1. Introduction
Pakistan, a country renowned for its rich agricultural heritage, faces the challenge of rehabilitating barren lands with poor nutritional and elemental status. As a developing nation, Pakistan requires cost-effective strategies to enhance agricultural productivity, particularly in response to the demands of a growing population and the need to bolster its economy. Researchers have been actively exploring innovative approaches to improve fertilizers, with a notable focus on utilizing agricultural waste as a sustainable resource for soil enrichment [
1,
2,
3].
Pressmud, a by-product of the sugarcane industry, represents a promising avenue for enhancing soil fertility [
4]. Pakistan, home to approximately 81 sugar mills with a crushing capacity of 6.1 million tons, produces around 1.2 to 1.8 million tons of sugarcane pressmud (SPM) annually [
5]. This by-product, rich in organic matter and essential nutrients, has gained attention as a valuable resource for biofortification in agriculture. Composting, a well-established method for stabilizing organic matter, plays a critical role in transforming pressmud into a nutrient-rich biofertilizer. The process involves the microbial decomposition of organic material, resulting in the formation of humic substances that improve soil health.
The use of pressmud, as a soil amendment has been widely studied for its ability to enhance soil fertility, particularly in terms of increasing phosphorus (P) and nitrogen (N) content. Several studies have demonstrated that when pressmud is applied in conjunction with conventional fertilizers, there is a significant improvement in the availability of P and N in the soil. For instance, research has shown that pressmud can act as a slow-release fertilizer, providing essential nutrients over time, thereby enhancing the soil’s nutrient profile [
6,
7]
However, there is limited literature focusing on the efficacy of pressmud in enhancing P and N levels through bacterial augmentation alone, without the addition of traditional fertilizers. Most studies on pressmud have primarily investigated its role as an organic amendment in combination with fertilizers rather than exploring its potential as a standalone biofertilizer when inoculated with specific bacterial strains. Bacterial augmentation, particularly with phosphorus-solubilizing and nitrogen-fixing Bacillus strains, is a promising area of research, as it could further enhance the bioavailability of these critical nutrients in the soil. Despite its potential, the research on this approach remains sparse, and more studies are needed to fully understand and optimize the use of pressmud with bacterial inoculants for soil fertility enhancement [
8,
9]. This literature provides a basis for understanding the role of pressmud in soil fertility enhancement, particularly in terms of P and N content, while also highlighting the need for further research on bacterial augmentation as a standalone approach.
2. Results
2.1. Moisture Content
Moisture content is a critical factor in maintaining the composting process. A 49% reduction in moisture content was observed in E2 after 60 days of composting, while E3 showed a 58% reduction over the same period, compared to a 73% decrease in the control (E5). Detailed moisture content measurements at 15, 30, 45, and 60 days is illustrated in
Figure 1.
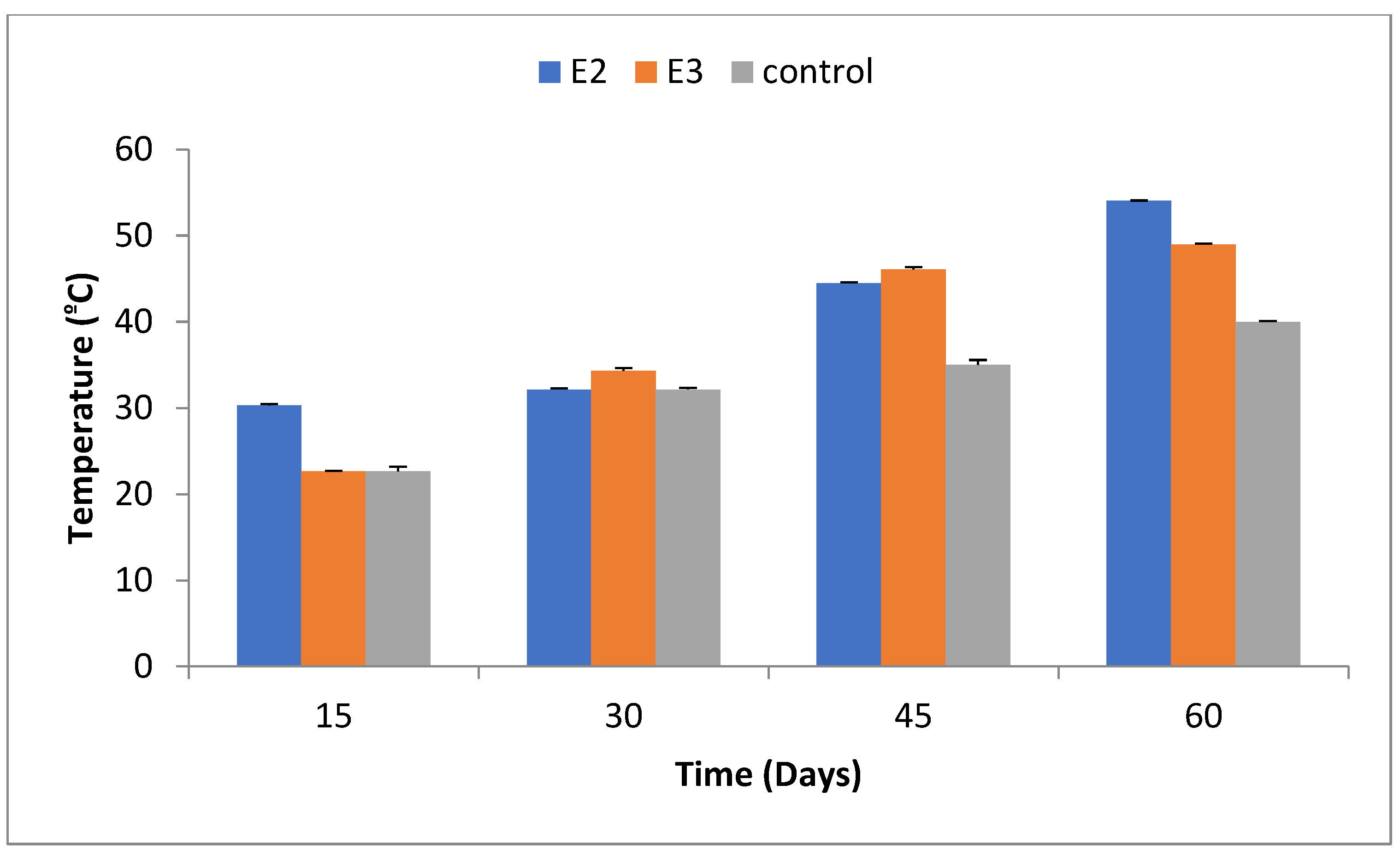
2.2. Temperature Variation
Temperature fluctuations were recorded at 15, 30, 45, and 60 days. At the end of the experiment, E2 exhibited a temperature of 54±0.02ºC, while E3 showed 49±0.09ºC, compared to 40±0.09ºC in the control group. These temperature values at each interval are shown in
Figure 2.
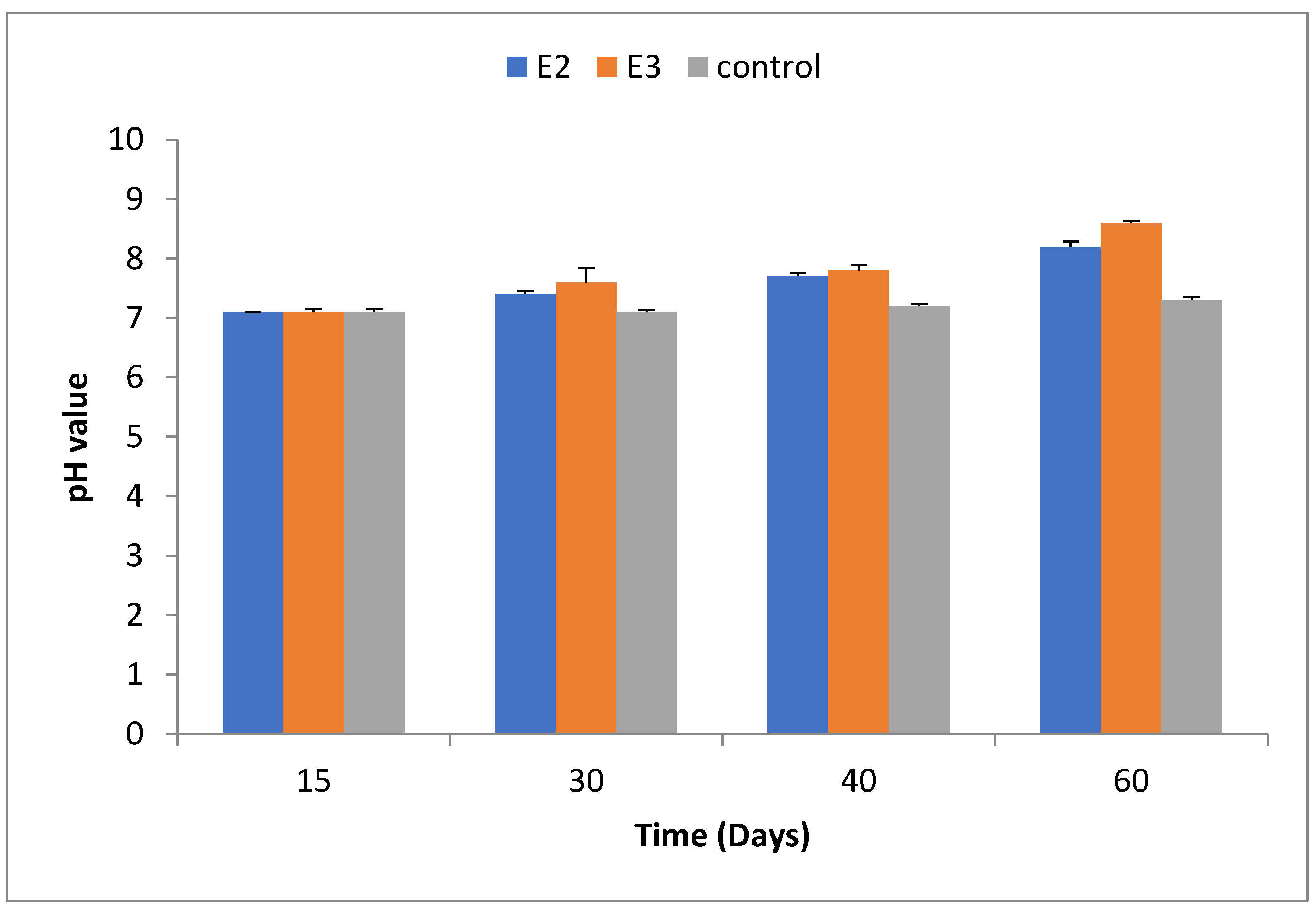
2.3. pH of the Compost
The pH of the compost was measured at 15, 30, 45, and 60 days. In E2, the pH values ranged from 7.1±0.05 at day 15 to 8.2±0.12 at day 60. For E3, pH increased from 7.1±0.05 to 8.6±0.03 over the same period, while the control group (E5) showed a pH of 7.3±0.06 after 60 days. The pH data are summarized in Table 3 and illustrated in
Figure 3.
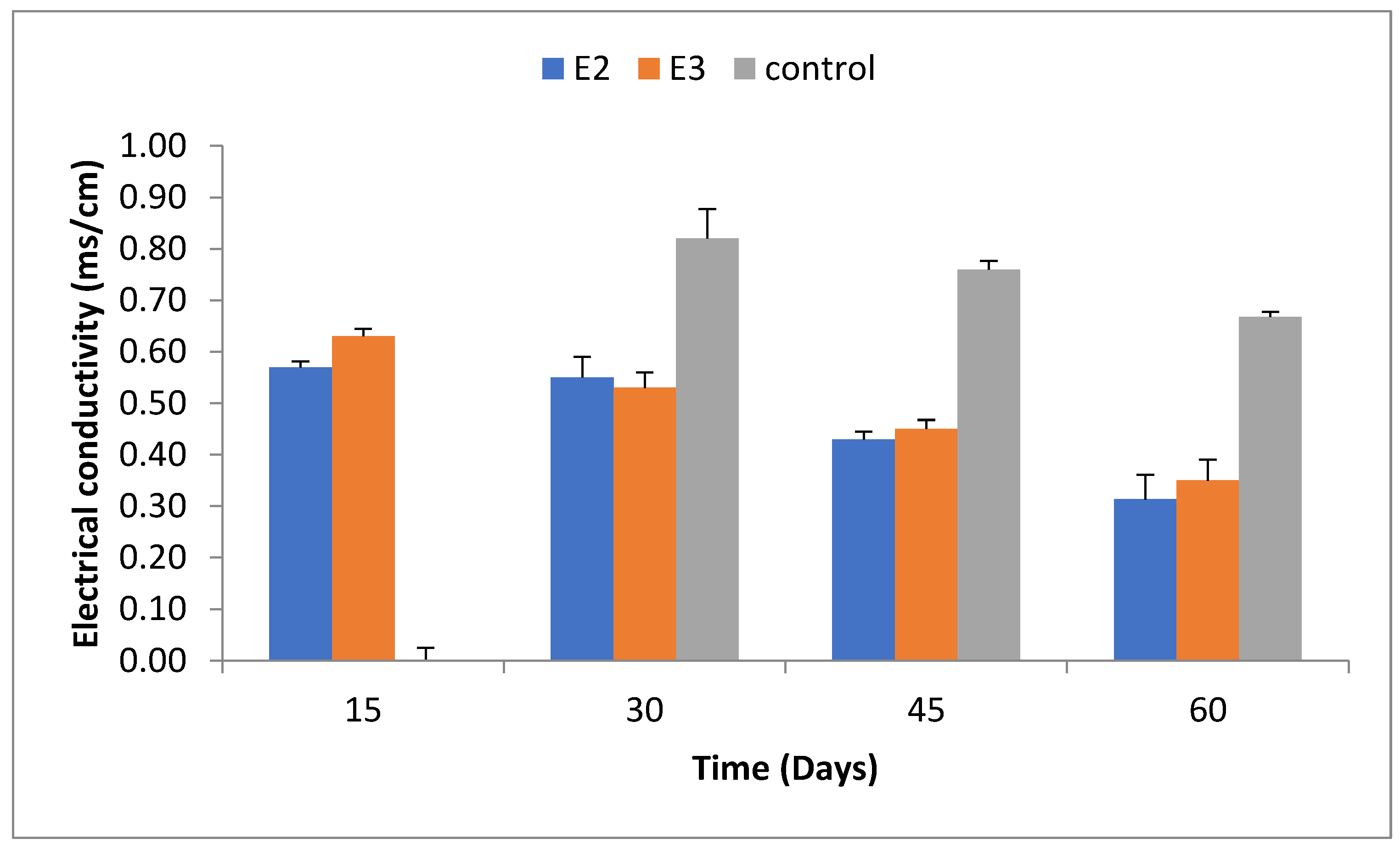
2.4. Electrical Conductivity (EC)
At the beginning of the experiment, EC increased due to the release of soluble salts. After 60 days, EC in the control group was 0.68±0.01 ms/cm, whereas in E2 and E3, EC was recorded as 0.31±0.05 ms/cm and 0.35±0.04 ms/cm, respectively. The data is illustrated in
Figure 4.
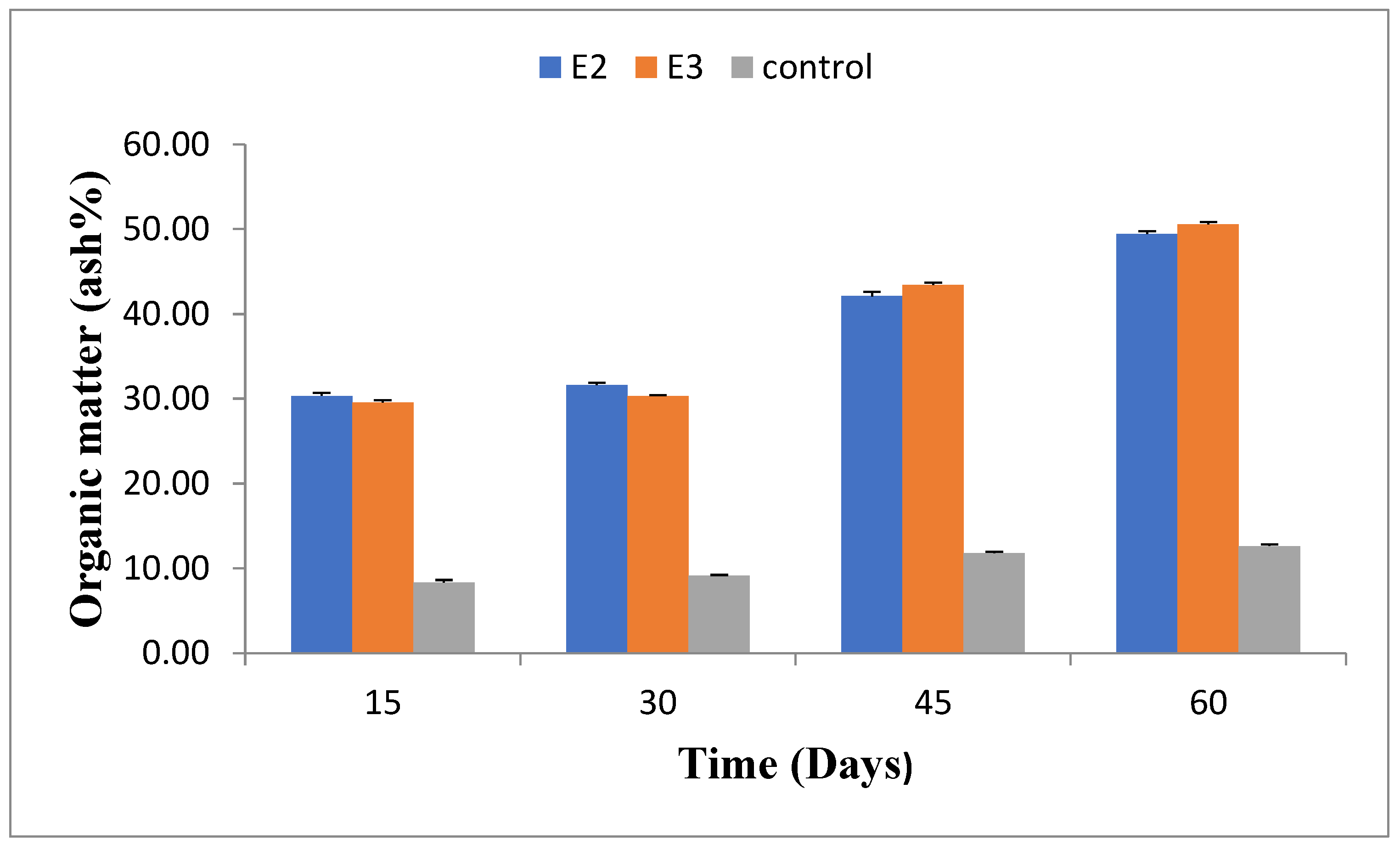
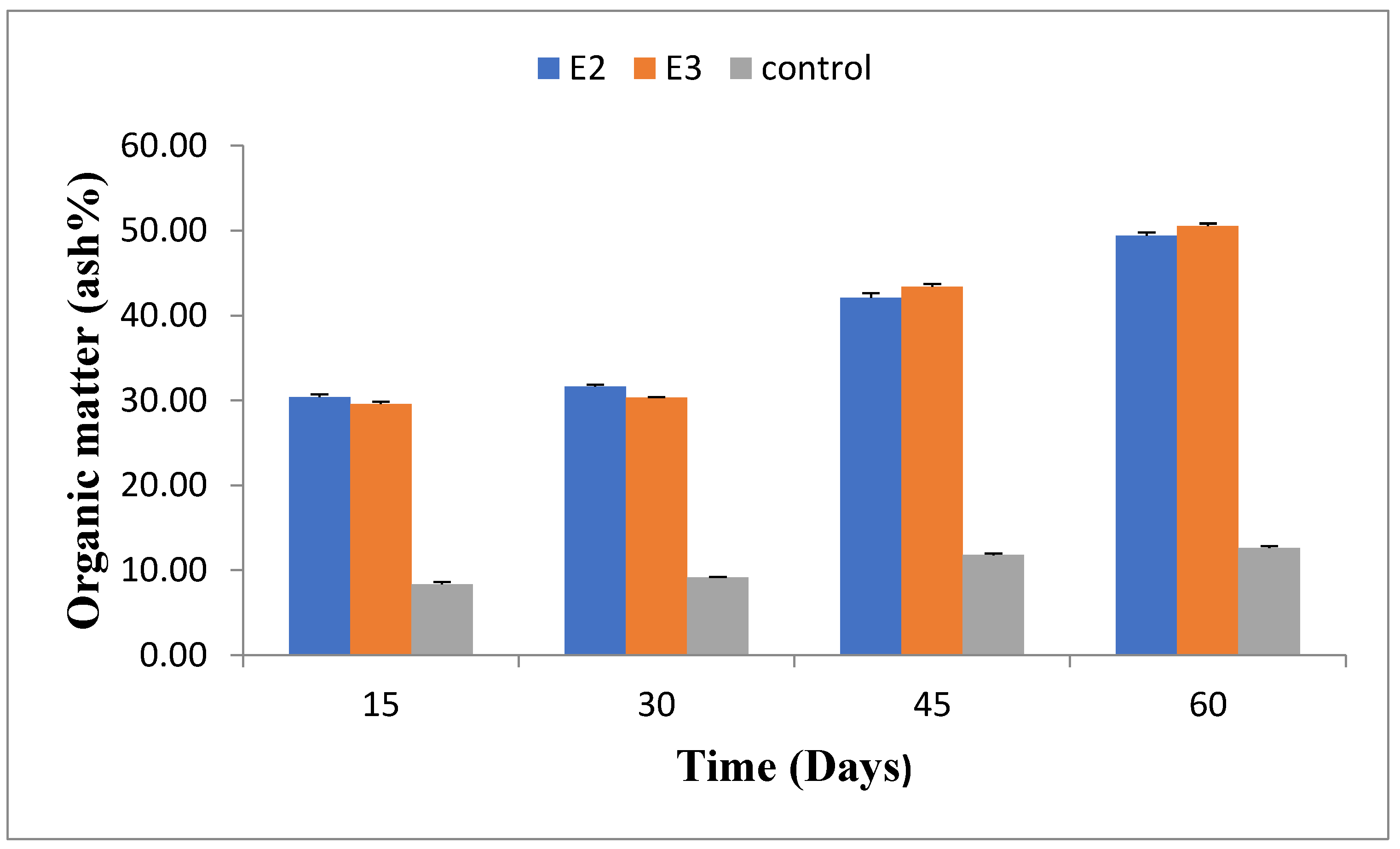
2.5. Organic Matter(%)
The organic matter content varied during the experiment, increasing gradually. After 60 days, E2 had 49.41±0.35% organic matter, and E3 had 50.55±0.28%, compared to 12.63±0.19% in the control group (E5). Detailed organic matter content at different stages is provided in
Figure 5.
2.6. Waste Mass Degradation
A rapid decrease in waste mass was observed across all experimental groups at the onset of the experiment. In particular, the E2 group exhibited the highest degradation, with approximately 60% of the mass degraded by day 45. This represents a 58% increase in degradation in the E2 group compared to the control group. Although the difference between the E2 and E3 groups was minimal, the control group showed only 0.3% mass degradation, likely due to the slower bacterial activity. Both E2 and E3 groups produced a blackish-brown material with an earthy, woody odor, which was markedly different from the control group (
Figure 6).
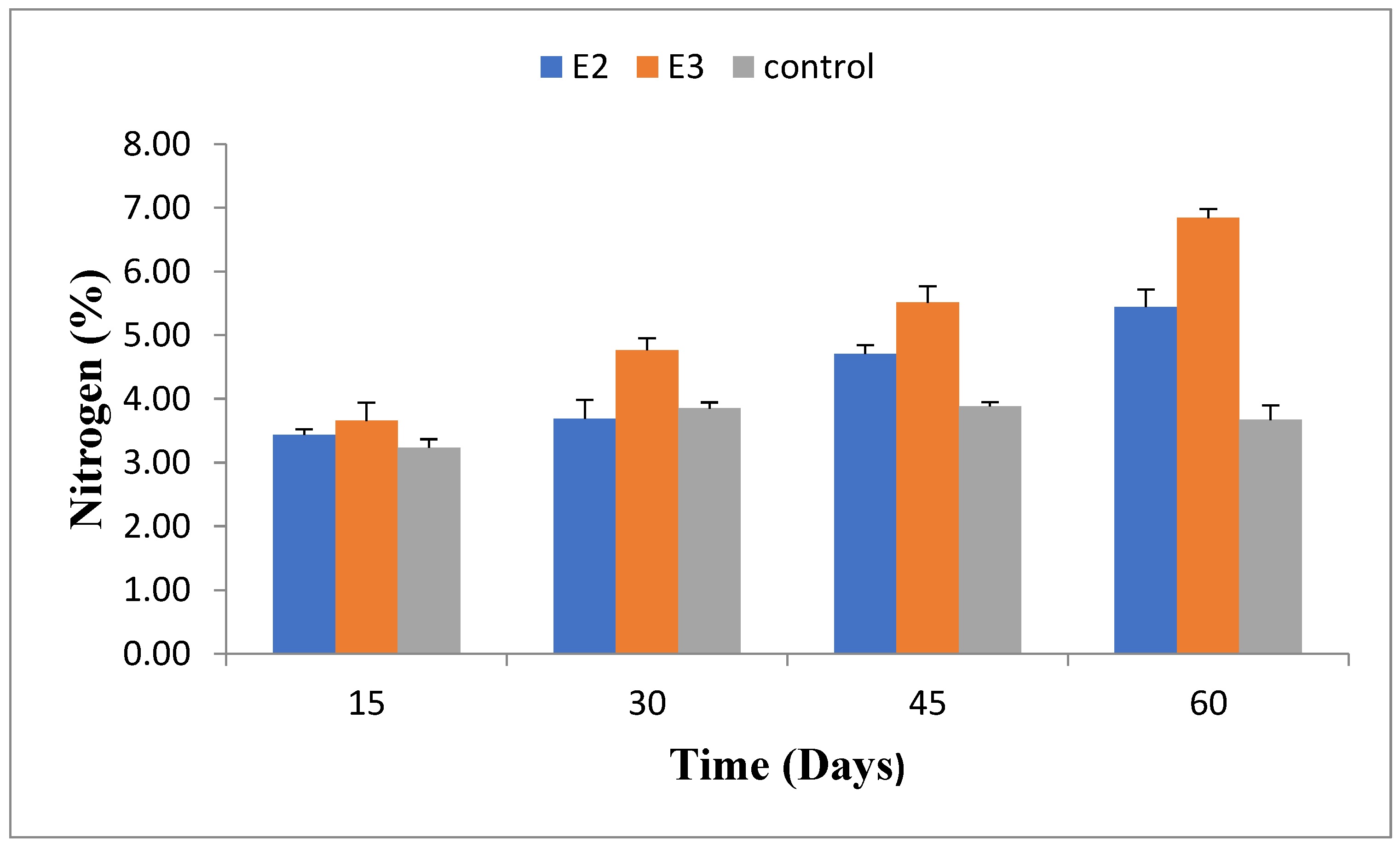
2.7. Total Nitrogen (N) Content
Nitrogen content increased as composting progressed. E2 and E3 showed a similar nitrogen content of approximately 1.48% after 60 days, significantly higher than the control group’s 0.98%. The detailed nitrogen content at different stages is provided in
Figure 7.
Figure 7.
Nitrogen content(%) of different pressmud compost groups enriched with or without phosphorus-solubilizing and nitrogen-fixing bacteria (E2: B. Amyloliquefaciens -ASK 11; E3: B. Megaterium-ANF3) after varying time intervals.
Figure 7.
Nitrogen content(%) of different pressmud compost groups enriched with or without phosphorus-solubilizing and nitrogen-fixing bacteria (E2: B. Amyloliquefaciens -ASK 11; E3: B. Megaterium-ANF3) after varying time intervals.
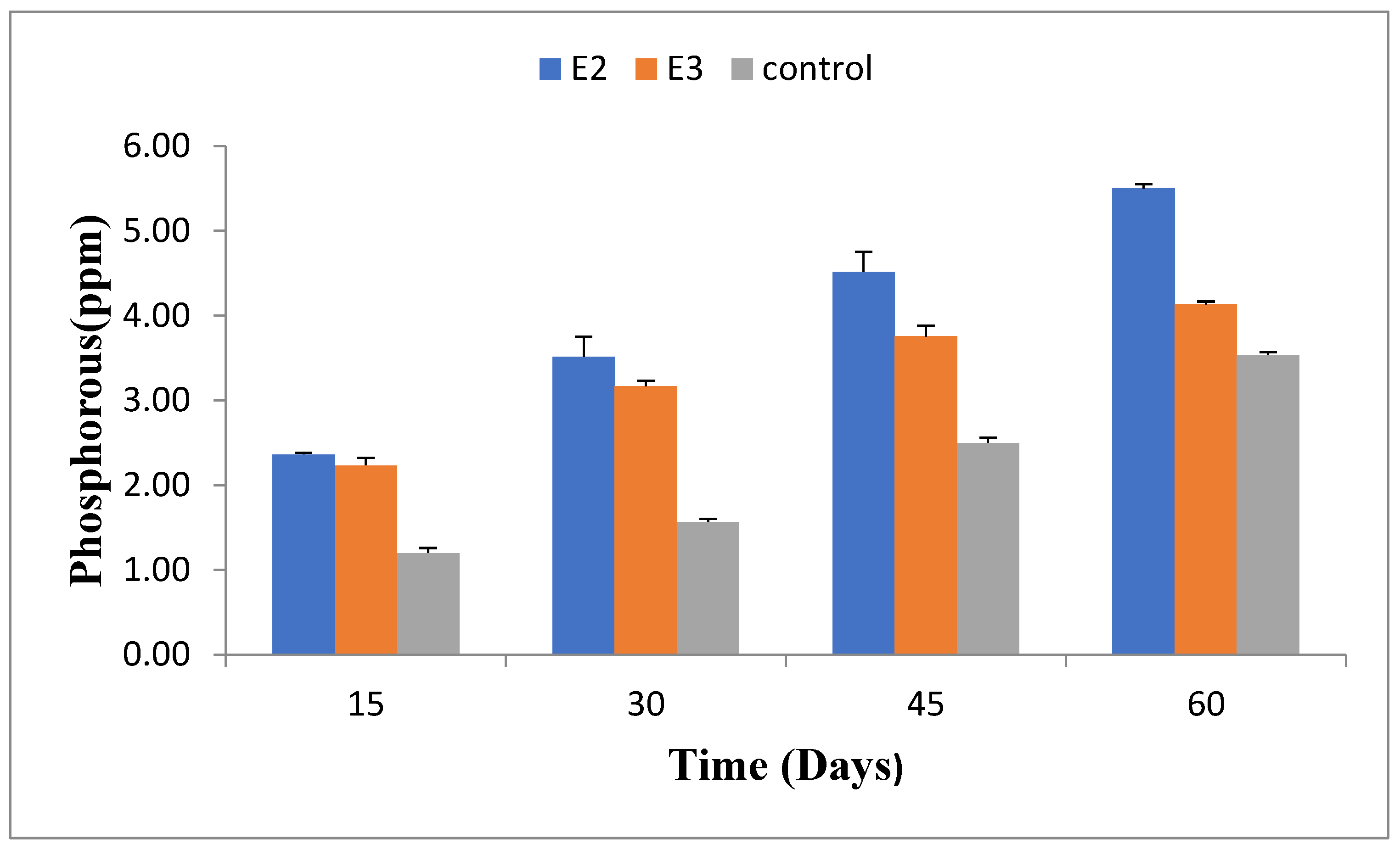
2.8. Phosphorus Concentration(ppm)
During the composting process, total phosphorus was measured at various intervals, specifically on days 15, 30, 45, and 60, and reported in parts per million (ppm). Among the experimental groups, E2 demonstrated the highest phosphorus concentration, reaching 5.41 ppm by day 60. In contrast, the E3 group had a phosphorus concentration of 4.1 ppm at the end of the experiment. The control group exhibited the lowest phosphorus concentration, with a value of 1.98 ppm.
Figure 7.
Total phosphorus concentration in different pressmud compost groups enriched with/without phosphorus-solubilizing and nitrogen-fixing bacteria (E2: Bacillus amyloliquefaciens- ASK 11; E3: Bacillus megaterium- ANF3) over various time intervals (days).
Figure 7.
Total phosphorus concentration in different pressmud compost groups enriched with/without phosphorus-solubilizing and nitrogen-fixing bacteria (E2: Bacillus amyloliquefaciens- ASK 11; E3: Bacillus megaterium- ANF3) over various time intervals (days).
2.9. FT-IR Analysis of Compost
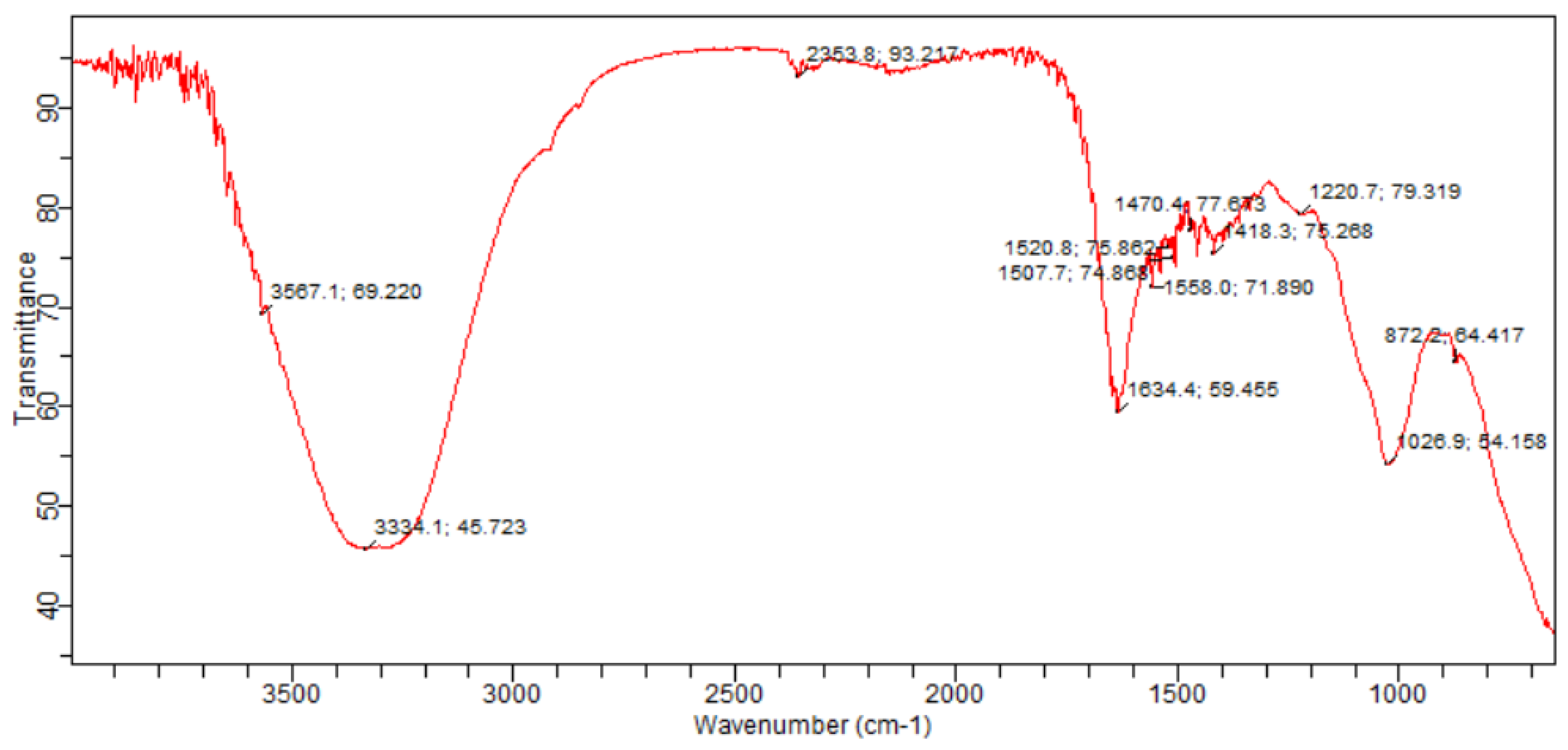
2.9.1. Experimental Setup E2 pressmud Compost Analysis
The FT-IR spectra of the biofortified compost sample from experimental setup E2 showed distinct absorbance peaks. The broad absorption bands observed at 3567 cm⁻¹ and 3334 cm⁻¹ correspond to O-H stretching vibrations, indicative of hydroxyl groups. Vibrations associated with hydrogen stretching bonds, such as H₂O, OH, and NH₃, were identified. A peak at 2353 cm⁻¹ was attributed to the C-C cyclic group, which also suggests the presence of graphite-like carbon bond vibrations. The narrow peaks at 1507 cm⁻¹ and 1520 cm⁻¹ were characteristic of aromatic rings with double bonds. A sharp peak at 1634 cm⁻¹ indicated unsaturated double and triple bonds. The fingerprint region showed peaks at 1470 cm⁻¹ and 1418 cm⁻¹, representing organic phosphate. The peak at 1025 cm⁻¹ was assigned to P-O-C stretching in organic phosphate compounds, while the peak at 872 cm⁻¹ indicated aromatic C-H stretching bonds.
Figure 8.
FT-IR spectra of mature press mud compost from experimental setup E2 bioaugumented with Bacillus amyloliquefaciens- ASK 11 after 60 days.
Figure 8.
FT-IR spectra of mature press mud compost from experimental setup E2 bioaugumented with Bacillus amyloliquefaciens- ASK 11 after 60 days.
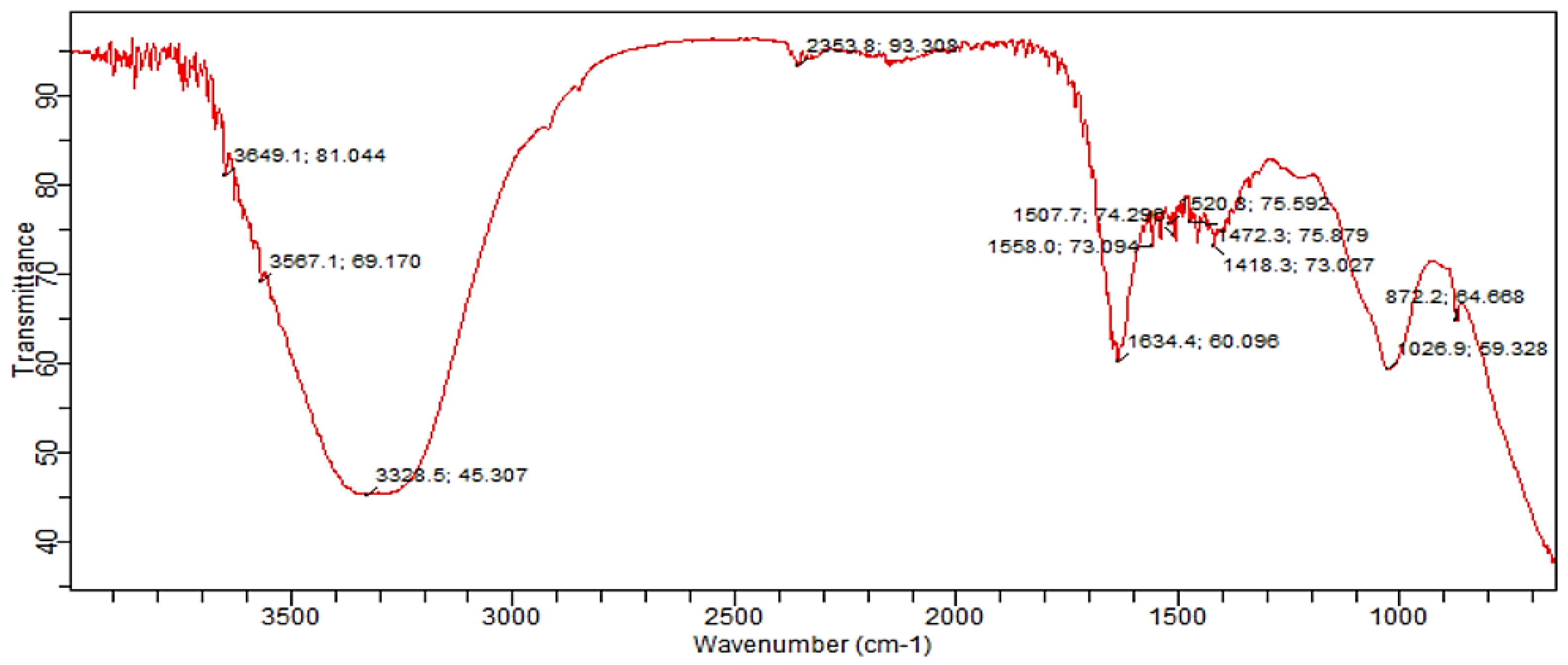
2.9.2. Experimental Setup E3: Compost Analysis
The FT-IR spectra of the compost sample from experimental setup E3 provided insights into the composting process and maturity. The O-H stretching vibrations at 3849 cm⁻¹ and 3567 cm⁻¹ indicated a continuous increase in band height, correlating with the maturation of the organic material. The wavenumber at 3328 cm⁻¹ was associated with carboxyl and hydrogen bonds in phenolic and alcoholic groups. A broad band at 2905 cm⁻¹ corresponded to C-H stretching vibrations in cellulose. Dual peaks identified at 2353 cm⁻¹ were indicative of C-C cyclic bonds and graphite-like structures. Several peaks at 1507 cm⁻¹, 1520 cm⁻¹, and 1558 cm⁻¹ were attributed to aromatic carbon rings (C-H). A sharp peak at 1634 cm⁻¹ was associated with unsaturated double and triple bonds, as well as the amide group. The fingerprint region contained peaks related to ethanol and C-C stretching bonds, with lignin present at 1472 cm⁻¹ and organic sulfate at 1418 cm⁻¹. An aliphatic compound was identified at 1026 cm⁻¹. Additionally, stretching bands at 1090-1110 cm⁻¹ suggested the presence of polysaccharides. The bands at 1680-1638 cm⁻¹were associated with protein origins, corresponding to amide I and II. The absorption bands at 2820 cm⁻¹ and 2950 cm⁻¹ were attributed to aliphatic methylene groups, indicating the presence of lipids and fats. The bands at 3750 cm⁻¹ and 3900 cm⁻¹ were representative of O-H stretching vibrations.
Figure 9.
FT-IR spectra of mature pressmud compost from experimental setup E3 bioaugumented with B. megaterium- ANF3 after 60 days.
Figure 9.
FT-IR spectra of mature pressmud compost from experimental setup E3 bioaugumented with B. megaterium- ANF3 after 60 days.
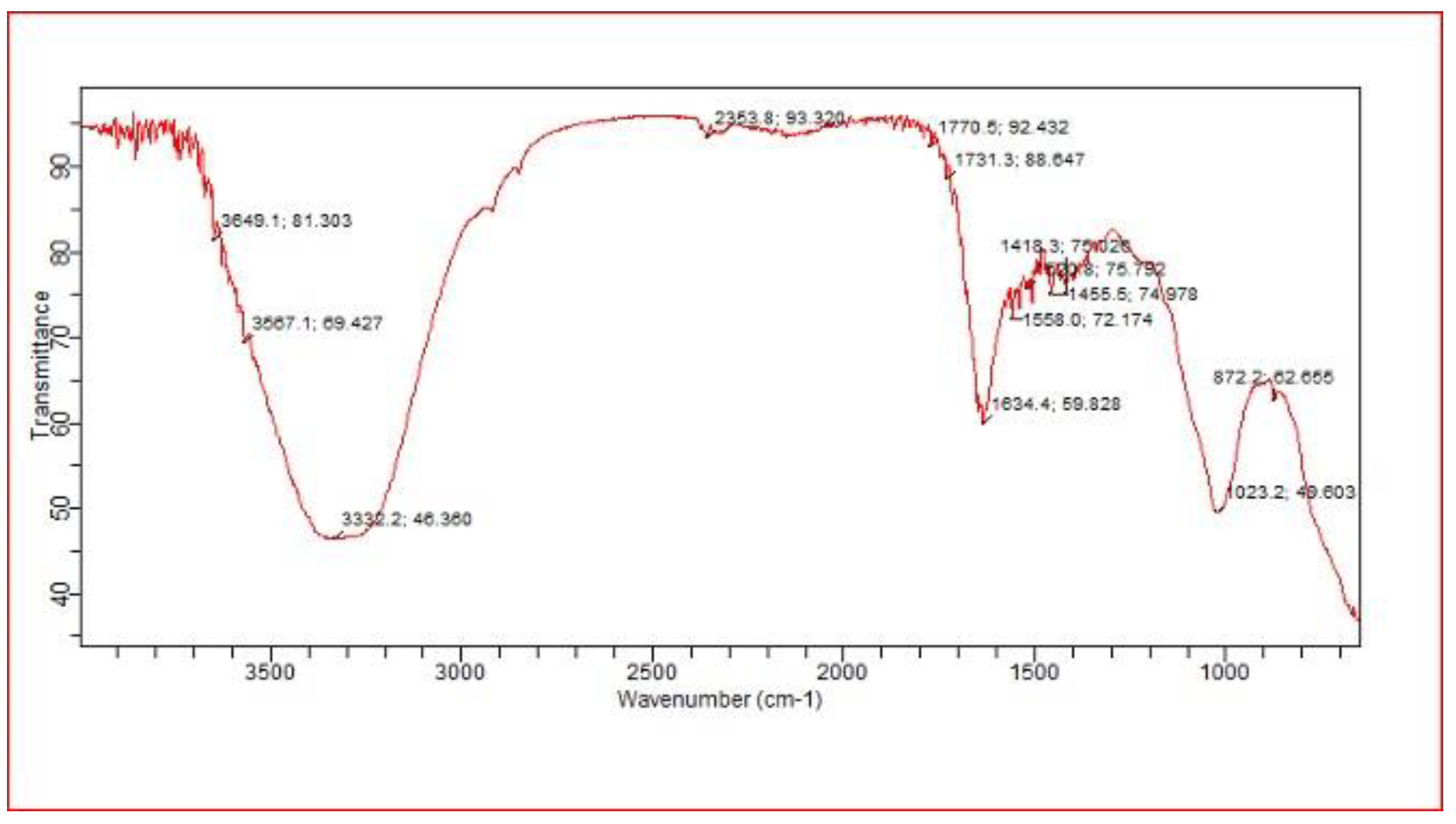
2.9.3. FT-IR Spectrum of Controll Pressmud Compost
The FT-IR spectra of the control sample (E5) revealed a peak at 3649 cm⁻¹ corresponding to C-H stretching bands, with no evidence of hydrogen-linked groups. A peak at 2353 cm⁻¹ was attributed to cyclic carbons (C-C), graphite, and alcoholic groups. The sharp peak at 1770 cm⁻¹ indicated the presence of aldehyde, ketone, and ester bonds. A sharp peak at 1558 cm⁻¹ was characteristic of aromatic ring compounds. Methyl and symmetric bands were observed at 1455 cm⁻¹ and 1418 cm⁻¹, while a narrow band at 872 cm⁻¹ was associated with aromatic C-H stretching bonds.
Figure 10.
FT-IR spectra of mature pressmud compost of control group without bioaugumented after 60 days.
Figure 10.
FT-IR spectra of mature pressmud compost of control group without bioaugumented after 60 days.
3. Discussion
This study aimed to biofortify sugarcane waste compost through the application of nitrogen-fixing and phosphorus-solubilizing bacterial strains, with the objective of developing a potent biofertilizer. The biofortified compost primarily consisted of essential nutrients, including calcium (Ca), nitrogen (N), phosphorus (P), and potassium (K), which are crucial for soil fertility and optimal plant growth [
10]
Regular amendments of compost to soil have been shown to enhance nutrient concentrations significantly; however, they may also increase levels of heavy metals, raising environmental concerns. Therefore, it is essential to develop compost formulations that not only provide nutrients in a controlled manner but also minimize the risk of metal accumulation. The introduction of metal-reducing bacteria, such as
Bacillus megaterium-ASNF3 and
Bacillus amyloliquefaciens-ASK11, into pressmud compost can produce a unique compost with dual benefits. These strains can enhance the bioavailability of nitrogen and phosphorus while simultaneously reducing the toxicity of heavy metals. By facilitating the reduction of metal ions, these microbial strains help mitigate the potential environmental risks associated with metal accumulation, ensuring a safer and more effective biofertilizer for agricultural use[
11,
12].
In the current study, the nitrogen content in the compost was measured at 6.98% when treated with nitrogen-fixing bacterial bioaugmentation, demonstrating a notable increase compared to the control group lacking such treatment [
13]. This aligns with findings from Wang et al. [
14], where thermophilic nitrogen-fixing strains, including *Bacillus subtilis* (NF1) and *Azotobacter chroococcum* (NF2), were employed in cow dung compost. Their research indicated that these strains effectively raised total Kjeldahl nitrogen concentrations by 38.43% to 55.35% and extended thermophilic conditions, thereby improving overall compost quality.
Furthermore, phosphorus solubilizing bacteria, specifically *Bacillus amyloliquefaciens* (ASK11), demonstrated an increase in phosphorus concentration of E2 by 5.41 ppm compared to compost without bioaugmentation. This release of bioavailable phosphorus from pressmud is crucial for plant utilization, as traditional composting methods may not sufficiently enhance phosphorus availability for crops.
A proper carbon-to-nitrogen (C/N) ratio is vital for effective composting, with an optimal ratio of approximately 30 [
15]. Deviations from this ratio can lead to anaerobic conditions and nutrient loss, impacting microbial activity and compost quality[
16]. In our study, Bacillus megaterium-ASNF3 bioaugumented pressmud compost exhibited a maximum temperature of 49 °C, while the compost with B. amyloliquefaciens- ASK11 reached up to 54 °C. The pH of the experimental compost cans changed from neutral to alkaline while a very slight shift of pH from neutral to slightly aklaline was observed. Consistent with findings from Rich and Bharti [
17], our results indicated a maximum pH value of 8.6 for the E3 treatment, suggesting the stability of microbial communities under these conditions.
Increased electrical conductivity at the experiment’s outset coorelated with microbial activity [
18]. This underscores the potential of microbial interventions as environmentally friendly techniques for effective waste degradation and nutrient recycling. The E2 treatment exhibited a waste mass degradation rate of 2.9%, while E3 achieved 3.5%, corroborating the biodegradation capabilities of *B. amyloliquefaciens* as noted by Ayilara et al. [
19]. Various physical and chemical parameters significantly influence biodegradation efficacy, highlighting the interdependence of these factors on microbial activity[
20].
Overall, this study demonstrates that the application of *Bacillus* strains can significantly enhance nitrogen and phosphorus contents in pressmud compost, making these nutrients more biologically available for plant uptake. The measured phosphorus concentration of 5.41 ppm in the E2 treatment at 60 days, along with the 6.98% nitrogen content attributed to the activity of B. megaterium, emphasizes the potential of these microbial agents in sustainable agriculture.
4. Material and Methods
4.1. Preparation of Consortia
Pure cultures of two bacterial strains named
Bacillus amyloliquefaciens-ASK11 and Bacillus amyloliquefaciens-ASNF3 with accession No. KC527054 and KC527057, respectively, were collected from Microbial Biotechnology Laboratory of the Punjab University, Lahore and used in this study. These strains are well known for their nitrogen fixing and cellulose degrading potential together with their heavy metals reduction ability [
21,
22]. The phosphorous solubilizing ability of
Bacillus amyloliquefaciens-ASK-11 was determined following the methods of Amri et al. [
23].
4.2. Inoculum Preparation
Small amount of the bacteria from the slants were revived in nutrient broth. For pure culturing pic up single colony and transfer on ager plate then added equal quantity of bacterial consortia into the compost. For the maturation of compost was left for 60 days, during maturation physiochemical parameters were checked regularly. Bacterial refresh in nutrient broth for 36 hrs at 38 ℃ temperature. Later for preparation of inoculum for compost the 0.1ml of the bacterial culture was inoculated into the 500ml flask containing 300ml of nutrient broth .The culture incubated for 48 hour at the optimum growth condition as described in [
21,
22]. The growth was then harvested by centrifugation at 6000rmp.The microbial pellets were then suspended in sterilized distilled water after repeated wash with water. This culture was use for the corresponding compost treatment as bacterial inoculum.
4.3. Compost Formation
The experiment for the compost formation was established in microbial Biotechnology and aquatoxicology laboratory of Zoology Department of GC Women University, Faisalabad in the plastic cans. The aeration was supplied with the help of sterilized piping connected to the aerator. The sterilization of the air was ensured by plugging with the cotton. Compost of sugar cane press mud was prepared by adding three basic ingredients i.e., (1) Pressmud (2) Brown material (3) Green material containing vegetable waste and fruit scraps from the kitchen and green grass clippings. Addition of these two ingredients in the press mud was done appropriately to manage the C: N ratio between 26- 36 [
24]. Two experimental setups designated as E2, E3 and a control group E5 were established on the basis of microbes processing the compost. Experimental group E2 contained
B.amyloliquificens-ASK11 while the experimental group E3 contained
B. megaterium-ASNF3. The controlled group was without bioaugumentation. All three setups were processed together with their indigenous microbes.
Figure 11.
The material used for composting explained as: (a) green material (b) brown material and (c) pressmud for compost formation.
Figure 11.
The material used for composting explained as: (a) green material (b) brown material and (c) pressmud for compost formation.
4.4. Physiochemical Characteristics of Compost
The physical and chemical characteristics of pressmud biofortified compost are crucial indicators of its quality and suitability for agricultural use. There were some physical and chemical properties associated with biofortified compost. There were following parameters EC, pH, organic matter, Moisture contents, waste mass degradation, temperature were determined in the compost.
4.4.1. Moisture Content
To determine the moisture contents 2g sample of compost was weighed on a weight machine W1. After it the sample was spread thinly and evenly on a tray and placed in an oven at -70°C (140-160°F) for -5 hour. After that the sample was cooled and weighed again as W2.The moisture content % was determined by following formula [
25].
4.4.2. Variation in Temperature
Maintaining optimal compost temperature is essential for efficient decomposition, pathogen reduction, and the production of high-quality compost. The temperature of the compost was maintained by carefully selected of the seasons as well as laboratory place. The temperature of the compost was monitored by using thermometer inserted into the compost. The compost was Turned or aerated if temperature was raised while moisture levels were adjusted moisture level and proper insulation was ensured to regulate compost temperatures and promote efficient decomposition throughout the composting process [
26].
4.4.3. Determination of pH Value
The value of pH was determined by using pH meter (inno TECH- pH 18 model).To determine the pH a compost suspension (water mixture) was prepared with 1g compost suspended into10mL of distilled water in a glass jar. The suspension was mixed and the pH was measured afterwards in the aqueous solution. The electrode of the pH meter was dipped into the suspension and noted reading of each experimental group on pH meter [
27].
4.4.4. Electrical Conductivity
To determine the EC, 50 mL fresh compost and 250 mL water was placed in an Erlenmeyer flask of 500 ml. The suspension was shaken for 1 hour with an agitator and filtered afterwards. The EC was measured in the water extract on EC conductivity meter [
28].
4.4.5. Organic Matter (OM)
The organic matter was determination by following the method of Masood et al. [29]. For determination of (OM) cleaned the crucible and placed into muffle furnace at 600oC for 1hour. Then it was cooled and weighed the empty crucible. The 2 gram compost sample was taken in crucible and notes the weight. The compost filled crucible was placed into muffle furnace at 550
oC for 4hours. This process indicate the completely oxidation of organic matter with appearing white grey matter called ash and weight the ash which is present in the crucible. The organic matter was calculated by using the following equation
(W1) = weight of empty crucible
(W2) = weight sample filled crucible
(W3) = weight the ash
Difference of weight in ash= (W1- W2)
4.4.6. Waste Mass Degradation
Applying density formula waste mass degradation was calculated at different time intervals 15, 30, 45 and 60 days [
29].
4.4.7. Determination of Nitrogen in Sample
The Kjeldahl metho [
30]was used to determine the nitrogen in the compost. The method was consists of three basic steps. Digestion, distillation and quantification[.
For digestion 1g sample of compost was added into a clean crucible. Five gram digestion mixture (Potassium sulphate 100g + Copper sulphate 10g + pherric sulphate 5g) and 25-30 ml sulphuric acid were then added into the compost sample then placed it in digester for one and half hour or until light green color appear later distilled water was added to make the volume 250 ml in the volumetric flask. 10 ml of sample solution was taken in Kjeldhal distillation apparatus and added 10 ml of 40% NaOH solution in it. Another flask containing 10 ml of 4% boric acid were taken. Allowed the water to boil so that steam enters into kjeldhal flask containing sample, fumes of ammonia gas were produced and passed through the condenser into 4% solution of boric acid until boric acid became colorless.
The colorless solution of boric acid was titrated with N/10 H
2SO
4 till color of boric acid reappeared. Recorded the volume of acid used. The % N in the sample was then determined using following formula.
4.4.8. Analysis of Phosphorus in Compost
Grind the sample (BFC) add a suitable acid, such as concentrated nitric acid (HNO3) or a mixture of nitric acid and perchloric acid (HNO3 + HClO4), in the sample for digestion. Heat the mixture in the incubator until the sample is completely digested and a clear solution is obtained. After digestion, cool it at room temperature. Filter the solution by using a filter paper to remove any undigested residue. Diluted the solution 10 times .Determine the phosphorus concentration into the sample by using by using spectrophotometer at UV- 1800nm via molybdenum blue method [
31,
32].
5. Conclusion
The biofortification of the pressmud generated from the sugarcane industry through the application of microbial strains is an environmentally friendly technique. In this study, Bacillus amyloliquefaciens and Bacillus megaterium were utilized to enhance nitrogen and phosphorus content after 60 days of composting. This approach effectively degrades waste mass and recycles nutrients, thereby improving compost efficacy. The use of these bacterial strains facilitates the efficient utilization of by-products from sugarcane factories. Consequently, this waste material can be transformed into biofertilizers, enhancing both the quality and quantity of crops, ultimately supporting increased agricultural yields to meet the demands of a growing population.
Author Contributions
Conceptualization, U.S. and S.A.; methodology, U.S. A.S; software, S.A.; validation, U.S., S.A. and T.M.; formal analysis, A.H.; investigation, S.K.; resources, U.S and T.M.; data curation, U.S. and S.K.; writing—original draft preparation, U.S. and S.A.; writing—review and editing, U.S. and S.A.; visualization, A.S.; supervision, S.A. and A.S; project administration, T.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was conducted without any external funding.
Acknowledgments
The authors are very thankful and acknowledge the Microbial Biotechnology Laboratory of institute of Zoology, University of the Punjab, Lahore for providing bacterial strains.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pawlak, K.; Kołodziejczak, M. The role of agriculture in ensuring food security in developing countries: Considerations in the context of the problem of sustainable food production. Sustainability. 2020, 12, 5488. [Google Scholar] [CrossRef]
- Skrzypczak, D.; Trzaska, K.; Mironiuk, M.; Mikula, K.; Izydorczyk, G.; Polomska, X.; Wisliewski, J.; Mielko-Nizialek, K.; Mustakas, K.; Chojnacka, K. Recent innovations in fertilization with treated digestate from food waste to recover nutrients for arid agricultural fields. Environ. Sci. Poll. Res. 2023, 1–23. [Google Scholar] [CrossRef]
- Perera, G. G.; Wijesekara, J. P.; Subhashini, P.; Ekanayake, K. V.; Rashid, M. R. EcoGrowAdviser: Organic fertiliser recommendation system to enhance organic fertilization process-a case from Sri Lankan farming industry. Int. J. Agric. Ext. 2024, 12, 35–49. [Google Scholar]
- Bokhtiar, S. M.; Roksana, S.; Moslehuddin, A. Z. M. Soil fertility and productivity of sugarcane influenced by enriched pressmud compost with chemical fertilizers. SAARC J. Agri, 2015, 13, 183–197. [Google Scholar] [CrossRef]
- Iqbal, M. A.; Iqbal, A. Sugarcane production, economics and industry in Pakistan. Am. J. Agric. Environ. Sci. 2014, 14, 1470–1477. [Google Scholar]
- Bokhtiar, S. M.; Sakurai, K. Effects of pressmud compost and chemical fertilizers on soil fertility and the yield and quality of sugarcane. Plant and Soil, 2005, 272, 207–217. [Google Scholar]
- Kumar, S.; Verma, N. K.; Bhardwaj, A. K. Recycling sugar industry waste through composting to produce organic fertilizer for sugarcane. In. J. Re. Organ. Waste. Agric. 2018, 7, 349–357. [Google Scholar]
- Tallapragada, P.; Gudimi, M. Phosphate solubility and biocontrol activity of Trichoderma harzianum. Turk. J. Biol. 2011, 35, 593–600. [Google Scholar] [CrossRef]
- Rakkiyappan, P.; Malathi, S.; Radhamani, S. Effect of pressmud on soil nutrient status and yield of sugarcane in Erode district of Tamil Nadu. Agricultural Reviews. 2012, 33, 46–49. [Google Scholar]
- Ahmed, T.; Noman, M.; Qi, Y.; Shahid, M.; Hussain, S.; Masood, H. A.; Li, B. Fertilization of Microbial Composts: A Technology for Improving Stress Resilience in Plants. Plants. 2023, 12, 3550. [Google Scholar] [CrossRef]
- Rashid, M. I. , Saleem, M., & Azam, F. Bioavailability of Nutrients in Biofortified Compost: The Role of Microbial Strains. Appli. Soil. Ecol. 2016, 107, 61–70. [Google Scholar]
- Alengebawy, A.; El-Masry, M. A.; El-Shafei, M. Composting of Organic Wastes: Heavy Metals and Nutrient Dynamics. Environ. Sci. Poll. Rese. 2021, 28, 18456–18468. [Google Scholar]
- Orr, C. H.; James, A.; Leifert, C.; Cooper, J. M.; Cummings, S. P. Diversity and activity of free-living nitrogen-fixing bacteria and total bacteria in organic and conventionally managed soils. Appl. Environ. Microbiol. 2011, 77, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, X.; Zhao, J. Thermophilic Nitrogen-Fixing Bacteria in Composting: Enhancements in Nutrient Dynamics and Soil Health. J. Soil. Scie. Plant. Nutr. 2024, 24, 199–213. [Google Scholar]
- Gonawala, M. K.; Jardosh, H. Influence of C/N Ratio on Composting Efficiency: A Review. J. Environ. Manage. 2018, 215, 127–135. [Google Scholar]
- Méndez-Matías, A.; Robles, C.; Ruiz-Vega, J.; Castañeda-Hidalgo, E. Composting agroindustrial waste inoculated with lignocellulosic fungi and modifying the C/N ratio. Rev. Mexicana. cienc. agríc. 2018, 9, 271–280. [Google Scholar]
- Rich, S. J.; Bharti, S. Effect of Compost on Soil pH and Nutrient Dynamics. Asian J.Agric. Biol. 2015, 3, 1–7. [Google Scholar]
- Obileke, T. A.; Akinyemi, O. A.; Olaniyi, A. A. Electrical Conductivity as an Indicator of Compost Quality: Implications for Microbial Activity. In. J. Recy. Org. Waste. Agric. 2021, 10, 1–9. [Google Scholar]
- Ayilara, M. S.; Babalola, O. O. Bioremediation of environmental wastes: the role of microorganisms. Front Agron. 2023, 5, 1183691. [Google Scholar] [CrossRef]
- Noorjahan, A. The Role of Environmental Factors in Microbial Biodegradation: A Comprehensive Review. J. Micro. Biode. 2014, 5, 85–92. [Google Scholar]
- Aslam, S.; Ali, H.; Javed, I. Q. Dual action of chromium-reducing and nitrogen-fixing, Bacillus megaterium-ASNF3 for improved agro-rehabilitation of chromium-stressed soils. 3Biotech. 2016, 6, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Ali, H.; Javed, I. Q. Production of Cellulases byBacillusamyloliquefaciens-ASK11 under high chromium Stress. Waste and Biomass Valorization. 2019, 10, 53–61. [Google Scholar] [CrossRef]
- Amri, M.; Rjeibi, M. R.; Gatrouni, M.; Mateus, D. M.; Asses, N.; Pinho, H. J.; Abbes, C. Isolation, identification, and characterization of phosphate-solubilizing bacteria from Tunisian soils. Microorganisms. 2023, 11, 783. [Google Scholar] [CrossRef]
- Salman, M.; Inamullah, Jamal, A. ; Mihoub, A.; Saeed, M. F.; Radicetti, E.; Pampana, S. Composting sugarcane filter mud with different sources differently benefits sweet maize. Agronomy. 2023, 13, 748. [Google Scholar] [CrossRef]
- Sarkar, P.; Chourasia, R. Bioconversion of organic solid wastes into biofortified compost using a microbial consortium. Int. J. Recycl. Org. Waste. Agric. 2017, 6, 321–334. [Google Scholar] [CrossRef]
- Bajko, J.; Fišer, J.; Jícha, M. Temperature measurement and performance assessment of the experimental composting bioreactor. In. EPJ. Web. Confer. 2018, 180, 02003, Vikram, N.; Sagar, A.; Gangwar, C.; Husain, R.; Kewat, R. N. Properties of humic acid substances and their effect in soil quality and plant health. In Humus and humic substances-recent advances. IntechOpen: India, 2022, 1, 1– 14. [Google Scholar] [CrossRef]
- Ameen, A. , Ahmad, J., & Raza, S. Effect of pH and moisture content on composting of Municipal solid waste. In. J. Sci.Res. 2016, 6, 35–37. [Google Scholar]
- Neves, A. C.; da Costa, P.; de Oliveira Silva, C. A.; Pereira, F. R.; Mol, M. P. G. Analytical methods comparison for pH determination of composting process from green wastes. Environ. Eng. Manag. J. 2021, 20, 133–139. [Google Scholar] [CrossRef]
- Masood, S.; Hussain, A.; Javid, A.; Rashid, M.; Bukahri, S. M.; Ali, W.; Aslam, K. Fungal conversion of chicken-feather waste into biofortified compost. Braz. J. Biol. 2022, 83, e248026. [Google Scholar]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M. J.; Asuero, A. G.; Wybraniec, S. An overview of the Kjeldahl method of nitrogen determination. Part I. Early history, chemistry of the procedure, and titrimetric finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Yokota, T.; Ito, T.; Saigusa, M. Measurement of total phosphorus and organic phosphorus contents of animal manure composts by the dry combustion method. Soil sci. plant. nutr. 2003, 49, 267–272. [Google Scholar] [CrossRef]
- Debicka, M.; Jamroz, E.; Bekier, J.; Ćwieląg-Piasecka, I.; Kocowicz, A. The Influence of Municipal Solid Waste Compost on the Tranformations of Phosphorus Forms in Soil. Agronomy. 2023, 13, 1234. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).