1. An Overview
Stem cell-based therapeutic products have immense potential for the treatment of a broad spectrum of degenerative diseases and injuries. Stem cells find applications at the forefront of this medical revolution because of their unique capacity for differentiation, immune response modulation, and tissue repair promotion. This review critically discusses a significant aspect that requires attention and innovation: the tumorigenic risks posed by these therapies. Among these, mesenchymal stem cells stand out as a source of reassurance and confidence. They have been widely studied because they are easily isolated from sources such as bone marrow, adipose tissue, and Wharton's jelly, and can support the repair of damaged tissue through paracrine signaling and immunomodulation. Embryonic Stem Cells (ESCs) have the highest potential for differentiation, derived from the inner mass of blastocysts, whereas induced Pluripotent Stem Cells (iPSCs), reprogrammed from adult cells, offer a patient-specific approach to regenerative medicine. In the end, however, stem cell therapies hold much clinical promise.
Tumorigenicity can nullify the safety and effectiveness of such treatments; however, the risk involved is enormous. Historical examples such as teratoma formation by ESCs and iPSCs underpin the importance of finding a solution. Other reported mechanisms of tumorigenicity include genomic instability, abnormal differentiation, and dysregulation of signaling pathways that normally regulate the growth and survival of cells. Much work needs to be completed to develop these issues; however, at the same time, the transformational potential of stem cell therapies for further regenerative medicine cannot be sacrificed. The ability to solve these issues of control tumorigenicity using any stem cell-derived product is essential for promoting patient safety and furthering regulatory acceptance. Direct health risks and ethical and regulatory concerns due to patient tumorigenic outcomes are major influential factors impacting the broader adoption of stem cell therapies in clinical practice. Preclinical safety requirements for international regulatory bodies are becoming increasingly stringent, and mandatory rigorous screening has been implemented to allay concerns related to potential tumorigenicity.
This review outlines the various challenges faced with the tumorigenic potential of stem cell-derived products. It also examines the mechanisms that drive tumorigenicity, current strategies to mitigate such risks, and advances in technology and methodology. This review aims to summarize the current understanding of the tumorigenic risks associated with stem cell therapies to point out the proactive measures that may need to be considered to ensure the safety and efficacy of these promising treatments. The manuscript strongly stresses that this is a critical area for continued vigilance and creative solutions, while inspiring confidence in how much success has already been accomplished.
2. Introduction
2.1. Background on Stem Cell-Derived Therapeutic Products
2.1.1. Definition and Types of Stem Cells
Mesenchymal Stem Cells (MSCs), also known as mesenchymal stromal cells or medicinal signaling cells, are multipotent stromal cells that can differentiate into various cell types. These cells differentiate into osteoblasts (bone cells), chondrocytes (cartilage cells), myocytes (muscle cells), and adipocytes (fat cells). MSCs are derived from various sources, such as bone marrow, peripheral blood, adipose tissue, and neonatal birth-associated tissues, including the placenta, umbilical cord, and cord blood. MSCs have shown promise in various medical treatments, such as bone regeneration, cartilage, muscle, and adipose tissues. They have been studied for their potential application in the treatment of osteoarthritis, bone fractures, and autoimmune diseases.
In contrast to multipotent MSCs, Embryonic Stem Cells (ESCs) exhibit a higher degree of plasticity. Derived from the inner cell mass of a blastocyst, which is an early stage pre-implantation embryo, they can develop into any of the 200+ human cell types. This unique potential has significantly advanced our understanding of human development and diseases, making ESCs a significant research tool and inspiring further exploration and discovery (
Table 1).
While ESCs provide invaluable insights into human biology, Induced Pluripotent Stem Cells (iPSCs) offer a more ethically acceptable and patient-specific alternative. Generated directly from somatic cells, they closely resemble ESCs in their ability to differentiate into any cell type in the body. Reprogramming of adult cells into iPSCs is achieved by introducing four specific genes, Sox2, Oct4, Klf4, and cMyc, into the cells. The advantage of iPSCs is that they can be directly derived from adult tissues, thereby bypassing the need for embryos. This makes iPSCs significant for their potential in personalized medicine, emphasizing their potential for patient-specific treatment. iPSCs are being utilized in personalized medicine because of their ability to be derived directly from adult tissues, enabling the creation of patient-specific stem cell lines, which hold great potential for tailoring treatments to individual patients. iPSCs have been studied for their applications in disease modeling, drug screening, and transplantation therapies, highlighting their potential in personalized medicine.
2.1.2. Therapeutic Applications and Clinical Potential
Owing to their remarkable potential, stem cells play a pivotal role in regenerative medicine, offering hope for the repair and regeneration of damaged tissues. A primary example of their promise is cardiac repair. Stem cells, particularly mesenchymal stem cells (MSCs) and induced pluripotent stem cells (iPSCs), have shown significant promise. According to a study by [
1,
2], MSCs and iPSCs can restore cardiac muscle function by targeting damaged proteins to reverse complex changes caused by heart attack. However, the specific challenges and limitations associated with using stem cells for cardiac repair include issues related to the survival, integration, and maturation of transplanted cells; potential immune rejection; and ethical considerations for certain types of stem cells. MSCs are known for their immunomodulatory effects and ability to differentiate into multiple cell types [
1]. Simultaneously, iPSCs are reprogrammed from adult cells and have the potential to form any cell type in the body, making them effective for treating cardiac conditions [
2]. Another study by [
3] reported similar outcomes but highlighted the need for improved methods to enhance cell survival and integration post-transplantation (
Table 2).
Another promising application of stem cells is in the treatment of neurodegenerative diseases. Stem cells, especially iPSCs, have been explored for treating conditions such as Alzheimer's and Parkinson's. [
4] demonstrated that iPSCs could differentiate into dopaminergic neurons, offering a potential treatment for Parkinson's disease. However, similar challenges arise, such as issues related to the survival, integration, and maturation of transplanted cells, potential immune rejection, and ethical considerations. MSCs and iPSCs have different properties that affect their effectiveness in treating neurodegenerative diseases. MSCs are known for their immunomodulatory effects and ability to differentiate into multiple cell types. Simultaneously, iPSCs are reprogrammed from adult cells and have the potential to form any cell type in the body [
5]. According to [
6], iPSCs can replace damaged or lost cells, provide a conducive environment for regeneration, or protect existing healthy neurons and glial cells from further damage.
In addition to their regenerative capabilities, stem cells also exhibit significant immunomodulatory properties. MSCs and hematopoietic stem cells (HSCs) can migrate to sites of inflammation and injury and modulate pathogenic immune responses by interacting with various immune cell types and secreting factors that can dampen inflammation and promote tolerance [
7,
17,
18]. A review by a few research groups discusses the potential of MSCs in modulating immune responses in conditions such as graft-versus-host disease (GVHD), a complication of allogeneic stem cell transplantation. Donor stem cells can attack recipient cells, causing GVHD. Their findings suggest that MSCs can mitigate these effects by interacting with T cells and secreting anti-inflammatory cytokines, thus reducing GVHD severity [
10,
11,
12,
13,
18].
Furthermore, stem cells have revolutionized drug screening and disease modeling. In particular, iPSCs are increasingly used in drug discovery and disease modeling [
14]. According to a previous study [
15,
16], iPSCs can generate unlimited disease-relevant cell types, which is invaluable for identifying novel molecular targets and performing large-scale phenotypic screens. Their study found that iPSCs derived from patients with specific genetic disorders could be used to model diseases in vitro, enabling researchers to test the efficacy and safety of new drugs in a controlled environment [
15]. This potential to generate patient-specific cell lines offers exciting prospects for personalized medicine and the future of drug discovery and disease modeling.
3. Tumorigenic Potential
3.1. Explanation of Tumorigenicity
Tumorigenicity refers to the potential of cells to form tumors when introduced into an organism. In the context of stem cell therapies, this term describes the propensity of stem cells to undergo uncontrolled growth and differentiation, leading to tumor formation. This is a critical concern in the development and clinical application of stem cell-based therapies, because the risk of tumorigenesis must be meticulously managed to ensure patient safety [
19].
Several factors contribute to tumorigenicity in stem cell therapies. Intrinsic factors include genetic mutations, epigenetic changes, and telomerase activity [
20]. A group of researchers discusses the genetic mutations that can arise during cell culture, reprogramming, or even after transplantation. noted that epigenetic changes, such as DNA methylation and histone modifications, can deregulate gene expression and contribute to tumorigenic transformation [
21]. Telomerase activity, which is often upregulated in stem cells to maintain their proliferative capacity, can also play a role in tumorigenesis by allowing cells to bypass normal cellular senescence and continue dividing. A few research groups have discussed the role of telomerase in stem cell biology and its implications for cancer and aging, further highlighting its significance in tumorigenicity [
22,
23,
24].
Extrinsic factors include microenvironment influences, immune interactions, and inflammation. The microenvironment or niche in which stem cells reside can significantly impact their behavior. [
25], highlight that inflammatory signals and interactions with immune cells within the microenvironment can either suppress or promote tumorigenicity, depending on the context. Chronic inflammation has been linked to an increased risk of cancer development because it can induce a pro-tumorigenic environment through the production of reactive oxygen species (ROS) and other inflammatory mediators. They provided an in-depth review of the links between inflammation and cancer, highlighting the impact of the tumor microenvironment on cancer progression.
3.2. Historical Context and Examples of Tumorigenic Outcomes
Embryonic stem cells (ESCs) have been shown to form teratomas, which are benign tumors of various tissue types. In an early study by Thomson et al.(1998), ESCs were injected into immunocompromised mice, resulting in teratoma formation. These teratomas contain differentiated cells from all three germ layers, highlighting the pluripotency of ESCs and their potential for uncontrolled growth. Teratoma formation highlights the need for stringent differentiation protocols and extensive preclinical testing before ESCs can be safely used in clinical settings. This has also been discussed in a review article by Golchin et al.(2021). This paper recently discussed research studies on clinical trials involving hESCs, outlining their benefits, drawbacks, and specific considerations. Some limitations of hESCs have been addressed, and numerous ongoing clinical trial studies have focused on enhancing their clinical applications, particularly in treating macular degeneration and neurodegenerative diseases. Nonetheless, further research and discourse are required to address the crucial issues related to hESC-based therapy.
Induced pluripotent stem cells (iPSCs) also exhibit tumorigenic potential, particularly due to the genetic instability introduced during reprogramming. Miura et al. (2009) demonstrated that iPSCs can form teratomas when injected into mice, similar to ESCs. Moreover, Okita et al. (2007) identified genetic mutations and chromosomal abnormalities in iPSCs that can lead to tumor formation. These mutations can arise from reprogramming factors or stress. To mitigate these risks, researchers are exploring safer reprogramming methods, such as non-integrative and chemical reprogramming, that do not alter the host genome. Yamanaka (2013) provides a comprehensive overview of iPSCs and discusses the advancements in reprogramming techniques to reduce tumorigenicity risks.
Mesenchymal stem cells (MSCs), although generally considered to have a lower tumorigenic potential than ESCs and iPSCs, have been shown to undergo malignant transformation under certain conditions. Tolar et al. (2007) reported instances in which MSCs exhibited malignant transformation after extensive in vitro expansion. Similarly, a study by Foudah et al. (2009) found that prolonged culture of MSCs can lead to chromosomal abnormalities and transformation into cancerous cells. These examples highlight the importance of monitoring MSCs for their genetic stability and limiting their expansion in culture to reduce the risk of malignant transformation. Additionally, Caplan (2017) emphasized the need to thoroughly characterize MSC populations and implement stringent quality control measures to ensure the safety and efficacy of MSC-based therapies. Neri (2019) further supported these findings by discussing the long-term safety and genetic stability of MSCs for therapeutic applications.
4. Importance of Addressing Tumorigenic Potential
4.1. Clinical Implications
Ensuring the safety of stem cell therapies is paramount for preventing patient harm. The potential for stem cells to form tumors poses significant risks, including the possibility of malignancies that could endanger the lives of patients. For instance, a study by Amariglio et al. (2009) reported a case in which a patient developed a brain tumor following stem cell therapy, highlighting the critical need for rigorous safety protocols. Comprehensive preclinical testing and long-term monitoring are essential to ensure patient safety. These measures involve advanced screening methods to detect genetic abnormalities and ensure that stem cells are fully differentiated before transplantation, thereby reducing the likelihood of tumor formation.
Additionally, tumorigenic risks undermine the therapeutic potential of stem cells by compromising their safety and effectiveness. If the risk of tumor formation is not adequately addressed, it can lead to complications that negate the benefits of therapy. According to Lee et al. (2013), undifferentiated or partially differentiated cells can form tumors, potentially outweighing the regenerative benefits of treatment. Therefore, ensuring that stem cell therapies are safe and effective requires meticulous control of cell differentiation and proliferation. This includes optimizing culture conditions, implementing strict quality control measures, and conducting thorough preclinical and clinical evaluations to verify the safety and efficacy of stem cell products.
4.1. Regulatory Concerns and Patient Safety
Regulatory agencies, such as the U.S. The Food and Drug Administration (FDA) [
36] and the European Medicines Agency (EMA) [
37] have stringent requirements for the safety of stem cell therapies. These regulations ensure that stem cell treatments meet high safety, efficacy, and quality standards before being approved for clinical use. For instance, the FDA’s guidelines on human cell- and tissue-based products mandate extensive preclinical testing, including assessments of tumorigenic potential, to demonstrate that therapies do not pose unacceptable risks to patients. Similarly, the EMA guidelines emphasize the need for comprehensive risk assessments and robust quality control measures. Compliance with these regulations is crucial for gaining approval for stem cell therapies and maintaining public trust in innovative treatments.
In addition to regulatory requirements, there is an ethical imperative to prevent tumorigenic outcomes in stem cell treatments. The potential for harm from tumorigenic cells raises significant ethical concerns, particularly regarding the informed consent process and the responsibility of researchers and clinicians to ensure patient safety. Hyun (2010) discussed the ethical challenges of stem cell research and the importance of transparency and accountability in addressing these risks. Ethical guidelines require that patients be fully informed about the potential risks and benefits of stem cell therapies and that researchers take all the necessary steps to minimize the risk of tumorigenesis. This includes implementing rigorous safety protocols, conducting thorough risk assessments, and ensuring that all research and clinical practices adhere to the highest ethical standards. Daley et al. (2016) provided a comprehensive set of guidelines for stem cell research and clinical translation. It outlines the ethical responsibilities of researchers and clinicians, including the prevention of tumorigenic outcomes in stem cell treatments and ensuring patient safety. The most recent update features a comprehensive discussion of the stringent safety protocols outlined in the guidelines. They briefly introduced the types and characteristics of stem cells and summarized the current clinical applications and market developments of SCT. The results focused on the current development status of SCT-related standards at three levels: the International Organization for Standardization (ISO), important international organizations, and national organizations. Finally, they offer perspectives and conclusions on the significance and challenges of SCT standardization. [
40,
41].
5. Mechanisms of Tumorigenicity
5.1. Intrinsic Factors
5.1.1. Genetic Mutations and Instability
Genetic mutations and chromosomal instability are the primary intrinsic factors that contribute to the tumorigenic potential of stem cells. During in vitro expansion and manipulation, stem cells acquire genetic mutations that may lead to oncogenesis. Studies have shown that long-term culture of stem cells, particularly embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs), can result in chromosomal abnormalities and mutations in oncogenes and tumor suppressor genes. For instance, chromosomal instability (CIN) is a hallmark of many cancers and is often observed in stem cells cultured for extended periods [
42,
43]. This instability can lead to an imbalance in chromosome number, known as aneuploidy, and increased rates of loss of heterozygosity, which drives tumorigenesis [
44].
5.1.2. Epigenetic Changes
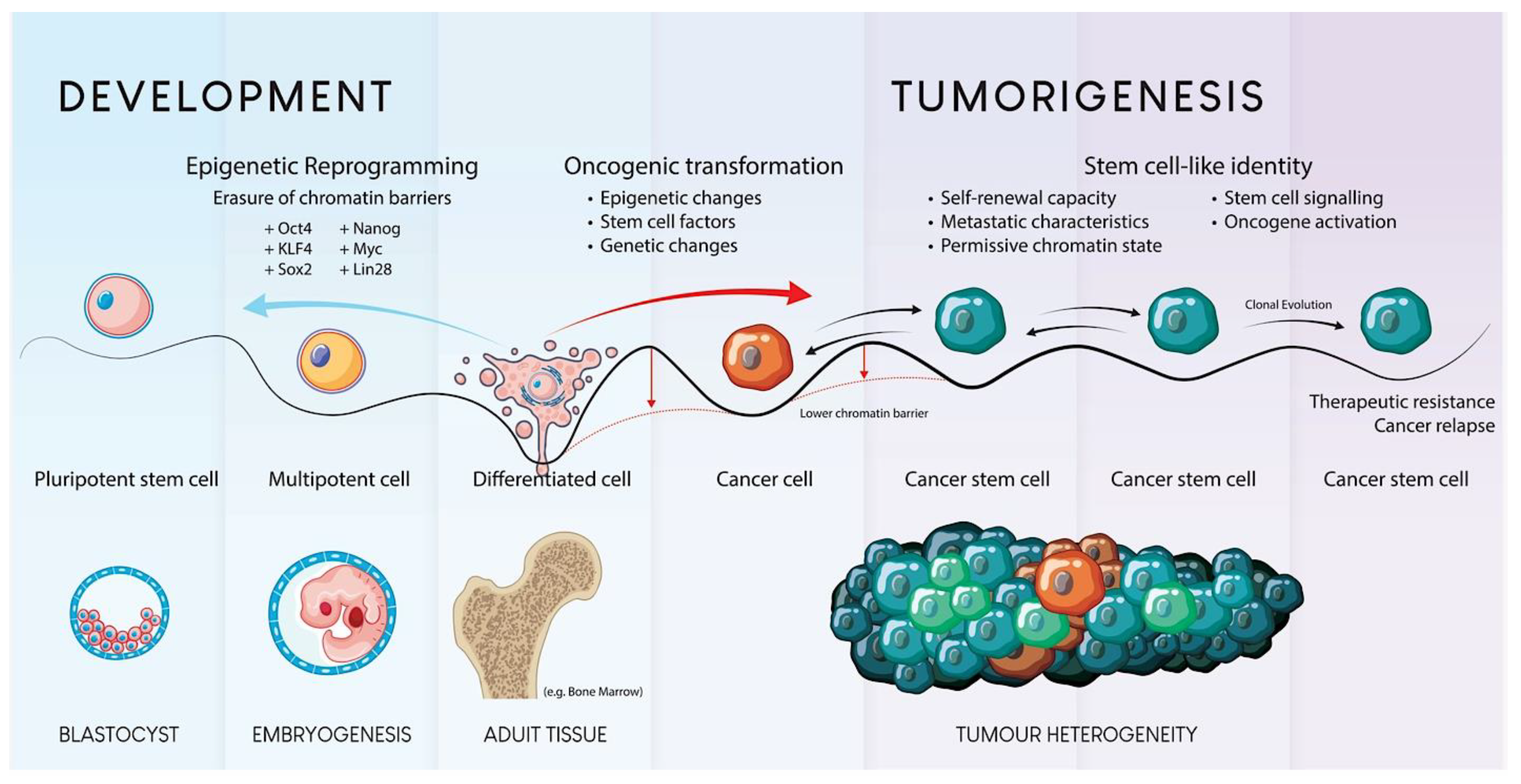
Epigenetic modifications are crucial for regulating gene expression and maintaining cellular identity. Aberrant epigenetic changes such as DNA methylation, histone modification, and chromatin remodeling can lead to gene dysregulation during cell proliferation and differentiation. Research has indicated that epigenetic dysregulation in stem cells can promote tumorigenesis by activating oncogenes and silencing tumor suppressor genes (
Figure 1). For instance, hypermethylation of tumor suppressor genes, such as p16INK4a, has been observed in mesenchymal stem cells (MSCs) undergoing malignant transformation [
45]. Epigenetic instability can result in the activation of oncogenes and repression of tumor suppressor genes, further driving the tumorigenic process [
46].
5.1.3. Telomerase Activity and Cellular Immortality
Telomerase activity is another intrinsic factor that contributes to the tumorigenic potential of stem cells. Telomerase, an enzyme that maintains telomere length, is typically active in stem cells to maintain their long-term proliferative capacity. However, dysregulated telomerase activity can lead to cellular immortality, which is a hallmark of cancer. Studies have demonstrated that heightened telomerase activity in iPSCs increases the risk of tumor formation. For example, enhanced telomerase activity can prevent the normal shortening of telomeres, allowing cells to bypass senescence and continue dividing, thus increasing their potential for malignant transformation [
47].
5.2. Extrinsic Factors
5.2.1. Microenvironment Influences
The microenvironment or niche is pivotal in modulating stem cell behavior and influencing tumorigenic potential. The extracellular matrix, neighboring cells, and soluble factors collectively create a microenvironment that can support normal differentiation or promote tumorigenesis. Studies have shown that an altered microenvironment can induce the malignant transformation of stem cells. For instance, exposure to a fibrotic or inflammatory microenvironment can drive MSCs toward a tumorigenic phenotype [
48]. The tumor microenvironment is characterized by regions of fluctuating hypoxia, low pH, and nutrient deprivation, which can induce genetic mutations and increase the mutation frequency within tumor cells.
5.2.2. Immune System Interactions
The interaction between stem cells and the immune system is a critical extrinsic factor that influences tumorigenicity. Although MSCs are known for their immunomodulatory properties, which are beneficial for therapeutic applications, these properties can also lead to immune evasion, a characteristic of cancer cells. Research indicates that the immunosuppressive environment created by MSCs can facilitate tumor growth by inhibiting antitumor immune responses. For example, immunosuppressive cytokines produced by MSCs can suppress the activity of cytotoxic T-cells and natural killer cells, allowing tumor cells to evade immune surveillance [
49].
5.2.3. Inflammation and Cytokines Milieu
Inflammation and the associated cytokine milieu are significant extrinsic factors that influence the tumorigenic potential of stem cells. Chronic inflammation creates a pro-tumorigenic environment by providing growth factors, cytokines, and chemokines that promote cellular proliferation and survival. Studies have shown that pro-inflammatory cytokines, such as IL-6 and TNF-α, can enhance the tumorigenic potential of stem cells by activating oncogenic signaling pathways, such as NF-κB and STAT3 [
50]. These cytokines promote the survival and proliferation of the transformed cells, thereby enhancing their tumorigenic potential.
5.2.4. Comparative Analysis with Related Studies
Comparative analysis with related studies revealed that intrinsic and extrinsic factors contribute to the tumorigenic potential of stem cell-derived products. For instance, a review by Nam et al. (2022) demonstrated that long-term culture of iPSCs results in genetic mutations that increase their tumorigenic potential [
51]. Similarly, a group of researchers highlighted the role of the microenvironment in modulating MSC behavior, showing that a pro-inflammatory environment significantly increased the risk of malignant transformation [
52]. These findings emphasize the multifactorial nature of tumorigenicity and the need for comprehensive strategies to address intrinsic and extrinsic risks.
6. Strategies to Mitigate Tumorigenic Risks in Stem Cell Therapies
6.1. Quality Control Measures
6.1.1. Stringent Culture and Expansion Protocols
Adhering to GMP standards is crucial for stem cell production to ensure safety, efficacy, and consistency. GMP guidelines require controlled environments, thorough documentation, and quality assurance during production. This is exemplified by the production of clinical-grade T-cell or stem cell gene therapy products, where stringent environmental controls and quality checks are mandatory before administering them to patients [
53]. Similarly, implementing GMP to produce mesenchymal stem cells (MSCs) ensures low immunogenicity and high safety standards [
54]. Continuous genetic and phenotypic monitoring is essential to detect and eliminate any abnormalities that could lead to tumorigenicity. Regular checks include karyotyping and PCR-based methods to detect common genetic changes and monitoring phenotypic stability to ensure that the cells retain their intended functions [
55]. Several studies have demonstrated successful implementation of stringent quality control measures. For example, using a microfluidic platform for longitudinal monitoring significantly reduces heterogeneity in stem cell cultures, ensuring consistent phenotypic outcomes [
56]. Another study highlighted the development of GMP-compliant human embryonic stem cell lines and underscored the importance of adhering to GMP standards for clinical applications [
57].
6.1.2. Monitoring Genetic Stability
Genetic instability in stem cells can be detected using karyotyping, next-generation sequencing (NGS), and comparative genomic hybridization (CGH) techniques. These methods help identify and eliminate cells with mutations that could lead to tumor formation. For example, karyotyping can reveal chromosomal aberrations, whereas NGS can detect specific gene mutations [
58]. It is important to screen stem cells regularly using these techniques to enable the early detection of mutations. Studies have shown that stem cells in culture can undergo genetic changes over time, highlighting the need for frequent monitoring [
59]. PCR-based methods have also been developed to rapidly detect common genetic variants and ensure timely intervention [
60]. Several studies have highlighted the effectiveness of genetic screening in preventing tumorigenicity. For instance, research comparing different culture conditions found that certain methods increased genetic stability, thereby reducing the risk of tumor formation [
61]. Furthermore, a study has demonstrated that mesenchymal stem cells maintain chromosomal stability over multiple passages, thereby validating the importance of continuous genetic monitoring [
62].
6.2. Refinement of Differentiation Protocols
6.2.1. Improved Differentiation Techniques
Advancements in differentiation protocols have significantly reduced the number of undifferentiated cells, thus mitigating tumorigenic risks. Efficient protocols involve using specific culture conditions, growth factors, and co-culturing with other cell types to promote the desired differentiation while suppressing undesired lineages. For example, co-culturing human pluripotent stem cells (hPSCs) with neural stem cells or precursor cells (NPCs) under neural differentiation conditions results in the rapid and complete disappearance of undifferentiated cells through neural conversion [
63]. Ensuring complete differentiation is crucial for minimizing the risk of tumorigenic cells. Strategies include preconditioning cultures under terminal differentiation conditions, using specific growth factors and inhibitors, and employing multistage differentiation protocols. For instance, a four-stage differentiation protocol for mouse embryonic stem cells to insulin-producing cells minimizes neuronal differentiation, resulting in a pure population of insulin-producing cells [
64].
Moreover, the use of viral vector-based approaches to directly target and kill tumorigenic cells has been proposed to improve the safety of stem cell therapies [
65]. Developing efficient protocols for directing cardiomyogenic differentiation of stem cells in vitro demonstrated higher engraftment efficiency and enhanced myocardial regeneration, reducing the risk of teratoma formation [
66]. Additionally, protocols for differentiating human embryonic stem cells into the definitive endoderm using activinA/nodal signaling and GSK3 inhibition have shown high efficiency and robustness, ensuring a defined starting condition for differentiation [
67].
6.2.2. Minimizing Residual Undifferentiated Cells
In stem cell research, various techniques such as flow cytometry and magnetic-activated cell sorting (MACS) have been employed to ensure the purity of differentiated cells by removing undifferentiated cells. Flow cytometry can sort cells based on specific surface markers, allowing for the isolation of differentiated cells while simultaneously removing undifferentiated cells. MACS utilizes magnetic beads conjugated with antibodies to specifically target and remove undifferentiated cells. An example of this is the separation of undifferentiated human embryonic stem cells (hESCs) using MACS and FACS, which demonstrated its effectiveness in reducing teratoma risks [
68]. These techniques significantly enhance the safety and efficacy of stem cell therapies by mitigating the risk of tumor formation. For instance, MACS combined with cytotoxic antibody treatment resulted in near-complete removal of undifferentiated cells, providing a safer therapeutic product [
69].
Moreover, approaches that induce differentiation of cancer stem cells have been shown to reduce their tumorigenic potential [
70]. It is worth noting that different methods for eliminating undifferentiated cells have varying levels of effectiveness. Although flow cytometry is precise, it is labor-intensive and costly. In contrast, MACS offers a faster and more scalable solution, albeit with potentially lower precision than that of flow cytometry. A study comparing these methods found that fluorescence-activated cell sorting (FACS) is more effective in eliminating undifferentiated cells. It was also noted that MACS, followed by cytotoxic antibody treatment, achieved near-complete removal with the added benefit of scalability [
71]. Additionally, the use of machine learning algorithms to detect and eliminate undifferentiated cell clusters has shown promise in improving the efficiency of cell sorting methods [
72].
6.3. Genomic and Epigenomic Screening Techniques
6.3.1. Advanced Screening Methodologies
High-throughput sequencing (HTS) is essential for detecting genetic and epigenetic aberrations in stem cells. HTS technology enables comprehensive genome-wide analysis and the identification of mutations, copy number variations, and other genetic changes. For instance, it has been pivotal to identify gene mutations such as TP53, which is critical for maintaining genomic stability in human pluripotent stem cells (hPSCs) [
73]. Additionally, HTS allows for more sensitive monitoring of minimal residual disease in leukemia than traditional methods [
74]. Moreover, epigenomic profiling is crucial for identifying tumorigenic cells by examining DNA methylation, histone modifications, and chromatin accessibility. Techniques such as bisulfite sequencing and methylation arrays enable the detection of epigenetic changes that may contribute to tumorigenicity. Notably, epigenetic modifications in cancer-related genes have been identified using RNA sequencing, underscoring the need for routine quality control of hPSCs [
75]. Furthermore, high-throughput methylation profiling has revealed gene body signatures correlating with gene expression, thereby aiding the identification of epigenetic markers for cancer diagnosis and treatment [
76].
In addition, various genomic and epigenomic screening techniques have been compared for their effectiveness in detecting genetic and epigenetic aberrations. Research has demonstrated that High-throughput sequencing can detect low-frequency genetic events that other methods may miss [
77]. Furthermore, comparing high-throughput sequencing with other techniques, such as single nucleotide polymorphism (SNP) arrays, has highlighted the superiority of HTS in identifying copy number aberrations and subclonal markers in heterogeneous tumors [
78].
6.3.2. Detection and Elimination of Tumorigenic Cells
Specific approaches are being pursued in targeted cancer research to identify and eliminate tumorigenic cells. These methods involve the use of specific markers and genetic targets to screen for and remove potentially dangerous cells. Techniques such as targeted sequencing and high-throughput single-cell sequencing are being utilized to detect genetic aberrations in individual cells, thus allowing for the precise identification and elimination of tumorigenic cells. For example, high-throughput single-cell sequencing has been instrumental in uncovering low-level mosaicism in hPSCs, which is a crucial step in ensuring the safety of these cells in clinical applications [
78]. Numerous studies have documented successful instances of detecting and eliminating potentially tumorigenic cells. For example, RNA sequencing has been employed to identify cancer-related mutations in hPSCs, ensuring that only genetically stable cells can be used for therapeutic purposes [
79]. Furthermore, in a separate study, integrative high-throughput sequencing of patients with advanced cancer contributed to biomarker-driven clinical trials, ultimately leading to tailored treatment strategies [
80].
7. Innovative Approaches to Enhance Safety
7.1. Gene Editing Technologies
7.3.1. Application of CRISPR/Cas9
The CRISPR/Cas9 system is a powerful gene editing tool derived from the bacterial immune system. It consists of the Cas9 enzyme, which acts as a molecular scissor, and a guide RNA (gRNA) that directs Cas9 to a specific location in the genome. This system allows precise editing of genetic sequences by introducing double-strand breaks at targeted sites, which can be repaired by natural mechanisms, either through non-homologous end joining (NHEJ) or homology-directed repair (HDR) [
81]. Furthermore, CRISPR/Cas9 is widely used for correcting genetic mutations in stem cells, offering a promising approach for treating genetic disorders [
82]. Gene editing can significantly enhance the safety profile of stem cell therapies by precisely correcting disease-causing mutations, thereby reducing the risk of introducing tumorigenic cells. By targeting specific mutations and ensuring accurate genetic correction, CRISPR/Cas9 minimizes off-target effects and potentially unintended consequences [
83].
In addition, using CRISPR/Cas9 in hematopoietic stem cells (HSCs) has shown promise in maintaining engraftment and multilineage repopulation potential while correcting genetic defects [
84,
85]. Several case studies have highlighted the successful application of CRISPR/Cas9 in mitigating tumorigenic risks. For example, CRISPR/Cas9 has been used to correct dystrophin mutations in muscle stem cells in a mouse model of Duchenne muscular dystrophy, restoring normal function and reducing tumorigenic potential [
86,
87]. Furthermore, another study demonstrated the correction of the sickle cell mutation in hematopoietic stem cells, producing normal hemoglobin and reducing the risk of cancerous cell growth [
88,
89].
7.3.2. Other Gene Editing Tools
In addition to CRISPR/Cas9, other gene editing tools are available, such as Transcription Activator-Like Effector Nucleases (TALENs) and Zinc Finger Nucleases (ZFNs). TALENs are designed to target specific DNA sequences to create double-strand breaks at the desired locations. In contrast, ZFNs use zinc finger domains to recognize and bind to specific DNA sequences for targeted gene modification. Each of these gene editing tools has unique advantages and limitations. CRISPR/Cas9 is known for its simplicity, cost-effectiveness, and high efficiency in simultaneously targeting multiple sites. Although TALENs and ZFNs are more complex and labor-intensive, they offer higher specificity and potentially fewer off-target effects. Studies comparing these technologies have shown that CRISPR/Cas9 often provides efficient and versatile gene editing. However, TALENs and ZFNs are preferred in cases requiring the utmost precision [
90,
91].
7.2. Modulation with Small Molecules
Small molecules, with their ability to precisely target specific molecular pathways within cells, can modulate signaling pathways to promote safe differentiation and reduce tumorigenicity. This precision allows for a high level of control over specific outcomes. For instance, small molecules can activate signaling pathways that induce osteogenesis during embryological stages. They can also target intracellular signaling pathways, epigenetic modifications, and other cellular processes to regulate cell development, fate, and function.
7.2.1. Examples of Small Molecules Used in Stem Cell Research
Various small molecules play crucial roles in stem cell research and therapy. ROCK Inhibitors are used to increase the efficiency of reprogramming human fibroblasts to induced pluripotent stem cells (iPSCs). ROCK inhibitors reduce cell stress and promote cell survival during reprogramming. Studies have shown that ROCK inhibitors can enhance the efficiency of iPSC generation and improve cell viability during culture transitions. For example, [
92] demonstrated that adding ROCK inhibitors significantly improves the differentiation potential of stem cells into endothelial cells, highlighting the importance of reducing cellular stress. [
93,
94] found that ROCK inhibitors enhanced the reprogramming efficiency of adult cells into iPSCs, thereby improving the yield and quality of iPSC cultures.
Other examples include epigenetic modulators, small molecules targeting specific signaling pathways, epigenetic processes, and other cellular processes. Epigenetic modulators, such as histone deacetylase inhibitors (e.g., Trichostatin A) and DNA methyltransferase inhibitors (e.g., 5-Azacytidine), can influence gene expression by modifying the epigenetic landscape of stem cells. This promotes differentiation and reduces the risk of tumorigenicity. [
93,
94] highlighted that molecules like valproic acid and 5-azacytidine can significantly enhance the differentiation potential of mesenchymal stem cells by altering their epigenetic state. [
95] discussed the importance of epigenetic stability in stem cells and suggested that epigenetic modifiers could maintain genetic stability during stem cell culture, enhancing their therapeutic potential.
7.2.2. Other Small Molecules
More exciting possibilities exist for exploring various molecular approaches to manipulate stem cell behavior for therapeutic applications. Scientists have studied the roles of key molecular agents in stimulating differentiation, promoting reprogramming, and maintaining pluripotency in stem cells. For instance, statins inhibit the mevalonate pathway, promoting osteogenic differentiation and enhancing bone repair, whereas metformin facilitates differentiation into osteogenic and adipogenic lineages through AMPK signaling modulation. Adenosine and dexamethasone are utilized for specific lineage differentiation, such as osteoblasts and chondrocytes, and T63 and tetrahydroquinolines enhance fibroblast reprogramming into iPSCs, thereby improving the yield and quality.
Additionally, CHIR99021, a GSK3 inhibitor, activates Wnt signaling to enhance self-renewal and pluripotency maintenance [
96]. Furthermore, forskolin elevates cAMP levels, thereby influencing stem cell proliferation and survival. Tranylcypromine and valproic acid serve as histone deacetylase inhibitors, enhancing reprogramming efficiency and maintaining pluripotency. [
97] reported that these inhibitors can replace some reprogramming factors, thus simplifying the reprogramming process. [
98] showed that valproic acid promotes neuronal differentiation of stem cells by altering their epigenetic state. Lastly, 3-Deazaneplanocin A reduces H3K27me3 levels via EZH2 inhibition, promoting stem cell differentiation, and reducing tumorigenic potential through epigenetic modification. These diverse approaches represent exciting prospects for manipulating stem cell behavior for therapeutic applications.
7.3. Studies and Applications
Small molecules have also been shown to enhance the therapeutic potential of stem cells. For example, a four-part small-molecule cocktail was found to protect iPSCs from stress and maintain normal stem cell structure and function. [
99] demonstrated that this cocktail, consisting of chroman 1, emricasan, polyamines, and trans-ISRIB (CEPT), significantly improved the viability of genetically stable hPSCs and their differentiated progeny by blocking several stress mechanisms. Nanotechnology combined with small molecules can enhance targeted cancer treatment. For instance, nanoparticles have been engineered to deliver small molecules that induce the differentiation of cancer stem cells (CSCs), making them more susceptible to conventional therapies. [
100] discussed the use of nanocarriers to deliver differentiation-inducing agents to CSCs, thereby improving the efficacy of cancer treatments. [
101] showed that epigenetic modulation in liver cancer stem cells could increase the representation of highly tumorigenic cells, facilitating their isolation and targeted treatment.
Small molecules such as CHIR99021 and PD0325901 have been shown to stimulate the self-renewal of embryonic stem cells (ESCs) and iPSCs by modulating the GSK3/WNT and MEK/ERK signaling pathways. These pathways are crucial for maintaining pluripotency and directing cell differentiation. [
102] discussed the selection of small molecules that promote differentiation while maintaining the self-renewal capability of stem cells. [
103] found that transient inhibition of specific kinases can enhance iPSC generation, demonstrating the potential of small molecules to improve reprogramming efficiency.
References
- Carvalho, T. Stem Cell-Derived Heart Cells Injected into First Patient. Nat Med 2023, 29. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Zickler, A.M.; El Andaloussi, S. Dosing Extracellular Vesicles. Adv Drug Deliv Rev 2021, 178. [Google Scholar] [CrossRef]
- Guo, Y.; Yu, Y.; Hu, S.; Chen, Y.; Shen, Z. The Therapeutic Potential of Mesenchymal Stem Cells for Cardiovascular Diseases. Cell Death Dis 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized IPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. New England Journal of Medicine 2020, 382. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, S. Pluripotent Stem Cell-Based Cell Therapy—Promise and Challenges. Cell Stem Cell 2020, 27. [Google Scholar] [CrossRef]
- Sierra-Sánchez, Á.; Kim, K.H.; Blasco-Morente, G.; Arias-Santiago, S. Cellular Human Tissue-Engineered Skin Substitutes Investigated for Deep and Difficult to Heal Injuries. NPJ Regen Med 2021, 6. [Google Scholar] [CrossRef]
- Burnham, A.J.; Daley-Bauer, L.P.; Horwitz, E.M. Mesenchymal Stromal Cells in Hematopoietic Cell Transplantation. Blood Adv 2020, 4. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.G. Intestinal Epithelial Plasticity and Regeneration via Cell Dedifferentiation. Cell Regeneration 2020, 9. [Google Scholar] [CrossRef]
- Wu, H.H.; Symersky, J.; Lu, M. Structure and Mechanism of a Redesigned Multidrug Transporter from the Major Facilitator Superfamily. Sci Rep 2020, 10. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory Properties of Mesenchymal Stem Cells/Dental Stem Cells and Their Therapeutic Applications. Cell Mol Immunol 2023, 20. [Google Scholar] [CrossRef]
- Müller, L.; Tunger, A.; Wobus, M.; von Bonin, M.; Towers, R.; Bornhäuser, M.; Dazzi, F.; Wehner, R.; Schmitz, M. Immunomodulatory Properties of Mesenchymal Stromal Cells: An Update. Front Cell Dev Biol 2021, 9. [Google Scholar] [CrossRef]
- Shi, M.; Liu, Z.W.; Wang, F.S. Immunomodulatory Properties and Therapeutic Application of Mesenchymal Stem Cells. Clin Exp Immunol 2011, 164. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Song, S.U. Immunomodulatory Properties of Mesenchymal Stem Cells and Their Therapeutic Applications. Arch Pharm Res 2012, 35, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Qiu, L.; Veeraraghavan, V.P.; Sheu, C.-L.; Mony, U. Advances in IPSC Technology in Neural Disease Modeling, Drug Screening, and Therapy. Curr Stem Cell Res Ther 2024, 19, 809–819. [Google Scholar] [CrossRef]
- Elitt, M.S.; Barbar, L.; Tesar, P.J. Drug Screening for Human Genetic Diseases Using IPSC Models. Hum Mol Genet 2018, 27. [Google Scholar] [CrossRef]
- Rowe, R.G.; Daley, G.Q. Induced Pluripotent Stem Cells in Disease Modelling and Drug Discovery. Nat Rev Genet 2019, 20, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal Stem Cells and Their Microenvironment. Stem Cell Res Ther 2022, 13. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal Stromal Cell Therapies: Immunomodulatory Properties and Clinical Progress. Stem Cell Res Ther 2020, 11. [Google Scholar] [CrossRef]
- Lee, A.S.; Tang, C.; Rao, M.S.; Weissman, I.L.; Wu, J.C. Tumorigenicity as a Clinical Hurdle for Pluripotent Stem Cell Therapies. Nat Med 2013, 19. [Google Scholar] [CrossRef]
- Sato, Y.; Bando, H.; Di Piazza, M.; Gowing, G.; Herberts, C.; Jackman, S.; Leoni, G.; Libertini, S.; MacLachlan, T.; McBlane, J.W.; et al. Tumorigenicity Assessment of Cell Therapy Products: The Need for Global Consensus and Points to Consider. Cytotherapy 2019, 21, 1095–1111. [Google Scholar] [CrossRef]
- Keyvani-Ghamsari, S.; Khorsandi, K.; Rasul, A.; Zaman, M.K. Current Understanding of Epigenetics Mechanism as a Novel Target in Reducing Cancer Stem Cells Resistance. Clin Epigenetics 2021, 13, 120. [Google Scholar] [CrossRef]
- Günes, C.; Rudolph, K.L. The Role of Telomeres in Stem Cells and Cancer. Cell 2013, 152. [Google Scholar] [CrossRef] [PubMed]
- Lateef, H.B.; Suresh, P.M.; Bharathi, P.; Pathak, S.; Banerjee, A. A Brief Overview of Telomeres and Telomerase in Aging and Cancer. Curr Appl Sci Technol 2023, 23. [Google Scholar]
- Lansdorp, P.M. Telomeres, Aging, and Cancer: The Big Picture. Blood 2022, 139. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Chen, X.; Zhang, S.; Fang, J.; Chen, M.; Xu, Y.; Chen, X. Mesenchymal Stem Cells as a Double-Edged Sword in Tumor Growth: Focusing on MSC-Derived Cytokines. Cell Mol Biol Lett 2021, 26. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science (1979) 1998, 282, 1145–1147. [Google Scholar] [CrossRef] [PubMed]
- Golchin, A.; Chatziparasidou, A.; Ranjbarvan, P.; Niknam, Z.; Ardeshirylajimi, A. Embryonic Stem Cells in Clinical Trials: Current Overview of Developments and Challenges. In Advances in Experimental Medicine and Biology; Springer, 2021; Vol. 1312, pp. 19–37.
- Miura, K.; Okada, Y.; Aoi, T.; Okada, A.; Takahashi, K.; Okita, K.; Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Ohnuki, M.; et al. Variation in the Safety of Induced Pluripotent Stem Cell Lines. Nat Biotechnol 2009, 27. [Google Scholar] [CrossRef]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of Germline-Competent Induced Pluripotent Stem Cells. Nature 2007, 448. [Google Scholar] [CrossRef]
- Yamanaka, S. The Winding Road to Pluripotency (Nobel Lecture). Angewandte Chemie International Edition 2013, 52, 13900–13909. [Google Scholar] [CrossRef]
- Tolar, J.; Nauta, A.J.; Osborn, M.J.; Panoskaltsis Mortari, A.; McElmurry, R.T.; Bell, S.; Xia, L.; Zhou, N.; Riddle, M.; Schroeder, T.M.; et al. Sarcoma Derived from Cultured Mesenchymal Stem Cells. Stem Cells 2007, 25. [Google Scholar] [CrossRef]
- Foudah, D.; Redaelli, S.; Donzelli, E.; Bentivegna, A.; Miloso, M.; Dalprà, L.; Tredici, G. Monitoring the Genomic Stability of in Vitro Cultured Rat Bone-Marrow-Derived Mesenchymal Stem Cells. Chromosome Research 2009, 17. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to Change the Name! Stem Cells Transl Med 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Neri, S. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int J Mol Sci 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L.; et al. Donor-Derived Brain Tumor Following Neural Stem Cell Transplantation in an Ataxia Telangiectasia Patient. PLoS Med 2009, 6. [Google Scholar] [CrossRef] [PubMed]
- Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue-Based Products: Minimal Manipulation and Homologous Use | FDA Available online:. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/regulatory-considerations-human-cells-tissues-and-cellular-and-tissue-based-products-minimal (accessed on 2 July 2024).
- Stem Cell-Based Medicinal Products - Scientific Guideline | European Medicines Agency Available online:. Available online: https://www.ema.europa.eu/en/stem-cell-based-medicinal-products-scientific-guideline (accessed on 2 July 2024).
- Hyun, I. The Bioethics of Stem Cell Research and Therapy. Journal of Clinical Investigation 2010, 120. [Google Scholar] [CrossRef]
- Daley, G.Q.; Hyun, I.; Apperley, J.F.; Barker, R.A.; Benvenisty, N.; Bredenoord, A.L.; Breuer, C.K.; Caulfield, T.; Cedars, M.I.; Frey-Vasconcells, J.; et al. Setting Global Standards for Stem Cell Research and Clinical Translation: The 2016 ISSCR Guidelines. Stem Cell Reports 2016, 6. [Google Scholar] [CrossRef]
- Turner, L. ISSCR’s Guidelines for Stem Cell Research and Clinical Translation: Supporting Development of Safe and Efficacious Stem Cell-Based Interventions. Stem Cell Reports 2021, 16. [Google Scholar] [CrossRef]
- Zhang, J.; Suo, M.; Wang, J.; Liu, X.; Huang, H.; Wang, K.; Liu, X.; Sun, T.; Li, Z.; Liu, J. Standardisation Is the Key to the Sustained, Rapid and Healthy Development of Stem Cell-based Therapy. Clin Transl Med 2024, 14. [Google Scholar] [CrossRef]
- Nowak, M.A.; Komarova, N.L.; Sengupta, A.; Jallepalli, P.V.; Shih, I.-M.; Vogelstein, B.; Lengauer, C. The Role of Chromosomal Instability in Tumor Initiation; 2002. [Google Scholar]
- Carloni, V.; Morganti, E.; Galli, A.; Mazzocca, A. The Adaptability of Chromosomal Instability in Cancer Therapy and Resistance. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Giam, M.; Rancati, G. Aneuploidy and Chromosomal Instability in Cancer: A Jackpot to Chaos. Cell Div 2015, 10. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Geens, M.; Spits, C. Genetic and Epigenetic Instability in Human Pluripotent Stem Cells. Hum Reprod Update 2013, 19, 187–205. [Google Scholar] [CrossRef] [PubMed]
- Raptis, S.; Bapat, B. Genetic Instability in Human Tumors. EXS 2006.
- Michor, F. Chromosomal Instability and Human Cancer. In Proceedings of the Philosophical Transactions of the Royal Society B: Biological Sciences; Royal Society, March 29 2005; Vol. 360; pp. 631–635. [Google Scholar]
- Reynolds, T.Y.; Rockwell, S.; Glazer, P.M. Genetic Instability Induced by the Tumor Microenvironment. Cancer Res 1996, 56. [Google Scholar] [CrossRef]
- Gronroos, E.; Lopez-García, C. Tolerance of Chromosomal Instability in Cancer: Mechanisms and Therapeutic Opportunities. Cancer Res 2018, 78. [Google Scholar] [CrossRef]
- He, Q.; Au, B.; Kulkarni, M.; Shen, Y.; Lim, K.J.; Maimaiti, J.; Wong, C.K.; Luijten, M.N.H.; Chong, H.C.; Lim, E.H.; et al. Chromosomal Instability-Induced Senescence Potentiates Cell Non-Autonomous Tumourigenic Effects. Oncogenesis 2018, 7. [Google Scholar] [CrossRef]
- Nam, H.; Lee, I.; Sa, J.K.; Kim, S.S.; Pyeon, H.; Lee, K.H.; Lee, K.; Lee, S.; Joo, K.M. Correction to: Effects of Long-Term In Vitro Expansion on Genetic Stability and Tumor Formation Capacity of Stem Cells. Stem Cell Rev Rep 2022, 18. [Google Scholar] [CrossRef]
- Ferguson, L.P.; Diaz, E.; Reya, T. The Role of the Microenvironment and Immune System in Regulating Stem Cell Fate in Cancer. Trends Cancer 2021, 7. [Google Scholar] [CrossRef]
- Seet, W.T.; Afandi, M.A.M.; Shamsuddin, S.A.; Lokanathan, Y.; Ng, M.H.; Maarof, M. Current Good Manufacturing Practice (CGMP) Facility and Production of Stem Cell. In Stem Cell Production: Processes, Practices and Regulations; 2022.
- Aghayan, H.R.; Payab, M.; Mohamadi-Jahani, F.; Aghayan, S.S.; Larijani, B.; Arjmand, B. GMP-Compliant Production of Human Placenta-Derived Mesenchymal Stem Cells. In Methods in Molecular Biology; 2021; Vol. 2286.
- Ozkan, E.; Lacerda, M.P. Genetics, Cytogenetic Testing And Conventional Karyotype; 2021. [Google Scholar]
- Jackson-Holmes, E.L.; McDevitt, T.C.; Lu, H. A Microfluidic Trap Array for Longitudinal Monitoring and Multi-Modal Phenotypic Analysis of Individual Stem Cell Aggregates. Lab Chip 2017, 17. [Google Scholar] [CrossRef]
- Vitillo, L.; Durance, C.; Hewitt, Z.; Moore, H.; Smith, A.; Vallier, L. GMP-Grade Neural Progenitor Derivation and Differentiation from Clinical-Grade Human Embryonic Stem Cells. Stem Cell Res Ther 2020, 11. [Google Scholar] [CrossRef]
- Miura, T.; Yasuda, S.; Sato, Y. A Simple Method to Estimate the In-House Limit of Detection for Genetic Mutations with Low Allele Frequencies in Whole-Exome Sequencing Analysis by next-Generation Sequencing. BMC Genom Data 2021, 22. [Google Scholar] [CrossRef]
- Mulligan, S.P. Karyotype and Outcome in CLL. Blood 2023, 142. [Google Scholar] [CrossRef]
- Laing, O.; Halliwell, J.; Barbaric, I. Rapid PCR Assay for Detecting Common Genetic Variants Arising in Human Pluripotent Stem Cell Cultures. Curr Protoc Stem Cell Biol 2019, 49. [Google Scholar] [CrossRef] [PubMed]
- Bielski, C.M.; Taylor, B.S. Homing in on Genomic Instability as a Therapeutic Target in Cancer. Nat Commun 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in Phenotype and Differentiation Potential of Human Mesenchymal Stem Cells Aging in Vitro. Stem Cell Res Ther 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Chen, H.; Huang, D.; Chen, H.; Fei, L.; Cheng, C.; Huang, H.; Yuan, G.C.; Guo, G. Mapping Human Pluripotent Stem Cell Differentiation Pathways Using High Throughput Single-Cell RNA-Sequencing. Genome Biol 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Naujok, O.; Francini, F.; Picton, S.; Jörns, A.; Bailey, C.J.; Lenzen, S. A New Experimental Protocol for Preferential Differentiation of Mouse Embryonic Stem Cells into Insulin-Producing Cells. Cell Transplant 2008, 17. [Google Scholar] [CrossRef]
- Mitsui, K.; Ide, K.; Takahashi, T.; Kosai, K. ichiro Viral Vector-Based Innovative Approaches to Directly Abolishing Tumorigenic Pluripotent Stem Cells for Safer Regenerative Medicine. Mol Ther Methods Clin Dev 2017, 5. [Google Scholar]
- Afjeh-Dana, E.; Naserzadeh, P.; Moradi, E.; Hosseini, N.; Seifalian, A.M.; Ashtari, B. Stem Cell Differentiation into Cardiomyocytes: Current Methods and Emerging Approaches. Stem Cell Rev Rep 2022, 18. [Google Scholar]
- Naujok, O.; Diekmann, U.; Lenzen, S. The Generation of Definitive Endoderm from Human Embryonic Stem Cells Is Initially Independent from Activin A but Requires Canonical Wnt-Signaling. Stem Cell Rev Rep 2014, 10. [Google Scholar] [CrossRef]
- Fong, C.Y.; Peh, G.S.L.; Gauthaman, K.; Bongso, A. Separation of SSEA-4 and TRA-1-60 Labelled Undifferentiated Human Embryonic Stem Cells from a Heterogeneous Cell Population Using Magnetic-Activated Cell Sorting (MACS) and Fluorescence-Activated Cell Sorting (FACS). Stem Cell Rev Rep 2009, 5. [Google Scholar] [CrossRef]
- Li, Y.; Shen, Z.; Chai, Z.; Zhan, Y.; Zhang, Y.; Liu, Z.; Liu, Y.; Li, Z.; Lin, M.; Zhang, Z.; et al. Targeting MS4A4A on Tumour-Associated Macrophages Restores CD8+ T-Cell-Mediated Antitumour Immunity. Gut 2023, 72. [Google Scholar] [CrossRef] [PubMed]
- Buccarelli, M.; Beninati, S.; Tabolacci, C. Editorial: Cancer Stem Cell Differentiation: A Realistic Potential Therapeutic Option? Front Oncol 2023, 13. [Google Scholar] [CrossRef]
- Sutermaster, B.A.; Darling, E.M. Considerations for High-Yield, High-Throughput Cell Enrichment: Fluorescence versus Magnetic Sorting. Sci Rep 2019, 9. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.; Deng, H.; Woodworth, C.D.; Kaya, M. An Unsupervised Machine Learning Algorithm to Detect Undifferentiated Cell Clusters of Immortalized Human Cervical Epithelial Cell. In Proceedings of the BioSMART 2021 - Proceedings: 4th International Conference on Bio-Engineering for Smart Technologies; 2021. [Google Scholar]
- Marei, H.E.; Althani, A.; Afifi, N.; Hasan, A.; Caceci, T.; Pozzoli, G.; Morrione, A.; Giordano, A.; Cenciarelli, C. P53 Signaling in Cancer Progression and Therapy. Cancer Cell Int 2021, 21. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wood, B.L. Minimal Residual Disease in Acute Lymphoblastic Leukemia: Techniques and Application. In Clinical Management of Acute Lymphoblastic Leukemia: From Bench to Bedside; 2022.
- Yang, X.; Liu, M.; Li, M.; Zhang, S.; Hiju, H.; Sun, J.; Mao, Z.; Zheng, M.; Feng, B. Epigenetic Modulations of Noncoding RNA: A Novel Dimension of Cancer Biology. Mol Cancer 2020, 19. [Google Scholar] [CrossRef]
- Wang, Q.; Xiong, F.; Wu, G.; Liu, W.; Chen, J.; Wang, B.; Chen, Y. Gene Body Methylation in Cancer: Molecular Mechanisms and Clinical Applications. Clin Epigenetics 2022, 14. [Google Scholar] [CrossRef]
- De Cario, R.; Kura, A.; Suraci, S.; Magi, A.; Volta, A.; Marcucci, R.; Gori, A.M.; Pepe, G.; Giusti, B.; Sticchi, E. Sanger Validation of High-Throughput Sequencing in Genetic Diagnosis: Still the Best Practice? Front Genet 2020, 11. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, K.; Wang, W.L.; Yin, T.T.; Dong, W.Q.; Xu, C.J. A High-Throughput SNP Discovery Strategy for RNA-Seq Data. BMC Genomics 2019, 20. [Google Scholar] [CrossRef]
- Hong, M.; Tao, S.; Zhang, L.; Diao, L.T.; Huang, X.; Huang, S.; Xie, S.J.; Xiao, Z.D.; Zhang, H. RNA Sequencing: New Technologies and Applications in Cancer Research. J Hematol Oncol 2020, 13. [Google Scholar] [CrossRef]
- Roychowdhury, S.; Iyer, M.K.; Robinson, D.R.; Lonigro, R.J.; Wu, Y.M.; Cao, X.; Kalyana-Sundaram, S.; Sam, L.; Balbin, O.A.; Quist, M.J.; et al. Personalized Oncology through Integrative High-Throughput Sequencing: A Pilot Study. Sci Transl Med 2011, 3. [Google Scholar] [CrossRef]
- Khoshandam, M.; Soltaninejad, H.; Mousazadeh, M.; Hamidieh, A.A.; Hosseinkhani, S. Clinical Applications of the CRISPR/Cas9 Genome-Editing System: Delivery Options and Challenges in Precision Medicine. Genes Dis 2024, 11. [Google Scholar] [CrossRef]
- Li, T.; Li, S.; Kang, Y.; Zhou, J.; Yi, M. Harnessing the Evolving CRISPR/Cas9 for Precision Oncology. J Transl Med 2024, 22, 749. [Google Scholar] [CrossRef] [PubMed]
- Chehelgerdi, M.; Chehelgerdi, M.; Khorramian-Ghahfarokhi, M.; Shafieizadeh, M.; Mahmoudi, E.; Eskandari, F.; Rashidi, M.; Arshi, A.; Mokhtari-Farsani, A. Comprehensive Review of CRISPR-Based Gene Editing: Mechanisms, Challenges, and Applications in Cancer Therapy. Mol Cancer 2024, 23. [Google Scholar]
- Bak, R.O.; Dever, D.P.; Porteus, M.H. CRISPR/Cas9 Genome Editing in Human Hematopoietic Stem Cells. Nat Protoc 2018, 13. [Google Scholar] [CrossRef]
- Ugalde, L.; Fañanas, S.; Torres, R.; Quintana-Bustamante, O.; Río, P. CRISPR/Cas9-Mediated Gene Editing. A Promising Strategy in Hematological Disorders. Cytotherapy 2023, 25. [Google Scholar] [CrossRef] [PubMed]
- Chemello, F.; Bassel-Duby, R.; Olson, E.N. Correction of Muscular Dystrophies by CRISPR Gene Editing. Journal of Clinical Investigation 2020, 130. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Shi, H.; Gou, S.; Wang, X.; Li, L.; Jin, Q.; Wu, H.; Zhang, H.; Li, Y.; Wang, L.; et al. In Vivo Genome Editing in Mouse Restores Dystrophin Expression in Duchenne Muscular Dystrophy Patient Muscle Fibers. Genome Med 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- DeWitt, M.A.; Magis, W.; Bray, N.L.; Wang, T.; Berman, J.R.; Urbinati, F.; Muñoz, D.P.; Kohn, D.B.; Walters, M.C.; Carroll, D.; et al. Efficient Correction of the Sickle Mutation in Human Hematopoietic Stem Cells Using a Cas9 Ribonucleoprotein Complex. bioRxiv 2016. [Google Scholar]
- Rheney, J. Using Crisper in the Treatment of Sickle Cell Disease. Journal of Student Research 2023, 12. [Google Scholar] [CrossRef]
- Kits, E. CRISPR-Cas9, TALENs and ZFNs - the Battle in Gene Editing. ProteinTech 2018. [Google Scholar]
- Castro, N.G.; Bjelic, J.; Malhotra, G.; Huang, C.; Alsaffar, S.H. Comparison of the Feasibility, Efficiency, and Safety of Genome Editing Technologies. Int J Mol Sci 2021, 22. [Google Scholar]
- Zentelytė, A.; Žukauskaitė, D.; Jacerytė, I.; Borutinskaitė, V.V.; Navakauskienė, R. Small Molecule Treatments Improve Differentiation Potential of Human Amniotic Fluid Stem Cells. Front Bioeng Biotechnol 2021, 9. [Google Scholar] [CrossRef]
- Schugar, R.C.; Robbins, P.D.; Deasy, B.M. Small Molecules in Stem Cell Self-Renewal and Differentiation. Gene Ther 2008, 15. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, W.; Liu, S.; Chen, C. Targeting Breast Cancer Stem Cells. Int J Biol Sci 2023, 19. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Bhonde, R. Genetic and Epigenetic Stability of Stem Cells: Epigenetic Modifiers Modulate the Fate of Mesenchymal Stem Cells. Genomics 2020, 112. [Google Scholar] [CrossRef]
- Tran, K.A.; Jackson, S.A.; Olufs, Z.P.G.; Zaidan, N.Z.; Leng, N.; Kendziorski, C.; Roy, S.; Sridharan, R. Collaborative Rewiring of the Pluripotency Network by Chromatin and Signalling Modulating Pathways. Nat Commun 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Huangfu, D.; Osafune, K.; Maehr, R.; Guo, W.; Eijkelenboom, A.; Chen, S.; Muhlestein, W.; Melton, D.A. Induction of Pluripotent Stem Cells from Primary Human Fibroblasts with Only Oct4 and Sox2. Nat Biotechnol 2008, 26. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Chen, X.; Yu, D.; Li, T.; Cui, J.; Wang, G.; Hu, J.F.; Li, W. Histone Deacetylase Inhibitor Valproic Acid Promotes the Induction of Pluripotency in Mouse Fibroblasts by Suppressing Reprogramming-Induced Senescence Stress. Exp Cell Res 2015, 337. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tristan, C.A.; Chen, L.; Jovanovic, V.M.; Malley, C.; Chu, P.H.; Ryu, S.; Deng, T.; Ormanoglu, P.; Tao, D.; et al. A Versatile Polypharmacology Platform Promotes Cytoprotection and Viability of Human Pluripotent and Differentiated Cells. Nat Methods 2021, 18. [Google Scholar] [CrossRef]
- Geng, Y.; Amante, J.J.; Goel, H.L.; Zhang, X.; Walker, M.R.; Luther, D.C.; Mercurio, A.M.; Rotello, V.M. Differentiation of Cancer Stem Cells through Nanoparticle Surface Engineering. ACS Nano 2020, 14. [Google Scholar] [CrossRef]
- Marquardt, J.U.; Raggi, C.; Andersen, J.B.; Seo, D.; Avital, I.; Holczbauer, A.; Factor, V.M.; Thorgeirsson, S.S. Abstract 4252: Mechanistic and Clinical Implications of Epigenetic Modulation in Liver Cancer Stem Cells. Cancer Res 2010, 70. [Google Scholar] [CrossRef]
- KalantarMotamedi, Y.; Peymani, M.; Baharvand, H.; Nasr-Esfahani, M.H.; Bender, A. Systematic Selection of Small Molecules to Promote Differentiation of Embryonic Stem Cells and Experimental Validation for Generating Cardiomyocytes. Cell Death Discov 2016, 2. [Google Scholar] [CrossRef] [PubMed]
- Kevin, W.-H.L.; Jenny, C.-Y.H.; Kong, C.-W.; Lee, Y.-K.; Ng, K.-M.; Au, K.-W.; Chan, Y.-C.; Lau, C.-P.; Tse, H.-F.; Siu, C.-W. P135. Transient Rho Kinase Inhibition Enhance the Generation of Human Induced Pluripotent Stem Cells in Feeder-Independent, Serum-Free Culture System. Differentiation 2010, 80. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).