1. Introduction
Cardiofaciocutaneous (CFC) syndrome belongs to the group of RASopathies, a collection of rare genetic disorders. Initially documented in 1986, CFC syndrome is exceptionally uncommon[1]. RASopathies encompass a spectrum of genetic syndromes caused by mutations in genes encoding components of the RAS-MAPK signaling pathway. These disorders affect various organ systems and are characterized by a range of clinical features, including facial dysmorphisms (such as hypertelorism, low-set ears, and short stature), cardiac anomalies (such as hypertrophic cardiomyopathy and pulmonary valve stenosis), neurocognitive impairments, musculoskeletal abnormalities, and an increased predisposition to certain malignancies.RASopathies share a common underlying molecular pathway dysregulation, making them a significant area of research and clinical interest in medical genetics[2,3,4]. Costello and Noonan syndromes exhibit similarities with CFC syndrome, possibly stemming from the biochemical connections among the mutated genes in each condition. The genes affected in all three syndromes play roles in the MAP kinase pathway[1,2,3,4].
Mutations implicated in cardiofaciocutaneous (CFC) syndrome are located in genes such as KRAS, BRAF, MEK1, and MEK2. These mutations disrupt the normal functioning of the RAS-MAPK signaling pathway, leading to the characteristic features of the syndrome [5,6].
Currently, there is no specific therapy available for cardiofaciocutaneous (CFC) syndrome. Treatment primarily focuses on managing the symptoms and complications associated with the condition. This may involve a multidisciplinary approach, including interventions such as physical therapy, occupational therapy, speech therapy, and educational support to address developmental delays and cognitive impairments. Additionally, symptomatic management of cardiac abnormalities, gastrointestinal issues, and other medical concerns may be necessary. Research efforts continue to explore potential targeted therapies or interventions to address the underlying molecular mechanisms of CFC syndrome[1,2,3,4].
2. Material and Methods
Crystal structure of BRAF target. The B-RAF proto-oncogene serine/threonine-protein kinase, commonly referred to as B-RAF, is a protein kinase that plays a crucial role in the RAS-MAPK signaling pathway. This structure was taken by Protein Data Bank, (PDB Code: 1UWH) and was evaluated by Autodock Vina with Pyrx program, using the following coordienate: Grid box Coordinates of binding Center X ( 72.7339569655 Å), Y( 43.3583608556 Å), Z(65.4433627357 Å) with these dimensions: size_x = 15.9381936398Å, size_y = 17.8877913107Å, size_z = 15.9190885074 Å
Cristal structure of Serine/threonine-protein kinase commonly known as B-RAF, is a protein kinase that plays a crucial role in cell signaling pathways involved in cell growth, proliferation, and differentiation. It is a member of the RAF family of kinases, which also includes A-RAF and C-RAF (RAF-1).
This structure was taken by Protein Data Bank, (PDB Code: 6V2U) and was evaluated by Autodock Vina with Pyrx program, using the following coordienate: Grid box coordinates of binding Center X ( -21.8904333387 Å), Y( 2.5165693616 Å), Z(3.86251123716Å) with these dimensions: size_x = 18.6840073104Å, size_y = 13.2757498727Å, size_z = 15.3563413366Å
Crystal structure of Dual specificity mitogen-activated protein kinase kinase 1 (MEK-1) .It is a crucial component of the RAS-MAPK signaling pathway. It acts as an intermediate kinase, relaying signals from activated RAS proteins to downstream targets, including ERK (Extracellular signal-regulated kinase) proteins. This structure was taken by Protein Data Bank, (PDB Code: 3DV3) and was evaluated by Autodock Vina with Pyrx program, using the following coordinates: Grid box Coordinates of binding Center X ( 39.7557617112 Å), Y( -13.6367545795Å), Z(-1.38867059557 Å) with these dimensions: size_x = 12.4215691793Å, size_y = 15.3563413366Å, size_z = 15.3563413366 Å
- part A of BRAF
The crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB). This structure consists of 805 amino acids. Docking analysis was performed targeting specific amino acids within the BRAF protein sequence that have been implicated in cardio-facio-cutaneous syndrome (CFC). Based on research findings from several papers, the docking analysis focused on amino acids within BRAF that are believed to be key residues contributing to CFC syndrome-associated changes [6,12,13,14].These amino acids were likely identified through functional studies, genetic analyses, and structural modeling[6,12,13,14].
Position 467-S, Position 468-F, Position 469-G, Position 485-L, Position 499 -K , Position 501- E Position 525-L,Position 580- N,Position 581-N,Position 595-F,Position 596-G,Position 599-T,Position 601- K,Position 638- D, Position 709- Q . Docking simulations were evaluated by Autodock Vina with Pyrx program, using the following coordinates: Grid box Coordinates of binding Center X ( 133.560427876Å), Y( 111.426389374 Å), Z(144.367042643Å) with these dimensions: size_x = 24.7421442473Å, size_y = 33.3557394974Å, size_z = 22.6784331074Å
First, docking was performed on the non-mutated amino acids on the BRAF protein, and then on those mutated responsible for CFC:
VAR_035096: Position 467, S>A (Serine to Alanine) [6]
VAR_035097: Position 468, F>S (Phenylalanine to Serine) [6]
VAR_018621: Position 469, G>E (Glycine to Glutamic Acid) [14]
VAR_026115: Position 485, L>F (Leucine to Phenylalanine) [6]
VAR_026116: Position 499, K>E (Lysine to Glutamic Acid) [6]
VAR_026117: Position 501, E>G (Glutamic Acid to Glycine) [6]
VAR_058626: Position 525, L>P (Leucine to Proline) [13]
VAR_065173: Position 580, N>D (Asparagine to Aspartic Acid) [12]
VAR_026119: Position 581, N>D (Asparagine to Aspartic Acid) [6]
VAR_018625: Position 595, F>L (Phenylalanine to Leucine) [14]
VAR_035098: Position 596, G>V (Glycine to Valine) [6]
VAR_058628: Position 599, T>R (Threonine to Arginine) [13]
VAR_058629: Position 601, K>Q (Lysine to Glutamine) [13]
VAR_058630: Position 638, D>E (Aspartic Acid to Glutamic Acid)[13]
VAR_058631: Position 709, Q>R (Glutamine to Arginine)[13]
- part B of BRAF
The crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB). This structure consists of 805 amino acids. Docking analysis was performed targeting specific amino acids within the BRAF protein sequence that have been implicated in cardio-facio-cutaneous syndrome (CFC). Based on research findings from several papers, the docking analysis focused on amino acids within BRAF that are believed to be key residues contributing to CFC syndrome-associated changes [6,12-14].These amino acids were likely identified through functional studies, genetic analyses, and structural modeling[6,12-14].
Position 241- T, Position 244-T, Position 245,-L, Position 246- A, Position 257- Q ,Position 262-Q Position 275-, E. Docking simulations were evaluated by Autodock Vina with Pyrx program, using the following coordinates: Grid box Coordinates of binding Center X ( 126.700644084Å), Y( 132.3250793 Å), Z(113.819859488Å) with these dimensions: size_x = 27.831363389Å, size_y = 27.7795813891Å, size_z = 23.6216084532Å
First, docking was performed on the non-mutated amino acids on the BRAF protein, and then on those mutated responsible for CFC:
R_058621: Position 241, T>P (Threonine to Proline) [12]
VAR_065171: Position 244, T>P (Threonine to Proline) [12]
VAR_058623: Position 245, L>F (Leucine to Phenylalanine) [13]
VAR_026113: Position 246, A>P (Alanine to Proline) [12,13]
VAR_026114: Position 257, Q>R (Glutamine to Arginine) [6]
VAR_065172: Position 262, Q>K (Glutamine to Lysine) [12]
VAR_058624: Position 275, E>K (Glutamic Acid to Lysine) [13]
-Crystal structure of the GTPase KRAS, often referred to simply as KRAS, is a member of the Ras family of small GTPases. It plays a critical role in cell signaling pathways that regulate cell proliferation, differentiation, and survival. KRAS acts as a molecular switch, cycling between an active (GTP-bound) state and an inactive (GDP-bound) state, depending on its nucleotide-binding state. This structure was taken by Protein Data Bank, (PDB Code: 4WA7) and was evaluated by Autodock Vina with Pyrx program, using the following coordienate: Grid box Coordinates of binding Center X ( 39.7557617112 Å), Y( -13.6367545795Å), Z(-1.38867059557 Å) with these dimensions: size_x = 12.4215691793Å, size_y = 15.3563413366Å, size_z = 15.3563413366 Å
3. Results and Discussion
Cardiofaciocutaneous (CFC) syndrome, a member of the RASopathies, represents a group of rare genetic disorders initially documented in 1986. This syndrome is characterized by its rarity and encompasses various clinical features affecting multiple organ systems. These features include facial dysmorphisms such as hypertelorism and low-set ears, cardiac anomalies like hypertrophic cardiomyopathy, neurocognitive impairments, musculoskeletal abnormalities, and an increased predisposition to certain malignancies. RASopathies, including CFC syndrome, stem from mutations in genes encoding components of the RAS-MAPK signaling pathway, highlighting a common molecular dysregulation underlying these conditions [1,2,3,4,5]
Costello and Noonan syndromes, closely related to CFC syndrome, share similarities possibly due to the biochemical connections among the mutated genes involved. Notably, all three syndromes involve genes playing roles in the MAP kinase pathway. Mutations implicated in CFC syndrome reside in genes such as KRAS, BRAF, MEK1, and MEK2, which disrupt the normal functioning of the RAS-MAPK signaling pathway, leading to the characteristic features of the syndrome. Currently, there is no specific therapy for CFC syndrome, and treatment primarily revolves around managing associated symptoms and complications[1,2,3,4,5].
This study utilized molecular docking simulations[7,8,9], a powerful computational technique, to screen natural compounds against mutated KRAS, BRAF, MEK1, and MEK2 genes implicated in Cardiofaciocutaneous (CFC) syndrome. Through this approach, several promising compounds are identified with strong binding affinities to the mutated sites, suggesting their potential as targeted therapies for CFC syndrome.
One key implication of our findings is the potential repurposing of natural compounds as therapeutic agents for CFC syndrome.
Amentoflavone and Hypericin, among others, emerged as lead candidates with promising binding properties.These compounds have previously been studied for their pharmacological activities[10,11], offering a foundation for further preclinical and clinical investigations in the context of CFC syndrome.
Several papers reported in Literature, showed potential mutations in Serine/threonine-protein kinase BRAF of CFC pathology[6,12,13,14]:
Here is a summary of the natural variants identified in the Cardiofaciocutaneous syndrome 1 (CFC 1) gene:
VAR_058621: Position 241, T>P (Threonine to Proline) [12]
VAR_065171: Position 244, T>P (Threonine to Proline) [12]
VAR_058623: Position 245, L>F (Leucine to Phenylalanine) [13]
VAR_026113: Position 246, A>P (Alanine to Proline) [12,13]
VAR_026114: Position 257, Q>R (Glutamine to Arginine) [6]
VAR_065172: Position 262, Q>K (Glutamine to Lysine) [12]
VAR_058624: Position 275, E>K (Glutamic Acid to Lysine) [13]
VAR_035096: Position 467, S>A (Serine to Alanine) [6]
VAR_035097: Position 468, F>S (Phenylalanine to Serine) [6]
VAR_018621: Position 469, G>E (Glycine to Glutamic Acid) [14]
VAR_026115: Position 485, L>F (Leucine to Phenylalanine) [6]
VAR_026116: Position 499, K>E (Lysine to Glutamic Acid) [6]
VAR_026117: Position 501, E>G (Glutamic Acid to Glycine) [6]
VAR_058626: Position 525, L>P (Leucine to Proline) [13]
VAR_065173: Position 580, N>D (Asparagine to Aspartic Acid) [12]
VAR_026119: Position 581, N>D (Asparagine to Aspartic Acid) [6]
VAR_018625: Position 595, F>L (Phenylalanine to Leucine) [14]
VAR_035098: Position 596, G>V (Glycine to Valine) [6]
VAR_058628: Position 599, T>R (Threonine to Arginine) [13]
VAR_058629: Position 601, K>Q (Lysine to Glutamine) [13]
VAR_058630: Position 638, D>E (Aspartic Acid to Glutamic Acid)[13]
VAR_058631: Position 709, Q>R (Glutamine to Arginine)[13]
These variants represent natural genetic variations within the CFC1 gene and are associated with CFC1-related conditions, including cardiofaciocutaneous syndrome (CFC) and other disorders. They have been documented in various publications and databases such as dbSNP and Uniprot ( BRAF - Serine/threonine-protein kinase B-raf - Homo sapiens (Human) | UniProtKB | UniProt).
The basic idea of this molecular docking-based study was initially to perform an initial screening of a library of natural molecules in the active site of the main proteins mutated in CFC (in this case, BRAF, MEK-1, and KRAS). Subsequently, to perform an even more accurate docking, in the areas of BRAF amino acids that easily mutate in the CFC syndrome, as reported in various studies ([6-12-14]), taking into account the best natural molecules selected that have been (Amentoflavone and Hypericin). From all these investigations, Hypericin and Amentoflavone obtained excellent binding energies, both in the active site of the investigated proteins and in the areas of amino acids that tend to mutate, hypothesizing that these molecules could be a potential weapon of cure against this disease, blocking the mutated genes, although further biological studies are needed to confirm these computational findings.
However, it's important to acknowledge the limitations of this study. Molecular docking simulations provide valuable insights into potential interactions between compounds and target proteins, but experimental validation is essential to confirm the predicted binding affinities and assess the compounds' efficacy and safety in vivo. Additionally, the complexity of the RAS-MAPK signaling pathway and the heterogeneity of CFC syndrome may necessitate a multi-target approach or combination therapy to achieve optimal therapeutic outcomes.
Future research directions may involve in vitro and in vivo studies to validate the identified lead compounds and elucidate their mechanisms of action. Furthermore, structural optimization and medicinal chemistry efforts can be pursued to enhance the potency, selectivity, and pharmacokinetic properties of the lead molecules.
| Compounds |
Binding
Energies (1) with
BRAF
( Kcal/mol)
|
Binding
Energies with
MEK-1
( Kcal/mol)
|
Binding Energies with KRAS
(Kcal/mol)
|
Binding
Energies (2)
with
BRAF
( Kcal/mol)
|
| Crystal Ligand Bax * |
-12.1 |
-9.4 |
-8.1 |
-9 |
| Amentoflavone |
-10.3 |
-9.5 |
-10 |
-9.5 |
| Diosmin |
-10.2 |
-8.7 |
-8 |
-8.8 |
| Emodin |
-10.5 |
-8 |
-8 |
-9.1 |
| Hypericin |
-9.8 |
-10.4 |
-7.1 |
-12.7 |
| Crystal Ligand MEK ** |
/ |
-8.9 |
|
/ |
| Crystal Ligand GDP*** |
/ |
/ |
-10.1 |
/ |
| Crystal Ligand Dabrafenib |
|
|
|
-11.9 |
| *4-{4-[({[4-CHLORO-3-(TRIFLUOROMETHYL)PHENYL]AMINO}CARBONYL)AMINO]PHENOXY}-N-METHYLPYRIDINE-2-CARBOXAMIDE. **3,4-difluoro-2-[(2-fluoro-4-iodophenyl)amino]-N-(2-hydroxyethoxy)-5-[(2-hydroxyethoxy)methyl]benzamide ***GUANOSINE-5'-DIPHOSPHATE. |
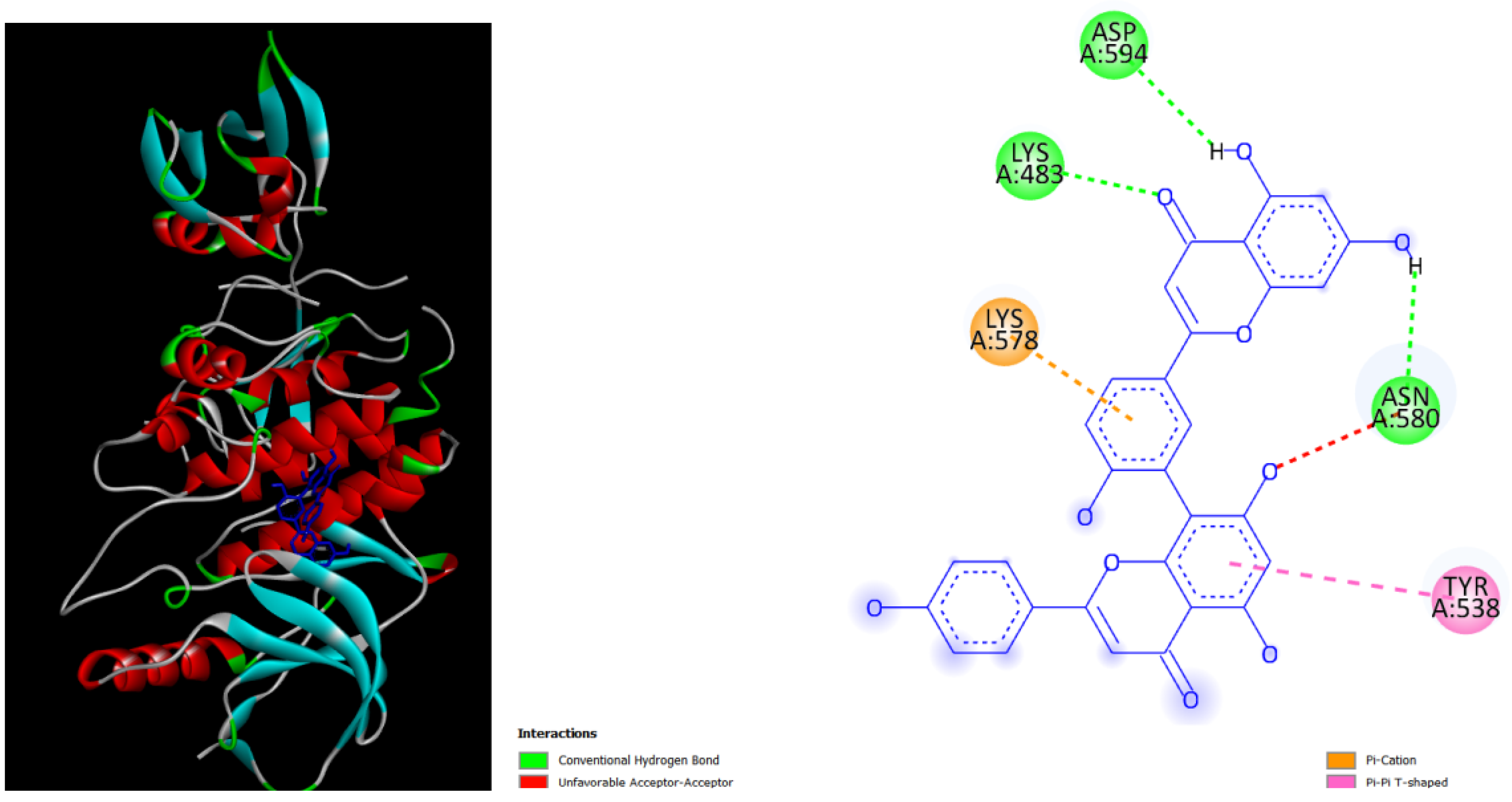
Figure 1.
displays the docking outcomes of Part A of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB), in conjunction with Amentoflavone -7.3 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Amentoflavone .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
Figure 1.
displays the docking outcomes of Part A of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB), in conjunction with Amentoflavone -7.3 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Amentoflavone .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
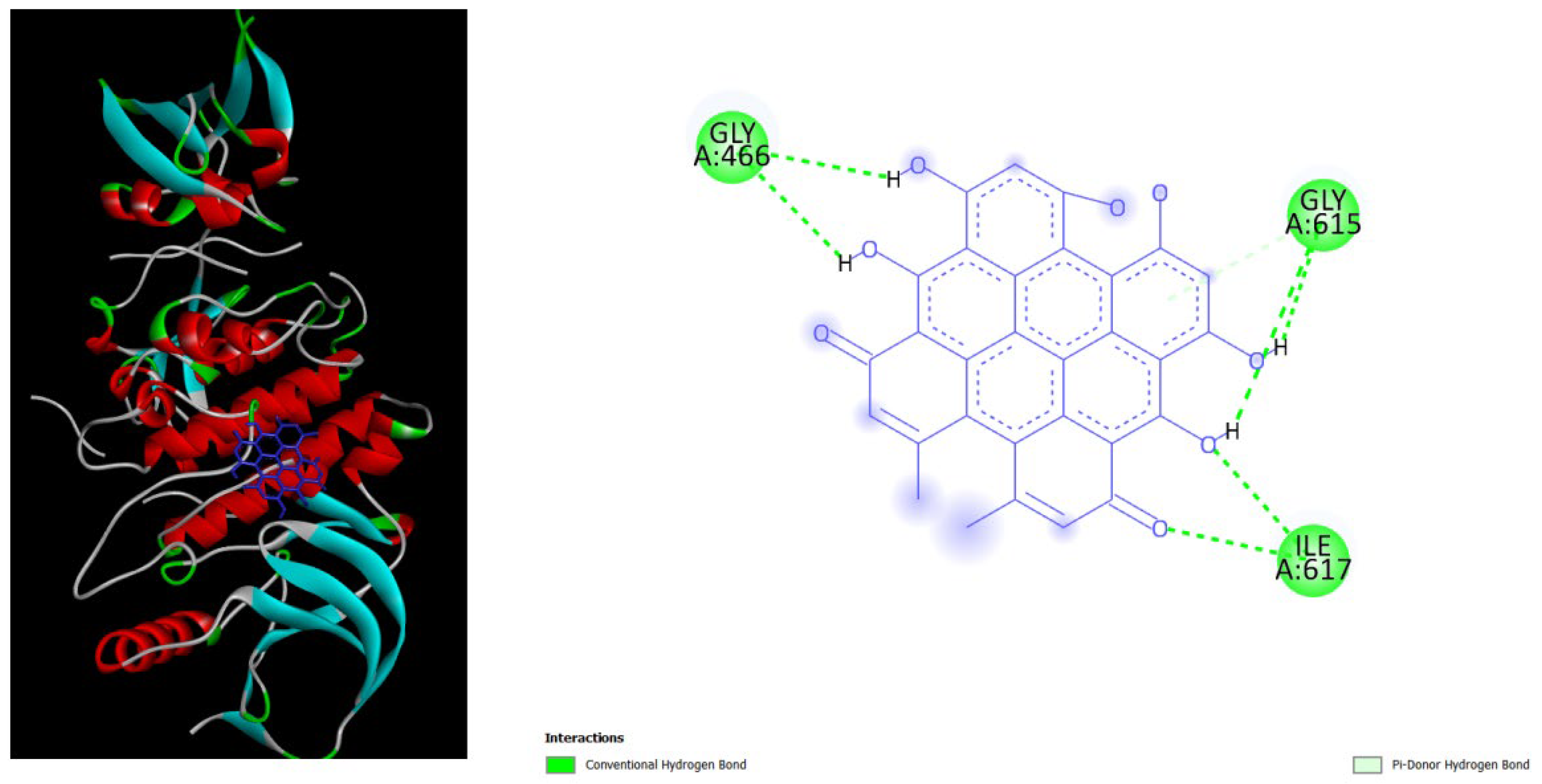
Figure 2.
displays the docking outcomes of Part A of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB),in conjunction with Hypericin -5.8 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Hypericin .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
Figure 2.
displays the docking outcomes of Part A of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB),in conjunction with Hypericin -5.8 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Hypericin .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
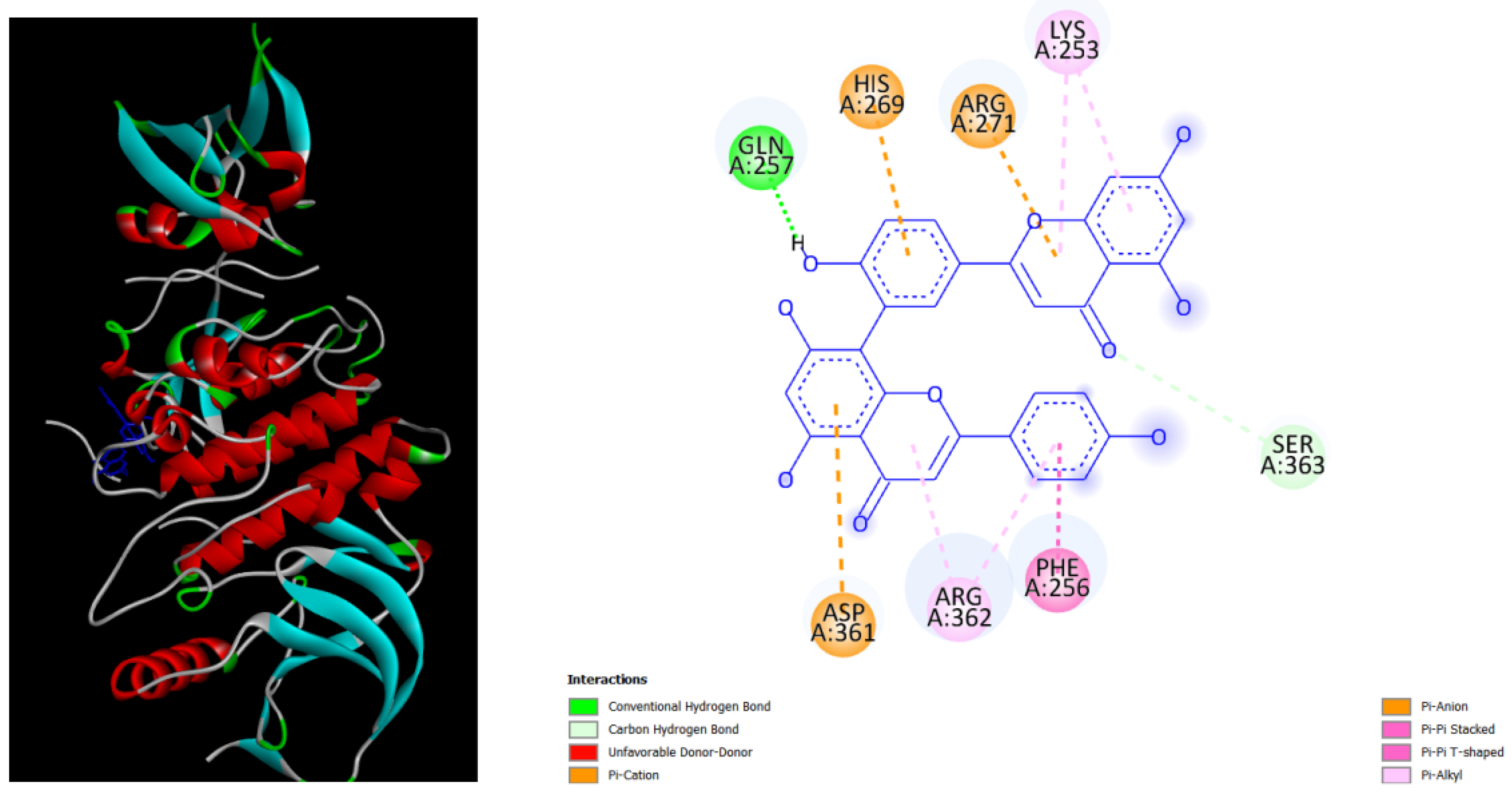
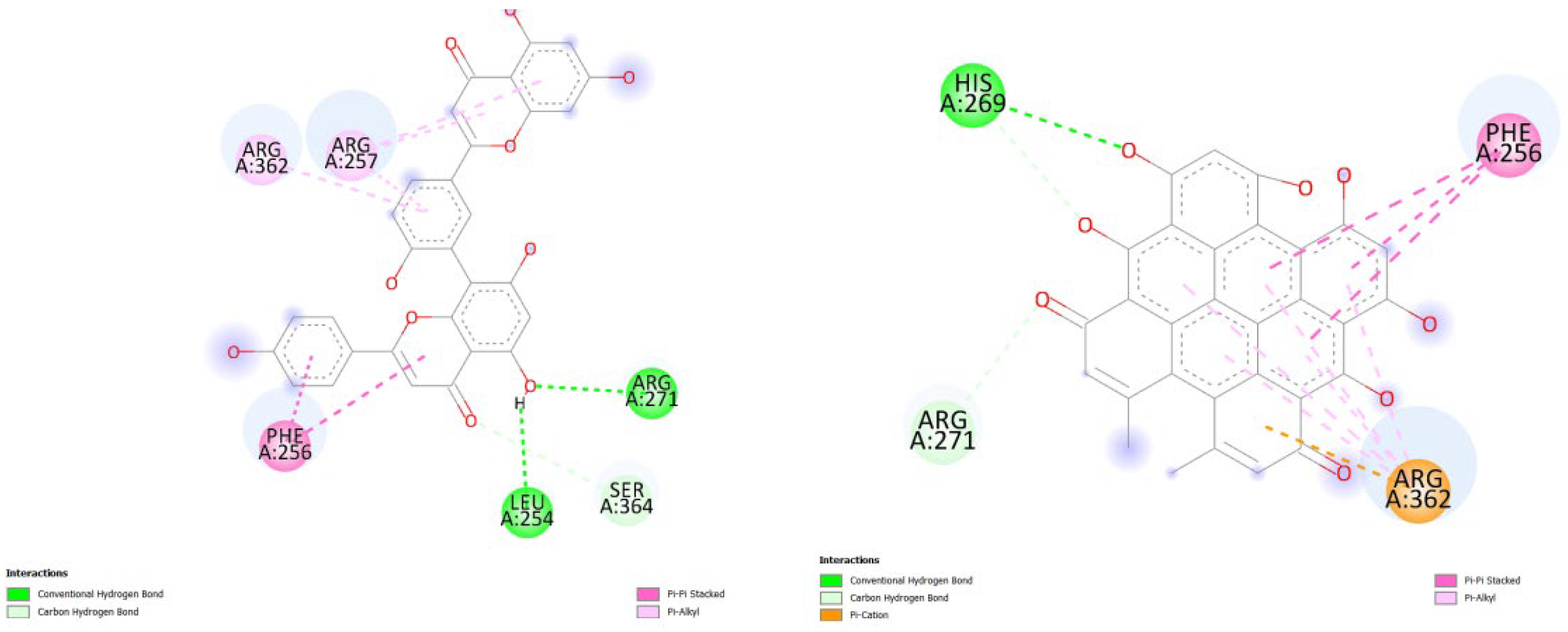
Figure 3.
displays the docking outcomes of Part B of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB), in conjunction with Amentoflavone -9.2 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Amentoflavone .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
Figure 3.
displays the docking outcomes of Part B of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB), in conjunction with Amentoflavone -9.2 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Amentoflavone .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
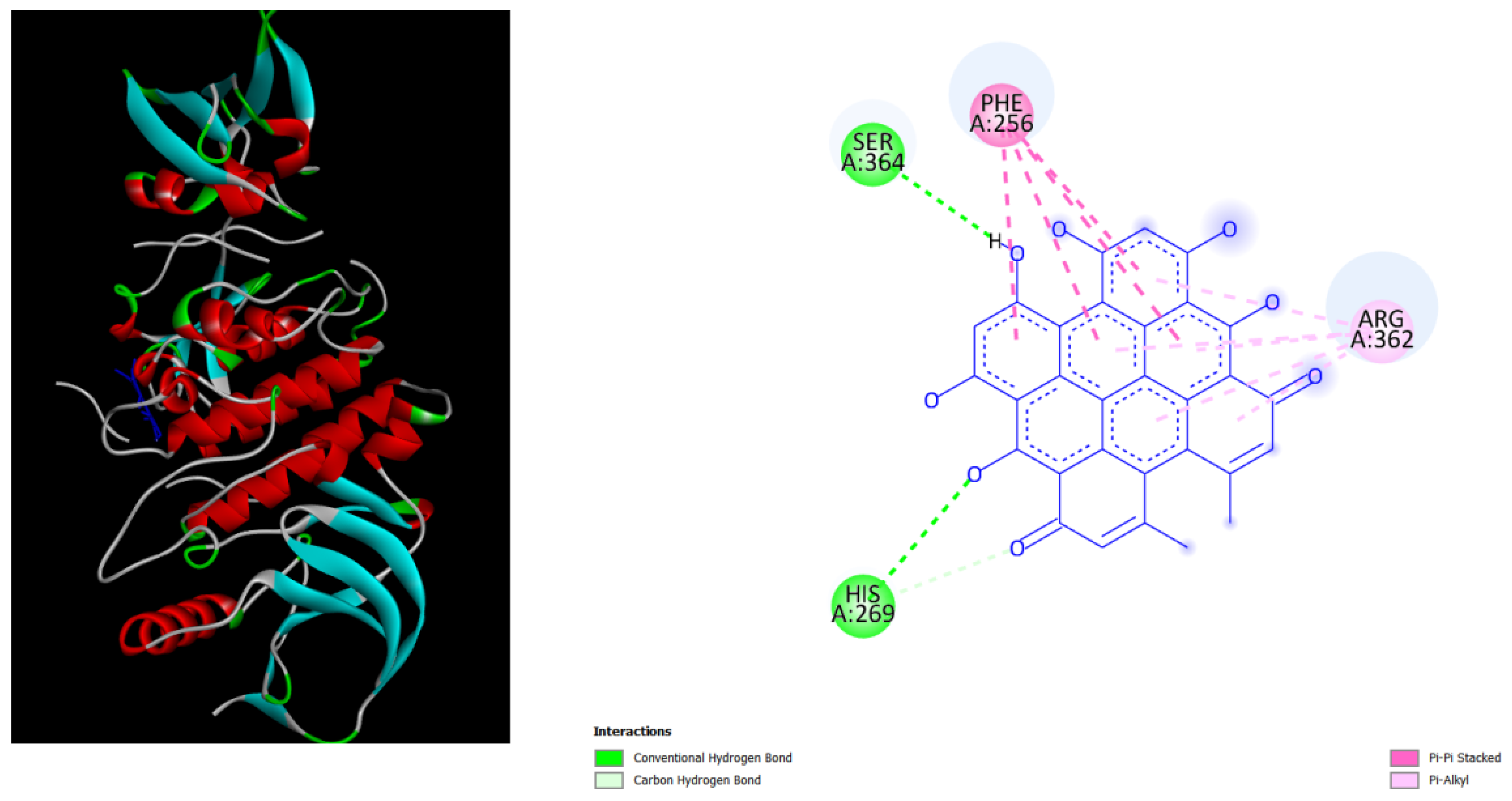
Figure 4.
displays the docking outcomes of Part B of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB), in conjunction with Hypericin -9.9 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Hypericin .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
Figure 4.
displays the docking outcomes of Part B of crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT was obtained from the Protein Data Bank (PDB), in conjunction with Hypericin -9.9 kcal/mol, analyzed by Autodock Vina with Pyrx program. On the left side, 2D diagrams illustrate the residue interactions between the protein and Hypericin .On the right side, potential area of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome are highlighted. .
In summary, our study underscores the potential of computational approaches in drug discovery and highlights the promise of natural compounds as targeted therapies for CFC syndrome. While further research and clinical validation are needed, our findings offer hope for improved management of this rare genetic disorder and pave the way for future therapeutic advancements.
From all the docking experiments, it was understood that hypericin and amentoflavone could be potential molecules against CFC because they showed a good ability to bind to the BRAF protein. Additionally, this study identified mainly on which amino acids they could bind better, which are responsible for CFC, namely:
VAR_058621: Position 241, T>P (Threonine to Proline) [
12]
VAR_065171: Position 244, T>P (Threonine to Proline) [
12]
VAR_058623: Position 245, L>F (Leucine to Phenylalanine) [
13]
VAR_026113: Position 246, A>P (Alanine to Proline) [
12,
13]
VAR_026114: Position 257, Q>R (Glutamine to Arginine) [
6]
VAR_065172: Position 262, Q>K (Glutamine to Lysine) [
12]
VAR_058624: Position 275, E>K (Glutamic Acid to Lysine) [
13]
In particular, it has been understood that Hypericin appears to bind better to the BRAF protein, whereas Amentoflavone also binds to BRAF but with a slightly lower capacity compared to hypericin. Hypericin has a binding energy of -10.1 kcal/mol, while amentoflavone has a binding energy of -9.2 kcal/mol. However, Amentoflavone also binds well to the KRAS protein (-10 kcal/mol vs. -7 kcal/mol of Hypericin). In the case of the MEK-1 protein, both molecules bind well, with Hypericin having a binding energy of -10.5 kcal/mol compared to -9.5 kcal/mol of Amentoflavone. Overall, both molecules could be useful against Cardio-facio-cutaneous (CFC) syndrome.
Figure 5.
illustrates the docking results from Part B of the crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT. The 2D diagrams depict the residue interactions between the protein and Amentoflavone/Hypericin, obtained from the Protein Data Bank (PDB). Specifically, the left side shows the interactions with Amentoflavone (-9.2 kcal/mol), while the right side shows the interactions with Hypericin (-10.1 kcal/mol), analyzed using Autodock Vina with the Pyrx program. The diagrams highlight the potential zone of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome.
Figure 5.
illustrates the docking results from Part B of the crystal structure of Serine/threonine-protein kinase B-RAF (BRAF) with PDB code 8DGT. The 2D diagrams depict the residue interactions between the protein and Amentoflavone/Hypericin, obtained from the Protein Data Bank (PDB). Specifically, the left side shows the interactions with Amentoflavone (-9.2 kcal/mol), while the right side shows the interactions with Hypericin (-10.1 kcal/mol), analyzed using Autodock Vina with the Pyrx program. The diagrams highlight the potential zone of key residues of the protein that could be mutated in cardio-facio-cutaneous (CFC) syndrome.
4. Conclusions
In conclusion, our study represents a significant step forward in the quest for therapeutic interventions for Cardiofaciocutaneous (CFC) syndrome, a rare genetic disorder with limited treatment options. By employing molecular docking simulations, we screened a library of natural compounds against mutated KRAS, BRAF, MEK1, and MEK2 genes, key players in the dysregulated RAS-MAPK signaling pathway underlying CFC syndrome. Our findings identified several promising compounds, including Amentoflavone and Hypericin, exhibiting strong binding affinities and favorable interactions with mutated sites. These compounds hold potential as lead molecules for further development as targeted therapies. Through this research, we highlight the feasibility of utilizing computational approaches to expedite the drug discovery process and offer new avenues for therapeutic intervention in CFC syndrome. Further experimental validation and clinical studies are warranted to assess the efficacy and safety of these compounds, paving the way for improved management of this challenging genetic disorder.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Scorrano, G., David, E., Calì, E., Chimenz, R., La Bella, S., Di Ludovico, A., ... & Ceravolo, G. (2023). The Cardiofaciocutaneous Syndrome: From Genetics to Prognostic–Therapeutic Implications. Genes, 14(12), 2111. [CrossRef]
- Musto, E., Gambardella, M. L., Perulli, M., Quintiliani, M., Veredice, C., Verdolotti, T., ... & Battaglia, D. I. (2024). Status epilepticus in BRAF-related cardio-facio-cutaneous syndrome: Focus on neuroimaging clues to physiopathology. Epilepsia Open, 9(1), 258-267. [CrossRef]
- Feng, B., Li, X., Zhang, Q., Wang, Y., Gu, S., Yao, R. E., ... & Wang, X. (2023). Molecular and phenotypic spectrum of cardio-facio-cutaneous syndrome in Chinese patients. Orphanet Journal of Rare Diseases, 18(1), 284. [CrossRef]
- Onesimo, R., Sforza, E., Giorgio, V., Viscogliosi, G., Kuczynska, E. M., Margiotta, G., ... & Zampino, G. (2023). The “FEEDS (FEeding Eating Deglutition Skills)” over Time Study in Cardiofaciocutaneous Syndrome. Genes, 14(7), 1338. [CrossRef]
- Kenney-Jung, D. L., Collazo-Lopez, J. E., Rogers, D. J., Shanley, R., Zatkalik, A. L., Whitmarsh, A. E., ... & Pierpont, E. I. (2024). Epilepsy in cardiofaciocutaneous syndrome: Clinical burden and response to anti-seizure medication. American Journal of Medical Genetics Part A, 194(2), 301-310. [CrossRef]
- Rodriguez-Viciana, Pablo, Osamu Tetsu, William E. Tidyman, Anne L. Estep, Brenda A. Conger, Molly Santa Cruz, Frank McCormick, and Katherine A. Rauen. "Germline mutations in genes within the MAPK pathway cause cardio-facio-cutaneous syndrome." Science 311, no. 5765 (2006): 1287-1290. [CrossRef]
- Azad, I. (2023). Molecular Docking in the Study of Ligand-Protein Recognition: An Overview. Molecular Docking-Recent Advances.
- Mohanty, M., & Mohanty, P. S. (2023). Molecular docking in organic, inorganic, and hybrid systems: A tutorial review. Monatshefte für Chemie-Chemical Monthly, 154(7), 683-707.
- Ding, J., Tang, S., Mei, Z., Wang, L., Huang, Q., Hu, H., ... & Wu, J. (2023). Vina-GPU 2.0: Further Accelerating AutoDock Vina and Its Derivatives with Graphics Processing Units. Journal of Chemical Information and Modeling, 63(7), 1982-1998. [CrossRef]
- Ferrari, Ivan Vito. "Exploring the Interaction of E3 Ubiquitin-Protein Ligase Parkin with Natural Compound Amentoflavone: Implications for Parkinson’s Disease Therapy." (2024). Preprint.
- Ferrari, Ivan Vito."Exploring the Therapeutic Potential of Hypericin: Molecular Docking with ABAD and BACE1 targets in Alzheimer's Disease Pathogenesis." (2023). Preprint.
- Schulz, Anna Leana, Beate Albrecht, Cumhur Arici, Ineke Van Der Burgt, Annegret Buske, Gabriele Gillessen-Kaesbach, Raoul Heller et al. "Mutation and phenotypic spectrum in patients with cardio-facio-cutaneous and Costello syndrome." Clinical genetics 73, no. 1 (2008): 62-70. [CrossRef]
- Sarkozy, Anna, Claudio Carta, Sonia Moretti, Giuseppe Zampino, Maria C. Digilio, Francesca Pantaleoni, Anna Paola Scioletti et al. "Germline BRAF mutations in Noonan, LEOPARD, and cardiofaciocutaneous syndromes: molecular diversity and associated phenotypic spectrum." Human mutation 30, no. 4 (2009): 695-702. [CrossRef]
- Davies, Helen, Graham R. Bignell, Charles Cox, Philip Stephens, Sarah Edkins, Sheila Clegg, Jon Teague et al. "Mutations of the BRAF gene in human cancer." Nature 417, no. 6892 (2002): 949-954. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).