1. Introduction
Parasitoid flies represent one of the threats to honeybee (
Apis mellifera) pollinators worldwide. These flies are known to be present in many countries based on reports of sporadic detection, whereas their prevalence and the extent to which they contribute to honeybee colony weakening are still little known. Diptera able to attack honeybees are the scuttle flies, family Phoridae, mainly represented by
Megaselia scalaris [
1,
2,
3,
4,
5,
6], and
Senotainia tricuspis, a viviparous species of the flesh fly family Sarcophagidae, found in Europe, Mediterranean countries and Middle East [
7]. Other Phoridae able to parasitize honeybees were described in particular geographic regions such as
Apocephalus borealis, possibly involved in the colony collapse disorder (CDD) in North America [
8], and
Melaloncha spp. in Brazil [
9] and other
Megaselia species could exert parasitism towards honeybees [
10,
11,
12].
M. scalaris is a generalist species able to cause myiases in a variety of living hosts, including humans [
13], animals [
14], and different arthropod species [
15,
16,
17,
18]. Indeed, it was proposed as a biocontrol agent against other pests [
14,
19]. It also infests inanimate organic matter and food commodities [
20]. It was found to frequently infest honeybees in Central Italy with high infestation rates expressed as the ratio of fly larvae number on number of infested bees [
2]. Reports on honeybee infestation by this or other
Megaselia species regard different European and extra-European countries [
3,
4,
5,
6,
10,
12]. Modeling of
M. scalaris expansion favored by the ongoing climate changes indicated that this species currently finds optimal living conditions in regions of North and Sub-Saharan Africa, Southern Europe, and France with the potential to invade Internal zones of Balkans and Eastern Europe [
21].
S. tricuspis was reported to cause heavy infestations with high colony mortality in apiaries. It can lead to the collapse of a honeybee colony when it reaches an infestation rate above 70% [
22]. These flies lie in wait for honeybees leaving or returning to the hive and attack them repeatedly according to a behavior that has been recently described in detail [
22,
23]. Since each female harbors several hundreds of larvae, it can infest a high number of bees [
7]. In Central Italy, adults of
S. tricuspis emerge during late spring and begin to infest honeybees at the end of May [
22]. Larvae formed on bees finally leave the host and pupate in the soil. Adults can emerge from soil either after overwintering in early spring or June-July since pupae formed in spring develop into adults within 15-20 days giving rise to a new generation in the same year [
22].
The extent of the detrimental effects of the parasitoid flies on honeybee health is not yet fully known and requires further investigation. This can also depend on the laborious and time-consuming methods of detection most often used that are based on the observation of fly larvae development on the body of honeybees captured and kept alive in controlled conditions of temperature and humidity for a few days [
2,
7].
Therefore, this study was aimed at developing molecular detection methods based on quantitative polymerase chain reaction (qPCR) to speed up the detection of parasitoid flies on apparently healthy honeybees. This could allow the detection of the infestation in an early phase and adopt countermeasures such as placing traps to reduce the fly infestation level [
22]. The detection methods developed in this study were characterized for specificity, inclusivity, and limit of detection (LOD) and were preliminarily applied to samples of both adult bees and hive debris collected in Central Italy and Kosovo.
In addition, thirteen isolates of M. scalaris obtained from apiaries of Abruzzo and Molise were genotyped by sequencing a region of the mitochondrial cytochrome oxidase I gene COI and compared to biotypes from other world regions to obtain indications on genetic relatedness. This was not possible for S. tricuspis because of the unavailability of sequences for comparison.
2. Materials and Methods
2.1. Design of qPCR Tests Specific for M. scalaris and S. tricuspis
PCR primers and probes used in this study were selected after a search of the nucleotide sequences available for
M. scalaris and
S. tricuspis in the public domain database accessed through the National Center of Biotechnology Information (
https://www.ncbi.nlm.nih.gov/, accessed on 30 August 2024). Representatives of the gene sequences available were aligned by Blastn (
https://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearch&LINK_LOC=blasthome, accessed on 31 August 2024) to the nucleotide database limiting the search to
M. scalaris or
S. tricuspis taxa, respectively, so conserved regions could be identified for the species. Then all the oligonucleotides that could be designed in the conserved regions were aligned by Blastn to verify inclusivity for the highest number of biotypes and exclusivity towards other insect species. The Blastn alignments were carried out for up to 1,000 database entries. Oligonucleotides and probes were provided by Eurofins Genomics (Ebersberg, Germany). Those used in the test specific for
M. scalaris were targeted on the mitochondrial
COI gene and were MegaF2: 5′-ACTCTTTTATTAGCAAGAAGTA-3′, MegaR2: 5′-ATAGAAGAAATTCCGGCAA-3′ and MegaP2: 5′Cy5-TTCTAGAATTGCYCATAG-MBGEQ-3′, while those used in the test specific for
S. tricuspis were targeted on the mitochondrial
cytB gene and were StrF1: 5′- GTTACTCCCGTTCACATT-3′, StrR: 5′-TATTGTTCAGAAATAGATTTTATTAATT-3′ and StrP3: 5′FAM-CTTCGTTCAATCCCCAAT-MBGEQ-3′. Other primers were designed for sequencing purposes on the same genes were MegaF3: 5′-TAAGTATTATAATTCGAGCTGAA-3′ for
M. scalaris and senseqF: 5′-AGATAATGCAACACTTACC-3′/senseqR: 5′-GTAAATTATCTCACCATTTAAC-3′ for
S. tricuspis. For amplification of the DNA extraction control the primers specific for
Tenebrio molitor reported by Rossi et al. [
24] were used.
2.2. DNA and RNA Extraction
DNA extraction was carried out by two procedures differing in the amount of sample processed and, consequently, amounts of reagents and number of phases. In particular, two or twenty bees and 200 μl or 3 ml of hive debris were examined to ensure acceptable sensitivity. The hive debris was measured approximately in volume because of the difficulty of manipulation for precise weighing and for the great variability in density among samples. The extraction procedure from two bees or 200 μl of hive debris was designated as “small-scale” and that from 20 bees or 3 ml of hive debris as “large-scale”. In the small-scale procedure, the sample was transferred into a 2 ml safe-lock eppendorf tube (Eppendorf, Milan, Italy) containing approximately 200 μl of unwashed glass beads of 200 µm diameter (Merck, Darmstadt, Germany) previously sterilized by autoclaving. The bee sample was crushed with a sterile pipette to facilitate the subsequent mechanical disruption by bead beating. To the sample 200 μl of Macherey-Nagel lysis buffer T1 (Carlo Erba, Cornaredo, Italy) and 25 µg of DNA extraction control constituted by homogenized
T. molitor as described by Rossi et al. [
24], were added and the sample was disrupted in TissueLyser II (Qiagen, Milan, Italy) at 30 Hz for 3 min. The sample homogenate was centrifuged at 12,000 × g for 10 min and the supernatant was transferred into a 2 ml safe-lock eppendorf tube containing 200 μl of Macherey-Nagel B3 buffer. After mixing 210 μl of pure molecular biology grade ethanol was added and the DNA was allowed to precipitate for 10 min at room temperature. The whole sample suspension was transferred on a Macherey-Nagel NucleoSpin Tissue kit spin column (Carlo Erba) and DNA purification was completed according to the instructions. The DNA was eluted in two steps in a final volume of 60 μl by adding in each step 30 μl of elution buffer to the column.
In the large-scale procedure, the sample was transferred in a 5 ml screw cap tube (Sarstedt Srl., Trezzano sul Naviglio, MI, Italy) containing approximately 1 ml of sterile unwashed glass beads of 200 µm diameter (Merck). To the 20 bees, 1 ml of Macherey-Nagel lysis buffer T1 (Carlo Erba) was added and the bees were crushed with a sterile pipette. An additional 500 μl of lysis buffer and 25 μg of the DNA extraction control were added and the sample was mechanically disrupted in the TissueLyser II instrument equipped with a 5 ml tube adapter. To the hive debris, 1.5 ml of T1 buffer and 25 μg of the DNA extraction control were added prior to disruption. The homogenate was then centrifuged at 12,000 × g for 10 min and the supernatant was transferred into a 5 ml Eppendorf tube containing 1.5 ml of B3 buffer. For the hive debris, the centrifugation was repeated by transferring the supernatant in a 5 ml Eppendorf tube if the supernatant was not clear before mixing with B3 buffer. The sample suspension was mixed and 1.575 ml of pure ethanol was added. The DNA was allowed to precipitate for 10 min at room temperature and the sample was centrifuged at 14,000 × g for 10 min. Most of the supernatant was discarded by gently aspiring with a micropipette and about 600 μl of it was left at the bottom of the tube. After pellet resuspension this was loaded on a spin column and DNA purification was completed as for the small-scale extraction method.
The RNA was extracted from six
M. scalaris flies or one
S. tricuspis fly by the same procedure applied to honeybees for virus detection by Rossi et al. [
24].
2.3. Determination of the Limit of Detection (LOD)
The minimum number of target gene copies spiked in samples of bees and hive debris and detectable in 95% extraction replicates, i.e., the limit of detection (LOD) [], was determined by using plasmid pUC57 containing synthetic DNA fragments (GenScript Biotech, Rijswijk, The Netherlands) with sequences identical to gene regions between the primers. These fragments were quantified by the Qubit 3 Fluorometer (Thermo Fisher Scientific, Rodano, Italy) and the Qubit DNA HS Assay Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions and were used to spike honeybee or hive debris samples, which, in preliminary assays, tested negative in the qPCR assays to be evaluated in this study.
The LOD was determined also in terms of weight of fly per g of sample by spiking honeybee and hive debris samples previously found to be negative for the fly-specific tests, with known amounts of M. scalaris or S. tricuspis homogenate obtained from the isolates made available in this study. Twenty replicates of spiked samples were used for DNA extraction with the two procedures described above.
2.4. qPCR Conditions
Quantitative PCR reactions were carried out in a QuantStudio 5 instrument (Thermo Fisher Scientific) with a PCR program comprising initial denaturation at 95°C for 5 min and 50 cycles of denaturation at 95°C for 15 sec, and annealing/elongation at 52°C for 1 min. The qPCR reaction contained 1× Takara Premix Ex Taq Probe qPCR (Diatech LabLine, Jesi, AN, Italy), bovine serum albumin (Merck) 0.1 μg/μl, primers and probes for the target 0.2 μM, primers and probes for the DNA extraction control 0.1 μM and 10 μl of DNA extract in a total reaction volume of 50 μl. Quantitative PCR tests were carried out to detect microbial pathogens of honeybees in fly isolates by previously reported tests. These comprised specific tests for
Paenibacillus larvae,
Melissococcus plutonius,
Vairimorpha ceranae and
V. apis and viruses acute paralysis bee virus (ABPV), black queen cell virus (BQCV), chronic bee paralysis virus (CBPV), deformed wings virus A and B (DWVA and DWVB), and sacbrood virus (SBV) [
24,
25,
26,
27]. Synthetic DNA or RNA were used as positive controls in PCR reactions and the negative amplification controls were represented by extracts obtained without adding a sample.
2.5. Collection of Bees and Hive Debris
Samples of honeybees and hive debris were collected by beekeepers from 16 apiaries in Abruzzo and Molise regions, Central Italy, and 16 apiaries in Kosovo during June-July 2021. Sample sites were uniformly distributed in the two geographical districts. Only one hive per apiary was sampled. At least 30 foraging bees to be examined by molecular tests were captured at the hive entrance and introduced in sterile 50 ml centrifuge tubes (Biosigma, Cona, VE, Italy). The same tubes were used to collect hive debris picked with sterile disposable spoons (Biosigma) from the hive bottom drawer. Samples were transported to the laboratory in refrigerated conditions within 48 h and stored at -80°C before analysis. Samples collected in Kosovo were soaked in RNAlater solution (Thermo Fisher Scientific, Rodano, Italy) before being transported by hand to the Istituto Zooprofilattico Sperimentale dell’Abruzzo e del Molise (IZSAM), branch of Campobasso, Italy, and analyzed.
Viable honeybees were collected in apiaries of Abruzzo and Molise in July 2022 (the sampling of viable bees could not be carried out in the year 2021 because of the COVID-19 pandemic activity limitations) and analyzed for the development of parasitoid flies as described by Ricchiuti et al. [
2].
2.6. Sanger Sequencing
Sanger sequencing was carried out by Eurofins Genomics on amplification products from the M. scalaris COI gene obtained with primers MegaF3: 5′-TAAGTATTATAATTCGAGCTGAA-3′ and MegaR2 and from the cytB gene of S. tricuspis obtained with primers senseqF: 5′-AGATAATGCAACACTTACC-3′/senseqR: 5′-GTAAATTATCTCACCATTTAAC-3′. The same oligonucleotides were used as sequencing primers. The obtained sequences received the following GenBank accession numbers: M. scalaris n9, PQ242635; n10, PQ242636; n11, PQ242637; n15, PQ242638; n17, PQ242639; n19, PQ242640; n20, PQ242641; n21, PQ242642; n22, PQ242643; n24, PQ242644; n27, PQ242645; n28, PQ242646; n33, PQ242647; S. tricuspis n13, PQ255542; n14, PQ255543; n32, PQ255544.
2.7. Construction of a Phylogenetic Tree
For
M. scalaris a phylogenetic tree was constructed including the sequences obtained in this study and one representative for each sequence variant retrieved by Blastn. The sequence variants to be included were selected by visually observing each pairwise matching in Blastn. For the tree construction the online tool “advanced phylogeny analysis” available at
http://www.phylogeny.fr/simple_phylogeny.cgi, accessed on 31 August 2024, was used.
3. Results
3.1. Performance of the qPCR Methods in the Detection of M. scalaris and S. tricuspis
The GenBank database search for nucleotide sequences from the species
M. scalaris and
S. tricuspis retrieved 170 partial or complete sequences of the
COI gene, one complete mitochondrion genome, and a whole genome in the stage of unassembled and not annotated scaffolds for
M. scalaris. For
S. tricuspis only four partial coding sequences for genes elongation factor 1-alpha (Ef1a) and mitochondrial genes
cytB,
COI, and NADH dehydrogenase subunit 4,
ND4, obtained in a phylogenetic study [
28], were available.
For M. scalaris the first half of the COI gene was elected as a region for primer design since it exhibits higher variability among the species of Diptera as well as intra-species variability. Moreover, the high number of sequences available for this gene could allow a reliable assessment of the inclusivity of the oligonucleotides designed. It was not possible to evaluate the inclusivity of the nucleotides designed for S. tricuspis because of the unavailability of sequences from different individuals.
None of the oligonucleotides evaluated for M. scalaris was specific only for this species but the combination of MegaF2 with MegaR2 can ensure specificity since these primers differ in the fly species different from matched beyond M. scalaris. Primers targeted on S. tricuspis mitochondrial cytB, StrF1, and StrR appeared to be specific for S. tricuspis in in silico evaluation.
The sensitivity of the PCR tests was evaluated with DNA extracts from honeybee and hive debris samples contaminated with known copy numbers of plasmid pUC57 containing the synthetic DNA fragments identical to the target regions. In the small-scale extraction procedure, 100 copies of the target per g of sample were detected in all 20 replicates for both target organisms and both sample types. The lowest copy number detected in all replicates of the sample types with the large-scale extraction was 10 copies of the target sequence per g of sample. The cycle threshold (Ct) values at the LOD varied between 30.26 and 32.16 for M. scalaris and between 28.36 and 30.02 for S. tricuspis.
3.2. Analysis of Samples of Honeybees and Hive Debris for the Presence of M. scalaris and S. tricuspis
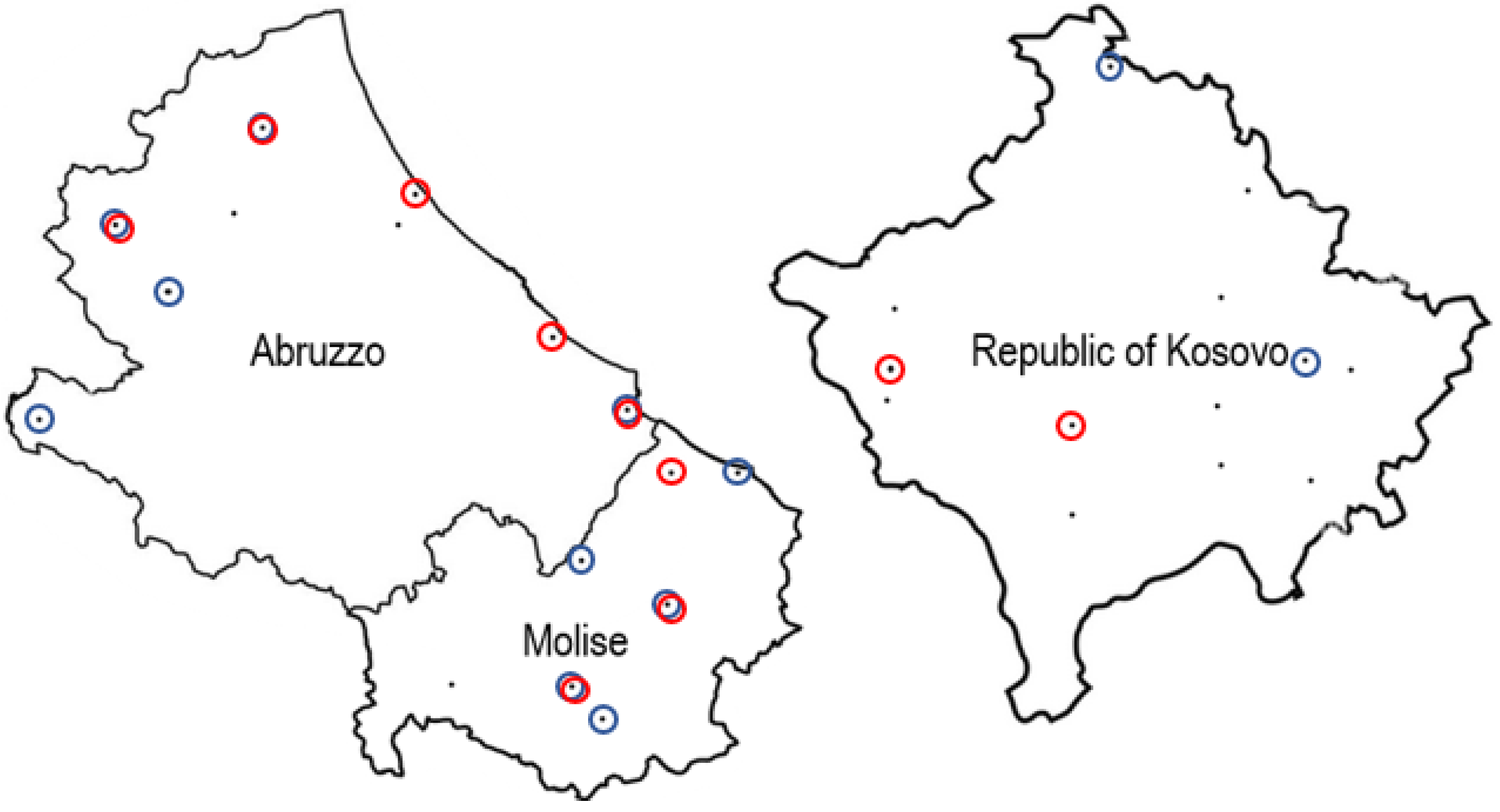
The samples used for the detection of
M. scalaris and
S. tricuspis naturally present in honeybees and hive debris were obtained from the sampling sites shown as black dots in
Figure 1 in Abruzzo e Molise and Kosovo. The sites in which the target organisms were detected are circled.
The samples were analyzed by using the large-scale extraction procedure and the Ct values were in most cases below the range of those observed at the LOD, indicating a low amount of fly material present on apparently healthy honeybees and hive debris. The Ct values obtained for samples are shown in
Table 1 which also reports the towns of collection and sample type.
It can be observed that both flies were more frequently detected in Abruzzo and Molise with a percentage of positive samples of bees of 52.25% for M. scalaris and 31.25% for S. tricuspis. In Abruzzo and Molise the flies were also detected in hive debris in percentages of 37.5% for M. scalaris and 12.5% for S. tricuspis. In half of the sites positive for M. scalaris this fly was detected both in bees and hive debris, while this was observed only for one among eight sites positive for S. tricuspis. For the samples from Kosovo the flies were only detected in bees at percentage of 12.5% each. Results indicated that these organisms are more reliably detected using bees as sample type.
3.3. Isolation of Flies from Honeybees Captured in Positive Sampling Sites
Samples of viable bees were collected one year later from the molecular analyses in Abruzzo and Molise sites in which M. scalaris and S. tricuspis were detected. Emerging fly larvae were observed for five of the samples, namely those taken in Oratino, Termoli, Montenero di Bisaccia and Trivento for M. scalaris and Vasto for S. tricuspis. For each sample four individual flies of those developed in adults were used for DNA extraction, amplification and sequencing of a COI gene region and a cytB region for M. scalaris and S. tricuspis, respectively. A total of thirteen sequences could be obtained for M. scalaris and three for S. tricuspis. The results showed sequence variability for M. scalaris isolates from the same sample in two instances, though most of the isolates shared identical sequences. The three sequences obtained for S. tricuspis were identical but divergent from the only database entry available.
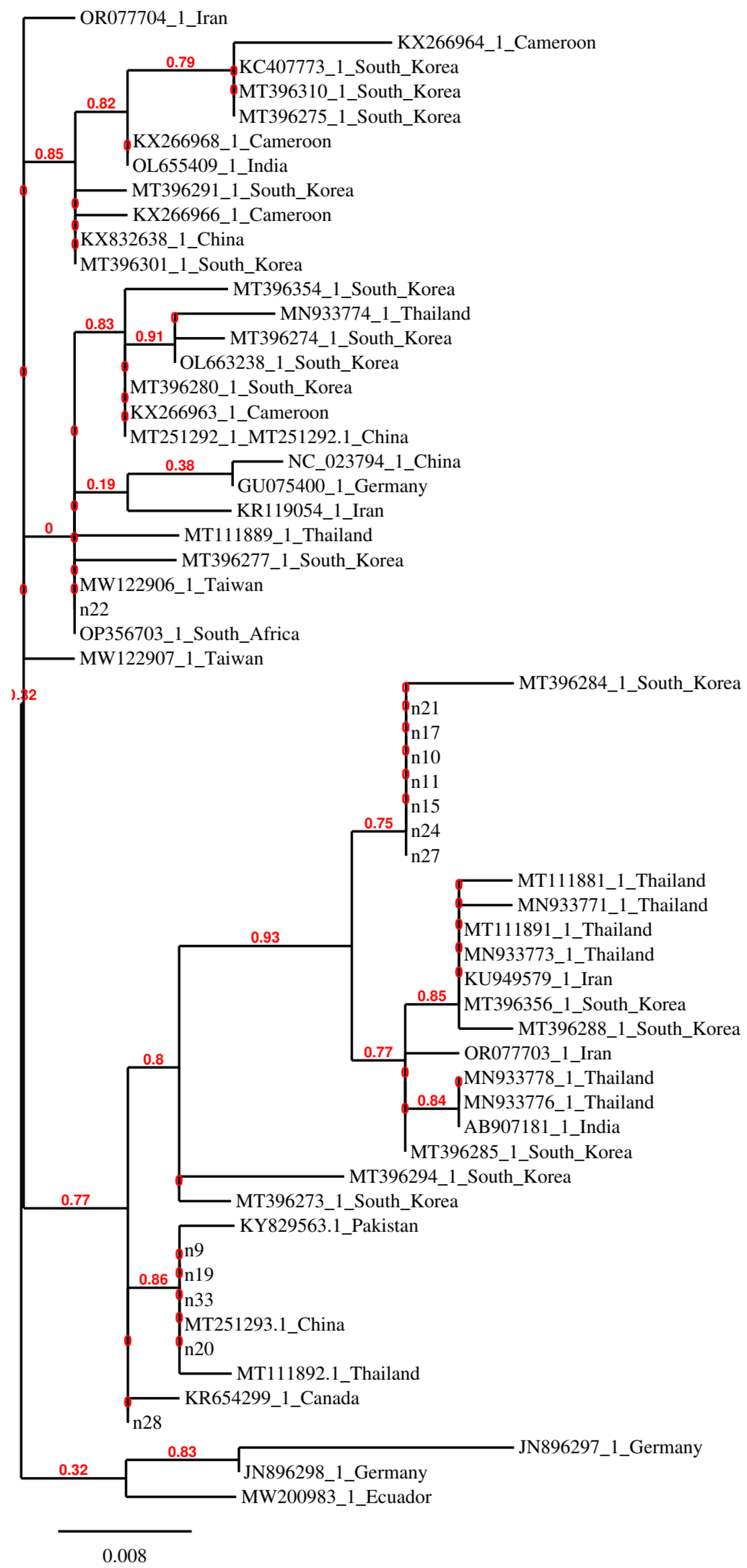
Figure 2 shows a phylogenetic tree constructed with the
COI gene regions of
M. scalaris in which the isolates from Abruzzo and Molise are compared with isolates from other countries.
It can be observed that some isolates from this study, i.e., those that do not have an accession number in
Figure 2, do not cluster closely and some of them are identical to biotypes from very distant geographical regions. Identity was also observed among other isolates from distant geographical regions, indicating the possibility that
M. scalaris flies might be transferred among countries through infested materials.
3.4. Re-Assessment of LOD in Terms of Weight of Fly Material per G of Sample
The availability of fly isolates allowed a better simulation of real sample analysis by using known weights of fly material to spike bee and hive debris samples negative for the fly-specific qPCR tests. In this case, the 10 µg of fly per g of bees or hive debris could be detected in 90% of replicates processed with the small-scale extraction method with Ct values between 30.56 and 33.24 for M. scalaris and 27.91 and 30.02 for S. tricuspis and 1 µg per g of bees or hive debris was detected in 95% of samples processed with the large-scale extraction method with Ct values between 31.37 and 34.01 for M. scalaris and 29.71 and 30.45 for S. tricuspis. Therefore, the large-scale extraction method is more sensitive and reliable than the small-scale extraction method for the detection of parasitoid flies in hive matrices.
3.5. Detection of Honeybee Microbial Pathogens on Parasitoid Flies
DNA and RNA extracted with the small-scale extraction method from pools of adult flies that emerged from bees were used to analyze the presence of honeybee microbial pathogens in the parasites.
P. larvae was detected in one
M. scalaris sample, DWVA in four
M. scalaris samples, BQCV and CBPV in two
M. scalaris samples and DWVB in one
M. scalaris sample.
N. ceranae was detected in the
S. tricuspis sample. All these pathogens were present at low levels, close to or below the respective LODs [
24,
25,
26,
27], in the flies but their presence indicates that parasitoid flies can participate in the spread of microbial pathogens among apiaries.
4. Discussion
This study made available experimental procedures suitable to detect parasitoid flies M. scalaris and S. tricuspis in hive matrices. For M. scalaris a qPCR test was described before qPCR but it was targeted on the mitochondrial ATP6 gene for which only one representative is available in the GenBank database. Therefore, no analysis of inclusivity and exclusivity was possible. Moreover, the previously available test was evaluated for specificity toward insect contaminating food commodities and not toward hive-associated insects. For these reasons, it was decided to design a new test targeted on the COI gene for which inclusivity and specificity could be evaluated in silico. No qPCR tests specific for S. tricuspis were available before this study and, at this time, in silico evaluation excluded possible cross-reactions of the designed primer/probe system with other organisms.
The preliminary application of the developed methods to real samples indicated that the early detection of the infestation on apparently healthy honeybees is possible but the low levels of contamination observed, often below the LOD of 1 µg of fly material per g of sample, indicated that the large-scale DNA extraction method provides more reliable results by reducing the number of false negative results. The low levels of fly material detected on real samples are explainable by the fact that when the infestation is still not manifest only fly eggs or very small fly larvae are present on the bee body.
Though the contamination levels were low, the developed methods showed some value in real sample analysis from distinct geographical contexts. A high percentage of sites infested by the flies was identified in Central Italy. The lower positive sample percentage identified in Kosovo agrees with the distribution model of the infestation of apiaries by
M. scalaris proposed by Abou-Shaara and Darwish [
21], which predicts a lower current prevalence of this pest in the internal Balkans countries. On the other hand, the presence of
S. tricuspis in that geographical region was detected so attempts for its isolation in that region are worthwhile to understand if it is opportune to adopt measures to contrast its detrimental effects and further spread.
Methods suitable for phylogenetic analyses of M. scalaris and S. tricuspis that can be applied to a large number of isolates were used in this study. The phylogenetic analysis based on the COI gene sequence of M. scalaris showed that this generalist species is locally represented by different biotypes and that some of the biotypes are distributed worldwide. This indication could be exploited to investigate the reasons for the high adaptability of the most widespread biotypes by making available viable representatives and studying their behavior.
The detection of honeybee microbial pathogens in the parasitoid flies agrees with previous findings regarding
A. borealis and
M. scalaris [
3,
8] confirming the implication of these pests in facilitating the spread of bee diseases of microbial nature.
In conclusion, this study made available methods of rapid detection of parasitoid flies in honeybees and hive debris that can be exploited to increase knowledge of their prevalence and trends in geographic distribution. The application of these methods for early detection at the apiary level will allow measures such as positioning of soil types that enhance fly larvae mortality rate around the apiary and chromotropic traps on the roof of the hives [
22].
Author Contributions
Conceptualization, methodology, investigation, project administration, data curation, writing, review and editing F.R.; formal analysis, investigation, data curation, review and editing M.I., B.H., P.M., F.G., I.D.M., M.P. and L.M.; conceptualization, supervision, funding acquisition, review and editing L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, grant number IZS AM 420 RC.
Data Availability Statement
Data obtained in the experiments carried out in this study are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Fernández, P.; Alverez, S.C.; Moraga, Q.E. Primera cita de Megaselia scalaris (Loew, 1866), (Diptera: Phoridae) en Apis mellifera iberiensis. Revista Ibero-latinoamericana de parasitología 2010, 69, 72–76. [Google Scholar]
- Ricchiuti, L.; Miranda, M.; Venti, R.; Bosi, F.; Marino, L.; Mutinelli, F. Infestation of Apis mellifera colonies by Megaselia scalaris (Loew, 1866) in Abruzzo and Molise regions, central-southern Italy. J. Apic. Res. 2016, 55, 187–192. [Google Scholar] [CrossRef]
- Menail, A.H.; Piot, N.; Meeus, I.; Smagghe, G.; Loucif-Ayad, W. Large Pathogen Screening Reveals First Report of Megaselia Scalaris (Diptera: Phoridae) Parasitizing Apis Mellifera Intermissa (Hymenoptera: Apidae). J. Invertebr. Pathol. 2016, 137, 33–37. [Google Scholar] [CrossRef]
- Cham, D.T.; Fombong, A.T.; Ndegwa, P.N.; Irungu, L.W.; Nguku, E.; Raina, S.K. Megaselia scalaris (Diptera: Phoridae), an opportunist parasitoid of honey bees in Cameroon. Afr. Entomol. 2018, 26, https://hdl.handle.net/10520/EJC–df2838c22. [Google Scholar] [CrossRef]
- Debnath, P.; Roy, D. First Record of Megaselia Scalaris (Loew) as a Potential Facultative Parasitoid of Apis Mellifera in India. Asian J. Biol. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Pérez-Mata, J.Á.; Obregón-Zúñiga, J.A.; Vergara-Pineda, S. Nuevo registro de Megaselia scalaris Loew, 1866 (Diptera: Phoridae) en el estado de Querétaro asociados a colmenas de Apis mellifera Linnaeus, 1758 (Hymenoptera: Apidae). Entomología Mexicana, 2020, 7, 329–333. [Google Scholar]
- Haddad, N.; Adjlane, N.; Loucif-Ayad, W.; Shebl, M.A.; Saba, M.; Albaba, I.; El-Obeid, D.; Sabah, M.; Giusti, M.; Felicioli, A. Presence and infestation rate of Senotainia tricuspis (Meigen) (Diptera, Sarcophagidae) on honey bees in the Mediterranean Region. J. Apic. Res. 2015, 54, 121–122. [Google Scholar] [CrossRef]
- Core, A.; Runckel, C.; Ivers, J.; Quock, C.; Siapno, T.; Denault, S.; Brown, B.; Derisi, J.; Smith, C.D.; Hafernik, J. A New Threat to Honey Bees, the Parasitic Phorid Fly Apocephalus Borealis. PLoS ONE 2012, 7, e29639. [Google Scholar] [CrossRef]
- Brown, B.V. Two New Bee-Killing Flies from Brazil (Insecta: Diptera: Phoridae: Melaloncha). Biodivers. Data J. 2016, 4, e7715. [Google Scholar] [CrossRef]
- Dutto, M.; Ferrazzi, P. Megaselia Rufipes (Diptera: Phoridae): A New Cause of Facultative Parasitoidism in Apis Mellifera. J. Apic. Res. 2014, 53, 141–145. [Google Scholar] [CrossRef]
- Kiprijanovska, H.; Lazarevska, S.; Golubovski, M.; Uzunov, A. First Report on the Presence of Megaselia Rufipes (Meigen, 1804) and Megaselia Praeacuta (Schmitz, 1919) in Honey Bee Colonies in the Republic of Macedonia. J. Apic. Res. 2019, 58, 114–116. [Google Scholar] [CrossRef]
- Sabo, R.; Legáth, J.; Staroň, M.; Sabová, L. The First Record of Facultative Parasitism of Megaselia Spp. (Diptera: Phoridae) in a Honeybee Colony in Slovakia. Folia Vet. 2020, 64, 44–48. [Google Scholar] [CrossRef]
- Ghavami, M.B.; Djalilvand, A. First Record of Urogenital Myiasis Induced by Megaselia Scalaris (Diptera: Phoridae) from Iran. J. Arthropod Borne Dis. 2015, 9, 274–280. [Google Scholar]
- El-Hawagry, M.S.A.; Ebrahim, A.M.E.; Nada, M.S.E. First Detection of Megaselia Scalaris (Loew) (Diptera: Phoridae) as a Facultative Endoparasitoid of Nezara Viridula (L.) (Hemiptera: Pentatomidae). Egypt. J. Biol. Pest Contr. 2021, 31. [Google Scholar] [CrossRef]
- Costa, J.; Almeida, C.E.; Esperança, G.M.; Morales, N.; Dos S Mallet, J.R.; Gonçalves, T.C.M.; do Prado, A.P. First Record of Megaselia Scalaris (Loew) (Diptera: Phoridae) Infesting Laboratory Colonies of Triatoma Brasiliensis Neiva (Hemiptera: Reduviidae). Neotrop. Entomol. 2007, 36, 987–989. [Google Scholar] [CrossRef] [PubMed]
- Koch, N.M.; Fontanarrosa, P.; Padro, J.; Soto, I.M. First record of Megaselia scalaris (Loew) (Diptera: Phoridae) infesting laboratory stocks of mantids (Parastagmatoptera tessellate, Saussure). Arthropods 2013, 2, 1–6. [Google Scholar]
- Zhang, X.S.; Liu, G.C.; Zhang, D.X.; Shi, C.M. Novel trophic interaction: The scuttle fly Megaselia scalaris (Diptera: Phoridae) is a facultative parasitoid of the desert scorpion Mesobuthus eupeus mongolicus (Scorpiones: Buthidae). Journal of Natural History 2016, 51, 1–15. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Q.; Xiang, L.; Gu, R.;, Y.; Zhang, Y.; Bai, X.; Niu, X.; Li, T.; Wei, J.; et al. First Report on Megaselia scalaris Loew (Diptera: Phoridae) Infestation of the Invasive Pest Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) in China. Insects 2021, 12, 65. [CrossRef]
- Arafat, E.A.; El-Samad, L.M.; Hassan, M.A. Scuttle Fly Megaselia Scalaris (Loew) (Diptera: Phoridae) Endoparasitoid as a Novel Biocontrol Agent against Adult American Cockroaches (Periplaneta Americana). Sci. Rep. 2024, 14, 9762. [Google Scholar] [CrossRef]
- Furui, S.; Miyanoshita, A.; Imamura, T. Real-time PCR-based identification methods for Megaselia scalaris (Loew)(Diptera: Phoridae) targeting mitochondrial DNA. Food Sci. Technol. Res. 2022, 28, 119–122. [Google Scholar] [CrossRef]
- Abou-Shaara, H.F.; Darwish, A.A.E. Expected Prevalence of the Facultative Parasitoid Megaselia Scalaris of Honey Bees in Africa and the Mediterranean Region under Climate Change Conditions. Int. J. Trop. Insect Sci. 2021, 41, 3137–3145. [Google Scholar] [CrossRef]
- Bedini, G.; Boni, C.B.; Coppola, F.; Sagona, S.; Giusti, M.; Pinzauti, M.; Felicioli, A. Host-Parasitoid Relationship between Apis mellifera (Linnaeus, 1758) and Senotainia tricuspis (Meigen, 1838) (Diptera, Sarcophagidae): Fly Aggression Behavior and Infestation Rates of Senotainiosis. Insects 2023, 14, 415. [Google Scholar] [CrossRef] [PubMed]
- Polidori, C.; Piwczynski, M.; Ronchetti, F.; Johnston, N.P.; Szpila, K. Host-Trailing Satellite Flight Behaviour Is Associated with Greater Investment in Peripheral Visual Sensory System in Miltogrammine Flies. Sci. Rep. 2022, 12, 2773. [Google Scholar] [CrossRef]
- Rossi, F.; Del Matto, I.; Ricchiuti, L.; Marino, L. Selection and Multiplexing of Reverse Transcription–Quantitative PCR Tests Targeting Relevant Honeybee Viral Pathogens. Microorganisms 2024, 12, 1105. [Google Scholar] [CrossRef] [PubMed]
- Piwczyński, M.; Pape, T.; Deja-Sikora, E.; Sikora, M.; Akbarzadeh, K.; Szpila, K. Molecular Phylogeny of Miltogramminae (Diptera: Sarcophagidae): Implications for Classification, Systematics and Evolution of Larval Feeding Strategies. Mol. Phylogenet. Evol. 2017, 116, 49–60. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; Vandesompele, J.; Wittwer, C.T. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Rossi, F.; Amadoro, C.; Ruberto, A.; Ricchiuti, L. Evaluation of Quantitative PCR (qPCR) Paenibacillus larvae Targeted Assays and Definition of Optimal Conditions for Its Detection/Quantification in Honey and Hive Debris. Insects 2018, 9, 165. [Google Scholar] [CrossRef]
- A Roetschi, H Berthoud, R Kuhn, A Imdorf. Infection rate based on quantitative real-time PCR of Melissococcus plutonius, the causal agent of European foulbrood, in honeybee colonies before and after apiary sanitation. Apidologie 2008, 39, 362–371. [Google Scholar] [CrossRef]
- Babin, A.; Schurr, F.; Rivière, M.-P.; Chauzat, M.-P.; Dubois, E. Specific Detection and Quantification of Three Microsporidia Infecting Bees, Nosema Apis, Nosema Ceranae, and Nosema Bombi, Using Probe-Based Real-Time PCR. Eur. J. Protistol. 2022, 86, 125935. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).