1. Introduction
Faba bean is an important legume species grown for human food and animal feed from ancient times. Like all crops, faba bean is susceptible to abiotic stresses, including temperature extremes; however relatively little research has examined the impact of high and low temperatures on flowering and pod formation: both important determinants of yield.
The influence of high temperature and moisture stress on faba bean and other legumes are often studied together, as plant responses to high temperature are linked to plant water status. High temperatures affect various growth processes which may significantly reduce yield in legumes [
1]. Sehgal et al. [
2]. studied heat stress in lentil and found that high temperatures (30/20 °C day/night) inhibited yield and yield components (seed number and seed weight per plant) more than drought stress, while drought stress reduced individual seed weights more than heat stress. The combined stresses were found to severely reduce yield.
El Nadi [
3]. studied the influence of different temperatures on flower abortion in faba bean and found that flower abortion was higher at 29.5 °C compared to 18.3 °C. Abdalla and Fischbeck [
4] used three day/night temperature regimes in controlled conditions; 15/11, 20/15 and 30/23 °C, and found that the 30/23 °C regime significantly reduced seed yield compared to 20/15 °C. More recently, Bishop [
5] observed plants exposed to 18/10, 22/14, 26/18, 30/22, 34/26 °C day/night temperatures for five days and concluded that yield per plant was highest at 18/10 °C.
Faba beans produce excess flowers and many are not retained even under ideal growing conditions [
6]. This adaptation provides a defence against biotic and abiotic stresses such as frost which may prevent flowers from forming pods. Relatively little research has been conducted on the effect of frost and low temperatures on the reproductive structures of faba bean in Europe as the crop flowers in autumn when frost is absent. However, frost is a major abiotic stress of faba bean production in Australia, particularly in the flowering, early pod formation and pod-filling stages of development [
7,
8].
Liu et al. [
9] compared average yields of faba bean in one province of China to weather records and observations of flowering in the field. They found that temperatures below 1 °C were damaging to the reproductive structures of faba bean and that seven days with minimums below 2 °C caused significant yield loss. Loss and Siddique [
10], based on field observations of the cultivar Fiord at different planting dates, concluded that faba bean was able to tolerate mild spring frosts down to -2.0 °C.
This study aimed to determine the temperature ranges for optimal faba bean flowering and pod development in the field by assessment of flowering and podding at individual nodes at different temperatures.
2. Materials and Methods
2.1. Genotypes, Sites and Experiments
Experiments were conducted in 2012 and 2013 to compare reproductive development of three different genotypes at three different sowing dates (
Table 1) at Breeza (31°10’S and 150° 25’E, elevation 285m) and Narrabri (30°16’S and 149°48’E, elevation 212 m) in northwest New South Wales (NSW). A randomised complete block design with four replications was used at both sites. Three genotypes were evaluated; Cairo, Doza and IX148f. Cairo is a commercial variety with the latest maturity of the three genotypes. Doza, another commercial variety, is slightly earlier than Cairo. IX148f is a breeding line that has performed well in yield trials and has the shortest maturity of the three.
In both years and at both sites, experiments were sown using a four-row cone seeder at a seeding density of 26 seeds/m2. Plots of three rows at 60 cm spacing and eight rows at 50 cm spacing were sown at Breeza and Narrabri, respectively. Seeds were inoculated in furrows at planting with liquid Rhizobium leguminosarum (group F) and no mineral fertilizer was applied. In 2012, in-crop weed control was achieved by hand chipping, whereas in 2013 a post-sow/pre-emergent herbicide 70 g/ha Spinnaker® (700 g a.i./kg Imazethapyr, Group B) was used. One application of Dithane™ (750 g a.i./kg Mancozeb) was made at both sites in both years to control foliar diseases and in 2012 400 g/ha Aphidex® (500 g/kg Pirimicarb) was applied at Breeza to control aphids (Aphis sp.).
2.2. Data Capture
At the commencement of flowering, three random plants per plot were selected from the centre row at Breeza and the 6th row at Narrabri and marked with a plastic peg. The main stem of the plant was subsequently selected and flowering and podding recorded at individual nodes. In order to identify nodal positions on the plant, a leaf subtending from each node was numbered with a permanent marker pen counting from the bottom up as plants grew. The dates at which individual nodes opened flowers and produced pods were recorded. In some cases, the main stem ceased to grow due to frost or insect attack in which case the strongest branch from the same plant was selected and the process continued. This procedure continued approximately twice weekly until flowering ceased. Approximately 3,700 nodes were examined. As 3 – 5 flowers appeared on each node, it was not possible to record if individual flowers on a node developed into a pod due to time constraints. Flowering and podding information was recorded only for the nodal position and nodes were deemed to have podded if both a flower and a pod greater than 10 mm length were produced at a node. Thus, the number of pods produced on the individual nodes were not recorded.
Daily temperatures were recorded throughout the growing season at each site in both years. Average minimum and maximum temperatures seven and ten days after flowering and average temperature on the day of flowering were calculated.
2.3. Statistical Analysis
Flowering and podding were analysed using the REML function of Genstat (16th edition). Year, irrigation and sowing date were considered fixed effects for each genotype and individual nodes on individual plants within replicates, sowing dates, irrigation regimes and years were considered random effects.
The adjusted means for each temperature parameter and the associated podding proportion were obtained from the linear mixed model analysis of each genotype x site combination. These points were plotted with error bars and a polynomial trendline fitted. The equation for each trendline was used to predict podding proportion over a wide range of temperatures. These plots were subsequently used to identify the temperature at which maximum podset occurred for each of the temperature parameters.
Pairwise comparisons of genotype podding proportion within defined temperature ranges were tested using Fisher’s least significant difference test in Genstat. Comparisons were considered significant at P<0.05.
3. Results
Growing conditions in 2012 were wetter, cooler and more favourable than 2013 (
Table 2). The number of days with minimum temperatures below 0
0C were higher in 2012 than in 2013 and conversely the number of days with maximum temperatures over 25
0C were lower in 2012 than in 2013. Narrabri was a warmer site than Breeza in both years (
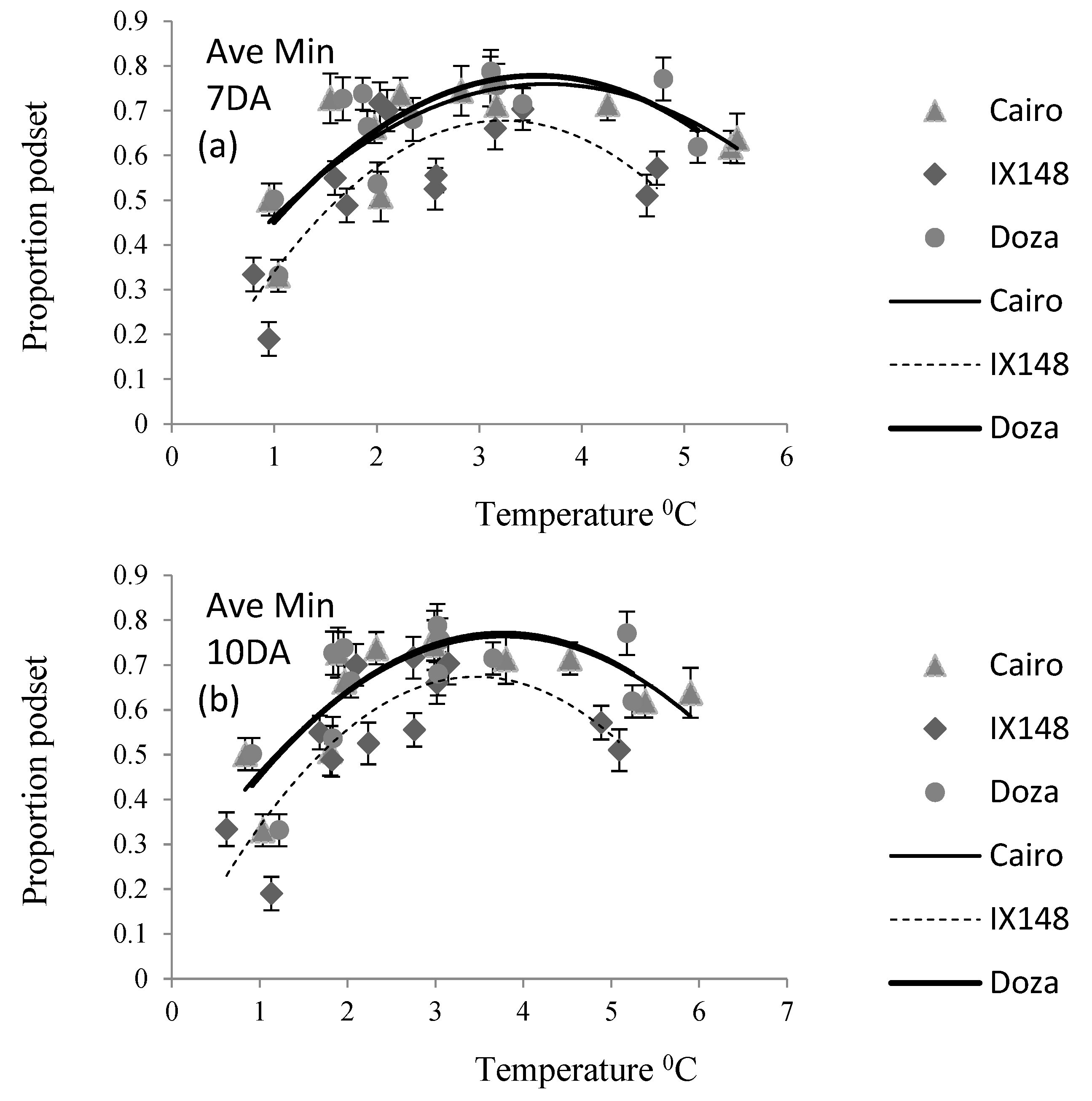
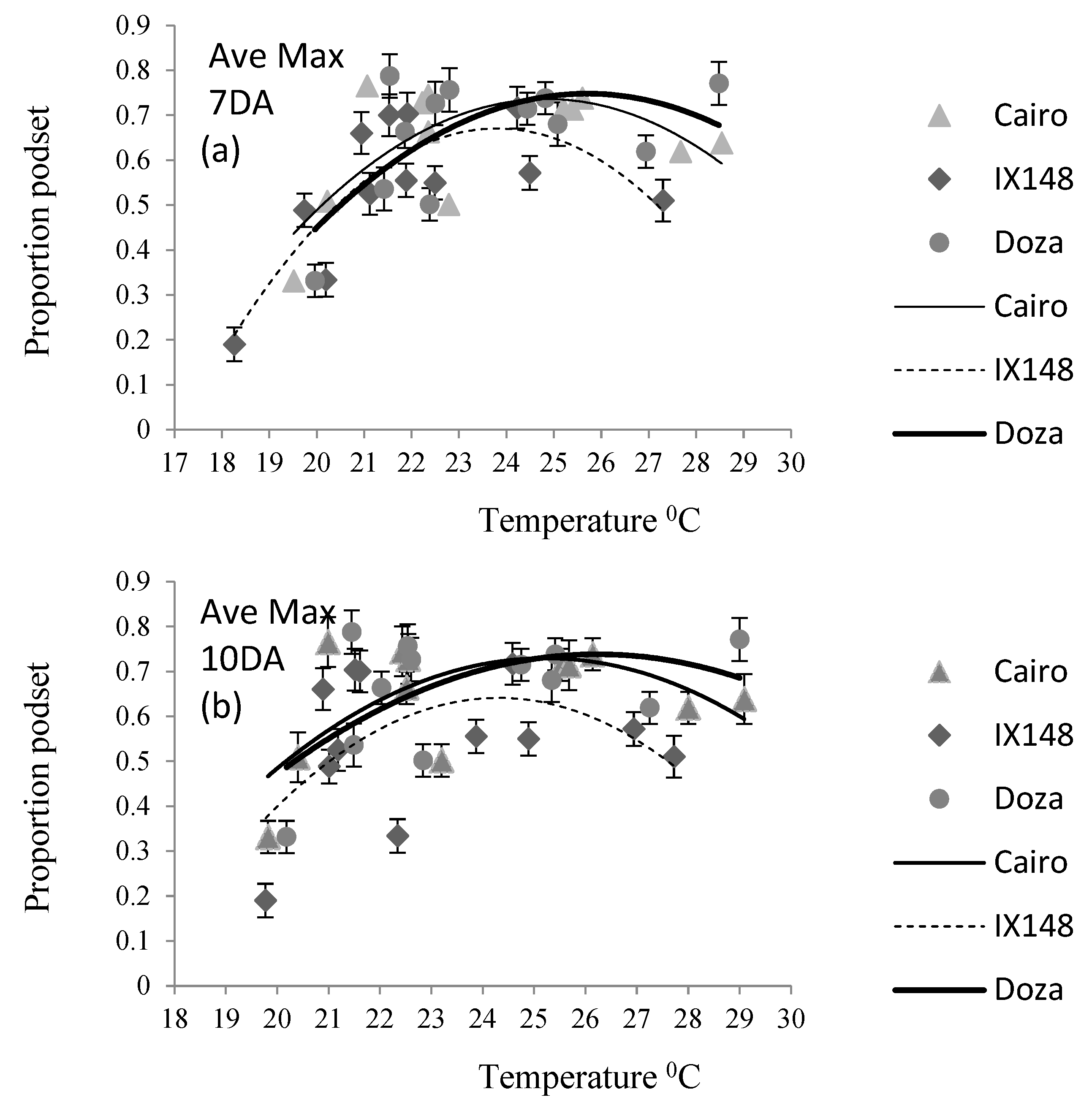
Table 2). The relationship between pod set and temperature was described as non-linear (
Figure 1,
Figure 2 and
Figure 3). In all cases, podding increased with increasing temperature to a maximum and this was followed by a decline at higher temperatures. Differences between genotypes were minimal.
The average minimum temperature for podding ranged from 1 - 6
0C for Cairo, 1 - 5
0C for IX148f and 1- 5.5
0C for Doza. The genotype IX148f had a steeper rise and fall than either Doza or Cairo indicating a narrower range of minimum temperatures for podding (
Figure 1).
In the 7-day period post flowering, podding occurred in the maximum temperature range of 19.5-29.0
0C for Cairo and Doza, and 18-27.5
0C for IX148f. The corresponding temperatures 10-days after flowering were 20.0-29.0
0C for Cairo and Doza and 20-28
0C for IX148f (
Figure 2).
When average daily temperature 7-days after flowering was assessed, podding occurred in the range 9.5-16
0C for Cairo, 9-14.5
0C for IX148f and 9.5-15
0C for Doza. Ten days after flowering the respective ranges were 10.0-16.5
0C, 9-15.5
0C and 10.0-16
0C. (
Figure 3). Average daily temperature on the day of flowering resulted in podding ranges of 8.5-15
0C, 8.0-14
0C and 8.5-14
0C for Cairo, IX148F and Doza, respectively. The curves showed a less distinct fall after the optimum was reached compared to average temperature 7 and 10-days after flowering.
Genotypes had similar responses to average minimum temperatures 7-days (Ave Min 7DA) and 10-days after flowering (Ave Min10DA) (
Table 3). IX148f had a significantly lower podding proportion than Cairo which in turn was lower than Doza, which were similar.
The trend in optimum maximum temperature amongst genotypes was similar at both 7 (Ave Max 7DA) and 10 (Ave Max 10DA) days following flowering (
Table 4). IX148f had a significantly lower optimum than Cairo which in turn had a lower optimum than Doza. The optimum maximum temperature was in the range of 23.7-26.1
0C. The lower optimum of Cairo compared to Doza is harder to explain as Doza has a shorter maturity than Cairo. The lower optimums in IX148f for both minimum and maximum temperatures may be a result of its earlier maturity resulting in flowering at lower temperatures.
The R2 values for 7-days after flowering (0.42- 0.70) were generally higher than at 10-days (0.32 –0.42), particularly in IX148f which gave a high R2 (0.70) at 7 days and low (0.25) at 10-days post flowering.
Optimum average daily temperatures 7- and 10-days following flowering were significantly lower for IX148f than Cairo or Doza (
Table 5). Doza had a significantly higher optimum 7-days after flowering and a similar optimum 10-days after flowering compared to Cairo. Surprisingly, when average temperature on the day of flowering was assessed, IX148f had a higher optimum than Cairo, which in turn was higher than Doza. Overall, the optimums were in the range 11.7-13.9
0C.
4. Discussion
The highest correlations between temperature and podding were observed for minimum temperatures, indicating that minimum temperature may be more relevant to faba bean yield than average and maximum temperatures. High temperatures occurred towards the end of the season, when soil moisture was more limited, and this may have partially confounded estimations of the impact of high temperature on podding. Nevertheless, there was sufficient soil moisture at both locations in both years late in the season to discount severe moisture stress as an influential factor.
The effect of temperature on pod set under field conditions was significant over both years and locations. The average temperature at which podding was maximised was 12.5 - 13.5 °C. This is consistent with the observations of Bishop et al. [
5] who observed maximum seed yield at day/night temperatures of 18/10 °C, which corresponds to an average daily temperature of 14 °C, like the present study. The minimum temperature at which podding was highest was 3.5 °C. This was consistent with Liu et al. [
9] who found seven days at minimum temperatures below 2.0 °C caused yield loss. The maximum temperature at which optimal podding was maintained was approximately 25 °C, after which it declined; this was somewhat lower than observed by El Nadi [
3] although this study did not include a treatment of 25°C. Others reported that pod set was reduced at day/night temperatures of 30/23 °C compared to 20/15 °C [
4]. However, these authors did not attempt to identify the critical temperature beyond which podding starts to diminish.
Genotypes varied in their response to temperature. The genotype IX148f had consistently lower optimum temperatures for maximum pod set compared to Doza and Cairo across all temperature regimes except daily average. This may be a result of the earlier maturity of IX148f or may reflect a greater tolerance of low temperatures during flowering and podset. The higher daily average temperature optimum of IX148f may be an anomaly of the short period of temperature recording (i.e. one day).
IX148f had a consistently lower maximum podding proportion compared to Doza and Cairo, despite producing the highest yield. This indicates that podding started earlier in IX148f and continued for longer compared to Doza and Cairo. Doza and Cairo had similar optimums for both minimum temperature regimes and average 10-day temperature. For other temperature regimes, Doza had higher optimums than Cairo despite its shorter maturity. This indicated possible adaptation of Doza to higher temperatures.
Pulse grain production in Australia is limited by autumn sowing as flowering commences during winter when temperatures are generally too low for effective pod set [
11]. However, temperatures rise quickly in the spring and seed set is then limited by high temperatures and moisture stress. The trend in northern agricultural environments of Australia has been to develop early season varieties to avoid heat and moisture stress [
12,
13]. The ability of the earlier maturing genotype IX148f to set seed when exposed to lower temperatures compared to the longer maturing Cairo and Doza provided a convincing example. The optimum time for planting faba bean in the northern grain growing region of Australia is early May [
11], which results in flowering in July when temperatures are still cold. They found the earliest flowering genotype IX148f achieved the highest yield of the three varieties and the current study suggests that faba bean has the ability to flower and set seed at lower temperatures.
Much of the previous work in faba bean and other pulse crops was completed in growth chambers and involved tagging of individual nodes and flowers, techniques not used in the current study. The use of growth chambers allows greater control of temperature, however as reported by Porter et al. [
14], they introduced other artefacts that influence plant responses. Experiments in field conditions with imposed temperature treatments have been used in a smaller number of studies in other crops [
15,
16]. However, the control of temperature in the field is difficult and the imposition of specific temperature regimes is not possible. Our results are based on field environments and are therefore more relevant than artificial environments. If the environmental conditions are accurately assessed, then plant responses to different temperatures can be determined.
5. Conclusions
This study identified temperature limits for pod set in faba bean and provided evidence that these optimums can be manipulated genetically to expand the adaptation of the crop. However, further research is needed to more accurately identify and confirm the lower temperature limits of flowering and pod set in faba bean, and to explore genetic diversity for pod set at lower temperature. The northern grain growing region of Australia depends upon stored soil moisture from summer rain. If flowering and podset can commence earlier in the season when stored moisture is less depleted, more moisture would be available to the crop in the reproductive phase. Earlier studies found that a greater proportion of crop water used post flowering was associated with higher yield [
17,
18]. The current study clearly demonstrated that early flowering variety sets seeds at low temperature and has potential to produce high yield. Faba bean breeders should continue to target earlier maturing varieties for this region that can set pods at lower temperatures and avoid terminal heat and drought. This may also be applicable to other winter legumes, such as field pea in the moisture limited environment.
Author Contributions
Conceptualization, K.N.A.; Methodology, K.N.A.; Formal analysis, R.T.; Investigation, B.M.; Data curation, B.M.; Writing—original draft, B.M.; Writing—review & editing, K.N.A. and R.T.; Supervision, K.N.A.; All authors have read and agreed to the published version of the manuscript.
Funding
Physical Resources were made available from the Grains Research and Development Corporation (GRDC) pathology project (DAN 00176) and the GRDC faba bean breeding project (UA000127).
Data Availability Statement
The data are available from main author (BM).
Acknowledgments
The first author wishes to thank former supervisor Dr Carina Moeller for assistance in planning these trials. I would like to acknowledge the NSW Department of Primary Industries (DPI) and Northwest Local Land Services (NWLLS) for allowing me to pursue this study and use of resources at the Liverpool Plains Field Research Station (LPFRS). I would also like thank Mr Joop van Leur for his assistance in trial design and data processing and for making physical resources available from his pathology project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- McDonald, G.K.; Paulsen, G.M. High temperature effects on photosynthesis and water relations of grain legumes. Plant Soil. 1997, 196, 47–57. [Google Scholar] [CrossRef]
- Sehgal, A.; Sita, K.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K. H. M.; Nayyar, H. Effects of drought, heat and their interaction on the growth, yield and photosynthetic function of lentil (Lens culinaris Medikus) genotypes varying in heat and drought sensitivity. Front. Plant Sci. 2017, Volume 8, Article 1776. [CrossRef]
- El Nadi, A.H. 1969. Water relations of beans I. Effects of water stress on growth and flowering Vicia faba L.). Exp. Agric. 1969, 5, 195–207. [Google Scholar]
- Abdalla, M.M.F.; Fischbeck, G. Growth and fertility of five stocks of field beans grown under three temperature regimes and the effect of natural water stress on seed index of a collection of Vicia faba L. Zeitschrift fur Acker- und Pflanzenbau 1978, 147, 81–91. [Google Scholar]
- Bishop, J.; Potts, S.G.; Jones, H.E. Susceptibility of faba bean (Vicia faba L.) to heat stress during floral development and anthesis. J. Agron. Crop. Sci. 2016, 202, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Patrick, J.W.; Stoddard, F.L. Physiology of flowering and grain filling in faba bean. Field Crops Res. 2010, 115, 234–242. [Google Scholar] [CrossRef]
- Maqbool, A.; Shafiq, S.; Lake, L. Radiant frost tolerance in pulse crops-a review. Euphytica. 2010, 172, 1–12. [Google Scholar] [CrossRef]
- Alharbi, N.H.; Alghamdi, S.S.; Migdadi, H.M.; El-Harty. E.H and Adhikari, K.N. Evaluation of frost damage and pod set in faba bean (Vicia faba L.) under field conditions. Plants 2021, 10, 1925. [CrossRef]
- Liu, Z.S.; Zhao, Y.Z.; Bao, S.Y.; Guang, W. Low temperature and faba bean (Vicia faba L.) yield. Fabis Newsletter. 1987, 18, 14–17. [Google Scholar]
- Loss, S.P.; Siddique, K.H.M. Adaption of faba bean (Vicia faba L.) to dryland Mediterranean-type environments 1. Seed yield and yield components. Field Crops Res. 1997, 52, 17–28. [Google Scholar] [CrossRef]
- Manning, B.K.; Adhikari, K.N.; Trethowan, R. Impact of sowing time, genotype, environment and maturity on biomass and yield components in faba bean (Vicia faba). Crop Pasture Sci. 2020, 71, 147–154. [Google Scholar] [CrossRef]
- Adhikari, K.N. Breeding faba bean for sub-tropical region of Australia. Legume Perspectives 2023, 24, 10–12. [Google Scholar]
- Adhikari, K. N., Khazaei, H., Ghaouti, L., Maalouf, F., Vandenberg, A., Link, W. and O'Sullivan, D. M. Conventional and molecular breeding tools for accelerating genetic gain in faba bean (Vicia faba L.). Frontiers in Plant Science 2021, 12(2174).
- Porter, A.S.; Evans-Fitz Gerald, C.; McElwain, J.C.; Yiotis, C.; Elliott-Kingston, C. How well do you know your growth chambers? Testing for chamber effect using plant traits. Plant Methods. 2015, 11:44. [CrossRef]
- Thistlethwaite, R.J.; Tan, D.K.Y.; Bokshi, A.I.; Ullah, S.; Trethowan, R.M. A phenotyping strategy for evaluating the high-temperature tolerance of wheat. Field Crops Res. 2020, 255. [Google Scholar] [CrossRef]
- Pattison, A.L.; Mohammad, N.U.; Trethowan, R.M. Use of field based chambers to quantify the influence of heat stress in chickpea in-situ. Field Crops Res. 2021, 270.
- Siddique, K. H. M.; Regan, K. L.; Tennant, D.; and Thomson, B.D. Water use and water use efficiency of cool season grain legumes in low rainfall Mediterranean-type environments. Eur. J. Agron. 2001, 15, 267–280. [Google Scholar] [CrossRef]
- Mwanamwenge, J.; Loss, S.P.; Siddique, K.H.M.; Cocks, P.S. Growth, seed yield and water use of faba bean (Vicia faba L). in a short-season Mediterranean-type environment. Aust. J. Exp. Agric. 1998, 38, 171–180. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).