Submitted:
31 August 2024
Posted:
02 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
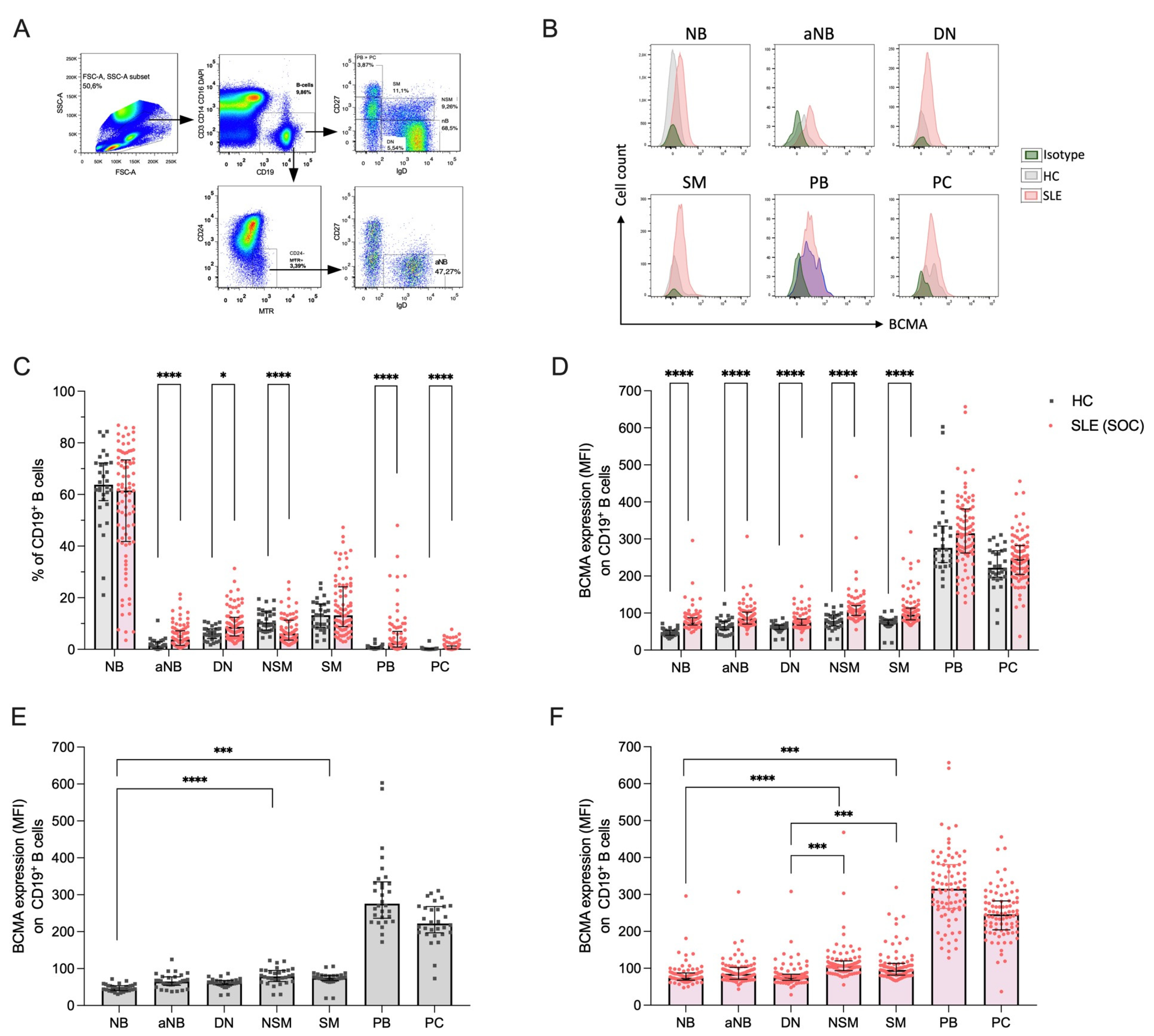
2.1. B Cell Subset Distribution and their BCMA Expression
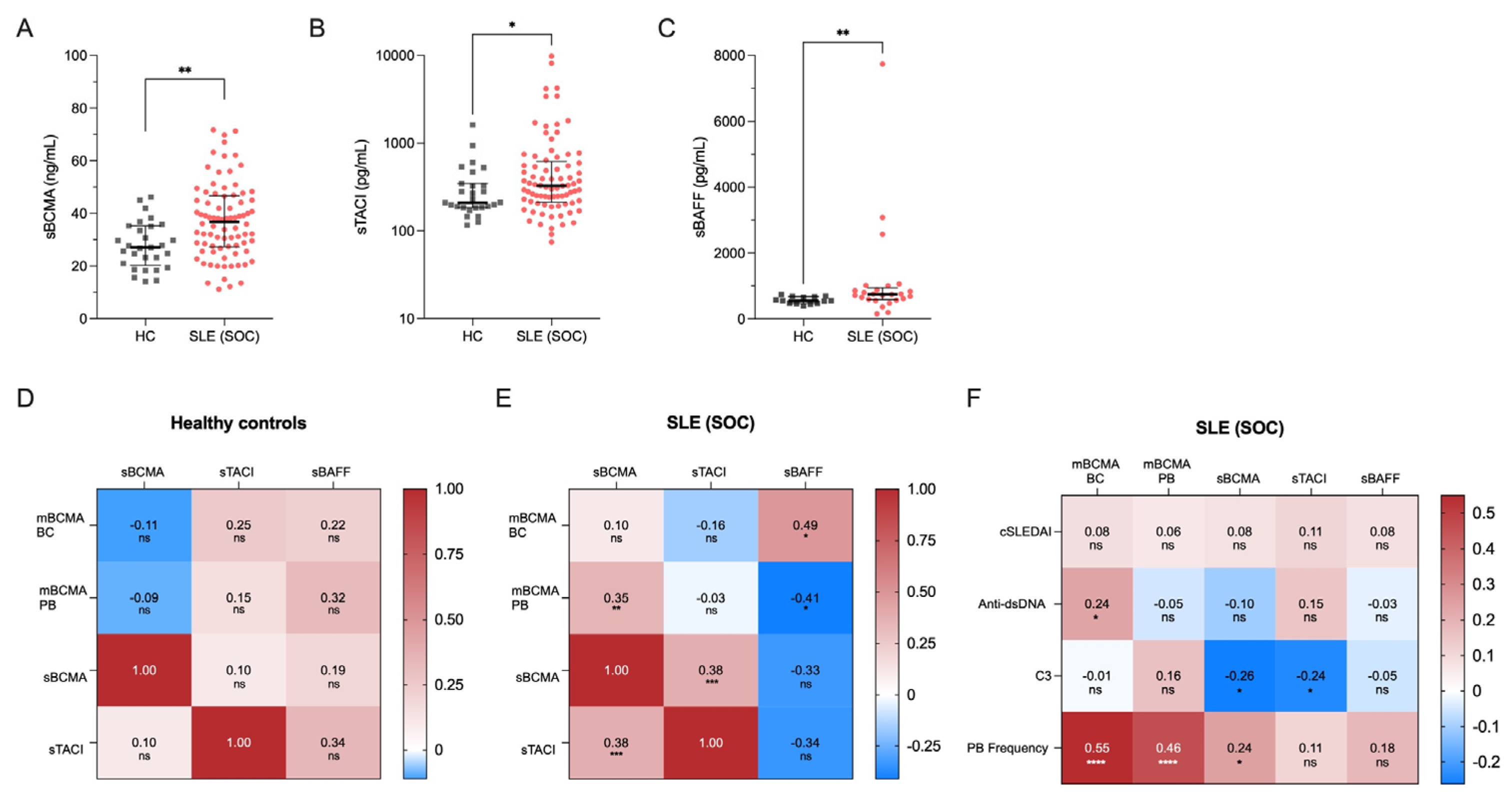
2.2. Soluble Markers of the BAFF-APRIL System and Their Correlations
2.3. Correlations of BCMA with Clinical and Serologic Variables and Plasmablast Frequency
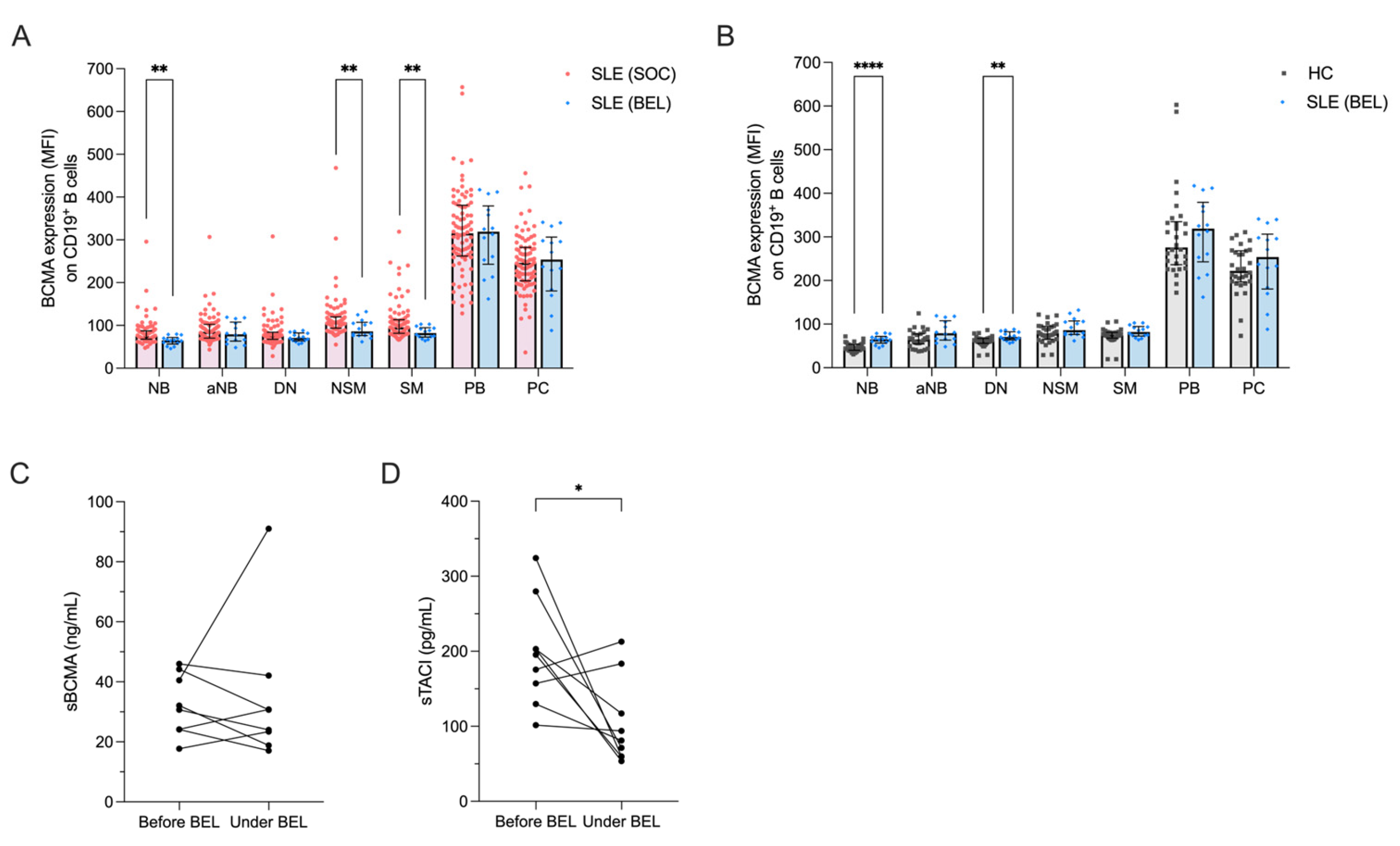
2.4. Impact of Belimumab Treatment on the BAFF/APRIL System
3. Discussion
4. Materials and Methods
4.1. Patient and Control Blood Samples
4.2. Cell Isolation and Flow Cytometry
4.3. ELISA
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsokos, G.C. Systemic lupus erythematosus. N Engl J Med 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Arbuckle, M.R.; McClain, M.T.; Rubertone, M.V.; Scofield, R.H.; Dennis, G.J.; James, J.A.; Harley, J.B. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003, 349, 1526–1533. [Google Scholar] [CrossRef]
- Iwata, S.; Tanaka, Y. B-cell subsets, signaling and their roles in secretion of autoantibodies. Lupus 2016, 25, 850–856. [Google Scholar] [CrossRef]
- Hiepe, F.; Dörner, T.; Hauser, A.E.; Hoyer, B.F.; Mei, H.; Radbruch, A. Long-lived autoreactive plasma cells drive persistent autoimmune inflammation. Nat Rev Rheumatol 2011, 7, 170–178. [Google Scholar] [CrossRef]
- Alexander, T.; Sarfert, R.; Klotsche, J.; Kühl, A.A.; Rubbert-Roth, A.; Lorenz, H.M.; Rech, J.; Hoyer, B.F.; Cheng, Q.; Waka, A.; et al. The proteasome inhibitior bortezomib depletes plasma cells and ameliorates clinical manifestations of refractory systemic lupus erythematosus. Ann Rheum Dis 2015, 74, 1474–1478. [Google Scholar] [CrossRef]
- Ostendorf, L.; Burns, M.; Durek, P.; Heinz, G.A.; Heinrich, F.; Garantziotis, P.; Enghard, P.; Richter, U.; Biesen, R.; Schneider, U.; et al. Targeting CD38 with Daratumumab in Refractory Systemic Lupus Erythematosus. N Engl J Med 2020, 383, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Roccatello, D.; Fenoglio, R.; Caniggia, I.; Kamgaing, J.; Naretto, C.; Cecchi, I.; Rubini, E.; Rossi, D.; De Simone, E.; Del Vecchio, G.; et al. Daratumumab monotherapy for refractory lupus nephritis. Nat Med 2023, 29, 2041–2047. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; He, S.; Zhang, W.; Zhang, H.; DeStefano, V.M.; Wada, M.; Pinz, K.; Deener, G.; Shah, D.; Hagag, N.; et al. BCMA-CD19 compound CAR T cells for systemic lupus erythematosus: A phase 1 open-label clinical trial. Ann Rheum Dis 2024. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; Garfall, A.L.; van de Donk, N.; Nahi, H.; San-Miguel, J.F.; Oriol, A.; Nooka, A.K.; Martin, T.; Rosinol, L.; Chari, A.; et al. Teclistamab in Relapsed or Refractory Multiple Myeloma. N Engl J Med 2022, 387, 495–505. [Google Scholar] [CrossRef]
- Presley, A.D.; Fuller, K.M.; Arriaga, E.A. MitoTracker Green labeling of mitochondrial proteins and their subsequent analysis by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B Analyt Technol Biomed Life Sci 2003, 793, 141–150. [Google Scholar] [CrossRef]
- Wirths, S.; Lanzavecchia, A. ABCB1 transporter discriminates human resting naive B cells from cycling transitional and memory B cells. Eur J Immunol 2005, 35, 3433–3441. [Google Scholar] [CrossRef]
- Tipton, C.M.; Fucile, C.F.; Darce, J.; Chida, A.; Ichikawa, T.; Gregoretti, I.; Schieferl, S.; Hom, J.; Jenks, S.; Feldman, R.J.; et al. Diversity, cellular origin and autoreactivity of antibody-secreting cell population expansions in acute systemic lupus erythematosus. Nat Immunol 2015, 16, 755–765. [Google Scholar] [CrossRef] [PubMed]
- Jacobi, A.M.; Mei, H.; Hoyer, B.F.; Mumtaz, I.M.; Thiele, K.; Radbruch, A.; Burmester, G.R.; Hiepe, F.; Dörner, T. HLA-DRhigh/CD27high plasmablasts indicate active disease in patients with systemic lupus erythematosus. Ann Rheum Dis 2010, 69, 305–308. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, A.V.; Rubtsova, K.; Fischer, A.; Meehan, R.T.; Gillis, J.Z.; Kappler, J.W.; Marrack, P. Toll-like receptor 7 (TLR7)-driven accumulation of a novel CD11c⁺ B-cell population is important for the development of autoimmunity. Blood 2011, 118, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Sachinidis, A.; Xanthopoulos, K.; Garyfallos, A. Age-Associated B Cells (ABCs) in the Prognosis, Diagnosis and Therapy of Systemic Lupus Erythematosus (SLE). Mediterr J Rheumatol 2020, 31, 311–318. [Google Scholar] [CrossRef]
- Jenks, S.A.; Cashman, K.S.; Zumaquero, E.; Marigorta, U.M.; Patel, A.V.; Wang, X.; Tomar, D.; Woodruff, M.C.; Simon, Z.; Bugrovsky, R.; et al. Distinct Effector B Cells Induced by Unregulated Toll-like Receptor 7 Contribute to Pathogenic Responses in Systemic Lupus Erythematosus. Immunity 2018, 49, 725–739. [Google Scholar] [CrossRef]
- Wei, C.; Anolik, J.; Cappione, A.; Zheng, B.; Pugh-Bernard, A.; Brooks, J.; Lee, E.H.; Milner, E.C.; Sanz, I. A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007, 178, 6624–6633. [Google Scholar] [CrossRef]
- Torigoe, M.; Iwata, S.; Nakayamada, S.; Sakata, K.; Zhang, M.; Hajime, M.; Miyazaki, Y.; Narisawa, M.; Ishii, K.; Shibata, H.; et al. Metabolic Reprogramming Commits Differentiation of Human CD27(+)IgD(+) B Cells to Plasmablasts or CD27(-)IgD(-) Cells. J Immunol 2017, 199, 425–434. [Google Scholar] [CrossRef]
- Jacobi, A.M.; Reiter, K.; Mackay, M.; Aranow, C.; Hiepe, F.; Radbruch, A.; Hansen, A.; Burmester, G.R.; Diamond, B.; Lipsky, P.E.; et al. Activated memory B cell subsets correlate with disease activity in systemic lupus erythematosus: Delineation by expression of CD27, IgD, and CD95. Arthritis Rheum 2008, 58, 1762–1773. [Google Scholar] [CrossRef]
- Szelinski, F.; Stefanski, A.L.; Schrezenmeier, E.; Rincon-Arevalo, H.; Wiedemann, A.; Reiter, K.; Ritter, J.; Lettau, M.; Dang, V.D.; Fuchs, S.; et al. Plasmablast-like Phenotype Among Antigen-Experienced CXCR5-CD19(low) B Cells in Systemic Lupus Erythematosus. Arthritis Rheumatol 2022, 74, 1556–1568. [Google Scholar] [CrossRef]
- Moore, P.A.; Belvedere, O.; Orr, A.; Pieri, K.; LaFleur, D.W.; Feng, P.; Soppet, D.; Charters, M.; Gentz, R.; Parmelee, D.; et al. BLyS: Member of the tumor necrosis factor family and B lymphocyte stimulator. Science 1999, 285, 260–263. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.A.; Vilas-Boas, A.; Isenberg, D.A. B-cell survival factors in autoimmune rheumatic disorders. Ther Adv Musculoskelet Dis 2015, 7, 122–151. [Google Scholar] [CrossRef]
- Yap, D.Y.; Lai, K.N. Cytokines and their roles in the pathogenesis of systemic lupus erythematosus: From basics to recent advances. J Biomed Biotechnol 2010, 2010, 365083. [Google Scholar] [CrossRef]
- Bossen, C.; Cachero, T.G.; Tardivel, A.; Ingold, K.; Willen, L.; Dobles, M.; Scott, M.L.; Maquelin, A.; Belnoue, E.; Siegrist, C.A.; et al. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood 2008, 111, 1004–1012. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Gross, J.A.; Dillon, S.R.; Min, J.K.; Elkon, K.B. Increased BCMA expression in lupus marks activated B cells, and BCMA receptor engagement enhances the response to TLR9 stimulation. Autoimmunity 2011, 44, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; MacKay, F.; Steiner, V.; Hofmann, K.; Bodmer, J.L.; Holler, N.; Ambrose, C.; Lawton, P.; Bixler, S.; Acha-Orbea, H.; et al. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 1999, 189, 1747–1756. [Google Scholar] [CrossRef]
- Vincent, F.B.; Morand, E.F.; Schneider, P.; Mackay, F. The BAFF/APRIL system in SLE pathogenesis. Nat Rev Rheumatol 2014, 10, 365–373. [Google Scholar] [CrossRef]
- Avery, D.T.; Kalled, S.L.; Ellyard, J.I.; Ambrose, C.; Bixler, S.A.; Thien, M.; Brink, R.; Mackay, F.; Hodgkin, P.D.; Tangye, S.G. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest 2003, 112, 286–297. [Google Scholar] [CrossRef]
- Moreaux, J.; Legouffe, E.; Jourdan, E.; Quittet, P.; Rème, T.; Lugagne, C.; Moine, P.; Rossi, J.F.; Klein, B.; Tarte, K. BAFF and APRIL protect myeloma cells from apoptosis induced by interleukin 6 deprivation and dexamethasone. Blood 2004, 103, 3148–3157. [Google Scholar] [CrossRef]
- Darce, J.R.; Arendt, B.K.; Wu, X.; Jelinek, D.F. Regulated expression of BAFF-binding receptors during human B cell differentiation. J Immunol 2007, 179, 7276–7286. [Google Scholar] [CrossRef]
- O’Connor, B.P.; Raman, V.S.; Erickson, L.D.; Cook, W.J.; Weaver, L.K.; Ahonen, C.; Lin, L.L.; Mantchev, G.T.; Bram, R.J.; Noelle, R.J. BCMA is essential for the survival of long-lived bone marrow plasma cells. J Exp Med 2004, 199, 91–98. [Google Scholar] [CrossRef]
- Laurent, S.A.; Hoffmann, F.S.; Kuhn, P.H.; Cheng, Q.; Chu, Y.; Schmidt-Supprian, M.; Hauck, S.M.; Schuh, E.; Krumbholz, M.; Rübsamen, H.; et al. γ-Secretase directly sheds the survival receptor BCMA from plasma cells. Nat Commun 2015, 6, 7333. [Google Scholar] [CrossRef] [PubMed]
- Meinl, E.; Thaler, F.S.; Lichtenthaler, S.F. Shedding of BAFF/APRIL Receptors Controls B Cells. Trends Immunol 2018, 39, 673–676. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Camarena, D.C.; Palafox-Sánchez, C.A.; Cruz, A.; Marín-Rosales, M.; Muñoz-Valle, J.F. Analysis of the receptor BCMA as a biomarker in systemic lupus erythematosus patients. Sci Rep 2020, 10, 6236. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Gómez, J.A.; Salazar-Camarena, D.C.; Román-Fernández, I.V.; Ortiz-Lazareno, P.C.; Cruz, A.; Muñoz-Valle, J.F.; Marín-Rosales, M.; Espinoza-García, N.; Sagrero-Fabela, N.; Palafox-Sánchez, C.A. BAFF system expression in double negative 2, activated naïve and activated memory B cells in systemic lupus erythematosus. Front Immunol 2023, 14, 1235937. [Google Scholar] [CrossRef]
- Zhao, L.D.; Li, Y.; Smith, M.F., Jr.; Wang, J.S.; Zhang, W.; Tang, F.L.; Tian, X.P.; Wang, H.Y.; Zhang, F.C.; Ba, D.N.; et al. Expressions of BAFF/BAFF receptors and their correlation with disease activity in Chinese SLE patients. Lupus 2010, 19, 1534–1549. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Camarena, D.C.; Ortiz-Lazareno, P.C.; Cruz, A.; Oregon-Romero, E.; Machado-Contreras, J.R.; Muñoz-Valle, J.F.; Orozco-López, M.; Marín-Rosales, M.; Palafox-Sánchez, C.A. Association of BAFF, APRIL serum levels, BAFF-R, TACI and BCMA expression on peripheral B-cell subsets with clinical manifestations in systemic lupus erythematosus. Lupus 2016, 25, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Fujioka, K.; Kida, T.; Omura, S.; Sofue, H.; Sakashita, A.; Sagawa, T.; Isoda, Y.; Kasahara, A.; Sagawa, R.; et al. Association between early immunophenotypic changes and therapeutic response of belimumab in patients with systemic lupus erythematosus. Lupus 2023, 32, 63–73. [Google Scholar] [CrossRef]
- Ramsköld, D.; Parodis, I.; Lakshmikanth, T.; Sippl, N.; Khademi, M.; Chen, Y.; Zickert, A.; Mikeš, J.; Achour, A.; Amara, K.; et al. B cell alterations during BAFF inhibition with belimumab in SLE. EBioMedicine 2019, 40, 517–527. [Google Scholar] [CrossRef]
- Huang, W.; Quach, T.D.; Dascalu, C.; Liu, Z.; Leung, T.; Byrne-Steele, M.; Pan, W.; Yang, Q.; Han, J.; Lesser, M.; et al. Belimumab promotes negative selection of activated autoreactive B cells in systemic lupus erythematosus patients. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Shah, N.; Chari, A.; Scott, E.; Mezzi, K.; Usmani, S.Z. B-cell maturation antigen (BCMA) in multiple myeloma: Rationale for targeting and current therapeutic approaches. Leukemia 2020, 34, 985–1005. [Google Scholar] [CrossRef]
- Batten, M.; Groom, J.; Cachero, T.G.; Qian, F.; Schneider, P.; Tschopp, J.; Browning, J.L.; Mackay, F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med 2000, 192, 1453–1466. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, F.S.; Kuhn, P.H.; Laurent, S.A.; Hauck, S.M.; Berer, K.; Wendlinger, S.A.; Krumbholz, M.; Khademi, M.; Olsson, T.; Dreyling, M.; et al. The immunoregulator soluble TACI is released by ADAM10 and reflects B cell activation in autoimmunity. J Immunol 2015, 194, 542–552. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Aljaro, P.; Montes-Cano, M.A.; García-Lozano, J.R.; Aquino, V.; Carmona, R.; Perez-Florido, J.; García-Hernández, F.J.; Dopazo, J.; González-Escribano, M.F. Protein and functional isoform levels and genetic variants of the BAFF and APRIL pathway components in systemic lupus erythematosus. Sci Rep 2022, 12, 11219. [Google Scholar] [CrossRef]
- Smulski, C.R.; Kury, P.; Seidel, L.M.; Staiger, H.S.; Edinger, A.K.; Willen, L.; Seidl, M.; Hess, H.; Salzer, U.; Rolink, A.G.; et al. BAFF- and TACI-Dependent Processing of BAFFR by ADAM Proteases Regulates the Survival of B Cells. Cell Rep 2017, 18, 2189–2202. [Google Scholar] [CrossRef]
- Sanchez, E.; Li, M.; Kitto, A.; Li, J.; Wang, C.S.; Kirk, D.T.; Yellin, O.; Nichols, C.M.; Dreyer, M.P.; Ahles, C.P.; et al. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. Br J Haematol 2012, 158, 727–738. [Google Scholar] [CrossRef]
- Stohl, W.; Hiepe, F.; Latinis, K.M.; Thomas, M.; Scheinberg, M.A.; Clarke, A.; Aranow, C.; Wellborne, F.R.; Abud-Mendoza, C.; Hough, D.R.; et al. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum 2012, 64, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Piantoni, S.; Regola, F.; Masneri, S.; Merletti, M.; Lowin, T.; Airò, P.; Tincani, A.; Franceschini, F.; Andreoli, L.; Pongratz, G. Characterization of B- and T-Cell Compartment and B-Cell Related Factors Belonging to the TNF/TNFR Superfamily in Patients With Clinically Active Systemic Lupus Erythematosus: Baseline BAFF Serum Levels Are the Strongest Predictor of Response to Belimumab after Twelve Months of Therapy. Front Pharmacol 2021, 12, 666971. [Google Scholar] [CrossRef]
- Parodis, I.; Sjöwall, C.; Jönsen, A.; Ramsköld, D.; Zickert, A.; Frodlund, M.; Sohrabian, A.; Arnaud, L.; Rönnelid, J.; Malmström, V.; et al. Smoking and pre-existing organ damage reduce the efficacy of belimumab in systemic lupus erythematosus. Autoimmun Rev 2017, 16, 343–351. [Google Scholar] [CrossRef]
- Aringer, M.; Costenbader, K.; Daikh, D.; Brinks, R.; Mosca, M.; Ramsey-Goldman, R.; Smolen, J.S.; Wofsy, D.; Boumpas, D.T.; Kamen, D.L.; et al. 2019 European League Against Rheumatism/American College of Rheumatology Classification Criteria for Systemic Lupus Erythematosus. Arthritis Rheumatol 2019, 71, 1400–1412. [Google Scholar] [CrossRef]
- Gladman, D.D.; Ibañez, D.; Urowitz, M.B. Systemic lupus erythematosus disease activity index 2000. J Rheumatol 2002, 29, 288–291. [Google Scholar] [PubMed]
- Cossarizza, A.; Chang, H.D.; Radbruch, A.; Akdis, M.; Andrä, I.; Annunziato, F.; Bacher, P.; Barnaba, V.; Battistini, L.; Bauer, W.M.; et al. Guidelines for the use of flow cytometry and cell sorting in immunological studies. Eur J Immunol 2017, 47, 1584–1797. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | SLE patients (n=100) | Healthy controls (n=30) | P-Value |
|---|---|---|---|
| Age, median (range) | 38.5 (19–80) | 28.7 (22–59) | 0.11 |
| Gender, female, n (%) | 90 (90) | 26 (86.7) | 0.61 |
| Ethnicity, n (%) Caucasian Asian African Latin American |
90 (90) 1 (1) 6 (6) 3 (3) |
26 (86.7) 2 (6.7) 2 (6.7) 0 (0) |
0.61 0.07 0.89 0.34 |
| Disease duration, median years, (range) | 6.5 (6–40) | ||
| Disease activity SLEDAI-2K, median (range) Clinically active, n (%) DORIS Remission n (%) |
4 (0–26) 46 (46) 54 (54) |
||
| Active clinical manifestations at time of presentation, n (%) Musculoskeletal Mucocutaneous Polyserositis Nephritis CNS Cytopenia |
33 (33) 18 (18) 3 (3) 5 (5) 2 (2) 43 (43) |
||
| Serology Anti-dsDNA positive, n (%) C3-deficiency, n (%) |
71 (71) 71 (71) |
||
| Medication, n (%) Prednisolone Prednisolone dosage (mg/d), median Prednisolone ≥ 7.5 mg/d Hydroxychloroquine Methotrexate Azathioprine Mycophenolate mofetil Calcineurin inhibitors Belimumab |
75 (75) 5.0 30 (30) 78 (78) 11 (11) 34 (34) 14 (14) 5 (5) 14 (14) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





