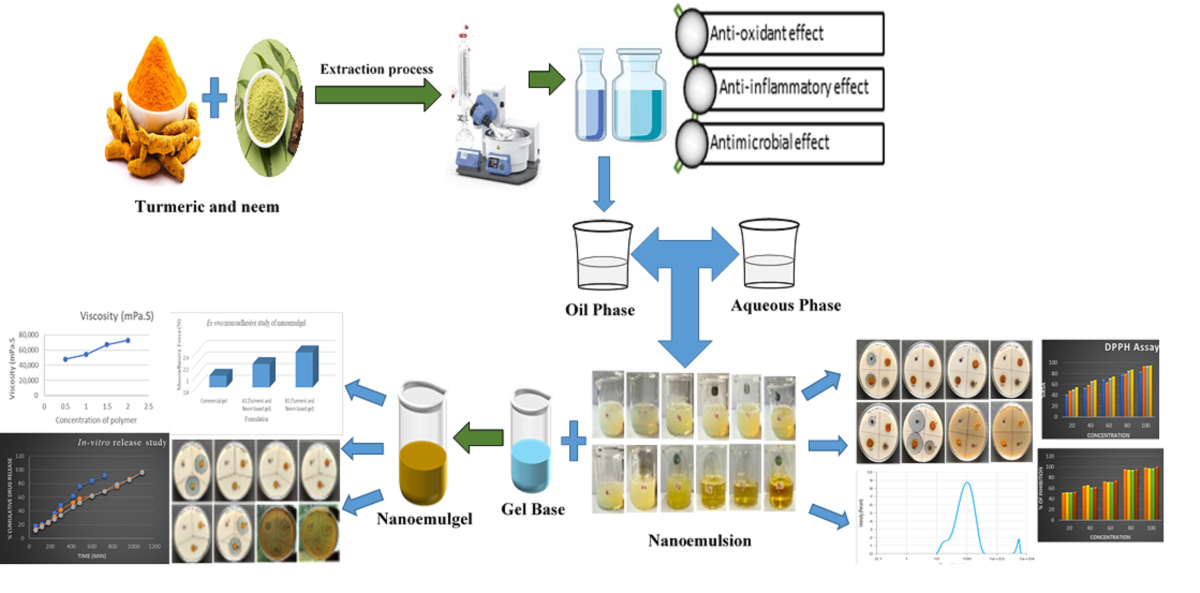
1. Introduction
The skin, the largest organ in the body, provides vital support to the defense mechanism of the body [
1]. Skin layers have the most significant ability to shield the body from microbial communities, ultraviolet (UV) radiation, and direct sunlight [
2]. Since it serves as the body’s initial line of defense against the infiltration of harmful substances like medications and other chemical compounds, the researchers must focus on addressing this issue [
3]. Skin diseases are becoming increasingly more dangerous since millions of people are reported to be suffering from a number of them [
4]. Skin infections can be caused by several pathogenic organisms, but mostly by bacteria and fungi [
5]. Skin infections and illnesses may expose people to danger if they remain untreated. Fortunately, there are several advantages to using this route of drug delivery over other conventional methods, including reduced systemic drug interactions, reduced invasiveness, simplicity in administration, avoidance of first-pass effects, extended duration of medication release, and improved patient compliance [
6].
In recent years, the healthcare research community has focused more on topical and transdermal application strategies because of their potential to treat systemic or skin disorders, their ability to carry a specific drug when in contact with skin, their increase bioavailability, their low side effects, and their regulated drug delivery profiles [
7,
8]. Transdermal drug delivery aims to enhance percutaneous absorption by crossing the stratum corneum [
9]. Molecules can be encapsulated in nanocarriers, including nanoemulsions, which may be rendered soluble as a formulation strategy. A nanoemulsion is made up of three components: water, oil and an emulsifying agent (surfactant). It is a kind of colloidal carrier system as well as a lipid-based delivery nanosystem [
10,
11]. It has been claimed that the use of nanoemulsion can result in improved drug penetration through the skin. These biphasic nanosystems provide enhanced skin penetration via drug-loaded nanoscale droplets- (<100nm) that readily permeate skin layers, because of the medicine’s high concentration gradient which reduces toxicity and causes no irritation [
12]. By increasing the surface area accessible for interaction with biological membranes, these nano-sized droplets improve absorption and bioavailability. They tend to require external energy to prepare, and the mixture that is turned out is usually transparent [
13]. Co-surfactant and surfactant (o/w or w/o type) work together to stabilize nanoemulsion globules [
14,
15]. Water and alcohol are examples of aqueous solvents that comprise the nanoemulsion’s aqueous phase, whereas synthetic or natural lipids are often utilized as the oil phase unless the oil itself serves as an active agent [
16].
Hydrated skin permits greater payloads, and gel networks provide easy and regulated migration of nanovesicles from the matrix to the skin’s surface. Carbomers, Pluronic’s, xanthan gum, and carrageenan are examples of biocompatible polymers that have been used as gel systems to transdermally administer emulsified medications [
17]. Its low viscosity limits the application of the nanoemulsion in treating a range of skin conditions. [
18]. Nanosized emulsions combined into a gel-based system make up nanoemulgels. Using a gelling ingredient that’s used to prepare the gel basis, nanoemulgel transforms nanoemulsions into a denser, more stable, and non-sticky system [
19]. To increase the designed nanoemulsion’s viscosity for transdermal administration, Carbopol 934 are reported to be frequently utilized [
18]. The nanoemulsion is changed into a nanoemulgel by adding a gel matrix to it indicating that the transdermal applications benefitted more from the nanoemulgel.
Natural products have a significant impact on treating a variety of ailments with greater safety and fewer side effects, which is the reason they are widely used as alternative medications today [
20]. Turmeric and neem are two of the most notable herbal plants utilized for their abundance of bioactive components and centuries-old traditional medical use [
21]. Turmeric, belonging to the Zingiberaceae family and well-known for its active constituent Curcumin, possesses a wide range of biological properties such as antimicrobial, and antioxidant properties [
22,
23]. Neem, belonging to the family of Meliaceae, is native to the Indian subcontinent and a few African countries. Neem is a multipurpose tree having excellent antibacterial, antifungal, antioxidant, anti-inflammatory, and insecticidal characteristics due to the plethora of active phytoconstituents such as nimbidin, nimbin, salannin, margolone and azadirachtin [
24,
25]. Based on their pharmacological properties reported, it can be assumed that combination of turmeric and neem would show a synergistic effect.
These naturally occurring compounds enhance the antimicrobial activity of the nanoemulgels and augment their capacity to cure acute infections by permitting a deeper penetration into the epidermal layers. Comparing the antimicrobial properties of turmeric and neem nanoemulgels to traditional antimicrobial medications is a crucial step in understanding their therapeutic potential and synergistic effects against infections caused by microbes [
26]. Development and assessment of topical nanoemulgels based on a combination of neem and turmeric extract is one possible substitute for antimicrobial therapy since the synergistic effect of neem and turmeric nanoemulgel has not been studied previously. This is in reaction to the growing concern about the emergence of antibiotic resistance and the pressing need to find efficient medicines to fight microbial infections. The combination of neem and turmeric extract used to make the nanoemulgel is also reasonably priced and widely accessible. The present study aims to utilise the synergistic antimicrobial properties of neem and turmeric extracts in a nanogel delivery system for topical application, improving local retention, stability, and therapeutic efficacy.
2. Results and Discussion
2.1. Antioxidant Activity of Turmeric and Neem Leaf Extract
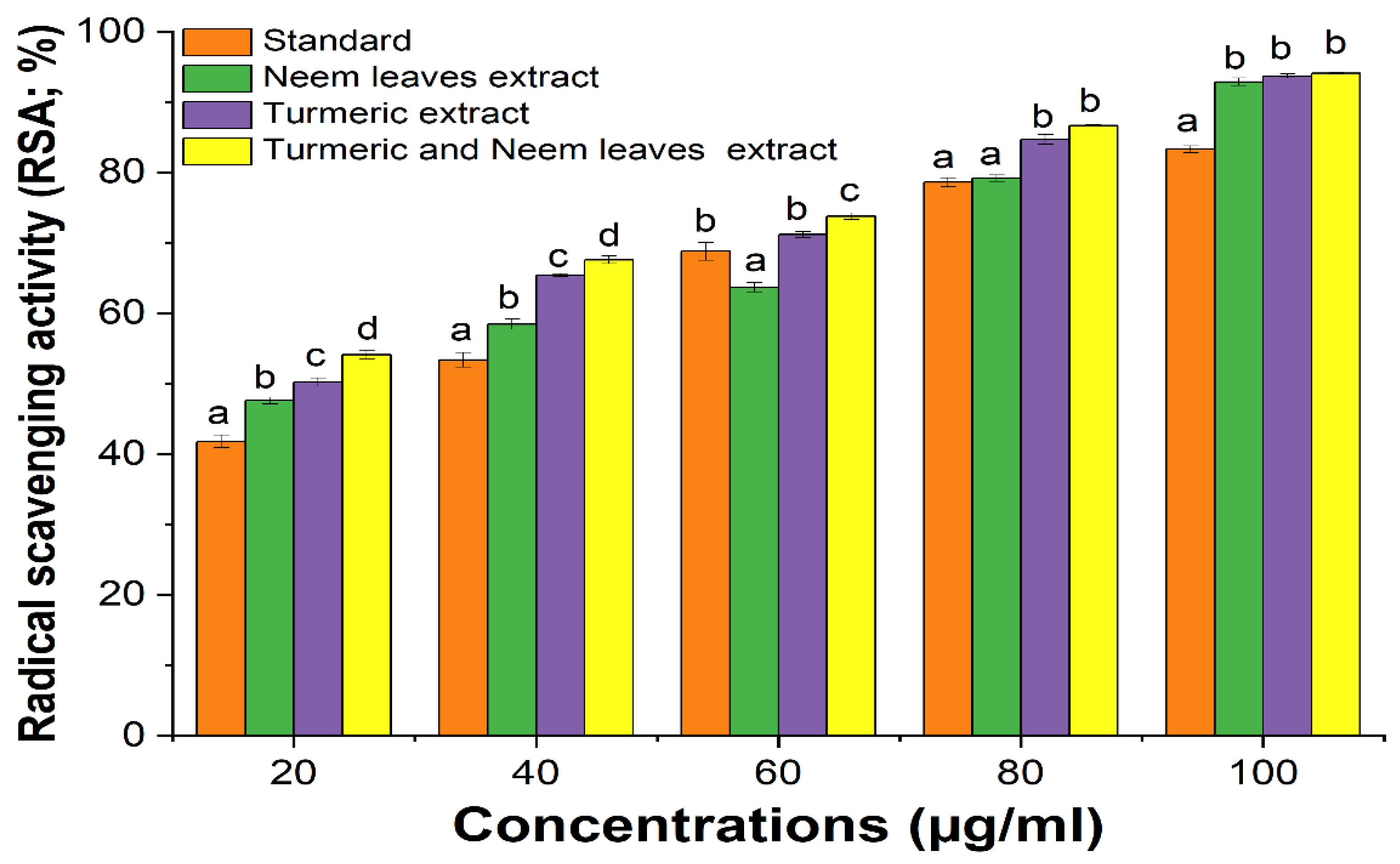
The ability of DPPH to scavenge the free radical was observed by assessing the color change from purple to yellow measuring at a wavelength of 517 nm. The result was reported as radical scavenging activity (RSA). Significant scavenging capabilities were observed in the combination of turmeric and neem extract than in the separate extract of turmeric and neem at different concentrations taking ascorbic acid as standard. Due to its synergistic effect, the turmeric-neem extract mixture’s antioxidant properties demonstrated noteworthy results, suggesting that the components of the extract are potent and effective antioxidants and when combined, it enhances the activity (
Figure 1,
Table 1) [
27,
28].
2.2. Anti-Inflammatory of Turmeric and Neem Leaf Extract
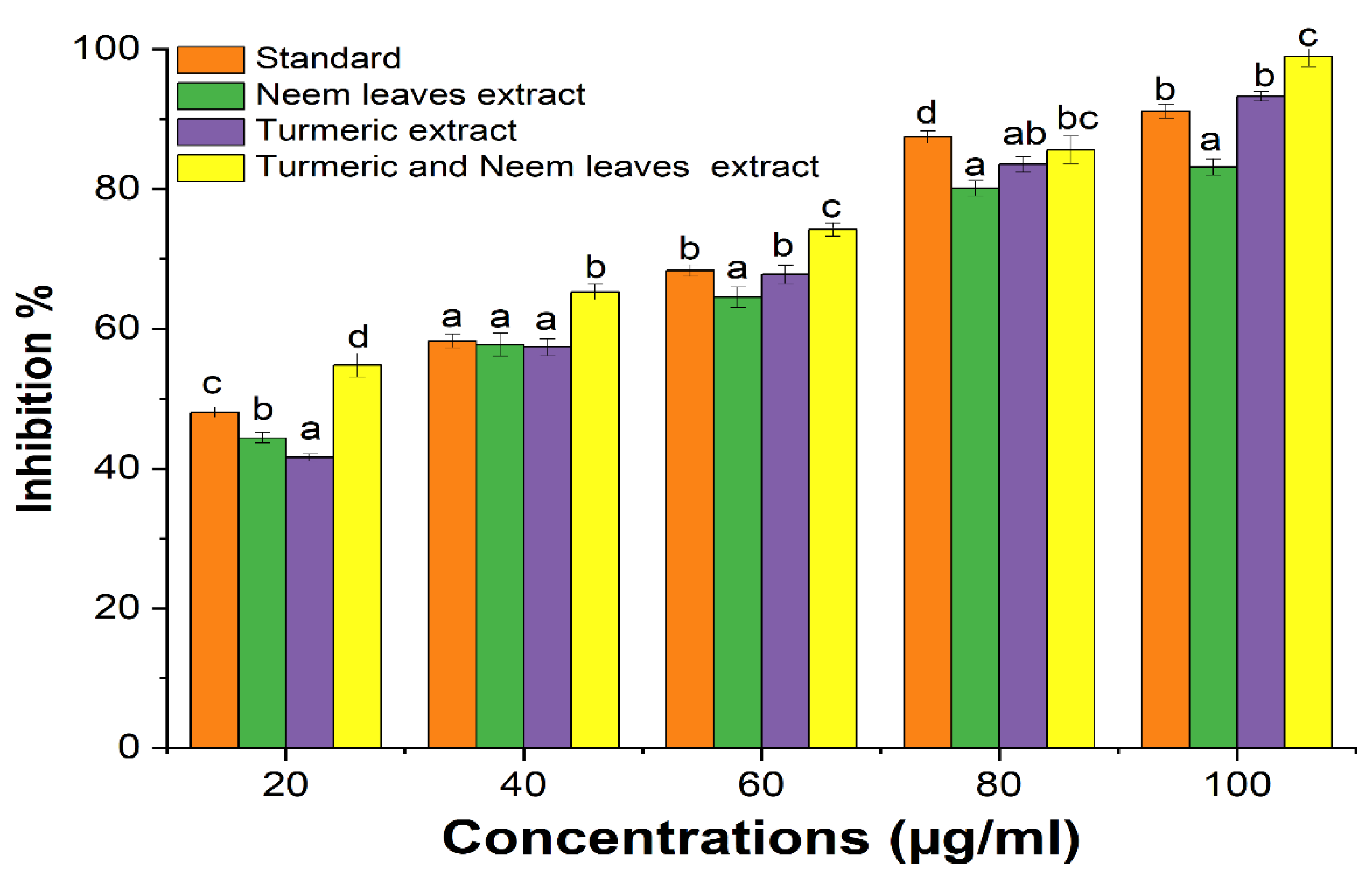
The anti-inflammatory activity of the turmeric and neem leaf extract was evaluated and compared with the standard Diclofenac sodium. It was observed that both extracts showed notable anti-inflammatory properties when compared to the standard (
Figure 2). The current investigation also shows that a mixture of turmeric and neem plant extract shows a synergistic effect where the anti-inflammatory property of the mixture was more significant than the individual extract’s ability to reduce inflammation depending on the concentration. Also, the plant extract may prevent haemolysis and more effectively stabilize the erythrocyte membrane at higher concentrations [
27,
29].
2.3. Antimicrobial Properties of Turmeric and Neem Leaf Extract
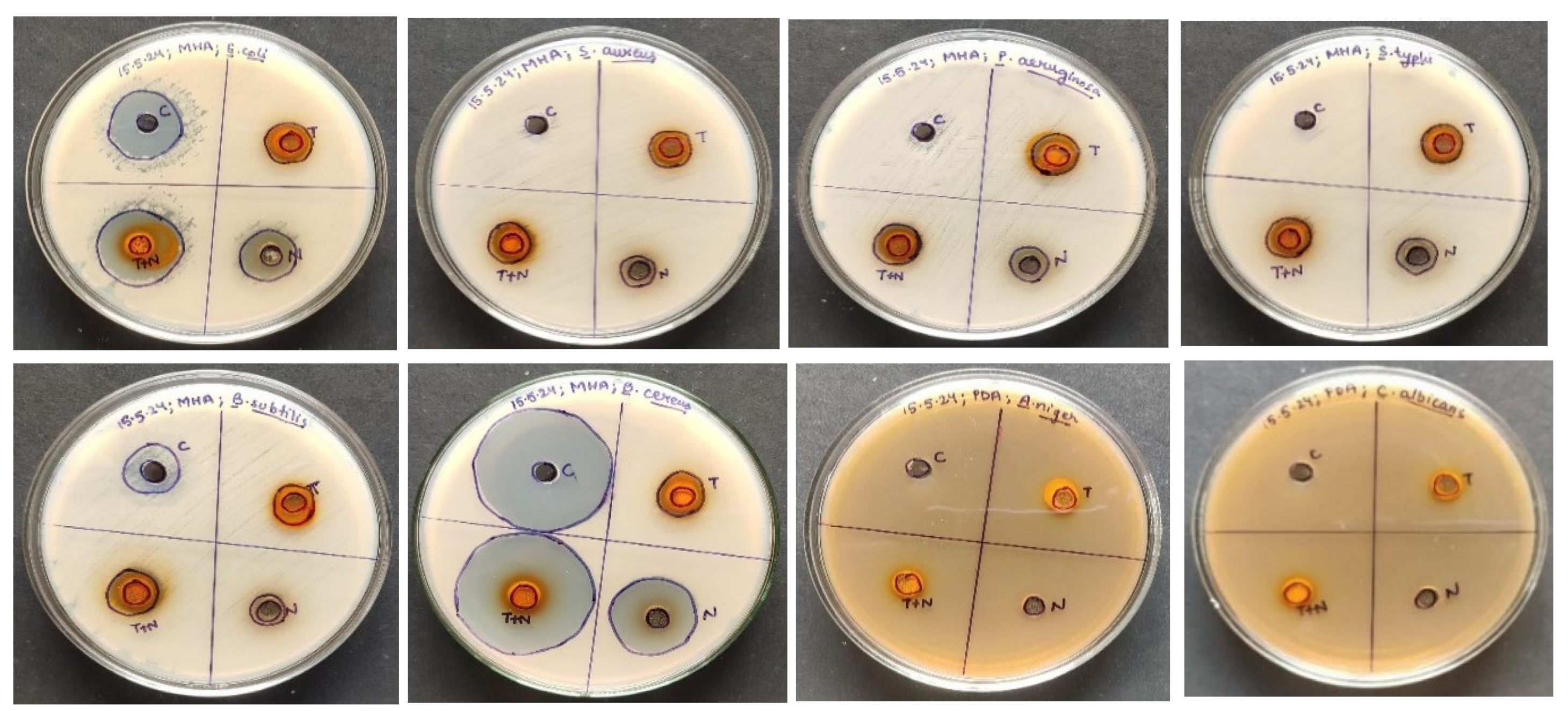
Using the agar well diffusion method, the antimicrobial capacity of the hydroalcoholic extract of neem leaves and turmeric was tested against a range of microorganisms, with Amoxycillin serving as a reference for bacterial pathogens and amphotericin B for fungal pathogens. The zone of inhibition indicates that there is a synergism between the turmeric and neem leaf extract mixture since it demonstrated considerably more antimicrobial activity than either extract alone (
Table 2,
Figure 3). Therefore, the extract’s antimicrobial action can be linked to the presence of phytochemicals with anti-pathogenic effects, including flavonoids, alkaloids, tannins, saponins, steroids, and phenolic compounds [
30].
2.4. Characterization of Turmeric and Neem Based Nanoemulsion
2.4.1. Physicochemical Evaluation and pH
Physical parameters such as color, homogeneity, phase separation, and appearance were examined by visual inspection. No particle matter was found in either of the formulations because they were all clear and transparent (
Table 3) [
31]. Each product’s pH was measured using a digital pH instrument that was also validated; the results showed that the range of pH values was between 5 and 6, which is close to the pH range of the skin (
Table 3) [
32].
2.4.2. Particle Characterization
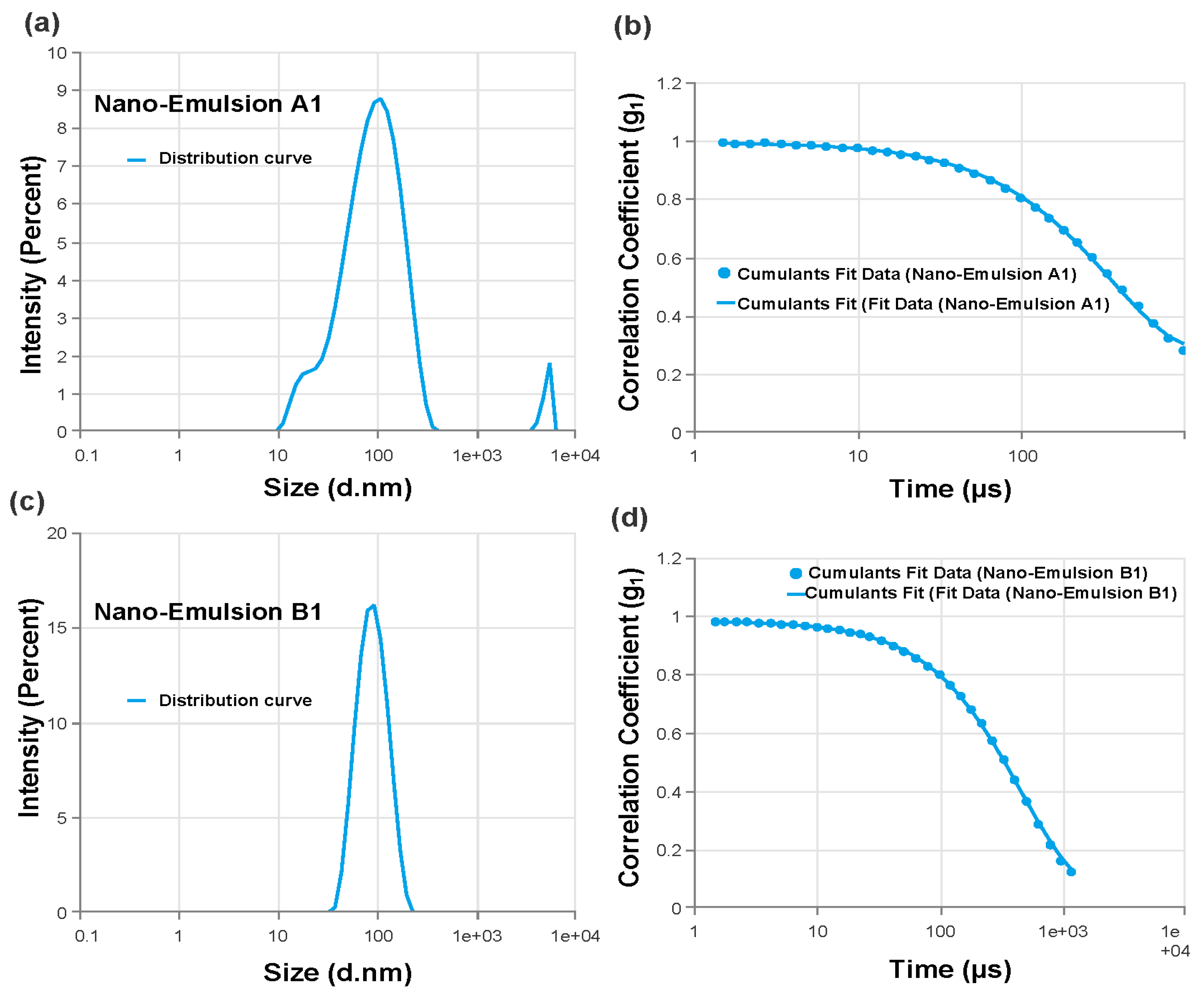
Each batch of nanoemulsion had droplet sizes ranging from 70 nm to 1768 nm. Formulations A1 (78.87 nm) and B1 (80.4 nm) had the smallest droplets. Every formulation under investigation had PDI values less than 0.691 (
Table 4). The PDI values were also noticeably low (0.414 and 0.156, respectively) for the formulations with the smallest droplet sizes (A1 and B1), showing excellent stability and monodispersity as illustrated through the size distribution curves (
Figure 4). A1 and B1 were the two formulations that were selected as the best ones for further research based on particle size and PDI values [
32,
33,
34,
35].
2.4.3. Instrumental Analysis
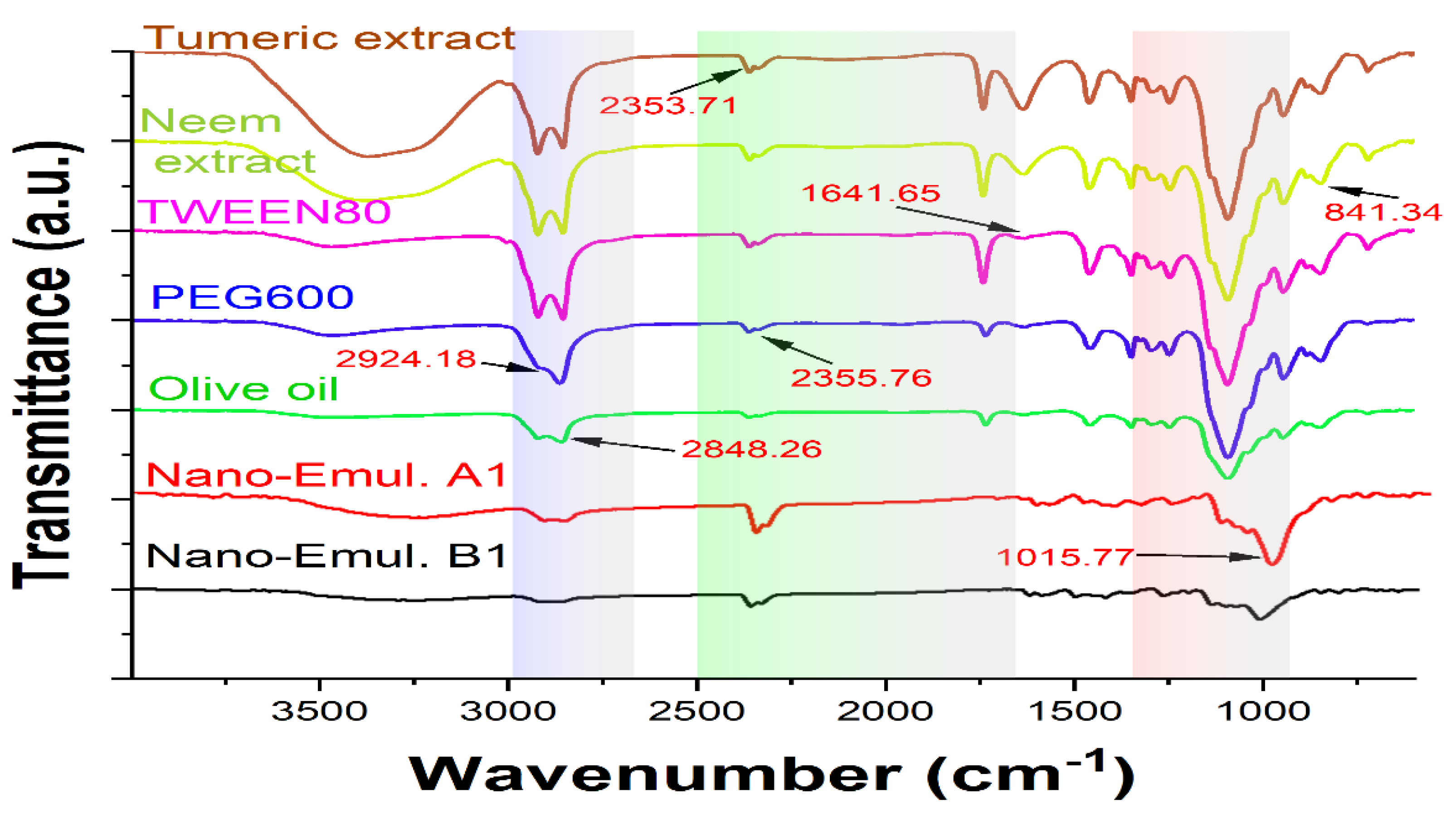
An FTIR analysis investigated the compatibility of turmeric and neem extract with olive oil, Tween 80, and PEG600 (
Figure 5). The stretching of the benzene ring at 1600 cm
−1, the O-H bonds at 3510 cm
−1, the combination of C=O and C is equivalent to C oscillations at 1600 cm
−1, the C–H groups bending at 1430 cm
−1, the C–O stretching at 1280 cm
−1, and the C- oxygen-carbon stretch at 1025 cm
−1 were the top values for both neem and turmeric [
36]. The presence of water molecules causes an extra-wide band at around 3400 cm
−1. Using the PEG 600, the 1105 cm
-1 (C–O–C bond), the 3389–3420 cm
−1 (OH stretch), and the C–H stretching band (2870–2890 cm
−1) were detected and for Tween 80 and olive oil, the 2920 cm
−1 (CH
3 band), 2864 cm
−1 (CH
2-stretch), and the 1095 cm
−1 (C–O–C band) were detected. However, the O-H bonds extension at 3447.10 cm
−1 was the greatest of the formulation spectra, 2870–2890 cm
−1 (C–H stretch), 2864 cm−1 (CH
2-stretch), 1025 cm
−1 (C-O-C stretching vibrations).
The retention of the polymer’s and other excipients’ characteristic peaks in the nanoemulsion formulations is confirmed by FTIR spectrum data, suggesting that the polymer and extracts’ constituent parts interact with one another on a molecular level. Moreover, the peaks exhibit no variation in the functional groups, indicating outstanding stability and the absence of phase separation in the formulation free from issues with extract-polymer compatibility [
37].
2.5. Characterization of Turmeric and Neem-Based Nanoemulgel
2.5.1. Physicochemical Properties
Physical characteristics including consistency and colour, and homogeneity were examined by physical examination (
Table 5) [
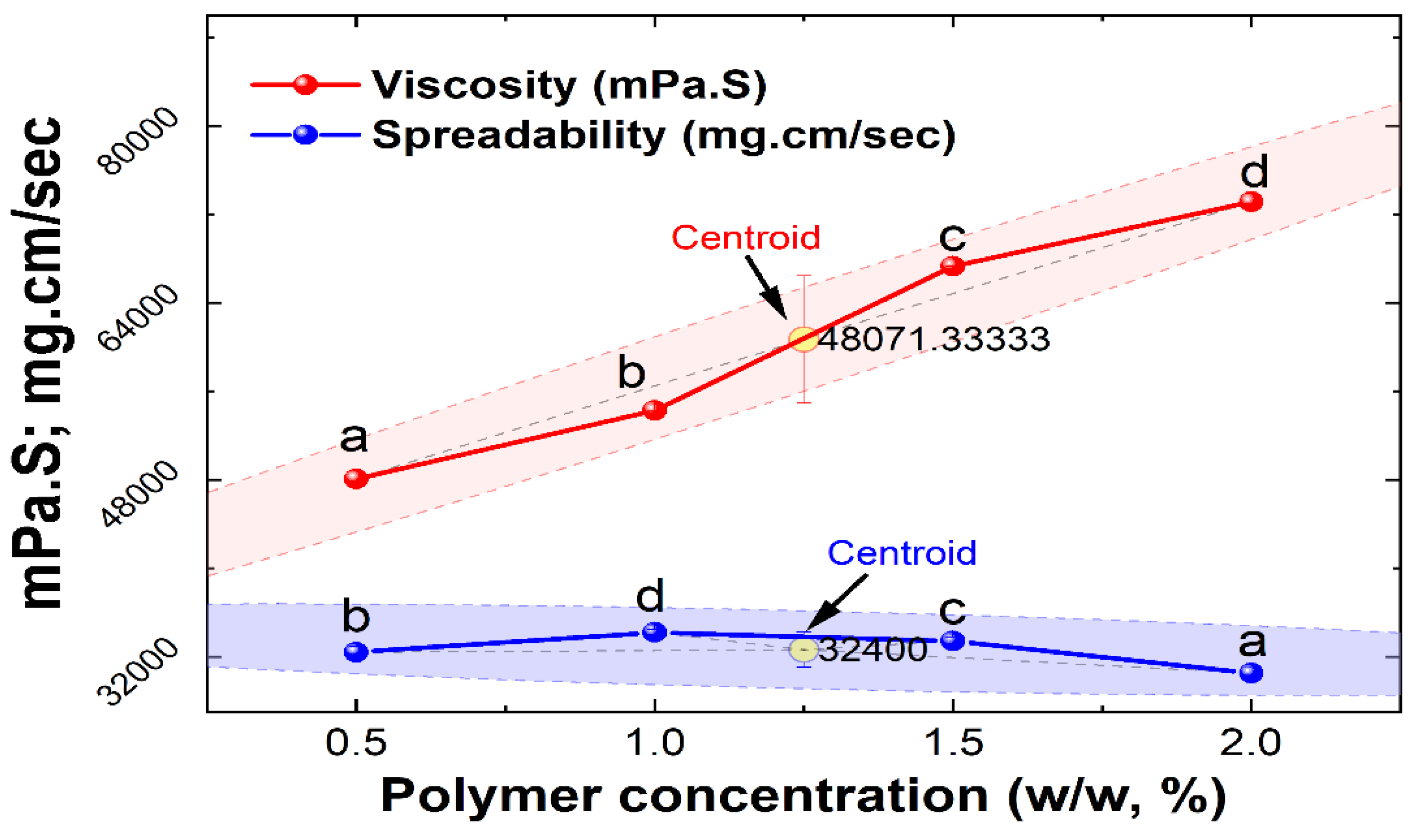
38]. Based on viscosity and spreadability, Optimization was done on the nanoemulgel formulations., and it was found that when the polymer content increased, the nanoemulgels spreadability reduced (
Table 5). In contrast to formulations F3 and F4, which both had viscosities of 67,3094 ± 3 and 73,127 ± 8 mPa·S respectively with low spreadability on another way F1 showed a viscosity of 48,072 ± 5 mPa·S with good spreadability 32 ± 0.5. Because of their ideal spreadability of 34 ± 0.2 and viscosity of 54,284 ± 7 mPa·S (
Figure 6), formulations F2 and F1 were deemed excellent. Carbopol 934 is a synthetic polymer with a viscosity of 30,000–50,000 centipoises is high and is commonly used in topical or transdermal formulations [
39]. The viscosity of the nanoemulgel increased with the concentration of the gelling ingredient has increased (Carbopol 934), and as a result, the formulation’s spreadability decreased. This is because the two properties of the formulation are inversely correlated [
40]. Based on the nanoemulgel formulation optimization, it was observed that the F2 formulation was best optimized. The F2 formulation of the nanoemulgel was then incorporated with the best formulation of nanoemulsion (A1 and B1) prepared from herbal extracts for further studies (
Table 6).
2.6. Drug–Excipient Interaction Studies
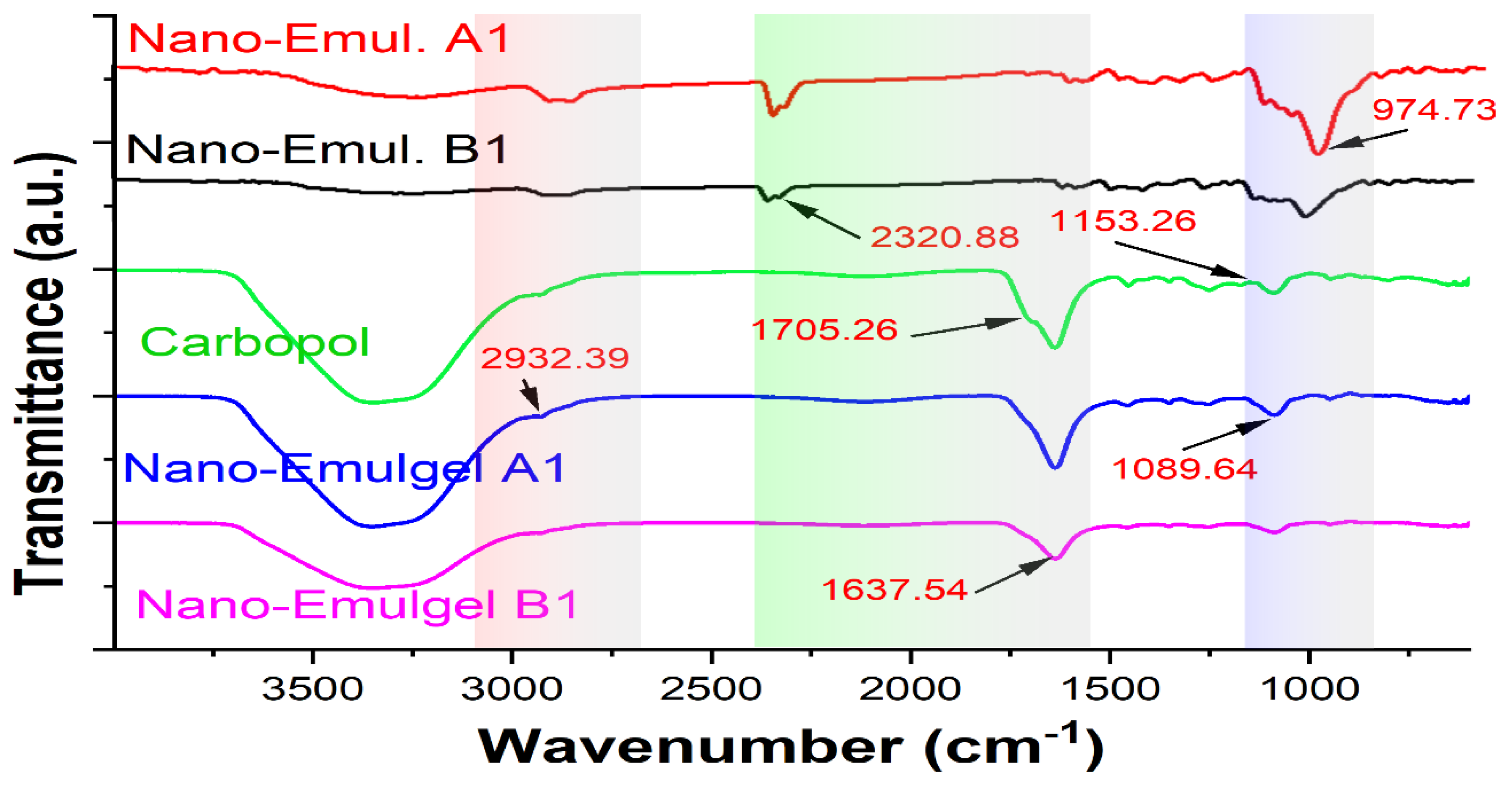
The drug-excipient compatibility in nanoemulgel formulations was determined using FTIR analysis (
Figure 7). The C=O stretching vibration peaks of Nanoemulgel A1 and B1 shifted to 1552 and 1545 cm
−1, respectively showing that hydrogen bonds are forming. Furthermore, the formulation’s wide OH peak moved to 3367 and 3344 cm
−1, corresponding to the OH groups and hydrogen bonding between molecules. The C-H stretching was correlated with peaks that were found between 2853 and 2924 cm
−1. Additionally, the formulation’s observation of the C-O-C stretching peak at 1049 cm
−1 was changed to 1042–1043 cm
−1. In Carbopol 934’s FTIR spectrum, the peak at 2927 cm
−1 is associated with the compound CH
2 stretching, the COOH group is connected to a peak at 1767 cm
−1, and the COOH group is linked to peaks at 1451 cm
−1 and 1246 cm
−1.
It is verified by FTIR spectrum data that the distinctive peaks of the polymer and other excipients are retained in the nanoemulgel formulations, indicating that there is intermolecular interaction between the components of the extracts and polymer. Furthermore, the peaks show no change in the functional groups depicting excellent stability and the absence of phase separation of the formulation devoid of extract-polymer compatibility issues [
40].
2.7. In-Vitro Study
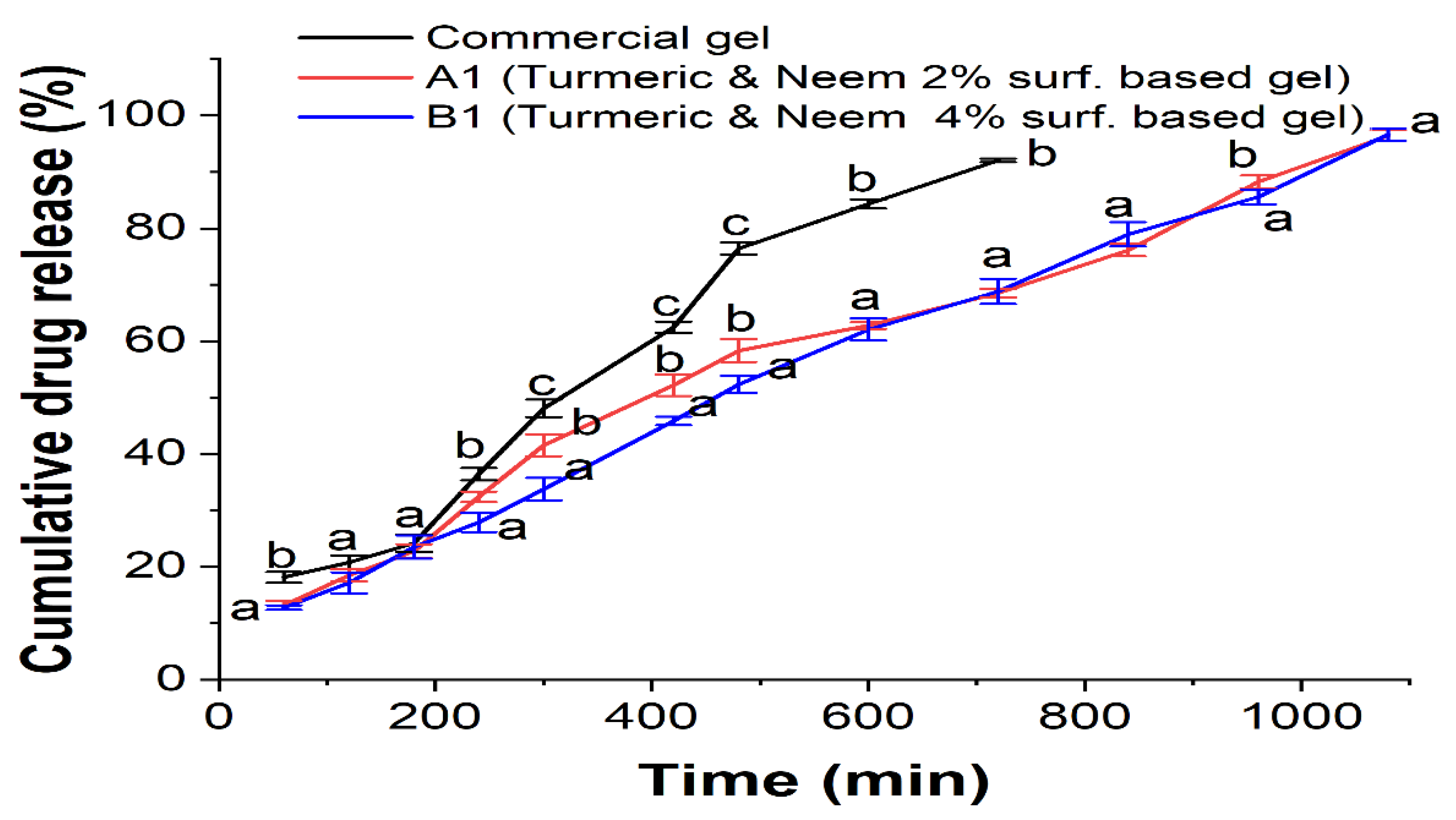
In the drug permeation study, a dialysis membrane was employed in conjunction with the commercial hydrogel formulation to examine the release of the herbal extract from the nanoemulgel formulations (A1 and B1) (
Figure 8). This is particularly noteworthy as the commercial gel releases less and the extract was released for eighteen hours. With an increase in the content of Carbopol 934 in the nanoemulgels, nanoemulsions A1 and B1, the aggregate drug release falls from 96.4% (A1) to 96.6% (B1). The hydrophilic gel layer typically allows nanoemulgel to be released, and the rate of release is determined by polymer swelling, degradation, and diffusion. Factors influencing drug release from carriers include the composition, ratio, and physical and chemical interactions between the extracts and the carriers. With a reduced release rate, enhanced skin retention can be a more effective treatment for localized skin infections. The developed formulation path was complex and prolonged, which may have contributed to the decrease in the release rate of the nanoemulgels.
The released media that used was consist of ethanol and distilled water (1:1 v/v) at pH 6.8. Oil droplets in nanoemulgel enter the skin’s subcutaneous layer directly after being released into the gel matrix, bypassing the hydrophilic phase change that occurs in nanoemulsion. Consequently, in subsequent formulations, formulation release patterns were reduced [
41,
42,
43].
2.8. Ex-Vivo Mucoadhesive Study
Among the most important elements in the route of administration for topical delivery is mucoadhesion, which encourages prolonged release. Low adherence of Carbopol nanoemulgel was found. However, with the concentration of commercially available hydrogel, prepared nanoemulgel demonstrated superior mucoadhesive qualities (
Table 7). Furthermore, when soaked in water, Carbopol swells a thousand times larger and has the strongest mucoadhesive property. This behaviour might be explained by a longer polymeric chain and a more robust cohesive force between the goat skin and the specified nanoemulgel. Rheological considerations and mucoadhesive strength have a linear connection when compared to the product’s viscosity and mucoadhesive strength values. Within the bounds of our trials, it can be interpreted that there was a correlation between the formulation’s increased viscosity and its increased mucoadhesive strength [
44]. Furthermore, it can be assumed that the swelling property of Carbopol might have helped in absorbing the exudates from the affected area.
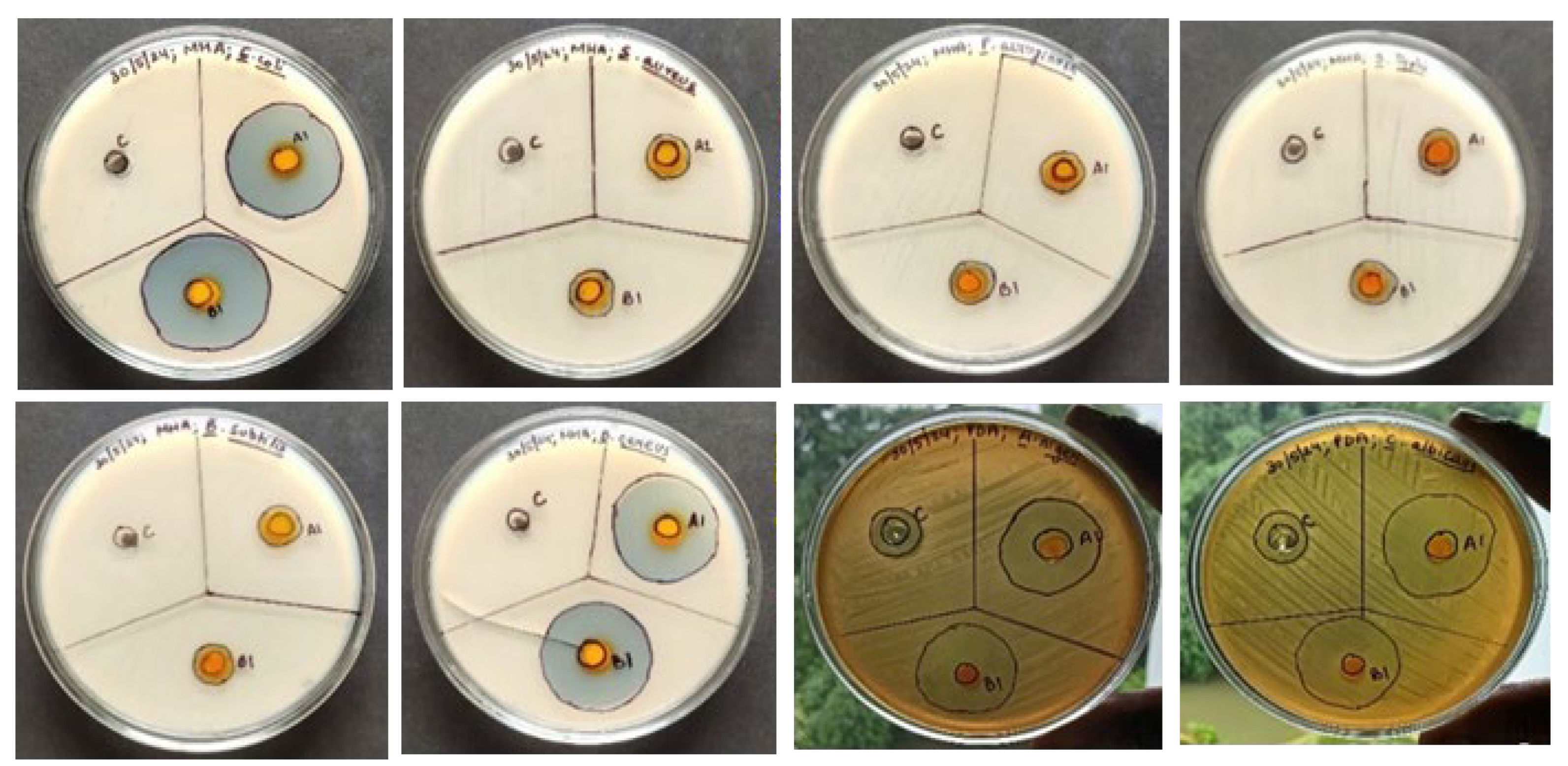
2.9. Antimicrobial Properties of Nanoemulgel
The zone of inhibition indicates that the mixture of turmeric and neem leaves-based nanoemulgel substantial antibacterial activity was shown by the plant materials than the commercially marketed hydrogel against different microorganisms taking marketed hydrogel formulation (Megaheal) as reference (
Figure 9,
Table 8) [
45].
2.10. Stability Study
Characterization was done on the optimized nanoemulgel formulation to ascertain its colour, pH, homogeneity, viscosity and spreadability. The optimized nanoemulgel formulation did not exhibit any notable alterations in its organoleptic qualities, and for three months it stayed uniform at different temperature such as temperature in the refrigerator (4°C ± 1°C), ambient temperature (22-25° C ± 1°C), and incubated temperature (37 ± 2°C) respectively as shown in
Table 9 [
46].
3. Conclusions
Currently, there has been an increased interest in preparing effective medication using natural products. In particular, the active constituents of herbal plants are used as potential sources having pharmacological properties including antimicrobial, anti-inflammatory, and antioxidant. Numerous researches have been published that describe the formulation of nanoemulgel for transdermal delivery, such as bigels loaded with ciprofloxacin and chitosan-laden nanoemulgel containing 5-fluorouracil. The preparation and evaluation of the nanoemulgel loaded with neem and turmeric extract for topical delivery of drugs against microbial infection marks the conclusion of this investigation. According to reports, because of their target selectivity, non-toxicity, biocompatibility, and biodegradability, nanoemulsions are a promising choice for antimicrobial nanosystems. The improved nanoemulsion in this work was successfully blended with the gel foundation to develop a topical nanoemulgel formulation. The properties of the nanoemulgel loaded with neem and turmeric extract were successfully assessed to determine whether it was suitable for topical application. Effectiveness was observed in both formulations A1 and B1, suggesting that the proportion utilized is suitable for producing a useful nanoemulgel. The formulation that was optimized demonstrated a small droplet size and PDI within an ideal range. Following a three-month evaluation of the preparation for various stability tests, it was discovered that the formulations were stable, had excellent homogeneity, and showed no phase separation. Viscosity, spreadability, and drug content analysis were all found to be within an acceptable range. The formulations were next assessed for antimicrobial properties, and it was discovered that they had a significant zone of inhibition and were effective against a variety of pathogens. This zone of inhibition demonstrates how the neem and turmeric-loaded nanoemulgel outperforms the separate extracts in terms of effectiveness and synergy. Furthermore, because of its mucoadhesive characteristics and prolonged release profile, it was determined that converting nanoemulsion into nanoemulgel would be more beneficial because of the latter’s prolonged release and increased duration of contact. Thus, the present study has indicated that turmeric and neem extract-loaded nanoemulgel can be promising alternatives for microbial infection particularly associated with microorganisms via topical application but for verification later on, an in-vivo skin absorption study and cell-based assay might be helpful.
4. Materials and Methods
4.1. Chemicals, Plants, and Microbial Strains
All the chemicals, including Tween 80 (surfactant), PEG 600 (co-surfactant), olive oil, Carbopol 934 (polymer), ethanol, methanol, 2,2-Diphenul-1-Picrylhydrazyl (DPPH), triethanolamine, Amoxicillin, and Amphotericin – B, were of analytical grade. Megaheal gel was purchased from the market. Mueller Hinton Agar and Potato Dextrose Agar were obtained from HiMedia, Mumbai, India. The herbal plant sample was obtained from the Brainware University herbal garden which has been maintained by plants procured from the West Bengal State Medicinal Plants Board, Kalyani, India. The microbial strains, Bacillus subtilis (ATCC 6633), Staphylocccus aureus (ATCC 6538), Escherichia coli (ATCC 8739), Pseudomonas aeruginosa (ATCC 9027), Bacillus cereus (ATCC11778), Salmonella typhi (NCTC 786), Aspergillus niger (ATCC 16404), and Candida albicans (ATCC 10231), gathered from the Central Drugs Laboratory (CDL) located in Kolkata, West Bengal, India.
4.2. Methods
4.2.1. Extraction from Neem Leaves
Before ground into a powder, the neem leaves were thoroughly cleansed in distilled water and allowed to dry in the shade for 10 days. The extraction was done using methanol and distilled water in a proportion of 80:20 (v/v). The powdered sample was extracted for one hour at 25°C and 150 rpm in a 500 mL litre scale using the hydroalcoholic mixture. After filtering the mixture using 125mm Whatman filter paper, the extracts were further dried in a CHILLER SLIM-CHIL-300 rotary evaporator set to low pressure and 35 °C. In the culmination, a pale green residue was obtained and stored in an airtight container in a cool, dark place [
47,
48].
4.2.2. Extraction from Turmeric Rhizomes
In a glass container containing 50 gm of powdered turmeric that had been bleached, 30 mL of ethanol and 70 mL of distilled water were added to produce a hydroalcoholic extract of the turmeric. Then, for 7 days it was left to stand at room temperature (24 ± 1°C) with frequent stirring. In order to extract the powder from the liquid, the suspension was filtered and then dried using the rotary evaporator. The extracts were thereafter stored in a refrigerator till it was needed [
49].
4.2.3. Antioxidant Activity of Hydroalcoholic Extract of Turmeric and Neem Leaves
DPPH Free Radical Scavenging Activity
The extracts capacity to scavenge free radicals was assessed using the DPPH test. A 0.1 mM DPPH solution was prepared in methanol. 1.6 ml of hydroalcoholic extract of turmeric, neem, and a mixture of turmeric and neem at various mixture of concentrations (20, 40, 60, 80, and 100µl) was prepared. with 2.4 ml of the DPPH solution prepared. After carefully mixing the concoction, it was allowed to sit at room temperature for about half an hour in the dark place. The absorbance of the combination was measured at 517 nm taking the standard as ascorbic acid. The percentage of inhibition was calculated with the following formula [
27].
Where A0 and A1 is the absorbance of the control and extract respectively.
4.2.4. Anti-Inflammatory Activity of Hydroalcoholic Extract of Turmeric and Neem Leaves
bovine Serum Albumin Denaturation Activity
Inhibition of protein denaturation was ascertained to determine the anti-inflammatory efficacy. In this study, the test sample comprised 1% of bovine serum albumin (BSA) and various quantities of hydroalcoholic extracts of neem, turmeric, and a mixture of turmeric and neem at various concentrations (20, 40, 60, 80, and 100µl). To correct the pH, 1N of HCl was added. The reaction mixture was incubated in a water bath at 37ºC for 20 minutes, and following that heated at 57ºC for 20 minutes. The reaction mixture was allowed to cool and then its absorbance was measured at 570 nm using a UV-Vis spectrometer (UV-1900i, Shimadzu) taking Diclofenac sodium as standard. Using the following formula, the percentage inhibition of protein denaturation was calculated
Where A
c is the absorbance of the control and A
s are the absorbance of sample [
27].
4.2.5. Anti-Microbial Activity of Hydroalcoholic Extract of Turmeric and Neem Leaves
The antimicrobial activity of the hydroalcoholic extract of turmeric, neem and a mixture of turmeric and neem was tested using agar well diffusion method by Muller-Hinton agar for antibacterial activity and Potato Dextrose agar to measure the antifungal activity, against microorganisms like
Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus cereus, Salmonella typhi, Aspergillus niger and
Candida albicans [
50]. Using sterile cotton swabs, the microorganisms were jabbed over the prepared Muller-
Hinton and Potato Dextrose agar plate. Then, 10 µl of the produced hydroalcoholic extracts of turmeric, neem, and a mixture of turmeric and neem were placed into each well. The antibacterial and antifungal activity was assessed by measuring the zone of inhibition in triplicates during a 24-hour incubation period at 37°C [
51].
4.2.6. Preparation and Optimization of Turmeric and Neem-Based Nanoemulsion
Using a magnetic stirrer, the components of the oil phase are olive oil (1% v/v) and 1% w/v extracts of neem and turmeric. The mixture is first mixed for 15 minutes. When the extracts in the oil phase had completely dissolved, twelve distinct combinations (
Table 10 and
Table 11) of oil (olive oil) and a mixture of surfactant (Tween 80) and co-surfactant (PEG 600) (Smix) were formed in different volume ratios. Both plant extracts in desired concentration combined with the oil phase, on another hand distilled water combined with a 2:1 and 4:1 ratio of Smix [
52,
53]. With constant stirring, the aqueous phase was added drop-by-drop to the oil phase at 25°C to produce the pre-emulsion (
Figure 10). For ten minutes, high-shear homogenization (OV 625 Digital Disperser, Antylia Scientific) was done at 10,000 rpm [
54].
4.2.7. Characterization of Turmeric and Neem-Based Nanoemulsion
Physicochemical Properties and pH
Visual evaluations were conducted of the nanoemulsion’s colour, homogeneity, consistency, and phase separation [
55]. Using a digital validated pH meter at room temperature, the pH of the prepared nanoemulsion was ascertained [
33].
Particle Characterization
The produced nanoemulsion formulation’s globule size was measured at 25°C using Zetasizer ZTS1240 (Malvern Panalytical Ltd.) [
33,
34].
FTIR Analysis
A Fourier Transform Infrared spectrophotometer (Nicolet 380 FT-IR Spectrometer) can be used for qualitative analysis and interaction investigations. This study aimed to evaluate the potential physiochemical interactions between the formulation ingredients used for preparing the nanoemulsion [
56].
4.2.8. Formulation of Turmeric and Neem-Based Nanoemulgel
After being dissolved in water and allowed to swell overnight, Carbopol 934 was neutralized with triethanolamine. To transform the gel into emulgel, it was properly incorporated with the Nanoemulsion using a magnetic stirrer and allowed to sit at room temperature (25°C) for around 20 minutes [
54]. Based on the gelling agent concentrations, spreadability, and viscosity the formulation of nanoemulgel was optimized. Four distinct formulations with variable gelling agent concentrations (0.5-2%) were produced to optimize the formulation (
Table 12). The final gel composition which contained 1% (w/w) of turmeric-neem leaf extract, was created by mixing the components in the proper amounts.
4.2.9. Characterization of Turmeric and Neem Based Nanoemulgel
Physical Examination
Colour, homogeneity, and consistency of the resulting nanoemulgel were examined visually [
57]. A digital validated pH instrument was used to confirm the pH of the nanoemulgel that was developed [
33].
Rheological Studies
To determine the viscosity of the prepared nanoemulgel, a Brookfield viscometer (Brookfield Engineering Laboratories, USA) was utilized. At 30 °C, the viscosity of the formulations was examined using spindles 1 and 2 rotated at 30 rpm for one minute, before the readings were recorded in triplicates [
58].
4.2.10. Spreadability of Nanoemulgel
The spreadability of the formulations was assessed using the Drag and Slip methodology [
58] where two glass slides were used (7.5 cm long and 2.5 cm wide). The setup was composed of a wooden block with two glass slides, one slide is fixed and the other one is movable, and a pulley fastened to a terminal. Subsequently, the formulation was mounted on the top fixed slide and pressed between the two plates to measure the spreadability of the mixture. To establish a homogenous layer of the formulation and release trapped air between the slides, The pulley’s upper glass slide has a 100 g weight fastened to it. The duration required for the upper/top slide to move was then recorded. The spreadability of the nanoemulgel was calculated using the formula below
Where spreadability (S), weight or mass (M) placed on the movable slide, glass slide length (L), and time (T) taken to cover distance.
4.2.11. FTIR Analysis
The FTIR analysis aimed to figure out for any incompatibilities between the active ingredients and excipients in the formulation of nanoemulgel, as previously mentioned [
54]. An FTIR study was carried out for the nanoemulsion, nanoemulgel, as well as Carbopol 934.
4.2.12. In-Vitro Study
The release apparatus from Hanson Research, which has been used for in-vitro release and has a working exterior area of 1.767 cm
2 and a receptor capacity of 7.0 mL. As the releasing membrane, a dialysis cellulose membrane was used. The membrane was allowed to equilibrate with the receptor medium as equal volume of ethanol and distilled water (1:1 v/v) at pH 6.8 for one hour before 1g of nanoemulgel was gently added to it. Megaheal, a commercially available hydrogel (Aristo Pharmaceuticals Pvt. Ltd, Mumbai, India), was used as the control gel. The composition of the commercially marketed hydrogel includes silver colloid (reported to have antimicrobial properties), carbomer (polymer), propylene glycol (to maintain the moisture), and triethanolamine (pH adjuster). A constant 37 ± 0.5°C and 350 rpm were maintained for the releasing medium. At predefined intervals, after extracting the 2 mL sample, the same volume of media was added in its place [
54]. At an effective wavelength of maximum absorbance of 431 nm, UV-visible spectrophotometry (UV-1900i, Shimadzu) was employed to estimate the extracted amount in every component [
59].
4.2.13. Ex-Vivo Mucoadhesive Study
Determining the strength needed to extract the product from goat skin, an ex vivo mucoadhesive experiment was carried out using the Texture analyzer (Brookfield QTS). A nearby abattoir provided the fresh goat skin that was gathered. Once the skin had been attached to the lower probe, the top probe received nanoemulgel around 250 μL. When the top probe dropped at a constant rate of 0.3 mm/s, an acceleration of 0.4 N was generated at skin contact. The probe moved up after two minutes, measuring the detachment force [
60].
4.2.14. Antimicrobial Activity of Turmeric and Neem-Based Nanoemulgel
Using Muller-Hinton agar and Potato Dextrose agar, the antibacterial activity was examined using the agar well diffusion method of the neem- and turmeric-based nanoemulgel, against microorganisms like
Bacillus subtilis, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Bacillus cereus, Salmonella typhi, Aspergillus niger and
Candida albicans. Using sterile cotton swabs, the microorganisms were jabbed over the Muller-Hinton and Potato Dextrose agar plate. Then, 10 µl of the produced turmeric and neem-based nanoemulgel, taking a marketed hydrogel (Megaheal, from Aristo Pharmaceutical Pvt. Ltd.) as control, were placed into each well. During a 24-hour incubation period at 37°C, to assess the antibacterial activity, the diameter of the zone of inhibition was measured three times [
51,
61].
4.2.15. Stability Study
The physical stability of the formulation under accelerated storage circumstances was ascertained by conducting a stability analysis for the in-situ formulation following the International Conference on Harmonization recommendations. The finished formulation was exposed to high temperatures and humidity levels. It was then stored for one to three months at various temperatures, including 4°C ± 1°C in the refrigerator, 22–25°C ± 1°C in the room, and 37 ± 2°C in the incubator. Organoleptic alterations, physical stability, viscosity, and spreadability of the formulation were assessed at the end of the 30, 60, and 90-day periods [
62].
4.2.16. Statistical Analysis
All the results were averaged in triplicates and reported as mean ± standard deviation by conducting a one-way analysis of variance (ANOVA). When the p-value < 0.05, the difference was considered to be statistically significant.
Author Contributions
Conceptualization, S.G.; and Z.I.; methodology, S.G.; and J.H.; software, and validation, S.G.; T.K.R.; and J.H..; formal analysis, S.G.; T.K.R.; and J.H.; investigation, S.G.; A.C.; T.K.R. Z.I. and C.M.; resources, S.G.; and J.H.; data curation, S.G.; A.C.; C.M.; T.K.R.; and Z.I.; writing—original draft preparation, S.G.; and A.C.; writing—review and editing, Z.I.; and M.G.; visualization, S.G.; and M.G.; supervision, S.G.; and M.G.; project administration, Z.I.; funding acquisition, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
The current work was funded by the “Belt and Road” joint project fund between Zhejiang University, China, and National Research Centre, Egypt (Project No: SQ2023YFE0103360).
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The original contributions presented in the study are included in the article.
Conflicts of Interest
The authors declare no conflicts of interest in this work.
References
- Siafaka, P.I.; Özcan Bülbül, E.; Okur, M.E.; Karantas, I.D.; Üstündağ Okur, N. The application of nanogels as efficient drug delivery platforms for dermal/transdermal delivery. Gels 2023, 9, 753. [Google Scholar] [CrossRef]
- Nawaz, A.; Latif, M.S.; Alnuwaiser, M.A.; Ullah, S.; Iqbal, M.; Alfatama, M.; Lim, V. Synthesis and characterization of chitosan-decorated nanoemulsion gel of 5-fluorouracil for topical delivery. Gels 2022, 8, 412. [Google Scholar] [CrossRef]
- Hamed, R.; Abu Alata, W.A.; Abu-Sini, M.; Abulebdah, D.H.; Hammad, A.M.; Aburayya, R. Development and comparative evaluation of ciprofloxacin nanoemulsion-loaded bigels prepared using different ratios of oleogel to hydrogels. Gels 2023, 9, 592. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Rasul, A.; Zafarullah, M.; Abbas, G.; Rasool, M.; Ali, F.; Ahmed, S.; Javaid, Z.; Abid, Z.; Riaz, H.; Mahmood Arshad, R.K.; Maryam, S.; Amna, N.; Asif, K. Nanoemulsions as novel nanocarrieres for drug delivery across the skin: In-vitro, in-vivo evaluation of miconazole nanoemulsions for treatment of Candidiasis albicans. Des. Monomers Polym. 2021, 24, 240–258. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Wang, J.Y.; Wu, Y.H. Application of cellular resolution full-field optical coherence tomography in vivo for the diagnosis of skin tumours and inflammatory skin diseases: A pilot study. Dermatol. 2022, 238, 121–131. [Google Scholar] [CrossRef]
- Alkilani, A.Z.; Nasereddin, J.; Hamed, R.; Nimrawi, S.; Hussein, G.; Abo-Zour, H.; Donnelly, R.F. Beneath the Skin: A Review of Current Trends and Future Prospects of Transdermal Drug Delivery Systems. Pharmaceutics 2022, 14, 1152. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Bülbül, E.Ö.; Mutlu, G.; Okur, M.E.; Karantas, I.D.; Okur, N.Ü. Transdermal Drug Delivery Systems and their Potential in Alzheimer’s Disease Management. CNS Neurol. Disord. Drug Targets 2020, 19, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhu, X.; Ji, S.; Huang, Z.; Zang, Y.; Ding, Y.; Zhang, J.; Ding, Z. Transdermal delivery of colchicine using dissolvable microneedle arrays for the treatment of acute gout in a rat model. Drug Deliv. 2022, 29, 2984–2994. [Google Scholar] [CrossRef]
- Sarheed, O.; Dibi, M.; Ramesh, K.V.; Drechsler, M. Fabrication of alginate-based O/W nanoemulsions for transdermal drug delivery of lidocaine: Influence of the oil phase and surfactant. Molecules 2021, 26, 2556. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An overview of micro-and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Wang, Y.; Li, M.; Wen, X.; Tao, H.; Wang, K.; Fu, R.; Tao, H.; Wang, F.; Chen, N.; Ni, Y. Conformational changes and the formation of new bonds achieving robust nanoemulsions by electrostatic interactions between whey protein isolate and chondrioitin sulfate. Food Hydrocoll. 2023, 136, 108263. [Google Scholar] [CrossRef]
- Yuan, L.; Pan, M.; Shi, K.; Hu, D.; Li, Y.; Chen, Y.; Qian, Z. Nanocarriers for Promoting Skin Delivery of Therapeutic Agents. Appl. Mater. Today 2022, 27, 101438. [Google Scholar] [CrossRef]
- Falleh, H.; Jemaa, M.B.; Neves, M.A.; Isoda, H.; Nakajima, M.; Ksouri, R. Formulation, physicochemical characterization, and anti - E. coli activity of food-grade nanoemulsions incorporating clove, cinnamon, and lavender essential oils. Food Chem. 2021, 359, 129963. [Google Scholar] [CrossRef] [PubMed]
- Hosny, K.M.; Alhakamy, N.A.; Sindi, A.M.; Khallaf, R.A. Coconut oil nanoemulsion loaded with a statin hypolipidemic drug for management of burns: Formulation and in vivo evaluation. Pharmaceutics 2020, 12, 1061. [Google Scholar] [CrossRef] [PubMed]
- Ojha, B.; Jain, V.K.; Gupta, S.; Talegaonkar, S.; Jain, K. Nanoemulgel: A promising novel formulation for treatment of skin ailments. Polym. Bull. 2022, 79, 4441–4465. [Google Scholar] [CrossRef]
- Sengupta, P.; Chatterjee, B. Potential and future scope of nanoemulgel formulation for topical delivery of lipophilic drugs. Int. J. Pharm. 2017, 526, 353–365. [Google Scholar] [CrossRef]
- Roy, A.; Nishchaya, K.; Rai, V.K. Nanoemulsion-based dosage forms for the transdermal drug delivery applications: A review of recent advances. Expert Opin. Drug Deliv. 2022, 19, 303–319. [Google Scholar] [CrossRef]
- Rai, V.K.; Mishra, N.; Yadav, K.S.; Yadav, N.P. Nanoemulsion as pharmaceutical carrier for dermal and transdermal drug delivery: Formulation development, stability issues, basic considerations and applications. J. Control. Release 2018, 270, 203–225. [Google Scholar] [CrossRef]
- Rai, V.K.; Sharma, A.; Thakur, A. Quality Control of Nanoemulsion: By PDCA Cycle and 7QC Tools. Curr. Drug Deliv. 2021, 18, 1244–1255. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Hossain, M.E.; Mannan Mithi, F.; Ahmed, M.; Saldías, M.; Akkol, E.K.; Sobarzo-Sánchez, E. Multifunctional therapeutic potential of phytocomplexes and natural extracts for antimicrobial properties. Antibiotics 2021, 10, 1076. [Google Scholar] [CrossRef]
- Dabas, A.; Yadav, P.; Geetanjali, Singh, R. Role of Herbal Medicine in Boosting Immune System. In Role of Herbal Medicines: Management of Lifestyle Diseases; Dhara, A.K., Mandal, S.C., Eds.; Springer Nature: Singapore, Singapore, 2024; pp. 389–401. [Google Scholar] [CrossRef]
- Srivastava, B.B.; Ripanda, A.S.; Mwanga, H.M. Ethnomedicinal, phytochemistry and antiviral potential of turmeric (Curcuma longa). Compounds 2022, 2, 200–221. [Google Scholar] [CrossRef]
- Bui, V.C.; Le, T.T.; Nguyen, T.H.; Pham, N.T.; Vu, H.D.; Nguyen, X.C.; De Tran, Q.; Hoang, T.; Le Dang, Q.; Lam, T.D. Curcumin-removed turmeric oleoresin nano-emulsion as a novel botanical fungicide to control anthracnose (Colletotrichum gloeosporioides) in litchi. Green Process. Synth. 2021, 10, 729–741. [Google Scholar] [CrossRef]
- Prakash, V.; Akhtar, S.; Kumar, J.; Mishra, S.K.; Pandey, R.R. Neem: The multifaceted and versatile tree. In Curr. Trends Med Chem., Pandey, R.R.; Kumar, J., Ed.; Thanuj International Publishers: India, 2022; pp. 127–138. [Google Scholar]
- Kumar, S.; Singh, N.; Devi, L.S.; Kumar, S.; Kamle, M.; Kumar, P.; Mukherjee, A. Neem oil and its nanoemulsion in sustainable food preservation and packaging: Current status and future prospects. J. Agric. Food Res. 2022, 7, 100254. [Google Scholar] [CrossRef]
- Algahtani, M.S.; Ahmad, M.Z.; Shaikh, I.A.; Abdel-Wahab, B.A.; Nourein, I.H.; Ahmad, J. Thymoquinone loaded topical nanoemulgel for wound healing: formulation design and in-vivo evaluation. Molecules 2021, 26, 3863. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.Y.; Parimalam, M.; Kumar, D.; Joseph, E.; David, D.; Vinolia, R. Screening of phytochemicals, Invitro assessment of antioxidant, anti-inflammatory, Tlc profiling and anticancer activity of Aegle Marmelos (L.) leaves. Ann. Rom. Soc. Cell Biol. 2021, 18061–18071. [Google Scholar]
- Ahmed, M.; Marrez, D.A.; Mohamed Abdelmoeen, N.; Abdelmoneem Mahmoud, E.; Ali, M.A.; Decsi, K.; Tóth, Z. Studying the antioxidant and the antimicrobial activities of leaf successive extracts compared to the green-chemically synthesized silver nanoparticles and the crude aqueous extract from Azadirachta indica. Processes 2023, 11, 1644. [Google Scholar] [CrossRef]
- Kaddour, S.M.; Arrar, L.; Baghiani, A. Anti-Inflammatory Potential Evaluation (In-Vitro and In-Vivo) of Arthrophytum scoparium Aerial Part. J. Drug Deliv. Ther. 2020, 10, 213–218. [Google Scholar] [CrossRef]
- Saleem, W.; Sarfraz, B.; Mazhar, S. Combined Effect of Honey, Neem (Azadirachta Indica), and Turmeric against Staphylococcus Aureus and E. Coli Isolated from a Clinical Wound Sample. BioScientific Review 2022, 4, 21–44. [Google Scholar] [CrossRef]
- Elsewedy, H.S.; Shehata, T.M.; Soliman, W.E. Tea tree oil nanoemulsion-based hydrogel vehicle for enhancing topical delivery of neomycin. Life 2022, 12, 1011. [Google Scholar] [CrossRef]
- Thomas, L.; Zakir, F.; Mirza, M.A.; Anwer, M.K.; Ahmad, F.J.; Iqbal, Z. Development of Curcumin loaded chitosan polymer based nanoemulsion gel: In vitro, ex vivo evaluation and in vivo wound healing studies. Int. J. Biol Macromol. 2017, 101, 569–579. [Google Scholar] [CrossRef]
- Deore, S.K.; Surawase, R.K.; Maru, A. Formulation and evaluation of o/w nanoemulsion of ketoconazole. Res. J. Pharma. Dosage Forms Tech. 2019, 11, 269–264. [Google Scholar] [CrossRef]
- Alkhanjaf, A.A.; Athar, M.T.; Ullah, Z.; Umar, A.; Shaikh, I.A. In Vitro and In Vivo Evaluation of a Nano-Tool Appended Oilmix (Clove and Tea Tree Oil) Thermosensitive Gel for Vaginal Candidiasis. J. Funct. Biomater. 2022, 13, 203. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Guo, G.; Wang, F.; Tao, H.; Yu, B.; Zhao, H.; Li, J.; Xu, M.; Tao, H.; Cui, B.; Wang, Y. Recombinant microgels utilized to formulate green Pickering emulsions. Food Hydrocoll. 2024, 150, 109678. [Google Scholar] [CrossRef]
- Saab, M.; Raafat, K.; El-Maradny, H. Transdermal delivery of capsaicin nanoemulgel: optimization, skin permeation and in vivo activity against diabetic neuropathy. Adv. Pharm. Bull. 2022, 12, 780. [Google Scholar] [CrossRef] [PubMed]
- Sunthar, T.P.; Marin, E.; Boschetto, F.; Zanocco, M.; Sunahara, H.; Ramful, R.; Kamei, K.; Zhu, W.; Pezzotti, G. Antibacterial and Antifungal properties of composite Polyethylene Materials reinforced with Neem and turmeric. Antibiotics 2020, 9, 857. [Google Scholar] [CrossRef]
- Poorani, G.; Uppuluri, S.; Uppuluri, K.B. Formulation, characterization, in vitro and in vivo evaluation of castor oil based self-nano emulsifying levosulpiride delivery systems. J. Microencapsul. 2016, 33, 535–543. [Google Scholar] [CrossRef]
- Soliman, W.E.; Shehata, T.M.; Mohamed, M.E.; Younis, N.S.; Elsewedy, H.S. Enhancement of curcumin anti-inflammatory effect via formulation into myrrh oil-based nanoemulgel. Polymers 2021, 13, 577. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Hussain, A.; Alshehri, S.; Imam, S.S.; Alnemer, U.A. Development and evaluations of transdermally delivered luteolin loaded cationic nanoemulsion: In vitro and ex vivo evaluations. Pharmaceutics 2021, 13, 1218. [Google Scholar] [CrossRef]
- Rehman, A.; Iqbal, M.; Khan, B.A.; Khan, M.K.; Huwaimel, B.; Alshehri, S.; Alamri, A.H.; Alzhrani, R.M.; Bukhary, D.M.; Safhi, A.Y.; Hosny, K.M. Fabrication, in vitro, and in vivo assessment of eucalyptol-loaded nanoemulgel as a novel paradigm for wound healing. Pharmaceutics 2022, 14, 1971. [Google Scholar] [CrossRef]
- Jain, P.; Soni, R.; Paswan, S.K.; Soni, P.K. Ketoconazole laden microemulsion based gel formulation against skin fungal infection. Int. J. App. Pharm. 2023, 15, 49–60. [Google Scholar] [CrossRef]
- Gul, U.; Khan, M.I.; Madni, A.; Sohail, M.F.; Rehman, M.; Rasul, A.; Peltonen, L. Olive oil and clove oil-based nanoemulsion for topical delivery of terbinafine hydrochloride: in vitro and ex vivo evaluation. Drug Deliv. 2022, 29, 600–612. [Google Scholar] [CrossRef]
- Chin, L.Y.; Tan, J.Y.; Choudhury, H.; Pandey, M.; Sisinthy, S.P.; Gorain, B. Development and optimization of chitosan coated nanoemulgel of telmisartan for intranasal delivery: A comparative study. J. Drug Deliv. Sci. Technol. 2021, 62, 102341. [Google Scholar] [CrossRef]
- Shome, S.; Talukdar, A.D.; Upadhyaya, H. Antibacterial activity of curcumin and its essential nanoformulations against some clinically important bacterial pathogens: A comprehensive review. Biotechnol. Appl. Biochem. 2022, 69, 2357–2386. [Google Scholar] [CrossRef]
- Arun, K.J.; Khan, A.; Mahato, N.; Devi, J.; Jawahar, S.S. Sulindac-Loaded Topical Nanoemulgel Formulation and Optimization. Bull. Env. Pharmacol. Life Sci. 2023, 12, 237–245. [Google Scholar]
- Ali, J.; ur Rehman, I.; Bangash, J.A. Phytochemicals content and in-vitro antioxidant properties of Azadirachta indica seeds, leaves and twigs prepared from different extraction techniques. Int. J. Eng. Sci. Technol. 2022, 14, 12–20. [Google Scholar] [CrossRef]
- Khoshraftar, Z.; Shamel, A.; Safekordi, A.A.; Zaefizadeh, M. Chemical composition of an insecticidal hydroalcoholic extract from tea leaves against green peach aphid. Int. J. Environ. Sci. Technol. 2019, 16, 7583–90. [Google Scholar] [CrossRef]
- Salem, W.H.; Moussa, T.A.; Abou Abouraya, N.L. Cytotoxicity and antibacterial activity of bleached turmeric hydro-alcoholic extract versus sodium hypochlorite as an endodontic irrigant: in vitro study. Braz. Dent. Sci. 2022, 25, e3566. [Google Scholar] [CrossRef]
- Rafiq, M.; Tunio, A.A.; Qureshi, A.S.; Rehman, T.; Bhutto, M.A.; Lasharı, Z. Determination of Phytochemicals, Antimicrobial, Antioxidant and Allelopathic Effects of Fagonia cretica L., collected from Jamshoro, Pakistan. Yuzuncu Yıl University J. Agric. Sci. 2022, 32, 785–794. [Google Scholar] [CrossRef]
- Donio, M.B.; Remya, R.; Michaelbabu, M.; Uma, G.; Citarasu, T. Characterization and biomedical application of Tetradecamethylcycloheptasiloxane, a silicone-type biosurfactant produced by Streptomyces castaneoglobisporus AJ9 isolated from solar salt works. Indian J. Geomarine Sci. 2022, 51, 694–704. [Google Scholar] [CrossRef]
- Khan, T.A.; Azad, A.K.; Fuloria, S.; Nawaz, A.; Subramaniyan, V.; Akhlaq, M.; Safdar, M.; Sathasivam, K.V.; Sekar, M.; Porwal, O.; Meenakshi, D.U. Chitosan-coated 5-fluorouracil incorporated emulsions as transdermal drug delivery matrices. Polymers 2021, 13, 3345. [Google Scholar] [CrossRef]
- Malik, M.R.; Al-Harbi, F.F.; Nawaz, A.; Amin, A.; Farid, A.; Mohaini, M.A.; Alsalman, A.J.; Hawaj, M.A.; Alhashem, Y.N. Formulation and characterization of chitosan-decorated multiple nanoemulsion for topical delivery in vitro and ex vivo. Molecules 2022, 27, 3183. [Google Scholar] [CrossRef]
- Ullah, N.; Amin, A.; Farid, A.; Selim, S.; Rashid, S.A.; Aziz, M.I.; Kamran, S.H.; Khan, M.A.; Rahim Khan, N.; Mashal, S.; Mohtasheemul Hasan, M. Development and evaluation of essential oil-based nanoemulgel formulation for the treatment of oral bacterial infections. Gels 2023, 9, 252. [Google Scholar] [CrossRef]
- Le, T.T.; Nguyen, T.K.; Nguyen, V.M.; Dao, T.C.; Nguyen, H.B.; Dang, C.T.; Le, T.B.; Nguyen, T.K.; Nguyen, P.T.; Dang, L.H.; Doan, V.M. Development and characterization of a hydrogel containing curcumin-loaded nanoemulsion for enhanced in vitro antibacterial and in vivo wound healing. Molecules 2023, 28, 6433. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Azad, A.K.; Safdar, M.; Nawaz, A.; Akhlaq, M.; Paul, P.; Hossain, M.K.; Rahman, M.H.; Baty, R.S. , El-Kott, A.F.; Kamel, M. Synthesis and characterization of acrylamide/acrylic acid Co-polymers and glutaraldehyde crosslinked pH-sensitive hydrogels. Gels 2022, 8, 47. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, M.; Mlynarczyk, D.T.; Dlugaszewska, J. Wondrous Yellow Molecule: Are Hydrogels a Successful Strategy to Overcome the Limitations of Curcumin? Molecules 2024, 29, 1757. [Google Scholar] [CrossRef] [PubMed]
- Latif, M.S.; Azad, A.K.; Nawaz, A.; Rashid, S.A.; Rahman, M.H.; Al Omar, S.Y.; Bungau, S.G.; Aleya, L.; Abdel-Daim, M.M. Ethyl cellulose and hydroxypropyl methyl cellulose blended methotrexate-loaded transdermal patches: in vitro and ex vivo. Polymers 2021, 13, 3455. [Google Scholar] [CrossRef]
- Scomoroscenco, C.; Teodorescu, M.; Raducan, A.; Stan, M.; Voicu, S.N.; Trica, B.; Ninciuleanu, C.M.; Nistor, C.L.; Mihaescu, C.I.; Petcu, C.; Cinteza, L.O. Novel gel microemulsion as topical drug delivery system for curcumin in dermatocosmetics. Pharmaceutics 2021, 13, 505. [Google Scholar] [CrossRef] [PubMed]
- Yeo, E.; Chieng, C.J.; Choudhury, H.; Pandey, M.; Gorain, B. Tocotrienols-rich naringenin nanoemulgel for the management of diabetic wound: Fabrication, characterization and comparative in vitro evaluations. Curr. Res. Pharmacol. Drug Discov. 2021, 2, 100019. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M. Comparison of antibacterial activity of nanocurcumin with bulk curcumin. ACS omega. 2022, 7, 46494–46500. [Google Scholar] [CrossRef]
- Sohail, M.; Naveed, A.; Abdul, R.; Khan, H.M.; Khan, H. An approach to enhanced stability: Formulation and characterization of Solanum lycopersicum derived lycopene based topical emulgel. Saudi Pharm. J. 2018, 26, 1170–1177. [Google Scholar] [CrossRef]
Figure 1.
Antioxidant activity of the extracts. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same concentration. Also, ascorbic acid was used as standard.
Figure 1.
Antioxidant activity of the extracts. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same concentration. Also, ascorbic acid was used as standard.
Figure 2.
Anti-inflammatory activity of the extracts. Antioxidant activity of the extracts. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same concentration. Also, ascorbic acid was used as standard.
Figure 2.
Anti-inflammatory activity of the extracts. Antioxidant activity of the extracts. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same concentration. Also, ascorbic acid was used as standard.
Figure 3.
Antimicrobial Activity of the Extracts.
Figure 3.
Antimicrobial Activity of the Extracts.
Figure 4.
Size distribution by intensity of the nanoemulsion understudy. (a) Nano-Emulsion A1, that contains 2% surfactant, distribution curve. (b) The correlation cofficient of Nano-Emulsion A1 that shows the cumulants data fit. (c) Nano-Emulsion B1, that contains 4% surfactant, distribution curve. (b) The correlation cofficient of Nano-Emulsion B1 that shows the cumulants data fit.
Figure 4.
Size distribution by intensity of the nanoemulsion understudy. (a) Nano-Emulsion A1, that contains 2% surfactant, distribution curve. (b) The correlation cofficient of Nano-Emulsion A1 that shows the cumulants data fit. (c) Nano-Emulsion B1, that contains 4% surfactant, distribution curve. (b) The correlation cofficient of Nano-Emulsion B1 that shows the cumulants data fit.
Figure 5.
FT-IR spectra of Turmeric, neem extract, Tween 80, PEG600, Olive oil, Nanoemulsion A1 and Nanoemulsion B1 respectively.
Figure 5.
FT-IR spectra of Turmeric, neem extract, Tween 80, PEG600, Olive oil, Nanoemulsion A1 and Nanoemulsion B1 respectively.
Figure 6.
Impact of polymer content on nanoemulgel viscosity and spreadability. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same analysis.
Figure 6.
Impact of polymer content on nanoemulgel viscosity and spreadability. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same analysis.
Figure 7.
FT-IR spectra of Nanoemulsion A1, Nanoemulsion B1, Carbopol 934, Nanoemulgel A1, Nanoemulgel B1 respectively.
Figure 7.
FT-IR spectra of Nanoemulsion A1, Nanoemulsion B1, Carbopol 934, Nanoemulgel A1, Nanoemulgel B1 respectively.
Figure 8.
In-vitro release study of nanoemulgel. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same analysis.
Figure 8.
In-vitro release study of nanoemulgel. Different alphabet superscript indicate that values differ significantly (p < 0.05) at the same analysis.
Figure 9.
Antimicrobial activity of turmeric and neem leaves based nanoemulgel.
Figure 9.
Antimicrobial activity of turmeric and neem leaves based nanoemulgel.
Figure 10.
Formulation of Nanoemulsion using turmeric and neem leaf.
Figure 10.
Formulation of Nanoemulsion using turmeric and neem leaf.
Table 1.
Antioxidant Activity of the Extract.
Table 1.
Antioxidant Activity of the Extract.
| Concentration (µg/ml). |
Standard |
% RSA (Neem leaves extract) |
% RSA (Turmeric extract) |
% RSA (Turmeric and Neem leaves extract) |
| 20 |
41.8±0.92 |
47.6±0.47 |
50.2±0.59 |
54.1±0.57 |
| 40 |
53.4±1.02 |
58.45±0.7 |
65.4±0.21 |
67.6±0.53 |
| 60 |
68.8±1.26 |
63.71±0.69 |
71.2±0.42 |
73.8±0.49 |
| 80 |
78.6±0.65 |
79.2±0.45 |
84.7±0.68 |
86.7±0.11 |
| 100 |
83.3±0.47 |
92.87±0.54 |
93.7±0.28 |
94.1±0.11 |
Table 2.
Antimicrobial Activity of the Extracts. .
Table 2.
Antimicrobial Activity of the Extracts. .
| Sr. No. |
Pathogens |
Zone of Inhibition (cm) |
| Standard* |
Neem leaves extract |
Turmeric extract |
Turmeric and Neem leaf extract |
| 1 |
Escherichia coli |
1.9± 1.041c
|
1.6± 0.194b
|
1.3± 1.053a
|
2.4± 1.064d
|
| 2 |
Staphylococcus aureus |
- |
1.2± 0.364a
|
2.5± 0.684c |
1.6± 0.931b
|
| 3 |
Pseudomonas aeruginosa |
- |
1.8± 0.743a
|
2.4± 0.674a
|
2.0± 1.041b
|
| 4 |
Salmonella typhi |
- |
1.2± 0.764a
|
1.2± 0.640a
|
1.4± 0.131b
|
| 5 |
Bacillus subtilis |
1.5± 0.661b
|
1.2± 1.163b
|
1.0± 0.319a
|
1.4± 0.452b
|
| 6 |
Bacillus cereus |
3.4± 0.423c
|
1.3± 0.390a
|
2.4± 0.043b
|
3.6± 0.672d
|
| 7 |
Aspergillus niger |
1.0± 0.981c
|
0.7± 0.714ab
|
0.5± 0.762a
|
0.8± 0.925ab
|
| 8 |
Candida albicans |
1.1± 0.584c
|
0.7± 0.261ab
|
0.6± 0.431a
|
0.9± 0.335ab
|
Table 3.
Physical evaluations of turmeric and neem leaf based nanoemulsion.
Table 3.
Physical evaluations of turmeric and neem leaf based nanoemulsion.
| Formulation Batch |
Colour |
Phase Separation |
Appearance |
Homogeneity |
pH Range |
| A1 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
5.4± 0.13 |
| A2 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
5± 0.91 |
| A3 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
5.7± 0.62 |
| A4 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
5.7± 0.73 |
| A5 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
6.1± 0.44 |
| A6 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
6.3± 0.19 |
| B1 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
5± 0.53 |
| B2 |
Light Yellowish |
Nil |
Pellucid |
Homogeneous |
5± 0.69 |
| B3 |
Yellowish |
Nil |
Pellucid |
Homogeneous |
5.2± 0.33 |
| B4 |
Yellowish |
Nil |
Pellucid |
Homogeneous |
6.2± 0.78 |
| B5 |
Yellowish |
Nil |
Pellucid |
Homogeneous |
6.6± 0.57 |
| B6 |
Yellowish |
Nil |
Pellucid |
Homogeneous |
6.8± 0.84 |
| *Values are expressed as Mean ± SD. |
Table 4.
Droplet sizes and PDI values of nanoemulsions.
Table 4.
Droplet sizes and PDI values of nanoemulsions.
| Formulation Batch |
Average droplet size (nm) |
PDI |
| A1 |
78.87± 2.67 |
0.4148± 0.13 |
| A2 |
102.3± 1.83 |
0.4355± 0.098 |
| A3 |
119.6± 1.15 |
0.3261± 0.055 |
| A4 |
139.3± 0.81 |
0.256± 0.032 |
| A5 |
242.3± 2.56 |
0.4787± 0.023 |
| A6 |
278.1± 2.31 |
0.2664± 2.21 |
| B1 |
80.4± 2.35 |
0.1569± 0.17 |
| B2 |
244.8± 0.89 |
0.65772 ± 2.38 |
| B3 |
308.9± 2.53 |
0.2542± 2.01 |
| B4 |
472.1± 2.82 |
0.7557± 1.12 |
| B5 |
811.5± 1.32 |
0.661± 0.79 |
| B6 |
1768± 1.89 |
0.3541± 0.41 |
| *Values are expressed as Mean ± SD. |
Table 5.
Physical evaluations of nanoemulgel.
Table 5.
Physical evaluations of nanoemulgel.
| Formulation Batch |
Color |
Consistency |
Homogeneity |
pH |
Viscosity (mPa·S) |
Spreadability (g·cm/s |
| F1 |
Yellowish |
Good |
Excellent |
6.13± 0.01 |
48,072 ± 5 |
32 ± 0.5 |
| F2 |
Yellowish |
Good |
Excellent |
6.3 ± 0.05 |
54,284 ± 7 |
34 ± 0.2 |
| F3 |
Yellowish |
Good |
Excellent |
6.8 ± 0.05 |
67,3094± 3 |
33 ± 0.3 |
| F4 |
Yellowish |
Good |
Excellent |
6.6 ± 0.03 |
73,127± 8 |
30 ± 0.5 |
Table 6.
Formulation of extract-loaded Nanoemulgel.
Table 6.
Formulation of extract-loaded Nanoemulgel.
| SI no |
Oil phase(% v/v)
|
Turmeric extract(%w/v)
|
Neem leaves extract(%w/v)
|
Surfactant (% v/v) |
Co surfactant (% v/v) |
Carbopol 934 (% w/w) |
Triethanolamine |
Distilled Water(% v/v)
|
| A1 |
1 |
1 |
1 |
2 |
1 |
1 |
q.s. |
Upto 50 ml |
| B1 |
1 |
1 |
1 |
4 |
1 |
1 |
q.s. |
Upto 50 ml |
Table 7.
Ex-vivo mucoadhesive study of nanoemulgel.
Table 7.
Ex-vivo mucoadhesive study of nanoemulgel.
| Formulation |
Viscosity (mPa·S) |
Mucoadhesive force (N) |
| Commercial gel |
34,284 ± 7 |
0.748 ± 0.033 |
| A1 (Turmeric and Neem based gel) |
51,317 ± 8 |
2.235 ± 0.120 |
| B1 (Turmeric and Neem based gel) |
54,251 ± 4 |
2.421 ± 0.128 |
Table 8.
Antimicrobial activity of nanoemulgel.
Table 8.
Antimicrobial activity of nanoemulgel.
| Sr. No. |
Pathogens |
Zone of Inhibition (cm) |
| Commercial Gel |
Turmeric & Neem based nanoemulgel (A1) |
Turmeric & Neem nanoemulgel (B1) |
| Antibacterial activity |
| 1 |
Escherichia coli |
- |
3.2 ± 0.672a
|
3.4± 0.762b
|
| 2 |
Staphylocccus aureus |
- |
3.4± 0.661a
|
3.3± 0.931a
|
| 3 |
Pseudomonas aeroginosa |
- |
1.3± 0.473a
|
1.3 ± 0.431a
|
| 4 |
Salmonella typhi |
- |
1.3± 0.264a
|
1.3± 0.440a
|
| 5 |
Bacillus subtilis |
- |
1.2± 0.163a
|
1.1± 0.319b
|
| 6 |
Bacillus cereus |
- |
3.4± 0.290a
|
3.2± 0.843a
|
| Antifungal activity |
| 7 |
Aspergillus niger |
1.4± 0.294 a
|
2.4± 0.416a
|
2.7± 0.649b
|
| 8 |
Candida albicans |
1.4± 0.682 a
|
3.2± 0.068b
|
2.8± 0.301a
|
Table 9.
Stability studies.
Table 9.
Stability studies.
| Formulation Batch |
Colour |
Consistency |
Homogeneity |
pH |
Viscosity (mPa·S) |
Spreadability (g·cm/s) |
| A1 |
Yellowish |
Good |
Excellent |
6.1±0.4 |
58,10± 2.00 |
31 ±3 |
| B1 |
Yellowish |
Good |
Excellent |
6.3±0.2 |
61,11±0.04 |
33± 2 |
Table 10.
Preparation and optimization of Nanoemulsion using turmeric and neem leaf extract.
Table 10.
Preparation and optimization of Nanoemulsion using turmeric and neem leaf extract.
| SI no |
Oil phase(% v/v) |
Turmeric extract (%w/w) |
Neem leaves extract(%w/w) |
Surfactant + Co-surfactant (2:1) |
Distilled Water (% v/v) |
| Surfactant (% v/v) |
Co surfactant (% v/v) |
| A1 |
1 |
1 |
1 |
2 |
1 |
Upto 50 ml |
| A2 |
1 |
1 |
1 |
3 |
1.5 |
Upto 50 ml |
| A3 |
1 |
1 |
1 |
4 |
2 |
Upto 50 ml |
| A4 |
1 |
1 |
1 |
5 |
2.5 |
Upto 50 ml |
| A5 |
1 |
1 |
1 |
6 |
3 |
Upto 50 ml |
| A6 |
1 |
1 |
1 |
7 |
3.5 |
Upto 50 ml |
Table 11.
Preparation & optimization of Nanoemulsion using turmeric and neem leaf extract.
Table 11.
Preparation & optimization of Nanoemulsion using turmeric and neem leaf extract.
| SI no |
Oil phase(% v/v) |
Turmeric extract (%w/w) |
Neem leaves extract(%w/w) |
Surfactant + Co-surfactant(4:1) |
Distilled Water(% v/v) |
| Surfactant (% v/v) |
Co surfactant (% v/v) |
| B1 |
1 |
1 |
1 |
4 |
1 |
Upto 50 ml |
| B2 |
1 |
1 |
1 |
6 |
1.5 |
Upto 50 ml |
| B3 |
1 |
1 |
1 |
48 |
2 |
Upto 50 ml |
| B4 |
1 |
1 |
1 |
10 |
2.5 |
Upto 50 ml |
| B5 |
1 |
1 |
1 |
12 |
3 |
Upto 50 ml |
| B6 |
1 |
1 |
1 |
14 |
3.5 |
Upto 50 ml |
Table 12.
Composition of nanoemulgel.
Table 12.
Composition of nanoemulgel.
| Constituents |
F1 |
F2 |
F3 |
F4 |
| Carbopol 934 (% w/w) |
0.5 |
1 |
1.5 |
2 |
| Triethanolamine |
0.1 ml |
0.1 ml |
0.1 ml |
0.1 ml |
| Distilled water |
q.s. |
q.s. |
q.s. |
q.s. |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).