster mix of the right consistency was achieved (Supplementary Figure S3, left panel). The plaster mix was then smeared onto the inside walls of the huts with gloved hands in circular motions by trained personnel (referred to as smearers). The smearers donned personal protective equipment that included coveralls, face masks, sturdy gloves and gumboots (Supplementary Figure S3, right panel). All female smearers received a β-HCG urine test to rule out pregnancy due to concerns about possible health/exposure risks to the unborn child. After 2-3 hours the smeared walls dried, and the occupants returned their properties into the hut. All used materials and waste generated were treated as described in the environmental compliance section.
Hut Wall Treatment by IRS
Indoor residual spraying (IRS) was conducted by trained and experienced entomologists comprising the district vector control officer and personnel from the Vector Control Division, Ministry of Health-Uganda. The necessary planning, procurement and training was completed before the actual house spraying. The households were sprayed using 1 g/m2 of Actellic 300 CS using WHO standard procedures with minor adaptations [17]. Spraying started in the innermost part of the house and worked outwards. IRS was applied in the villages of Oleroi and Otujai.
Control Huts with No Wall Treatment
The villages of Abwokodia, Akworo and Ongema were negative controls and 120 huts in these villages did not receive any intervention. Hut occupants were supplied with insecticide treated bednets.
Assessment of HD4MC and IRS Efficacy
The impact of the interventions on mosquito density and feeding rate were evaluated using PSC to collect and count wild Anopheles mosquitoes caught from control huts, HD4MC, and IRS-treated huts. PSC was undertaken as described in the baseline entomologic survey section. Twelve huts were randomly chosen per treatment arm. The surveys were carried out in October 2017 (1.5 months post-intervention), December 2017 (3.0 months post-intervention), and March 2018 (6.0 months post-intervention).
Assessment of Residual Insecticidal Activity on Treated Walls
The post-intervention residual insecticidal activity of HD4MC and IRS-treated hut walls were carried out using WHO cone bioassays [18,19] in October (1.5 months post-intervention) and December 2017 (3 months post-intervention) and March 2018 (6 months post-intervention). We employed a 2–5-days old susceptible adult female An. gambiae ss. Kisumu strain mosquitoes that were reared at the Vector Control Division-MoH insectary. Three huts were randomly selected from each of the treatment arms (Control, HD4MC and IRS). Three cones were mounted at different heights (upper level (1.5m), middle level (1.0m) and lower level (0.5m) on the wall surfaces for each hut. Ten (10) female An. gambiae ss Kisumu strain were introduced in each of the cones and exposed for 60 minutes. The knockdown time for female An. gambiae ss Kisumu strain was recorded at 0, 10, 20, 30,40, 50 and 60 minutes. After 60 minutes, the exposed mosquitoes were transferred into paper cups where they were fed with 10 % glucose solution and monitored up to 24 hours. The knock down rate of mosquitoes was determined by recording the number of mosquitoes lying down every 10 minutes for the first one hour and final mortality rate was recorded at the end of the twenty-four-hour holding period.
Environmental Safety Compliance
Reusable wears were washed and air dried. All solid waste were swept off the floor, collected, and disposed into a pit latrine or a hole dug in the garden in the absence of a latrine. Liquid waste was disposed of in a communal soak pit dug in the village according to strict World Health Organization [20,21] specifications. Used empty bottles were collected, transported, and incinerated by a local service provider licensed by the Uganda National Environment Management Authority.
Safety Assessment
The safety of HD4MC and IRS were assessed using different but complementary approaches including biomonitoring of dust samples from treated huts for organophosphate content, measuring pseudocholinesterase activity levels in blood collected from intervention applicators (smearers and sprayers), and clinical monitoring of subjects living in the treated huts as described below.
Biomonitoring of organophosphate levels in dust samples from treated huts: Actellic 300CS, the insecticide employed in HD4MC and IRS, is an organophosphate (OP) known by the chemical name, pirimiphos-methyl (PM). Household dust samples were collected from the sweepings of 9 village huts, HD4MC huts (N =3), IRS huts (N= 3) and control huts (N= 3). These samples were shipped to the laboratory of Dr. Mark Paine (Liverpool School of Tropical Medicine) for OP analysis. Briefly, dust samples (approximately 1 g each) were weighed and transferred to 10 mL glass tubes. One millilitre of acetonitrile, spiked with 100 µg of both primiphos methyl (PM) and dicyclohexyl phthalate (DCP) as internal standards, was added to each tube. The mixture was vigorously vortexed for 2-3 minutes, and then 0.5 mL aliquots were centrifuged at 15,000 rpm for 15 minutes to remove debris. Sample extracts and analytical standards were analyzed using reversed-phase high-performance liquid chromatography (RP-HPLC) following the method described in Fuseini et al. (2020) [22] with minor modification. The HPLC system was equipped with a Hichrom ACE 5C18 column (250 x 4.6 mm id), and a mobile phase consisting of 90% acetonitrile in water with 0.1% phosphoric acid. UV detection was employed at 232 nm. Injection volume was 10 µL per sample, and run time was 20 minutes. Both PM and DCP concentrations in samples were determined by comparing their respective peak areas to those of the corresponding analytical standards (PESTANAL®, analytical standard, Sigma-Aldrich, UK). The values were normalized against the corresponding internal standard (DCP) readings. The calculated PM concentrations were further adjusted based on the recovery of the spiked PM levels (100 µg/mL). The final PM content was expressed in parts per million (ppm, weight/weight), calculated based on the corrected amount detected per 1 gram of dust sample.
Measurement of acetyl cholinesterase activity in blood: The effect of organophosphate on acetyl cholinesterase activity among applicators of HD4MC was assessed using Test-mate (Model 400) erythrocyte acetylcholinesterase (AChE) test V.2 (EQM Research, Inc., Cincinnati, OH) as per the manufacturers’ guidelines. The test system is based on the Ellman colorimetric method in which acetylthiocholine is hydrolyzed by AChE, producing carboxylic acid and thiocholine, which reacts with the Ellman reagent (dithionitrobenzoic acid) and turns yellow. The rate of color formation is proportional to the amount of AChE. Measurements were performed in duplicates on samples collected at 3 time points (pre-exposure, and at 1.5- and 24-hours post exposure). Classically, a fall in AChE activity by 40 % following exposure is considered as toxic/severe adverse event and the one should stop handling the insecticide [23]. Administration of atropine or pralidoxime is recommended for a reduction by > 80 % below baseline [24]
Clinical monitoring: The occupants of intervention households were closely followed up for severe adverse events or signs of organophosphate poisoning. Severe organophosphate toxicity presents as unresponsiveness, pinpoint pupils, muscle fasciculations, and diaphoresis. Additional symptoms can include emesis, diarrhea, excessive salivation, lacrimation, and urinary incontinence [25]. Home visits were conducted daily during the first week, weekly during the first month, and subsequently monthly.
Statistical Analysis
All statistical analysis were performed using GraphPad Prism (version 10.1.2 for Windows, GraphPad Software, Boston, Massachusetts USA, www.graphpad.com). Categorical variables including sex, age and use of ITNs were summarized and tabulated as proportions and/or percentages and the median [interquartile range (IQR)] for non-normally distributed continuous variables. The number and/or proportion of mosquitoes collected in the different treatment arms were used to assess the efficacy of HD4MC and IRS. For each method, the number of species, Anopheles density (number of Anopheles per person and per night) were tabulated as proportions/percentages and compared using chi square analysis. The difference in mean AChE activity in ‘smearers’ at baseline and 1.5 and 24 hours post exposure was compared using one-way ANOVA. Baseline/pre-exposure blood samples from ‘smearers’ serve as a reference for post-intervention AChE levels in the same subject due to lack of normal reference ranges and high variability in AChE levels. The difference in mean AChE activity levels by sex was analysed using paired t test, while difference in AChE activity levels in ‘female smearers’ at baseline and at 24 hours post exposure was compared using a one sample t test.
Results
Participants Demographics
A total of 360 (120 per treatement arm) randomized households were enrolled in the study. The demographic characteristics of the household inhabitants are as shown in Table 1. There was no difference in participant composition by biological sex or age across the three treatment arms. About 51% of the household inhabitants were female, while 29% were children under 5 years old (Table 1). Insecticide treated bednets were the only vector control tool used in the study households. The proportion of participants using insecticide treated bednets was higher in the ‘control’ (47%) compared to HD4MC (24.7%) or IRS (28.3%) treated groups (Table 1).
Baseline Malaria Vector Composition, Density and Distribution
We conducted a baseline survey to characterize the malaria vector densities, species composition, biting and resting behavior, and infectivity in the study area prior to the intervention. A total of One thousand and seventy seven (1,077) malaria vectors/mosquitoes were collected using 3 different entomological techniques. The techniques employed served as proxies for investigating different vectoral behaviours (PSC = feeding and resting behaviour, CDC LT = indoor feeding behavior, HLC = human seeking and biting behaviour). About 20% (219/1077), 23% (247/1077) and 57% (611/1077) of the mosquitoes were collected using PSC, CDC LT and HLC respectively (Table 2). Out of 1,077 total vectors collected, 76% (823/1077) belonged to the Anopheles gambiae sensu lato (An. gambiae s.l) while 24% (254/1077) were Anopheles funestus sensu lato (An. funestus s.l). Malaria vectors exhibited a spatial distribution in the study area as no An. funestus s.l mosquitoes were caught using PSC and CDC LT methods in the villages of Ongema and Otujai and only 4 were caught using HLC method in the village of Otujai (HLC was not done in Ongema village due to logistical/operational issues). Of the 254 An. funestus s.l, 88% (224/254) were caught in Acurun. Similarly, the majority (42.2%) of the An.gambiae s.l. were from Acurun. Vector density was highest and more than double in the village of Acurun (52.4%) compared to the other 3 villages (Table 2). Feeding rate was evaluated for all the vectors collected by PSC in the 4 villages. About 96.8% (212/219) of the mosquitoes collected by PSC from the 4 villages had taken a blood meal (Supplementary Table 1), implying high rate of vector-human contact. Similarly, 95% (579/611) of mosquitoes collected by HLC were caught indoors (data not shown). This data indicates a high risk of malaria transmission in these villages and possible inadequacy of available treated bednets as a malaria vector control tool. The feeding pattern of species-specific female anopheles vectors were assessed using indoor and outdoor HLC starting at 6: 00pm to 6: 00am. The peak indoor feeding time for both An. gambiae and An. funestus was between 1:00 am to 3: 00am (Supplementary Table 2). No An. funestus was caught outdoors in the villages of Otujai and Ongema (Table 2).
Impact of HD4MC Intervention on Malaria Vector Density and Feeding Rate
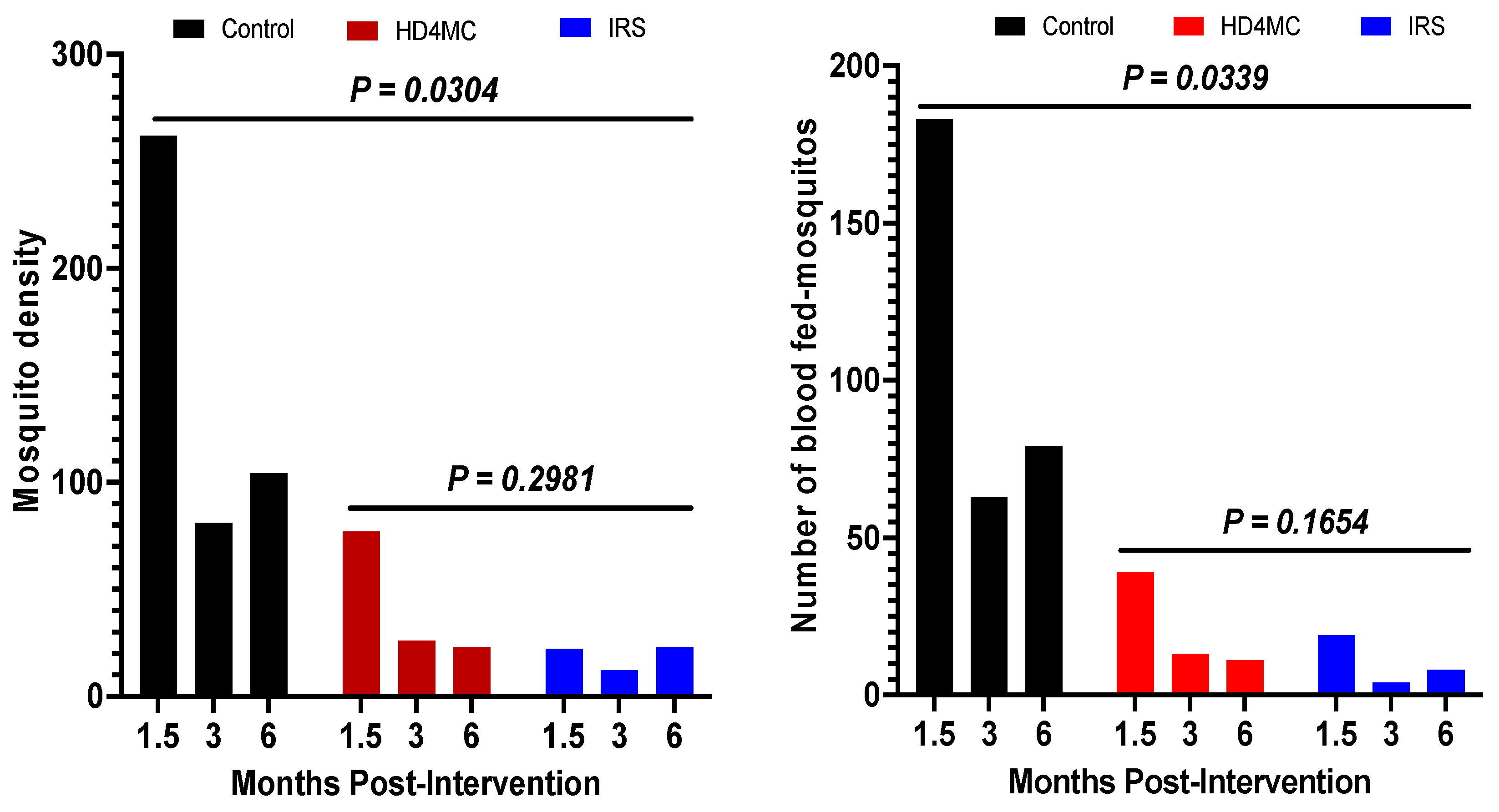
Impact on vector density: Malaria vector density estimated by pyrethrum spray catches (PSC) is used as an indicator for the efficacy of IRS and LLINs [26]. The efficacy of HD4MC intervention was assessed against IRS as the gold standard and non-treated huts as negative control at 1.5, 3, and 6 months post-intervention. This was done by comparing the proportions of female anopheles mosquitoes collected by PSC in selected households in the different treatment arms (Fig 1A). During the rainy season in October, 1.5 months after implementation, 72.6% (262/361), 21.3% (77/361), and 6.1% (22/361) female Anopheles mosquitoes were collected from huts in the control, HD4MC and IRS arms, respectively (Fig 1A). These data demonstrate that female Anopheles malaria vectors were reduced by 71 % and 92 % by HD4MC and IRS, respectively. At the end of the rainy season in late December, 3 months after implementation, 68.1% (81/119), 21.8% (26/119), and 6.1% (12/119) of female Anopheles mosquitoes were collected from huts in the control, HD4MC and IRS arms, respectively (Fig 1A). The intense heat and dry conditions that prevailed at the time appeared to have caused a significant drop in the number of caught mosquitoes even in the control arm. These data demonstrate that female Anopheles malaria vectors were reduced by 68 % and 85 % by HD4MC and IRS, respectively. The proportion of mosquitoes collected from the Control, HD4MC, and IRS huts were 69.4% (104/150), 15.3% (23/150), 15.3% (23/150) at six months, respectively. This translates to a 85% reduction in vector density in either the HD4MC or IRS treated households. Overall, there was a significant reduction in vector density between the intervention and control huts (P = 0.0304). There was no significant difference in vector density between HD4MC and IRS at all three time points post-intervention (P = 0.2981).
Impact on vector feeding: To better understand the vectoral transmission potential and the impact of HDMC on human exposure to malaria vector bites, the proportion of blood-fed vectors collected by PSC in the 3 experimental arms were compared. At 1.5 months post intervention, the proportion of fed female Anopheles mosquitoes was 76% (183/241), 16% (39/241) and 8% (19/241) in the control, HD4MC and IRS arms, respectively (Fig 1B). These translates into a 78.7% reduction in blood feeding by HD4MC, and 89.6 % by IRS compared to control. The number of fed female Anopheles mosquitoes was 63, 13 and 4 in the control, HD4MC and IRS arms at 3 months post intervention, translating to 79 % and 94 %, reduction in blood feeding by HD4MC and IRS respectively. HD4MC and IRS reduced female Anopheles mosquito blood feeding by 60.5 % and 53.5 % at six months, respectively (Fig 1B). Overall, there was a significant reduction in vector feeding rate between the intervention huts and untreated huts (P = 0.0339) but no difference between HD4MC and IRS treated huts (P = 0.1654).
Figure 1.
Post-intervention vector density. Figure 1B: Number of blood-fed mosquitoes.
Figure 1.
Post-intervention vector density. Figure 1B: Number of blood-fed mosquitoes.
Durability of insecticidal activity on treated walls: Three entomological assessments were carried out to determine the residual insecticidal activity on HD4MCand IRS-treated walls in October (1.5 months post-intervention), December 2017 (3 months post-intervention) and March 2018 (6 months post-intervention) using WHO cone bioassays. The major objective was to determine the residual mosquito killing ability of treated walls at the three time points after HD4MC and IRS. The target mosquitoes employed were 2-5-day old susceptible female adult An. gambiae ss Kisumu strain reared in an insectary at the Vector Control Division, Ministry of Health-Uganda. At 1.5 months post-intervention, 100 % of mosquitoes exposed to HD4MC and IRS treated walls were knocked down within 60 min of exposure and 100 % died within 24 hours after exposure. The results for all three study time points are shown in Table 3. The 24-hour mortality of mosquitoes exposed to HD4MC, and IRS treated walls after 6 months was 90 and 97.5 %, respectively.
Safety of HD4MC Intervention
Acetylcholinesterase Activity Levels in HD4MC Applicators
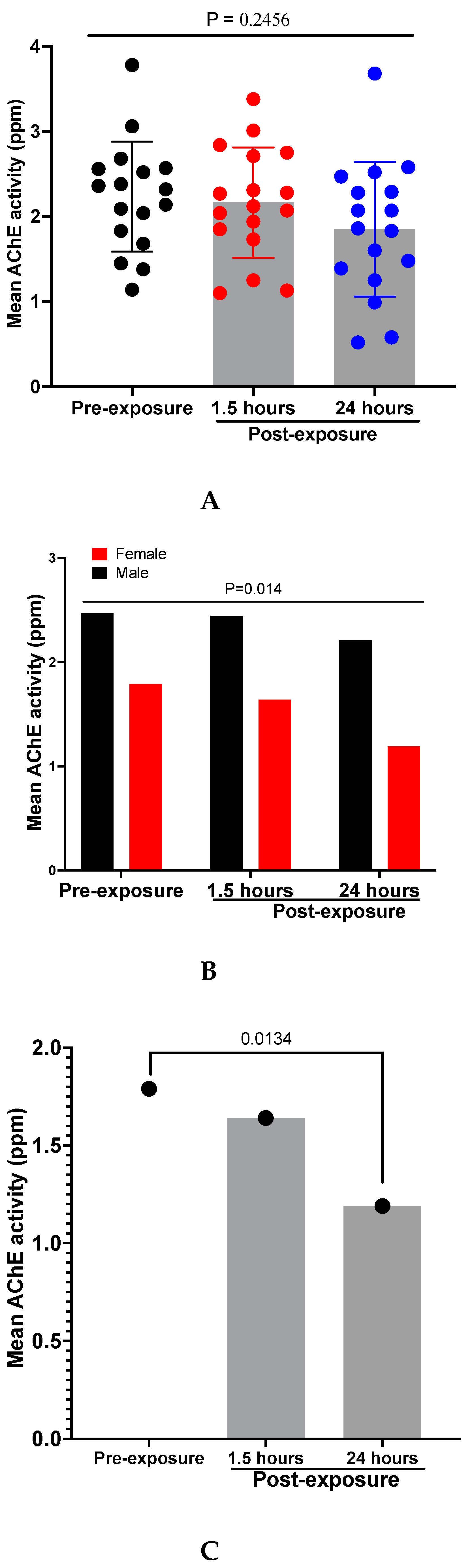
OP insecticide toxicity results from inhibition of acetylcholinesterase (AChE) activity, causing accumulation of acetylcholine and overstimulation at cholinergic synapses throughout the body [27]. This results in an ‘acute cholinergic crisis’ with bradycardia, hypotension, coma, and acute respiratory failure which requires immediate medical intervention [27]. The effect of organophosphate on acetyl cholinesterase activity was assessed in smearers before and after application of HD4MC using a one-way ANOVA. Although there was a visual decline in the mean AChE activity level at the different timepoints following exposure (pre-exposure, and at 1.5 and 24 hours post-exposure), this was not statistically significant (P = 0.2456 ) (Fig 2A). However, we observed a striking and significant reduction in AChE activity in females compared to males even under pre-exposure conditions (P = 0.014) (Fig 2B). In comparison to baseline, AChE activity level significantly redued at 24 hours post-exposure among female smearers (P = 0.0134) (Fig 2C).
Figure 2.
(A) Mean AChE activity levels in HD4MC applicators; Mean AChE activity levels in HD4MC applicators by biological Sex (B); Mean AChE activity levels in Females applicators only(C).
Figure 2.
(A) Mean AChE activity levels in HD4MC applicators; Mean AChE activity levels in HD4MC applicators by biological Sex (B); Mean AChE activity levels in Females applicators only(C).
Organophosphate Levels in the House Dust as a Proxy of House Occupant Exposure
The level of OP in dust samples collected from households assigned to the 3 treatment arms are as shown in Figure 3. No organophosphate was detected in dust samples from non-treated households. There was no significant difference in OP levels in HD4MC and IRS treated households. The median level of OP in house dust was 27.1 (IQR) and 24.2 (IQR) parts per million (ppm) in the HD4MC and IRS treated households respectively. There is no guideline available to define the maximum residue limit (MRL) for OP in house dust. The Australian MRL in Bran is 20 ppm [28]. If we take this value as guideline and comparator, the PM content of the house dust samples remains within the safe range for both HD4MC and IRS. A maximum residue level (MRL) is the highest level of a pesticide residue that is legally tolerated in or on food or feed when pesticides are applied correctly.
Clinical Adverse Events in House Occupants in Treated Households
Household occupants were closely followed up for adverse events or signs of OP poisoning. An adverse event was defined as any undesirable experience following the application of HD4MC or IRS. The visits were conducted daily during the first week, weekly during the first month, and subsequently monthly. Serious adverse events (OP toxicity) were defined as unresponsiveness, pinpoint pupils, muscle fasciculations, and diaphoresis. Adverse event symptoms can include emesis, diarrhea/abdominal pain, excessive salivation, lacrimation, headache, body itching and rash and urinary incontinence [25]. Home visits were conducted daily during the first week, weekly during the first month, and subsequently monthly. Table 4 shows the proportion of adverse events diagnosed during follow up. There was no serious adverse event associated with either HD4MC or IRS treatment. However, other adverse events registered headache (14.7%), itching (13.8%) and body rash (10.1%) which resolved during follow up. However, a major complaint by 36.7 % of house occupants was the strong smell of PM in both HD4MC and IRS arms. There was no significant difference in the number of adverse events registered in IRS versus HD4MC. Results represent complaints recorded within the first week. No IRS or HD4MC associated complaints were registered after a month post intervention.
Discussion
Millions of poor rural villagers in malaria-endemic regions live in mud-walled grass-thatched or iron-roofed huts. In this pilot study we investigated the effect of incorporating a WHO-approved insecticide into soil plaster customarily used to decorate hut mud walls to give them a smoother and pleasant or colorful appearance. We have termed this innovative approach Hut Decoration for Malaria Control (HD4MC). HD4MC had a knock down and killing activity on adult female Anopheles mosquitoes raised in an insectary and reduced the population density and feeding capacity of wild type An. gambiae and An. funestus in treated huts in head-to-head comparisons with the gold standard IRS used in malaria control. Although IRS had higher efficacy, at some time points the difference was not significant. The insecticidal residual activity of HD4MC remained high for up to 6 months post-intervention at which time point it was comparable to that of IRS. HD4MC was generally safe for both occupants of treated huts and applicators (smearers) based on the outcomes of clinical follow up and biochemical and analytical chemistry assays for AChE in blood of smearers and insecticide in house dust, respectively. The frequency of the major complaint of smell and headache and other systemic side effects was comparable between HD4MC and IRS. Pre- and post-exposure AChE levels were comparable even after 45 days post-intervention in smearers indicating lack of OP toxicity. Lower pre-exposure AChE levels in blood of females must be explored in a follow up study and if confirmed then female smearers will need closer monitoring for risk of OP toxicity. Pirimiphos-methyl levels in floor dust from huts treated with HD4MC and IRS were within the acceptable maximum residue limit (MRL) for bran in Australia. Overall, these data suggest that HD4MC could be an effective and safe mosquito vector control tool whose effect lasts at least 6 months without the need to reapply the soil mud plaster.
There are other studies which have reported the anti-mosquito effect of paint and wall linings containing organophosphate (OP) insecticide. First, combining OP insecticides and an insect growth hormone in wall paint and pyrethroid-treated Long Lasting Insecticide Treated Nets (LLINs) resulted in a one year killing efficacy against An. coluzzii that were highly resistant to pyrethroids but susceptible to OPs in Burkina Faso [29]. Second, the combination of insecticide paint on doors and windows with LLINs led to high but short-lasting mosquito mortality rates in Burkina Faso [30]. Third, insecticidal durable wall lining, and net wall hangings treated with pirimiphos-methyl in combination with LLINs provided significant protection against an Anopheles gambiae ss population resistant to pyrethroids but susceptible to OPs [31]. These studies also provided evidence for the selection of insecticide resistance genes by single rather than multiple insecticide interventions [31]. Fourth, a study in Cote D'Ivoire reported that OP wall lining plus LLINs, by contrast, were not effective against multiple insecticide-resistant Anopheles [32]. The reason for the disparate results in two countries in West Africa is probably due to multiple resistance against both OP and pyrethroid insecticides. Finally, several studies reported that insecticide wall painting led to long term efficacy for controlling sand flies, visceral leishmaniasis vectors, in Bangladesh, India, and Nepal, thereby confirming the versatility and usefulness of low-technology insecticidal wall lining beyond malaria and mosquito control [33,34,35].
The major strength of this pilot study is that we confirmed the safety of HD4MC in house occupants and applicators including lack of evidence of OP toxicity nor exposure to harmful levels of OP in dust collected from floors of treated huts. However, the study has several shortcomings. First, we did not investigate the correlation between reduced female Anopheles population density and blood feeding and prevalence or incidence of malaria in house occupants. Second, we did not monitor residual insecticidal activity beyond 6 months, so it is unclear whether the insecticidal activity remained high or decayed with time after 6 months following wall smearing. This information is required to inform malaria vector control policy based on HD4MC whether smearing should be repeated every 6 months. Third, we did not genotypically and phenotypically assess insecticide resistance in the study region before and after HD4MC. These shortcomings will be addressed in a future study.
Our hypothesis was that incorporation of insecticide into soil wall plaster as part of mud wall decoration in rural Ugandan villages turns this customary practice into a safe and effective malaria mosquito vector control tool. Our pilot study demonstrated reduced female Anopheles mosquito populations and feeding in HD4MC treated huts and provided evidence of the safety and tolerability of HD4MC. This study is significant because millions of people in impoverished communities live and sleep in mud-walled huts whose walls are customarily decorated with colored soil. The study provides preliminary evidence that smearing insecticide-treated soil plaster onto mud hut walls could be an alternative mosquito vector control tool besides IRS at the grass root level where soil is a natural resource which is freely available in gardens. Community participation, after appropriate training in safe handling of insecticides and smearing walls, could result in widespread acceptance and adoption of HD4MC. However, there remains several unanswered questions about the effect of soil type and microbial composition on the texture and appearance of smeared walls (smooth versus cracked appearance on drying), stability of the insecticide and duration of residual insecticidal activity, and ultimately the duration of efficacy against mosquitoes and protection against malaria disease. Future research must address these outstanding questions and provide evidence through randomized controlled trials whether HD4MC protects villagers living in smeared huts against malaria and whether under certain conditions it might contribute to the malaria elimination agenda.
In conclusion, the HD4MC low-technology is uniquely adapted for mud walled brick or wattle-and-mud walls and earth floors which cannot be painted. This type of house construction is widespread in impoverished villages in Africa, Asia and South America. In these regions, HD4MC could be adapted for vector control for various local vector-borne diseases including malaria, visceral leishmaniasis, dengue and Chagas' disease, to mention a few examples, and for the control of Tunga penetrans, the causative agent of tungiasis or jiggers, whose off-host stages thrive in organic matter in earth floors [36]. Future research should address the generalizability of HD4MC low technology to vector control of diseases other than malaria.
Patents: No patent is associated with any part of the work in this publication.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: Components of HD4MC; Figure S2: mixing of HD4MC components; Figure S3: smearing of the hut walls with HD4MC intervention; Figure S4: hut wall before and after smearing with HD4MC intervention; Supplemental Table 1: Baseline malaria vector feeding capacity; Supplemental Table 2: Feeding pattern of species-specific female Anopheles collected using HLC indoors per hour
Author Contributions
TJO led the field study; TJO, EO, GA, WJO, AA, BC conducted the study; TJO performed the statistical analysis and drafted the manuscript; IH and MP performed the HMO laboratory analysis; TGE conceived and directed the study. All authors read and agreed to the published version of the manuscript.
Funding
This research was supported by funding from the Global Innovation Fund and Grand Challenges Canada awarded to Thomas G. Egwang. The funders played no role in the study design, data collection and analysis nor in the preparation of the manuscript and decision to publish this paper.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board the VCD-REC of the Vector Control Division (Ministry of Health) accredited by the Uganda National Council for Science and Technology.
Informed Consent Statement
Informed consent was obtained from participants performing HLC and CDC LT collections. Permission was sought from the household heads to undertake mosquito collections in their homes. Community consent was also obtained from all the villages and their administrations. Written informed consent has been obtained from the participants to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We are very grateful to the individual households and the general community of Usuk sub-county for their generosity and participation. We would like to thank the clinical and laboratory staff of Med Biotech Laboratories at St Anne HC III Usuk, Katakwi, Uganda.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- World Health Organization, 2023. World malaria report 2023. World Health Organization.
- Larkin, H.D. Updated Malaria Recommendations for Children and Pregnant People. JAMA 2022, 328, 234–234. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. (2017). Mass drug administration for falciparum malaria: a practical field manuåal.
- Ogunah, J.A. , Lalah, J.O. and Schramm, K.W., 2020. Malaria vector control strategies. What is appropriate towards sustainable global eradication? Sustainable chemistry and pharmacy, 18, p.100339.
- Gachelin, G.; Garner, P.; Ferroni, E.; Verhave, J.P.; Opinel, A. Evidence and strategies for malaria prevention and control: a historical analysis. Malar. J. 2018, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Alout, H.; Roche, B.; Dabiré, R.K.; Cohuet, A. Consequences of insecticide resistance on malaria transmission. PLOS Pathog. 2017, 13, e1006499. [Google Scholar] [CrossRef]
- Hogan, A.B.; Winskill, P.; Ghani, A.C. Estimated impact of RTS,S/AS01 malaria vaccine allocation strategies in sub-Saharan Africa: A modelling study. PLOS Med. 2020, 17, e1003377. [Google Scholar] [CrossRef]
- Trottier, H. , & Elliott, S. J. (2021). World Health Organization recommends first malaria vaccine. Canadian Journal of Public Health= Revue Canadienne de Sante Publique, 112(6), 967.
- A Nnaji, C.; A Amaechi, U.; Wiysonge, C.S. R21/Matrix-M vaccine: optimising supply, maximising impact. Lancet 2024, 403, 525. [Google Scholar] [CrossRef] [PubMed]
- Toé, K.H.; Jones, C.M.; N’fale, S.; Ismail, H.M.; Dabiré, R.K.; Ranson, H. Increased Pyrethroid Resistance in Malaria Vectors and Decreased Bed Net Effectiveness, Burkina Faso. Emerg. Infect. Dis. 2014, 20, 1691–1696. [Google Scholar] [CrossRef] [PubMed]
- Mackowski, M.D. , 2020. Factors Limiting Effective Coverage of Indoor Residual Spraying Campaigns in Luapula Province, Zambia (Master's thesis, Syracuse University).
- Oxborough, R.M.; Kitau, J.; Jones, R.; Feston, E.; Matowo, J.; Mosha, F.W.; Rowland, M.W. Long-lasting control of Anopheles arabiensis by a single spray application of micro-encapsulated pirimiphos-methyl (Actellic® 300 CS). Malar. J. 2014, 13, 37–37. [Google Scholar] [CrossRef] [PubMed]
- Rowland, M.; Boko, P.; Odjo, A.; Asidi, A.; Akogbeto, M.; N'Guessan, R. A new long-lasting indoor residual formulation of the organophosphate insecticide pirimiphos methyl for prolonged control of pyrethroid-resistant mosquitoes: an experimental hut trial in Benin. PLoS ONE 2013, 8, e69516. [Google Scholar] [CrossRef] [PubMed]
- Kigozi, S.P.; Kigozi, R.N.; Sebuguzi, C.M.; Cano, J.; Rutazaana, D.; Opigo, J.; Bousema, T.; Yeka, A.; Gasasira, A.; Sartorius, B.; et al. Spatial-temporal patterns of malaria incidence in Uganda using HMIS data from 2015 to 2019. BMC Public Heal. 2020, 20, 1–14. [Google Scholar] [CrossRef]
- World Health Organization. (2013). Malaria entomology and vector control.
- Gillies MT, Meillon B: The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region). 1968; 2, 343.
- Spraying, W. I. R. (2015). An operational manual for indoor residual spraying (IRS) for malaria transmission control and elimination. World Health Organization: Geneva, Switzerland.
- World Health Organization. (2006). Guidelines for testing mosquito adulticides for indoor residual spraying and treatment of mosquito nets (No. WHO/CDS/NTD/WHOPES/GCDPP/2006.3). World Health Organization.
- World Health Organization. (2022). Standard operating procedure for testing insecticide susceptibility of adult mosquitoes in WHO bottle bioassays.
- World Health Organization. (2001). WHO Pesticide Evaluation Scheme (WHOPES).
- World Health Organization. (2011). Guidelines on public health pesticide management policy for the WHO African region.
- Fuseini, G.; Ismail, H.M.; von Fricken, M.E.; Weppelmann, T.A.; Smith, J.; Logan, R.A.E.; Oladepo, F.; Walker, K.J.; Phiri, W.P.; Paine, M.J.I.; et al. Improving the performance of spray operators through monitoring and evaluation of insecticide concentrations of pirimiphos-methyl during indoor residual spraying for malaria control on Bioko Island. Malar. J. 2020, 19, 1–8. [Google Scholar] [CrossRef]
- Namba, T.; Nolte, C.T.; Jackrel, J.; Grob, D. Poisoning due to organophosphate insecticides. Am. J. Med. 1971, 50, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Walton, E.L. Pralidoxime and pesticide poisoning: A question of severity? Biomed. J. 2016, 39, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Robb, E.L. , Regina, A.C. and Baker, M.B., 2017. Organophosphate toxicity.
- Hamel, M.J.; Marwanga, D.; Kariuki, S.; Gimnig, J.; Slutsker, L.; Were, V.; Laserson, K.F.; Otieno, P.; Bayoh, N.; Williamson, J. The Combination of Indoor Residual Spraying and Insecticide-Treated Nets Provides Added Protection against Malaria Compared with Insecticide-Treated Nets Alone. Am. J. Trop. Med. Hyg. 2011, 85, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Aroniadou-Anderjaska, V.; Figueiredo, T.H.; Furtado, M.d.A.; Pidoplichko, V.I.; Braga, M.F.M. Mechanisms of Organophosphate Toxicity and the Role of Acetylcholinesterase Inhibition. Toxics 2023, 11, 866. [Google Scholar] [CrossRef]
- Pesticide Residues in Food, 1977 Evaluations, WHO Expert Group on Pesticide Residues, Food and Agriculture Org., 1978-Technology & Engineering.
- Mosqueira, B.; Soma, D.D.; Namountougou, M.; Poda, S.; Diabaté, A.; Ali, O.; Fournet, F.; Baldet, T.; Carnevale, P.; Dabiré, R.K.; et al. Pilot study on the combination of an organophosphate-based insecticide paint and pyrethroid-treated long lasting nets against pyrethroid resistant malaria vectors in Burkina Faso. Acta Trop. 2015, 148, 162–169. [Google Scholar] [CrossRef]
- Poda, S.B.; Soma, D.D.; Hien, A.; Namountougou, M.; Gnankiné, O.; Diabaté, A.; Fournet, F.; Baldet, T.; Mas-Coma, S.; Mosqueira, B.; et al. Targeted application of an organophosphate-based paint applied on windows and doors against Anopheles coluzzii resistant to pyrethroids under real life conditions in Vallée du Kou, Burkina Faso (West Africa). Malar. J. 2018, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Ngufor, C.; Tchicaya, E.; Koudou, B.; N'Fale, S.; Dabire, R.; Johnson, P.; Ranson, H.; Rowland, M. Combining Organophosphate Treated Wall Linings and Long-lasting Insecticidal Nets for Improved Control of Pyrethroid Resistant Anopheles gambiae. PLOS ONE 2014, 9, e83897. [Google Scholar] [CrossRef]
- Ngufor, C.; Chouaïbou, M.; Tchicaya, E.; Loukou, B.; Kesse, N.; N’guessan, R.; Johnson, P.; Koudou, B.; Rowland, M. Combining organophosphate-treated wall linings and long-lasting insecticidal nets fails to provide additional control over long-lasting insecticidal nets alone against multiple insecticide-resistant Anopheles gambiae in Côte d’Ivoire: an experimental hut trial. Malar. J. 2014, 13, 396–396. [Google Scholar] [CrossRef]
- Alim, A.; Huda, M.M.; Ghosh, D.; Halleux, C.M.; Almahmud; Olliaro, P. L.; Matlashewski, G.; Kroeger, A.; Aseffa, A.; Mondal, D. Long-Term Efficacy of Insecticidal Wall Painting for Controlling Visceral Leishmaniasis Vectors in Bangladesh. Am. J. Trop. Med. Hyg. 2023, 109, 1022–1027. [Google Scholar] [CrossRef]
- Huda, M.M.; Kumar, V.; Das, M.L.; Ghosh, D.; Priyanka, J.; Das, P.; Alim, A.; Matlashewski, G.; Kroeger, A.; Alfonso-Sierra, E.; et al. Entomological efficacy of durable wall lining with reduced wall surface coverage for strengthening visceral leishmaniasis vector control in Bangladesh, India and Nepal. BMC Infect. Dis. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Mondal, D.; Das, M.L.; Kumar, V.; Huda, M.M.; Das, P.; Ghosh, D.; Priyanka, J.; Matlashewski, G.; Kroeger, A.; Upfill-Brown, A.; et al. Efficacy, Safety and Cost of Insecticide Treated Wall Lining, Insecticide Treated Bed Nets and Indoor Wall Wash with Lime for Visceral Leishmaniasis Vector Control in the Indian Sub-continent: A Multi-country Cluster Randomized Controlled Trial. PLOS Neglected Trop. Dis. 2016, 10, e0004932. [Google Scholar] [CrossRef] [PubMed]
- Matharu, A.K.; Ouma, P.; Njoroge, M.M.; Amugune, B.L.; Hyuga, A.; Mutebi, F.; Krücken, J.; Feldmeier, H.; Elson, L.; Fillinger, U. Identification of tungiasis infection hotspots with a low-cost, high-throughput method for extracting Tunga penetrans (Siphonaptera) off-host stages from soil samples–An observational study. PLOS Neglected Trop. Dis. 2024, 18, e0011601. [Google Scholar] [CrossRef] [PubMed]
Figure 3.
Median (interquartile range-IQR) PM levels in house dust collected from HD4MC and IRS treated households.
Figure 3.
Median (interquartile range-IQR) PM levels in house dust collected from HD4MC and IRS treated households.
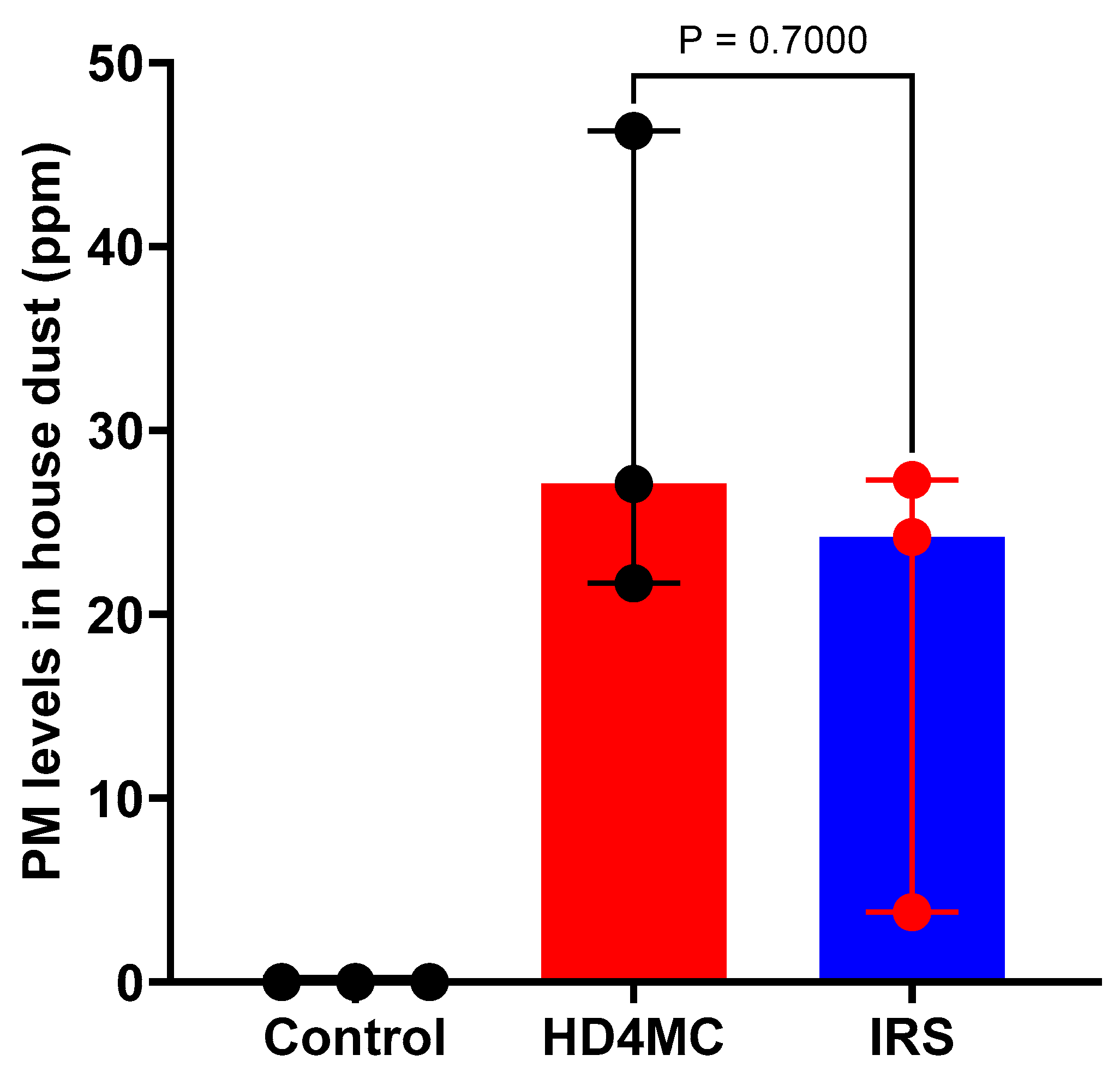
Table 1.
Participants demographic characteristics.
| Intervention | ||||
|---|---|---|---|---|
| Participants | Control | HD4MC | IRS | Total |
| Sex | ||||
| Male, n (%) | 282 (35.4) | 289 (36.3) | 226 (28.4) | 797 |
| Female, n (%) | 304 (35.5) | 267 (31.2) | 284 (33.2) | 855 |
| Age | ||||
| <5 years old, n (%) | 166 (35.0) | 156 (33.0) | 151 (32.0) | 473 |
| >5 years old, n (%) | 395 (34.2) | 400 (34.7) | 359 (31.1) | 1154 |
| Vector control tool, n (%) | ||||
| Use ITNs | 400 (47.0) | 210 (24.7) | 241 (28.3) | 851 |
% = percent, n = number, HD4MC = house decoration for malaria control, IRS = indoor residual spraying, sex = biological sex assigned at birth.
Table 2.
Baseline malaria vector composition, density, and distribution.
| Villages | PSC, n | CDC LT, n | HLC, n | Overall, n (%) | ||||
|---|---|---|---|---|---|---|---|---|
| An. gambiae | An. funestus | An. gambiae | An. funestus | An. gambiae | An. funestus | An. gambiae | An. funestus | |
| Ongema | 76 | 0 | 62 | 0 | ND | ND | 138 (100) | 00 (0.0) |
| Otujai | 34 | 0 | 67 | 0 | 108 | 4 | 209 (98.1) | 04 (1.9) |
| Acurun | 30 | 60 | 60 | 11 | 257 | 153 | 347 (60.8) | 224 (39.2) |
| Abwokodia | 16 | 3 | 38 | 9 | 75 | 14 | 129 (83.2) | 26 (16.7) |
| Total | 156 63 | 227 20 | 440 171 | 823 (76.4) | 254 (23.6) | |||
ND = Not done; n = number of mosquitoes collected per sub-category; % = percent.
Table 3.
Knock down and 24-hour mortality of lab-reared mosquitoes exposed to treated walls.
| Intervention | Residual insecticidal activity | |||||
| KD60 | Mortality | |||||
| 1.5 months | 3 months | 6 months | 1.5 months | 3 months | 6 months | |
| Control | 0 | 0 | 0 | 0 | 0 | 0 |
| HD4MC, % (n) | 100 (90/90) |
90.0 (81/90) | 84.4 (76/90) | 100 (90/90) | 94.4 (85/90) | 90.0 (81/90) |
| IRS, % (n) | 100 (90/90) |
83.3 (75/90) | 80.0 (72/90) | 100 (90/90) | 100 (90/90) | 98.0 (88/90) |
KD60 = percent of mosquitoes knocked down after exposure on treated wall for 60 minutes; mortality = Percent of dead mosquitoes 24 hours after exposure to treated wall.
Table 4.
Adverse events.
| Interventions | ||||
|---|---|---|---|---|
| Adverse events | Total (N = 109) | IRS (N = 90) | HD4MC (N = 19) | P-value |
| Headache, n (%) | 16 (14.7) | 14 (15.6) | 2 (10.5) | 0.5735 |
| Itching, n (%) | 15 (13.8) | 13 (14.4) | 2 (10.5) | 0.6523 |
| Body rash, n (%) | 11 (10.1) | 9 (10.0) | 2 (10.5) | 0.9448 |
| Flue, n (%) | 8 (7.3) | 6 (6.7) | 2 (10.5) | 0.5577 |
| Cough, n (%) | 7 (6.4) | 4 (4.4) | 3 (15.8) | 0.0668 |
| Abdominal pain, n (%) | 2 (1.8) | 2 (2.2) | 0 (0.0) | 0.4903 |
| Fever, n (%) | 10 (9.2) | 8 (8.9) | 2 (10.5) | 0.8222 |
| Foul smell, n (%) | 40 (36.7) | 34 (37.8) | 6 (31.6) | 0.6104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated







