1. Introduction
Aging is a natural and exceedingly intricate biological phenomenon governed by an interplay of genomics, transcriptomics, proteomics, metabolomics, as well as dietary habits, lifestyle, and environmental factors[
1]. The burgeoning elderly population poses an escalating public health issue on a global scale, with its proportion rapidly increasing and projected to reach two billion by 2050 for individuals aged 60 and above[
2]. Moreover, the health status of the aging population is deteriorating, leading to a surge in the prevalence of age-related diseases, particularly in China, where it is estimated that by 2040, there will be approximately 402 million elderly individuals[
2]. The recent decline in fertility and mortality rates has catalyzed a swift aging of the population, raising concerns about the health and quality of life of the elderly, and presenting significant challenges to healthcare systems. Aging is a complex biological process involving the interaction of various molecular and cellular mechanisms, such as theories of programmed longevity, free radical immunity, wear and tear, cross-linking, endocrine, and basal metabolism to describe the aging process[
3,
4]. Despite significant advances in aging research over the past decades, a comprehensive understanding of metabolic changes during the aging process remains limited. In recent years, an increasing number of researchers have begun to utilize omics technologies to study normal aging due to their high-throughput characteristics in proteomics, genomics, transcriptomics, and metabolomics[
5,
6,
7,
8].Metabolomic as a branch of systems biology, offers a powerful tool for the study of aging by quantifying all metabolites within a biological system[
9].
In the field of agricultural science, understanding the aging process in animal models such as pigs is of significant importance for comprehending aging and age-related diseases, as well as optimizing breeding management strategies to enhance production efficiency and meat quality[
10]. Pigs as an important agricultural animal model with physiological and anatomical characteristics similar to humans, serve as an ideal subject for studying aging-related metabolic changes[
11]. With the rapid development of metabolomics technologies, especially the application of high-resolution mass spectrometry and nuclear magnetic resonance techniques, studying the metabolic changes in pigs at the molecular level has become feasible[
12,
13].The aim of this study is to utilize metabolomics methods to analyze the metabolite profiles of pigs of different age groups to identify metabolic changes associated with aging. By conducting non-targeted metabolomics analysis on blood and tissue samples from young, middle-aged, and elderly pigs, we aim to reveal age-related metabolites and their changes in biological pathways. This information will not only aid in understanding the aging mechanisms in pigs but also provide a reference for aging research in other species, including humans.
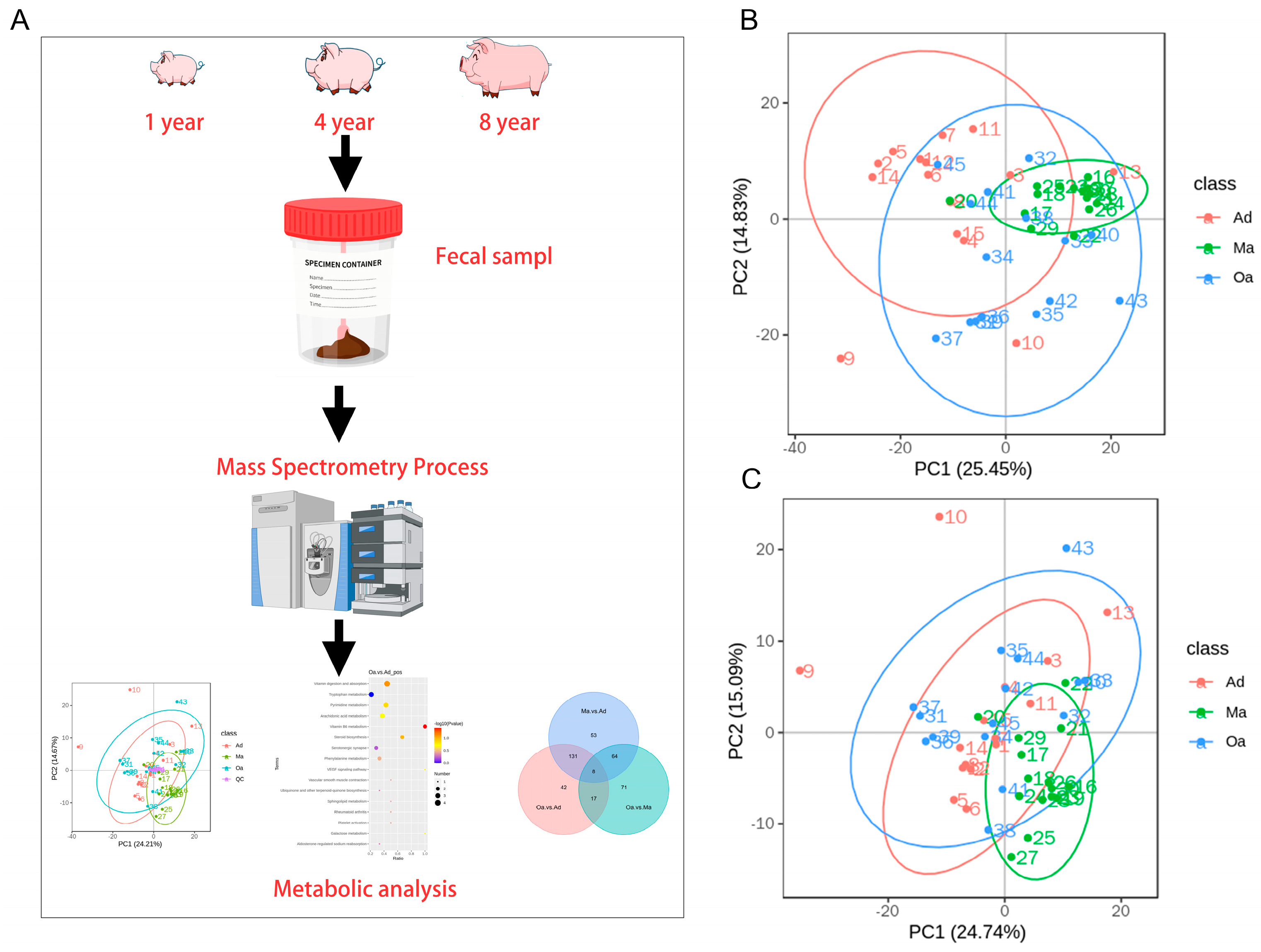
In this study, we initially collected feces samples from pigs of three different age groups: (1) one year old, (2) four years old, and (3) eight years old[
14]. Subsequently, non-targeted metabolomics analysis was performed using liquid chromatography-mass spectrometry (LC-MS) techniques. Through data analysis, we identified a series of age-related metabolites and observed significant metabolic changes in multiple biological pathways, including pyrimidine metabolism, vitamin metabolism, and amino acid metabolism, which play key roles in the aging process. Furthermore, we found that these metabolic changes are associated with known aging markers such as oxidative stress, inflammation, and cellular damage. These findings suggest that the changes of fecal metabolites can serve as a powerful tool to uncover molecular changes during the aging process in pigs, leading to the development of new biomarkers and therapeutic targets for age-related diseases. It may also provide new targets for the development of strategies to delay aging or improve human health and animal welfare.
3. Results
3.1. The Overall Characteristics of Feces Metabolomics
In metabolomics studies utilizing mass spectrometry, quality control (QC) samples are routinely employed to ensure the reliability and high quality of the acquired metabolomics data. Although QC samples are theoretically identical, systematic errors are often introduced during the processes of sample extraction, detection, and analysis. These errors can lead to variations among QC samples; the smaller the variations, the higher the method stability and data quality.
In the present study, the primary aim is to investigate the trajectory of changes in fecal metabolic profiles across various age stages in pigs, specifically at one year of age (Ad), four years of age (Ma), and eight years of age (Oa) (
Figure 1A). Principal component analysis (PCA) revealed a significant separation among the three age groups, indicating distinct metabolic profiles associated with aging. Additionally, the analysis highlighted a tight clustering of the QC samples, which suggests a high degree of stability and repeatability in the detection process. This dense distribution of QC samples reinforces the reliability of the data obtained (
Figure 1B,C).
3.2. The Trajectory of Dynamic Changes of Fecal Metabolites Associated with Aging
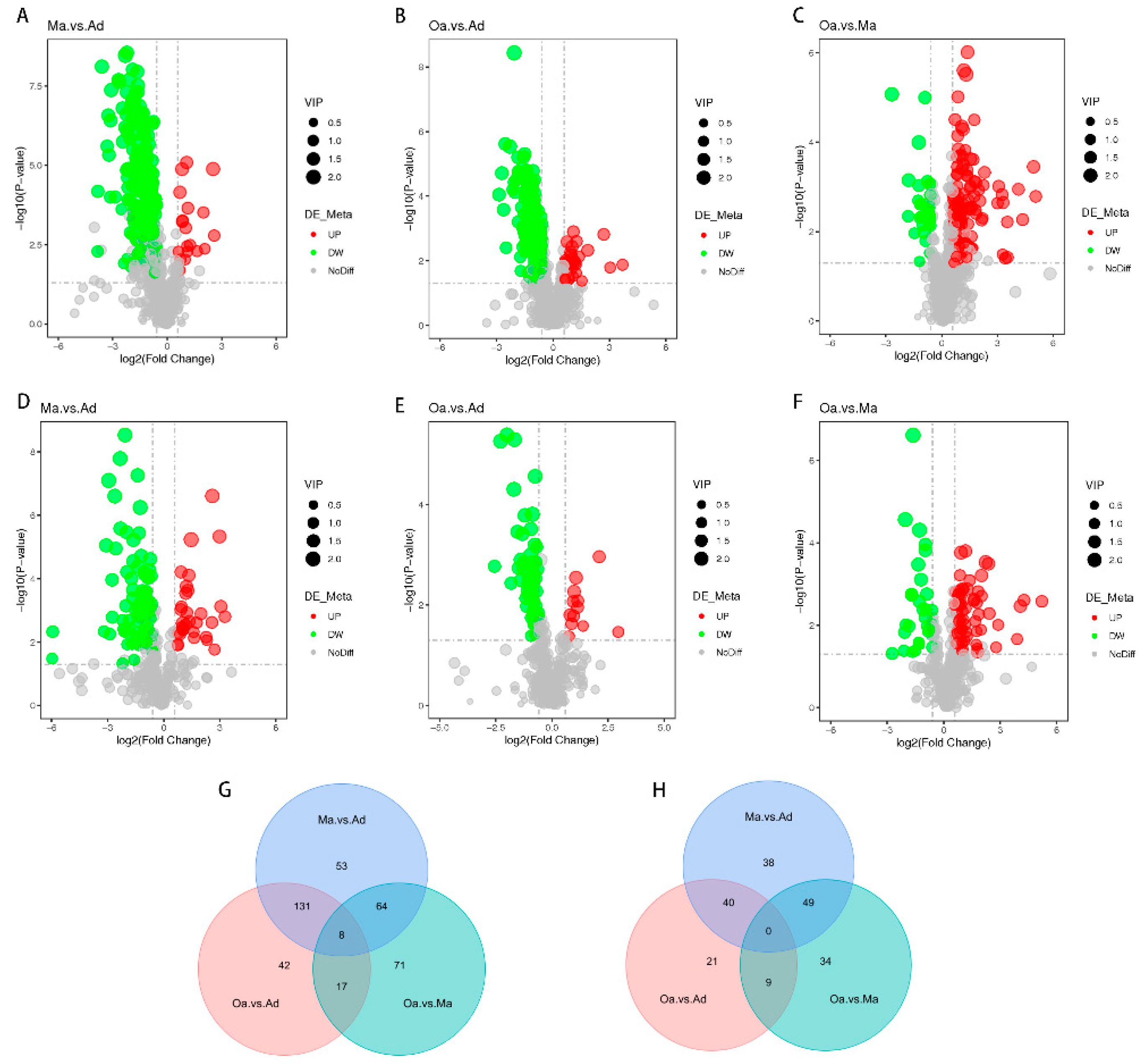
In the differential metabolomics analyses, a substantial number of metabolites were identified as significantly distinct among the swine in the middle-aged (Ma, four years old) group compared to the one-year-old (Ad) group, the elderly (Oa, eight years old) group compared to the Ad group, and the Oa group compared to the Ma group. Volcano plots indicated that a total of 383, 268, and 225 differentially expressed metabolites were detected in these comparisons, respectively (
Tables S1–S3). Among these metabolites, the upregulated counts were 333, 225, and 60, while the downregulated counts were 50, 43, and 192, respectively (
Figure 2A–F).
In positive ion mode, pairwise comparisons between the three groups revealed 53, 42, and 71 group-specific differential metabolites for the Ma vs. Ad, Oa vs. Ad, and Oa vs. Ma comparisons, respectively. Additionally, eight common differential metabolites were identified across these comparisons (
Figure 2G). In contrast, a total of 38, 21, and 34 group-specific differential metabolites were identified in negative ion mode; however, no overlapping differential metabolites were found in this mode (
Figure 2H). The eight common differential metabolites identified include: 4-(acetylamino)phenyl 3-chlorobenzoate, amoxicillin, 1-(4-nitrophenyl)piperidine, cyanidin, 4-guanidinobutanoic acid, ethyl 5-methoxy-2-methyl-1-phenyl-1H-indole-3-carboxylate, 5-(tert-butyl)-2-methyl-N-(4-nitrophenyl)-3-furamide, and valine.
3.3. Screening of Fecal Metabolites Intimately Linked to Aging
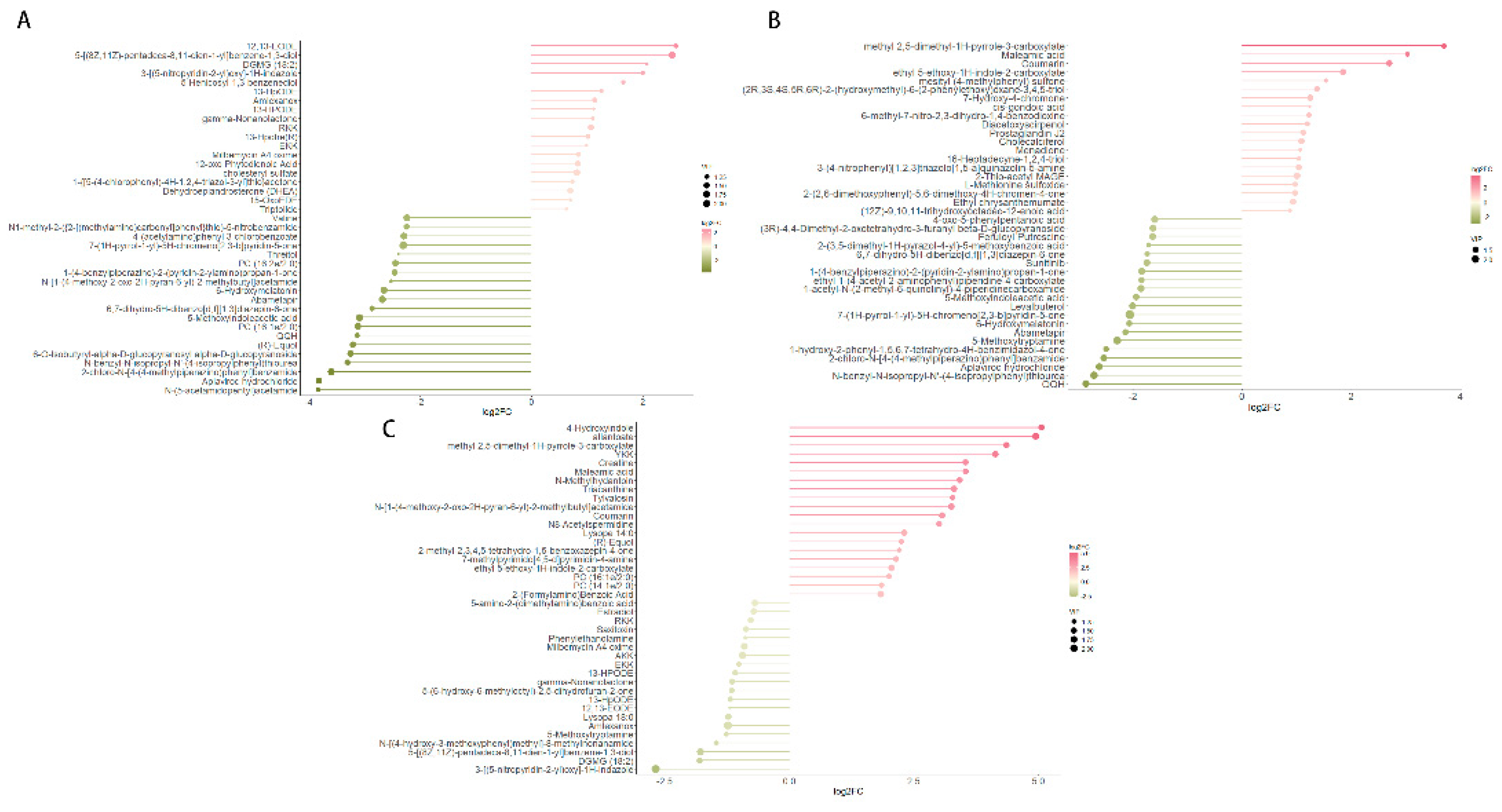
Differential metabolites were identified based on three key parameters: Variable Importance in Projection (VIP), fold change (FC), and p-value. To qualify as differential metabolites, a VIP value greater than 1 and a p-value less than 0.05 were required. As a result, metabolites were selected as differentially expressed according to the criteria of a p-value below 0.05 and a VIP value exceeding 1, with FC values either greater than 1 or less than 1. In pairwise comparisons among the one-year-old (Ad), four-year-old (Ma), and eight-year-old (Oa) groups, the top 20 upregulated and downregulated metabolites exhibiting the highest fold changes were selected for analysis. In the comparison between the Ma and Ad groups, 12,13-EODE demonstrated the highest level of upregulation, while N-(5-acetamidopentyl) acetamide showed the most significant downregulation (
Figure 3A). In the comparison between the Oa and Ad groups, methyl 2,5-dimethyl-AH-pyrrole-3-carboxylate exhibited the greatest upregulation, whereas QQH was the most pronounced downregulated metabolite (
Figure 3B). Finally, in the comparison between the Oa and Ma groups, 4-hydroxyindole was identified as the most upregulated metabolite, while 3-(G-nitropyridin-2-yl)oxy-1H-indazole exhibited the highest level of downregulation (
Figure 3C). Collectively, these findings highlight distinct metabolic alterations associated with aging and underscore the potential relevance of these metabolites in understanding the biochemical changes that occur across different life stages in pigs.
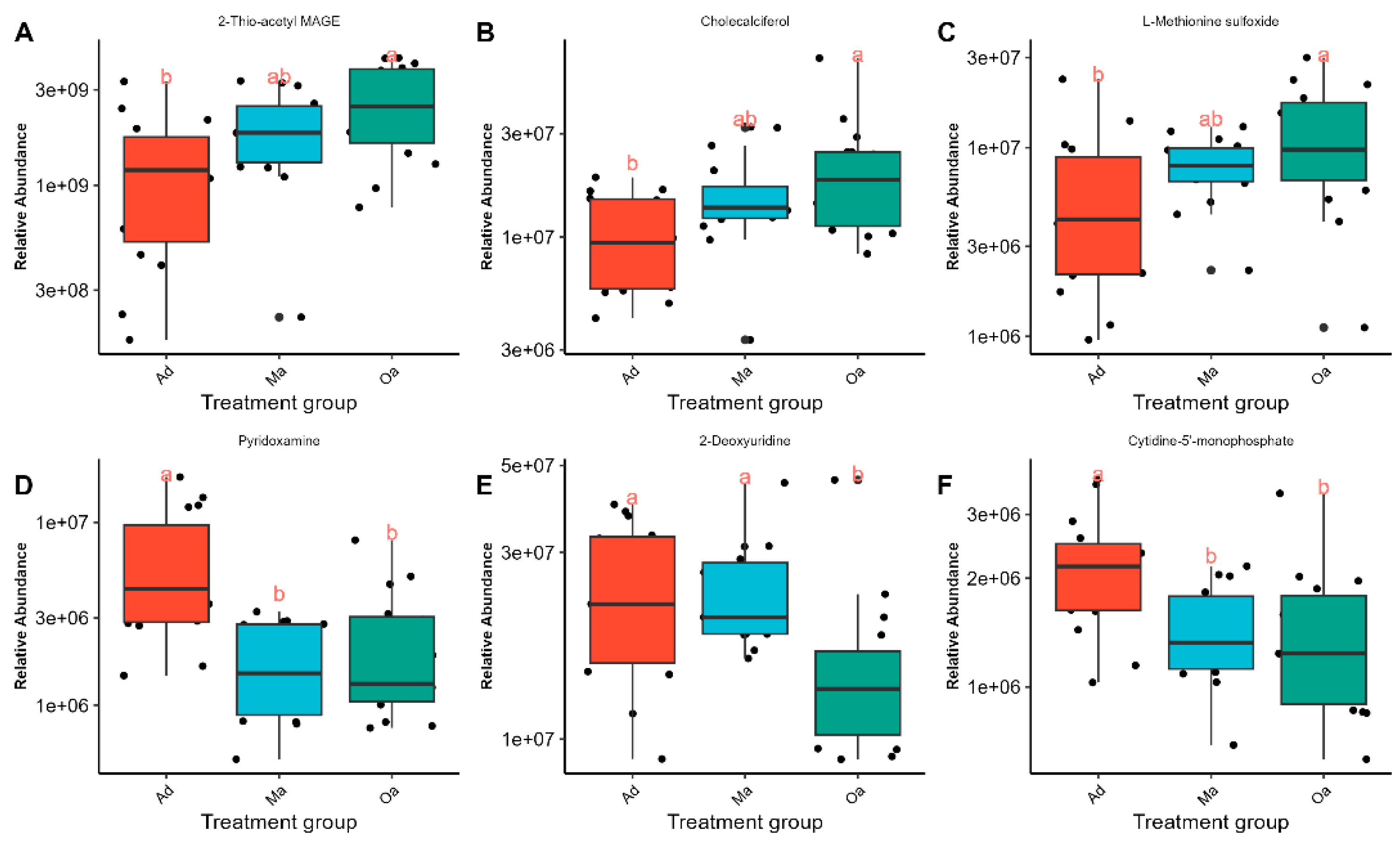
As the age of the pig population increases, 2-Thio-acetyl MAGE, Cholecalciferol, and L-Methionine sulfoxide have been observed to exhibit an increasing trend (
Figure 4A–C), while Pyridoxamine, 2-Deoxyuridine, and Cytidine-5-monophosphate have shown a decreasing trend (
Figure 4D–F). The identification of these down-regulated metabolites may provide insights into metabolic pathways that become less active over time. Together, these visual representations facilitate a clearer understanding of the dynamic metabolic shifts that accompany the aging process in pigs, underscoring the importance of both up-regulated and down-regulated metabolites in age-related research.
3.4. KEGG Enrichment Analysis Based on Differential Metabolites
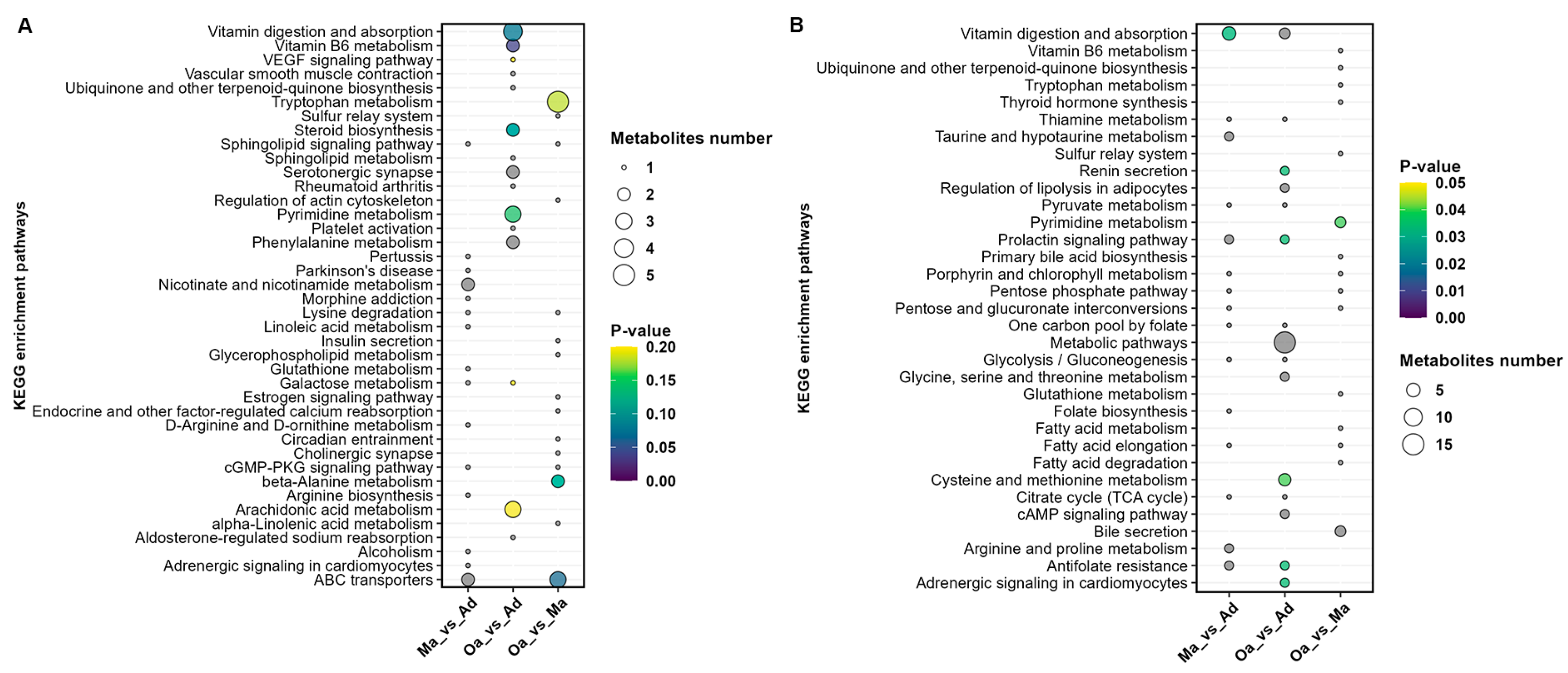
In the comparative analysis between the Ma (four years old) and Ad (one year old) groups, a total of 15 metabolic pathways were identified. These pathways predominantly included those related to vitamin digestion and absorption, taurine and hypotaurine metabolism, prolactin signaling, antifolate resistance, sphingolipid signaling, lysine degradation, linoleic acid metabolism, galactose metabolism, D-arginine and D-ornithine metabolism, the cGMP-PKG signaling pathway, and adrenergic signaling in cardiomyocytes.
When comparing the Oa (eight years old) group with the Ad (one year old) group, 12 metabolic pathways were identified, including Metabolic pathways, Cysteine and methionine metabolism, Renin secretion, Regulation of lipolysis in adipocytes, Prolactin signaling pathway, cAMP signaling pathway, Antifolate resistance, Adrenergic signaling in cardiomyocytes, Vitamin digestion and absorption, Pyrimidine metabolism, Vitamin B6 metabolism, and Steroid biosynthesis.
In the comparison between the Oa (eight years old) and Ma (four years old) groups, three metabolic pathways were identified, including Pyrimidine metabolism, ABC transporters, and beta-Alanine metabolism (
Figure 5A,B).
Furthermore, the analysis revealed that Pyrimidine metabolism and Metabolic pathways are significantly differential profiles common to all three groups (Tables 4–6). Pyrimidine metabolism plays a crucial role in cell division and DNA synthesis and is closely associated with the aging process. As age progresses, the rate of cellular division slows, and the capacity for DNA repair declines, which can affect the normal progression of pyrimidine metabolism. Abnormalities in pyrimidine metabolism may lead to the accumulation of abnormal metabolites within cells, potentially impacting cellular function and even leading to cell death.
4. Discussion
This study leverages metabolomics to uncover the aging related metabolomics and identify differences in metabolites composition among pigs aged 1 (Ad), 4 (Ma), and 8 years old (Oa). The aim is to identify specific biomarkers of the aging process and understand their underlying mechanisms, thereby formulating strategies to promote healthy aging and potentially prevent or treat age-related diseases[
15]. Results indicate that metabolites such as Cholecalciferol (Vitamin D), L-Methionine sulfoxide, Pyridoxamine, and Cytidine-5-monophosphate exhibit monotonic changes, either upregulated or downregulated, with increasing age. KEGG enrichment analysis revealed pathways such as Vitamin digestion and absorption, Cysteine and methionine metabolism, Vitamin B6 metabolism, and Pyrimidine metabolism as the dominant differential metabolic pathways.
Cholecalciferol, also known as Vitamin D, plays a multitude of crucial roles in the human body, including regulation of calcium and phosphorus metabolism and bone health[
16]. Adequate levels of Vitamin D are essential for maintaining bone strength and preventing osteoporosis. As age advances, the body's requirement for Vitamin D increases, as it helps maintain skeletal health, and deficiency is associated with an increased risk of osteoporosis and fractures in the elderly[
17]. Previous studies have suggested that Vitamin D may be related to various physiological processes, including immune function, cell growth and differentiation, and endocrine regulation, all of which are closely related to the aging process[
18]. Therefore, maintaining appropriate Vitamin D levels may help delay certain aspects of aging, such as the decline in muscle strength and cognitive function[
19]. Aging is a natural and inevitable process of molecular and cellular damage accumulation, leading to functional deficits in cells, tissues, and entire organs, thereby weakening the entire body[
20]. With the decline in overall immune capacity during aging, the relative number of senescent immune cells decreases[
21], reducing immune surveillance, such as the detection and destruction of tumor cells by cytotoxic T cells[
22]. Individuals with low immune function have a significantly higher risk of cancer than those with high immune function[
23]. The cancer-protective effect of adequate Vitamin D status may be primarily related to the ability to maintain a high level of immune capacity[
24]. Additionally, Souraya et al. reported that Vitamin D can enhance immunity during the aging process by partially blocking p38 MAPK signaling, thereby inhibiting the production of SASP (senescence-associated secretory phenotype) in senescent cells[
25]. Our study found that Cholecalciferol also shows an increasing trend with age, which is opposite to human aging, suggesting that its sufficiency is an essential component of healthy aging, not only helping to maintain the good condition of bones and skeletal muscles but also contributing to the homeostasis of the immune system, consistent with the views of Fantini et al.[
26].
L-Methionine sulfoxide (MetO) is a form of protein that has undergone a change in function and structure due to oxidation by reactive oxygen species (ROS) and hydrogen peroxide when exposed to methionine and methionyl residues under physiological or pathological conditions. In mammals, oxidative stress is usually accompanied by an inflammatory response, generating ROS and other molecules that cause tissue damage, report indicated L-Methionine sulfoxide can promote the initiation of inflammatory responses[
27]. Lee et al. proposed that the reduction of L-Methionine sulfoxide promotes mitophagy, which is involved in the process of mitophagy[
28]. Excessive ROS can damage mitochondria, and mitophagy can remove damaged mitochondria, protecting cells from apoptosis. Furthermore, the role of L-Methionine sulfoxide in health and disease has been studied in various organisms from bacteria to mammals, including humans. For example, in mammals, elevated levels of MetO are involved in the expression of markers related to neurodegenerative diseases[
29], mental health disorders[
30], hearing loss[
31], cystic fibrosis lung disease[
32], macular degeneration[
33], cardiovascular diseases[
34], liver and kidney toxicity[
35], and cancer[
36]. Our study shows that L-Methionine sulfoxide also exhibits an increasing trend with age. Catanesi et al. found that a diet rich in L-Methionine may demonstrate therapeutic potential for human aging and age-related diseases, protecting neurons from oxidative imbalance and maintaining mitochondrial function[
37]. Therefore, we believe that L-Methionine sulfoxide increases the risk of neurodegenerative diseases, including AD, and negatively regulates mitophagy, thereby accelerating cellular aging and apoptosis[
38].
Aging and age-related diseases, such as diabetes, atherosclerosis, and neurodegenerative diseases, are characterized by an increase in oxidative chemical modification of tissue proteins[
39]. Glyoxal or advanced glycation end-products (AGEs) are produced by secondary modification of proteins by carbohydrate oxidation products and are associated with the severity of diabetic complications in the kidneys, retina, and blood vessels[
40]. Onorato et al. provided insights into the mechanism of action of Pyridoxamine as an AGEs inhibitor and suggested that Pyridoxamine may help inhibit the increased chemical modification of tissue proteins in diabetes, atherosclerosis, and other chronic diseases, thereby alleviating age-related diseases[
41]. Additionally, Pyridoxamine reduces superoxide radicals produced by H2O2, possibly through a scavenging mechanism and reducing lipid peroxidation in cells[
42]. Our data indicate that Pyridoxamine shows a declining trend with age, thereby increasing the likelihood of diseases such as diabetes during the aging process. The reduction in its content increases the production of free radicals, accelerating the aging process.
According to the KEGG analysis based on differential metabolites, pyrimidine metabolism is a significant differential profile common to all three groups. Cytidine-5-monophosphate, a product in its metabolic process, is a nucleotide within the cell that participates in many important biochemical processes, including nucleic acid synthesis, phospholipid metabolism, and phosphorylation reactions[
43], playing an important role in the immunity of some adults, infants, and young mammals[
44]. Notably, Nakagawara et al. reported that Cytidine-5-monophosphate enhances the expression of PGC-1α in mouse C2C12 cells and promotes the formation of myotubes[
45], and PGC-1α can protect skeletal muscles and alleviate the occurrence of muscle atrophy[
46], which may suggest a molecular mechanism for muscle atrophy during the aging process in humans or animals. During the aging process, a series of changes occur in cellular metabolism and function, which may affect the level and role of Cytidine-5-monophosphate. However, there is currently no direct evidence to suggest that Cytidine-5-monophosphate itself promotes or delays the aging process. Our study indicates that Cytidine-5-monophosphate consistently shows a declining trend with age, suggesting its potentiality as a biomarker involved in the biological aging process, although its specific mechanism remains to be investigated.