Submitted:
30 August 2024
Posted:
31 August 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LSE
2.3. The Property Tests of LSE
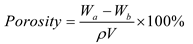
2.4. Characterizations
2.5. Assembly of SSC Device
2.6. Electrochemical Measurements
3. Results
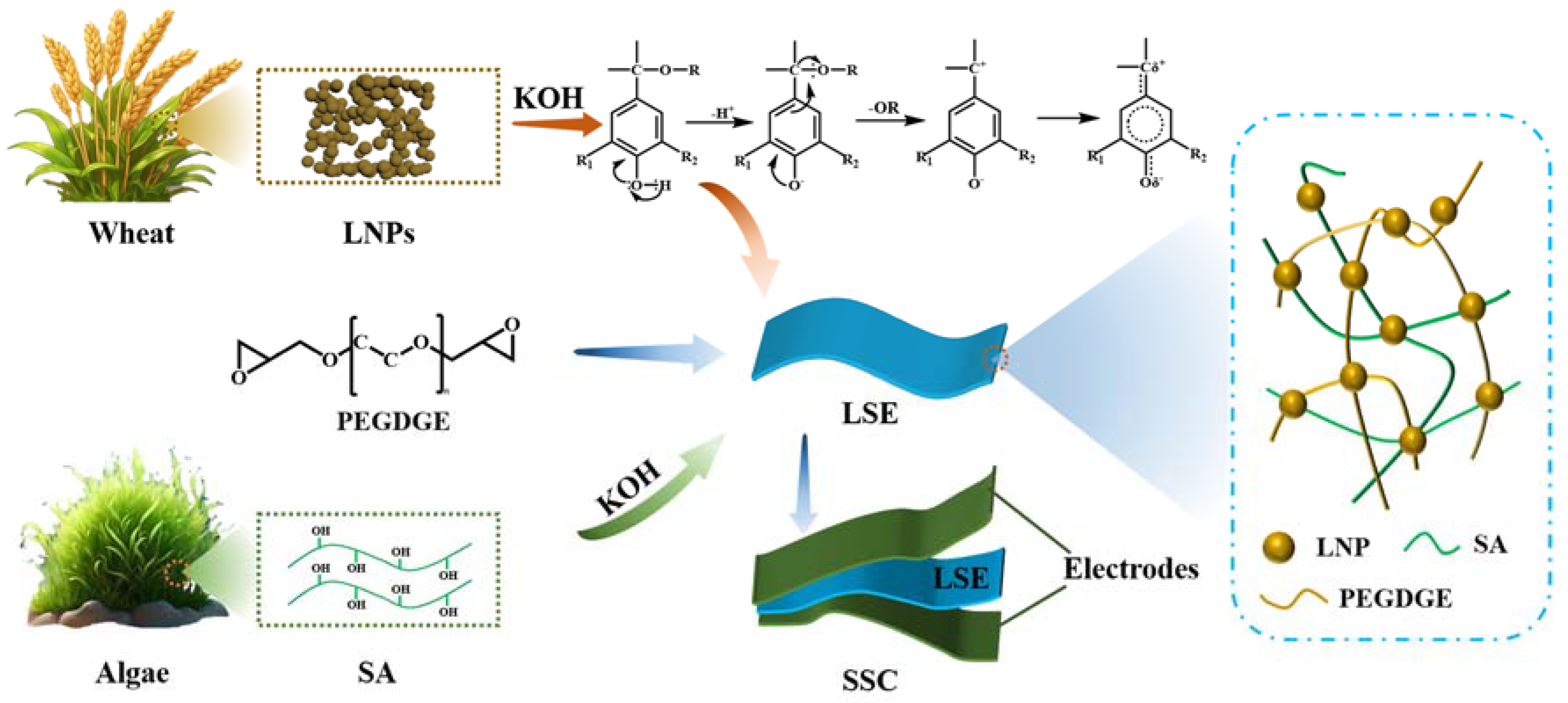
3.1. Structural Characterization of LSE
3.2. Physicochemical Performance of LSE
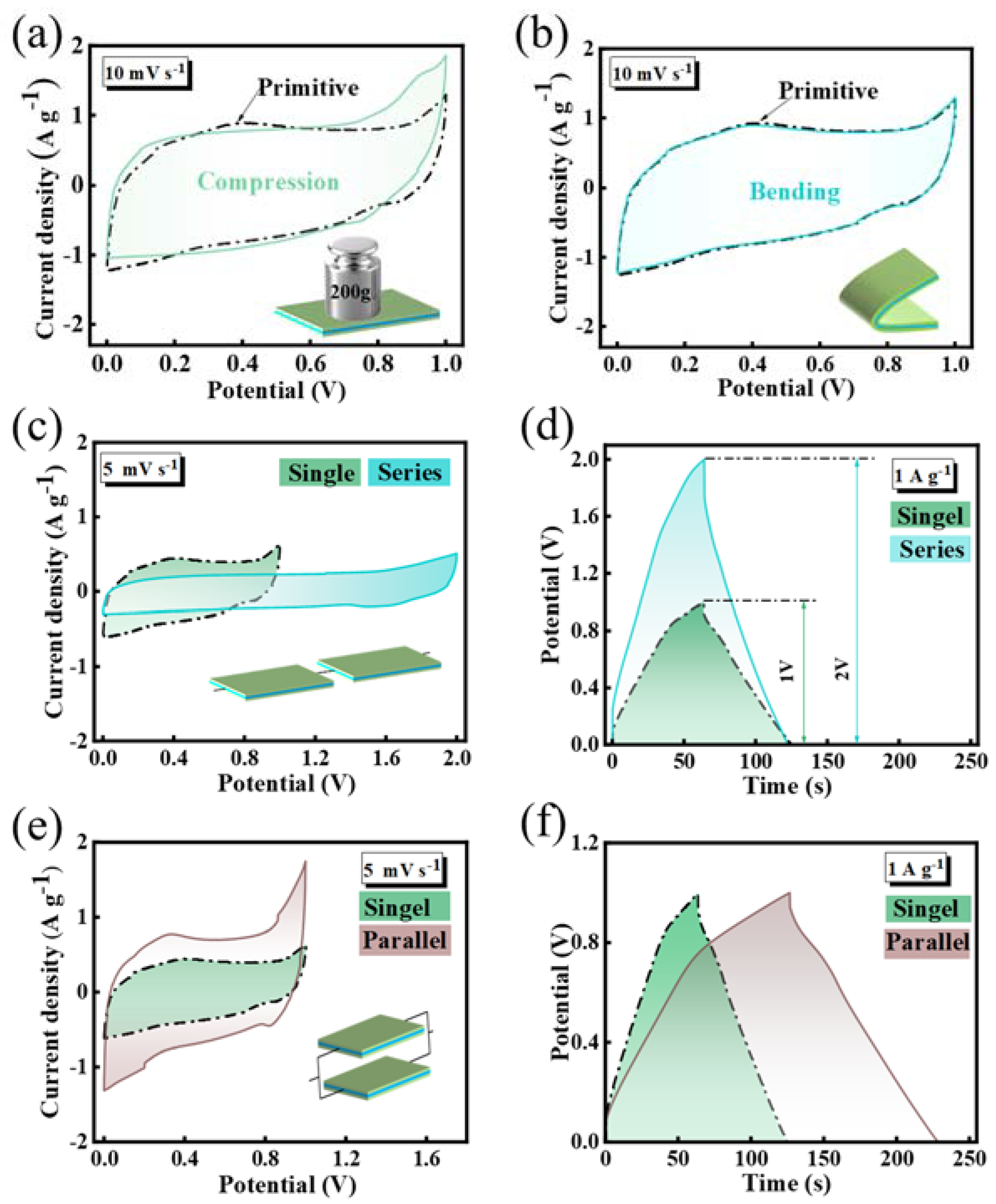
3.3. Electrochemical Performance of SSC Devices
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, J.; Hu, Y.; Wang, H.; Wang, T.; Wu, H.; Li, J.; Li, Y.; Wang, M.; Zhang, J. Lignin isolated from poplar wood for porous carbons as electrode for high-energy renewable supercapacitor driven by lignin/deep eutectic solvent composite gel polymer electrolyte. ACS Applied Energy Materials 2022, 5, 6393–6400. [Google Scholar] [CrossRef]
- Lamba, P.; Singh, P.; Singh, P.; Singh, P.; Bharti; Kumar, A. ; Gupta, M.; Kumar, Y. Recent advancements in supercapacitors based on different electrode materials: Classifications, synthesis methods and comparative performance. Journal of Energy Storage 2022, 48. [Google Scholar] [CrossRef]
- Poonam; Sharma, K. ; Arora, A.; Tripathi, S.K. Review of supercapacitors: Materials and devices. Journal of Energy Storage 2019, 21, 801–825. [Google Scholar] [CrossRef]
- Li, Z.; Fu, J.; Zhou, X.; Gui, S.; Wei, L.; Yang, H.; Li, H.; Guo, X. Ionic conduction in polymer-based solid electrolytes. Advanced Science 2023, 10. [Google Scholar] [CrossRef]
- Tan, S.-J.; Zeng, X.-X.; Ma, Q.; Wu, X.-W.; Guo, Y.-G. Recent advancements in polymer-based composite electrolytes for rechargeable lithium batteries. Electrochemical Energy Reviews 2018, 1, 113–138. [Google Scholar] [CrossRef]
- Jagan, M.; Vijayachamundeeswari, S.P. A comprehensive investigation of lithium-based polymer electrolytes. Journal of Polymer Research 2023, 30. [Google Scholar] [CrossRef]
- Yao, X.; Huang, B.; Yin, J.; Peng, G.; Huang, Z.; Gao, C.; Liu, D.; Xu, X. All-solid-state lithium batteries with inorganic solid electrolytes: Review of fundamental science. Chinese Physics B 2016, 25. [Google Scholar] [CrossRef]
- Zheng, F.; Li, C.; Li, Z.; Cao, X.; Luo, H.; Liang, J.; Zhao, X.; Kong, J. Advanced composite solid electrolytes for lithium batteries: Filler dimensional design and ion path optimization. Small 2023, 19. [Google Scholar] [CrossRef]
- Hou, P.; Gao, C.; Wang, J.; Zhang, J.; Liu, Y.; Gu, J.; Huo, P. A semi-transparent polyurethane/porous wood composite polymer electrolyte for solid-state supercapacitor with high energy density and cycling stability. Chemical Engineering Journal 2023, 467. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Zhang, Q.; Hou, C.; Shi, Q.; Wang, H. A highly ionic conductive poly(methyl methacrylate) composite electrolyte with garnet-typed Li6.75La3Zr1.75Nb0.25O12 nanowires. Chemical Engineering Journal 2019, 375. [Google Scholar] [CrossRef]
- Park, J.H.; Rana, H.H.; Lee, J.Y.; Park, H.S. Renewable flexible supercapacitors based on all-lignin-based hydrogel electrolytes and nanofiber electrodes. Journal of Materials Chemistry A 2019, 7, 16962–16968. [Google Scholar] [CrossRef]
- Cao, Q.; Lou, R.; Dong, L.; Niu, T.; Wei, G.; Lyu, G. Evaluation of gelation time affecting the self-assembled framework of lignin nanoparticle-based carbon aerogels and their electrochemical performances. ACS Applied Energy Materials 2023, 6, 10874–10882. [Google Scholar] [CrossRef]
- Qiu, F.; Huang, Y.; He, G.; Luo, C.; Li, X.; Wang, M.; Wu, Y. A lignocellulose-based neutral hydrogel electrolyte for high-voltage supercapacitors with overlong cyclic stability. Electrochimica Acta 2020, 363. [Google Scholar] [CrossRef]
- Mondal, A.K.; Xu, D.; Wu, S.; Zou, Q.; Huang, F.; Ni, Y. Design of Fe3+-rich, high-conductivity lignin hydrogels for supercapacitor and sensor applications. Biomacromolecules 2022, 23, 766–778. [Google Scholar] [CrossRef] [PubMed]
- Melro, E.; Filipe, A.; Sousa, D.; Valente, A.J.M.; Romano, A.; Antunes, F.E.; Medronho, B. Dissolution of kraft lignin in alkaline solutions. International Journal of Biological Macromolecules 2020, 148, 688–695. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Hou, P.; Liu, Y.; Zhao, J.; Huo, P. All-bio-based, adhesive and low-temperature resistant hydrogel electrolytes for flexible supercapacitors. Chemical Engineering Journal 2023, 455. [Google Scholar] [CrossRef]
- Zeng, J.; Dong, L.; Sha, W.; Wei, L.; Guo, X. Highly stretchable, compressible and arbitrarily deformable all-hydrogel soft supercapacitors. Chemical Engineering Journal 2020, 383. [Google Scholar] [CrossRef]
- Liu, T.; Ren, X.; Zhang, J.; Liu, J.; Ou, R.; Guo, C.; Yu, X.; Wang, Q.; Liu, Z. Highly compressible lignin hydrogel electrolytes via double-crosslinked strategy for superior foldable supercapacitors. Journal of Power Sources 2020, 449. [Google Scholar] [CrossRef]
- Pandey, G.P.; Hashmi, S.A.; Kumar, Y. Multiwalled carbon nanotube electrodes for electrical double layer capacitors with ionic liquid based gel polymer electrolytes. Journal of the Electrochemical Society 2010, 157, A105–A114. [Google Scholar] [CrossRef]
- Gorshkova, M.Y.; Volkova, I.F.; Grigoriyan, E.S.; Molchanov, S.P. Structure and properties of hydrogels based on sodium alginate and synthetic polyacids. Mendeleev Communications 2024, 34, 372–375. [Google Scholar] [CrossRef]
- Heng, Y.; Teng, G.; Chi, Y.; Hu, D. Construction of biomass-derived hybrid organogel electrodes with a cross-linking conductive network for high-performance all-solid-state supercapacitors. Biomacromolecules 2021. [Google Scholar] [CrossRef]
- Niu, T.; Lou, R.; Cao, Q.; Zhang, Y.; Zhang, Y.; Wei, G.; Wang, Z. In-situ growth of homogeneous δ-MnO2 within lignin based porous carbon to reassemble uniform mesoporous crosslinked 3D-network structure for supercapacitors. Materials Chemistry and Physics 2023, 305. [Google Scholar] [CrossRef]
- Cao, Q.; Zhu, M.; Chen, J.; Song, Y.; Li, Y.; Zhou, J. Novel lignin-cellulose-based carbon nanofibers as high-performance supercapacitors. ACS Applied Materials & Interfaces 2020, 12, 1210–1221. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Li, D.; Sun, S.; Peng, Z.; Komarneni, S.; Yang, D. Direct interfacial growth of MnO2 nanostructure on hierarchically porous carbon for high-performance asymmetric supercapacitors. ACS Sustainable Chemistry & Engineering 2018, 6, 633–641. [Google Scholar] [CrossRef]
- Li, Q.; Lu, T.; Wang, L.; Pang, R.; Shao, J.; Liu, L.; Hu, X. Biomass based N-doped porous carbons as efficient CO2 adsorbents and high-performance supercapacitor electrodes. Separation and Purification Technology 2021, 275. [Google Scholar] [CrossRef]
- Luo, N.; Wang, J.; Zhang, D.; Zhao, Y.; Wei, Y.; Liu, Y.; Zhang, Y.; Han, S.; Kong, X.; Huo, P. Inorganic nanoparticle-enhanced double-network hydrogel electrolytes for supercapacitor with superior low-temperature adaptability. Chemical Engineering Journal 2024, 479. [Google Scholar] [CrossRef]
- Lin, C.-H.; Wang, P.-H.; Lee, W.-N.; Li, W.-C.; Wen, T.-C. Chitosan with various degrees of carboxylation as hydrogel electrolyte for pseudo solid-state supercapacitors. Journal of Power Sources 2021, 494. [Google Scholar] [CrossRef]
- Sun, P.-P.; Zhang, Y.-H.; Shi, H.; Shi, F.-N. Controllable one step electrochemical synthesis of PANI encapsulating 3d-4f bimetal MOFs heterostructures as electrode materials for high-performance supercapacitors. Chemical Engineering Journal 2022, 427. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Han, X.; Li, Z.; Zhang, S.; Zong, M. A flexible Zinc-ion hybrid supercapacitor constructed by porous carbon with controllable structure. Applied Surface Science 2022, 579. [Google Scholar] [CrossRef]
- Sun, Y.; Xu, D.; Wang, S. Self-assembly of biomass derivatives into multiple heteroatom-doped 3D-interconnected porous carbon for advanced supercapacitors. Carbon 2022, 199, 258–267. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, X.; Wan, F.; Chen, K.; Niu, Z.; Chen, J. An all-freeze-casting strategy to design typographical supercapacitors with integrated architectures. Small 2018, 14. [Google Scholar] [CrossRef] [PubMed]
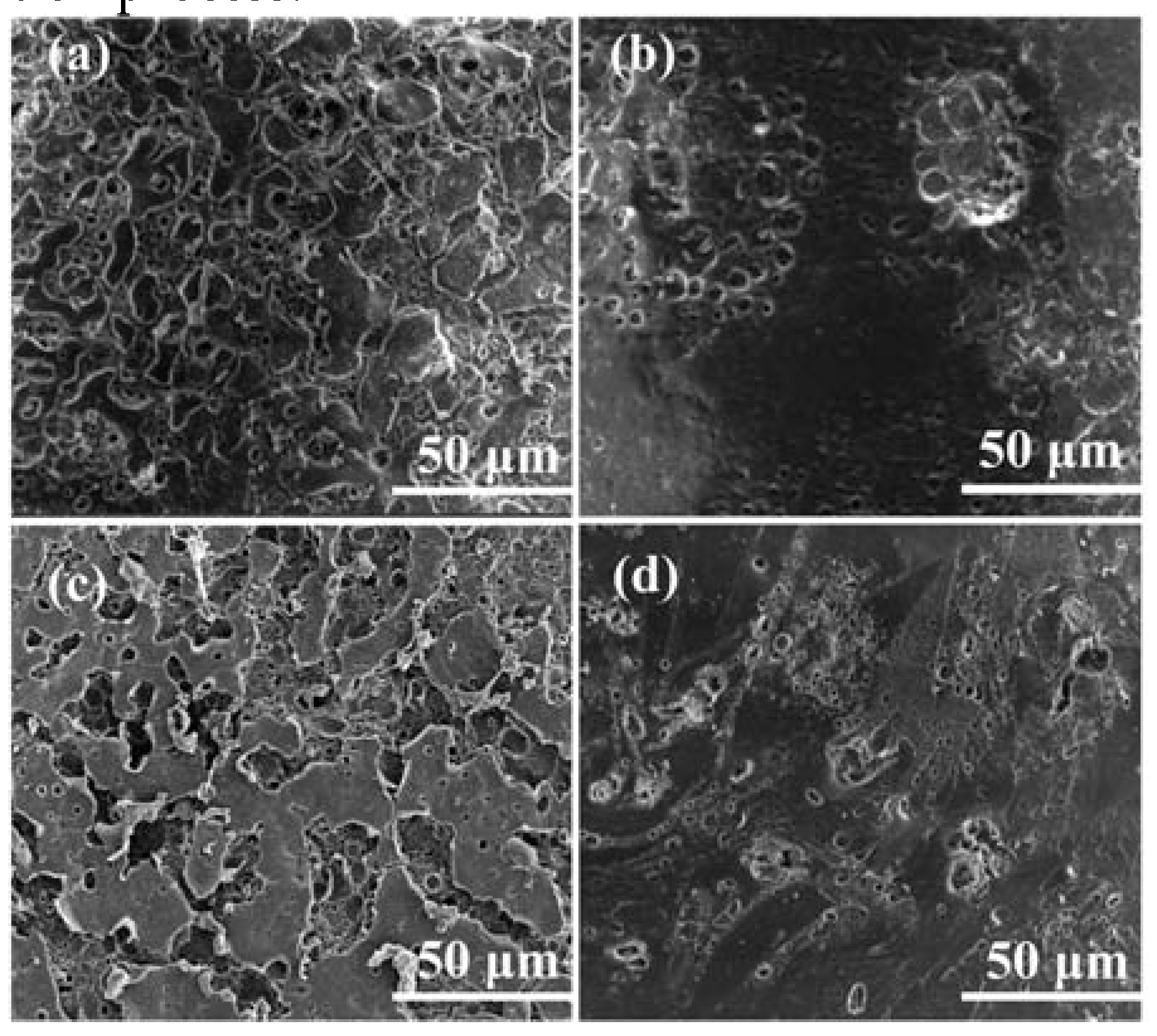
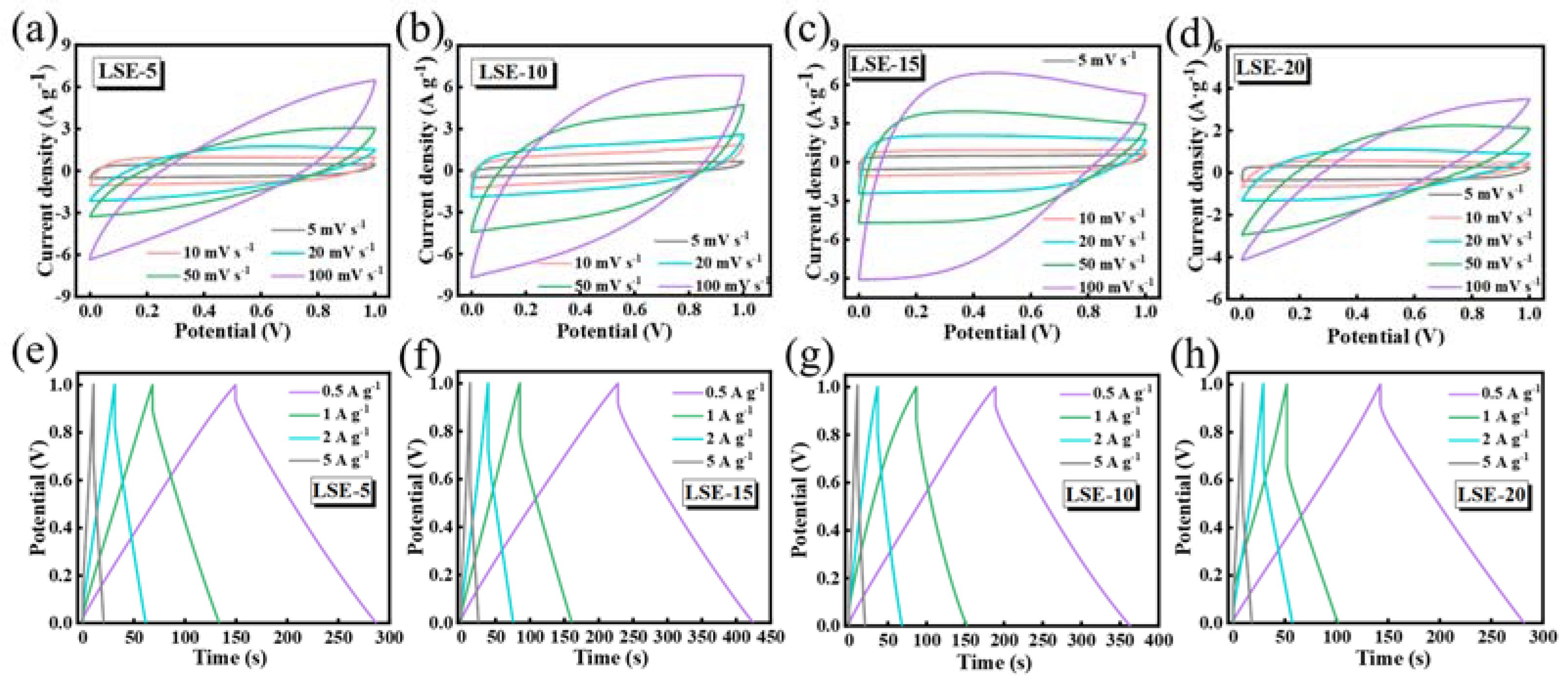
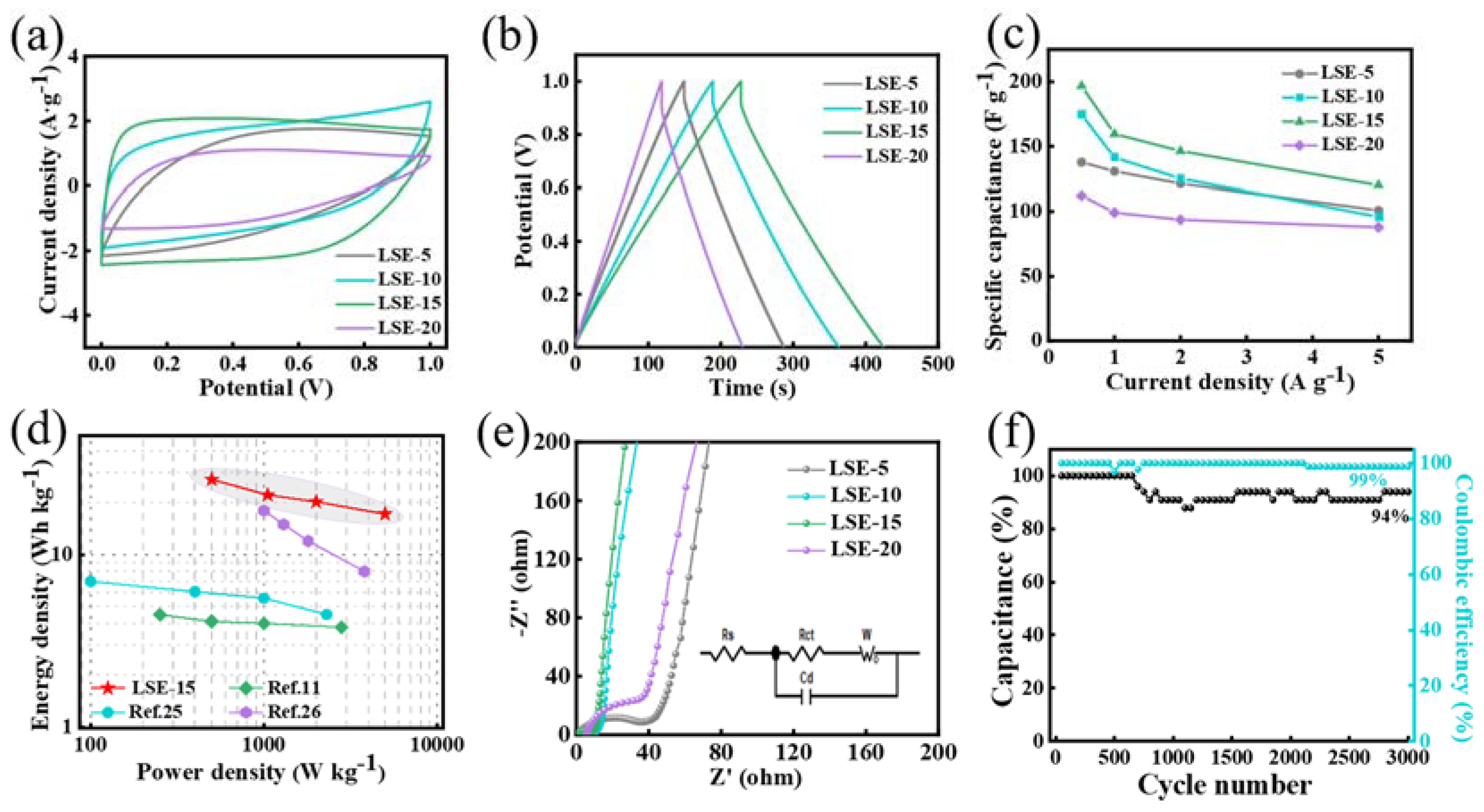
| C (F g−1) | Rs (Ω) | Rct (Ω) | |
|---|---|---|---|
| LSE-5 | 134 | 0.93 | 18.08 |
| LSE-10 | 175 | 0.43 | 9.89 |
| LSE-15 | 195 | 0.40 | 7.46 |
| LSE-20 | 115 | 5.64 | 25.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





