Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
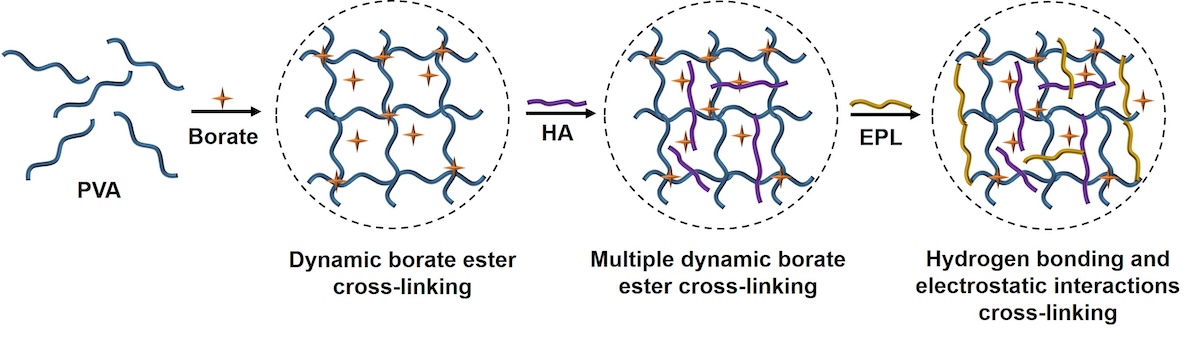
2.1. Fabrication of the Dynamic Double Network (DN) Hydrogels
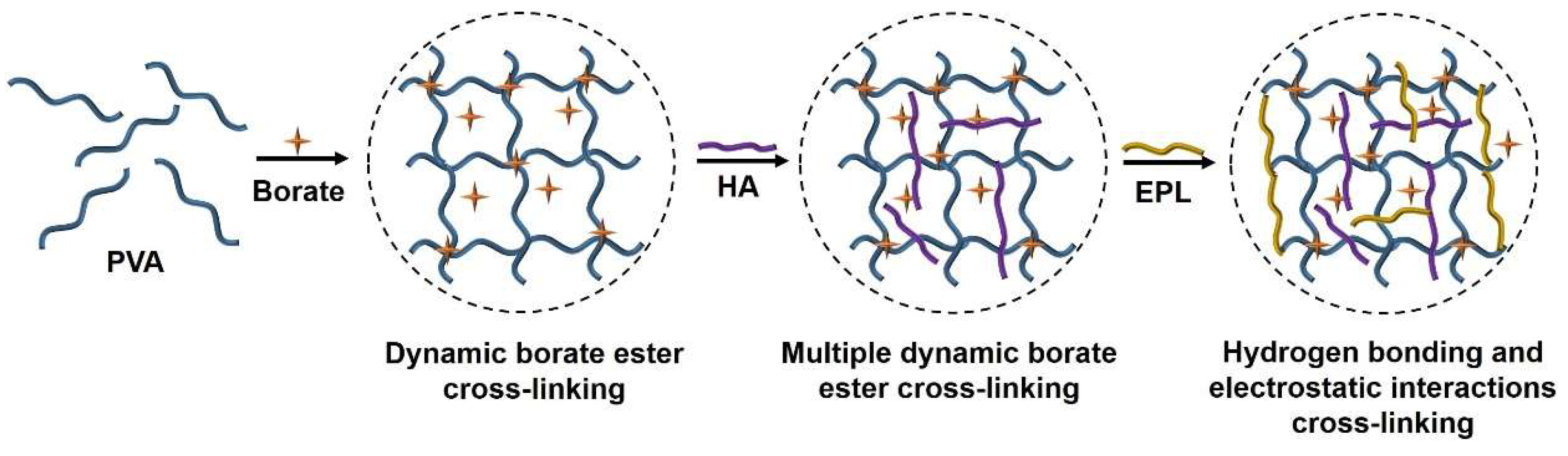
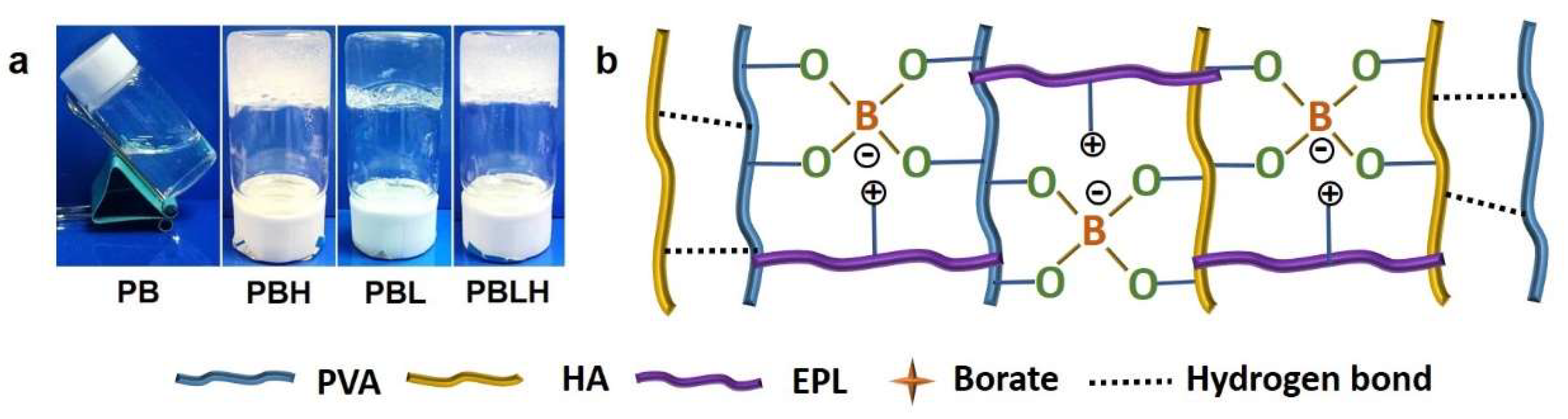
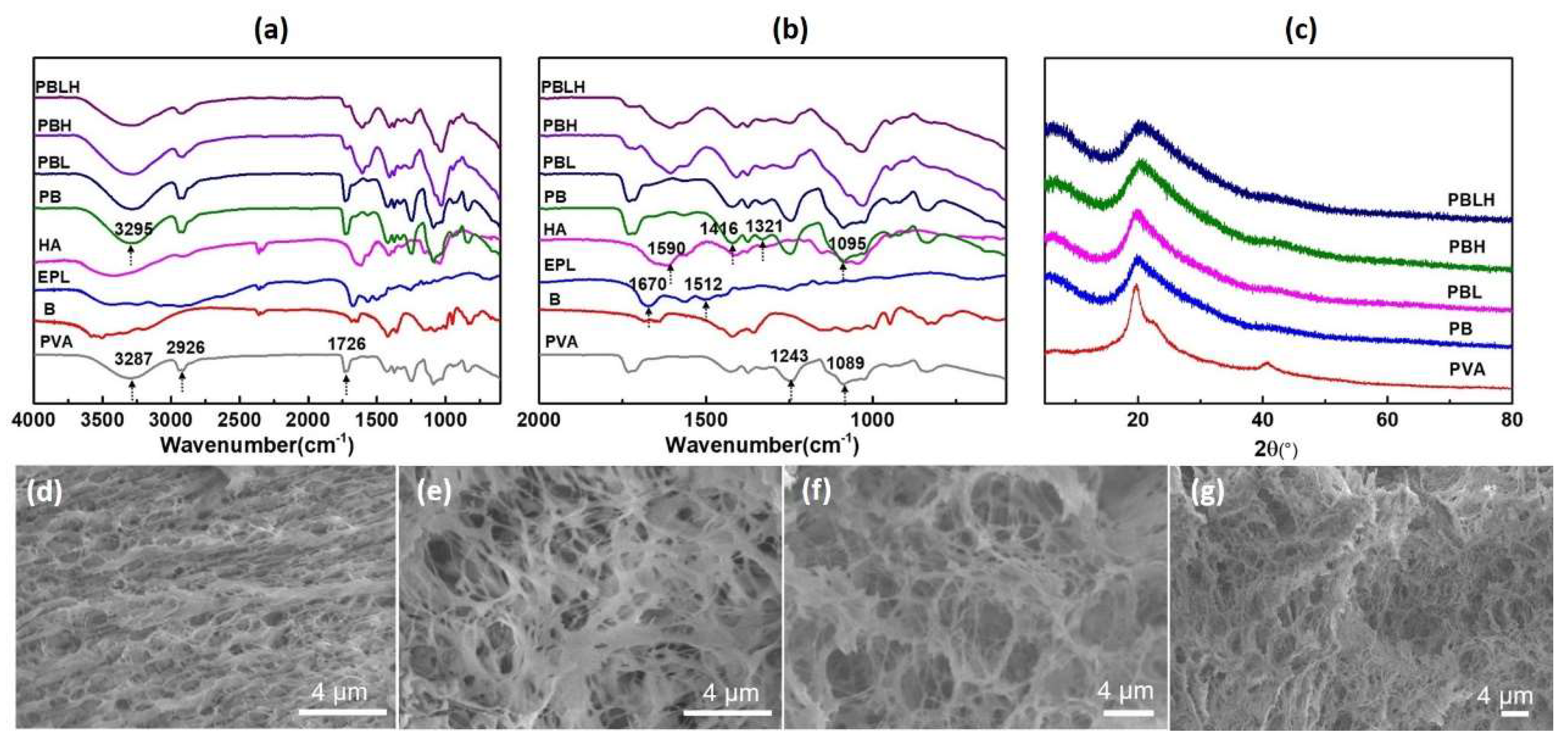
2.2. Structure and Micromorphology of DN Hydrogels
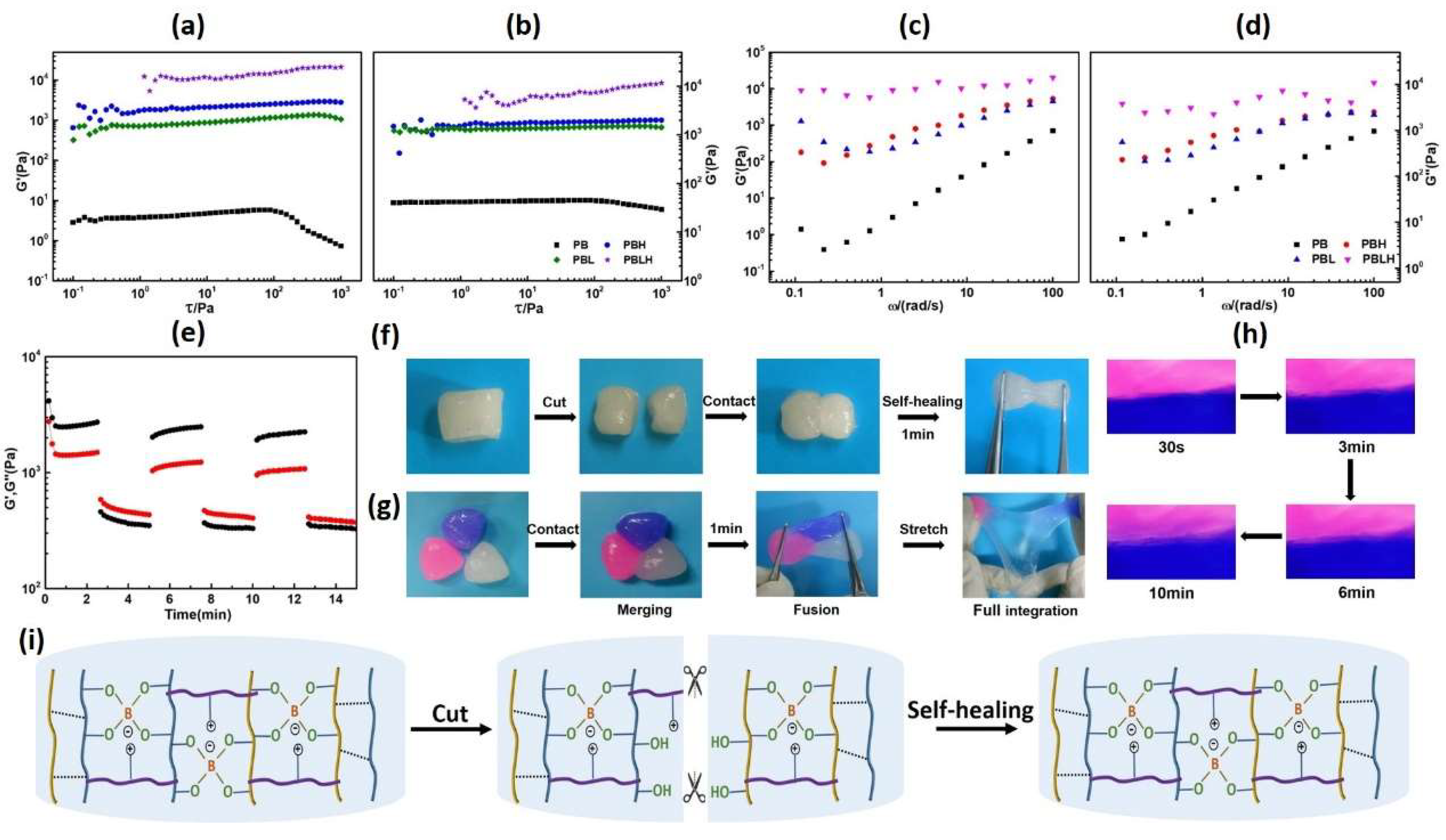
2.3. Rheology and Self-Healing Properties of the DN Composite Hydrogels
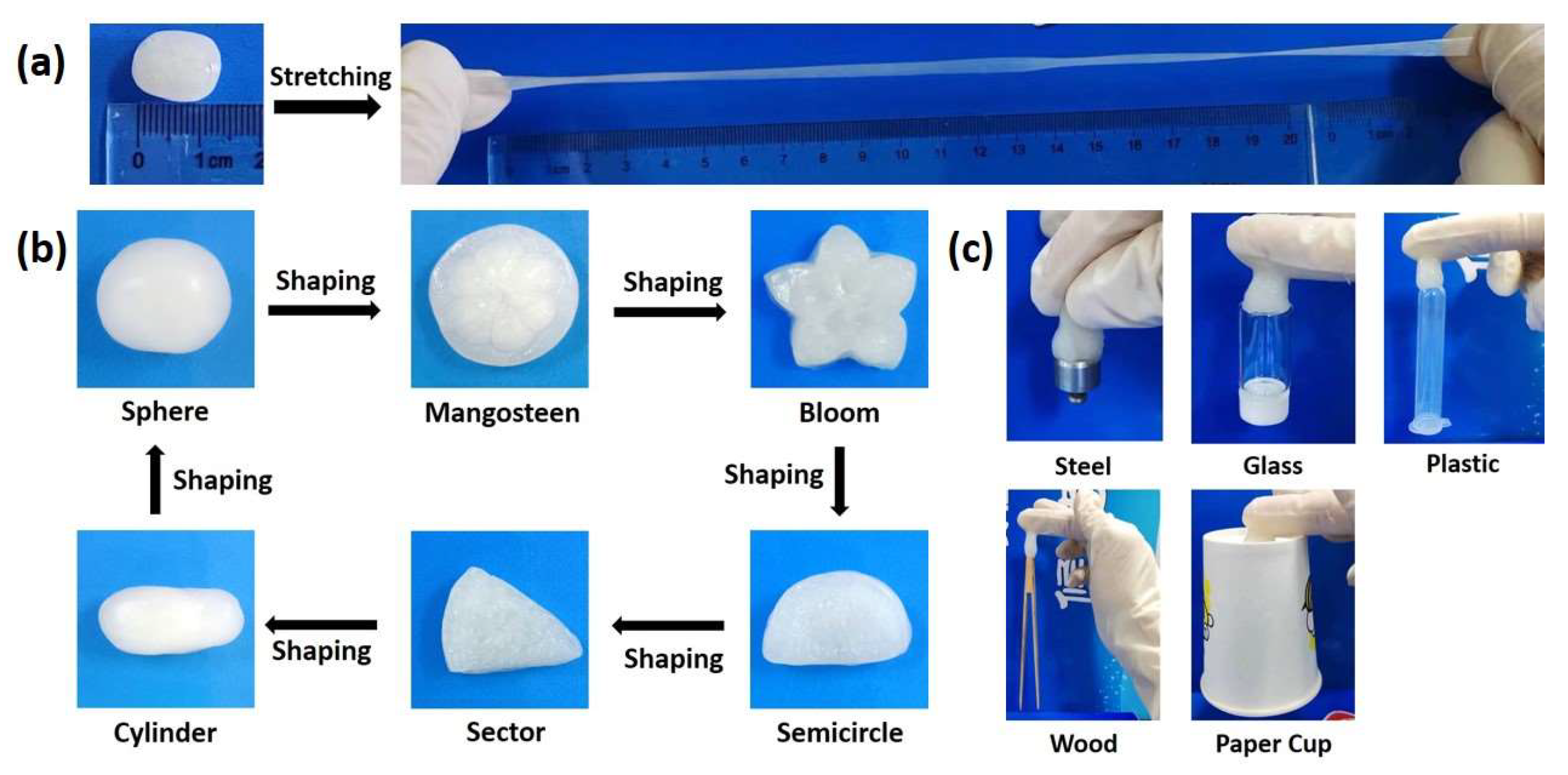
2.4. Stretchable, Shapeable and Adhesive Properties of PBLH Composite Hydrogels
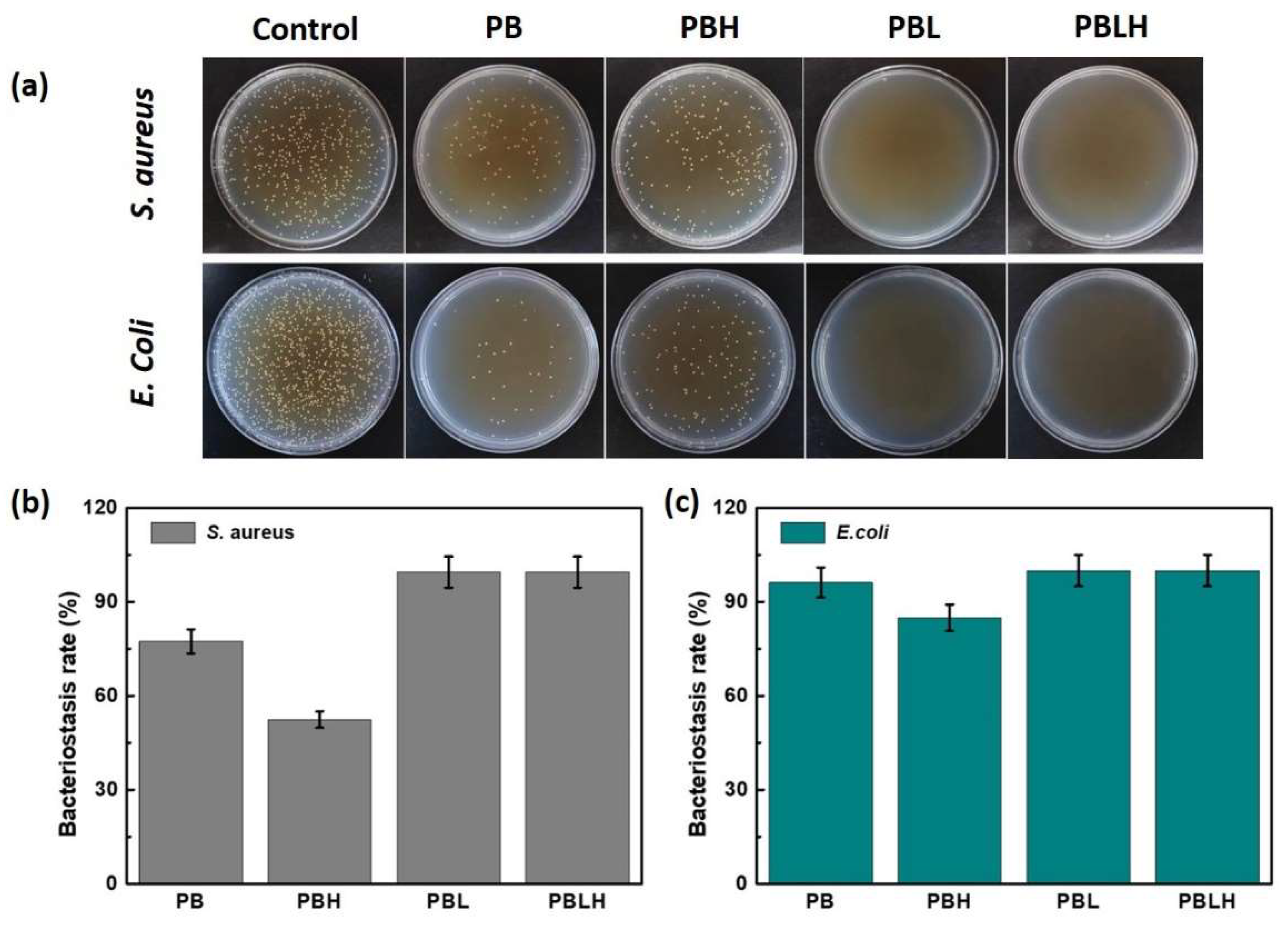
2.5. Antibacterial Activity of the Hydrogels
3. Materials and Methods
3.1. Materials
3.2. Hydrogels Preparation
3.3. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, D. Recent Advances in Hydrogels. Chem. Mater. 2022, 34, 1987–1989. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Z.; Li, T.; Wang, Y.; Zhang, K.; Wu, J.; Zhang, S.; Yuan, F.; Dong, W. Rapid Preparation of Highly Stretchable and Fast Self-Repairing Antibacterial Hydrogels for Promoting Hemostasis and Wound Healing. ACS Appl. Bio Mater. 2024, 7, 394–405. [Google Scholar] [CrossRef]
- Dehghan-Niri, M.; Vasheghani-Farahani, E.; Eslaminejad, M. Tavakol, M.; Bagheri, F. Preparation of Gum Tragacanth/Poly (vinyl alcohol)/Halloysite Hydrogel using Electron Beam Irradiation with Potential for Bone Tissue Engineering. Carbohyd. Polym. 2023, 305, 120548. [Google Scholar] [CrossRef] [PubMed]
- Guo, W-Y. Ma, M-G. Choi, W.; S. Kohane, D. Hybrid Nanoparticle–Hydrogel Systems for Drug Delivery Depots and Other Biomedical Applications. ACS Nano 2024, 18, 22780–22792. [Google Scholar] [CrossRef] [PubMed]
- Toufanian, S.; Mohammed, J.; Winterhelt, E.; Lofts, A.; Dave, R.; K. Coombes, B.; Hoare, T. A Nanocomposite Dynamic Covalent Cross-Linked Hydrogel Loaded with Fusidic Acid for Treating Antibiotic-Resistant Infected Wounds. ACS Applied Bio Mater. 2024, 7, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Liu, F.; Abdiryim, T.; Liu, X. Self-Healing Hydrogels: From Synthesis to Multiple Applications. ACS Mater. Lett. 2023, 5, 1787–1830. [Google Scholar] [CrossRef]
- Ding, X.; Fan, L.; Wang, L.; Zhou, M.; Wang, Y.; Zhao, Y. Designing Self-healing Hydrogels for Biomedical Applications. Mater. Horiz. 2023, 10, 3929–3947. [Google Scholar] [CrossRef]
- Rammal, H.; GhavamiNejad, A.; Erdem, A.; Mbeleck, R.; Nematollahi, M.; Emir Diltemiz, S.; Alem, H.; Ali Darabi, M.; Ertas, Y.; J. Catersonm, E.; Ashammakhi, N.. Advances in Biomedical Applications of Self-healing Hydrogels. Mater. Chem. Front., 2021, 5, 4368–4400. [Google Scholar] [CrossRef]
- Sun, P.; Ren, S.; Liu, F.; Wu, A.; Sun, N.; Shi, L.; Zheng, L. Smart low molecular weight hydrogels with dynamic covalent skeletons. Soft Matter, 2018, 14, 6678–6683. [Google Scholar] [CrossRef]
- Luo, C.; Guo, A.; Zhao, Y.; Sun, X. A High Strength, Low Friction, and Biocompatible Hydrogel from PVA, Chitosan and Sodium Alginate for Articular Cartilage. Carbohyd. Polym. 2022, 286, 119268. [Google Scholar] [CrossRef]
- Cho, S.; Hwang, S.; Dongyeop, X.; Park, J. Recent Progress in Self-healing Polymers and Hydrogels based on Reversible Dynamic B-O Bonds: Boronic/Boronate Esters, Borax, and Benzoxaborole. J. Mater. Chem. A, 2021, 9, 14630–14655. [Google Scholar] [CrossRef]
- Shui, T.; Pan, M.; Li, A.; Fan, H.; Wu, J.; Liu, Q.; Zeng, H. Poly (vinyl Alcohol) (PVA)-Based Hydrogel Scaffold with Isotropic Ultratoughness Enabled by Dynamic Amine-Catechol Interactions. Chem. Mater. 2022, 34, 8613–8628. [Google Scholar] [CrossRef]
- Yang, X.; Wang, B.; Sha, D.; Liu, Y.; Liu, Z.; Shi, K.; Liu, W.; Yu, C.; Ji, X. PVA/Poly(hexamethylene guanidine)/Gallic Acid Composite Hydrogel Films and Their Antibacterial Performance. ACS Appl. Polym. Mater. 2021, 3, 3867–3877. [Google Scholar] [CrossRef]
- Zhang, X.; Ren, K.; Xiao, C.; Chen, X. Guanosine-driven Hyaluronic Acid-based Supramolecular Hydrogels with Peroxidase-like Activity for Chronic Diabetic Wound Treatment. Acta Biomater. 2023, 172, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable Adhesive Self-Healing Multiple-Dynamic-Bond Crosslinked Hydrogel with Photothermal Antibacterial Activity for Infected Wound Healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Du, R.; Yang, Y.; Liu, Y.; Wan, Z.; Yang, X. Injectable Self-Healing Adhesive Natural Glycyrrhizic Acid Bioactive Hydrogel for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17562–17576. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, X.; Wang, Y.; Hao, J. Rapid-Forming and Self-Healing Agarose-Based Hydrogels for Tissue Adhesives and Potential Wound Dressings. Biomacromolecules. 2018, 19, 3980–3988. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, S.; Du, R.; Yang, Y.; Liu, Y.; Wan, Z.; Yang, X. Injectable Self-Healing Adhesive Natural Glycyrrhizic Acid Bioactive Hydrogel for Bacteria-Infected Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 17562–17576. [Google Scholar] [CrossRef]
- Fan, L.; He, Z.; Peng, X.; Xie, J; Su, F. ; Wei, D-X. Zheng, Y.; Yao, D. Injectable, Intrinsically Antibacterial Conductive Hydrogels with Self-Healing and pH Stimulus Responsiveness for Epidermal Sensors and Wound Healing. ACS Appl. Mater. Interfaces 2021, 13, 53541–53552. [Google Scholar] [CrossRef]
- Yan, B.; Huang, J.; Han, L.; Gong, L.; Li, L; N. Israelachvili, J.; Zeng, H. Duplicating Dynamic Strain-Stiffening Behavior and Nanomechanics of Biological Tissues in a Synthetic Self-Healing Flexible Network Hydrogel. ACS Nano 2017, 11, 11074–11081. [Google Scholar] [CrossRef]
- Li, P.; Zong, H.; Li, G.; Shi, Z.; Yu, X.; Zhang, K.; Xia, P.; Yan, S.; Yin, J. Building a Poly(amino acid)/Chitosan-Based Self-Healing Hydrogel via Host–Guest Interaction for Cartilage Regeneration. ACS Biomater. Sci. Eng. 2023, 9, 4855–4866. [Google Scholar] [CrossRef]
- Narasimhan, B. ; W. Dixon, A.; Mansel, B.; Taberner, A.; Mata, J.;Malmström, J. Hydrogen Bonding Dissipating Hydrogels: The Influence of Network Structure Design on Structure-Property Relationships. J. Colloid Interf. Sci. 2023, 630, 638–653. [Google Scholar]
- Wang, L.; Gao, G.; Zhou, Y.; Xu, T.; Chen, J.; Wang, R.; Zhang, R.; Fu, J. Tough, Adhesive, Self-Healable, and Transparent Ionically Conductive Zwitterionic Nanocomposite Hydrogels as Skin Strain Sensors. ACS Appl. Mater. Interfaces 2019, 11, 3506–3515. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Victor Jerca, V.; Hoogenboom, R. Bioinspired Double Network Hydrogels: from Covalent Double Network Hydrogels via Hybrid Double Network Hydrogels to Physical Double Network Hydrogels. Mater. Horiz., 2021, 8, 1173–1188. [Google Scholar] [CrossRef] [PubMed]
- B. Rodell, C.; N. Dusaj, N.; B. Highley, C.; A. Burdick, J. Injectable and Cytocompatible Tough Double-Network Hydrogels through Tandem Supramolecular and Covalent Crosslinking. Adv. Mater. 2016, 20160226. [Google Scholar]
- Yin, Y.; Gu, Q.; Liu, X.; Liu, F.; Julian McClement, D. Double network hydrogels: Design, fabrication, and application in biomedicines and foods. Adv Colloid Interface Sci. 2023, 320, 102999. [Google Scholar] [CrossRef]
- C. Ollier, R.; Xiang, Y.; M. Yacovelli, A.; J. Webber, M. Biomimetic Strain-Stiffening in Fully Synthetic Dynamic-Covalent Hydrogel Networks. Chem. Sci., 2023, 14, 4796–4805. [Google Scholar] [CrossRef]
- Rezanejade Bardajee, G.; Ghadimkhani, R.; Jafarpour, F. A Biocompatible Double Network Hydrogel based on Poly (acrylic acid) Grafted onto Sodium Alginate for Doxorubicin Hydrochloride Anticancer Drug Release. Int. J. Biol. Macromol. 2024, 260, 128871. [Google Scholar]
- Wang, Y.; Zhang, Y.; Ren, P.; Yu, S.; Cui, P.; B. Nielsen, C.; Abrahams, I.; Briscoe, J.; Lu, Y. Versatile and Recyclable Double-Network PVA/Cellulose Hydrogels for Strain Sensors and Triboelectric Nanogenerators under Harsh Conditions. Nano Energy 2024, 125, 109599. [Google Scholar] [CrossRef]
- Li, L.; Wu, P.; Yu, F.; Ma, J. Double Network Hydrogels for Energy/Environmental Applications: Challenges and Opportunities. J. Mater. Chem. A, 2022, 10, 9215–9247. [Google Scholar] [CrossRef]
- Xu, X.; Victor Jerca, V.; Hoogenboom, R. Bioinspired Double Network Hydrogels: from Covalent Double Network Hydrogels via Hybrid Double Network Hydrogels to Physical Double Network Hydrogels. Mater. Horiz., 2021, 8, 1173–1188. [Google Scholar] [CrossRef] [PubMed]
- Chen, W-P.; Hao, D-Z.; Hao, W-J.; Guo, X-L.; Jiang, L. Hydrogel with Ultrafast Self-Healing Property Both in Air and Underwater. ACS Appl. Mater. Interfaces 2018, 10, 1258–1265. [CrossRef] [PubMed]
- Ge, W.; Cao, S.; Shen, F.; Wang, Y.; Ren, J.; Wang, X. Rapid Self-healing, Stretchable, Moldable, Antioxidant and Antibacterial Tannic Acid-cellulose Nanofibril Composite Hydrogels. Carbohyd. Polym. 2019, 224, 115147. [Google Scholar] [CrossRef] [PubMed]
- Teng, J.; Zhao, W.; Zhang, S.; Yang, D.; Liu, Y.; Huang, R.; Ma, Y.; Jiang, L.; Wei, H.; Zhang, J.; Chen, J. Injectable Nanoparticle-Crosslinked Xyloglucan/ε-Poly-L-lysine Composite Hydrogel with Hemostatic, Antimicrobial, and Angiogenic Properties for Infected Wound Healing. Carbohyd. Polym. 2024, 336, 122102. [Google Scholar] [CrossRef]
- Xu, Q.; Dai, X.; Yang, L.; Liu, X.; Li, Y.; Gao, F. ε Polylysine-Based Macromolecules with Catalase-Like Activity to Accelerate Wound Healing by Clearing Bacteria and Attenuating Inflammatory Response. ACS Biomater. Sci. Eng. 2022, 8, 5018–5026. [Google Scholar] [CrossRef]
- Lee, J-H. ; Kwon, H-K.; Shin, H-J.; Nam, G-H.; Kim, J-H.; Cho, S. Quasi-Stem Cells Derived from Human Somatic Cells by Chemically Modified Carbon Nanotubes. ACS Appl. Mater. Interfaces 2018, 10, 8417–8425. [Google Scholar] [CrossRef]
- Meng, Z.; He, Y.; Wang, F.; Hang, R.; Zhang, X.; Huang, X.; Yao, X. Enhancement of Antibacterial and Mechanical Properties of Photocurable ε Poly L lysine Hydrogels by Tannic Acid Treatment. ACS Appl. Bio Mater. 2021, 4, 2713–2722. [Google Scholar] [CrossRef]
- Wei, P.; Duan, Y.; Wang, C.; Sun, P.; Sun, N. Co-Assembled Supramolecular Organohydrogels of Amphiphilic Zwitterion and Polyoxometalate with Controlled Microstructures. Molecules 2024, 29, 2286. [Google Scholar] [CrossRef]
- Dehghan-Niri, M.; Vasheghani-Farahani, E.; Eslaminejad, M.; Tavakol, M.; Bagheri, F. Preparation of Gum Tragacanth/Poly (vinyl alcohol)/Halloysite Hydrogel using Electron Beam Irradiation with Potential for Bone Tissue Engineering. Carbohyd. Poym. 2023, 305, 120548. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, J.; Lang, C.; Qiao, S.; An, G.; Fan, X.; Zhao, L.; Hou, C.; Liu, J. Enzyme-Regulated Fast Self-Healing of a Pillararene-Based Hydrogel. Biomacromolecules 2017, 18, 1885–1892. [Google Scholar] [CrossRef]
- Guo, H.; Huang, S.; Xu, A.; Xue, W. Injectable Adhesive Self-Healing Multiple-Dynamic-Bond Crosslinked Hydrogel with Photothermal Antibacterial Activity for Infected Wound Healing. Chem. Mater. 2022, 34, 2655–2671. [Google Scholar] [CrossRef]
- Huang, J.; Wu, C.; Yu, X.; Li, H.; Ding, S.; Zhang, W. Biocompatible Autonomic Self-healing PVA-TA Hydrogel with High Mechanical Strength, Macromol. Chem. Phys. 2021, 222, 2100061. [Google Scholar]
- Si, R.; Wang, Y.; Yang, Y.; Javeed, A.; Chen, J.; Han, B. Dynamic Dual-Crosslinking Antibacterial Hydrogel with Enhanced Bio-adhesion and Self-healing Activities for Rapid Hemostasis in Vitro and in Vivo. Mater. Des. 2023, 233, 112244. [Google Scholar] [CrossRef]
- Peng, Y-Y.; Cheng, Q.; Wang, W.; Wu, M.; Diaz-Dussan, D.; Kumarc, P.; Narain, R. Multi-Responsive, Injectable, and Self-healing Hydrogels based on Benzoxaborole-Tannic Acid Complexation. Polym. Chem. 2021, 12, 5623–5630. [CrossRef]
- Yang, X.; Liu, G.; Peng, L.; Guo, J.; Tao, L.; Yuan, J.; Chang, C.; Wei, Y.; Zhang, L. Highly Efficient Self-Healable and Dual Responsive Cellulose-Based Hydrogels for Controlled Release and 3D Cell Culture. Adv. Funct. Mater. 2017, 1703174. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



