1. Introduction
The benefits of dietary fiber have been well-recognized in the health and nutrition community. Naturally occurring dietary fibers have the advantage of including additional nutrients and bioactive compounds that support health and well-being [
1,
2]. Ranging from gut health to other chronic conditions, dietary fiber, along with complexed beneficial nutrients and bioactives, supports optimal physiological function [
3]. A recent review identified 17 sources of dietary fiber which collectively contained 64 bioactive compounds at detectable levels [
1]. The bioactive compounds included phenolic acids, flavonoids and non-flavonoid compounds such as tannins, tocopherols, tocotrienols, ascorbic acid, retinol equivalents, carotenoids, chlorophylls and betalains. This demonstrates the compositional complexity of naturally occurring dietary fibers, and the opportunity for these fibers to exert health benefits through multiple mechanisms.
One fiber included in the aforementioned review was hemp hull fiber. Hemp hull fiber is derived from the outer seed coat of hemp,
Cannabis sativa L., Cannabaceae. The seed hull is often viewed as a low-value side stream of hemp seed processing. However, this is a bioactive-rich fraction with high fiber content. Hemp hull has been identified through artificial intelligence as a key source of bioactives, N-trans caffeoyltyramine (NCT) and N-trans feruloyltyramine (NFT), that is also rich in dietary fiber [
4]. Proprietary hemp hull fiber (Bio Gut Fiber™) has been shown to act a prebiotic to support gut health, which is augmented with the bioactive benefits of NCT and NFT to reduce gut permeability [
5,
6]. Both NCT and NFT are acknowledged as key bioactives that also support liver function and other physiological processes, in addition to gut health through their influence on hepatic nuclear factor 4-alpha (HNF4a), a key regulator for liver, gut & pancreas [
7,
8].
Different sources of fiber exert benefits and challenges based on their physico-chemical properties. Solubility has long been used as the classification for fiber benefit, with soluble fibers gaining more attention over insoluble fibers. Attributes of fiber such as viscosity and fermentability, however, are more indicative of the health benefit. Solubility is an analytical definition that does not necessarily predict the physiological benefit of the dietary fiber [
1].
Fermentable fibers such as inulin, oligofructose, fructooligosacchrides, and galactooligosaccharides, support gut health through a prebiotic mechanism and generation of key metabolites such as short-chain fatty acids [
9]. While these fibers have gained significant attention for their prebiotic benefit for gut health, they are often accompanied by digestive side effects that can impact quality of life [
10,
11]. Digestive tolerance is indicated by the perceived gastrointestinal symptoms such as abdominal pain/cramping, bloating, burping, flatulence, nausea, rumbling noises (borborygmi), and acid reflux (heartburn) [
10]. Many of these perceived symptoms are tied directly to gas production in the gut, which is a by-product of fiber fermentation. While these symptoms typically do not pose long-term health concerns, they result in discomfort, reduced quality of life, and are socially unacceptable, and consumers often discontinue consuming the fiber.
Many fermentable fibers have low tolerance thresholds, which can negatively impact user experience. In a comprehensive review of gastrointestinal tolerance of dietary fibers, the lowest tolerance thresholds, based on human clinical studies, were 3.5 g/day alginate, 5 g/day inulin and 7.5 g/day fructooligosaccharide [
10]. This aligns with the low FODMAP (fermentable oligosaccharides, disaccharides, monosaccharides and polyols) dietary approach that suggests limitation of certain fermentable dietary fibers to manage digestive symptoms [
12]. Hemp hull fiber is low in FODMAPs, as it contains 50% cellulose, with the remaining fibers made up of hemicellulose and lignin [
4,
13]. While the recommended intakes for dietary fiber in the US (adequate intake) are 28 g/d for women and 35 g/d for men, these tolerance levels demonstrate that a mixture of dietary fibers is necessary to meet target intake levels [
14]. This is best addressed by including multiple sources of fiber that span the spectrum of fermentability.
The lesser-known benefits of naturally-occurring fibers need to be highlighted to increase consumer awareness and incorporation into the diet as a fiber source. Many of these fibers have higher digestive tolerance due to the metered bacterial fermentation rate in the gut [
10]. With a slower bacterial fermentation rate in the colon, the rate of gas production is also slower. This enables the digestive system to equilibrate the gas more readily, thus reducing the perceived symptoms. Insoluble fibers can provide benefits to gut health via prolonged fermentation in the gut, allowing beneficial metabolites such as short-chain fatty acids to be produced in the distal colon and thereby providing the prebiotic benefit, without the GI discomfort associated with rapidly fermenting soluble fiber [
15].
In the literature, much of the evaluation of dietary fibers and digestive tolerance is limited to single fiber interventions. The study reported here compared perceived quality of life, digestive symptoms, and anxiety while consuming one of two sources of dietary fiber, (inulin or hemp hull fiber) or a placebo (maltodextrin) over the course of 6 weeks. This completely virtual, randomized, placebo-controlled, parallel arm study is the first study to evaluate the perceived digestive comfort when consuming Brightseed’s Bio Gut Fiber product-- a proprietary hemp hull fiber and source of insoluble dietary fiber with AI discovered bioactive components [
4]. The inclusion of the comparison to inulin, a soluble fiber commonly consumed as a dietary supplement, was aimed at supporting the comparative effectiveness of commercially available products.
2. Materials and Methods
Study Subjects
This was a completely virtual, randomized, double-blinded, placebo-controlled study in adults aged 21 years or older living in the United States, administered by Radicle Science, Del Mar, California, USA. The protocol was reviewed and approved by Sterling Institutional Review Board (IRB protocol number 11062). Radicle GI HEALTHTM was registered on ClinicalTrials.gov [Identifier: NCT06009614]. It should be noted that the protocol registered is not this specific study, but rather is the templated study protocol utilized for all Radicle GI Health studies.
Since the study was conducted completely remotely, the study subjects were recruited online using several means of engagement: internet-based networks, retailers, and advocacy group distribution lists and email. This general study design and method of participant recruitment was shown to be successful in previous studies [
16,
17].
Subjects were recruited based on their intention to seek relief from GI related discomfort. The inclusion-exclusion criteria listed in
Table 1 were used for subject enrollment. Eligible subjects provided informed consent and were enrolled in the study.
Subjects were randomized to a study arm using a block randomization scheme that was stratified by sex assigned at birth and baseline Digestion-associated Quality of Life Questionnaire scores (Table 2).
Study Protocol
The study had an intervention duration of 6 weeks (42 days). Subject were randomly assigned to receive one of three study products:
Test product: Brightseed® Bio Gut Fiber (BGF), (Brightseed Inc, South San Francisco, CA, USA), a proprietary hemp hull fiber with bioactives at a dose providing 2.8 g fiber/day.
Comparator product: Inulin (Beneo, Mannheim, Germany), dose providing 2.8 g fiber/day. Maltodextrin was added to 4 g.
Placebo: Maltodextrin (Grain Processing Corporation, Washington, IN, USA), 4 g dose to approximately match total test and comparator product administered
All products were matched for weight and color. Fiber dose was chosen to provide 10% Daily Value or a “Good Source of Fiber” per United States fiber intake recommendations [
14]. Subjects were shipped enough study products for the 6-week intervention. The subjects were asked to confirm receipt of the products and confirm the identity of the product code, to ensure they received the correct study products. Subjects were instructed to consume one sachet of product each day, mixed into a commercial pudding mix, for the 6-week duration of the study. Subjects did not receive any financial compensation for participating in the study, but rather were provided with a personalized health report at the conclusion of the study based on the responses they provided.
Each week, subjects completed validated surveys through a secure, online platform. Automated reminders were sent to the subjects via text message and/or email.
Table 2 lists the validated surveys used in this study. The primary outcome was to detect significant differences in the perceived Digestion-associated Quality of Life Questionnaire (DQLQ). Secondary outcomes assessed perceived belly pain, gas and bloating, and reported side effects using Patient-Reported Outcomes Measurement Information System (PROMIS™) instruments.
A sample size calculation was conducted to ensure sufficient power to detect a significant difference in the effect on the primary outcome between each active product group and the control group. A Monte Carlo power analysis was conducted to determine the sample size required to detect a small effect size equal to a generalized Cohen’s D = 0.2 (which translates to a Cohen’s D of 0.302) at a two-sided p-value of 0.05 between the slopes of the control group and the active product groups, and the planned pairwise comparisons of the active product groups; Bonferroni correction was applied. For a power of 80% and accounting for up to 40% attrition, the required sample size is 250 participants per study arm.
3. Results
3.1. Subject Characteristics and Retention
A total of 948 subjects were randomized to a treatment arm or placebo and shipped the study products. In all treatment arms, the majority was female, white, and either overweight or obese, with a mean age of approximately 43 years (
Table 3).
The modified intent to treat (mITT) population was defined as the subjects (n=579) that completed at least one post baseline questionnaire at any time during the six-week intervention period. Drop-out rates were similar among the treatment arms. Twelve percent of the originally randomized subjects completed all six post-baseline questionnaires (n=114), which is the basis for the per-protocol analysis. As a fully remote/virtual study, this attrition rate was anticipated [
16]. The study was powered sufficiently such that this loss of subjects to follow-up did not impact statistical significance. Discontinuations tended to be from young males, which slightly increased the proportion and ages of females in the mITT and PP populations.
Table 4.
Subject demographics- mITT and PP Populations.
Table 4.
Subject demographics- mITT and PP Populations.
| Statistic |
mITT |
PP |
| Bio Gut Fiber |
Inulin |
Placebo |
Bio Gut Fiber |
Inulin |
Placebo |
| N |
198 |
188 |
193 |
38 |
30 |
46 |
Females, n
(%)
|
145
(73%) |
126
(67%) |
137
(71%) |
30
(79%) |
18
(60%) |
33
(72%) |
White, n
(%)
|
163
(82%) |
145 (77%) |
165
(85%) |
32
(84%) |
26
(87%) |
36
(78%) |
Mean Age (years)
(min, max)
|
44.4
(19, 77) |
43.7
(21, 72) |
44.2
(21, 78) |
46.7
(24, 71) |
46.3
(21, 72) |
46.7
(26, 72) |
BMI Category
Underweight
Normal Weight
Overweight
Obese |
4 (2%)
52 (26%)
63 (32%)
79 (40%) |
2 (1%)
52 (28%)
56 (30%)
78 (41%) |
3 (2%)
52 (28%)
57 (30%)
79 (41%) |
1 (3%)
9 (24%)
11 (29%)
17 (45%) |
2 (7%)
8 (27%)
7 (23%)
13 (43%) |
0
17 (37%)
14 (30%)
15 (33%) |
3.2. Statistical Analysis
In an effort to utilize all available data due to the high dropout rate, the modified intent-to-treat (mITT) population was used as the primary analysis population for assessing outcomes over time. A mixed model repeated measures analysis of covariance (MMRM) method was used to analyze survey total score changes over 6 weeks.
The MMRM model for DQLQ Score, Belly Pain Score, and Gas and Bloating Score, respectively, included effects for study arm, week, interaction between arm and week, and included baseline scores for the specific outcome as a covariate. Subjects were random blocking factors and a variance component option was used for the covariance structure of the R matrix. This same model was run for mITT and PP populations, for each outcome. All data are presented as least squares mean (LSM) to best address the level of missingness in the data set. All treatment means are reported with LSM and standard error by week.
For both populations, mITT and PP, the models converged indicating statistically significant differences among study arms and among weeks, but not for the interaction between arms and weeks. In both analyses, the baseline total score was a significant covariate) for all outcomes and was included in the analysis models.
3.3. Survey Results
All study arms, including placebo, resulted in notably decreased survey scores from baseline to week 1, irrespective of the outcome assessed. The scores reported during week 2 through week 6 never returned to the baseline level for any of the treatment arms (Figures 1-4), indicating an improvement in perceived digestive symptoms.
There were no study arm differences in LSM baseline for total DQLQ score, belly pain, or gas and bloating scores. The significant differences between treatment groups by week varied depending on the outcome assessed. These are described outcome by outcome in the next subsections.
3.3.1. Digestion Associated Quality of Life Questionnaire (DQLQ) Scores
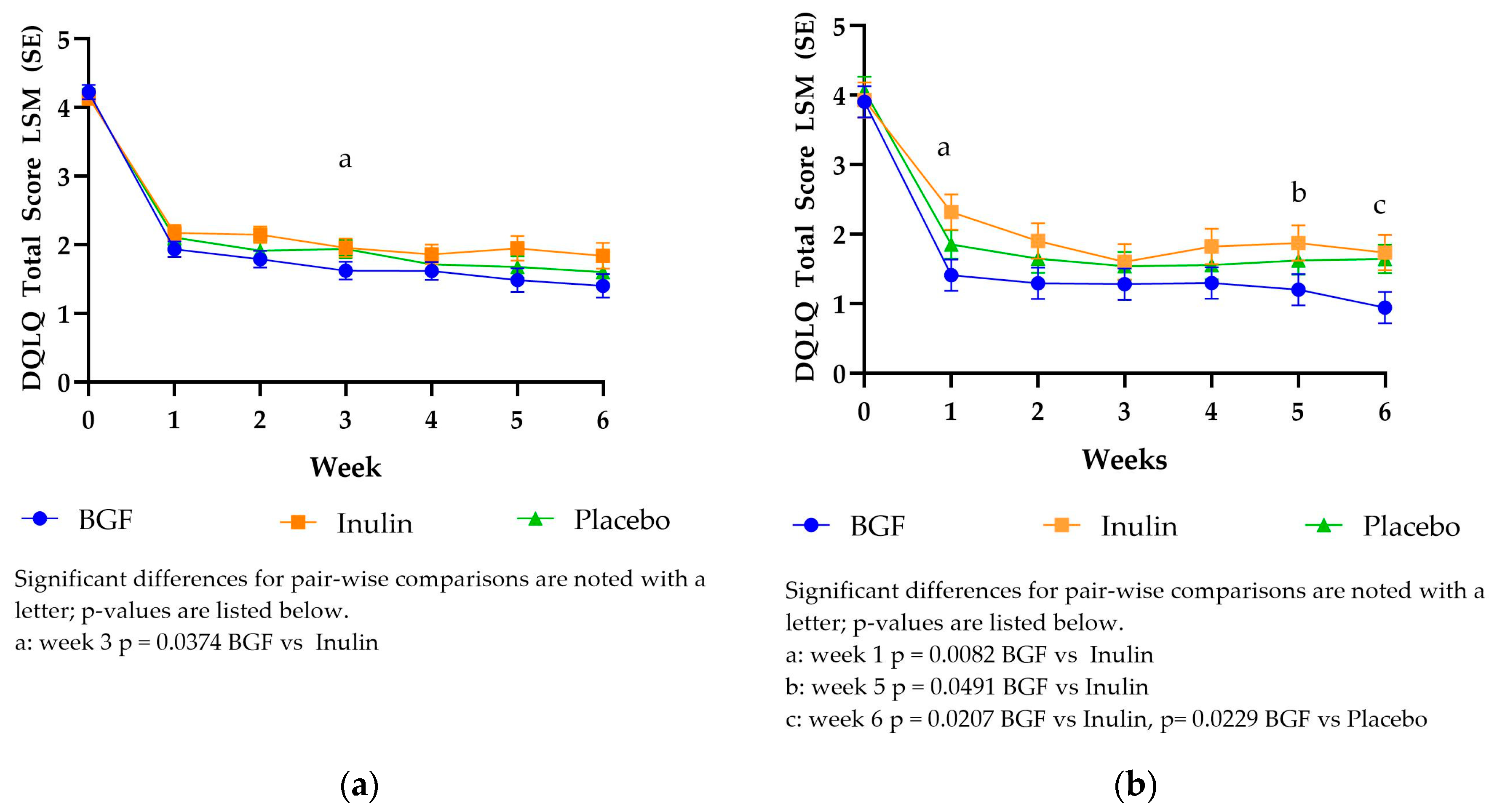
The DQLQ mITT LSM scores dropped from 4.14-4.22 to 1.93-2.17 between baseline and week 1 (
Figure 1) for both treatments and placebo. The DQLQ mITT scores were significantly different only at week 3, between BGF and inulin. The DQLQ PP scores exhibited a similar trend to the mITT scores, dropping from 3.91- 4.07 to 1.41-2.32 between baseline and week 1. Bio Gut Fiber consumption yielded significantly lower DQLQ PP scores compared to inulin at week 1, week 5, and week 6. Bio Gut Fiber yielded significantly lower DQLQ PP scores compared to placebo at week 6.
3.3.2. PROMIS Belly Pain Scores
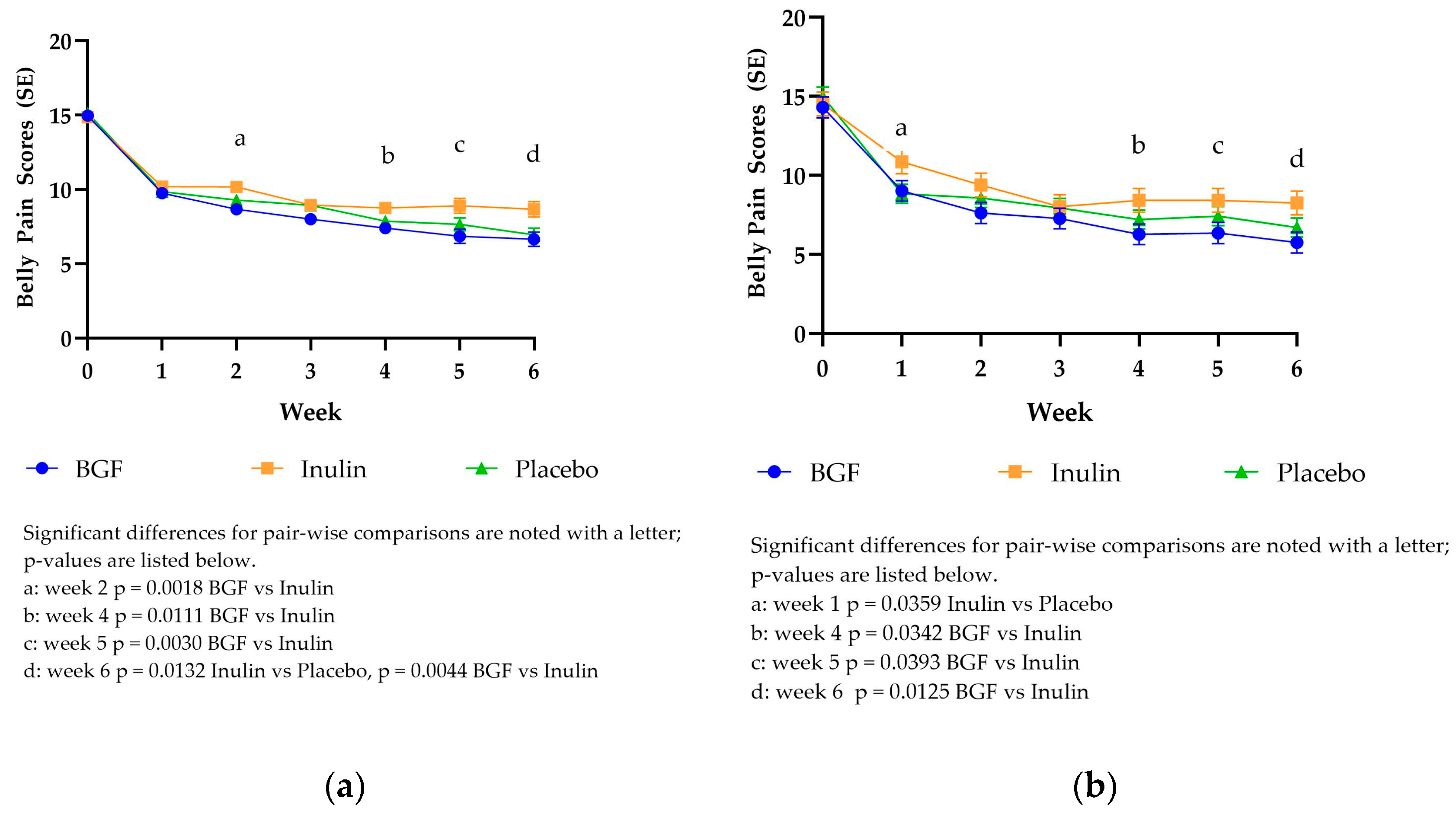
Over time, participants experienced, on average, a greater than 8-point decrease from baseline in total Belly Pain scores across all groups in both mITT and PP analyses (
Figure 2). This represents nearly a 50% reduction in total belly pain scores for all treatment arms. Similar to the trend seen with the DQLQ scores, Belly Pain mITT LSM scores dropped from 14.88-15.13 to 9.76-10.19 from baseline to week 1. Bio Gut Fiber consumption yielded significantly lower belly pain scores compared to inulin at week 2, week 4, week 5, and week 6. Placebo consumption yielded significantly lower belly pain scores compared to inulin at week 6. The Belly Pain PP LSM scores also dropped from 14.31-14.99 at baseline to 8.84-10.86 at week 1. The PP analysis exhibited a trend similar to the mITT analysis. Bio Gut Fiber consumption yielded significantly lower belly pain scores compared to inulin at weeks 4, 5, and 6. At week 1, placebo consumption resulted in significantly lower belly pain scores than inulin, while BGF consumption was trending toward being significantly lower (p=0.0662)
3.3.3. PROMIS Gas & Bloating Scores
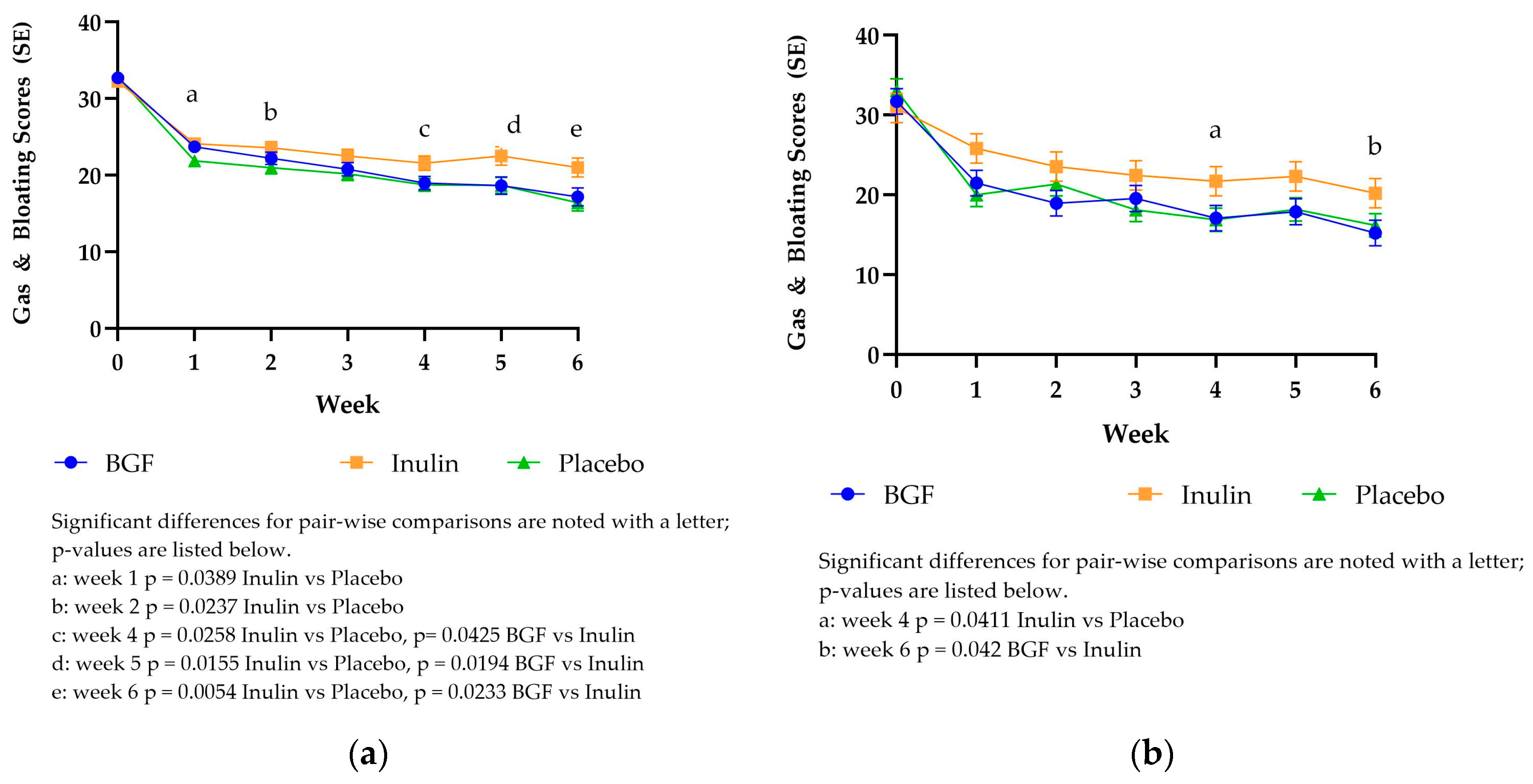
As seen with the DQLQ score and belly pain score, the gas and bloating score for all study arms exhibited the greatest decrease from baseline (32.26-32.80) to week 1 (21.87-24.13) in the mITT analysis as well as the PP analysis (30.87-33.09 baseline to 20.00-25.81 week 1,
Figure 3). Over time, participants experienced on average > 11-point decrease from baseline in total Gas and Bloating scores across all groups. This represents nearly a 35% reduction in total Gas and Bloating scores. In the mITT analysis, placebo intake resulted in significantly lower gas & bloating scores compared with inulin at weeks 1, 2, 4, 5 and 6. Bio Gut Fiber consumption also yielded significantly lower gas and bloating scores compared to inulin at weeks 4, 5, and 6. The PP scores exhibited a similar trend, but the pairwise differences were only significant at week 4 (placebo vs inulin) and week 6 (BGF vs inulin). At both time points, inulin resulted in significantly greater scores than the other treatments.
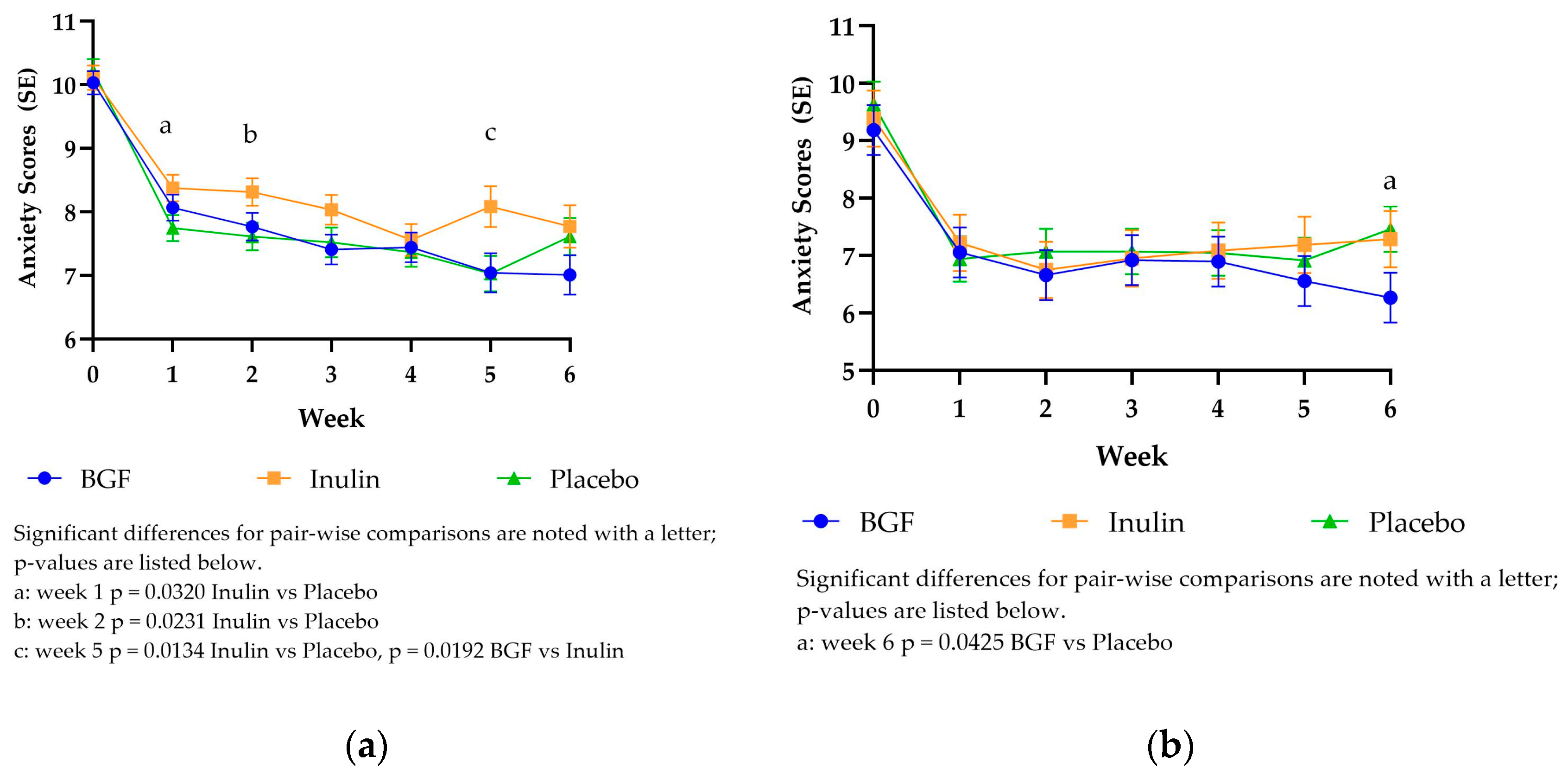
3.3.4. PROMIS Anxiety Scores
The PROMIS anxiety scores followed the same downward trend as the digestive comfort scores previously described. The baseline LSM scores were highest for all treatments in mITT (10.04-10.22) and PP (9.19-9.63) analysis, dropping at week 1 to 7.75-8.38 for mITT and 6.94-7.22 for PP (
Figure 4). Anxiety scores were significantly lower for placebo compared to inulin at weeks 1, 2, and 5 for the mITT analysis. Bio Gut Fiber consumption also resulted in significantly lower anxiety scores at week 5 compared to inulin. The only significantly different time point in the PP analysis was at week 6, with BGF consumption resulting in lower anxiety scores compared to placebo.
3.3.5. Side Effects
The incidence of reported side effects were similar for BGF (22.7%), Inulin (28.7%) and Placebo (29.0%) (Supplementary
Table 1). Most of the side effects were classified as gastrointestinal concerns (stomach cramps, diarrhea, and gas/bloating) followed by nervous system symptoms (headache and insomnia). Other side effects included changes in feeling of energy, caffeine sensitivity, urinary concerns, and respiratory concerns.
4. Discussion
Dietary fiber has been identified as a shortfall nutrient in the American diet for decades [
21]. On average, Americans consume only half of the recommended intake of dietary fiber. Fiber supplements and fiber fortified foods are approaches to close this gap. Its critical to acknowledge that not all fibers provide the same physiological benefits, and likewise, not all fibers have the same digestive tolerance. This study demonstrates that hemp hull fiber, when consumed at a “Good Source” of fiber level (2.8 grams per day), results in lower digestive distress than inulin, when consumed at the same level. Also, surprisingly, consumers perceive a difference in GI distress with a dose as low as 2.8 grams per day. Because our study included such a large group of participants, the study was uniquely able to tease out such a small effect.
The results of this randomized, placebo-controlled trial revealed that Bio Gut Fiber intake had a greater improvement in digestive symptom scores than inulin among a large sample of adults with digestive discomfort.
One differentiator between the two types of fiber administered is the inclusion of bioactive compounds. Hemp hull fiber intrinsically includes bioactive compounds that can support health and well-being, beyond the digestive tract. A recent review describes accompanying total phenolic compounds, total flavonoid content and antioxidant activity of sources of insoluble fiber, such as hemp hull [
1]. In addition to providing dietary fiber with improved digestive tolerance, insoluble fiber sources such as BGF, can also increase bioactive compound intake to support health. Highly fermentable fibers, such as inulin, have been associated with digestive wellness benefits (e.g., prebiotic effect) and also digestive discomfort [
22]. Inulin is among the sources of soluble fiber that increase abdominal pain, bloating, and flatulence, which can result in undue discomfort [
23]. The multi-faceted benefit of slowly fermented fibers reinforces their value in a health-promoting diet
This finding aligns with previous research suggesting that inulin has a relatively low digestive tolerance of 5 grams per day to avoid flatulence [
10]. In previous studies, both flatulence and bloating were reported at moderate intake of inulin from 2.0 to 7.5 grams per day. The findings in the present study, where the gas and bloating scores after consuming inulin were significantly higher than placebo and BGF at multiple time points, are supported by the current body of knowledge. This is likely due to inulin’s rapid fermentation rate which yields gas along with beneficial metabolites such as short-chain fatty acids [
18,
19]. While the short-chain fatty acids support digestive health, excessive and rapid gas production by the gut microbiota can yield discomfort. This may ultimately lead to an individual avoiding certain fibers to prevent digestive discomfort [
20].
Placebo intake also resulted in lower digestive symptom scores compared to baseline for all outcomes. It is unlikely that there was a physiological change in gut function when the placebo, a fully digestible, corn-based maltodextrin, was consumed. This indicates that participating in the study in and of itself resulted in a change in perceived digestive symptoms (a placebo effect). However, there were notably higher Gas and Bloating scores when inulin was consumed compared to placebo in the ITT analysis during 5 of the 6 weeks. Week 3 also trended towards a significant difference (p = 0.0576) between placebo and inulin. Interestingly, the increased Gas and Bloating scores after consuming inulin correspond with increased Anxiety scores for inulin, compared to placebo, at weeks 1, 2, and 5 in the mITT analysis. Itis conceivable that the digestive symptoms influenced the perceived anxiety for the participants.
The placebo effect noted in this study has been documented in other publications. Particularly when perceived gastrointestinal symptoms are measured, there can be a substantial placebo effect. Research on functional dyspepsia has reported placebo response rates ranging from 6% to 72% [
24]. When irritable bowel syndrome was studied, placebo response rates ranged from 3% to 84% across trials [
25]. While both of these situations involved the study of patients with a declared diseases and the present study examined healthy individuals that were experiencing self-reported gastrointestinal discomfort, it demonstrates that placebo effects in digestive symptom studies have been previously reported.
A unique attribute of this study is the decentralized nature of approach. Typically, clinical trials are site-based intervention studies that require an individual to attend study visits in-person. Decentralized study designs that employ digital tools to collect data in a remote environment became more common as a result of the COVID-19 pandemic [
26]. This provides an opportunity to recruit subjects that may otherwise be underrepresented in clinical research. However, compliance and subject follow-up remain challenges in decentralized trials due to the remote nature of the engagement. This was evident in the current study.
Participant compliance and retention can be challenging in a decentralized, remote study, such as this one. Based on the reported daily intake and weekly frequency, subject compliance for those that continued to stay enrolled was acceptable and met expectations for data analysis. In a recent remote study on sleep, 27% of subjects enrolled in the study dropped out prior to the first data collection [
16]. This study experienced a slightly higher drop-out rate, with 39% of enrolled subjects being lost to follow up and completing zero study visits. This may be a result of the lack of compensation for participating in the study or subject dislike of the study products.
A mITT analysis approach was taken to utilize the data collected from subjects who completed at least one study visit. This method is fairly robust in a study with multiple missing data points. However, approximately 48% of the expected outcomes were missing over the 6-week period. Therefore, to assess if estimated study arm differences are robust against this level of missingness, data were also analyzed using mixed model repeated measure (MMRM) method on the subset of PP population where there are no missing outcomes at any week.
Due to the attrition of participants over time, the final mITT dataset contained approximately 55% of the total expected data if all participants had completed all 6 weeks of questionnaires. However, this is far better than 12% of total expected data when a per-protocol definition is utilized, which is why a MMRM methodology for analyzing the data is preferred as it preserves all collected data. Even with the robust handling of missing data, problems in model convergence and adequate estimation of least squares means and intervention effects can arise. Therefore, it is prudent to compare the interpretation of outcomes using both the MMRM approach on the mITT population to the PP population which represents the purest subset of participants who completed all study requirements.
5. Conclusions
This study demonstrated that BGF, a source of naturally-occurring fiber and bioactives, is well tolerated by healthy adults when consumed in a practical dose. Digestive comfort after consuming BGF improved compared to baseline and was significantly better than inulin at multiple points during the intervention. This study supported the notion that inulin intake can result in noticeable digestive symptoms, which may deter individuals from consuming this type of fiber. Including BGF as a part of a balanced diet can enable an individual to meet dietary fiber intake goals while experiencing minimal digestive side-effects.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org. Table S1: Incidence of Side Effects Reported by Study Arm
Author Contributions
Conceptualization, methodology J-W.vK., S.K., E.K.P. and C.D., —study conduct oversight E.K.P.; — original data analysis, writing, draft preparation, J-W.vK.. and S.K.; —review and editing, C.D. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Sterling Institutional Review Board protocol number 11062, approved August 8, 2023.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets presented in this article are not readily available for proprietary reasons. Requests to access the datasets should be directed to the corresponding author.
Acknowledgments
The authors would like to acknowledge the contributions of Sophie Pinton (Scientist I, Process R&D Brightseed Inc.), Rob Grysen (Head of Food & Safety, Brightseed Inc.), Farai Machina (R&D, Commercial Product, Brightseed Inc.) for the preparation of the study materials, Susan Spruill (Applied Statistics and Consulting) for consultation on the statistical design and analysis, and Maria Stewart (Growing Brilliance LLC) for manuscript preparation.
Conflicts of Interest
B. J-W van Klinken and S Kalgaonkar are employees of Brightseed Inc.
References
- Timm, M.; Offringa, L.C.; Van Klinken, B.J.-W.; Slavin, J. Beyond Insoluble Dietary Fiber: Bioactive Compounds in Plant Foods. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Lattimer, J.M.; Haub, M.D. Effects of Dietary Fiber and Its Components on Metabolic Health. Nutrients 2010, 2, 1266–1289. [Google Scholar] [CrossRef]
- Dahl, W.J.; Stewart, M.L. Position of the Academy of Nutrition and Dietetics: Health Implications of Dietary Fiber. J Acad Nutr Diet 2015, 115, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- van Klinken, B.J.-W.; Stewart, M.L.; Kalgaonkar, S.; Chae, L. Health-Promoting Opportunities of Hemp Hull: The Potential of Bioactive Compounds. J Diet Suppl 2024, 1–15. [Google Scholar] [CrossRef]
- Bolster, D.; Chae, L.; van Klinken, J.-W.; Kalgaonkar, S. Impact of Selected Novel Plant Bioactives on Improvement of Impaired Gut Barrier Function Using Human Primary Cell Intestinal Epithelium. Journal of Food Bioactives 2022, 20, 11–16. [Google Scholar] [CrossRef]
- Flores Martinez, K.E.; Bloszies, C.S.; Bolino, M.J.; Henrick, B.M.; Frese, S.A. Hemp Hull Fiber and Two Constituent Compounds, N-Trans-Caffeoyltyramine and N-Trans-Feruloyltyramine, Shape the Human Gut Microbiome in Vitro. Food Chemistry: X 2024, 23, 101611. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Veeriah, V.; Levine, F. Liver Fat Storage Is Controlled by HNF4α through Induction of Lipophagy and Is Reversed by a Potent HNF4α Agonist. Cell Death Dis 2021, 12, 603. [Google Scholar] [CrossRef] [PubMed]
- Veeriah, V.; Lee, S.-H.; Levine, F. Long-Term Oral Administration of an HNF4α Agonist Prevents Weight Gain and Hepatic Steatosis by Promoting Increased Mitochondrial Mass and Function. Cell Death Dis 2022, 13, 89. [Google Scholar] [CrossRef]
- Carlson, J.L.; Erickson, J.M.; Lloyd, B.B.; Slavin, J.L. Health Effects and Sources of Prebiotic Dietary Fiber. Curr Dev Nutr 2018, 2, nzy005. [Google Scholar] [CrossRef]
- Mysonhimer, A.R.; Holscher, H.D. Gastrointestinal Effects and Tolerance of Nondigestible Carbohydrate Consumption. Adv Nutr 2022, 13, 2237–2276. [Google Scholar] [CrossRef]
- Grabitske, H.A.; Slavin, J.L. Low-Digestible Carbohydrates in Practice. J Am Diet Assoc 2008, 108, 1677–1681. [Google Scholar] [CrossRef] [PubMed]
- Gibson, P.R.; Shepherd, S.J. Evidence-Based Dietary Management of Functional Gastrointestinal Symptoms: The FODMAP Approach. J Gastroenterol Hepatol 2010, 25, 252–258. [Google Scholar] [CrossRef]
- Atzler, J.J.; Sahin, A.W.; Gallagher, E.; Zannini, E.; Arendt, E.K. Characteristics and Properties of Fibres Suitable for a Low FODMAP Diet- an Overview. Trends in Food Science & Technology 2021, 112, 823–836. [Google Scholar] [CrossRef]
- Dietary Reference Intakes: The Essential Guide to Nutrient Requirements |The National Academies Press. Available online: https://nap.nationalacademies.org/download/11537 (accessed on 15 May 2024).
- Wong, J.M.W.; de Souza, R.; Kendall, C.W.C.; Emam, A.; Jenkins, D.J.A. Colonic Health: Fermentation and Short Chain Fatty Acids. J Clin Gastroenterol 2006, 40, 235–243. [Google Scholar] [CrossRef]
- Saleska, J.L.; Bryant, C.; Kolobaric, A.; D’Adamo, C.R.; Colwell, C.S.; Loewy, D.; Chen, J.; Pauli, E.K. The Safety and Comparative Effectiveness of Non-Psychoactive Cannabinoid Formulations for the Improvement of Sleep: A Double-Blinded, Randomized Controlled Trial. J Am Nutr Assoc 2024, 43, 1–11. [Google Scholar] [CrossRef]
- Kolobaric, A.; Hewlings, S.J.; Bryant, C.; Colwell, C.S.; R. D’Adamo, C.; Rosner, B.; Chen, J.; Pauli, E.K. A Randomized, Double-Blind, Placebo-Controlled Decentralized Trial to Assess Sleep, Health Outcomes, and Overall Well-Being in Healthy Adults Reporting Disturbed Sleep, Taking a Melatonin-Free Supplement. Nutrients 2023, 15, 3788. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.L.; Timm, D.A.; Slavin, J.L. Fructooligosaccharides Exhibit More Rapid Fermentation than Long-Chain Inulin in an in Vitro Fermentation System. Nutr Res 2008, 28, 329–334. [Google Scholar] [CrossRef]
- Grabitske, H.A.; Slavin, J.L. Gastrointestinal Effects of Low-Digestible Carbohydrates. Crit Rev Food Sci Nutr 2009, 49, 327–360. [Google Scholar] [CrossRef]
- Mutuyemungu, E.; Singh, M.; Liu, S.; Rose, D.J. Intestinal Gas Production by the Gut Microbiota: A Review. Journal of Functional Foods 2023, 100, 105367. [Google Scholar] [CrossRef]
- Dietary Guidelines Advisory Committee Scientific Report of the 2020 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Agriculture and the Secretary of Health and Human Services; U.S. Department of Agriculture, Agricultural Research Service, 2020; p. 835.
- Eswaran, S.; Muir, J.; Chey, W.D. Fiber and Functional Gastrointestinal Disorders. Am J Gastroenterol 2013, 108, 718–727. [Google Scholar] [CrossRef]
- Barrett, J.S. Extending Our Knowledge of Fermentable, Short-Chain Carbohydrates for Managing Gastrointestinal Symptoms. Nutr Clin Pract 2013, 28, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Musial, F.; Klosterhalfen, S.; Enck, P. Placebo Responses in Patients with Gastrointestinal Disorders. World J Gastroenterol 2007, 13, 3425–3429. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-L.; Chang, F.-Y. Placebo Effect in Patients with Irritable Bowel Syndrome. J Gastroenterol Hepatol 2011, 26 Suppl 3, 116–118. [Google Scholar] [CrossRef]
- Dahne, J.; Hawk, L.W.J. Health Equity and Decentralized Trials. JAMA 2023, 329, 2013–2014. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).