Introduction
Since Charles Goodyear discovered the sulfur curing of Natural Rubber in 1839 [
1,
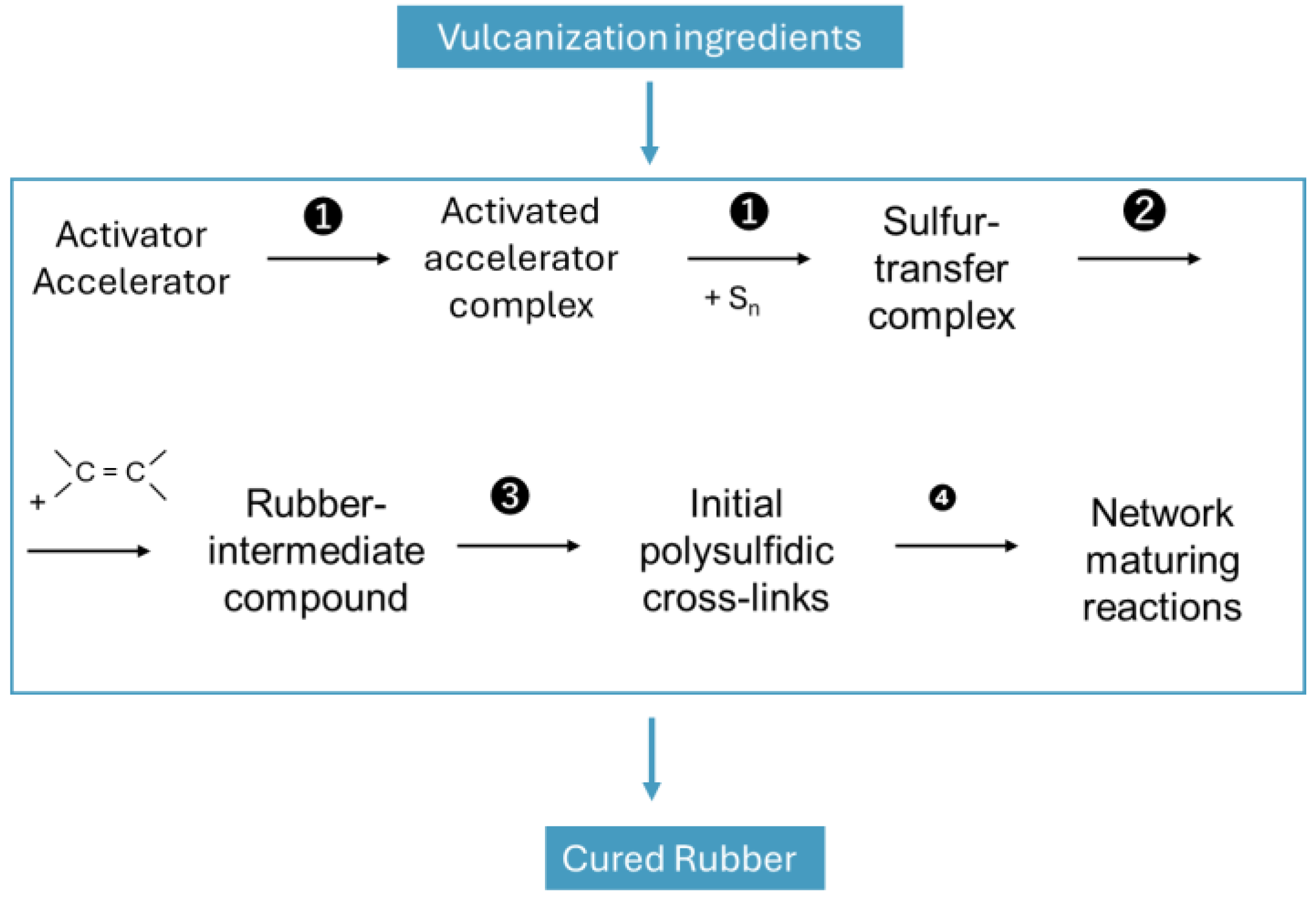
2] many studies were carried out to understand its mechanism. The broadly accepted mechanism was summarized from Morrison and Porter (
Figure 1) [
3].
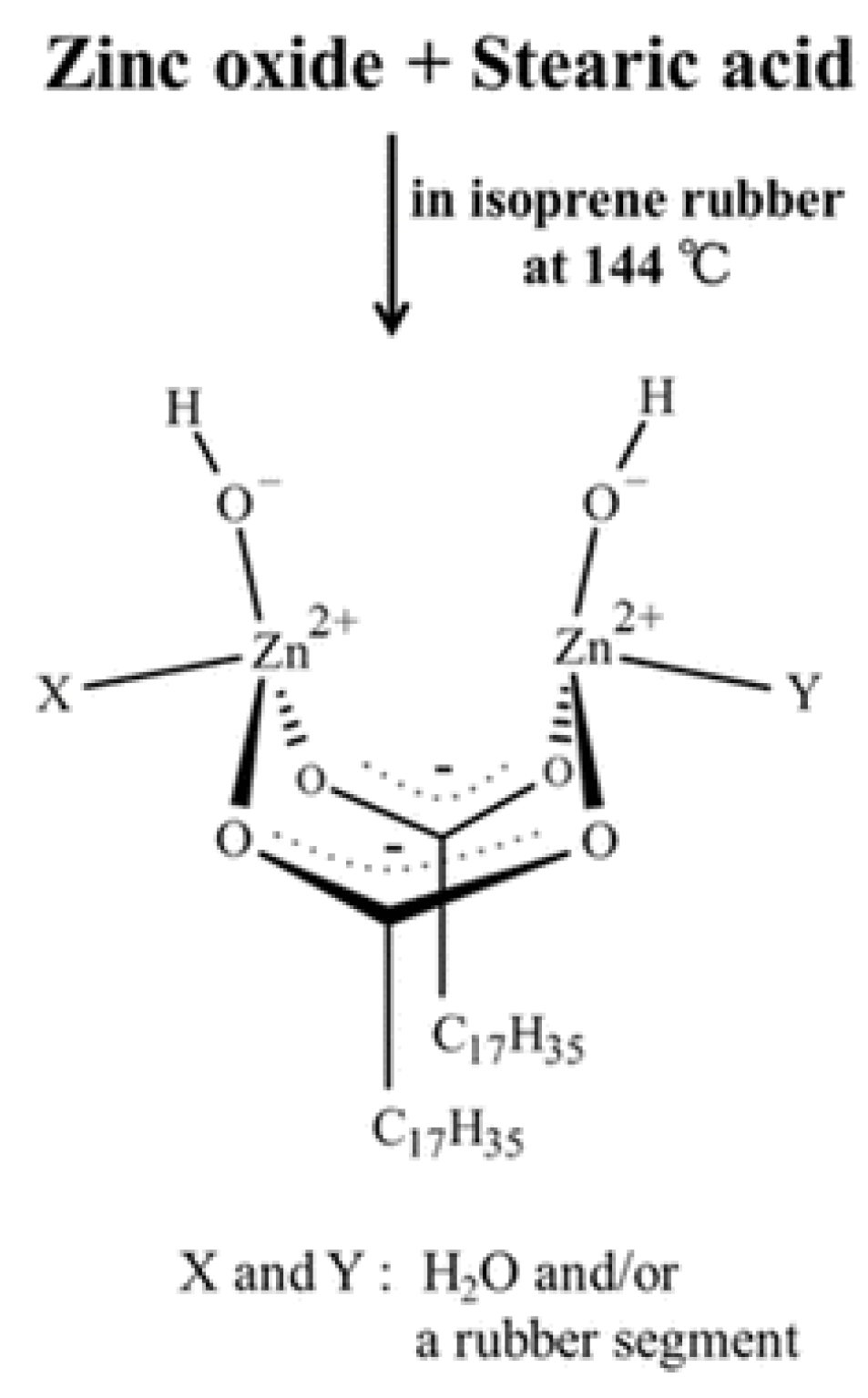
Sulfur attaches in general at the allylic position to the polymer. It is activated by the inclusion in an activated accelerator complex formed by Zinc oxide and stearic acid where accelerator fragments are attached as ligands. Ikeda [
5,
6] investigated this intermediate complex further and concluded that it is a dinuclear bridging bidentate Zinc/stearate complex with the following structure: (Zn
2(μ-O
2CC
17H
35)
2)
2+(OH
–)
2·XY, where X and Y are water and/or a rubber segment (
Figure 2). No information is given how the hydrogen atom is abstracted by this complex, to enable the final polymer-polymer coupling.
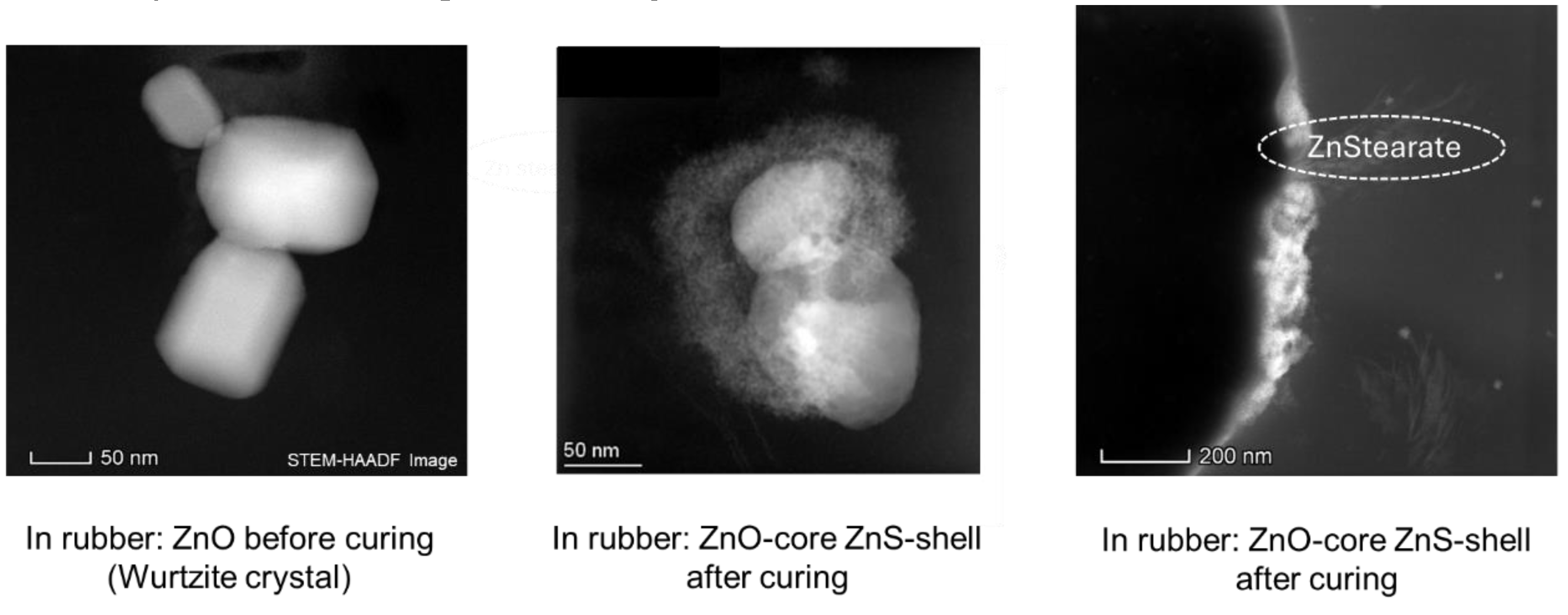
The place where the complex is formed was further investigated by Butuc. She found out that its formation takes place only at the surface of the ZnO crystal. At the end of the curing reaction where sulfur bridges between the polymer chains are formed, ZnS as well as Zn(stearate)
2 are additionally formed as side products (
Figure 3) [
7].
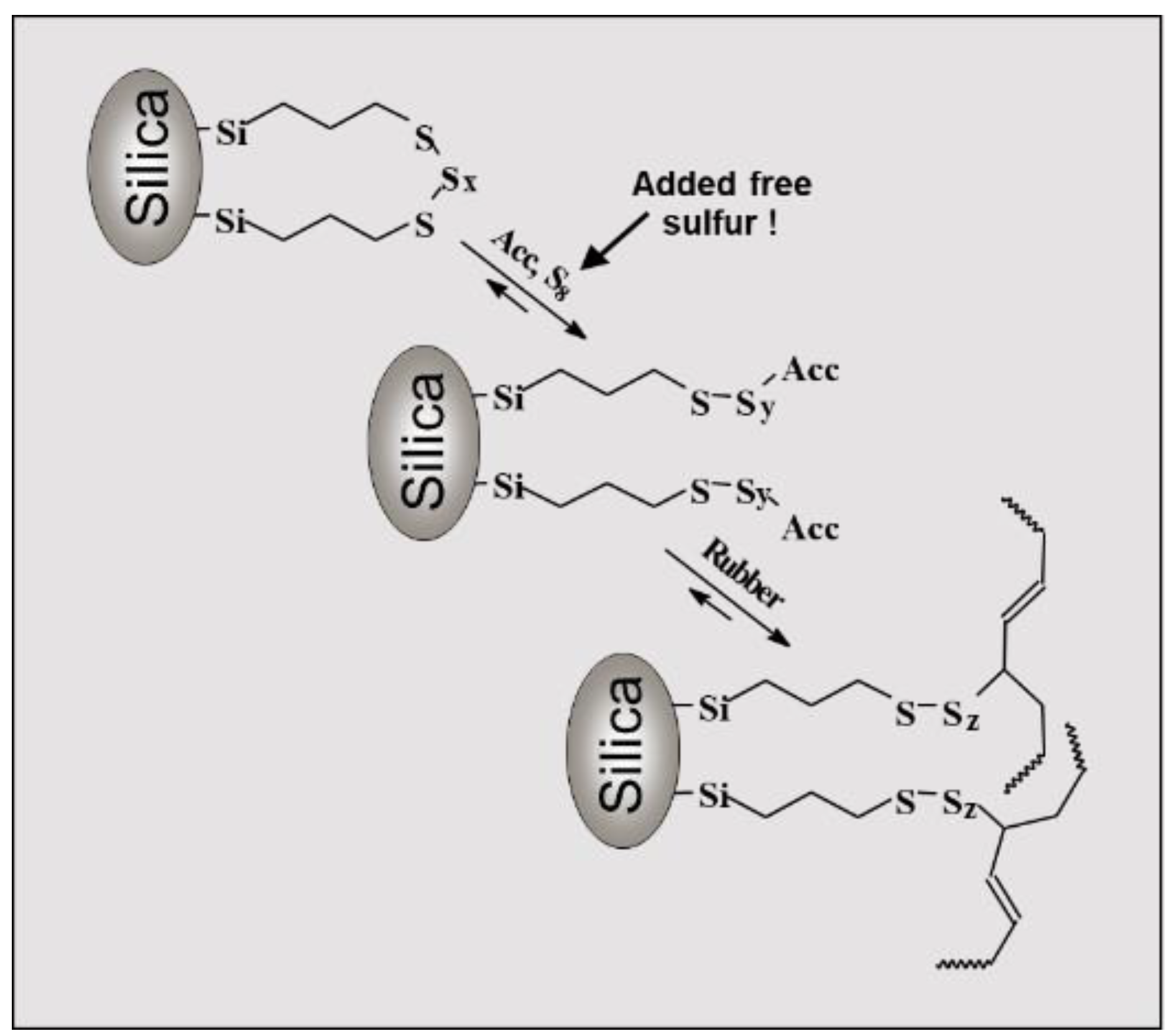
These more general findings can be used to understand the reactions during the production of a tire tread compound in a better way. A modern green tire tread compound is based on a silica / silane filled SSBR/BR rubber compound [
8]. Additionally to the coupling of the polymer chains via sulfur bridges, a new focus has to be put on the silica-silane-polymer bonding via the sulfur-function of the silane in such a compound. It was assumed that the sulfur function of the silane, typically a bis(triethoxysilylpropyl)tetrasulfide, is also connected to accelerators which are part of the active complex (
Figure 4) [
9].
Connecting this assumption to the latest findings of Ikeda and Butuc would lead to the conclusion that the active Zinc complex does not contribute to the formation of the polymer-filler bonds in the assumed way. If the complex is bound to the Zinc oxide surface where it supports the coupling of the sulfur to the mobile polymer chains it is unlikely to assume that the same supporting function takes place for the silane coupled to the non-mobile silica particle. Zinc oxide as well as silica particles are distributed in the polymer matrix, such a supporting function of the complex would have the pre-condition that both solid particles are in connection to each other which is usually not the case [
10].
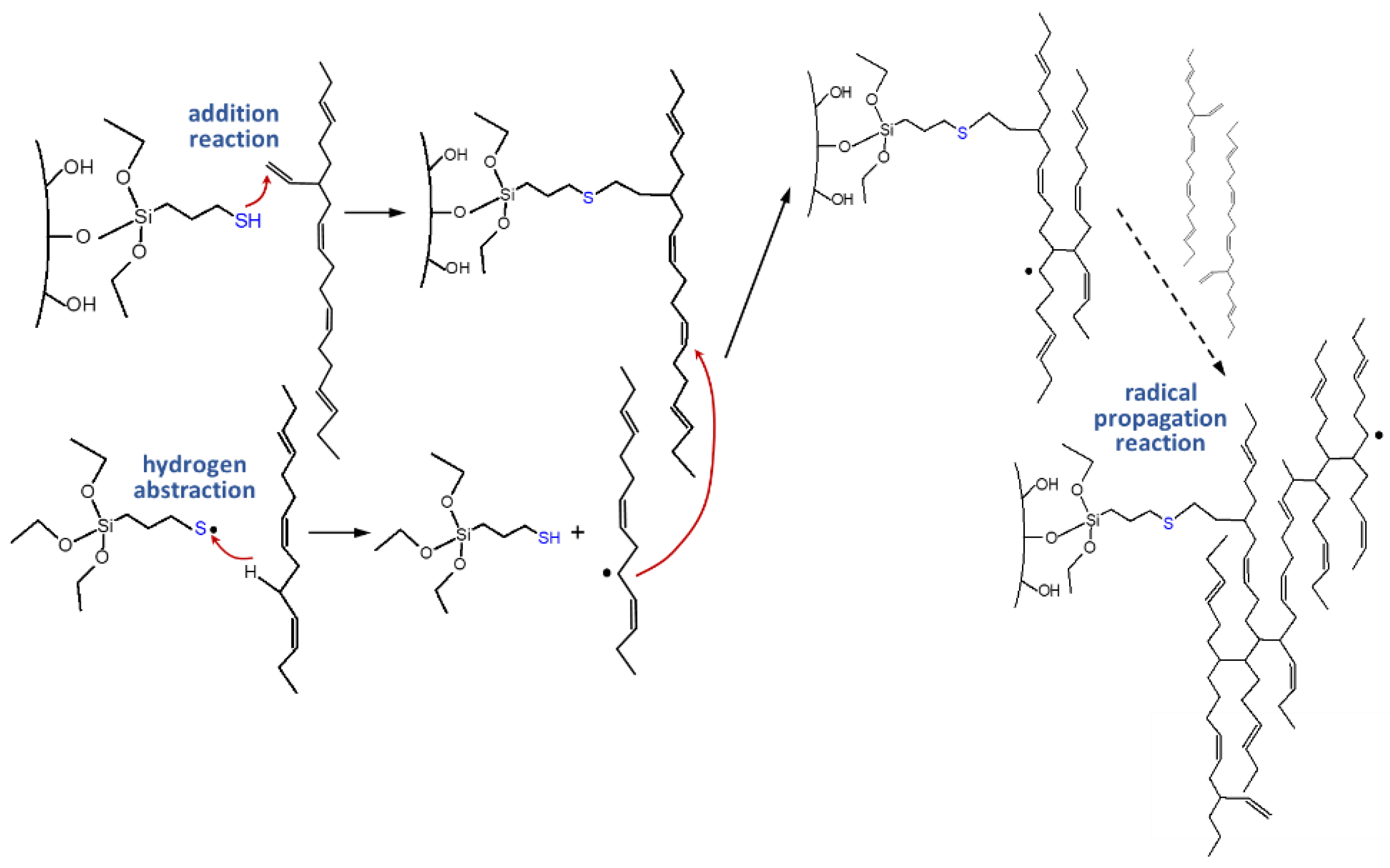
This polymer-filler crosslink reaction between silica and polymer via silane was further investigated by introducing an alternative group of sulfur silanes, the mercaptosilanes. The use of these mercaptosilanes as alternative to the traditional tetra- or disulfide silanes led to an improved dispersion behavior of the silica, an improved rolling resistance but also to a deteriorated processing behavior. Sato investigated their behavior in depth and could conclude that the observed effects were based on combination of addition reaction and radical propagation reaction where the sulfur radical of the mercaptosilane couples directly to the vinyl group of the main polymer, the SSBR, following an Anti-Markovnikov mechanism. This radical reaction leads to the quick generation of a large amount of dense chemical bound rubber, resulting in the observed good dispersion which causes the very low rolling resistance. Due to the fact that this coupling to the vinyl group takes already place during the mixing, the processing was worse and the viscosities increased (
Figure 5) [
11].
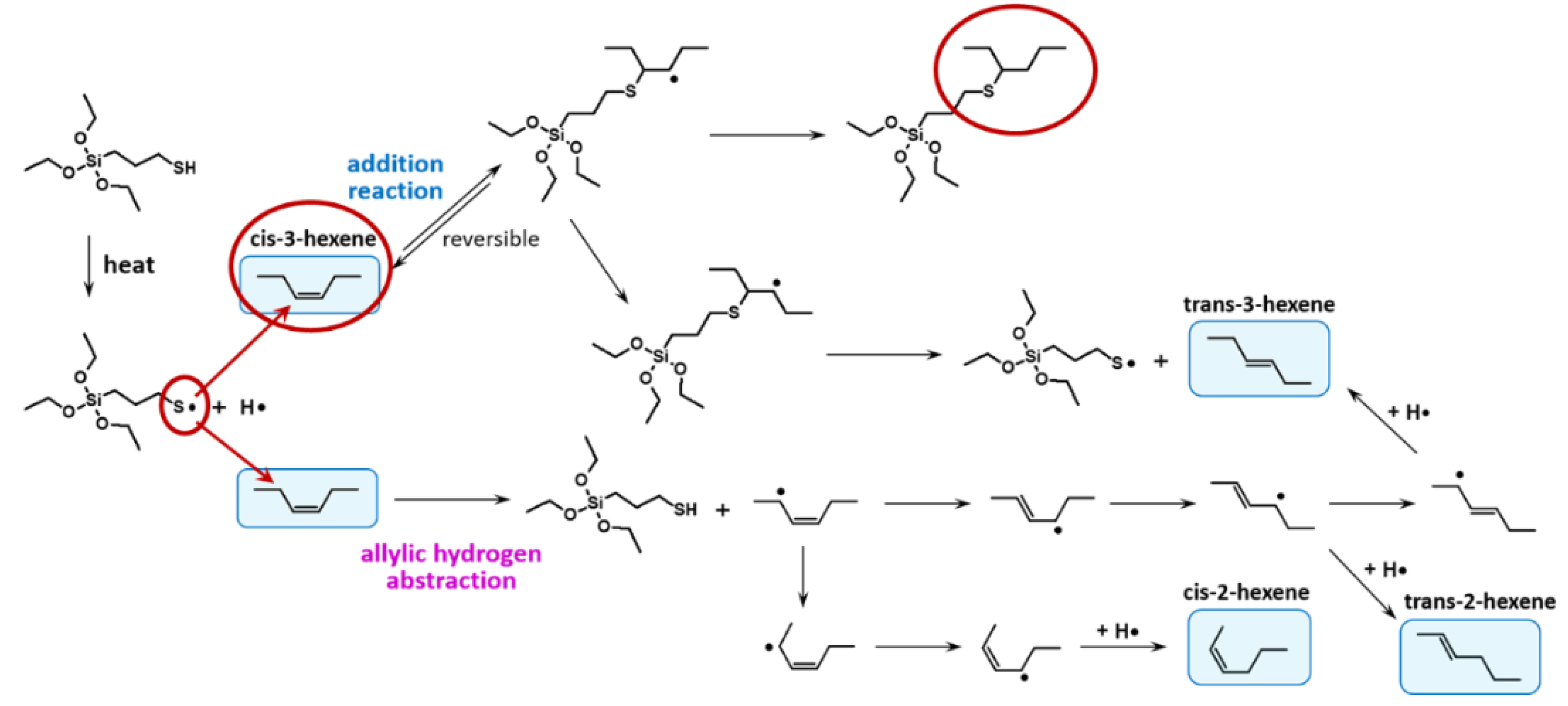
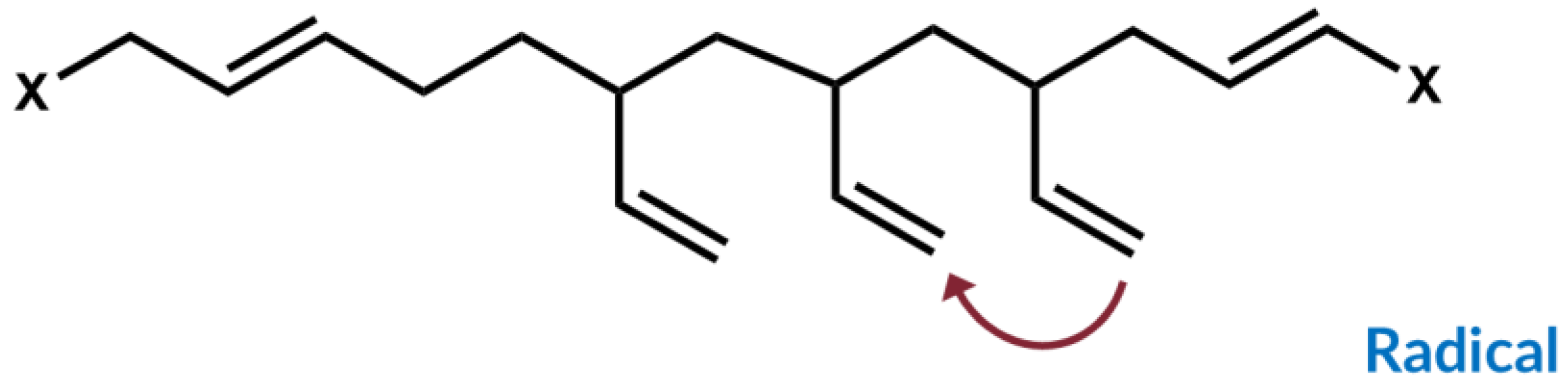
Furthermore, he investigated the possibility to also couple to cis-double bonds and could confirm by model studies with alkenes as well as oligomers that also a direct coupling from the sulfur radical of the mercaptosilane to the cis-double bond can occur, only with a lower yield than the coupling to the vinyl group (
Figure 6). Additionally, cis/trans isomerization could be observed [
11].
As a summary, it can be concluded that a sulfur radical of a mercaptosilane can couple directly to a vinyl double bond as well as to a cis double bond although at a lower yield. This means that the previous assumed support of the Zinc complex for a faster coupling reaction is not required in this system. This leads to the consideration that a similar process might also explain the coupling of the silane-modified silica to the polymer. If this coupling follows indeed a different mechanism as assumed it raises the question if the whole sulfur coupling in an SSBR/BR-based tire tread compound has to be re-considered.
Research Questions
Therefore, the following main research questions should be answered in the current study: If a sulfur radical from a mercaptosilane can couple directly preferably to a vinyl double bond can such direct coupling also occur from an opened sulfur ring to the vinyl group? Is this opening triggered only by temperature or is the presence of supporting molecules like accelerators required? Is it required to “re-think” the traditional coupling of the sulfur to the allylic position of the polymer as described before?
Re-Evaluation of Literature Data
To answer these questions, firstly, the curing behavior of sulfur-cured high-vinyl and low-vinyl SSBR was investigated. Such a study is already described in literature (
Figure 7) [
12]. The curing behavior of high and low vinyl SSBR was investigated in a model green tire tread compound with 2.5 phr DPG, 0.2 phr TBzTD, 1.6 phr CBS, 2 phr sulfur.
It can be observed that the presence of high vinyl having a lower styrene content leads to much faster curing and a higher final torque. The higher vinyl SSBR has also a higher amount of styrene. This means that it has less butadiene units which results in less allylic positions to couple to. Therefore, a lower torque should be expected when the torque is directly connected to the crosslink density without changing any other parameters like filler dispersion. Furthermore, the cure rate should be the same if the same coupling mechanism is assumed. This aspect was not yet considered in this study.
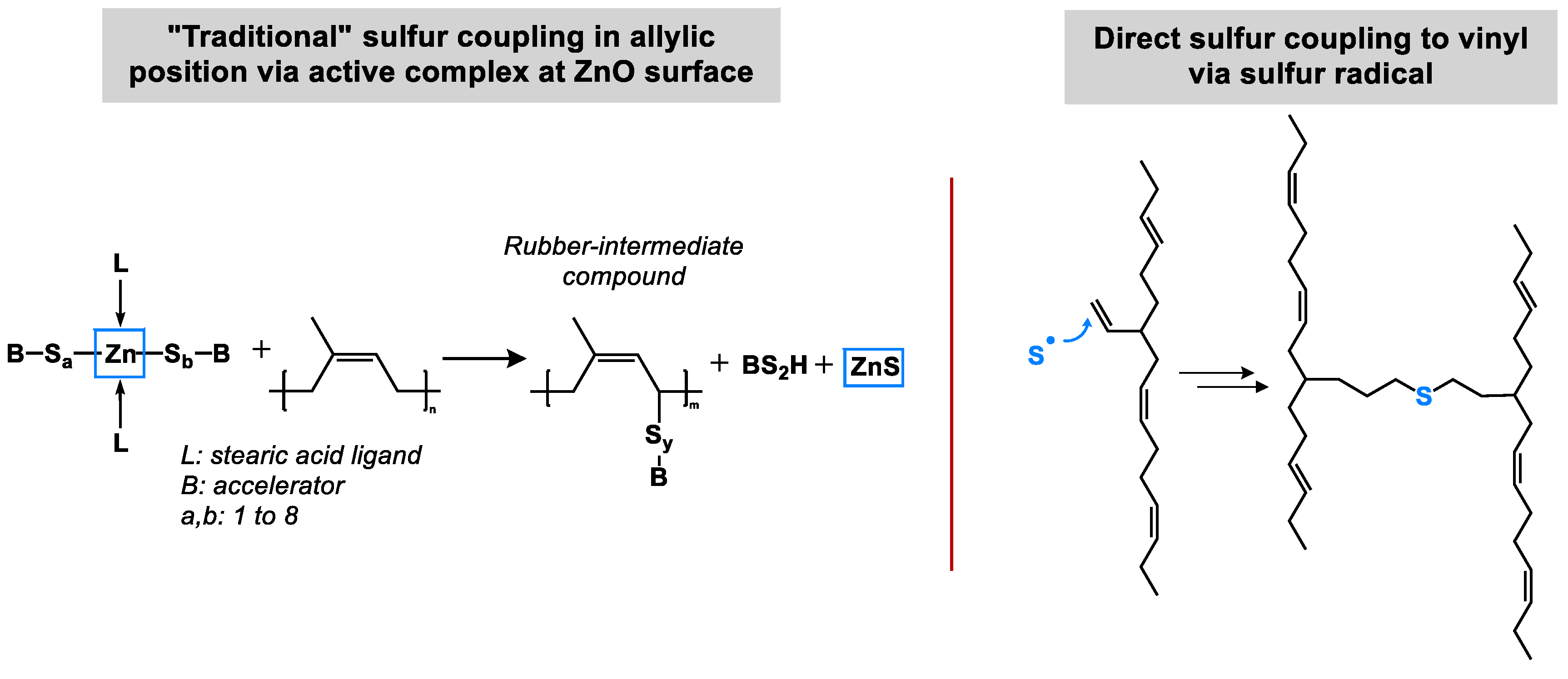
Proposal for an Alternative Sulfur Coupling Mechanism
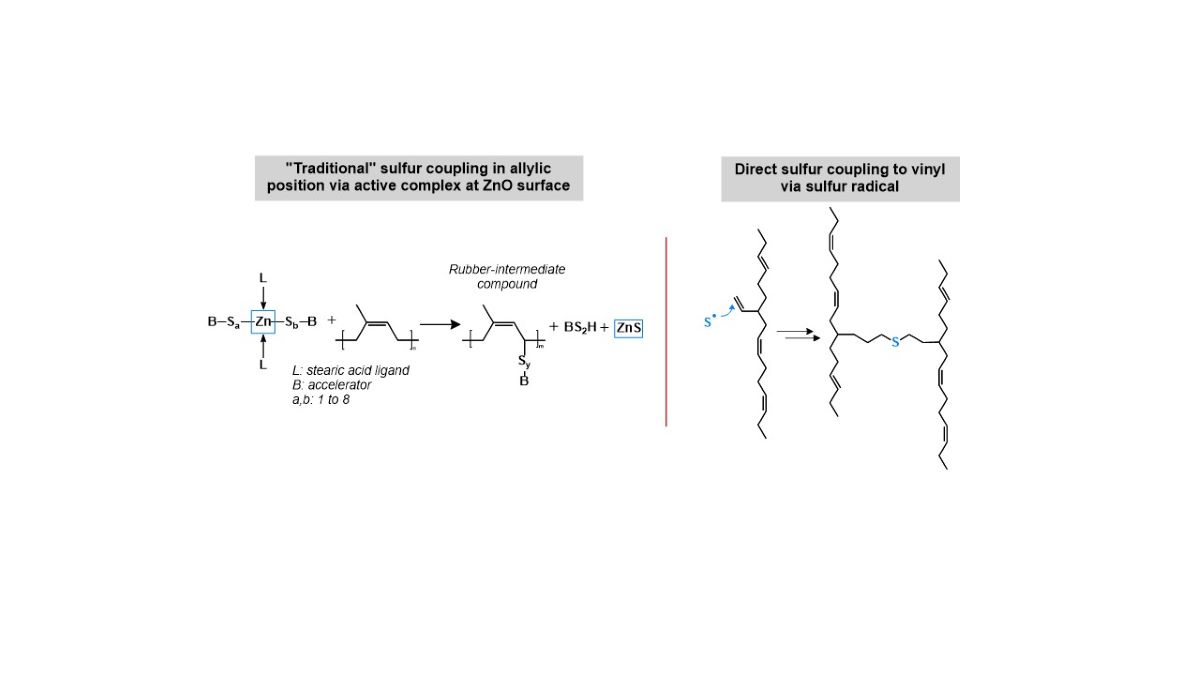
This is the first hint which can be found in literature when the already presented results are re-evaluated with a new focus. It can be assumed that two different mechanisms have to be considered for sulfur coupling. The „traditional“ sulfur coupling occurs in allylic position via an active complex at the ZnO surface. The alternative, a direct sulfur coupling, would occur by a direct binding of a sulfur radical to a vinyl double bond (
Figure 8). This direct coupling needs a trigger for the opening of the sulfur ring to form the sulfur radical. It can be the impact of the temperature or the accelerator or a combination of both. It is assumed that the Zinc complex does not play a role here.
The big difference (beside the position where the sulfur couples) between both mechanisms is that the “traditional” sulfur coupling needs activators but the direct coupling does not. Therefore, the logical approach to investigate this further is to cure high and low vinyl SSBR with and without activators.
Curing Behavior of High Vinyl SSBR versus Low Vinyl SSBR
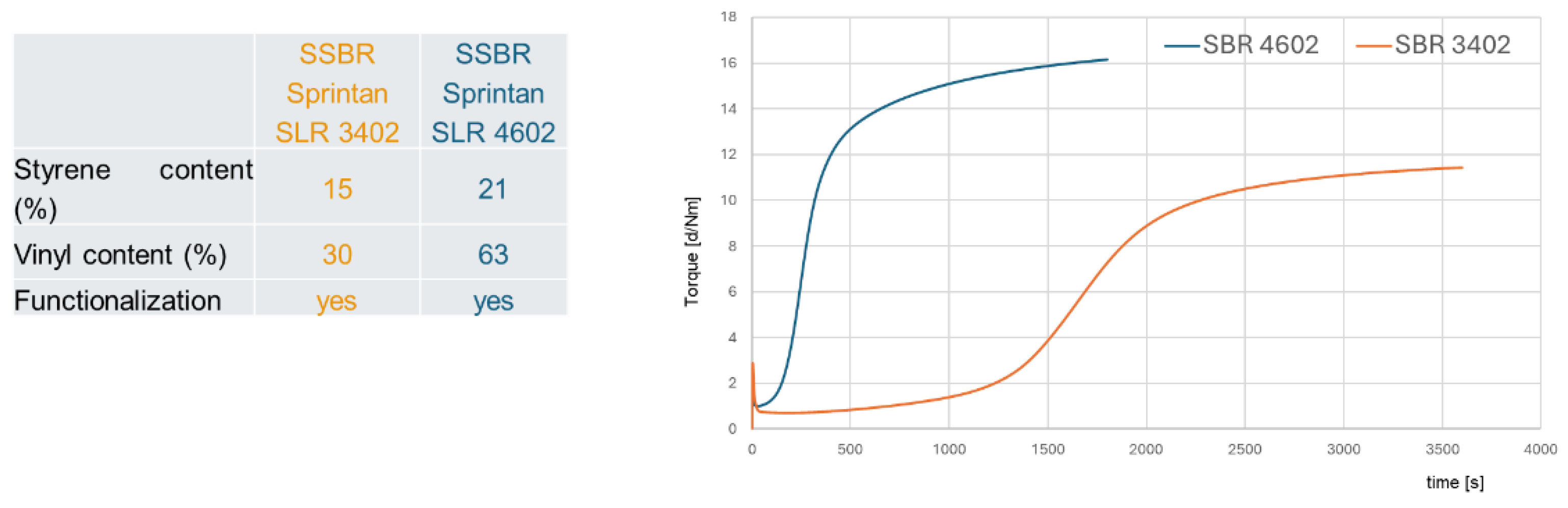
To answer the question if high vinyl SSBR follows a direct radical coupling mechanism and does not need any activators for this, two different SSBR types were investigated: The SSBR Sprintan SLR 3402 with a low and the SSBR Sprintan SLR 4601 with a high vinyl content (
Table 1).
These two types were tested each in a very basic unfilled compound with 2.0 phr TBBS and 1.4 phr sulfur and compared to the same compounds where additional 2.5 phr ZnO and 2.5 phr stearic acid (called in the following “activators”) were added.
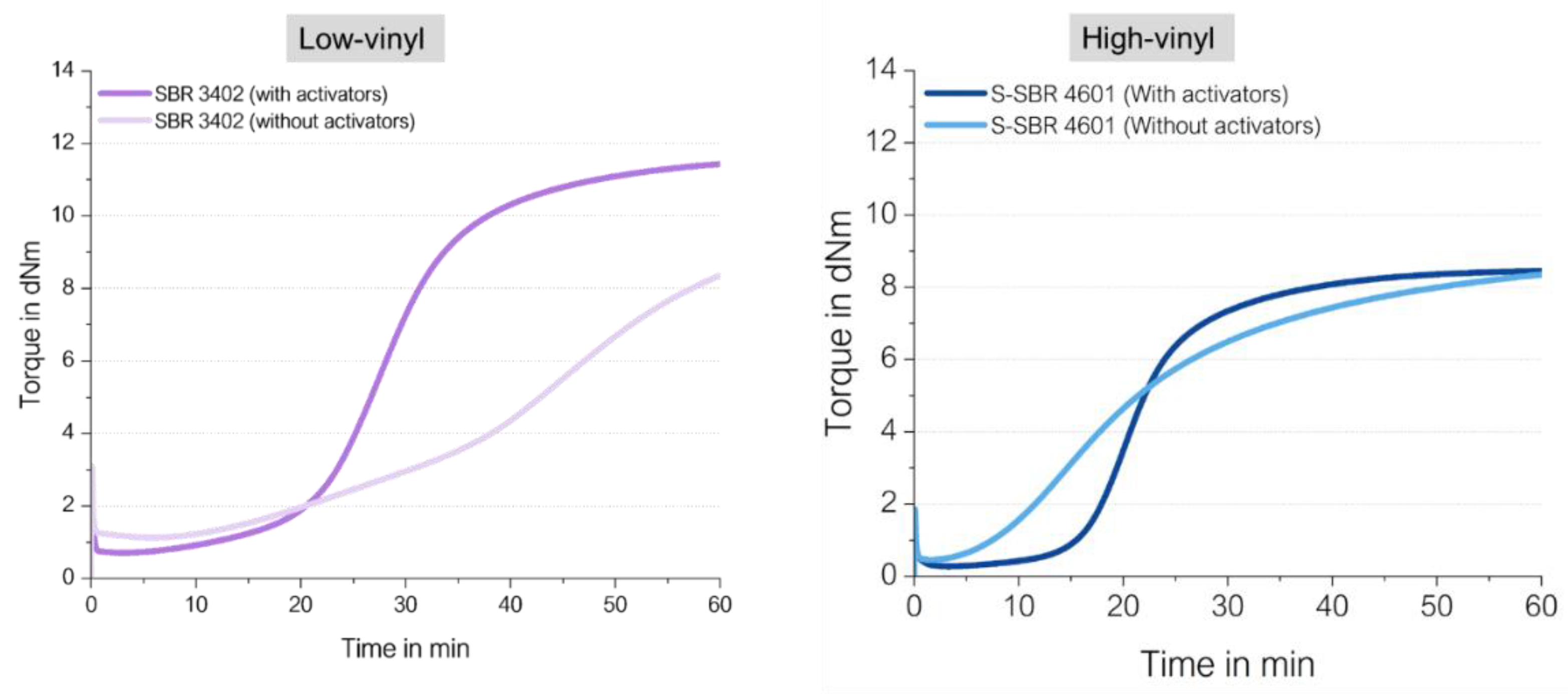
Figure 9 show their different curing behaviors.
There are several remarkable observations, starting from the graph on the right-hand side: the high vinyl SSBR shows the same final torque with and without activators. This delivers already the first hint that the above proposed direct radical mechanism from an opened S8 ring to a vinyl bond takes indeed place. Chapman and Johnson already considered this direct coupling as well, they proposed that “Zinc-accelerator complexes are either not needed or are acting catalytically” in an SSBR [
13].
Furthermore, the curing of high vinyl SSBR starts earlier without activators, the scorch time is significantly lower. This behavior can also be explained by the assumed radical process: a direct sulfur coupling to vinyl via sulfur radical starts earlier than the „traditional“ ionic process but has a lower reaction rate. Normally, radical processes can be assumed to be very fast, can even cause explosions [
14]. This assumes a chain reaction. In the current case of the direct coupling between a sulfur radical and the vinyl group, many different sulfur radicals need to be formed all over the matrix. The radical reaction can be terminated in many different ways, no chain reaction is assumed to take place all over the compound. This means as well that the supporting substance for this formation cannot be a catalyst but a substance which is well distributed in the compound, similar to sulfur, to initiate the radical formation spread all over the compound. The other option for such a trigger is the temperature.
The presence of activators in the high vinyl SSBR compound increases the scorch time. This leads to the conclusion, that ZnO and stearic acid do not act as activators in the system but as a retarder! They seem to interact with sulfur as well as with accelerators, capturing the sulfur radicals or avoiding even the formation of the radicals.
On the left-hand side, the low-vinyl containing SSBR cures much slower in the absence of activators, reaching after 60 min only 60% of the final torque of the compound in the presence of activators. Here, the amount of vinyl groups is too low to enable an efficient direct coupling.
The absolute value of the final torque of the low vinyl SSBR with activators is higher in comparison to the high vinyl SSBR. This is connected to the fact that the low vinyl SSBR contains also a significant lower amount of styrene, leading to a higher degree of butadiene units in the SSBR meaning a higher degree of crosslinkable sides. This higher degree of double bonds in the main chain seems to lead to a higher crosslink density indicated by the higher observed end torque.
Curing Behavior of NR
The above described surprising finding led directly to the next question: Why was this not yet reported in literature?
The sulfur curing mechanism described in the introduction (
Figure 1) was investigated for Natural Rubber (NR). NR does not contain any vinyl groups, therefore, it is considered that the direct radical coupling is not possible, which leads to the assumption that curing without activators should not lead to a sufficient torque increase.
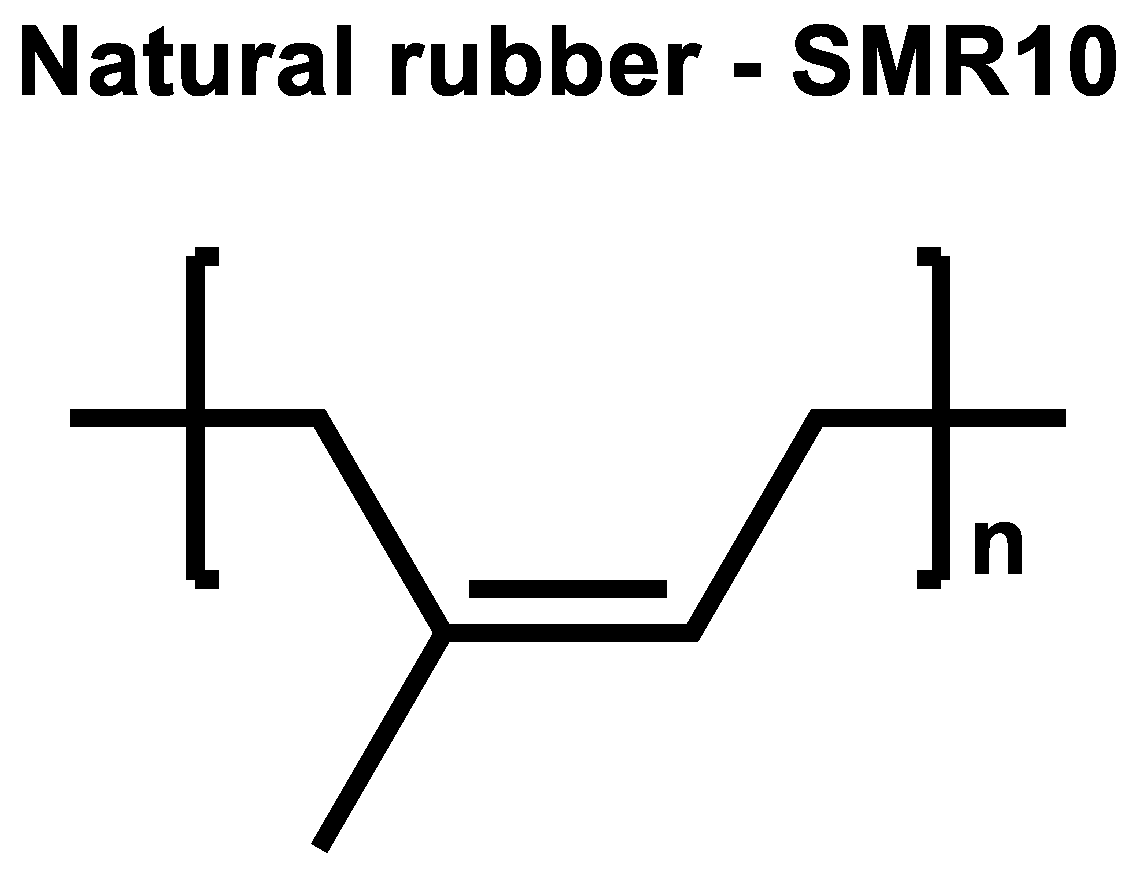
To proof this, SMR10 was selected as NR of choice (
Figure 10). Its curing behavior was investigated in an unfilled compound with 2.0 phr TBBS, 1.4 phr sulfur and compared to the same unfilled compound where additionally 2.5 phr ZnO and 2.5 phr stearic acid were added (
Figure 11).
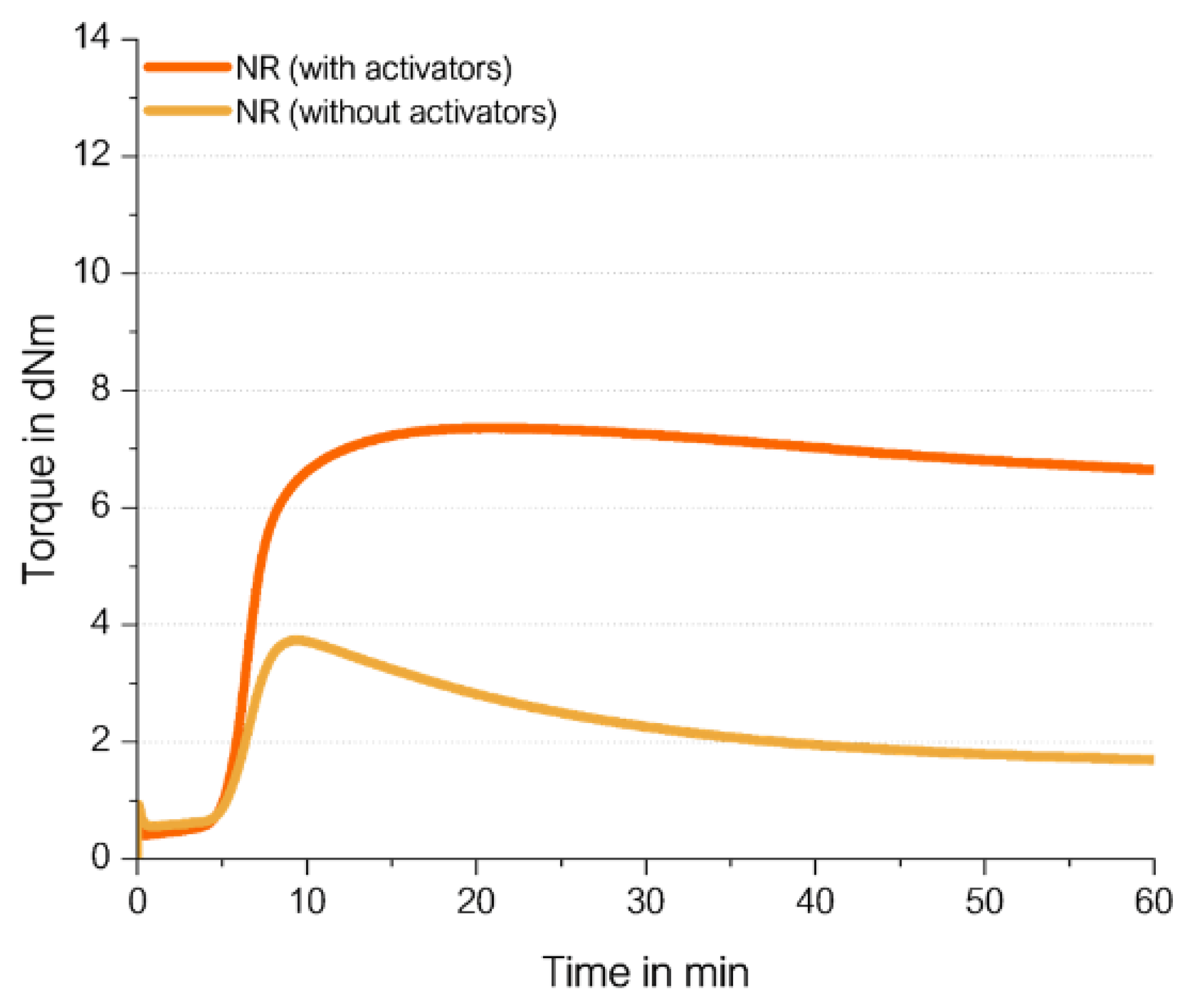
The curing of NR without activators results in a very low final torque. The torque increases but only to a low amount. This proves the theory that the curing in NR follows the ionic mechanism which was already described in literature due to the absence of vinyl groups in NR. It indicates that the presence of ZnO and stearic acid is essential to abstract the allylic hydrogen atom from the polymer chain to enable the coupling of sulfur.
The curing curve of the NR compound without activators has a maximum. The shape of the curve is similar to the curve with activators, but with a more pronounced maximum on a much lower level. The fact that NR-based compounds show a maximum caused by reversion is known from literature [
15,
16]. It can be caused by the breakage of the long polymer chains of NR. In case of the curing curve of the NR compound without activators, this breakage of the long NR polymer chains might generate radicals which can connect to another chains resulting first in a higher network density. When the breakage of the polymer chains continue, this formed higher network is weakened again, indicated by the maximum and the starting reversion. The observed maximum comes faster and is on a much lower level than that of the compound containing activators due to the assumption that the formation of polymer-polymer bonds via sulfur bridges does not occur in the absence of activators, that only the direct coupling of polymer chain fragments to polymer chains causes it.
Curing Behavior of High Vinyl SSBR versus Low Vinyl SSBR
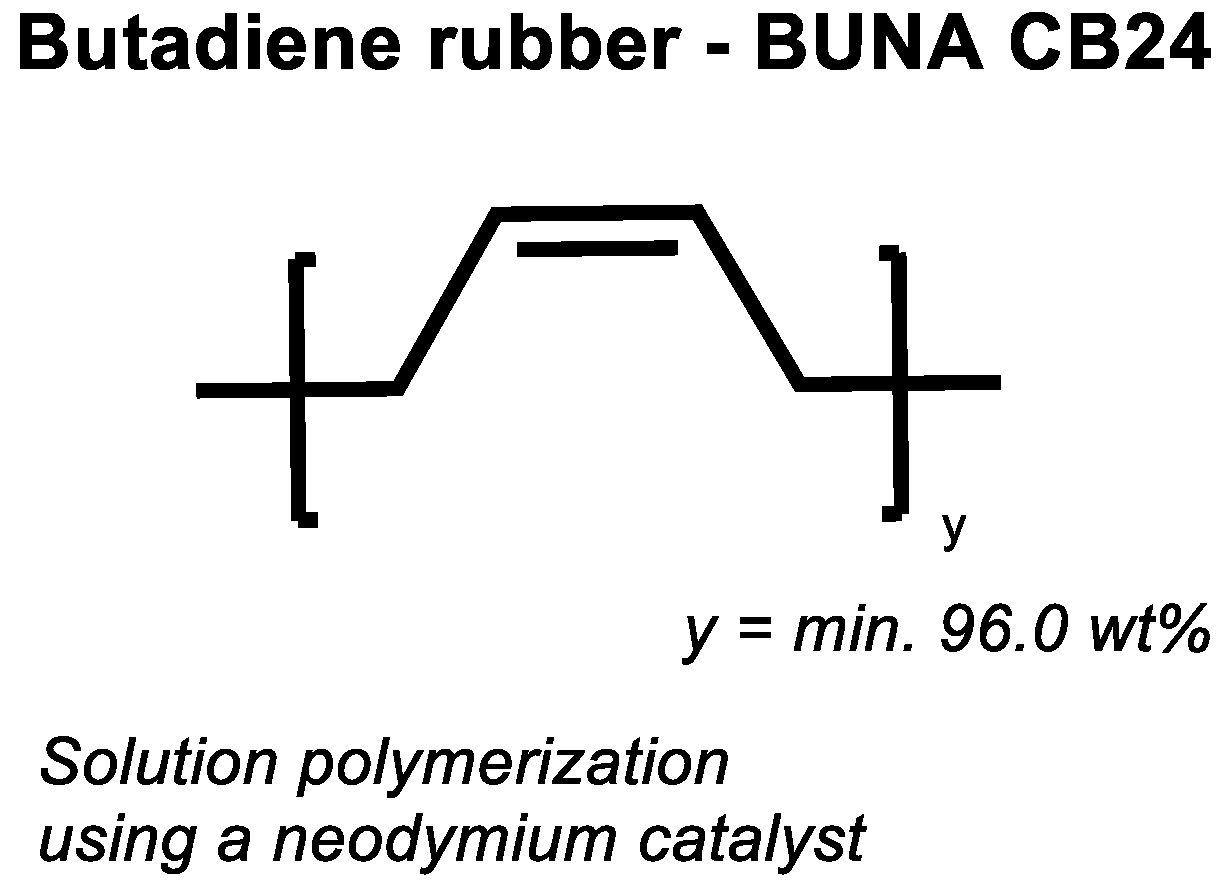
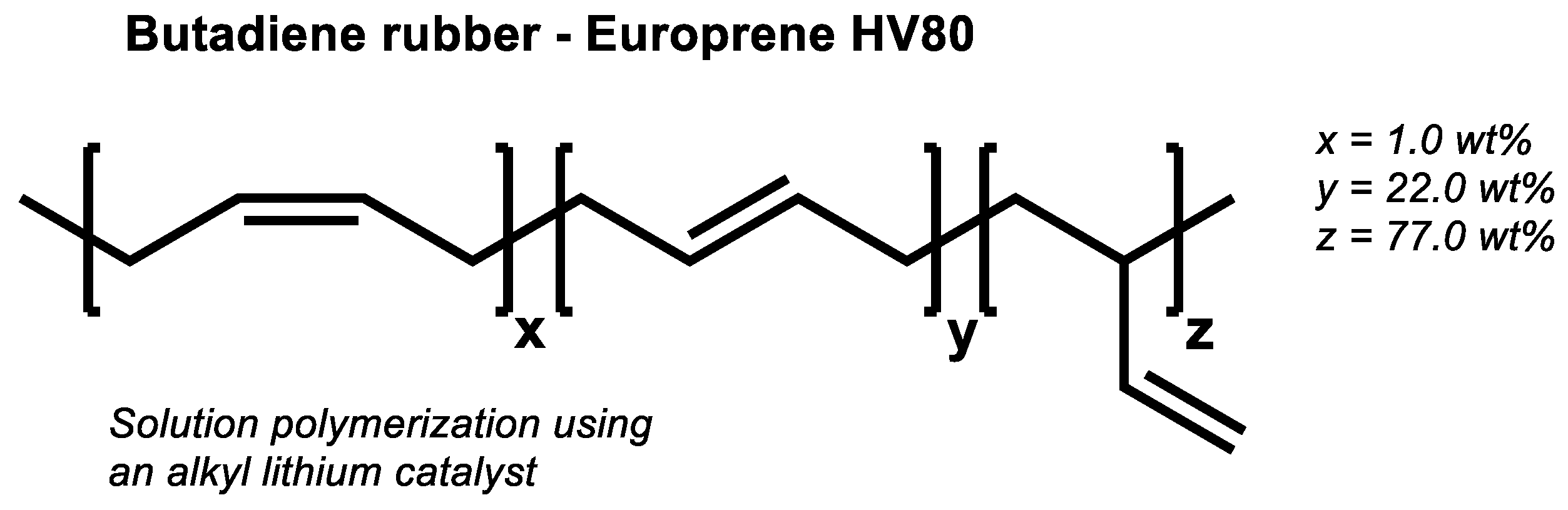
If the assumed direct sulfur coupling occurs in high vinyl SSBR it can be expected that the same mechanism will also occur in BR. To prove this, a low vinyl (CB 24) as well a high vinyl BR (Europrene HV80) were selected. Their cure behavior was tested in an unfilled compound with 2.0 phr TBBS and 1.4 phr sulfur and compared with unfilled compounds were additionally 2.5 phr ZnO and 2.5 phr stearic acid were added.
Figure 12 shows the chemical structure of the used high-cis BR, and
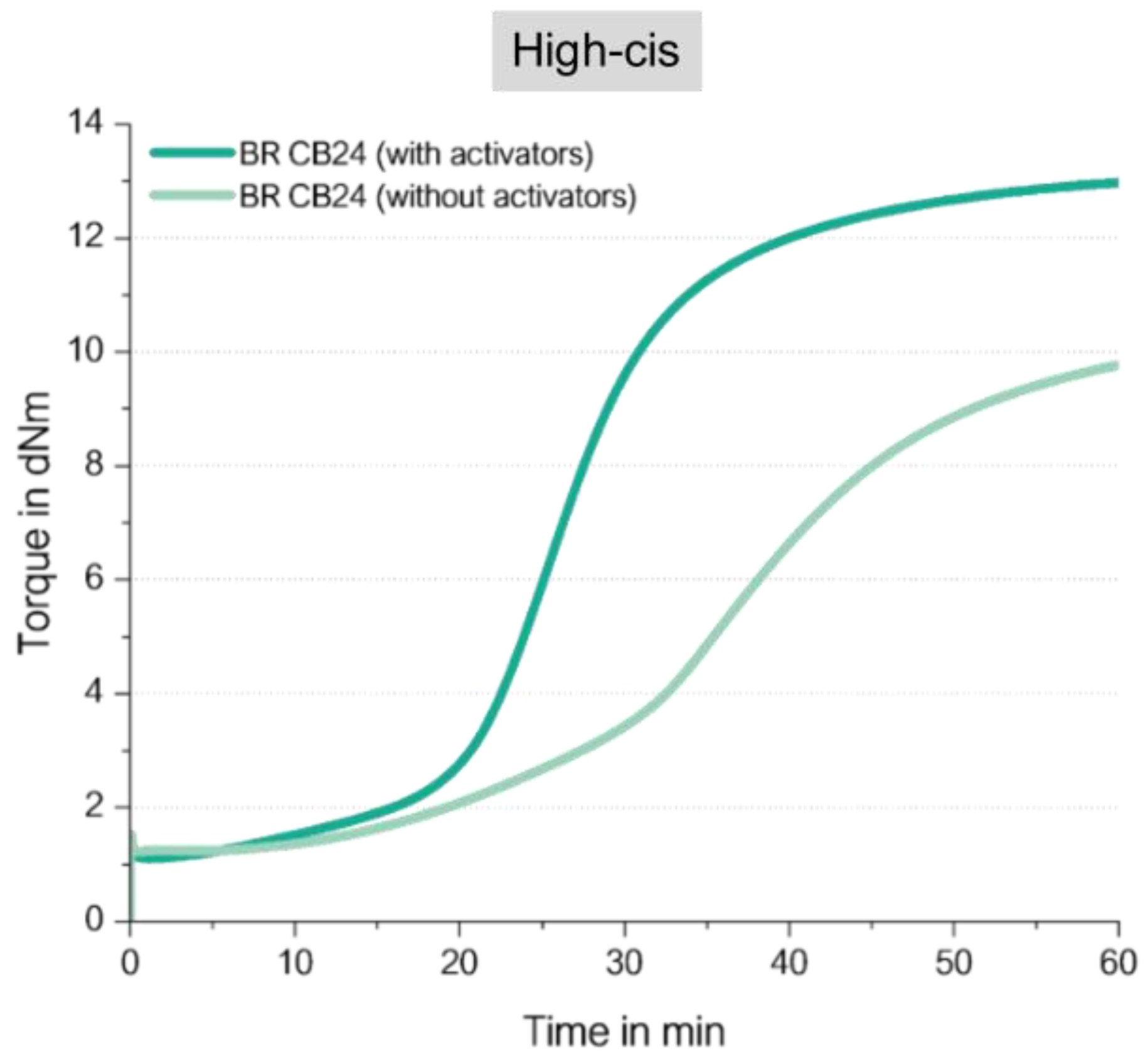
Figure 13 displays the curing behavior of the low vinyl BR with 96 wt% of cis double bonds.
It shows that the compound without any activator shows a significant increase in torque but results in a still lower final torque in comparison with the compound with activators. CB24 does contain 96 wt% of cis double bonds, therefore, this behavior was unexpected.
An answer for this can be found in literature. M. Sato [
11] reported that an alkene (cis-3-hexene) can react directly with mercaptosilane in a radical reaction and forms a variety of different reaction products (
Figure 6). The direct coupling product between mercaptosilane and the alkene was formed with a lower yield in comparison with that of the reaction between mercaptosilane and a vinyl-alkene. It clearly indicates that also the cis-double bond in SSBR can couple to sulfur via a direct mechanism.
The comparison with the observed NR which consists nearly 100% of cis-double bonds needs to consider one big difference. The cis double bond of NR has additionally a methyl group as a side group along the polymer chain. This methyl group adjacent to the double-bonded carbon atom in NR has an electropositive effect which enhances the activity of the unsaturated double bonds in the backbone of the polymer chains towards ionic reactions. A radical coupling might be hindered by this electron positive effect. Additionally, a steric hindrance might affect the reaction. Nevertheless, a small amount of a direct coupling to the cis-double bond might still occur, supporting the formation of the observed maximum in the cure curve (
Figure 11).
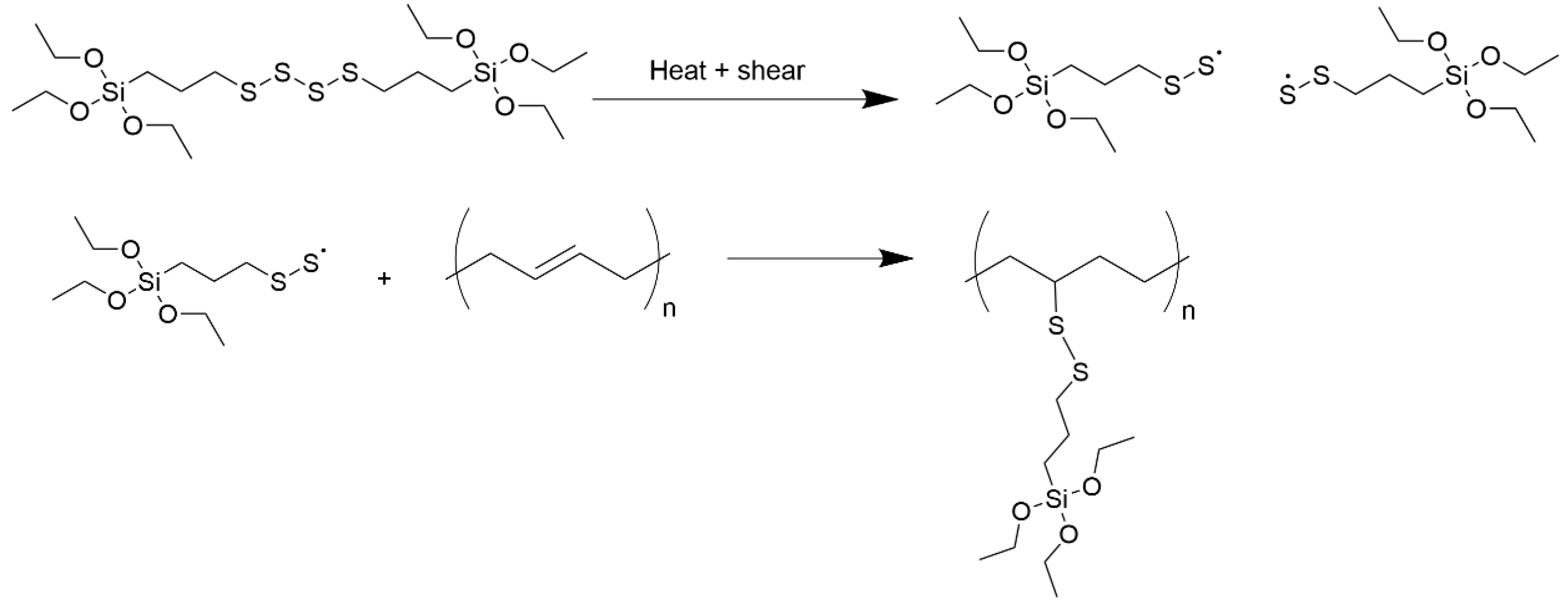
Another hint can be found in a study from Holstege [
18]. Here, the following mechanism for the reaction of TESPT with a double bond was proposed (
Figure 14). It shows that TESPT is cleaved first into fragments carrying a sulfur radical. These radicals can further couple chemically to the double bond of a polymeric chain.
Both examples from literature show that a direct addition reaction from a sulfur radical (formed from a silane) is possible to a cis-double bond in the main chain of an SSBR. For this reason, it can be considered again, that also the direct sulfur coupling in the case of the curing reaction of BR with sulfur is possible. As Sato [
11] already reported shows the reaction of cis-alkene with a sulfur radical a lower yield than the vinyl-alkene. This can explain the observed behavior in Figure13: the high-cis BR compound without activators shows a significant increase but still lower final torque than that one with activators. Meaning, that a direct coupling via S-radical is also possible to cis-double bonds but with lower yields.
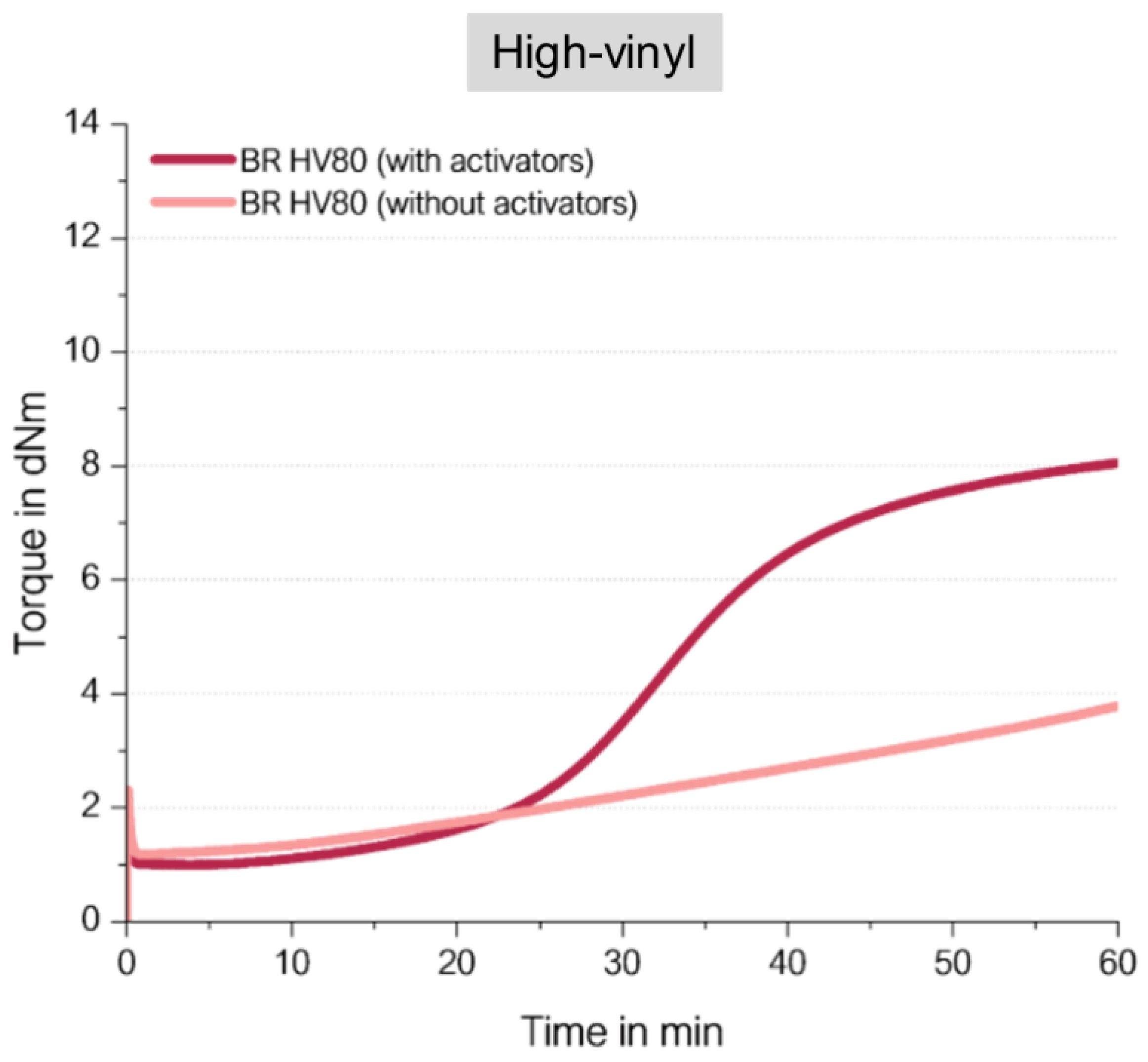
It was now expected that the BR with a high vinyl content (
Figure 15) shows also in the absence of activators the same final torques as observed before for the high- as well as for the low-vinyl SSBR. Surprisingly, a completely different behavior was observed (
Figure 16).
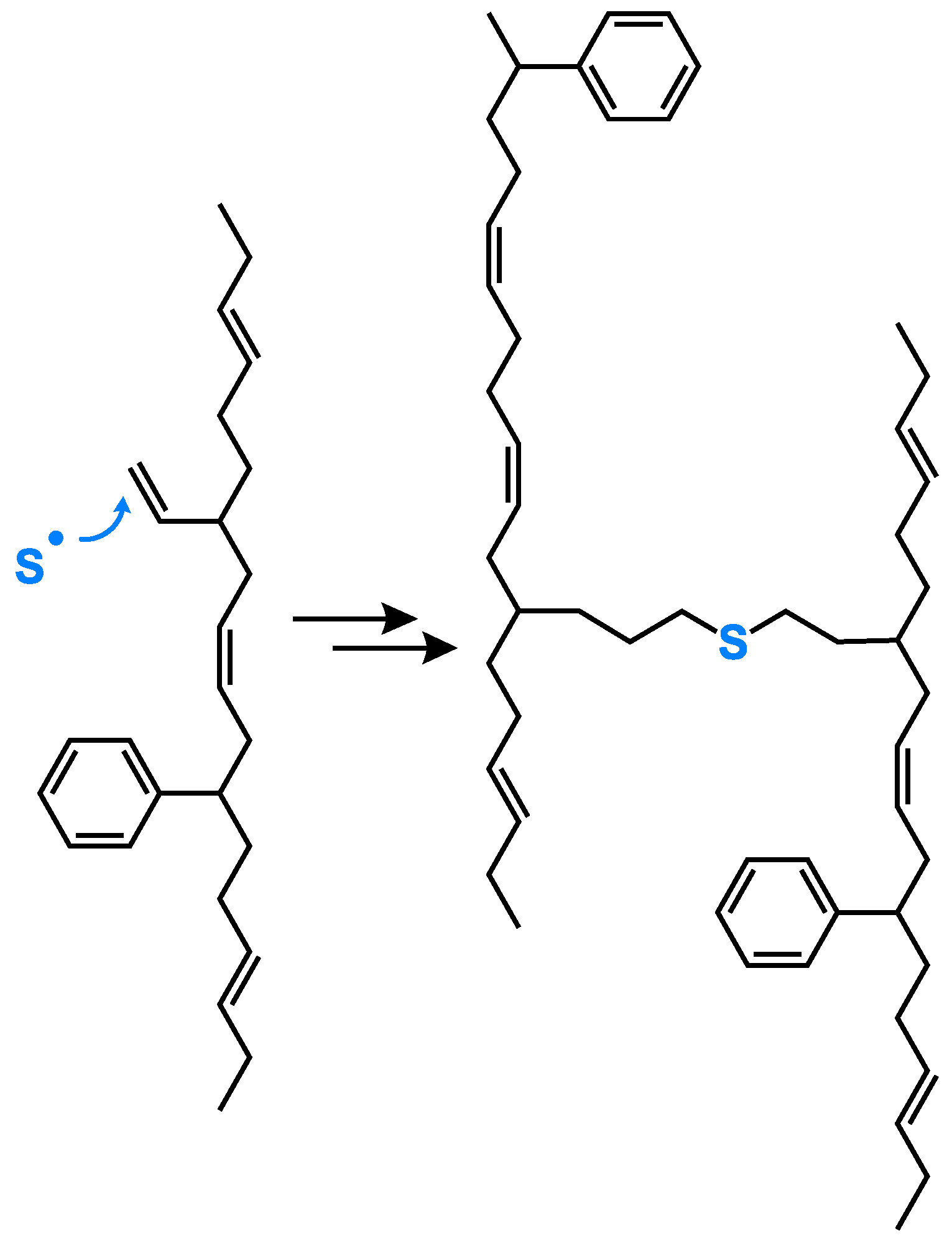
The high vinyl BR compound without activators ends up in a very low final torque. How can this unexpected behavior be explained?
Radicals are very reactive, they couple to the closest reaction partner. This means, having a polymeric structure with adjacent vinyl groups, they react preferably with each other (
Figure 17). The presence of such adjacent vinyl groups is with a vinyl content of 77% highly likely. Such an internal crosslink reaction is already known from curing reactions where triallyl cyanurate (TAC) or triallyl isocyanurate (TAIC) are used as co-agents [
21]. This means the assumed direct sulfur coupling can take place, but the formation of a radical along the polymeric chain triggers a further coupling reaction to the neighbored vinyl group. In the end, no crosslinks between different polymer chains are formed, which leads to the observed not pronounced increasing torque.
This leads to the conclusion that an effective direct sulfur coupling needs a “spacer” such as the styrene group in SSBR or a cis or trans BR unit in the low-vinyl BR to hinder such a coupling reaction to adjacent vinyl groups.
By comparing the finally achieved final torque in
Figure 13 and
Figure 16 for the BR-compounds with activators, another conclusion can be drawn. The high-vinyl BR shows even with activators an almost 30% lower final torque than the high-cis BR with activators. This indicates that even in the high-vinyl BR with activators such an adjacent crosslink reaction might take place which is ineffective for the increase in crosslink density and therefore also ineffective to cause an increase in torque. This explanation is also true for the observed lower torque for the high vinyl SSBR in comparison to the low vinyl SSBR (
Figure 9). Additionally, high vinyl BR or SSBR have much less allylic hydrogen atoms where the “traditional” sulfur coupling reaction via the activated Zn-complex can take place [
22]. For this reason, the addition reaction towards the double bond is much more likely.
Curing Behavior of High Vinyl SSBR Filled with Silica / Silane versus Filled with Carbon Black
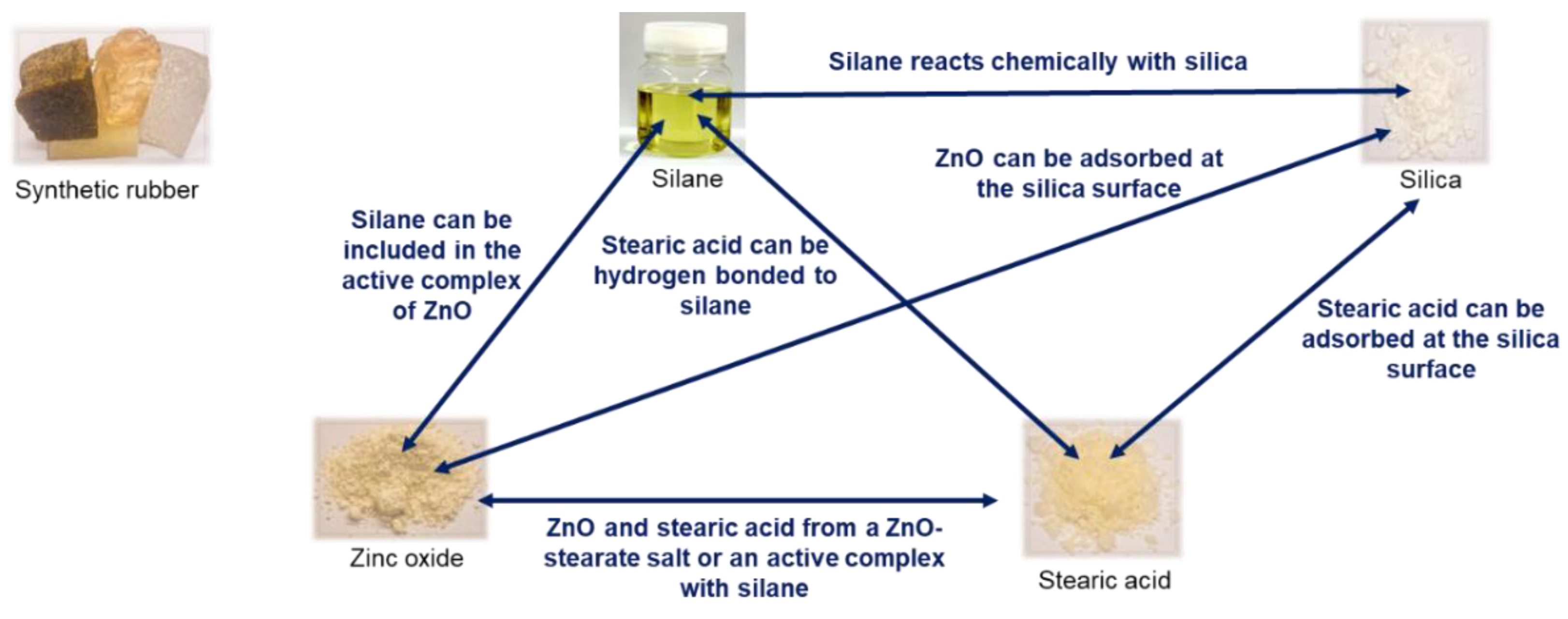
Up to now, all investigated compounds did not include any filler. In a silica/silane filled compound, many interactions are reported between the activators and silica as well as silane (
Figure 18) [
23].
Furthermore, the coupling of the silane-modified silica to the polymer was also reported to follow the „traditional“ sulfur coupling at the allylic position (
Figure 4). This coupling was assumed to be supported from the active Zinc complex. As discussed before, this support was considered to be unlikely due to the formation of the Zinc complex at the surface of the non-mobile Zinc oxide.
To find out which influence the silica/silane system has on the direct coupling reaction between a sulfur radical and a vinyl group, a high-vinyl SSBR filled with silica/silane (TESPD) was investigated by adding respectively leaving out the activators. For this, a model green tire tread compound with 1.5 phr DPG, 2.0 phr TBBS and 1.4 phr sulfur was mixed. These compounds were compared with carbon black (CB)-filled high-vinyl SSBR compounds where such manifold interactions between the different ingredients as described in
Figure 18 were not expected.
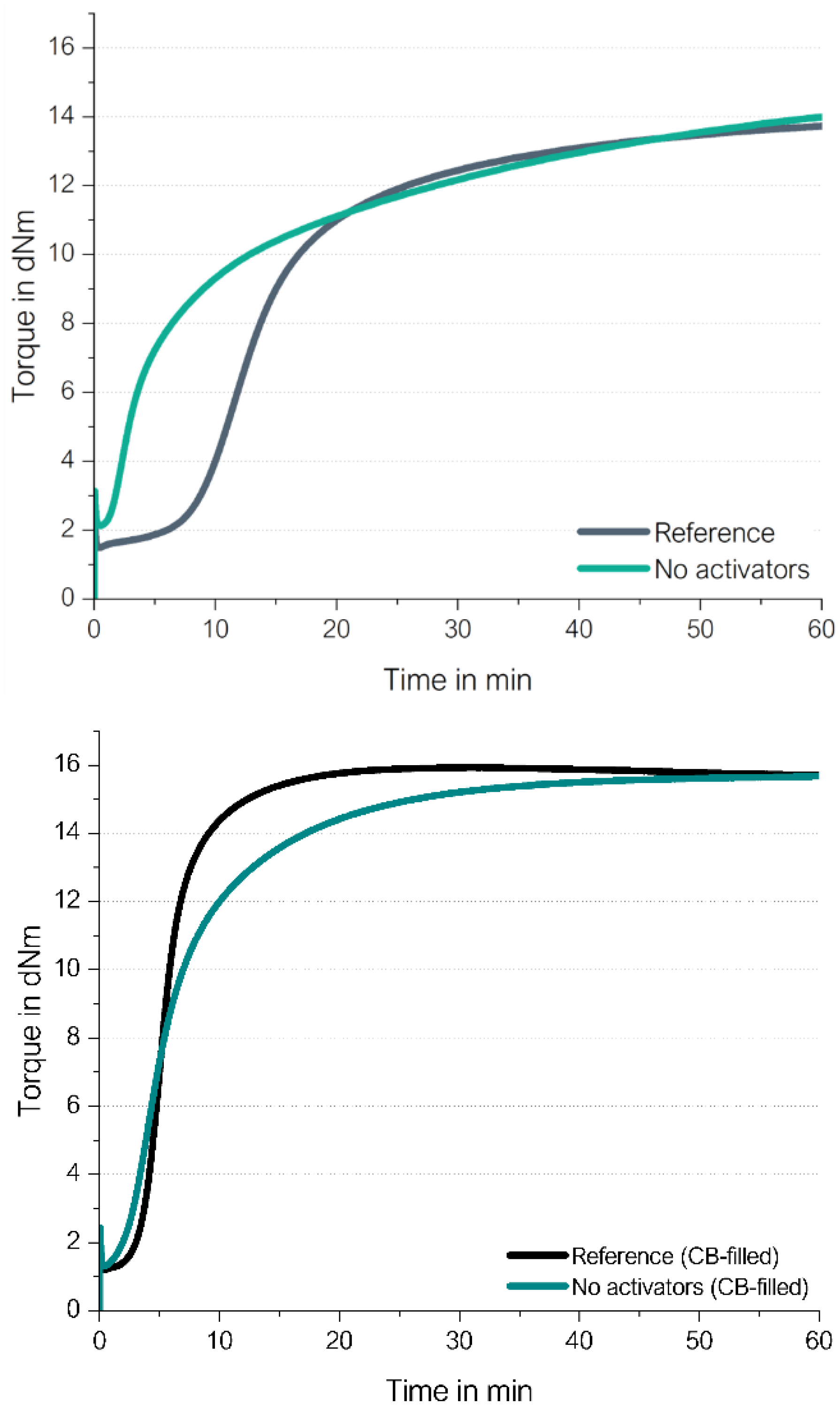
Figure 19 shows the curing behavior of the silica/silane on the left side as well as that of the CB-filled high-vinyl SSBR compounds on the right side.
The curing of high vinyl SSBR without activators (ZnO/stearic acid) starts also in CB-filled SSBR slightly earlier and leads to the same final torque. This means that the direct coupling of the sulfur radical to the vinyl group does occur also in a CB-filled system and the presence of CB influences the curing behavior only slightly.
The silica/silane-filled compounds show the same final torque, independent if activators are present or not. If the activators are left out, the scorch time is significantly reduced. This more significant reduction in the case of the silica/silane-filled system is due to the fact that the scorch time in the presence of the activators of the CB-filled compound is much shorter than for the silica/silane system which can absorb and in this way deactivate part of the curing system. Both cure curves of the filled compounds without activators look very similar, showing again that radical reaction starts very fast. As a conclusion, the direct sulfur coupling towards a vinyl group takes also place in filled compounds.
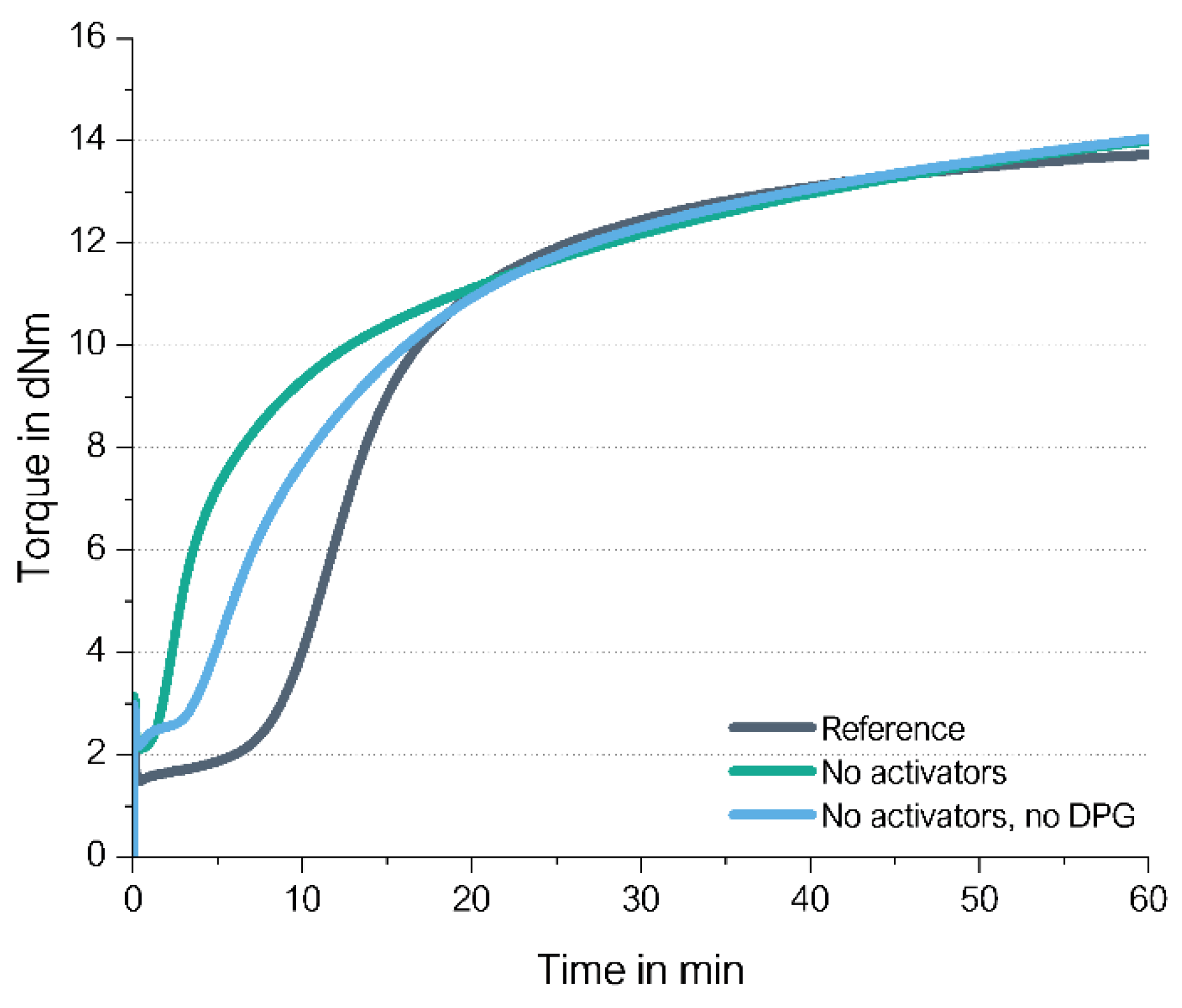
Curing Behavior of High Vinyl SSBR without DPG
It was shown in the previous described studies that a direct coupling of a sulfur radical towards a vinyl group can take place even in the absence of ZnO as well as stearic acid. If the assumed direct coupling mechanism shown in
Figure 8 on the right side is correct, it can be considered that also DPG can be left out.
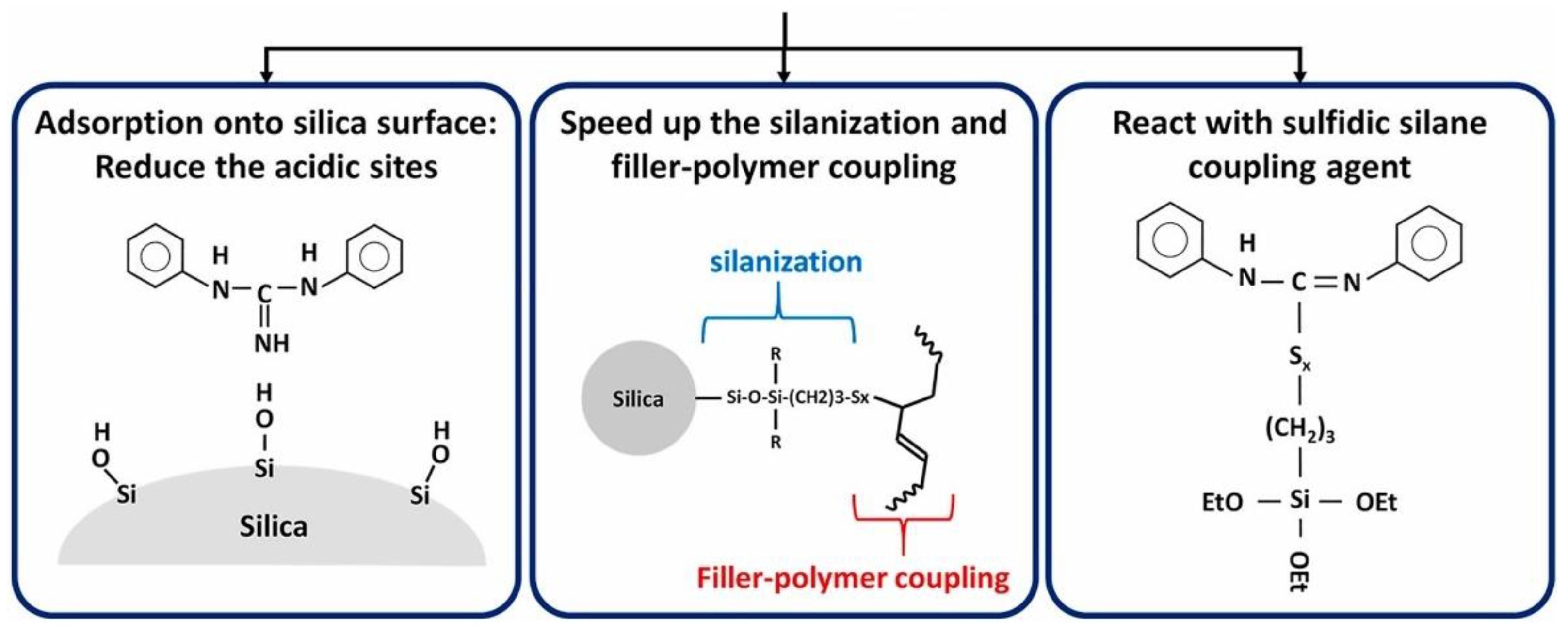
DPG plays a manifold role inside a silica/silane compound (
Figure 20) [
24]. It adsorbs at the silica surface and blocks it for the adsorption of the primary accelerators, it speeds up the silanization reaction and it can react directly with the silane.
DPG is for quite some time under discussion to be banned from use due to the release of harmful aniline [
25], therefore, it is desired to find a curing possibility especially in silica/silane compounds without DPG.
In order to prove if the curing of high-vinyl SSBR compounds allow the absence of DPG, a silica-filled high-vinyl SSBR-based model green tire tread compound without activators and without DPG was investigated and compared with the same compound but without activators. As reference, the compound with DPG as well as activators was taken (
Figure 21). A model green tire tread compound with 2.0 phr TBBS and 1.4 phr sulfur was selected as the basis for this study.
The curing of high vinyl SSBR without activators (ZnO/stearic acid) as well as without activators and DPG starts earlier and leads to the same (slightly higher) final torque. When DPG is left out additionally to the activators, the scorch time is slightly increased but still much shorter than for the reference. Amines are known for their accelerating effect on the breakage of the sulfur ring, TBBS with a higher potential than DPG [
26]. When DPG is left out the more active TBBS or the reaction product from TBBS, the amine, is assumed to be partly adsorbed at the silica surface, being in this way partly immobilized to support the opening of the sulfur ring. The result is the observed longer scorch time.
This means that in a high-vinyl compound not only the accelerators but also DPG can be left out to reach a sufficient crosslink density.
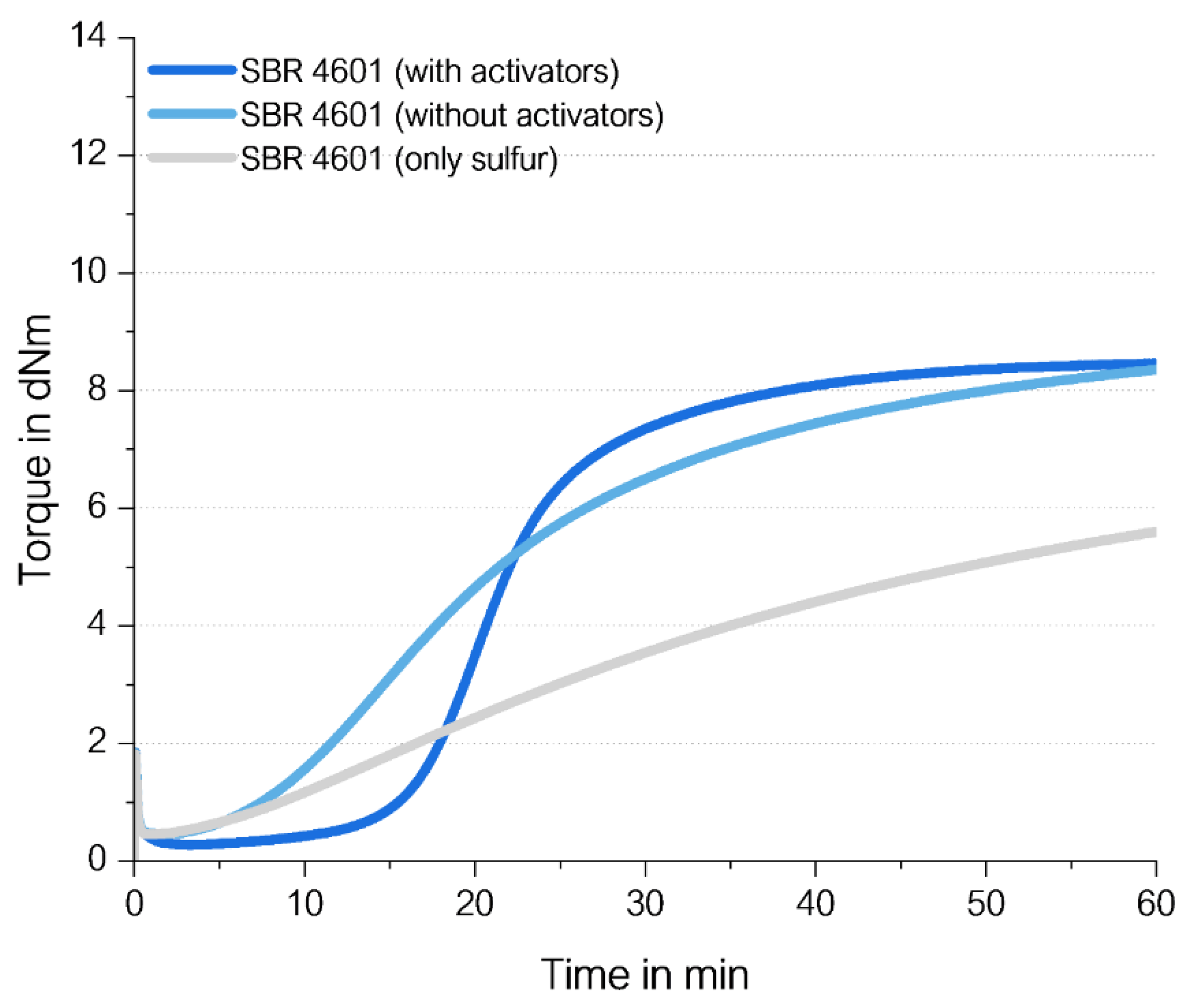
Curing Behavior of High Vinyl SSBR without Any Accelerator
After finding that for an effective curing of high-vinyl SSBR no activators as well as no DPG is required, the next open research question should be answered. Is any accelerator needed for the direct sulfur curing or is the presence of sulfur alone sufficient? In this case, the sulfur radical would be formed only from the sufficient high energy input due to the increase in temperature causing the opening of the sulfur ring.
For this, an unfilled high-vinyl SSBR with 1.4 phr sulfur without activators and without any accelerator was investigated and compared to a compound with 2.5 phr ZnO, 2.5 phr stearic acid as well as 2.0 phr TBBS (
Figure 22).
The direct sulfur coupling in a compound without activators and without accelerators (grey curve in
Figure 22) is slow but leads to 60% of the final torque containing the traditional curing system after 60 min. This means that TBBS seems to support the formation of S-radical but a direct coupling takes also place in the absence of any other ingredients, however, the formation of sulfur radicals by only heat takes longer.
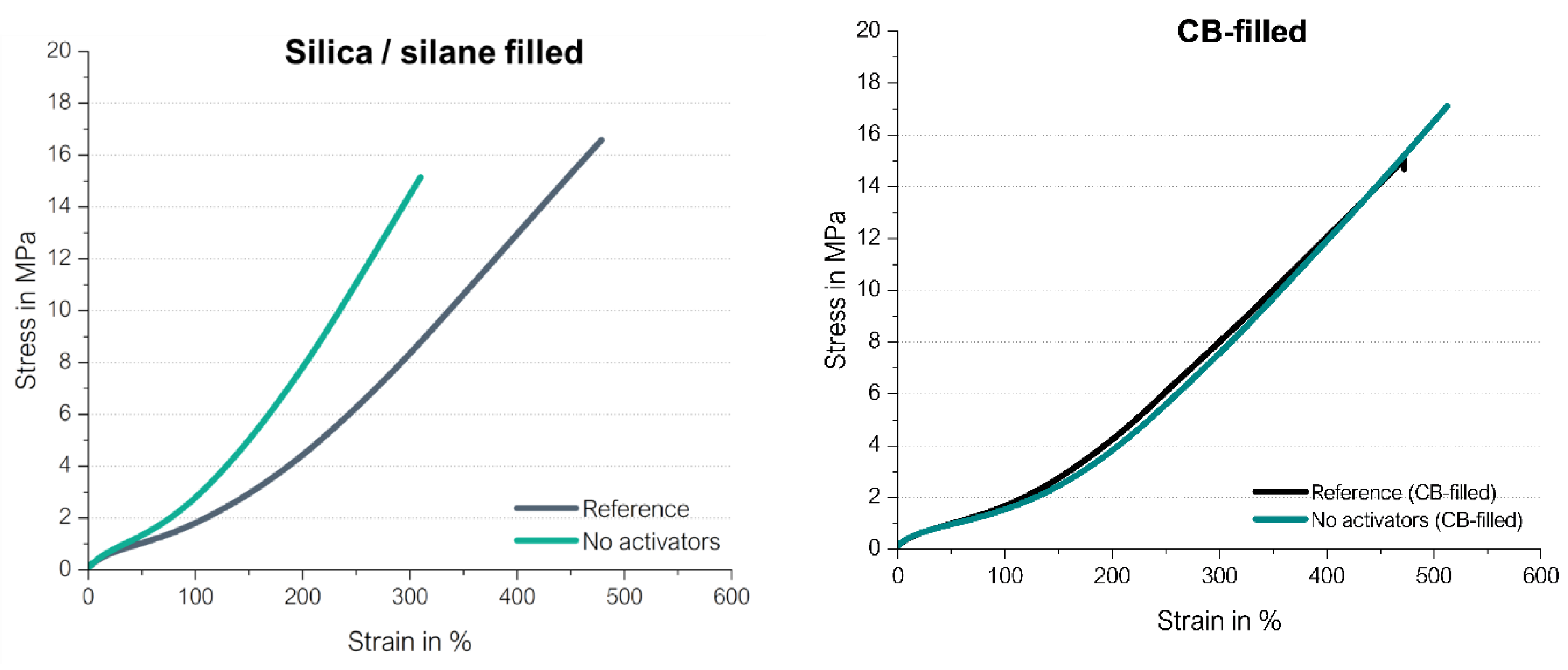
Reinforcing Behavior of Filled High Vinyl SSBR Compounds without Activators
Up to now, only the cure behavior was studied in detail. To get the final proof that the observed increase in the final torque is indeed related to a good reinforcing behavior, the stress-strain curves of the before described silica/silane filled as well as carbon black filled compounds were measured (
Figure 23). They were based on a model green tire tread compound with 1.5 phr DPG, 2.0 phr TBBS and 1.4 phr sulfur and compared with the same compound where additionally activators were added (called “reference” compound).
The carbon black-filled compounds show with and without activators the same reinforcing behavior, only the elongation at break as well as the tensile strength is higher for the compounds without activators. In the case of the silica/silane-filled SSBR, higher moduli but shorter elongation at break as well as tensile strength are achieved in the absence of activators. This shows that the sulfur is more effectively used for crosslinks in the absence of activators. If ZnO and stearic acid are present, manifold interactions can take place between the filler system, covering also unwanted reactions like the hydrogen bonding between silane and stearic acid. The sulfur curing can take place via the direct coupling as well as the “traditional” ionic pathway, leading to lower crosslink densities.
This assumption was confirmed from swelling tests: The silica-filled compound without activators results in a higher crosslink density than one with activators. For the shown example in
Figure 23, this is a difference in the crosslink density of 119 mol/cm³ for the reference compound with activators, versus that of the compound without activators of 166 mol/cm³.
All in all, cured high-vinyl SSBR compounds without activators show a high reinforcing behavior, similar to the reference when filled with carbon black, with increased moduli when filled with silica/silane.
Summary
NR couples via the well-investigated activated complex where ZnO, stearic acid as well as accelerators and sulfur are involved.
A different sulfur coupling mechanism occurs in high vinyl SSBR than in NR (
Figure 24).
The sulfur couples in a high amount to vinyl via a sulfur radical. The coupling to a cis-double bond in SBR can occur as well via a direct sulfur coupling but to a lower amount. For this coupling reaction, no activators (ZnO/stearic acid) are required. Their absence leads to a shorter scorch time. This means that ZnO and stearic acid act as retarder, not as activators in this system like in the traditional coupling system. The scorch time should be adjusted for practical use but is already sufficiently long without both, activators as well as DPG.
As an overall conclusion, a silica/silane filled tire tread compound based on high vinyl SSBR/BR shows the same curing performance even when the typically used activators as well as DPG are left out. It is only required to add one primary accelerator like TBBS plus sulfur as the whole curing system to the compound.
The absence of activators leads to similar (CB-filled) respectively higher (silica/silane-filled) reinforcement in a model tire tread compound.
Conclusion
Up to now, it was assumed that the sulfur coupling in a rubber compound followed a mechanism where the coupling to the polymer chain occurs in the allylic position, supported by a Zinc complex. This study showed that a direct coupling via a sulfur radical is the dominant mechanism in a high vinyl SSBR compound, the typical main elastomer for a tire tread compound. The generally assumed coupling to the allylic position is only valid e.g., in NR without any vinyl group.
The direct sulfur coupling in a high vinyl SSBR does not need any activators nor DPG, the presence of an activator for the sulfur ring opening like TBBS is sufficient. It can be considered that the coupling of the silane, attached already to the silica surface, to the polymer follows the same mechanism, a direct coupling via a sulfur radical.
Therefore, a new tire tread compound should be based on high-vinyl SSBR/high cis BR without ZnO, without stearic acid and without DPG. With this, the manifold interactions in the typical silica/silane filled green tire tread compound are significantly reduced, avoiding unwanted reactions like the interaction of stearic acid with silane which negatively influences the silica/silane coupling.
The abraded wear particles from such new tires will be less harmful for the environment. These Tire Wear Particles (TWP) do NOT contain any Zn-species which are toxic to aquatic systems. The use of DPG can also be avoided which releases the harmful aniline. This will have a very positive effect on the overall microplastic pollution caused by tire wear.
Furthermore, the big particles of ZnO are not well dispersed in the rubber matrix. By leaving them out, the compounds do not contain these inhomogeneous spots which can be the origin of abrasion. For this reason, the expected wear resistance from such tire tread compounds is expected to be better.
This new tire tread compound contains less ingredients which reduces also the production costs. It leaves two environmental critical substances out, Zinc species as well as DPG. This unique new approach of reducing the amount of substances without compromising the in-rubber performance enables the design of a more sustainable, more eco-friendly tire tread compound and paves the way for further innovations.
Figure 25.
Sustainable and plant-based tire [A. Blume created with AI].
Figure 25.
Sustainable and plant-based tire [A. Blume created with AI].
References
- Charles Goodyear and the Vulcanization of Rubber. Available online: https://connecticuthistory.org/charles-goodyear-and-the-vulcanization-of-rubber/ (accessed on 29 Aug., 2024).
- Goodyear, C. Improvement in the Mode of Preparing Caoutchouc with Sulphur for the Manufacture of Various Articles. US February, 1839.
- Morrison, N. J. , Porter, M. Temperature effects on the stability of intermediates and crosslinks in sulfur vulcanization. Rubber Chem. Technol. 1984, 57, 63–85. [Google Scholar] [CrossRef]
- Brydson, J.A. Rubber Chemistry, 1st ed.; Applied Sci. Pub.: London, United Kingdom, 1978; p. 21. [Google Scholar]
- Ikeda, Y. , Yasuda, Y., Ohashi, T., Yokohama, H., Minoda, S., Kobayashi, H., Honma, T. Dinuclear Bridging Bidentate Zinc/Stearate Complex in Sulfur Cross-Linking of Rubber. Macromolecules 2015, 48, 462–475. [Google Scholar] [CrossRef]
- Ikeda, Y. Sakaki, Y., Yasuda, Y., Junkong, P., Ohashi, T., Miyaji, K., Kobayashi, H. Roles of Dinuclear Bridging Bidentate Zinc/Stearate Complexes in Sulfur Cross-Linking of Isoprene Rubber. Organometallics 2019, 38, 2363–2380. [Google Scholar] [CrossRef]
- Butuc S., G. Novel Cyclic Polysulfide Based Blends: Elucidation of the Role of Zinc Oxide in Sulfur Crosslinking. PhD Thesis, University of Twente, The Netherlands, December 17, 2021. [Google Scholar]
- Rauline, R. Copolymer Rubber Composition with Silica Filler, Tires Having a Base of Said Composition and Method of Preparing Same. US522 July, 1993.
- Meon, W. , A., Luginsland, H.-D., Uhrlandt, S. Chapter 7: Silica and Silanes. In Rubber Compounding: Chemistry and Applications, 1st ed.; Rodgers, B., Ed.; Marcel Dekker, Inc.: New York, USA. 2004; p. 312. [Google Scholar]
- Nakanishi, Y. , Mita, K., Yamamoto, K., Ichino, K., Takenaka, M. Effects of Mixing Process on Spatial Distribution and Coexistence of Sulfur and Zinc in Vulcanized EPDM Rubber. Polymer 2021, 218, 123486. [Google Scholar] [CrossRef]
- Sato, M. Reinforcing Mechanisms of Silica/Sulfide-Silane vs. Mercapto-Silane Filled Tire Tread Compounds. PhD thesis, University of Twente, The Netherlands, , 2018. 18 July.
- Bisschop, B. Processing of Modern Passenger Car Tire Treads. Master thesis, University of Twente, The Netherlands, Feb. 12, 2020.
- Chapman, A. Johnson, T. The Role of Zinc in the Vulcanization of Styrene-Butadiene Rubbers. Proceedings of the International Rubber Conference, Maastricht, The Netherlands, -9, 2005. 7 June.
- Glarborg, G. , Halaburt, B., Marshall, P., Guillory, A., Troe, J., Thellefsen, M., Christensen, K. Oxidation of Reduced Sulfur Species: Carbon Disulfide. J. Phys. Chem. A. 2014, 118, 6798–6809. [Google Scholar] [CrossRef] [PubMed]
- Kaewsakul, W. Silica-Reinforced Natural Rubber for Low Rolling Resistance, Energy-Saving Tires: Aspects of Mixing, Formulation and Compatibilization. PhD thesis, University of Twente, The Netherlands, Apr 18, 2013.
- Bornstein, D. , Pazur, P.J. The Sulfur Reversion Process in Natural Rubber in Terms of Crosslink Density and Crosslink Density Distribution. Polymer Testing 2020, 88, 106524. [Google Scholar] [CrossRef]
- Lanxess, Buna CB 24. Product Data Sheet, Jan., 2008.
- Holstege, N. Design and Optimization of a Devulcanization Process for Butyl Rubber. Master thesis, University of Twente, The Netherlands, , 2024. 28 May.
- Rodríguez Garraza, A.L. , Mansilla, M.A., Depaoli, E.L., Macci, C., Cerveny, S., Marzocca, A.J., Somoza, A. Comparative Study of Thermal, Mechanical and Structural Properties of Polybutadiene Rubber Isomers Vulcanized Using Peroxide. Polymer Testing 2016, 52, 117–123. [Google Scholar] [CrossRef]
- Versalis, EUROPRENE® BR HV80. Technical Data Sheet, March, 2015.
- Muroga, S. , Takahashi, Y., Hikima, Y., Ata, S., Ohshima, M., Okazaki, T., Hata, K. New Evaluation Method for the Curing Degree of Rubber and its Nanocomposites Using ATR-FTIR Spectroscopy. Polymer Testing, 1069. [Google Scholar]
- Versloot, P. , Haasnoot, J.G., Nieuwenhuizen, P.J., Reedijk, J., Van Duin, M., Put, J. Sulfur Vulcanization of Simple Model Olefins, Part V: Double Bond Isomerization During Accelerated Sulfur Vulcanization as Studied by Model Olefins. Rubber Chem. Technol. 1997, 70, 114. [Google Scholar] [CrossRef]
- Blume, A. The Manifold Interactions of ZnO and Stearic Acid in a Modern Silica-Filled Tire Tread Compound. Proceedings of the Fall 200th Technical Meeting ACS Rubber Division, Pittsburgh, United States, Oct. 4-7, 2021.
- Jin, J. Influence of Compounding and Mixing on Filler Dispersion and Curing Behavior of Silica Compounds. PhD thesis, University of Twente, The Netherlands, , 2020. 11 March.
- Hayichelaeh, C. Reuvekamp, L.A.E.M., Dierkes, W.K., Blume, A., Noordermeer, J.W.M., Sahakaro, K., Enhancing the Silanization Reaction of the Silica-Silane System by Different Amines in Model and Practical Silica-Filled Natural Rubber Compounds. Polymers 2018, 10, 584. [Google Scholar] [CrossRef] [PubMed]
- Coran, A.Y. Chapter 7: Vulcanization. In The Science and Technology of Rubber, 4th ed.; Mark, J.E., Erman, B., Roland, C.M., Eds.; Academic Press: Cambridge, Massachusetts, USA, 2013; pp. 345–364. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).