Submitted:
30 August 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
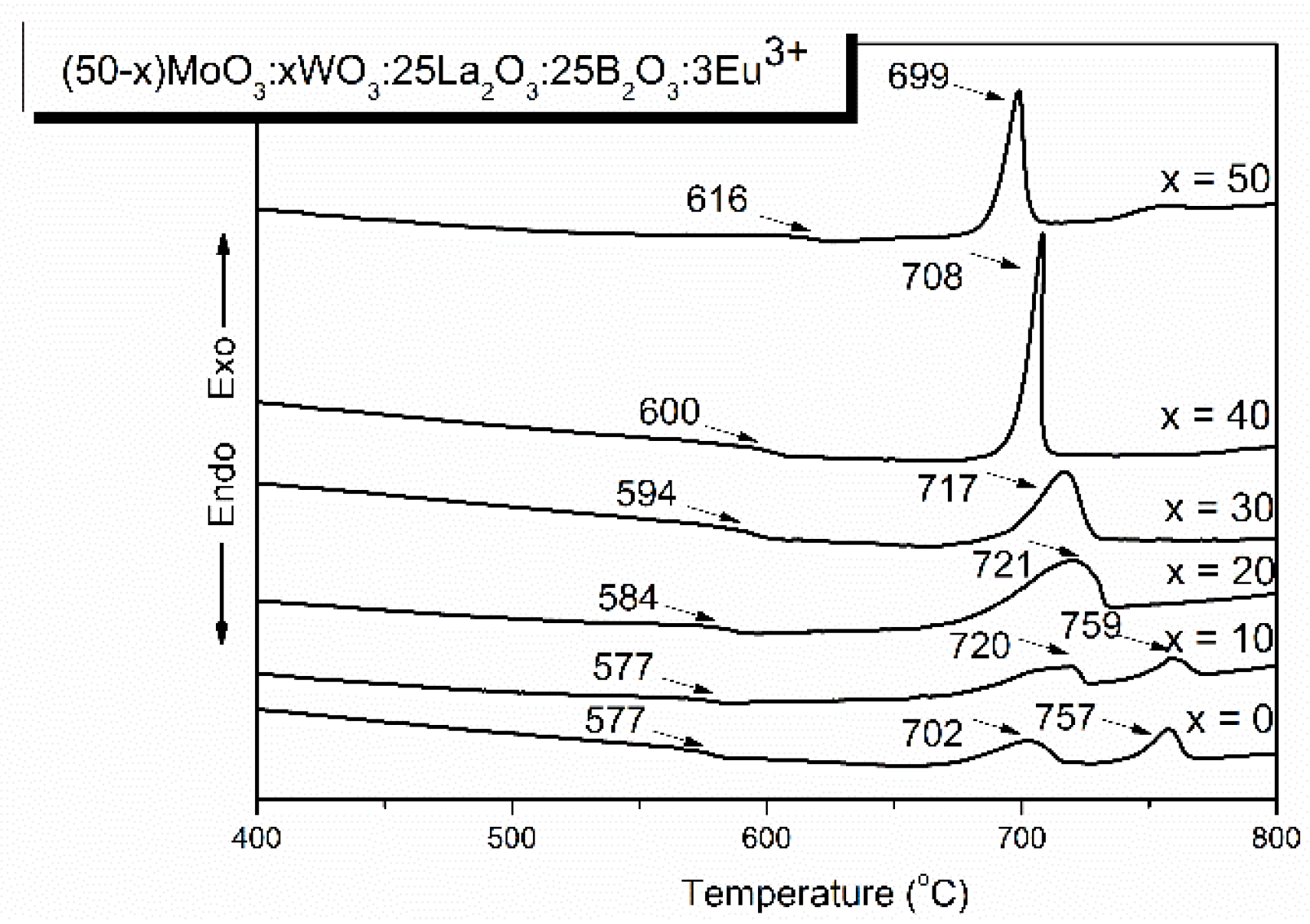
2.1. Thermal analysis
2.2. Structural investigations
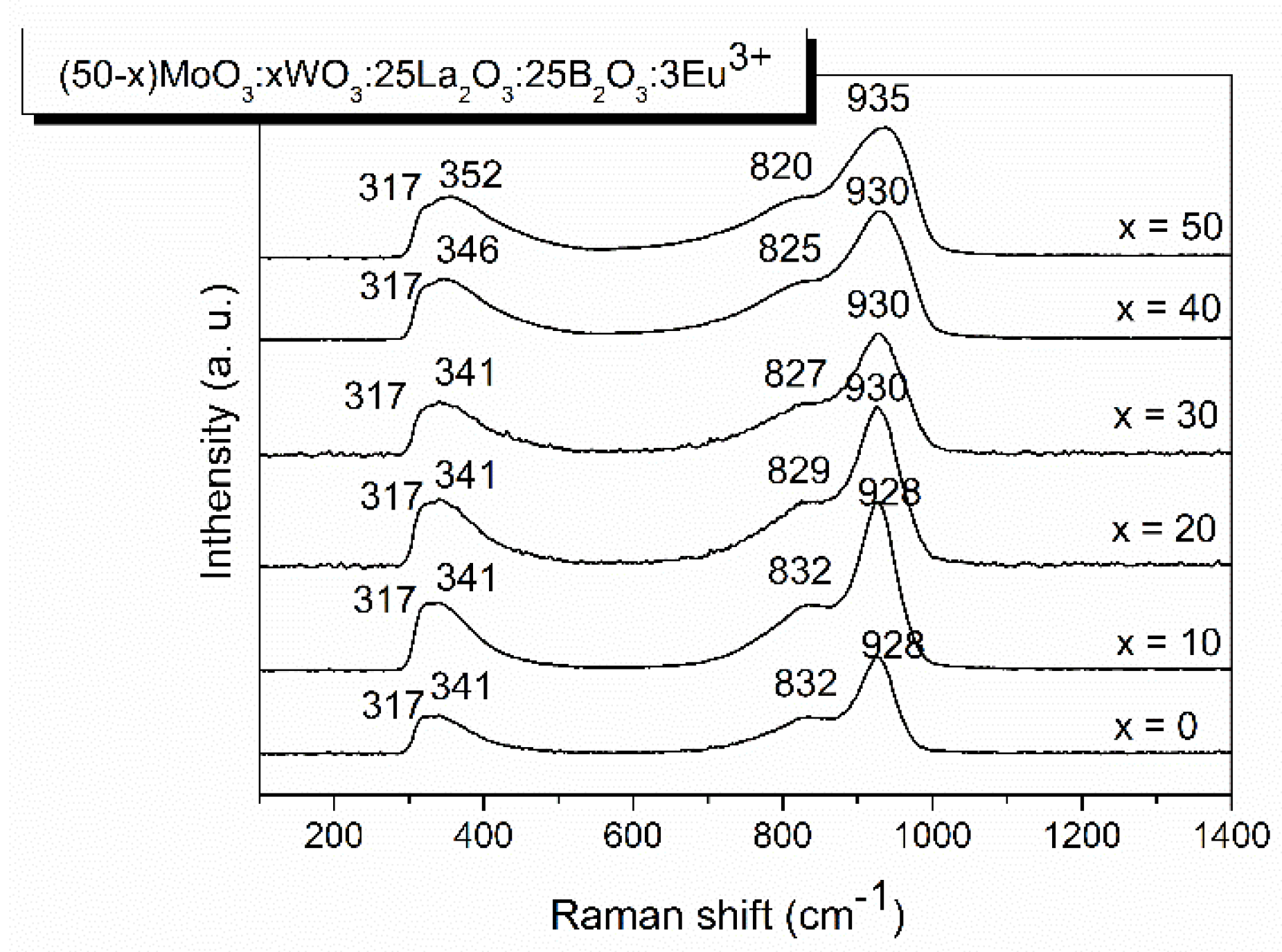
2.2.1. Raman analysis
2.2.2. Physical parameters
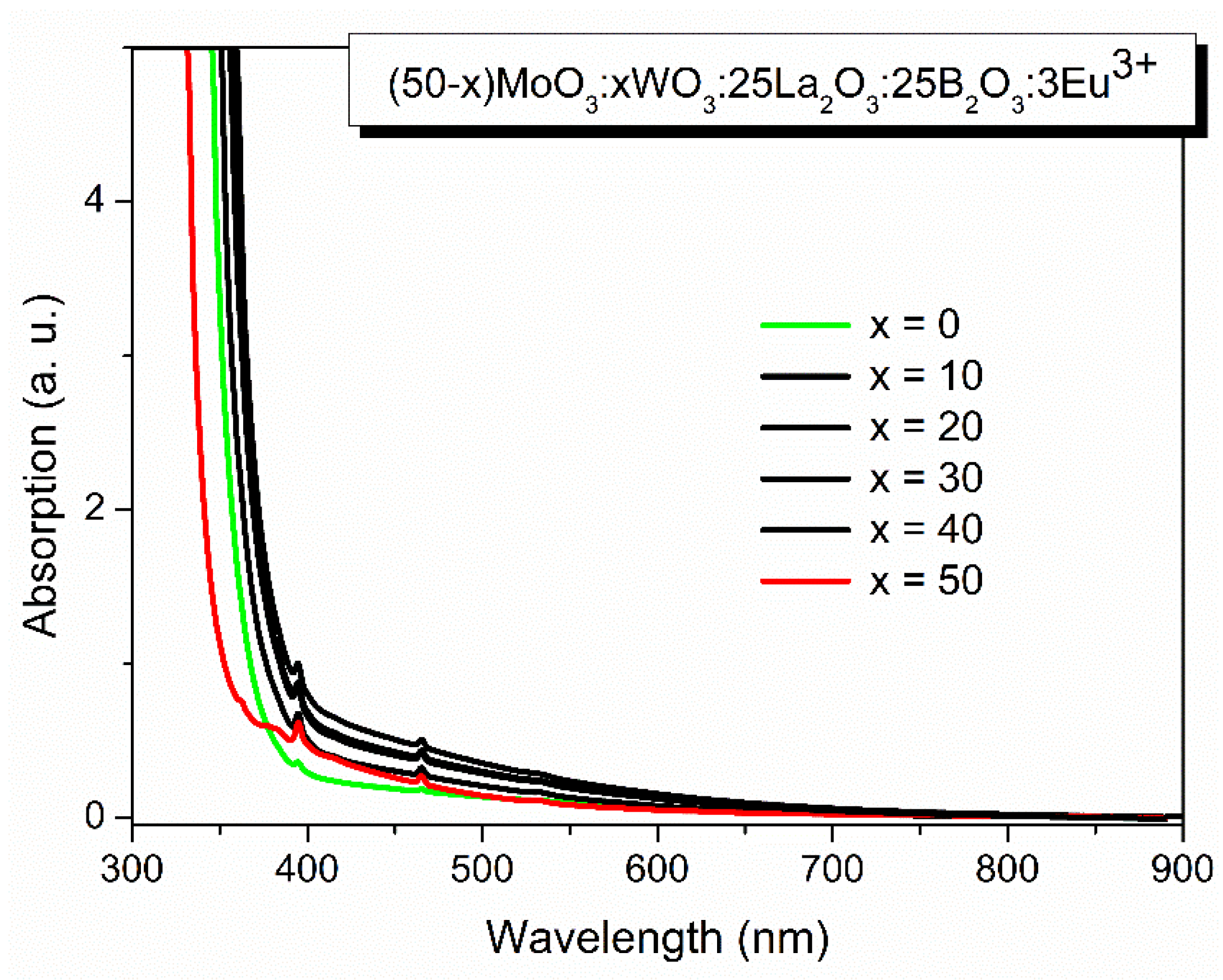
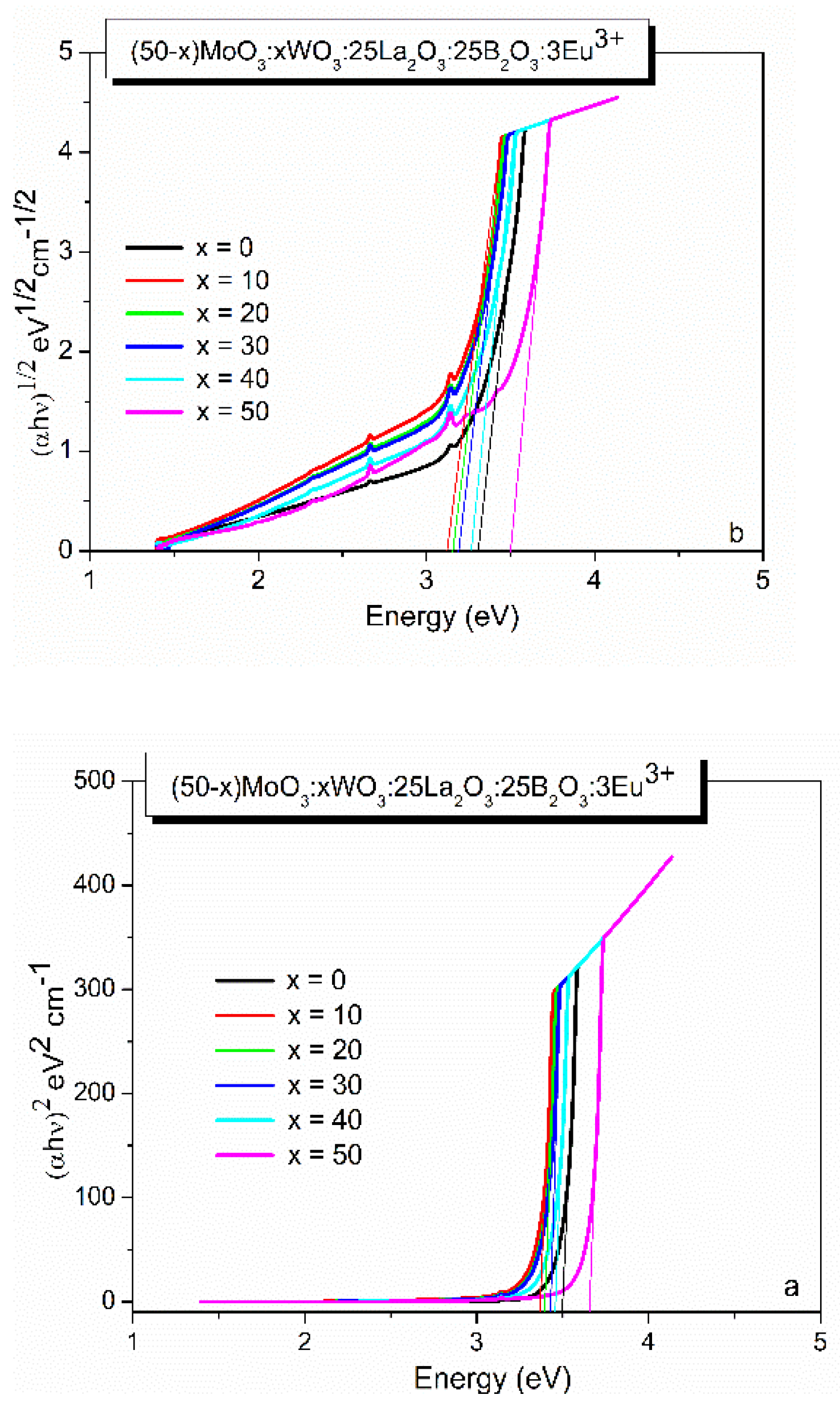
2.2.3. Optical studies
2.3. Luminescent properties
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J. The Role of Auxiliary Alkali Metal Ions on Scheelite Structure Double Molybdate and Tungstate Phosphors. Inorg. Chem. 2017, 56, 8078–8086. [Google Scholar] [CrossRef] [PubMed]
- Janulevicius, M.; Marmokas, P.; Misevicius, M.; Grigorjevaite, J.; Mikoliunaite, L.; Sakirzanovas, S.; Katelnikovas, A. Luminescence and luminescence quenching of highly efficient Y2Mo4O15:Eu3+ phosphors and ceramics. Scientific Reports 2016, 6, 26098. [Google Scholar] [CrossRef]
- Moreira, R.; Francisco, L.; Costa, I.; Barbosa, H.; Teotonio, E.; Felinto, M.; Malta, O.; Brito, H. Luminescence properties of BaMO4:Eu3+ (M: Mo or W) phosphors derived from co-precipitation reaction. J. Alloys Compd. 2023, 937, 168408. [Google Scholar] [CrossRef]
- Dutta, P.S.; Khanna, A. Eu3+ activated molybdate and tungstate based red phosphors with charge transfer band in blue region. ECS J. Solid State Sci. Technol. 2013, 2, R3153–R3167. [Google Scholar] [CrossRef]
- Yengkhom, D.; Ningombam, G.; Singh, T.; Chipem, F.; Singh, N. ; Luminescence enhancement and tunable color emission in Eu/Dy/Sm codoped CaW1- xMoxO4 phosphor. Inorg. Chem. Commun. 2022, 141, 109571. [Google Scholar] [CrossRef]
- Pier, T.; Jüstel, T. ; Application of Eu(III) activated tungstates in solid state lighting. Opt. Mater. X 2024, 22, 100299. [Google Scholar] [CrossRef]
- Vatsa, B.; Shafeeq, M.; Kesari, S. Triple molybdates and tungstates scheelite structures: Effect of cations on structure, band-gap and photoluminescence properties. J. Alloys Compd. 2021, 865, 158818. [Google Scholar] [CrossRef]
- Zhang, W.J.; Feng, W.L.; Nie, Y.M. Photoluminescence properties of red europium doped calcium tungstate phosphors for blue-pumped light-emitting diodes. Optik 2015, 126, 1341–1343. [Google Scholar] [CrossRef]
- Czajka, J.; Szczeszak, A.; Kaczorowska, N.; Lis, S. New Multicolor Tungstate-Molybdate Microphosphors as an Alternative to LED Components. Materials 2021, 14, 6608. [Google Scholar] [CrossRef]
- Wang, Y.; Honma, T.; Doi, Y.; Hinatsu, Y.; Komatsu, T. Magnetism of β′-Gd2(MoO4)3 and photoluminescence of β′-Eu2(MoO4)3 crystallized in rare-earth molybdenum borate glasses. J. Ceram. Soc. Jpn. 2013, 121, 230–235. [Google Scholar] [CrossRef]
- Devi, C.A.; Mahamuda, S.; Swapna, K.; Venkateswarlu, M.; Srinivasa Rao, A.; Vijaya Prakash, G. Compositional dependence of red luminescence from Eu3+ ions doped single and mixed alkali fluoro tungsten tellurite glasses. Opt. Mater. 2017, 73, 260–267. [Google Scholar] [CrossRef]
- Roy, J.S.; Messaddeq, Y. Photoluminescence study of Eu3+ doped zinc tungsten-antimonite glasses for red LED applications. J. Lumin. 2020, 228, 117608–4. [Google Scholar] [CrossRef]
- Wantana, N.; Kaewnuam, E.; Ruangtaweep, Y.; Kidkhunthod, P.; Kim, H.J.; Kothanf, S.; Kaewkhao, J. ; High density tungsten gadolinium borate glasses doped with Eu3+ ion for photonic and scintillator applications. Radiat. Phys. Chem. 2020, 172, 108868–7. [Google Scholar] [CrossRef]
- Dousti, M.R.; Poirier, G.Y.; de Camargo, A.S.S. Tungsten sodium phosphate glasses doped with trivalent rare earth ions (Eu3+, Tb3+, Nd3+ and Er3+) for visible and near-infrared applications. J. Non-Cryst. Solids 2020, 530, 119838–7. [Google Scholar] [CrossRef]
- Gandhi, Y.; Kityk, I.V.; Brik, M.G.; Raghav Rao, P.; Veeraiah, N. Influence of tungsten on the emission features of Nd3+, Sm3+ and Eu3+ ions in ZnF2–WO3–TeO2 glasses. J. Alloys Compd. 2010, 508, 278–291. [Google Scholar] [CrossRef]
- Babu, A.M.; Jamalaiah, B.C.; Suhasini, T.; Srinivasa Rao, T.; Rama Moorthy, L. Optical properties of Eu3+ ions in lead tungstate tellurite glasses. Solid State Sci. 2011, 13, 574–578. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Iordanova, R.; Dimitriev, Y.; Georgiev, N.; Komatsu, T. Eu3+ doped 1La2O3:2WO3:1B2O3 glass and glass-ceramic. Opt. Mater. 2014, 36, 1366–1372. [Google Scholar] [CrossRef]
- Milanova, M.; Aleksandrov, L.; Iordanova, R.; Petrova, P. A novel Eu3+- doped tungstate glasses for red emission: Preparation, structure and photoluminescence properties. J. Chem. Technol. Metall. 2022, 57, 247–257. [Google Scholar]
- Milanova, M.; Aleksandrov, L.; Iordanova, R. Structure and luminescence properties of tungsten modified zinc borate glasses doped with Eu3+ ions. Mater. Today: Proc. 2022, 61, 1206–1211. [Google Scholar] [CrossRef]
- Milanova, M.; Aleksandrov, L.; Yordanova, A.; Iordanova, R.; Tagiara, N.S.; Herrmann, A.; Gao, G.; Wondraczek, L.; Kamitsos, E.I. Structural and luminescence behavior of Eu3+ ions in ZnO-B2O3-WO3 glasses. J. Non-Cryst. Solids 2023, 600, 122006. [Google Scholar] [CrossRef]
- Yordanova, A.; Aleksandrov, L.; Milanova, M.; Iordanova, R.; Petrova, P.; Nedyalkov, N. Effect of WO3 addition on the structure and luminescent properties of ZnO-B2O3:Eu3+ glass. Molecules 2024, 29, 2470. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrov, L.; Komatsu, T.; Iordanova, R.; Dimitriev, Y. Study of molybdenum coordination state and crystallization behavior in MoO3-La2O3-B2O3 glasses by Raman spectroscopy. J. Phys. Chem. Solids 2011, 72, 263–268. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Komatsu, T.; Iordanova, R.; Dimitriev, Y. Raman spectroscopy study of WO3-La2O3-B2O3 glasses with no color and crystallization of LaBWO6. Opt. Mater. 2011, 34, 201–206. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Komatsu, T.; Shinozaki, K.; Honma, T.; Iordanova, R. Structure of MoO3-WO3-La2O3-B2O3 glasses and crystallization of LaMo1-xWxBO6 solid solutions. J. Non-Cryst. Solids 2015, 429, 171–177. [Google Scholar] [CrossRef]
- Villegas, M.A.; Fernández Navarro, J.M. Physical and structural properties of glasses in the TeO2–TiO2–Nb2O5 system. J Eur Ceram Soc. 2007, 27, 2715–2723. [Google Scholar] [CrossRef]
- Masuno, A.; Inoue, H.; Yoshimoto, K.; Watanabe, Y. Thermal and optical properties of La2O3 -Nb2O5 high refractive index glasses. Opt. Mater. Express 2014, 4, 710–717. [Google Scholar] [CrossRef]
- Tauc, J. Amorphous and Liquid Semiconductor; Plenum Press: London, UK; New York, NY, USA, 1974. [Google Scholar]
- Milanova, M.; Kostov, K.L.; Iordanova, R.; Aleksandrov, L.; Yordanova, A.; Mineva, T. Local structure, connectivity and physical properties of glasses in the B2O3- Bi2O3-La2O3-WO3 system. J. Non-Cryst. Solids 2019, 516, 35–44. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Ahlawat, N.; Agarwal, A. Influence of Bi2O3 on thermal, structural and dielectric properties of lithium zinc bismuth borate glasses. J. Alloys Compd. 2014, 597, 110–118. [Google Scholar] [CrossRef]
- Blasse, G.; Grabmaier, B.C. Luminescent Materials, 1st ed.; Springer Berlin: Heidelber, Germany, 1994; p. 18. [Google Scholar]
- Hoefdraad, H.E. The charge-transfer absorption band of Eu3+ in oxides. J. Solid State Chem. 1975, 15, 175–177. [Google Scholar] [CrossRef]
- Parchur, A.K.; Ningthoujam, R.S. Behaviour of electric and magnetic dipole transitions of Eu3+, 5D0→7F0 and Eu–O charge transfer band in Li+ co-doped YPO4:Eu3+. RSC Adv. 2012, 2, 10859–10868. [Google Scholar] [CrossRef]
- Mariselvam, K.; Juncheng, L. Synthesis and luminescence properties of Eu3+ doped potassium titano telluroborate (KTTB) glasses for red laser applications. J. Lumin. 2021, 230, 117735. [Google Scholar] [CrossRef]
- Dutta, P.S.; Khanna, A. Eu3+ activated molybdate and tungstate based red phosphors with charge transfer band in blue region. ECS J. Solid State Sci. Technol. 2013, 2, R3153–R3167. [Google Scholar] [CrossRef]
- Kotaka, M.; Honma, T.; Komatsu, T. Photoluminescence features of new Eu3+ -doped Gd4Mo7O27 phosphors synthesized using glass crystallization technique. J. Asian Ceram. Soc. 2018, 6, 314–321. [Google Scholar] [CrossRef]
- Thieme, C.; Herrmann, A.; Kracker, M.; Patzig, C.; Höche, T.; Rüssel, C. Microstructure investigation and fluorescence properties of Europium-doped scheelite crystals in glass-ceramics made under different synthesis conditions. J. Lumin. 2021, 238, 118244. [Google Scholar] [CrossRef]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev 2015, 295, 1–45. [Google Scholar] [CrossRef]
- Bünzli, J.C.G. Lanthanide luminescence: From a mystery to rationalization, understanding, and applications. In Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 2016; Volume 50, pp. 141–176. [Google Scholar]
- Sungpanich, J.; Thongtem, T.; Thongtem, S. Large-scale synthesis of WO3 nanoplates by a microwave-hydrothermal method. Ceram. Int. 2012, 38, 1051–1055. [Google Scholar] [CrossRef]
- Song, J.; Ni, X.; Zhang, D.; Zheng, H. Fabrication and photoluminescence properties of hexagonal MoO3 rods. Solid State Sci. 2006, 8, 1164–1167. [Google Scholar] [CrossRef]
- Devi, C.H.B.; Mahamuda, S.; Swapna, K.; Venkateswarlu, M.; Rao, A.S.; Prakash, G.V. Compositional dependence of red luminescence from Eu3+ ions doped single and mixed alkali fluoro tungsten tellurite glasses. Opt. Mater. 2017, 73, 260–267. [Google Scholar] [CrossRef]
- Nogami, M.; Umehara, N.; Hayakawa, T. Effect of hydroxyl bonds on persistent spectral hole burning in Eu3+ doped BaO-P2O5 glasses. Phys. Rev. B 1998, 58, 6166–6171. [Google Scholar] [CrossRef]
- Aleksandrov, L.; Milanova, M.; Yordanova, A.; Iordanova, R.; Nedyalkov, N.; Petrova, P.; Tagiara, N.S.; Palles, D.; Kamitsos, E.I. Synthesis, structure and luminescence properties of Eu3+-doped 50ZnO.40B2O3.5WO3.5Nb2O5 glass. Phys. Chem. Glas. Eur. J. Glass Sci. Technol. B 2023, 64, 101–109. [Google Scholar] [CrossRef]
- Iordanova, R.; Milanova, M.; Yordanova, A.; Aleksandrov, L.; Nedyalkov, N.; Kukeva, R.; Petrova, P. Structure and Luminescent Properties of Niobium-Modified ZnO-B2O3:Eu3+ Glass. Materials 2024, 17, 1415. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Aleksandrov, L.; Milanova, M.; Iordanova, R.; Petrova, P.; Nedyalkov, N. Effect of the Addition of WO3 on the Structure and Luminescent Properties of ZnO-B2O3: Eu3+ Glass. Molecules 2024, 29, 2470. [Google Scholar] [CrossRef] [PubMed]
- Yordanova, A.; Milanova, M.; Iordanova, R.; Fabian, M.; Aleksandrov, L.; Petrova, P. Network Structure and Luminescent Properties of ZnO–B2O3–Bi2O3–WO3: Eu3+ Glasses. Materials 2023, 16, 6779. [Google Scholar] [CrossRef]
- Bettinelli, M.; Speghini, A.; Ferrari, M.; Montagna, M. Spectroscopic investigation of zinc borate glasses doped with trivalent europium ions. J. Non-Cryst. Solids 1996, 201, 211–221. [Google Scholar] [CrossRef]
- Annapurna, K.; Das, M.; Kundu, M.; Dwivedhi, R.N.; Buddhudu, S. Spectral properties of Eu3+: ZnO–B2O3–SiO2 glasses. J. Mol. Struct. 2005, 741, 53–60. [Google Scholar] [CrossRef]
- Babu, A.M.; Jamalaiah, B.C.; Suhasini, T.; Rao, T.S.; Moorthy, L.R. Optical properties of Eu3+ ions in lead tungstate tellurite glasses. Solid State Sci. 2011, 13, 574–578. [Google Scholar] [CrossRef]
- Raju, B.D.P.; Reddy, C.M. Structural and optical investigations of Eu3+ ions in lead containing alkali fluoroborate glasses. Opt. Mater. 2012, 34, 1251–1260. [Google Scholar] [CrossRef]
- Manasa, P.; Jayasankar, C.K. Luminescence and phonon side band analysis of Eu3+-doped lead fluorosilicate glasses. Opt. Mater. 2016, 62, 139–145. [Google Scholar] [CrossRef]
- Oomen, E.W.J.L.; Dongen, A.M.A. Europium (III) in oxide glasses: Dependence of the emission spectrum upon glass composition. J. Non-Cryst. Solids 1989, 111, 205–213. [Google Scholar] [CrossRef]
- Kumar, A.; Rai, D.K.; Rai, S.B. Optical studies of Eu3+ ions doped in tellurite glass. Spectrochim Acta A Mol Biomol Spectrosc. 2002, 58, 2115–2125. [Google Scholar] [CrossRef]
- Song, H.; Chen, B.; Sun, B.; Zhang, J.; Lu, S. Ultraviolet light-induced spectral change in cubic nanocrystalline Y2O3: Eu3+. Chem. Phys. Lett. 2003, 372, 368–372. [Google Scholar] [CrossRef]
- Kabir, M.; Ghahari, M.; Afarani, M.S. Co-precipitation synthesis of nano Y2O3: Eu3+ with different morphologies and its photoluminescence properties. Ceram. Int. 2014, 40, 10877–10885. [Google Scholar] [CrossRef]
- Som, S.; Mitra, P.; Kumar, V.; Kumar, V.; Terblans, J.J.; Swart, H.C.; Sharma, S.K. The energy transfer phenomena and colour tunability in Y2O2S: Eu3+/Dy3+ micro-fibers for white emission in solid state lighting applications. Dalton Trans. 2014, 43, 9860–9871. [Google Scholar] [CrossRef]
- Binnemans, K.; Görller-Walrand, C. Application of the Eu3+ ion for site symmetry determination. J. Rare Earths 1996, 14, 173–180. [Google Scholar]
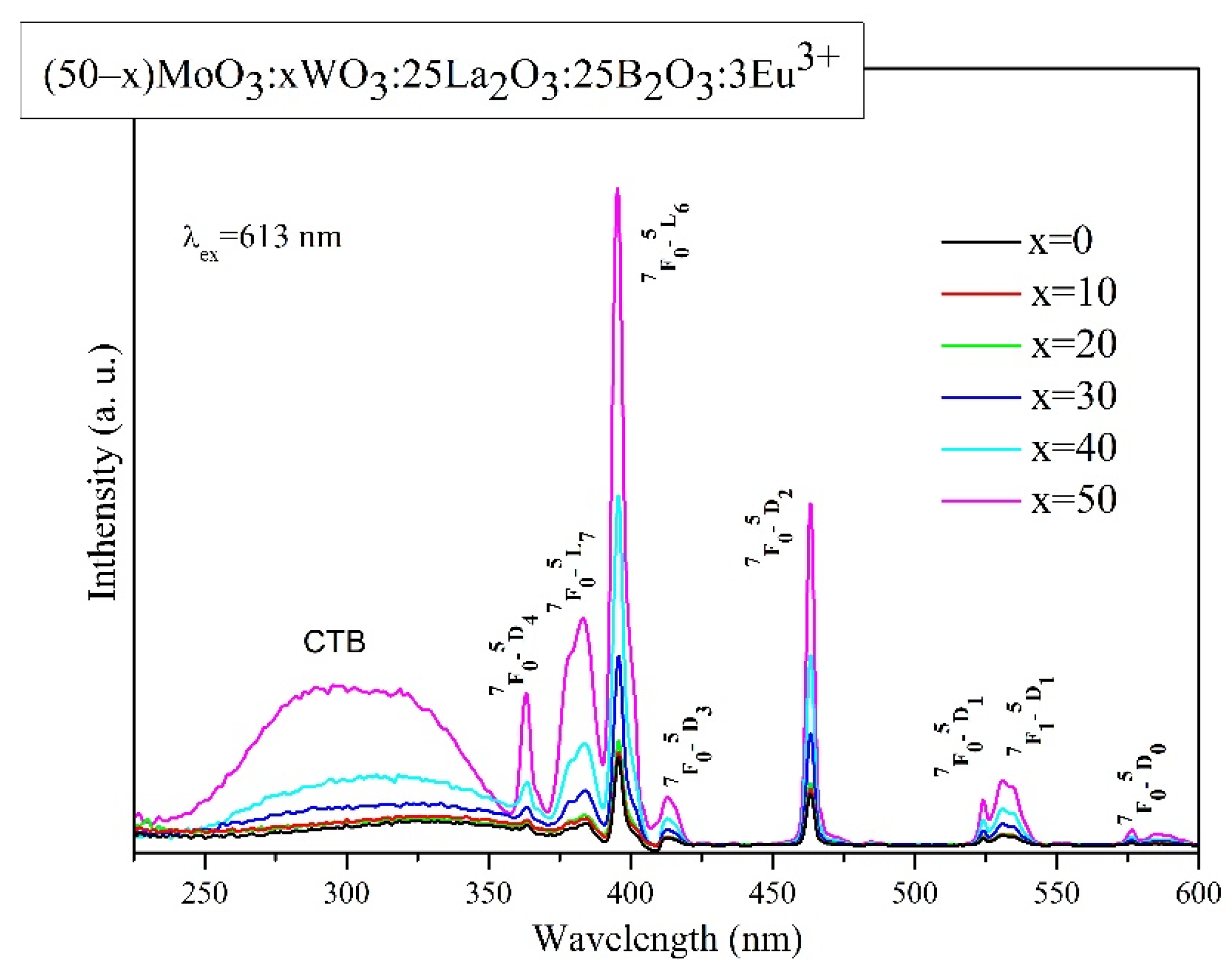
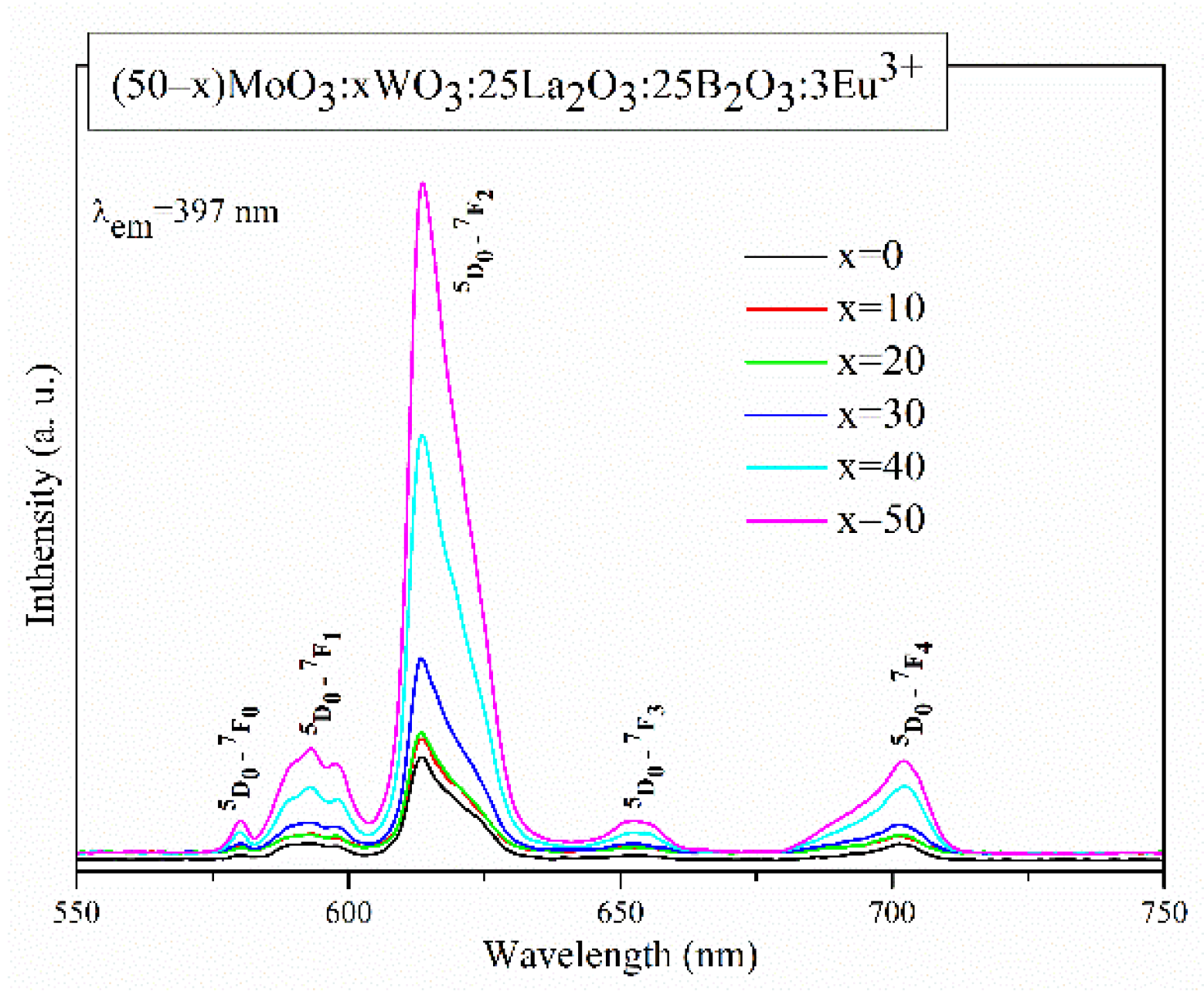
| Sample ID |
ρg (±0.01) (g/cm3) |
Vm (cm3/mol) |
Vo (cm3/mol) |
OPD (g atom/l) |
Eg direct (eV) |
Eg indirect (eV) |
А (nm) |
Refractive index, n |
|---|---|---|---|---|---|---|---|---|
| x = 0 | 4.756 | 38.14 | 12.34 | 81.02 | 3.50 | 3.31 | 350.7 | 1.93144 |
| x = 10 | 4.992 | 38.10 | 12.33 | 81.10 | 3.37 | 3.12 | 367.2 | 1.93452 |
| x = 20 | 5.439 | 36.58 | 11.84 | 84.47 | 3.39 | 3.16 | 364.1 | 1.94278 |
| x = 30 | 5.729 | 36.26 | 11.73 | 85.22 | 3.43 | 3.19 | 359.8 | 1.95236 |
| x = 40 | 6.064 | 35.71 | 11.56 | 86.53 | 3.46 | 3.27 | 353.7 | 1.96115 |
| x = 50 | 6.403 | 35.19 | 11.39 | 87.81 | 3.66 | 3.50 | 334.7 | 1.97066 |
| Glass composition | Relative Intensity Ratio, R | reference |
| 50MoO3:25La2O3:25B2O3:3Eu2O3 | 7.09 | Current work |
| 40MoO3:10WO3:25La2O3:25B2O3:3Eu2O3 | 7.15 | Current work |
| 30MoO3:20WO3:25La2O3:25B2O3:3Eu2O3 | 7.19 | Current work |
| 20MoO3:30WO3:25La2O3:25B2O3:3Eu2O3 | 7.42 | Current work |
| 10MoO3:40WO3:25La2O3:25B2O3:3Eu2O3 | 7.63 | Current work |
| 50WO3:25La2O3:25B2O3:3Eu2O3 | 7.82 | Current work |
| 50ZnO:40B2O3:10WO3:xEu2O3 (0≤x≤10) | 4.54÷5.77 | 20 |
| 50ZnO:40B2O3:5WO3:5Nb2O5:xEu2O3 (0≤x≤10) | 5.09÷5.76 | 43 |
| 50ZnO:(50–x)B2O3:xNb2O5:0.5Eu2O3:, x= 0, 1, 3 and 5 mol% | 4.31-5.16 | 44 |
| 50ZnO:(50−x)B2O3:0.5Eu2O3:xWO3, x = 0, 1, 3, 5. | 4.34-5.57 | 45 |
| 50ZnO:(49–x)B2O3:1Bi2O3:xWO3; x = 1, 5, 10, | 4.61-5.73 | 46 |
| 4ZnO:3B2O3 0.5–2.5 mol % Eu2O3 | 2.74-3.94 | 47 |
| 60ZnO:20B2O3:(20 − x)SiO2−xEu2O3 (x = 0 and 1) | 3.166 | 48 |
| 15PbF2:25WO3:(60–x)TeO2:xEu2O3 x = 0.1, 0.5, 1.0 and 2.0 mol% | 2.37-2.78 | 49 |
| 20PbO–5CaO–5ZnO–10LiF–59B2O3–1Eu2O | 2.320 | 50 |
| 45SiO2−(20−x)PbF2−20K2O−5Na2O−10LiF−1.0Eu2O3 | 2.44 | 51 |
| 89.5B2O3–10Li2O–0.5Eu2O3 | 2.41 | 52 |
| 57GeO2–40K2O–3Eu2O3 | 3.70 | 52 |
| 73P2O5–25CaO–2Eu2O3 | 3.95 | 52 |
| 79TeO2−20Li2CO3−1Eu2O3 | 4.28 | 53 |
| Eu3+:Y2O3 | 3.8-5.2 | 54, 55 |
| Eu3+:Y2O2S | 6.45-6.62 | 56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





