1. Introduction
It is established that nine isoforms, specifically NaV1.1-1.9 (or SCN1A-SCN5A and SCN8A-SCN11A), of voltage-gated Na+ (NaV) channels are distributed in mammalian excitable tissues, encompassing the central or peripheral nervous system, as well as the endocrine or neuroendocrine system (Catterall, 2012; de Lera Ruiz and Kraus, 2015; Shen et al., 2017; Bennett et al., 2019). These NaV channel proteins in eukaryotes have a single subunit, each containing four six-transmembrane pseudomains (Shen et al., 2017).
Upon rapid depolarization, NaV channels, which encode macroscopic voltage-gated Na+ currents (INa), undergo swift transitions from the resting (closed) state to the open state. Subsequently, there is a rapid shift to the inactivated state of the channel (Catterall, 2012; de Lera Ruiz and Kraus, 2015). The inactivation of INa can also accumulate before being triggered during repetitive brief depolarizing pulses (Taddese and Bean, 2002; Carter and Bean, 2011; Huang et al., 2015; Navarro et al., 2020). Once evoked, the increased magnitude of INa can depolarize the cell membrane through a positive feedback cycle, thereby eliciting the upstroke of the action potentials (APs) and governing the amplitude, frequency, and/or pattern of AP firing in different excitable cells such as sensory neurons (Catterall, 2012; de Lera Ruiz and Kraus, 2015; Bennett et al., 2019).
Tefluthrin (Tef) is a synthetic type-I pyrethroid insecticide that is characterized by the presence of a cyano group at the α-position of the alcohol moiety. It is recognized as an activator of INa and a slowing in current inactivation (So et al., 2018; Lin et al., 2022). Permethrin or λ-cyhalothrin, another pyrethroid, has been previously noticed to influence pain signaling (Jiang et al., 2013; Silwal et al., 2023). Telmisartan is a medication belonging to the class of angiotensin II receptor blockers. Despite its primary therapeutic effects on cardiovascular function, there is some evidence to reflect that Tel has additional effects on pain signaling (Hegazy et al., 2020). Whether the presence of Tef or Tel alters the amplitude and gating kinetics of INa in sensory or dorsal root ganglion (DRG) neurons is unclear. Alternatively, KB-R7943, an inhibitor of Na+-Ca2+ exchange process (Schröder et al., 1999; Amran et al., 2003), has been reported to cause antinociceptive effect in neuropathic pain model (Huang et al., 2019; Andreeva-Gateva et al., 2024). However, how KB-R7943 can cause any perturbations on INa linked to nociceptive signaling is unknown.
Therefore, the primary aim of this study was to examine the impact of three compounds―Tef, Tel, and KB-R7943―on cultured dorsal root ganglion (DRG) neurons. Specifically, the investigation focused on determing whether and how these compounds affect the magnitude and gating kinetics of INa. Additionally, a computational model of an interneuron, originally derived from the spinal dorsal horn (Ma et al., 2023), was utilized to investigate the influence of variations in INa inactivation and conductance, both individually and in combination, on dynamic changes in membrane potential. This investigation also employed the generation of bifurcation diagrams (White et al., 1995; Enns-Ruttan and Miurea, 2000; Chang et al., 2018).
2. Materials and Methods
2.1. Chemicals, Reagents, and Solutions Used in This Study
KB-R7943 (2-[2-[4-(4-nitrobenzyloxy)phenyl]ethyl]isothiourea, 2-[4-[(4-nitrophenyl)methoxy]phenyl]ethyl ester carbamimidothioic acid, C16H17N3O3S·CH3SO3H) was acquired from Cayman (Excel Biomedical, Tainan, Taiwan). Ranolazine (Ranexa®) and telmisartan (Micardis®, Tel) were supplied by Tocris (Bristol, UK), while tefluthrin (Tef), tetraethylammonium chloride (TEA), and tetrodotoxin (TTX) were by Sigma (Merck, Taipei, Taiwan).
The extracellular solution, specifically HEPES-buffered normal Tyrode’s solution, had the following compositions (mM): NaCl 136.5, KCl 5.4, CaCl2 1.8, MgCl2 0.53, glucose 5.5, and HEPES 5.5 (pH 7.4). In order to assess macroscopic K+ currents or membrane potential, we utilized a recording electrode filled with a solution containing (in mM): K-aspartate 130, KCl 20, MgCl2 1, Na2ATP 3, Na2GTP 0.1, EGTA 0.1, and HEPES 5 m (pH 7.2). For recording INa, K+ ions within the intracellular solution were substituted with equimolar Cs+ ions, and the pH was then adjusted to 7.2 with CsOH.
2.2. Cell Preparation
The rat dorsal root ganglion (DRG) neurons (neonatal) were high-quality sensory neuron suspension acquired from Lonza Walkersville, Inc. (R-DRG-505; Walkersville, MD) available at
https://bioscience.lonza.com/lonza_bs/TW/en/document/27607 (accessed on 15 July 2024). Following the recommended conditions, the cells were cultured using the PNBM
TM BulletKit
TM (Lonza), which includes a 200 ml bottle of primary neuron basal medium (PNBM
TM) and PNGM
TM SingleQuots
TM. The cells were incubated at 37 °C in monolayer cultures within a humidified environment containing 5% CO
2/95% air. Experiments were conducted five or six days after subculturing the cells, at a confluence of 60-80%.
2.3. Electrophysiological Measurements
Before the experiments, we dispersed DRG neurons using a 1% trypsin/EDTA solution. A small volume of cell suspension was transferred to a specially crafted chamber affixed on the stage of an inverted microscope. Cells were bathed at room temperature (20-25 °C) in the standard Tyrode’s solution. Prior to each measurement, the cells were allowed to settle at the chamber’s bottom. The patch pipettes were fashioned from Kimax®-51 borosilicate glass tube (#DWK34500-99; Kimble®, Merck, Tainan, Taiwan) and polished to attain a resistance ranging between 2 and 4 MΩ. Recordings of INa or other ionic currents were conducted in the whole-cell mode using a modified patch-clamp technique, employing an RK-400 amplifier (Bio-Logic, Claix, France) (Wu and Yu, 2023).
To nullify liquid junction potentials resulting from disparities between the composition of the pipette solution and that of the bath, adjustments were made before giga-Ω formation, and subsequent corrections were applied to the whole-cell data. The capacitive transients induced during
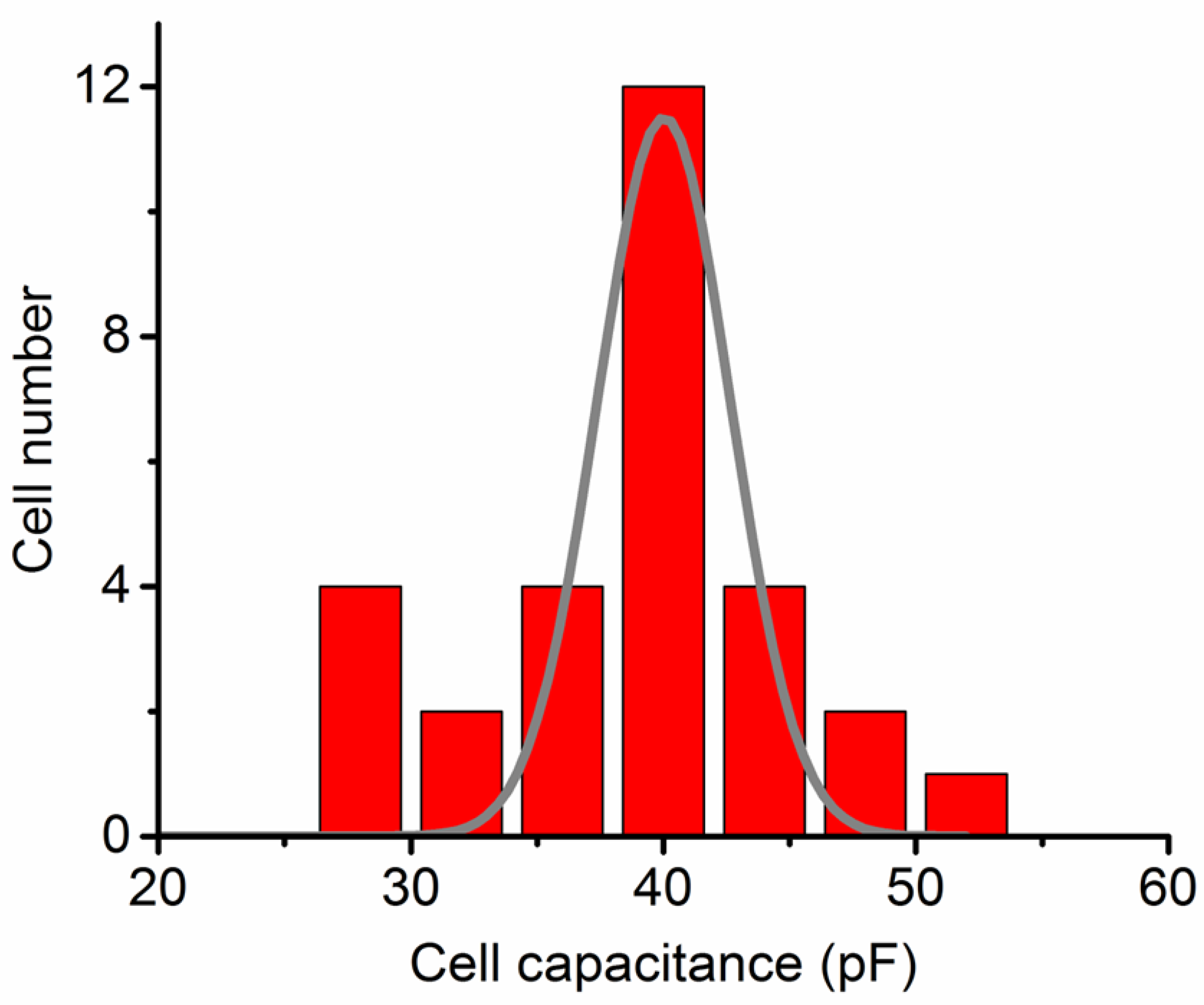
INa elicitation were counteracted by applying a hyperpolarizing pulse of equal magnitude. Cell-membrane capacitance of 28-49 pF (37.3 ± 5.6 pF; n = 29) was compensated.
Figure 1 illustrates a histogram showing the relationship between cell number and cell capacitance.
2.4. Data Recordings
The data were stored online on a laptop computer at 5 kHz or higher. The computer was equipped with Digidata® 1440A device (Molecular Devices; Bestgen Biotech, New Taipei City, Taiwan), facilitating analog-to-digital and digital-to-analog conversions controlled by pCLAMP® 10.6 (Molecular Devices). Off-line analyses of the acquired signals were performed using various analytical tools, including OriginPro® 2021 (OriginLab Corp.; Scientific Formosa, Kaohsiung, Taiwan), and custom-made macros developed in Excel® 2022 (Redmond, WA).
2.5. Curve-Fitting Procedures for INa Inactivation Time Course
The decay of the inactivation time course of
INa is elucidated through a family of exponential functions. This family can be defined as
Here, y(t) symbolizes the inactivation trajectory of INa over time, and the index i commonly takes on two values corresponding to the fast and slow components in the observed inactivation of INa. The nonlinear curve-fitting was performed to extract the parameters for the fast (τinact(F)) and slow component (τinact(S)) of the inactivation time constant (τinact).
2.6. Statistical Analyses
The experimental results are expressed as the means±SEM with sample sizes (n) indicating the cell number from which the data were taken. The paired or unpaired Student’s t-test and one- or two-way analysis of variance (ANOVA) followed by post-hic Fisher’s least-significant difference test were made for the evaluation of differences among means. Statistical analyses were performed using IBM SPSS Statistics 24.0 (Armonk, New York). The significance was determined at a P value of <0.05.
2.7. Computer Simulations
To explore the influence of changes in the amplitude and/or inactivation characteristics of
INa on action potential (AP) firing related to pain signaling, we utilized a theoretical model of AP firing derived previously (Ma et al., 2023). This model is based on the biophysical properties of parvalbumin-expressing interneurons (PVINs) situated within the dorsal horn of the spinal cord. Voltage-gated Ca
2+ current with concurrent changes in intracellular Ca
2+ concentrations ([Ca
2+]
i) were also included in the model (Brown, 2003; Ma et al., 2023). The detailed descriptions of the modeled neuron were provided previously (Ma et al., 2023). The mathematical model is given by the following:
To simulate changes in the rate of
INa inactivation, we mathematically constructed a modified Hodgkin-Huxley (HH) type model. The detailed framework utilized in this work was previously described (Ma et al., 2023), and the biophysical characteristics of
INa were noted to closely resembled those from earlier studies (Wu et al., 2015; Ghovanloo et al., 2024). For
INa, the modified HH scheme was used according to the following equation:
The rate constants for
INa activation used in the simulation are described by the following equations (Traub and Miles, 1991; Ma et al., 2023):
The inactivation variable
h in the paper of Ma et al. (2023) satisfies the dynamic equation
where
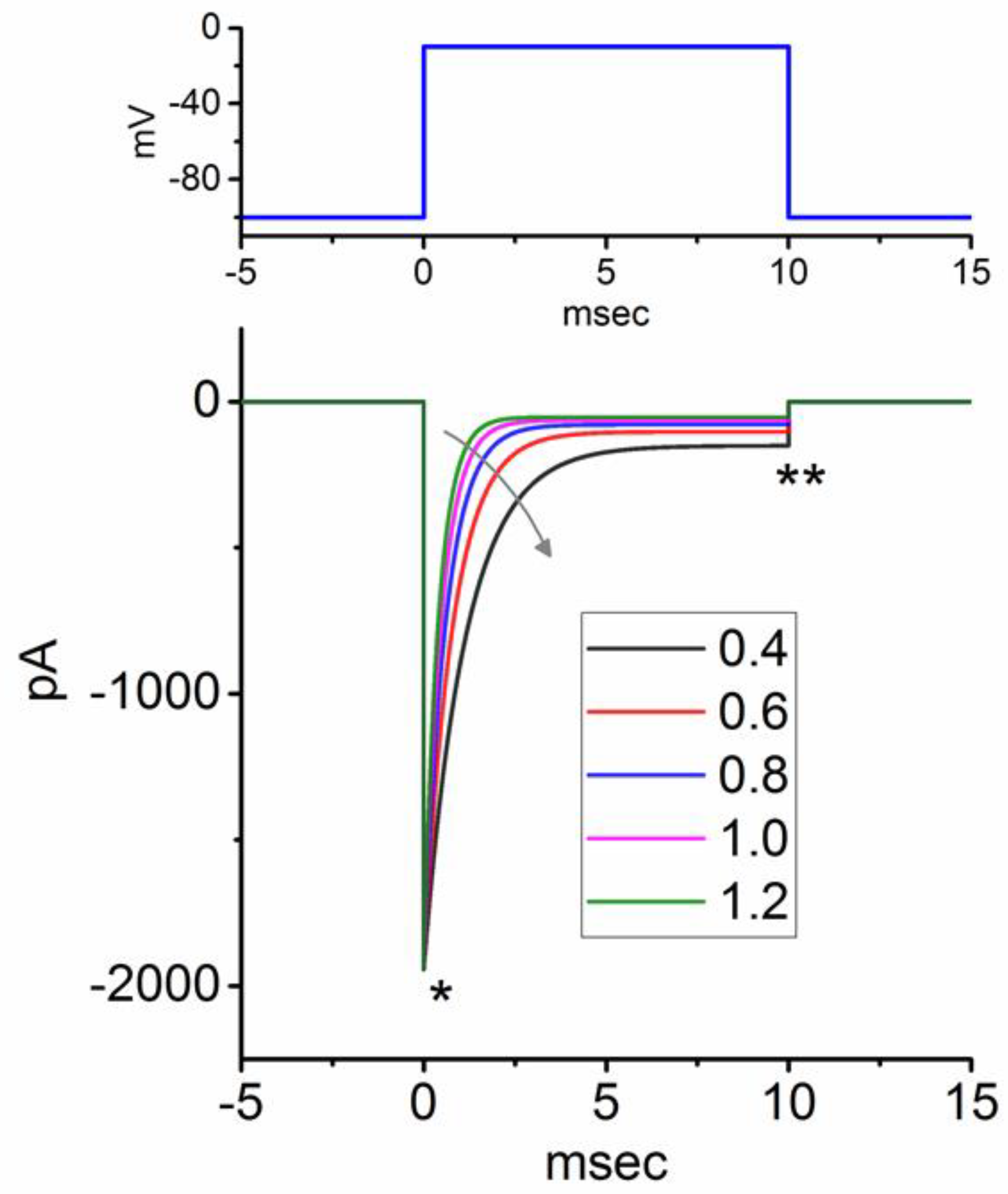
Moreover, an arbitrarily incorporated adjustable βh-inactivation parameter of INa, represented as ϕ, was integrated into the simulated neuron to mimic how variations in the inactivation time course of INa and current conductance (gNa) can influence the patterns of subthreshold oscillation (SO) or AP firing. This, in turn, leads to modifications in the bifurcation diagram through AUTO implementation (Winslow, 1989; White et al., 1995; Enns-Ruttan and Miura, 2000; Chang et al., 2018).
In particular, the inactivation parameter of
INa used for mimicking the increase or decrease in current inactivation is reformulated by
where
h is inactivation gating variable, α
h and β
h are the rate constants for inactivation gating variable, and the parameter ϕ represents the magnitude of Na
V-channel inactivation embedded in the β
h inactivation component. As the value of ϕ is decreased, the time course of Na
V channel inactivation elicited by membrane depolarization becomes slowed.
For detailed information on other onic currents involved in simulated changes in membrane potential, such as voltage-gated Ca2+ current, KV1.3 K+ current, KV3.1 K+ current, and small-conductance Ca2+-activated K+ current., please refer to the previous work by Ma et al., 2023.
2.8. Numerical Simulation Technique Employed in This Study
The XPPAUT simulation package, accessible at
https://sites.pitt.edu/~phase/bard/bardware/xpp/xpp.html (accessed on 2023, Dec 27), was utilized to conduct analyses related to linear stability and bifurcation. The term “bifurcation” refers to points in parameter space where the system undergoes a qualitative change in its dynamics, leading to the emergence of new solutions or the alteration of existing ones (Winslow, 1989; White et al., 1995). XPPAUT, a software tool for simulating and analyzing dynamical systems, provides a platform for conducting bifurcation analyses. The AUTO function in XPPAUT refers to a utility within the software that is used for bifurcation analysis (Winslow, 1989; White et al., 1995; Enns-Ruttan and Miura, 2000; Chang et al., 2018).
4. Discussion
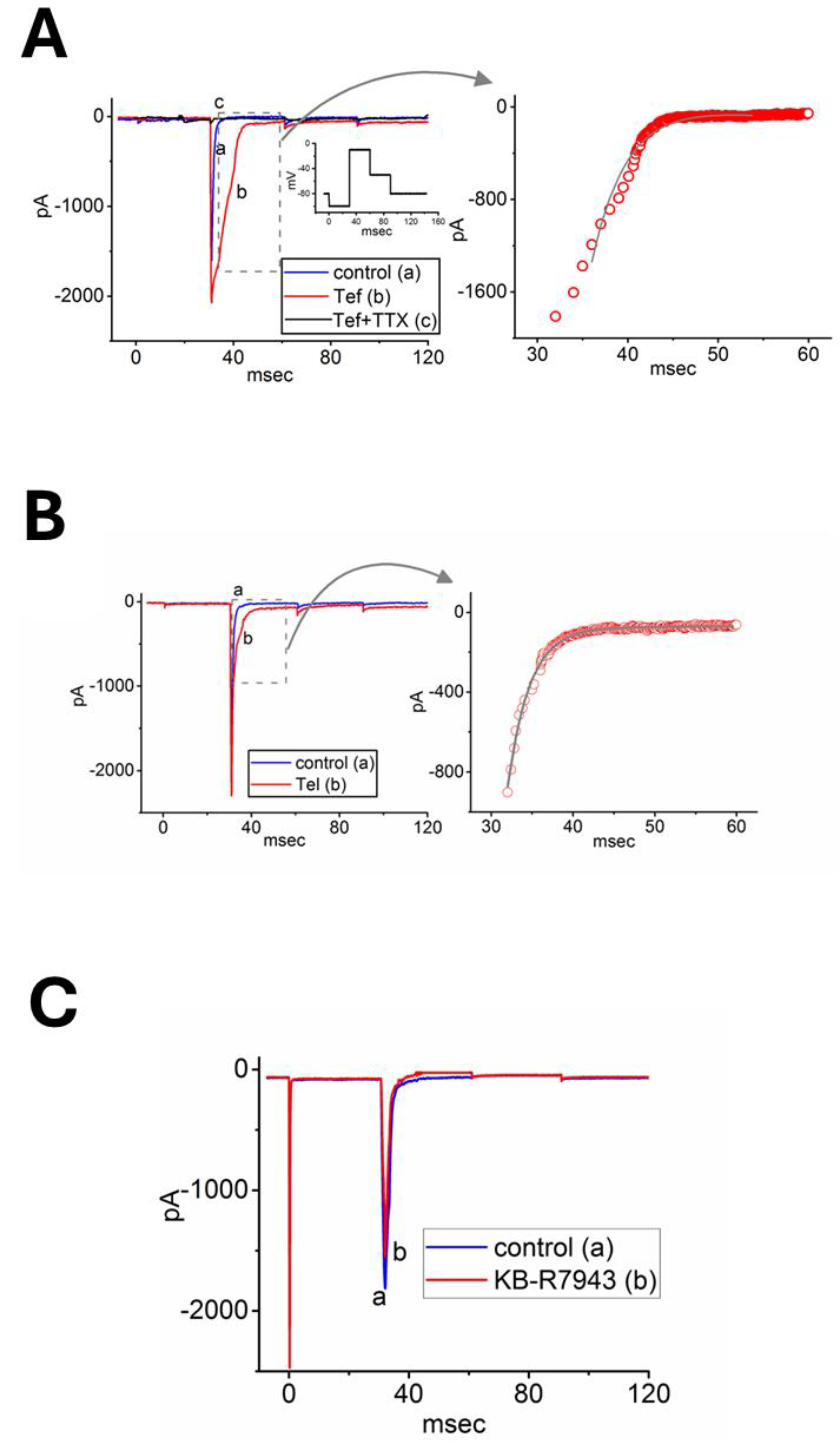
The results of this study demonstrate that in the presence of tefluthrin (Tef), telmisartan (Tel), or KB-R7943, the amplitude and gating of voltage-gated Na+ current (INa) in cultured dorsal root ganglion (DRG) neurons was affected. Tef or Tel increased the amplitude of INa while slowing down the inactivation time course of this current. The impact of Tel on INa is related to its effect on NaV channels and is not associated with its antagonistic action against angiotensin II receptors (Chang et al., 2018; Hegazy et al., 2020). Furthermore, in addition to suppression of the Na+-Ca2+ exchanging process (Schröder et al., 1999; Amran et al., 2003), KB-R7943 also inhibited the magnitude of INa while simultaneously accelerating the inactivation rate of INa in cultured DRG neurons.
This study shows that the presence of
INa can be detected in cultured DRG neurons. However, these
INa currents are blocked by tetrodotoxin (TTX). In these cells, we did not observe clear TTX-resistant (TTX-R)
INa (Roy and Narahashi, 1992; Lee et al., 2003). The Na
V1.6, Na
V1.7, Na
V1.8, and Na
V1.9 isoforms were noticed to be expressed in DRG neurons. It is thus possible that the Na
V1.8 expression in these cells (R-DRG-505 cells) could be relatively low (Szulczyk et al., 2022). However, we did observe that the current was enhanced by Tef or Tel and inhibited by KB-R7943, as reported previously (Kim et al., 2012; Chang and Wu, 2018; Chang et al., 2018; Lai et al., 2020; Lu and Wu, 2023). Additionally, the inactivation time course of these currents was also altered by these drugs (
Figure 1). Our in silico study proposed that the inactivation time course of
INa (indicated by the variable ϕ value), which is activated at depolarized potentials, contribute to the presence, amplitude and frequency of subthreshold oscillation (SO) and high-frequency spiking (SS) (White et al., 1995; Schweighofer et al., 1999; Taddese and Bean, 2002; Lee et al., 2003).
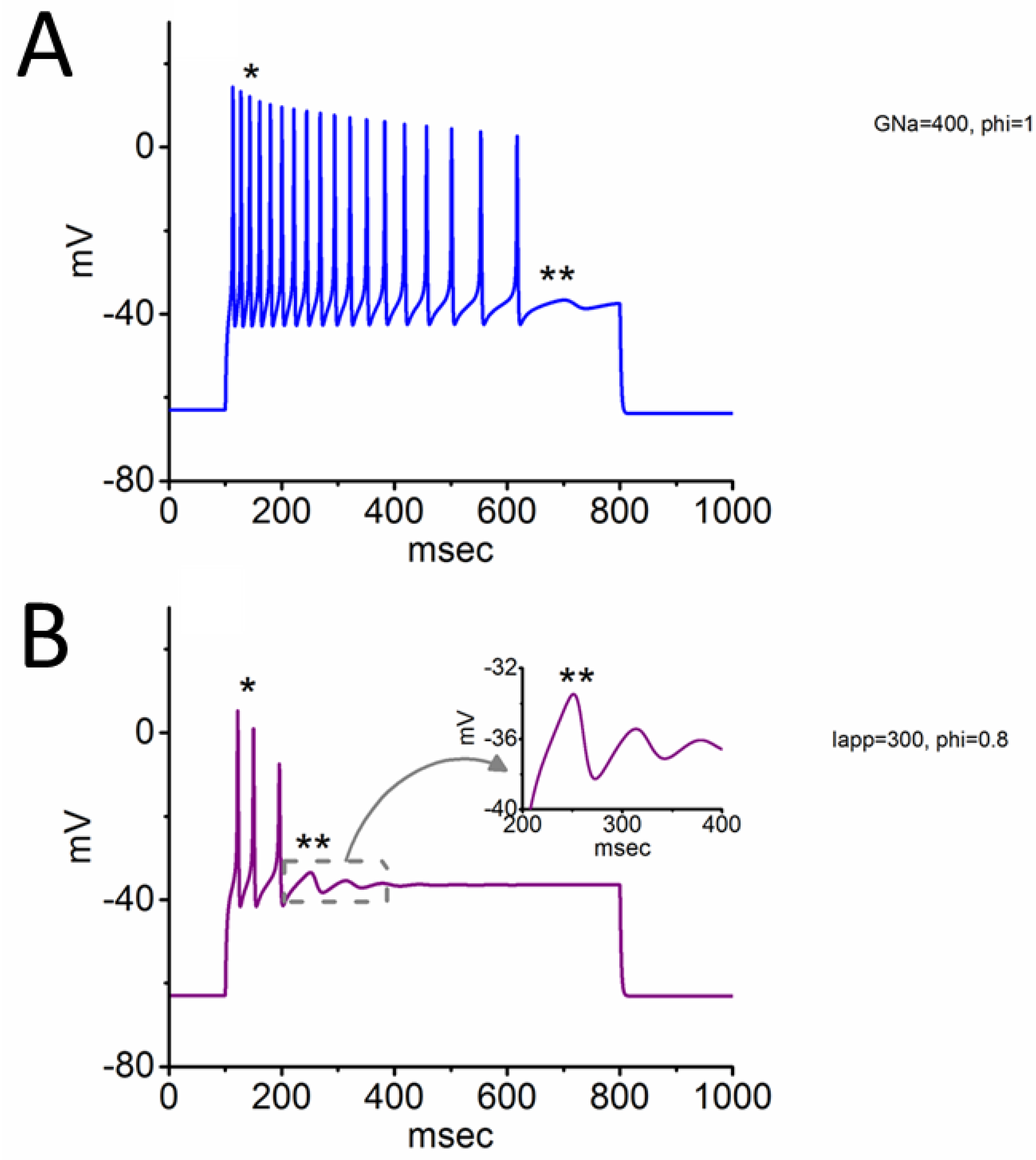
In our cultured DRG neurons, namely R-DRG-505 cells, we employed current-clamp voltage recordings to observe changes in membrane potential. However, we were unable to detect high-frequency spiking. Even in the presence of Tef or Tel, high-frequency spiking did not manifest. However, through our investigations using modeled interneurons in spinal dorsal horn (Ma et al., 2023), we found that enhancing the INa and slowing down the inactivation time course can simulate the exposure to Tef or Tel. The simulations suggest that these modeled neurons can generate SO and HS. Furthermore, when subjected to strong applied currents, HS with a rate of about 150 Hz was found to develop. The theoretical analyses of these scenarios are presented in the bifurcation diagrams provided herein.
In the modeled interneuron of this study, there were no parameters for the Na
+-Ca
2+ exchanging process. However, with the generation of AP firing, the level of [Ca
2+]
i also increased, as demonstrated in
Figure 6C. Therefore, at least in excitable cells such as neuronal cells, changes in the magnitude of
INa or the inactivation time course of the current might affect the level of [Ca
2+]
i without the need for the participation of the Na
+-Ca
2+ exchanging process. Indeed, KB-R7943, an inhibitor of Na
+-Ca
2+ exchanging process (Amran et al., 2003; Huang et al., 2019), was reported to inhibit the
INa responsible for these effects (Wu and Yu, 2023).
Previous reports have indicated that ranolazine, a blocker of late INa, has an inhibitory effect on the INa in DRG neurons, concurrently demonstrating relief in painful sensations (Gould et al., 2014; Wu et al., 2015; Gould and Diamond, 2016). This study confirms this phenomenon and additionally reveals that KB-R7943, an inhibitor of Na+-Ca2+ exchanging process, can inhibit INa magnitude while accelerating the inactivation time course of the INa, as described recently (Wu and Yu, 2023). Therefore, KB-R7943 may alleviate a certain type of pain signaling (Huang et al., 2019). Furthermore, further investigations are needed to explore how Tef or Tel influences the biophysical properties of INa in DRG neurons or sensory neurons associated with pain signaling, consequently modulating the nociceptive transmission (Jiang et al., 2013; Castellanos et al., 2018; Karádi et al., 2023; Silwal et al., 2023).
The results of this study demonstrated that when the INa in DRG neurons increased and its inactivation time course simultaneously slowed down, as mimicked by the presence of Tef or Tel, the cell membrane of these neurons is prone to generating the occurrence of SO. Moreover, excessive stimulation with strong electrical currents easily induced the HS emergence. Conversely, when INa decreased and the inactivation time course accelerated (as mimicked by cell exposure to KB-R7943), these cells were less likely to exhibit the pattern of SOs and even resulted in the HS development (White et al., 1995; Schweighofer et al., 1999; Chen et al., 2010; Ma et al., 2023). Therefore, alterations in the biophysical properties of INa impact the cell excitability of DRG neurons in vivo, subject to biophysical, pharmacological, or toxicological modulation of INa.
The modeled neuron used in this study includes two types of different delayed-rectifier K+ currents, namely KV1.3 and KV3.1 currents (Ma et al., 2023). Previous reports have indicated that changes in the magnitude and inactivation process of these K+ currents significantly impact the HS emergence (Klemic et al., 2001; Lin et al., 2008; Wang et al., 2008; Wu et al., 2008; Hsiao et al., 2022). KCNQ channels have been also reported to enable reliable presynaptic spiking occurring at high frequency (Zhang et al., 2022). Therefore, further investigations are needed to understand how the modulation of these K+ currents may have effects on the HS emergence in the presence of Tef or Tel.
In this study, we employed a theoretical model of action potential (AP) firing, which was adapted from a previous study by Ma et al. (2023). This model is based on the biophysical properties of parvalbumin-expressing interneurons (PVINs) located in the dorsal horn of the spinal cord. Although there are some differences between this model neuron and the dorsal root ganglion neuron, both of these types of neurons contain abundant NaV channels. We thus believe that it is valuable to investigate how changes in the amplitude and/or inactivation characteristics of INa affect the patterns of AP firing related to pain signaling. However, the effects of these compounds on the AP firing in dorsal root ganglion neurons occurring in vivo still require further analysis and research.
Although our study found that TTX-R INa was not detected in cultured DRG neurons (R-DRG-505), it is established that nociceptive DRG neurons (≤30 μm in a diameter) express TTX-R NaV channels, such as NaV1.8 and NaV1.9, and that previous studies have emphasized the role of these channels in nociceptive transmission (Wolf and Ma, 2007; Szulczyk et al., 2022; Nascimento de Lima et al., 2024). However, the modified PVINs in lamina III of the spinal cord are very heterogeneous and mainly express NaV1.1, NaV1.2, and NaV1.6, and they do not express NaV1.7 abundant in nociceptive DRG neruons (Fukuoka et al., 2010; Petitjean et al., 2015; Gradwellet al., 2022). Nevertheless, the effects of three compounds on TTX-R NaV channels should be tested, possibly using native DRG neurons or other types of spinal neurons linked to pain transmission.
Plasma Tel concentration after intravenous administration of a single dose of 40 mg Tel was noted to reach about 2.32 μM (Stangier et al., 2000). As for Tel, it is a pyrethroid insecticide. Its concentration in the blood of organisms varies greatly, reaching up to several micromolar levels. After intravenous administration, KB-R7943 reached a concentration of about 0.12 μM (Miyata et al., 2002), which is lower than the concentration used in this study. However, recent reports have found that KB-R7943 can cross the blood-brain barrier, with concentrations in brain tissue reaching 5 to 10 times higher (Andreeva-Gateva et al., 2024).
The effects of these compounds on the dorsal root ganglia may extend beyond the necessity of the blood-brain barrier. However, in nociceptive transmission, the dorsal root ganglia is one of the first primary neuron; the nerve transmission also needs to reach the dorsal horn of the spinal cord, then pass and conduct through long fibers upward to the medulla oblongata, further up to the thalamus, and then to the cerebral cortex. In these regions, the blood-brain barrier is often present. Therefore, if these compounds, such as telmisartan, tefluthrin, or KB-7943, can easily pass through the blood-brain barrier across those areas, it is believed that they could have a regulatory effect on these pain-linked transmission pathways. Furthermore, neurons of the dorsal horn of the spinal cord, including parbalbumin-expressing interneurons, were reported to exhibit greater heterogeneity than DRG neurons (Gradwell et al., 2022). Nevertheless, the effects of Tel, Tef, or KB-R7943 may have implications for biological activity in the nervous system (So et al., 2018; Lin et al., 2022). Among these effects, the regulation of ion channels in the cell membrane might be one of the important mechanisms.
Of note, the model used in the current study is not perfect. Indeed, there are many different formulations for Na
+ currents. The simulated firing of neuronal action potentials derived from dirrent regions linked to pain signaling can also be present in various possible forms, as detailed on the ModelDB website (
http://modeldb.sicence/, accessed on 14 July 2024).
To ensure our experimental results are more substantial and effective in presentations, and to observe potential action potential firing patterns, we further explored this through bifurcation analysis. We indeed spent a significant amount of time searching for developments of various types of simulation models. Thus, we found the model developed by Ma et al. (2023) in PVINs to be more suitable for our needs. However, we still believe it is not perfect, and in the future, we will continue to develop more suitable computational models so that the experimentally observed and theoretical results can be more consistent.
Tefluthrin has previously been reported to inhibit voltage-gated Ca2+ current and mildly suppress delayed-rectifier K+ currents (Wu et al., 2009). Additionally, telmisartan has been shown to suppress erg-mediated K+ currents (Chang and Wu, 2018). However, there are no reports indicating that KB-R7943 affects K+ currents. This paper focuses solely on the changes these compounds induce in the amplitude and gating of Na+ currents. Further analysis is required to understand the effects of these agents on other ion currents, such as K+ and Ca2+ currents, is necessary. Therefore, additional research is necessary to provide further clarification in this area.
Figure 1.
Histogram depicting the relationship between cell number and cell capacitance. Each red bar represents the number of cells used in this study. A smooth line overlaid on the histogram illustrates the Gaussian distribution used for curve fitting.
Figure 1.
Histogram depicting the relationship between cell number and cell capacitance. Each red bar represents the number of cells used in this study. A smooth line overlaid on the histogram illustrates the Gaussian distribution used for curve fitting.
Figure 2.
Effects of tefluthrin (Tef), telmisartan (Tel), and KB-R7943 on voltage-gated Na+ current (INa) in rat dorsal root ganglion (DRG) neurons using whole-cell current recordings. These experiments were conducted in Ca2+-free Tyrode’s solution containing CdCl2 (0.5 mM) and tetraethylammonium chloride (TEA, 10 mM). Panel (A), (B), or (C) displays INa traces under the conditions: in the absence (a, blue color) and in the presence (b, red color) of Tef (10 μM), Tel (10 μM), or KB-R7943 (10 μM), respectively. Inset in the left side of panel (A) indicates the voltage-clamp protocol applied. The right graph of panels (A) and (B) shows an expanded view of the dashed box from the left side, with the red data points fitted to a two-exponential function (gray curve). Please note that in pane (A), the current trace labeled c (black color) indicates further addition of tetrodotoxin (TTX, 1 μM), in the continued presence of Tef (10 μM).
Figure 2.
Effects of tefluthrin (Tef), telmisartan (Tel), and KB-R7943 on voltage-gated Na+ current (INa) in rat dorsal root ganglion (DRG) neurons using whole-cell current recordings. These experiments were conducted in Ca2+-free Tyrode’s solution containing CdCl2 (0.5 mM) and tetraethylammonium chloride (TEA, 10 mM). Panel (A), (B), or (C) displays INa traces under the conditions: in the absence (a, blue color) and in the presence (b, red color) of Tef (10 μM), Tel (10 μM), or KB-R7943 (10 μM), respectively. Inset in the left side of panel (A) indicates the voltage-clamp protocol applied. The right graph of panels (A) and (B) shows an expanded view of the dashed box from the left side, with the red data points fitted to a two-exponential function (gray curve). Please note that in pane (A), the current trace labeled c (black color) indicates further addition of tetrodotoxin (TTX, 1 μM), in the continued presence of Tef (10 μM).
Figure 3.
Simulated INa traces in response to short depolarization from -100 to -10 mV for 10 msec. Detailed profile was described in the Materials and Methods section and a previous paper (Ma et al., 2023). INa traces were created when the ϕ value in the inactivation process (βh) of h variable decreased from 1.2 to 0.4 with 0.2 decrements (as indicated by curved arrow). The term “βh” in the HH kinetics refers to the rate at which the NaV channel undergoes inactivation in the h gating variable. * and ** represent the transient and late components of INa activated by an abrupt depolarizing pulse. The upper part illustrates the voltage-clamp protocol applied.
Figure 3.
Simulated INa traces in response to short depolarization from -100 to -10 mV for 10 msec. Detailed profile was described in the Materials and Methods section and a previous paper (Ma et al., 2023). INa traces were created when the ϕ value in the inactivation process (βh) of h variable decreased from 1.2 to 0.4 with 0.2 decrements (as indicated by curved arrow). The term “βh” in the HH kinetics refers to the rate at which the NaV channel undergoes inactivation in the h gating variable. * and ** represent the transient and late components of INa activated by an abrupt depolarizing pulse. The upper part illustrates the voltage-clamp protocol applied.
Figure 4.
Two distinct spiking patterns simulated from the modeled neuron. When a current injection (700 msec in duration) was applied to this modeled neuron, two different spike patterns emerged, namely somatic spike (SS) and subthreshold oscillation (SO). In panel (A), gNa = 400 nS, Iapp = 300 pA, ϕ = 1.0. In panel (B), gNa = 300 nS, Iapp = 300 pA, ϕ = 0.8. The symbols * and ** represent the occurrence of SS and SO, respectively. The inset in panel (B) is a magnified view from the dashed box in (B), highlighting the SO emergence.
Figure 4.
Two distinct spiking patterns simulated from the modeled neuron. When a current injection (700 msec in duration) was applied to this modeled neuron, two different spike patterns emerged, namely somatic spike (SS) and subthreshold oscillation (SO). In panel (A), gNa = 400 nS, Iapp = 300 pA, ϕ = 1.0. In panel (B), gNa = 300 nS, Iapp = 300 pA, ϕ = 0.8. The symbols * and ** represent the occurrence of SS and SO, respectively. The inset in panel (B) is a magnified view from the dashed box in (B), highlighting the SO emergence.
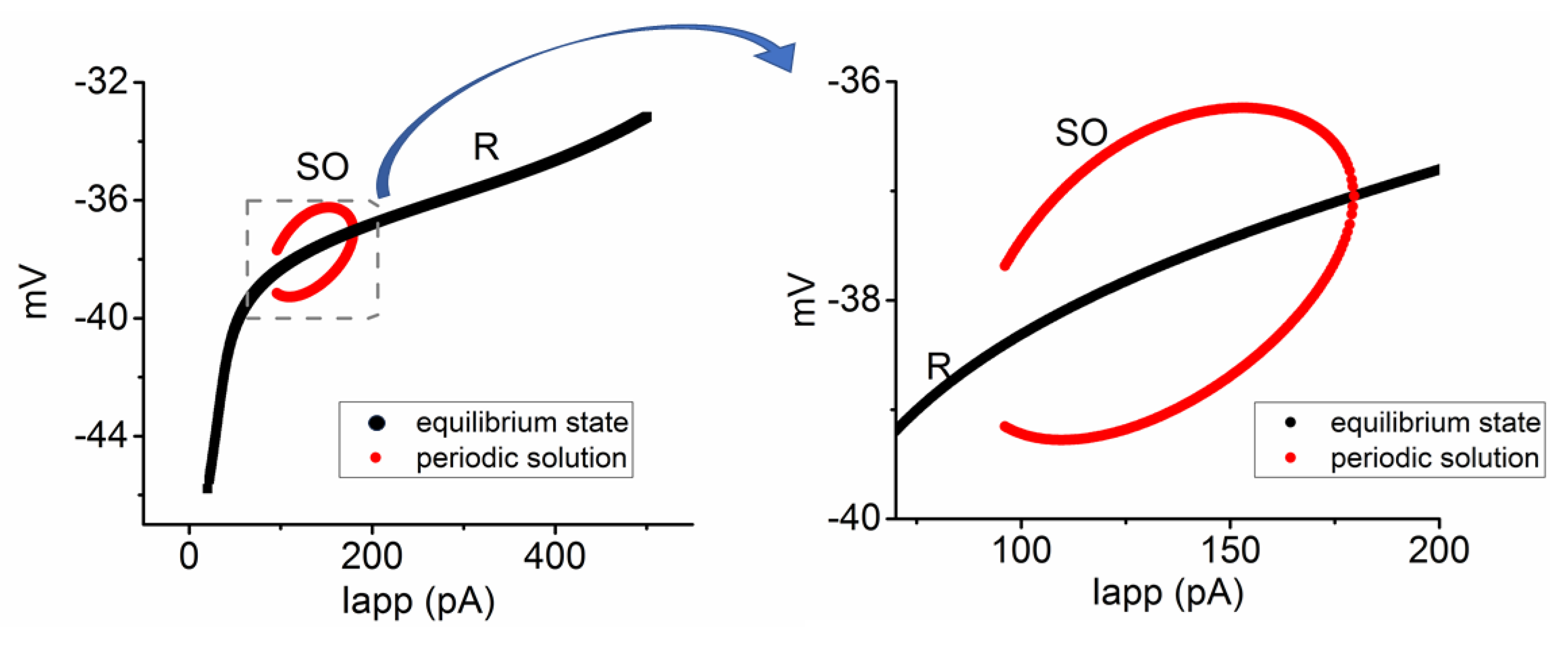
Figure 5.
Bifurcation diagram illustrating the relationship between Iapp and alterations in membrane potential in the modeled neuron. These simulations were conducted using default parameters detailed in
Table 1, with a ϕ value of 1.0. Two types of solutions in the system were identified. That is, one is the resting, steady-state, or equilibrium state (R, black color), while the other is the unstable and periodic solution indicative of subthreshold oscillation (SO, red color). The enlarged graph on the right corresponds to the highlighted region within the dashed box on the left.
Figure 5.
Bifurcation diagram illustrating the relationship between Iapp and alterations in membrane potential in the modeled neuron. These simulations were conducted using default parameters detailed in
Table 1, with a ϕ value of 1.0. Two types of solutions in the system were identified. That is, one is the resting, steady-state, or equilibrium state (R, black color), while the other is the unstable and periodic solution indicative of subthreshold oscillation (SO, red color). The enlarged graph on the right corresponds to the highlighted region within the dashed box on the left.
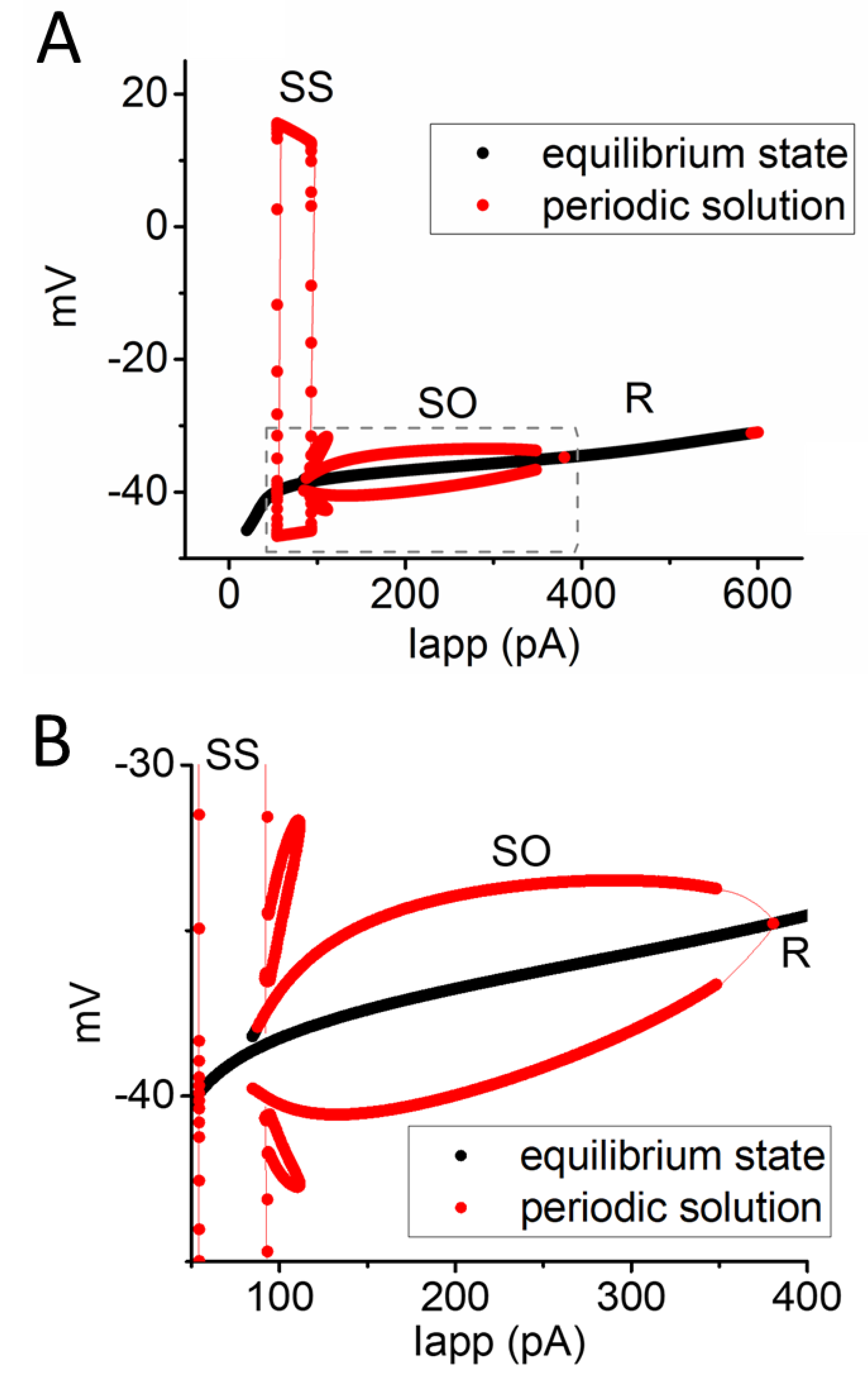
Figure 6.
Bifurcation diagram concerning the relationship between Iapp and membrane potential in the modeled neuron with a constant ϕ value of 0.8. Beside the equilibrium or resting state (R, in black color), two distinct types of periodic solutions (indicated in red color) were identified, namely somatic spiking (SS) occurring within the Iapp range of 52 to 97 pA and subthreshold oscillation (SO) at the Iapp range between 85 and 381 pA. Panel (B) is an enlarged view of the dashed box in panel (A).
Figure 6.
Bifurcation diagram concerning the relationship between Iapp and membrane potential in the modeled neuron with a constant ϕ value of 0.8. Beside the equilibrium or resting state (R, in black color), two distinct types of periodic solutions (indicated in red color) were identified, namely somatic spiking (SS) occurring within the Iapp range of 52 to 97 pA and subthreshold oscillation (SO) at the Iapp range between 85 and 381 pA. Panel (B) is an enlarged view of the dashed box in panel (A).
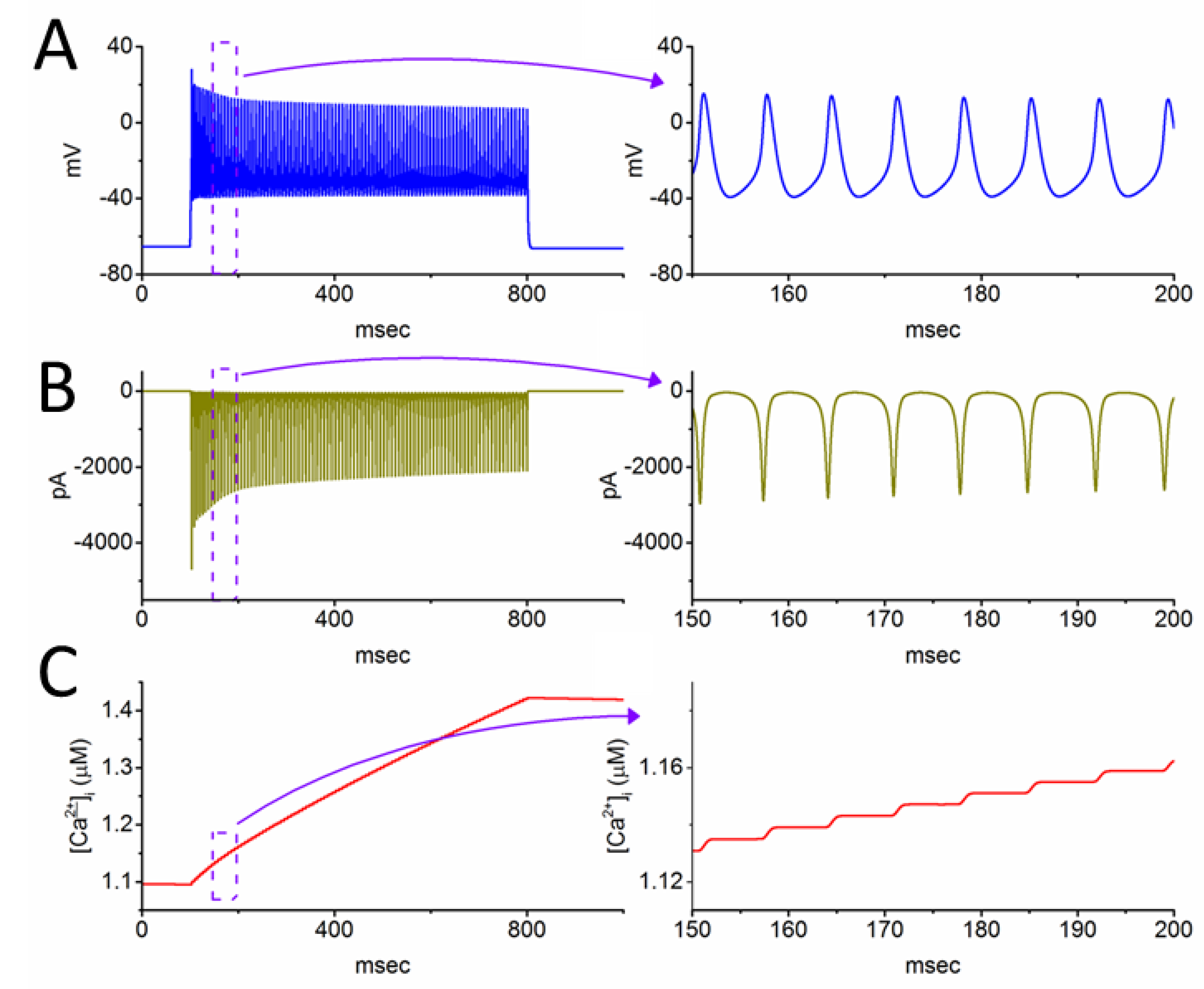
Figure 7.
Effect of elevated Iapp at 700 pA on spiking, INa, and intracellular Ca2+ concentration ([Ca2+]i) in the simulated neuron. Panels (A), (B), and (C) respectively depict membrane potential, INa amplitude, and [Ca2+]i induced by a 700-msec Iapp with 700 pA. Each corresponding panel on the right provides an enlarged view of the graph contained within the dashed box on the left.
Figure 7.
Effect of elevated Iapp at 700 pA on spiking, INa, and intracellular Ca2+ concentration ([Ca2+]i) in the simulated neuron. Panels (A), (B), and (C) respectively depict membrane potential, INa amplitude, and [Ca2+]i induced by a 700-msec Iapp with 700 pA. Each corresponding panel on the right provides an enlarged view of the graph contained within the dashed box on the left.
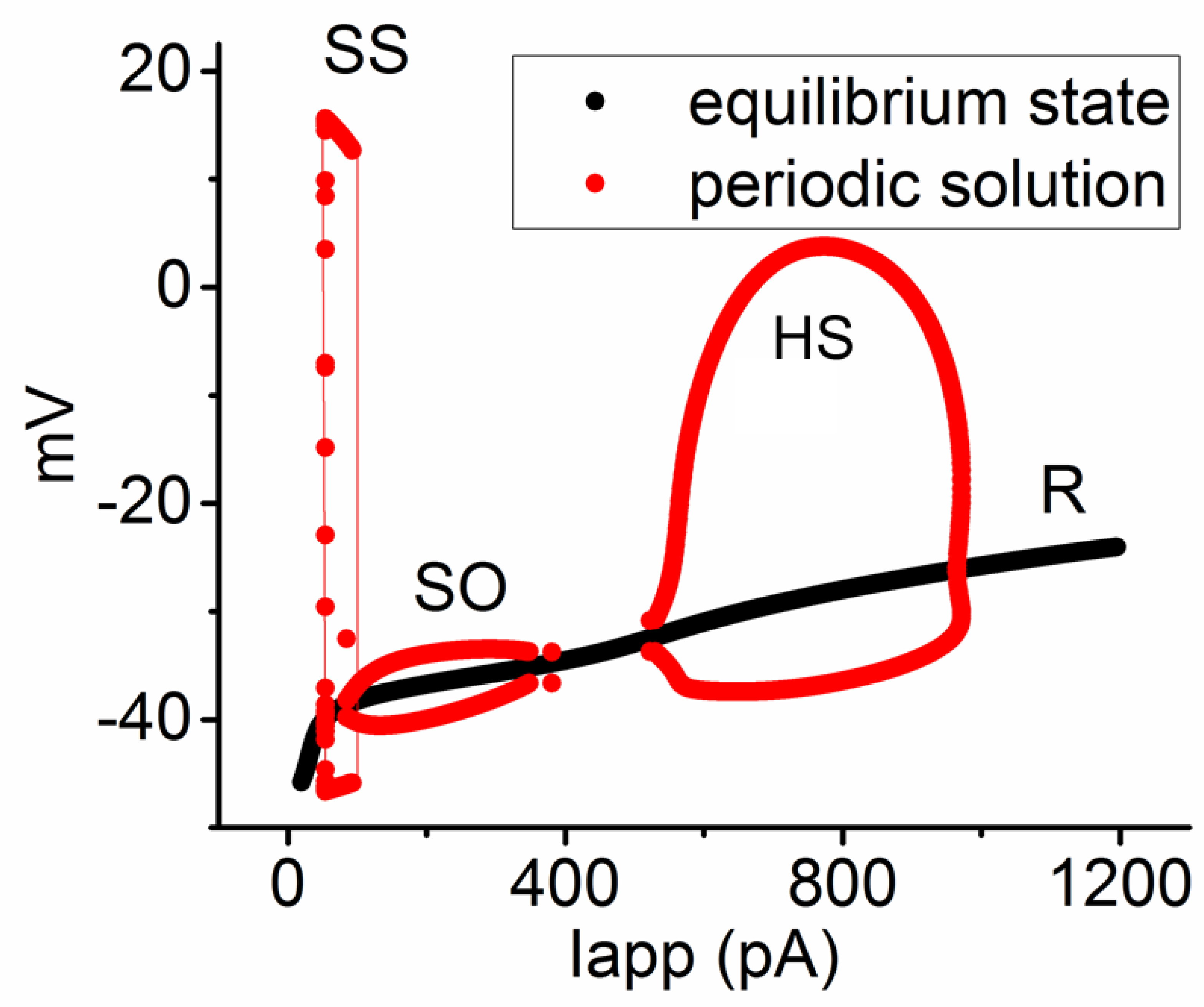
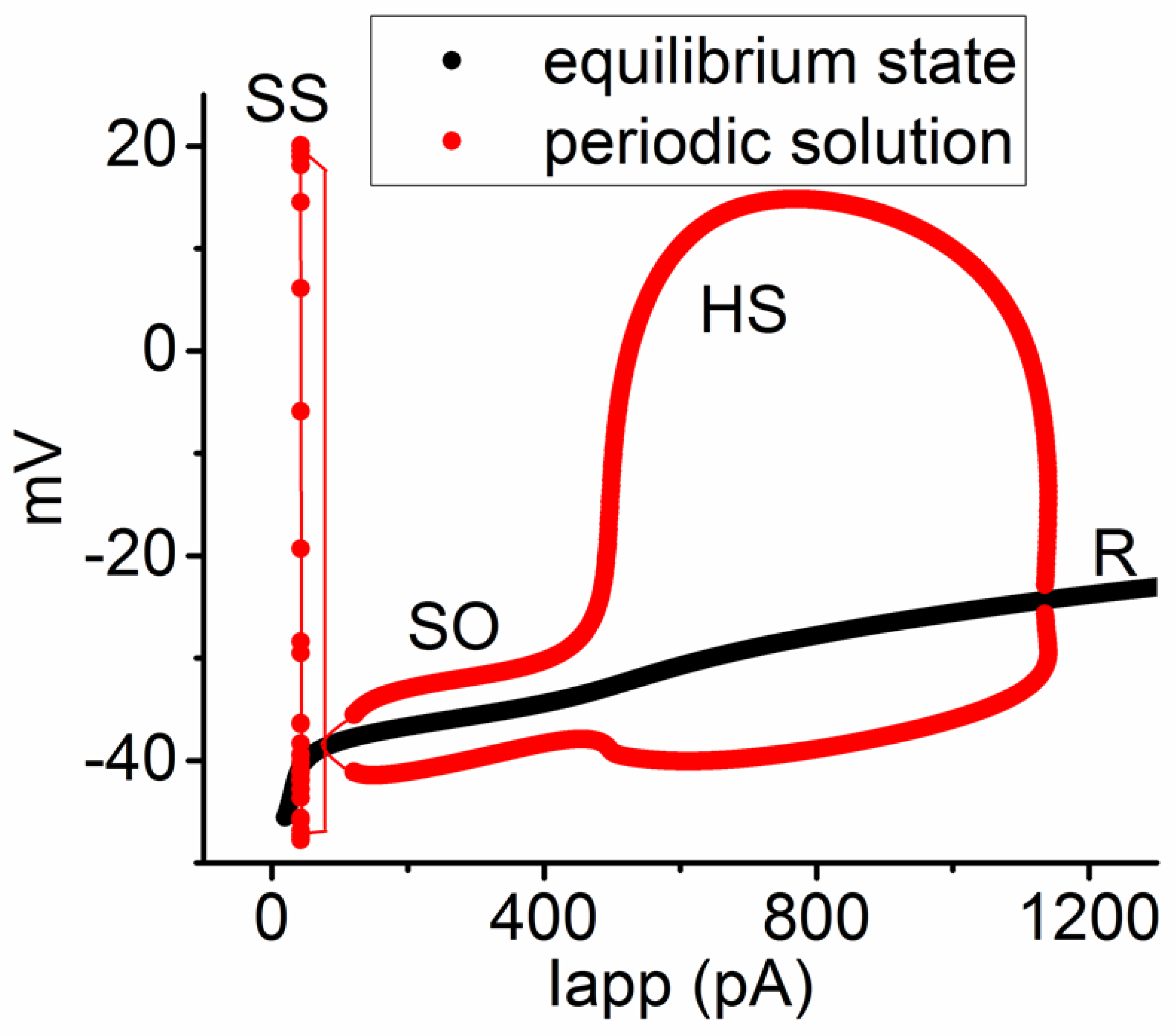
Figure 8.
Bifurcation diagram showing the relationship between the applied current (Iapp) and membrane potential in the modeled neuron. The parameters were set at gNa = 300 nS and ϕ = 0.8, with the Iapp range spanning from 20 to 1300 pA. Three unstable bifurcations (indicated in red color) occur, namely SS with low frequency, SO, and a high-frequency spiking pattern (HS). This caption suggests that, in the presence of compounds like tefluthrin (Tef) or telmisartan (Tel), the modeled neuron exhibited HS occurrence, especially at higher intensities of applied current ranging between 500 and 950 pA. Red and black colors in this and the following Figures denote the periodic solutions (SS, SO, and HS) and the equilibrium or resting state (R), respectively.
Figure 8.
Bifurcation diagram showing the relationship between the applied current (Iapp) and membrane potential in the modeled neuron. The parameters were set at gNa = 300 nS and ϕ = 0.8, with the Iapp range spanning from 20 to 1300 pA. Three unstable bifurcations (indicated in red color) occur, namely SS with low frequency, SO, and a high-frequency spiking pattern (HS). This caption suggests that, in the presence of compounds like tefluthrin (Tef) or telmisartan (Tel), the modeled neuron exhibited HS occurrence, especially at higher intensities of applied current ranging between 500 and 950 pA. Red and black colors in this and the following Figures denote the periodic solutions (SS, SO, and HS) and the equilibrium or resting state (R), respectively.
Figure 9.
Phase diagram illustrating the impact of enhanced gNa coupled with a reduced ϕon the relationship of Iapp versus membrane potential. In this diagram, we set ϕ = 0.8, and increased gNa to 320 nS. With the increased Iapp, the coalescence of SO and HS became evident. No existence in the stable steady-state for Iapp ranging between 20 and 1100 pA was noted.
Figure 9.
Phase diagram illustrating the impact of enhanced gNa coupled with a reduced ϕon the relationship of Iapp versus membrane potential. In this diagram, we set ϕ = 0.8, and increased gNa to 320 nS. With the increased Iapp, the coalescence of SO and HS became evident. No existence in the stable steady-state for Iapp ranging between 20 and 1100 pA was noted.
Table 1.
Default parametric values used for the modeling of interneurons in the dorsal horn of the spinal cord. For more detailed parameters of this model, please refer to Ma et al. (2023) paper.
Table 1.
Default parametric values used for the modeling of interneurons in the dorsal horn of the spinal cord. For more detailed parameters of this model, please refer to Ma et al. (2023) paper.
| Symbol or parameter |
Description*
|
Value |
| Cm
|
Membrane capacitance (pF) |
30 |
| gNa
|
Maximum Na+ current conductance (nS) |
300 |
| gCa
|
Maximum Ca2+ current conductance (nS) |
8 |
| gK1
|
Maximum KV1 current conductance (nS) |
15 |
| gK3
|
Maximum KV3 current conductance (nS) |
180 |
| gSK
|
Maximum SK K+ current conductance (nS) |
10 |
| gleak
|
Maximum leak current conductance (nS) |
8 |
| VNa
|
Na+ reversal potential (mV) |
58 |
| VK
|
K+ reversal potential (mV) |
-80 |
| VCa
|
Ca2+ reversal potential (mV) |
68 |
| VLeak
|
Reversal potential for leak current (mV) |
-50 |
| γ |
Ca2+ recovery rate (ms-1) |
0.01 |
| kSK
|
Ca2+ sensitivity of SK channel (μM) |
0.8 |
| A |
Cell surface area (μm2) |
3000 |
| ϕ |
Adjustable βh-inactivation parameter of h gating variable (dimensionless) |
1.0 |