Submitted:
01 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
Introduction
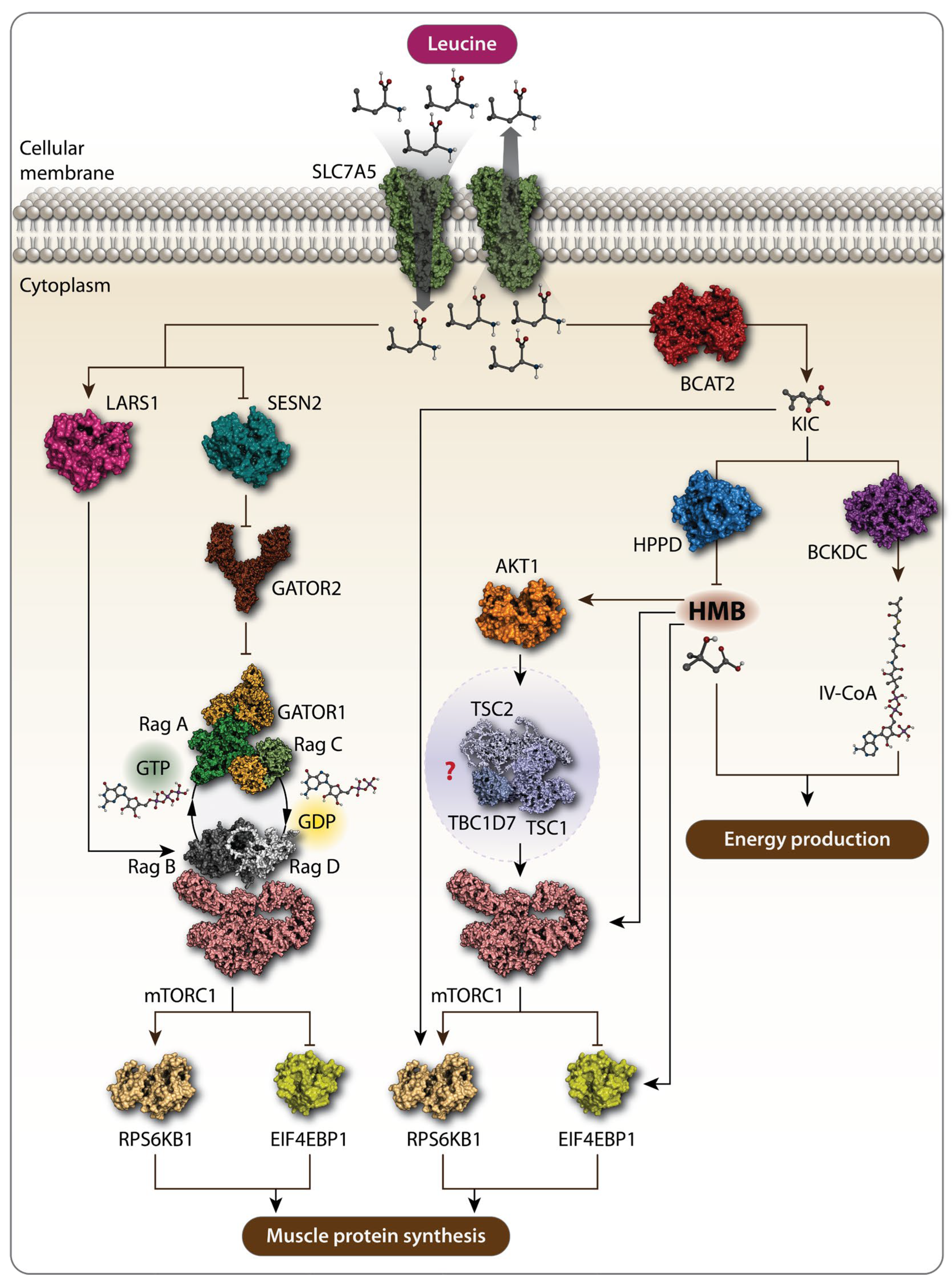
1. Leucine and Muscle Protein Synthesis: Mechanisms and Impacts
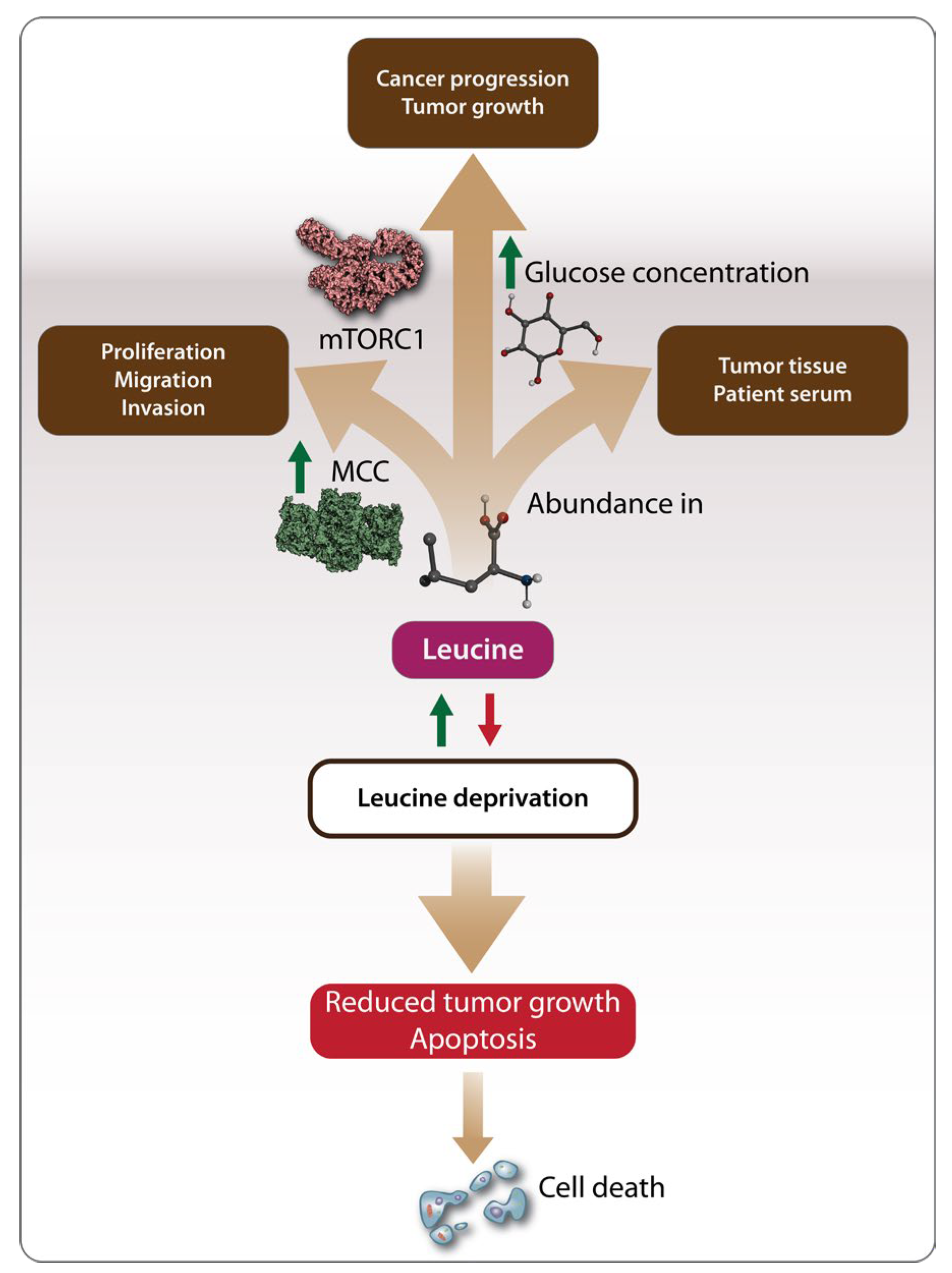
2. Leucine and Cancer Cachexia
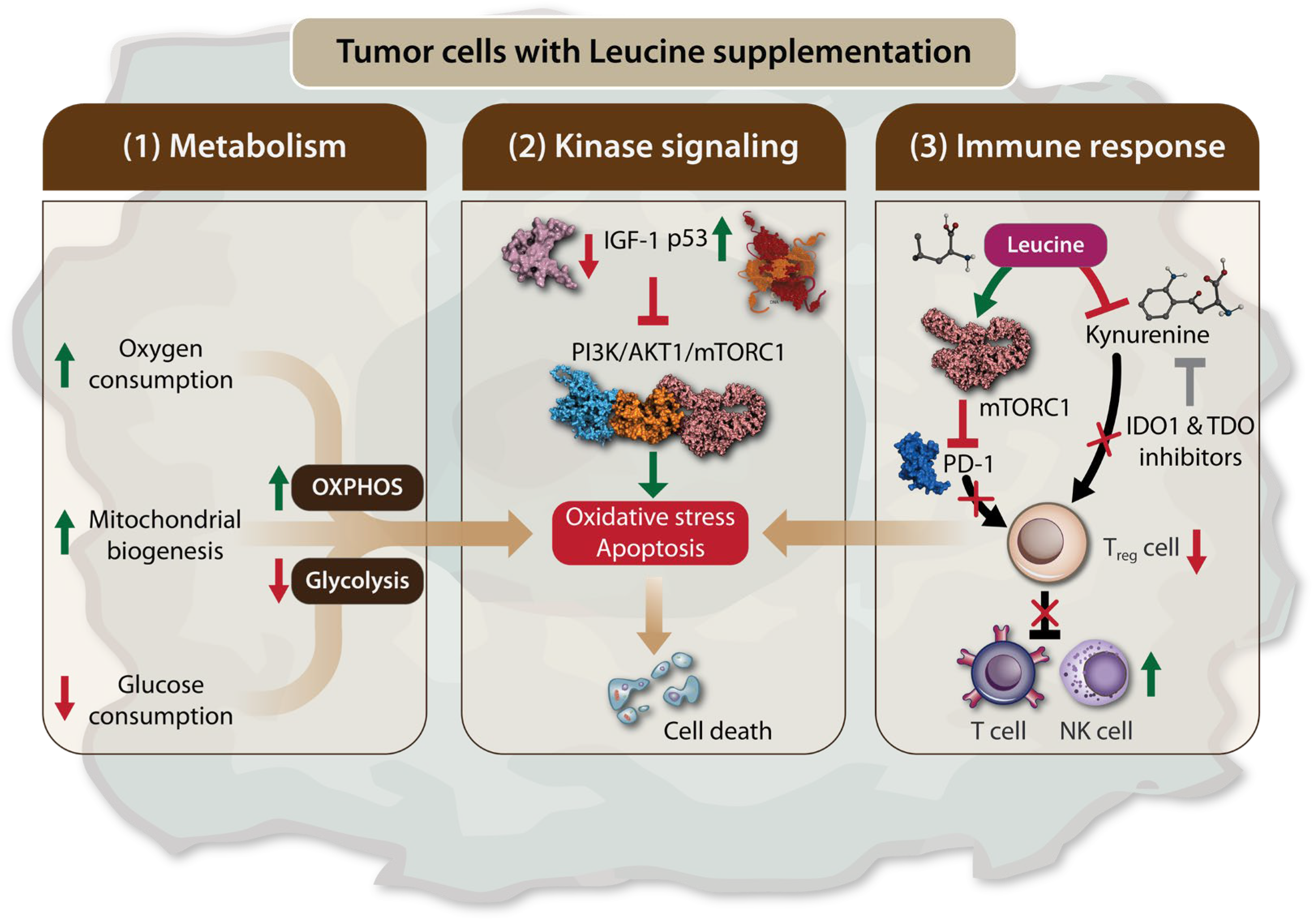
2.1. Anti-Tumor Effects of Leucine
2.2. Tumorigenic Effects of Leucine
Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary Requirements of “Nutritionally Non-Essential Amino Acids” by Animals and Humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Neinast, M.; Murashige, D.; Arany, Z. Branched Chain Amino Acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef] [PubMed]
- Lal, H.; Chugh, K. Metabolic and Regulatory Effects of Branched Chain Amino Acid Supplementation. Nutrition Research 1995, 15, 1717–1733. [Google Scholar] [CrossRef]
- May, M.E.; Buse, M.G. Effects of Branched-Chain Amino Acids on Protein Turnover. Diabetes Metab. Rev. 1989, 5, 227–245. [Google Scholar] [CrossRef] [PubMed]
- Nelson, A.R.; Phillips, S.M.; Stellingwerff, T.; Rezzi, S.; Bruce, S.J.; Breton, I.; Thorimbert, A.; Guy, P.A.; Clarke, J.; Broadbent, S.; et al. A Protein–Leucine Supplement Increases Branched-Chain Amino Acid and Nitrogen Turnover But Not Performance. Medicine & Science in Sports & Exercise 2012, 44, 57–68. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Burd, N.A.; Phillips, S.M. Nutritional Regulation of Muscle Protein Synthesis with Resistance Exercise: Strategies to Enhance Anabolism. Nutr Metab (Lond) 2012, 9, 40. [Google Scholar] [CrossRef]
- Jackman, S.R.; Witard, O.C.; Philp, A.; Wallis, G.A.; Baar, K.; Tipton, K.D. Branched-Chain Amino Acid Ingestion Stimulates Muscle Myofibrillar Protein Synthesis Following Resistance Exercise in Humans. Front. Physiol. 2017, 8, 390. [Google Scholar] [CrossRef]
- Wang, C.; Guo, F. Branched Chain Amino Acids and Metabolic Regulation. Chin. Sci. Bull. 2013, 58, 1228–1235. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-Chain Amino Acids in Metabolic Signalling and Insulin Resistance. Nat Rev Endocrinol 2014, 10, 723–736. [Google Scholar] [CrossRef]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-Chain Amino Acid Supplementation and Exercise-Induced Muscle Damage in Exercise Recovery: A Meta-Analysis of Randomized Clinical Trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef]
- Blomstrand, E. A Role for Branched-Chain Amino Acids in Reducing Central Fatigue. The Journal of Nutrition 2006, 136, 544S–547S. [Google Scholar] [CrossRef] [PubMed]
- Gervasi, M.; Sisti, D.; Amatori, S.; Donati Zeppa, S.; Annibalini, G.; Piccoli, G.; Vallorani, L.; Benelli, P.; Rocchi, M.B.L.; Barbieri, E.; et al. Effects of a Commercially Available Branched-Chain Amino Acid-Alanine-Carbohydrate-Based Sports Supplement on Perceived Exertion and Performance in High Intensity Endurance Cycling Tests. Journal of the International Society of Sports Nutrition 2020, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.R. Branched-Chain Amino Acids and Muscle Protein Synthesis in Humans: Myth or Reality? Journal of the International Society of Sports Nutrition 2017, 14, 30. [Google Scholar] [CrossRef]
- Global BCAA Market [2024-2032] | Advanced Research Report.
- Gleeson, M. Interrelationship between Physical Activity and Branched-Chain Amino Acids. The Journal of Nutrition 2005, 135, 1591S–1595S. [Google Scholar] [CrossRef]
- Marcon, M.; Zanella, P.B. The Effect of Branched-Chain Amino Acids Supplementation in Physical Exercise: A Systematic Review of Human Randomized Controlled Trials. Science & Sports 2022, S0765159721002008. [Google Scholar] [CrossRef]
- Plotkin, D.L.; Delcastillo, K.; Van Every, D.W.; Tipton, K.D.; Aragon, A.A.; Schoenfeld, B.J. Isolated Leucine and Branched-Chain Amino Acid Supplementation for Enhancing Muscular Strength and Hypertrophy: A Narrative Review. International Journal of Sport Nutrition and Exercise Metabolism 2021, 31, 292–301. [Google Scholar] [CrossRef]
- Han, L.; Dong, L.; Leung, K.; Zhao, Z.; Li, Y.; Gao, L.; Chen, Z.; Xue, J.; Qing, Y.; Li, W.; et al. METTL16 Drives Leukemogenesis and Leukemia Stem Cell Self-Renewal by Reprogramming BCAA Metabolism. Cell Stem Cell 2023, 30, 52–68.e13. [Google Scholar] [CrossRef]
- Lee, J.H.; Cho, Y.; Kim, J.H.; Kim, J.; Nam, H.Y.; Kim, S.W.; Son, J. Branched-Chain Amino Acids Sustain Pancreatic Cancer Growth by Regulating Lipid Metabolism. Exp Mol Med 2019, 51, 1–11. [Google Scholar] [CrossRef]
- Columbus, D.A.; Fiorotto, M.L.; Davis, T.A. Leucine Is a Major Regulator of Muscle Protein Synthesis in Neonates. Amino Acids 2015, 47, 259–270. [Google Scholar] [CrossRef]
- Kang, M.C. Muscle Protein Metabolism in Critically Illness. Surg Metab Nutr 2020, 11, 35–39. [Google Scholar] [CrossRef]
- Zaromskyte, G.; Prokopidis, K.; Ioannidis, T.; Tipton, K.D.; Witard, O.C. Evaluating the Leucine Trigger Hypothesis to Explain the Post-Prandial Regulation of Muscle Protein Synthesis in Young and Older Adults: A Systematic Review. Front. Nutr. 2021, 8, 685165. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of Leucine and Its Metabolite Β-hydroxy-β-methylbutyrate on Human Skeletal Muscle Protein Metabolism. The Journal of Physiology 2013, 591, 2911–2923. [Google Scholar] [CrossRef] [PubMed]
- Ananieva, E.A.; Powell, J.D.; Hutson, S.M. Leucine Metabolism in T Cell Activation: mTOR Signaling and Beyond. Advances in Nutrition 2016, 7, 798S–805S. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Li, F.; Song, B.; Zheng, C.; Zhong, Y.; Xu, K.; Kong, X.; Yin, Y.; Wang, W.; Shu, G. β-Hydroxy-β-Methyl Butyrate, but Not α-Ketoisocaproate and Excess Leucine, Stimulates Skeletal Muscle Protein Metabolism in Growing Pigs Fed Low-Protein Diets. Journal of Functional Foods 2019, 52, 34–42. [Google Scholar] [CrossRef]
- Escobar, J.; Frank, J.W.; Suryawan, A.; Nguyen, H.V.; Van Horn, C.G.; Hutson, S.M.; Davis, T.A. Leucine and α-Ketoisocaproic Acid, but Not Norleucine, Stimulate Skeletal Muscle Protein Synthesis in Neonatal Pigs. The Journal of Nutrition 2010, 140, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Pisati, R.; Rondanelli, M.; Caccialanza, R. Whey Protein, Leucine- and Vitamin-D-Enriched Oral Nutritional Supplementation for the Treatment of Sarcopenia. Nutrients 2022, 14, 1524. [Google Scholar] [CrossRef]
- Gielen, E.; Beckwée, D.; Delaere, A.; De Breucker, S.; Vandewoude, M.; Bautmans, I.; the Sarcopenia Guidelines Development Group of the Belgian Society of Gerontology and Geriatrics (BSGG); Bautmans, I. ; Beaudart, C.; Beckwée, D.; et al. Nutritional Interventions to Improve Muscle Mass, Muscle Strength, and Physical Performance in Older People: An Umbrella Review of Systematic Reviews and Meta-Analyses. Nutrition Reviews 2021, 79, 121–147. [Google Scholar] [CrossRef]
- Martínez-Arnau, F.M.; Fonfría-Vivas, R.; Buigues, C.; Castillo, Y.; Molina, P.; Hoogland, A.J.; van Doesburg, F.; Pruimboom, L.; Fernández-Garrido, J.; Cauli, O. Effects of Leucine Administration in Sarcopenia: A Randomized and Placebo-Controlled Clinical Trial. Nutrients 2020, 12, 932. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Bise, T.; Shimazu, S.; Tanoue, M.; Tomioka, Y.; Araki, M.; Nishino, T.; Kuzuhara, A.; Takatsuki, F. Effects of a Leucine-Enriched Amino Acid Supplement on Muscle Mass, Muscle Strength, and Physical Function in Post-Stroke Patients with Sarcopenia: A Randomized Controlled Trial. Nutrition 2019, 58, 1–6. [Google Scholar] [CrossRef]
- Bodine, S.C. The Role of mTORC1 in the Regulation of Skeletal Muscle Mass. Fac Rev 2022, 11. [Google Scholar] [CrossRef]
- Suryawan, A.; Rudar, M.; Fiorotto, M.L.; Davis, T.A. Differential Regulation of mTORC1 Activation by Leucine and β-Hydroxy-β-Methylbutyrate in Skeletal Muscle of Neonatal Pigs. Journal of Applied Physiology 2020, 128, 286–295. [Google Scholar] [CrossRef]
- Akbay, B.; Shmakova, A.; Vassetzky, Y.; Dokudovskaya, S. Modulation of mTORC1 Signaling Pathway by HIV-1. Cells 2020, 9, 1090–1090. [Google Scholar] [CrossRef]
- Nicklin, P.; Bergman, P.; Zhang, B.; Triantafellow, E.; Wang, H.; Nyfeler, B.; Yang, H.; Hild, M.; Kung, C.; Wilson, C.; et al. Bidirectional Transport of Amino Acids Regulates mTOR and Autophagy. Cell 2009, 136, 521–534. [Google Scholar] [CrossRef] [PubMed]
- Chantranupong, L.; Wolfson, R.L.; Orozco, J.M.; Saxton, R.A.; Scaria, S.M.; Bar-Peled, L.; Spooner, E.; Isasa, M.; Gygi, S.P.; Sabatini, D.M. The Sestrins Interact with GATOR2 to Negatively Regulate the Amino-Acid-Sensing Pathway Upstream of mTORC1. Cell Reports 2014, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Han, J.M.; Jeong, S.J.; Park, M.C.; Kim, G.; Kwon, N.H.; Kim, H.K.; Ha, S.H.; Ryu, S.H.; Kim, S. Leucyl-tRNA Synthetase Is an Intracellular Leucine Sensor for the mTORC1-Signaling Pathway. Cell 2012, 149, 410–424. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Ro, S.-H.; Kim, M.; Park, H.-W.; Semple, I.A.; Park, H.; Cho, U.-S.; Wang, W.; Guan, K.-L.; Karin, M.; et al. Sestrin2 Inhibits mTORC1 through Modulation of GATOR Complexes. Scientific Reports 2015, 5, 9502–9502. [Google Scholar] [CrossRef]
- Parmigiani, A.; Nourbakhsh, A.; Ding, B.; Wang, W.; Kim, Y.C.; Akopiants, K.; Guan, K.-L.; Karin, M.; Budanov, A.V. Sestrins Inhibit mTORC1 Kinase Activation through the GATOR Complex. Cell Reports 2014, 9, 1281–1291. [Google Scholar] [CrossRef]
- Saxton, R.A.; Knockenhauer, K.E.; Wolfson, R.L.; Chantranupong, L.; Pacold, M.E.; Wang, T.; Schwartz, T.U.; Sabatini, D.M. Structural Basis for Leucine Sensing by the Sestrin2-mTORC1 Pathway. Science 2016, 351, 53–58. [Google Scholar] [CrossRef]
- Wolfson, R.L.; Chantranupong, L.; Saxton, R.A.; Shen, K.; Scaria, S.M.; Cantor, J.R.; Sabatini, D.M. Sestrin2 Is a Leucine Sensor for the mTORC1 Pathway. Science 2016, 351, 43–48. [Google Scholar] [CrossRef]
- Yang, M.; Lu, Y.; Piao, W.; Jin, H. The Translational Regulation in mTOR Pathway. Biomolecules 2022, 12, 802. [Google Scholar] [CrossRef]
- Moghei, M.; Tavajohi-Fini, P.; Beatty, B.; Adegoke, O.A.J. Ketoisocaproic Acid, a Metabolite of Leucine, Suppresses Insulin-Stimulated Glucose Transport in Skeletal Muscle Cells in a BCAT2-Dependent Manner. American Journal of Physiology-Cell Physiology 2016, 311, C518–C527. [Google Scholar] [CrossRef]
- Kao, M.; Columbus, D.A.; Suryawan, A.; Steinhoff-Wagner, J.; Hernandez-Garcia, A.; Nguyen, H.V.; Fiorotto, M.L.; Davis, T.A. Enteral β-Hydroxy-β-Methylbutyrate Supplementation Increases Protein Synthesis in Skeletal Muscle of Neonatal Pigs. American Journal of Physiology-Endocrinology and Metabolism 2016, 310, E1072–E1084. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Hossain, T.; Limb, M.C.; Phillips, B.E.; Lund, J.; Williams, J.P.; Brook, M.S.; Cegielski, J.; Philp, A.; Ashcroft, S.; et al. Impact of the Calcium Form of β-Hydroxy-β-Methylbutyrate upon Human Skeletal Muscle Protein Metabolism. Clinical Nutrition 2018, 37, 2068–2075. [Google Scholar] [CrossRef] [PubMed]
- Girón, M.D.; Vílchez, J.D.; Salto, R.; Manzano, M.; Sevillano, N.; Campos, N.; Argilés, J.M.; Rueda, R.; López-Pedrosa, J.M. Conversion of Leucine to β-Hydroxy-β-Methylbutyrate by α-Keto Isocaproate Dioxygenase Is Required for a Potent Stimulation of Protein Synthesis in L6 Rat Myotubes: HMB Is a Potent Stimulator of Protein Synthesis. Journal of Cachexia, Sarcopenia and Muscle 2016, 7, 68–78. [Google Scholar] [CrossRef]
- Huang, H.; Long, L.; Zhou, P.; Chapman, N.M.; Chi, H. mTOR Signaling at the Crossroads of Environmental Signals and T-cell Fate Decisions. Immunological Reviews 2020, 295, 15–38. [Google Scholar] [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef]
- Cai, S.-L.; Tee, A.R.; Short, J.D.; Bergeron, J.M.; Kim, J.; Shen, J.; Guo, R.; Johnson, C.L.; Kiguchi, K.; Walker, C.L. Activity of TSC2 Is Inhibited by AKT-Mediated Phosphorylation and Membrane Partitioning. Journal of Cell Biology 2006, 173, 279–289. [Google Scholar] [CrossRef]
- Dibble, C.C.; Elis, W.; Menon, S.; Qin, W.; Klekota, J.; Asara, J.M.; Finan, P.M.; Kwiatkowski, D.J.; Murphy, L.O.; Manning, B.D. TBC1D7 Is a Third Subunit of the TSC1-TSC2 Complex Upstream of mTORC1. Molecular Cell 2012, 47, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Garlick, P.J. The Role of Leucine in the Regulation of Protein Metabolism. The Journal of Nutrition 2005, 135, 1553S–1556S. [Google Scholar] [CrossRef]
- Ham, D.J.; Caldow, M.K.; Lynch, G.S.; Koopman, R. Leucine as a Treatment for Muscle Wasting: A Critical Review. Clinical Nutrition 2014, 33, 937–945. [Google Scholar] [CrossRef]
- Beaudry, A.G.; Law, M.L. Leucine Supplementation in Cancer Cachexia: Mechanisms and a Review of the Pre-Clinical Literature. Nutrients 2022, 14, 2824. [Google Scholar] [CrossRef] [PubMed]
- Cruz, B.; Oliveira, A.; Gomes-Marcondes, M.C.C. L-Leucine Dietary Supplementation Modulates Muscle Protein Degradation and Increases pro-Inflammatory Cytokines in Tumour-Bearing Rats. Cytokine 2017, 96, 253–260. [Google Scholar] [CrossRef]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; De Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; Brunetto, S.Q.; et al. Leucine-Rich Diet Induces a Shift in Tumour Metabolism from Glycolytic towards Oxidative Phosphorylation, Reducing Glucose Consumption and Metastasis in Walker-256 Tumour-Bearing Rats. Sci Rep 2019, 9, 15529. [Google Scholar] [CrossRef] [PubMed]
- Maschke, J.; Kruk, U.; Kastrati, K.; Kleeberg, J.; Buchholz, D.; Erickson, N.; Huebner, J. Nutritional Care of Cancer Patients: A Survey on Patients’ Needs and Medical Care in Reality. Int J Clin Oncol 2017, 22, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.A.; Lashinger, L.M.; Rasmussen, A.J.; Hursting, S.D. Leucine Supplementation Differentially Enhances Pancreatic Cancer Growth in Lean and Overweight Mice. Cancer Metab 2014, 2, 6. [Google Scholar] [CrossRef]
- Schrems, E.R.; Haynie, W.S.; Perry, R.A.; Morena, F.; Cabrera, A.R.; Rosa-Caldwell, M.E.; Greene, N.P.; Washington, T.A. Leucine Supplementation Exacerbates Morbidity in Male but Not Female Mice with Colorectal Cancer-Induced Cachexia. Nutrients 2023, 15, 4570. [Google Scholar] [CrossRef]
- Xie, X.-L.; Wei, M.; Yunoki, T.; Kakehashi, A.; Yamano, S.; Kato, M.; Wanibuchi, H. Long-Term Treatment with l-Isoleucine or l-Leucine in AIN-93G Diet Has Promoting Effects on Rat Bladder Carcinogenesis. Food and Chemical Toxicology 2012, 50, 3934–3940. [Google Scholar] [CrossRef]
- Hassan, Y.A.; Helmy, M.W.; Ghoneim, A.I. Combinatorial Antitumor Effects of Amino Acids and Epigenetic Modulations in Hepatocellular Carcinoma Cell Lines. Naunyn-Schmiedeberg’s Arch Pharmacol 2021, 394, 2245–2257. [Google Scholar] [CrossRef]
- Ball, H.J.; Fedelis, F.F.; Bakmiwewa, S.M.; Hunt, N.H.; Yuasa, H.J. Tryptophan-Catabolizing Enzymes €“ Party of Three. Front. Immunol. 2014, 5. [Google Scholar] [CrossRef]
- Hoffmann, D.; Dvorakova, T.; Stroobant, V.; Bouzin, C.; Daumerie, A.; Solvay, M.; Klaessens, S.; Letellier, M.-C.; Renauld, J.-C.; Van Baren, N.; et al. Tryptophan 2,3-Dioxygenase Expression Identified in Human Hepatocellular Carcinoma Cells and in Intratumoral Pericytes of Most Cancers. Cancer Immunology Research 2020, 8, 19–31. [Google Scholar] [CrossRef]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of Tumoral Immune Resistance by Inhibition of Tryptophan 2,3-Dioxygenase. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Uyttenhove, C.; Pilotte, L.; Théate, I.; Stroobant, V.; Colau, D.; Parmentier, N.; Boon, T.; Van Den Eynde, B.J. Evidence for a Tumoral Immune Resistance Mechanism Based on Tryptophan Degradation by Indoleamine 2,3-Dioxygenase. Nat Med 2003, 9, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Tomek, P. Tryptophan: A Rheostat of Cancer Immune Escape Mediated by Immunosuppressive Enzymes IDO1 and TDO. Front. Immunol. 2021, 12, 636081. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, M.D.; Carlomagno, S.; Frumento, G.; Balsamo, M.; Cantoni, C.; Conte, R.; Moretta, L.; Moretta, A.; Vitale, M. The Tryptophan Catabolite L-Kynurenine Inhibits the Surface Expression of NKp46- and NKG2D-Activating Receptors and Regulates NK-Cell Function. Blood 2006, 108, 4118–4125. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, X.; Dong, W.; Fang, Y.; Lv, J.; Zhang, T.; Fiskesund, R.; Xie, J.; Liu, J.; Yin, X.; et al. Tumor-Repopulating Cells Induce PD-1 Expression in CD8+ T Cells by Transferring Kynurenine and AhR Activation. Cancer Cell 2018, 33, 480–494.e7. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An Interaction between Kynurenine and the Aryl Hydrocarbon Receptor Can Generate Regulatory T Cells. The Journal of Immunology 2010, 185, 3190–3198. [Google Scholar] [CrossRef]
- Rad Pour, S.; Morikawa, H.; Kiani, N.A.; Yang, M.; Azimi, A.; Shafi, G.; Shang, M.; Baumgartner, R.; Ketelhuth, D.F.J.; Kamleh, M.A.; et al. Exhaustion of CD4+ T-Cells Mediated by the Kynurenine Pathway in Melanoma. Sci Rep 2019, 9, 12150. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, H.; Liu, W.; Yan, S.-M.; Li, Y.; Tan, L.; Chen, Y.; Liu, J.; Peng, Z.; Yuan, Y.; et al. Amino Acids and RagD Potentiate mTORC1 Activation in CD8 + T Cells to Confer Antitumor Immunity. J Immunother Cancer 2021, 9, e002137. [Google Scholar] [CrossRef]
- Keenan, M.M.; Chi, J.-T. Alternative Fuels for Cancer Cells. The Cancer Journal 2015, 21, 49–55. [Google Scholar] [CrossRef]
- Chen, Y.-Y.; Zhang, X.-N.; Xu, C.-Z.; Zhou, D.-H.; Chen, J.; Liu, Z.-X.; Sun, Y.; Huang, W.; Qu, L.-S. MCCC2 Promotes HCC Development by Supporting Leucine Oncogenic Function. Cancer Cell Int 2021, 21, 22. [Google Scholar] [CrossRef]
- Morine, Y.; Utsunomiya, T.; Yamanaka-Okumura, H.; Saito, Y.; Yamada, S.; Ikemoto, T.; Imura, S.; Kinoshita, S.; Hirayama, A.; Tanaka, Y.; et al. Essential Amino Acids as Diagnostic Biomarkers of Hepatocellular Carcinoma Based on Metabolic Analysis. Oncotarget 2022, 13, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Xiao, F.; Wang, C.; Yin, H.; Yu, J.; Chen, S.; Fang, J.; Guo, F. Leucine Deprivation Inhibits Proliferation and Induces Apoptosis of Human Breast Cancer Cells via Fatty Acid Synthase. Oncotarget 2016, 7, 63679–63689. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, X.; Zou, Y.; Gong, J.; Ge, Z.; Lin, X.; Zhang, W.; Huang, H.; Zhao, J.; Saw, P.E.; et al. A High-Fat Diet Promotes Cancer Progression by Inducing Gut Microbiota–Mediated Leucine Production and PMN-MDSC Differentiation. Proc. Natl. Acad. Sci. U.S.A. 2024, 121, e2306776121. [Google Scholar] [CrossRef]
- Gi, M.; Wanibuchi, H. Roles of Leucine and Isoleucine in Experimental Models of Bladder Carcinogenesis. Food Safety 2015, 3, 136–142. [Google Scholar] [CrossRef]
- Aversa, Z.; Costelli, P.; Muscaritoli, M. Cancer-Induced Muscle Wasting: Latest Findings in Prevention and Treatment. Ther Adv Med Oncol 2017, 9, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhu, H.; Weng, M.; Zhang, H.; Wang, C.; Sun, L. CC-223, NSC781406, and BGT226 Exerts a Cytotoxic Effect Against Pancreatic Cancer Cells via mTOR Signaling. Front. Pharmacol. 2020, 11, 580407. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, X.; Cheng, C.; Yu, W.; Yi, P. Metabolism of Amino Acids in Cancer. Front. Cell Dev. Biol. 2021, 8, 603837. [Google Scholar] [CrossRef]
- Chen, J.; Cui, L.; Lu, S.; Xu, S. Amino Acid Metabolism in Tumor Biology and Therapy. Cell Death Dis 2024, 15, 42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




