Submitted:
02 September 2024
Posted:
03 September 2024
Read the latest preprint version here
Abstract
Keywords:
Introduction
Materials and Methods
Description of study area
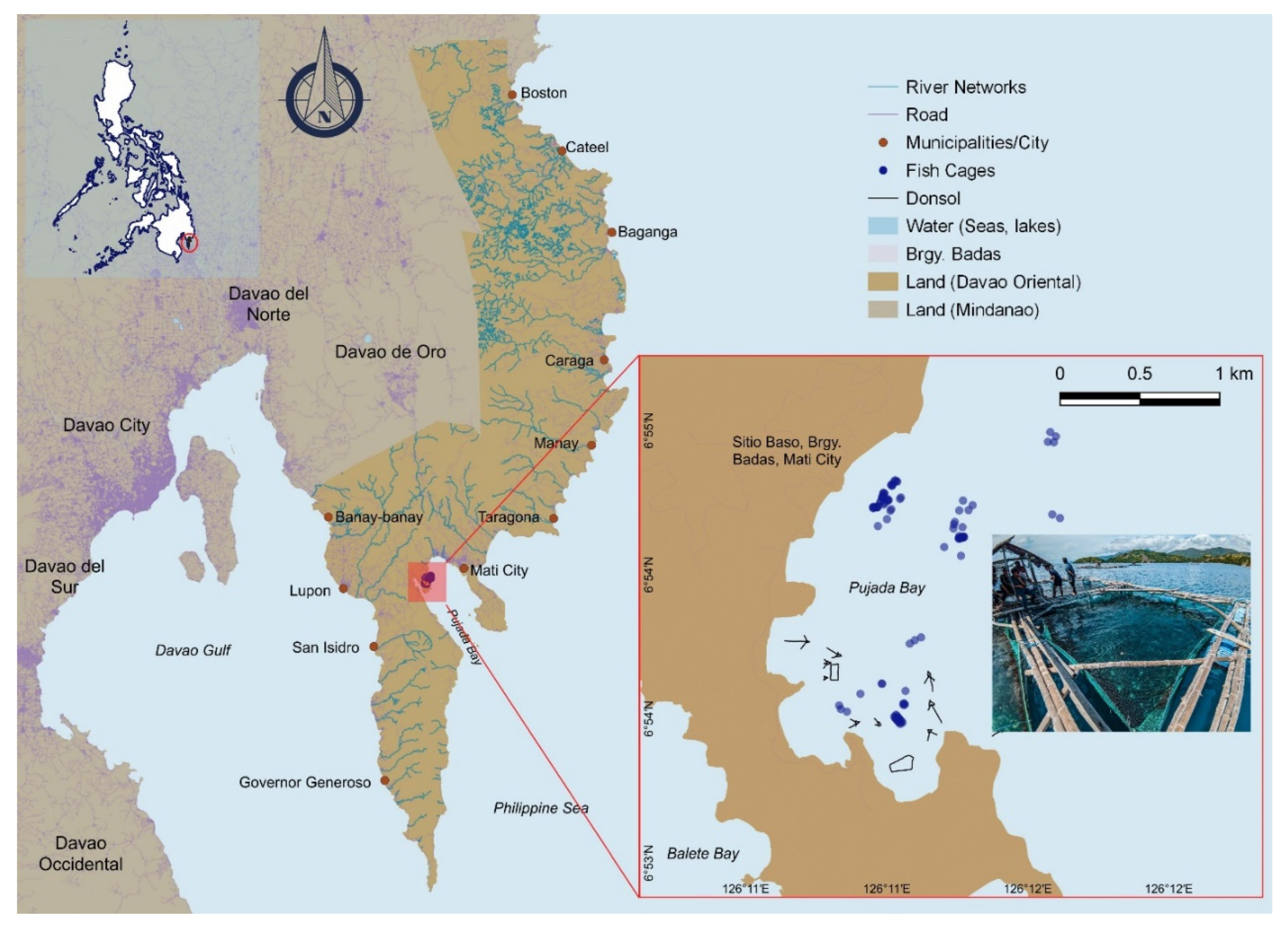
Description of treatments
Description of sampling
Data analyses
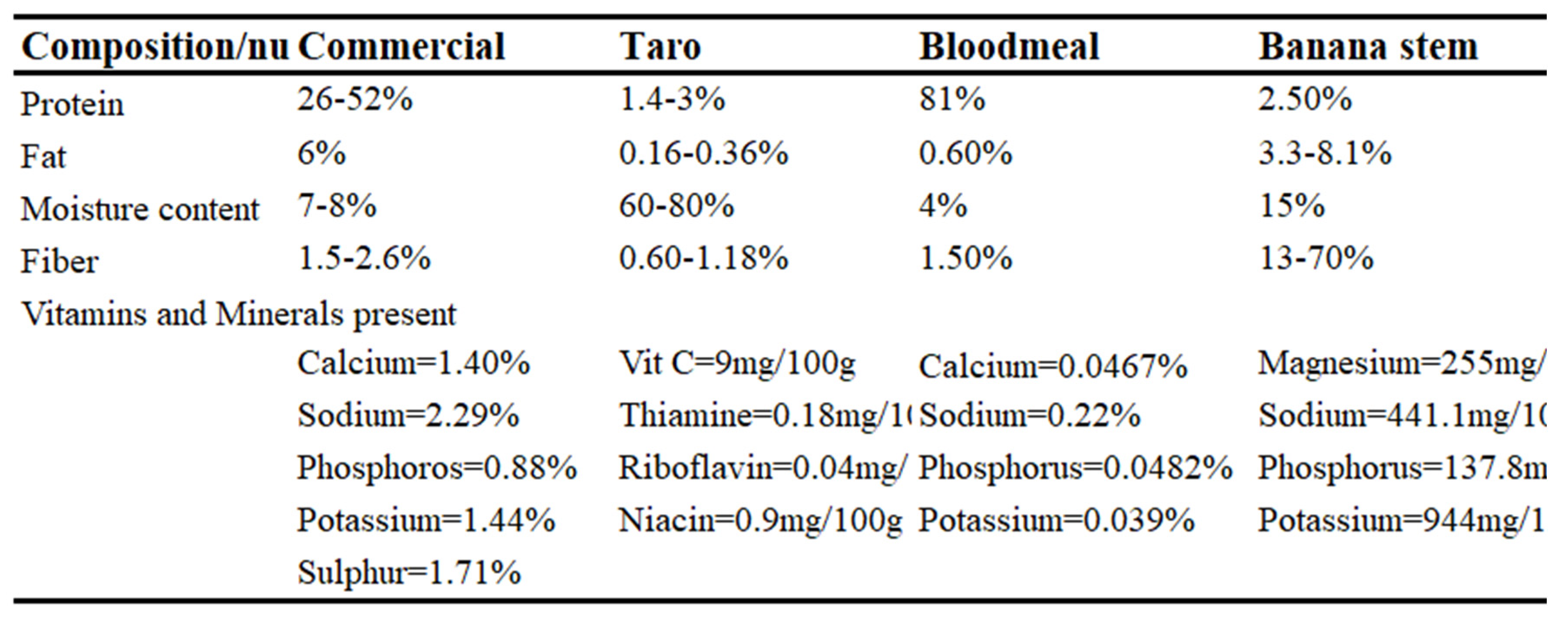
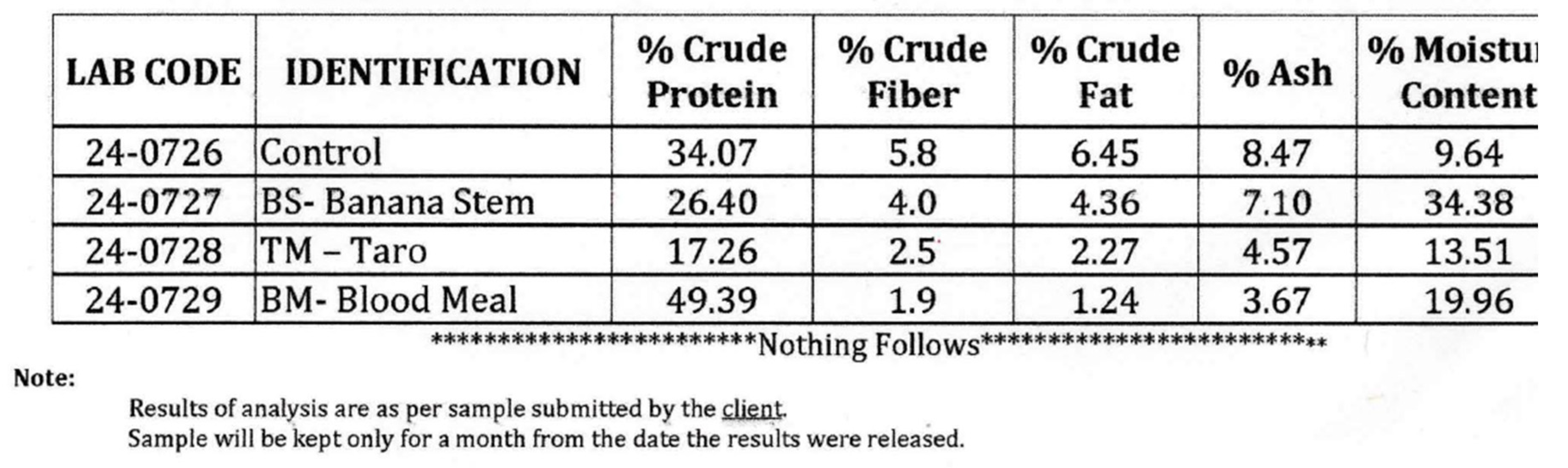
Feed ingredients and proximate analysis
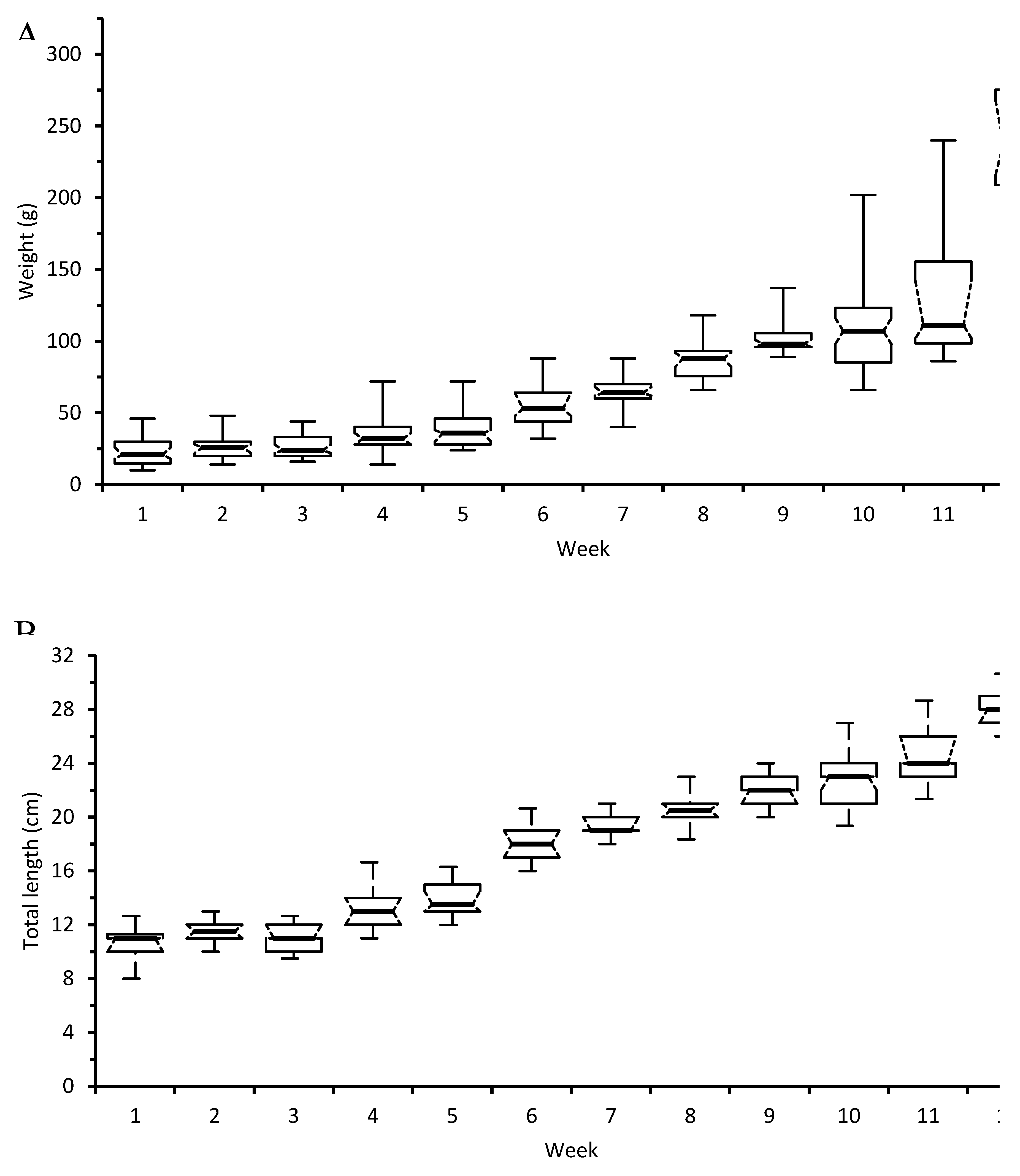
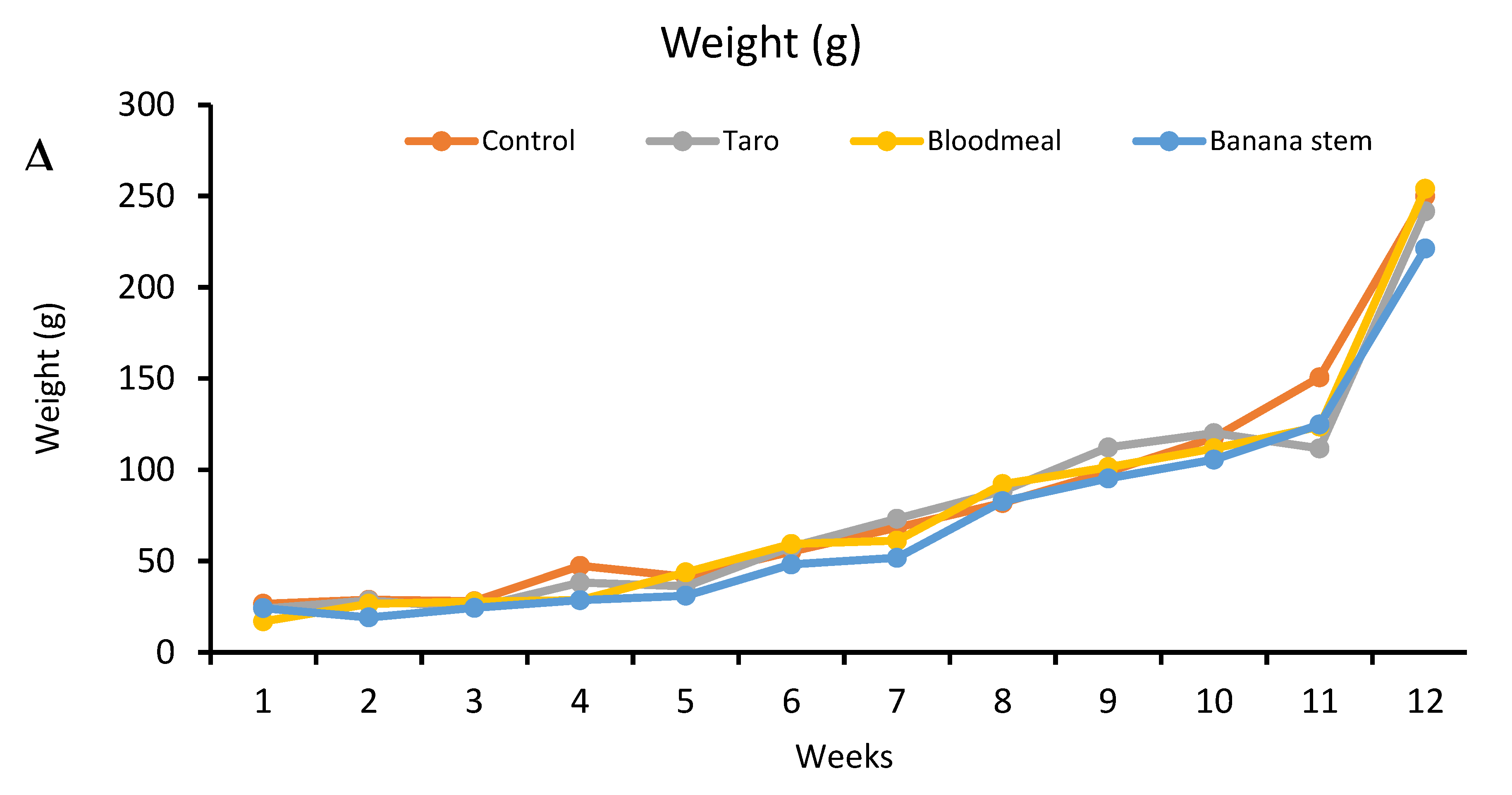
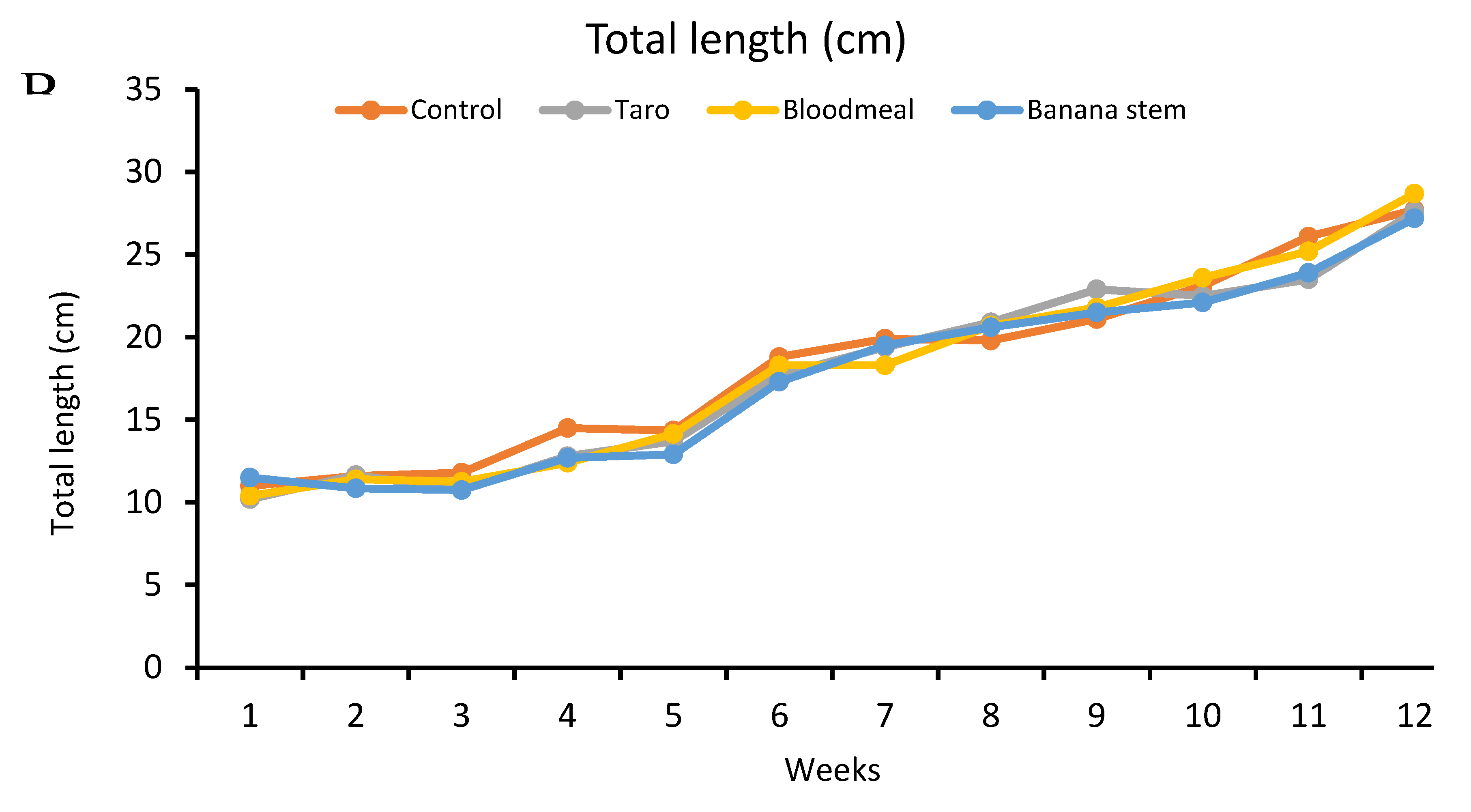
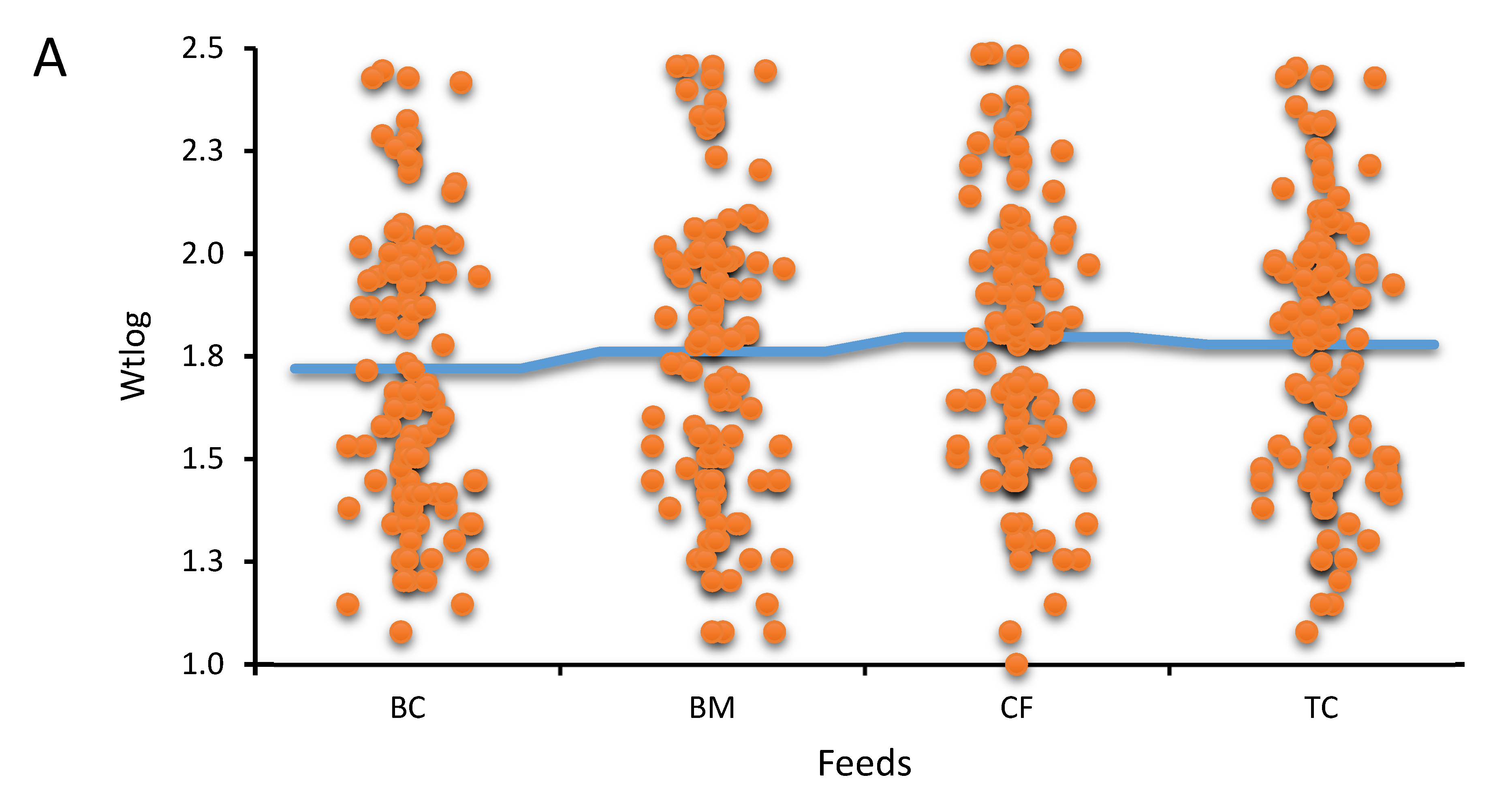
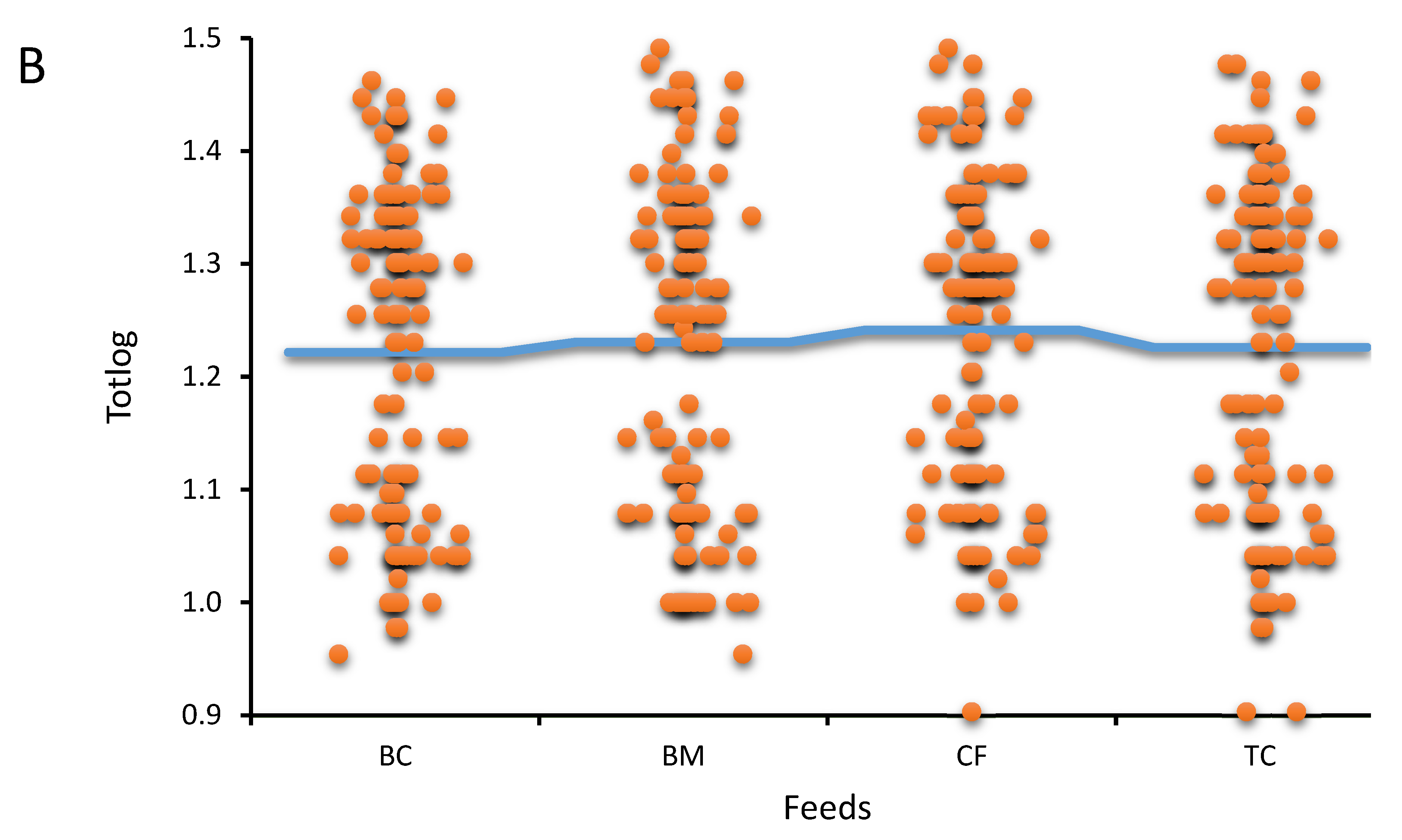
Results of the study
| Technical basis | Treatments | |||
| Commercial | Taro+Commercial | Bloodmeal | Banana Stem+Commercial | |
| Stocking density | 3000 | 3000 | 3000 | 3000 |
| Culture period (days) | 84 | 84 | 84 | 84 |
| Initial ABW (g) | 18 | 19.2 | 17 | 19.6 |
| Final ABW (g) | 249.93 | 241.67 | 253.965 | 221.185 |
| Weight gain (g) | 223.33 | 217.67 | 236.97 | 196.99 |
| % Ave. weight gain | 1053.34 | 1103.71 | 1476.35 | 898.62 |
| Ave daily weight gain (g) | 2.66 | 2.59 | 2.82 | 2.35 |
| Specific growth rate (%) | 3.93 | 3.96 | 4.55 | 3.72 |
| Survival Rate (%) | 90 | 89 | 87 | 77 |
| Feed Conversion Ratio | 1.65 | 1.71 | 1.60 | 2.18 |
| Protein Efficiency Ratio | 1.31 | 3.21 | 0.97 | 1.36 |
| Fulton's Condition Factor | 1.17 | 1.15 | 1.07 | 1.09 |
| Yield (kg) | 674.81 | 645.26 | 662.85 | 510.94 |
| Operational Cost (Php) | ||||
| Cost of fingerlings | 16,250 | 16,250 | 16,250 | 16,250 |
| Labor expense | 7,500 | 7,500 | 7,500 | 7,500 |
| Cost of feed consumed | 52,109.76 | 32,140.80 | 44,461.44 | 27,190.77 |
| Cost per kilogram of feeds | 52.53 | 32.4 | 44.82 | 27.41 |
| Total cost of production | 75,857.78 | 55,894.77 | 68,191.60 | 50,934.77 |
| Return (Php) | ||||
| Revenue | 101,221.65 | 96,788.84 | 99,427.30 | 76,641.00 |
| Gross Profit | 25,363.87 | 40,894.07 | 31,235.70 | 25,706.23 |
| Gross Profit Margin (%) | 25.06 | 42.25 | 31.42 | 33.54 |
| Cost-Benefit Ratio | 2.99 | 1.37 | 2.18 | 1.98 |
| *Note: Buying price at 150/kg | ||||
Cost-Benefit Analysis of Locally Sourced Alternative Feeds
Discussion
Feed comparison
Contribution to circular economy
Cost-effective alternative feeds
Conclusion
- Cheap food sources that can be used for animal feeds are widely available in the market such as taro;
- Agricultural waste product such as banana stem which are usually thrown away after harvesting banana fruits and used for burning can be recycled and used for animal feeds when mixed with existing commercial feeds at almost no costs;
- Poultry and cow-sourced bloodmeal together with soybean meal can be used as a substitute for formulated feeds as shown by its consistent ability to provide high protein feed to milkfish grow out culture;
- The results of the study showed that farmed milkfish under grow-out culture with adjusted feeding rates based on average body weight using the four treatments tested in this study demonstrates possible alternative usage for milkfish farmers who wanted to reduce their feed costs.
- The pursuit and push to use agricultural wastes products, towards its transformation into feed (and fiber and fuel) components can help realize the SDG goals of the government in the long-term and applies the circular economic principle.
- There is an improved economic performance with integration of taro and commercial feeds in milkfish diet. This demonstrates sustainability and viability of taro meal as a promising alternative source to minimize the feed cost in aquaculture industry.
References
- Abreo, N.A.S., Siblos, S.K.V., Macusi, E.D., 2020. Anthropogenic Marine Debris (AMD) in Mangrove Forests of Pujada Bay, Davao Oriental, Philippines. Journal of Marine and Island Cultures 9, 38-53. [CrossRef]
- Aditika, S., Kapoor, B., Singh, S., Kumar, P., 2021. Taro (Colocasia esculenta): Zero wastage orphan food crop for food and nutritional security. South African Journal of Botany 145, 1-13. [CrossRef]
- Agbo, N.W., Adjei-Boateng , D., Jauncey, K., 2011. The Potential of Groundnut (Arachis hypogaea L.) By-Products as Alternative Protein Sources in the Diet of Nile Tilapia (Oreochromis niloticus). Journal of Applied Aquaculture 23, 367-378. [CrossRef]
- Alzate Acevedo, S., Díaz Carrillo, Á.J., Flórez-López, E., Grande-Tovar, C.D., 2021. Recovery of Banana Waste-Loss from Production and Processing: A Contribution to a Circular Economy. Molecules 26, 5282. [CrossRef]
- Audu, R., Yola, I.A., 2020. Contemporary issues in fisheries and aquaculture: a review of conventional feed ingredients for fish feed in Nigeria. Bayero Journal of Pure and Applied Sciences 13, 22-28. [CrossRef]
- Aya, F.A., 2017. Utilizing alternative ingredients in aquafeeds for sustainable aquaculture. Fish for the People 15, 37-44.
- Bagarinao, T., 1999. Ecology and Farming of Milkfish. Southeast Asia Fisheries Aquaculture Department (SEAFDEC), Tigbauan, Iloilo.
- Dou, Z., Dierenfeld, E.S., Wang, X., Chen, X., Shurson, G.C., 2024. A critical analysis of challenges and opportunities for upcycling food waste to animal feed to reduce climate and resource burdens. Resources, Conservation and Recycling 203, 107418. [CrossRef]
- Dou, Z., Toth, J.D., Westendorf, M.L., 2018. Food waste for livestock feeding: Feasibility, safety, and sustainability implications. Global Food Security 17, 154-161. [CrossRef]
- El-Sayed, A.-F.M., 2004. Protein nutrition of farmed Tilapia: Searching for unconventional sources, in: Bolivar, R.B., Mair, G.C., Fitzsimmons, K. (Eds.), Proceedings of the 6th international symposium on tilapia in aquaculture ISTA Publications, Manila, Philippines, pp. 364–378.
- Ewald, N., Vidakovic, A., Langeland, M., Kiessling, A., Sampels, S., Lalander, C., 2020. Fatty acid composition of black soldier fly larvae (Hermetia illucens) - possibilities and limitations for modification through diet. Waste Management 102, 40-47. [CrossRef]
- FAO, 2009. State of world fisheries and aquaculture 2008. Food and Agriculture Organization of the United Nations, Rome, Italy.
- Ganesan, A.R., Mohan, K., Kandasamy, S., Surendran, R.P., Kumar, R., Rajan, D.K., Rajarajeswaran, J., 2024. Food waste-derived black soldier fly (Hermetia illucens) larval resource recovery: A circular bioeconomy approach. Process Safety and Environmental Protection 184, 170-189. [CrossRef]
- Gasco, L., Biancarosa, I., Liland, N.S., 2020. From waste to feed: A review of recent knowledge on insects as producers of protein and fat for animal feeds. Current Opinion in Green and Sustainable Chemistry 23, 67-79. [CrossRef]
- Gatto, A., Kuiper, M., van Middelaar, C., van Meijl, H., 2024. Unveiling the economic and environmental impact of policies to promote animal feed for a circular food system. Resources, Conservation and Recycling 200, 107317. [CrossRef]
- Gueco, L., 2022. The Philippine Banana Industry Roadmap (2021-2025). Department of Agriculture-Bureau of Agricultural Research, Quezon City, Philippines.
- Guerrero, R.D., 2019. Farmed tilapia production in the Philippines is declining: What has happened and what can be done. Philippine Journal of Science 148, XI-XV.
- Hoof, B.V., Solano, A., Riaño, J., Mendez, C., Medaglia, A.L., 2024. Decision-making for circular economy implementation in agri-food systems: A transdisciplinary case study of cacao in Colombia. J Clean Prod 434, 140307. [CrossRef]
- Lawal, M.O., Aderolu, A.Z., Ajayi, J.A., Soyinka, O.O., 2012. Dietary effects of yam peels on the growth and haematology of Clarias gariepinus (Burchell, 1822) juveniles. Zoologist 10, 13-17.
- Lebot, V., 2020. Tropical root and tuber crops: cassava, sweet potato, yams, and aroids, 2nd ed. CABI.
- Liguez, A.K., 2021. Engine size and proportion of catch kept affects fish catch in Pujada Bay, Davao Oriental. Davao Research Journal 12, 22–33. [CrossRef]
- Loy, T.H., Spriggs, M., Wickler, S., 1992. Direct evidence for human use of plants 28,000 years ago-starch residues on stone artifacts from the northern Solomon Islands. Antiquity 66, 898–912. [CrossRef]
- Lukuyu, B., Okike, I., Duncan, A., Beveridge, M., Blümmel, M., 2014. Use of cassava in livestock and aquaculture feeding programs. International Livestock Research Institute (ILRI) Addis Ababa, Ethiopia, pp. 1-83.
- Macusi, E.D., 2017. Fish Aggregating Devices and the Role of Socio-economic Factors in Driving Spatial Effort Allocation of Fishers. SOUTHEAST ASIAN REGIONAL CENTER FOR GRADUATE STUDY AND RESEARCH IN AGRICULTURE, Los Banos, Laguna Philippines.
- Macusi, E.D., Cayacay, M.A., Borazon, E.Q., Sales, A.C., Habib, A., Fadli, N., Santos, M.D., 2023a. Protein Fishmeal Replacement in Aquaculture: A Systematic Review and Implications on Growth and Adoption Viability. Sustainability 15, 12500. [CrossRef]
- Macusi, E.D., Macusi, E.S., Bongas, H.P., Cayacay, M.A., 2023b. Typology of milkfish (Chanos chanos) farms: Their operations, socio-economic viability, and challenges. Preprints (2023120507).
- Macusi, E.D., Macusi, E.S., Jimenez, L.A., Catam-isan, J.P., 2020. Climate change vulnerability and perceived impacts on small-scale fisheries in eastern Mindanao. Ocean & Coastal Management 189, 105143. [CrossRef]
- Macusi, E.D., Morales, I.D., Abreo, N.A.S., Jimenez, L.A., 2019. Perception of solid waste management and rate of accumulation in schools in Mati City, Mindanao island, Philippines. Journal of Marine and Island Cultures 8, 113-131. [CrossRef]
- Macusi, E.D., Siblos, S.K.V., Betancourt, M.E.S., Macusi, E.S., Calderon, M.N., Bersaldo, M.J.I., Digal, L.N., 2022. Impacts of COVID-19 on the catch of small-scale fishers and their families due to restriction policies in Davao Gulf, Philippines. Frontiers in Marine Science 8, 770543. [CrossRef]
- Macusi, E.S., Sales, A.C., Macusi, E.D., Bong-as, H.P., Cayacay, M.A., Omandam, J.L., Schüler, M., Vidal, C., 2023c. Indigenous Knowledge Systems and Practices (IKSP), Livelihood Resources and Aspirations of the Matigsalog and Ata Tribes. Sustainability 15, 1-15. [CrossRef]
- Matthews, P.J., Agoo, E.M.G., Tandang, D.N., Madulid, D.A. , 2012. Ethnobotany and ecology of wild taro (Colocasia esculenta) in the Philippines: implications for domestication and dispersal, in: Spriggs, M., Addison, D., Matthews, P.J. (Eds.), Irrigated Taro (Colocasia esculenta) in the Indo-Pacific. National Museum of Ethnology, Osaka, Japan, p. 363.
- Maynawang, I., Peralez, C., Lorenzo, H., Macusi , E., 2021. Role of women and coastal livelihood in the small-scale fisheries (SSF) of Lupon and Governor Generoso, Davao Oriental. Davao Research Journal 12, 10-21. [CrossRef]
- McLean, E., 2023. 4.23 - Feed Ingredients for Sustainable Aquaculture, in: Ferranti, P. (Ed.), Sustainable Food Science - A Comprehensive Approach. Elsevier, Oxford, pp. 392-423.
- Medhekar, A., 2024. Chapter 2 - Circular economy in agriculture and sustainable development, in: Machado, C.F., Davim, J.P. (Eds.), Circular Economy and Manufacturing. Woodhead Publishing, pp. 15-31.
- Molina-Poveda, C.I., edited by 2016. Nutrient requirements, in: Nates, S.F. (Ed.), Aquafeed formulation. Academic Press, Walthan, Massachussets, p. 279.
- Mujtaba, M., Fernandes Fraceto, L., Fazeli, M., Mukherjee, S., Savassa, S.M., Araujo de Medeiros, G., do Espírito Santo Pereira, A., Mancini, S.D., Lipponen, J., Vilaplana, F., 2023. Lignocellulosic biomass from agricultural waste to the circular economy: a review with focus on biofuels, biocomposites and bioplastics. J Clean Prod 402, 136815. [CrossRef]
- Munguti, J., Charo-Karisa, H., Opiyo, M.A., Ogello, E.O., Marijani, E., Nzayisenga, L., Liti, D., 2012. Nutritive value and availability of commonly use feed ingredients for farmed Nile Tilapia (Oreochromis niloticus) and African catfish (Clarias gariepinus) in Kenya, Rwanda and Tanzania. African Journal of Food, Agriculture, Nutrition and Development 12. [CrossRef]
- Najar, I.N., Sharma, P., Das, R., Tamang, S., Mondal, K., Thakur, N., Gandhi, S.G., Kumar, V., 2024. From waste management to circular economy: Leveraging thermophiles for sustainable growth and global resource optimization. J Environ Manage 360, 121136. [CrossRef]
- Onsay, E.A., Baltar, K.C., Galicia, E.R., Pesino, I.R.C., 2022. The Dynamics of Taro (Colocasia esculenta) through Value Chain Analysis and Crop Accounting in Partido District, Camarines Sur, the Philippines, in: Orhan, Ö. (Ed.), Sustainable Rural Development Perspective and Global Challenges. IntechOpen, Rijeka, p. Ch. 8.
- Pratiwi, D.Y., Andhikawati, A., 2021. Utilization of Water Hyacinth (Eichhornia crassipes) as Fish Feed Ingredient. Asian Journal of Fisheries Aquatic Resources, 35-42. [CrossRef]
- Provin, A.P., Medeiros d’Alva, A., de Aguiar Dutra, A.R., Salgueirinho Osório de Andrade Guerra, J.B., Leal Vieira Cubas, A., 2024. Closing the cycle: Circular economy strategies for the textile industry using banana farming waste. J Clean Prod 470, 143352. [CrossRef]
- Quintero-Herrera, S., Zwolinski, P., Evrard, D., Cano-Gómez, J.J., Rivas-García, P., 2023. Turning food loss and waste into animal feed: A Mexican spatial inventory of potential generation of agro-industrial wastes for livestock feed. Sustainable Production and Consumption 41, 36-48. [CrossRef]
- Rana, K.J., Siriwardena, S., Hasan, M.R., 2009. Impact of rising feed ingredient prices on aquafeeds and aquaculture production, FAO Fisheries and Aquaculture Technical Paper 541. Food and Agriculture Organization (FAO), Rome.
- Sehgal, H.S., Sharma, S., 1993. A note on evaluation of some wastes and by-products from agriculture and animal husbandry as feed ingredients for Cirrhina mrigala (Ham.). Bioresource Technology 44, 9-11. [CrossRef]
- Siddique, S., Grassauer, F., Arulnathan, V., Sadiq, R., Pelletier, N., 2024. A review of life cycle impacts of different pathways for converting food waste into livestock feed. Sustainable Production and Consumption. [CrossRef]
- Sun, X., Dou, Z., Shurson, G.C., Hu, B., 2024. Bioprocessing to upcycle agro-industrial and food wastes into high-nutritional value animal feed for sustainable food and agriculture systems. Resources, Conservation and Recycling 201, 107325. [CrossRef]
- Tahamtani, F.M., Ivarsson, E., Wiklicky, V., Lalander, C., Wall, H., Rodenburg, T.B., Tuyttens, F.A.M., Hernandez, C.E., 2021. Feeding live Black Soldier Fly larvae (Hermetia illucens) to laying hens: effects on feed consumption, hen health, hen behavior, and egg quality. Poultry Sci 100, 101400. [CrossRef]
- Ulloa, J.B., van Weerd, J.H., Huisman, E.A., Verreth, J.A.J., 2004. Tropical agricultural residues and their potential uses in fish feeds: the Costa Rican situation. Waste Management 24, 87-97. [CrossRef]
- Upadhyay, S.K., Singh, G., Rani, N., Rajput, V.D., Seth, C.S., Dwivedi, P., Minkina, T., Wong, M.H., Show, P.L., Khoo, K.S., 2024. Transforming bio-waste into value-added products mediated microbes for enhancing soil health and crop production: Perspective views on circular economy. Environmental Technology & Innovation 34, 103573. [CrossRef]
- van Huis, A., van Itterbeeck, J., Klunder, H., Mertens, E., Halloran, A., Muir, G., Vantomme, P., 2013. Edible Insects: Future Prospects for Food and Feed Security. FAO, Rome, Italy.
- Wanapat, M., Suriyapha, C., Dagaew, G., Prachumchai, R., Phupaboon, S., Sommai, S., Matra, M., 2024. The recycling of tropical fruit peel waste-products applied in feed additive for ruminants: Food manufacturing industries, phytonutrient properties, mechanisms, and future applications. Journal of Agriculture and Food Research 17, 101234. [CrossRef]
- Windarto, S., Nengsi, D.R., Putro, S.P., Widowati, W., Adi, S., Anggraeni, N., Herawati, V.E.,, 2023. Effect of Fish Meal Substitution Using Maggot Flour (Hermetia illucens) on Growth Performance, Feed Utilization Efficiency, Protein Retention and Body Composition of the Milkfish (Chanos chanos) Egyptian Journal of Aquatic Biology & Fisheries 27, 933 – 953.
- Wong, M.-H., Mo, W.-Y., Choi, W.-M., Cheng, Z., Man, Y.-B., 2016. Recycle food wastes into high quality fish feeds for safe and quality fish production. Environmental Pollution 219, 631-638. [CrossRef]


 |
 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




