Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
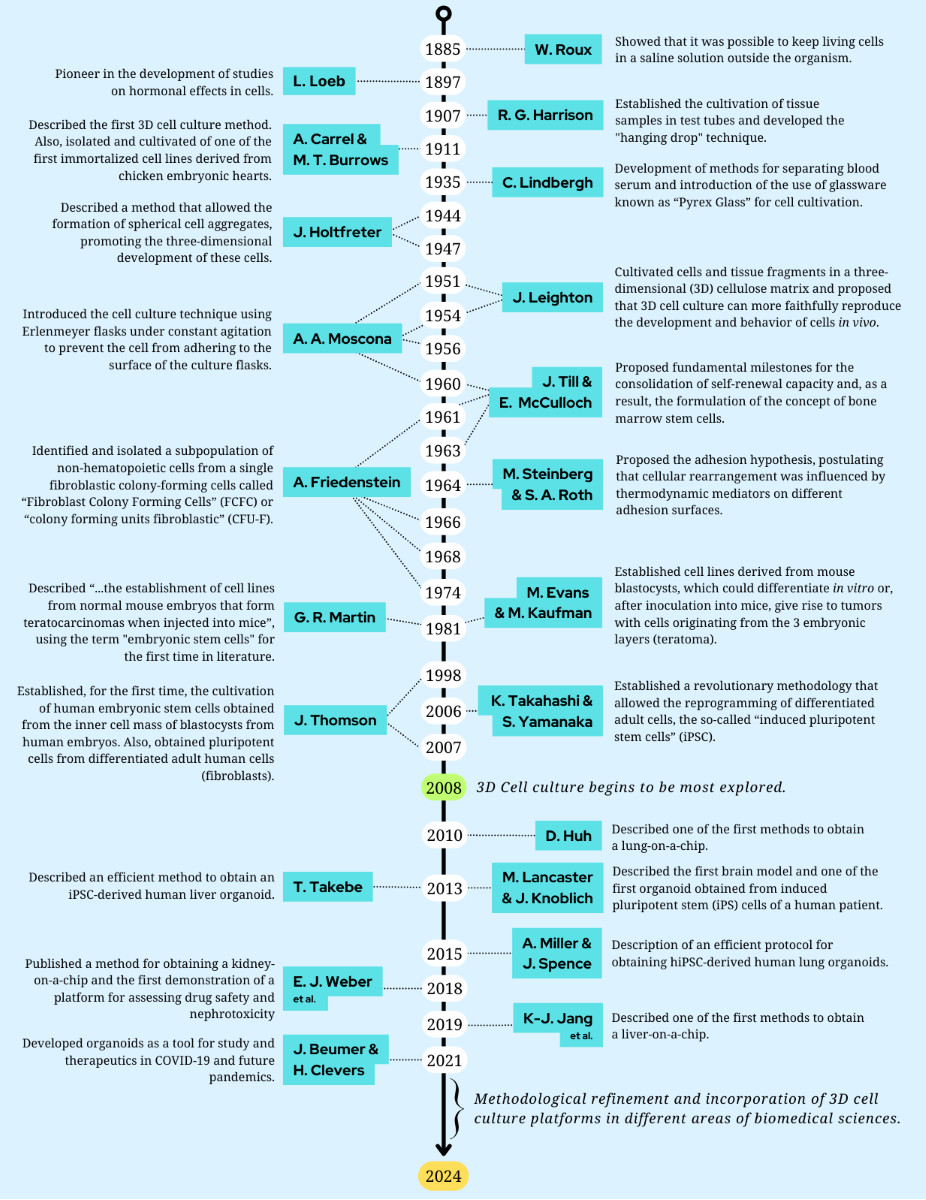
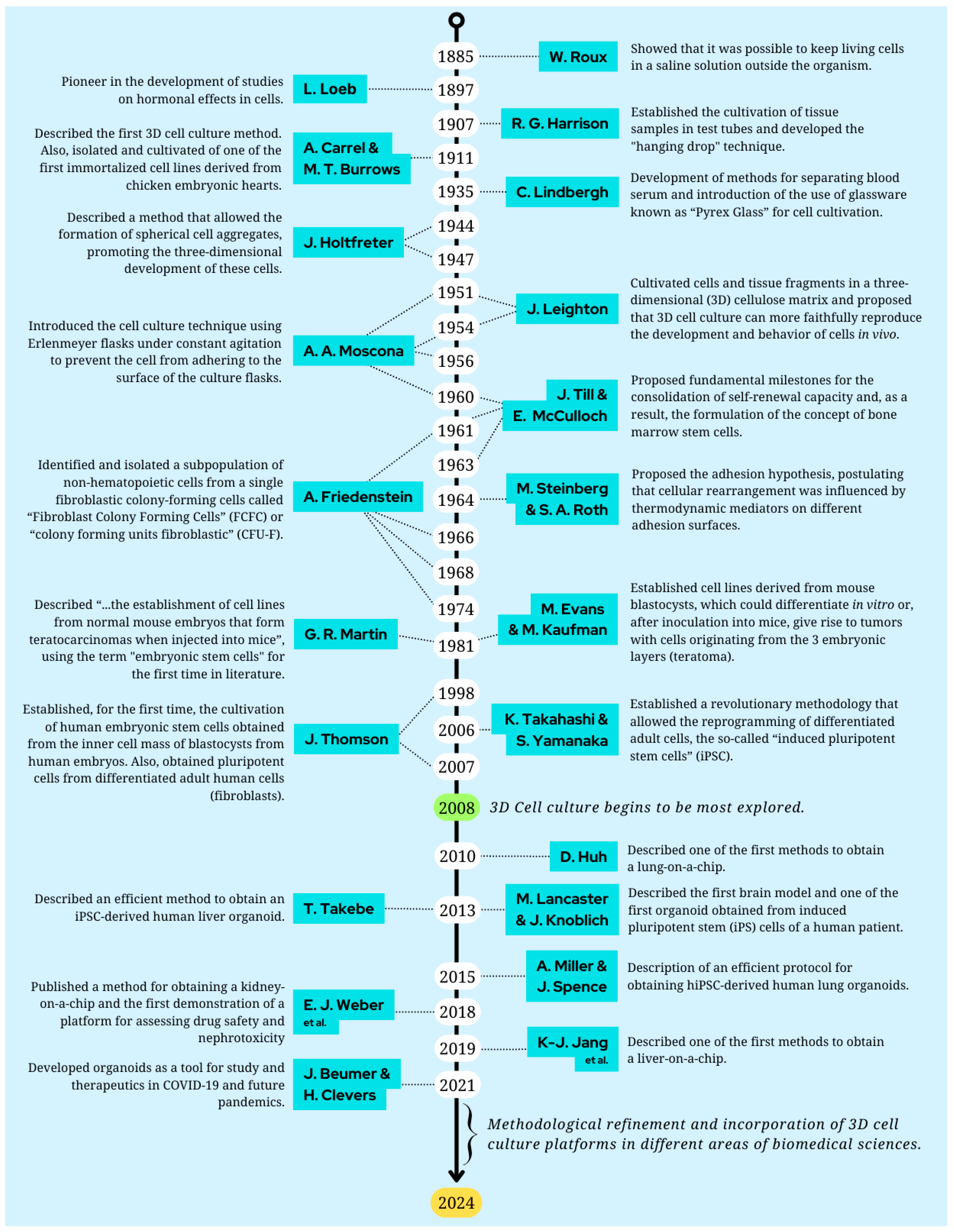
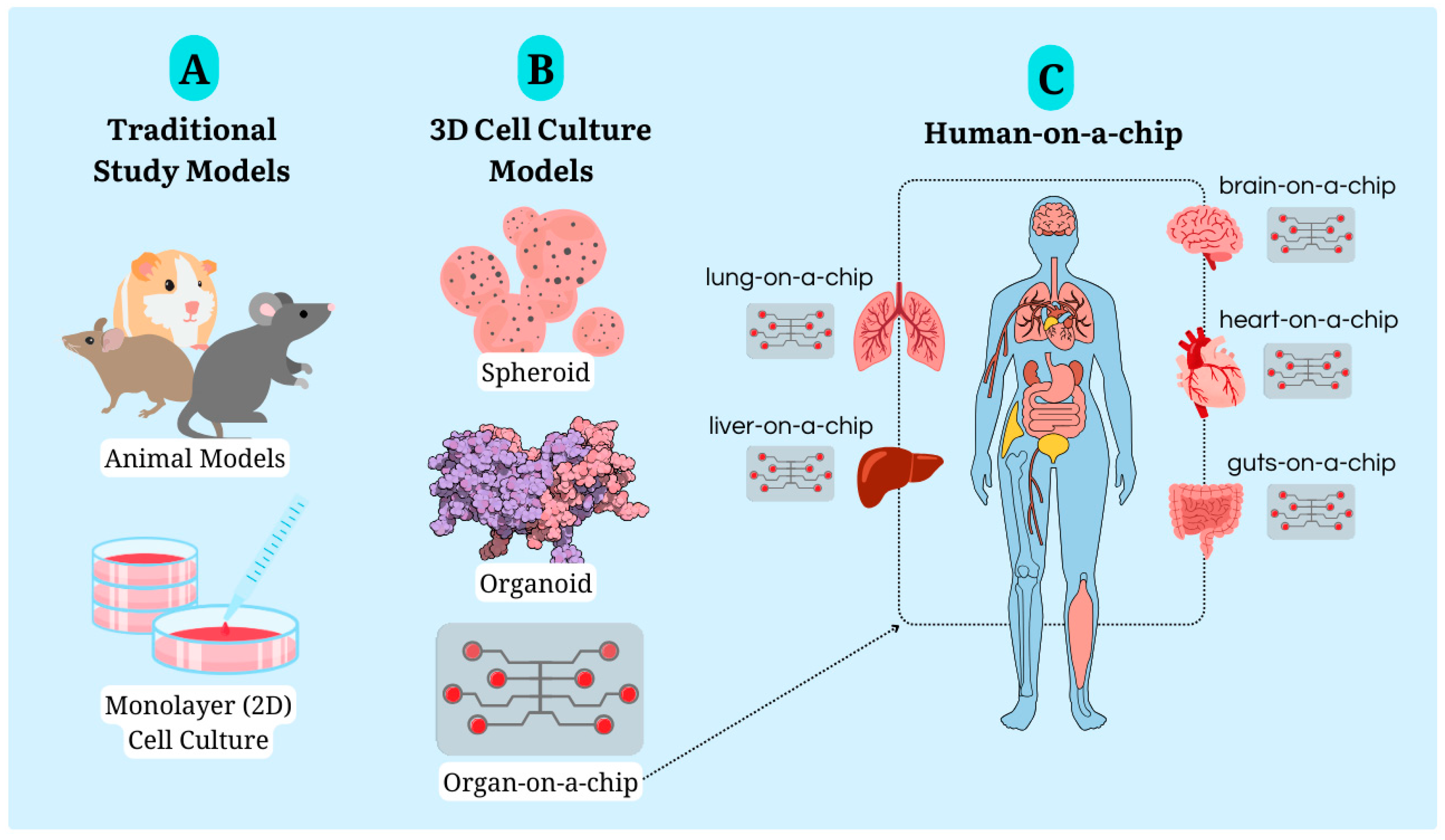
1. An Overview: The Cell Culture History
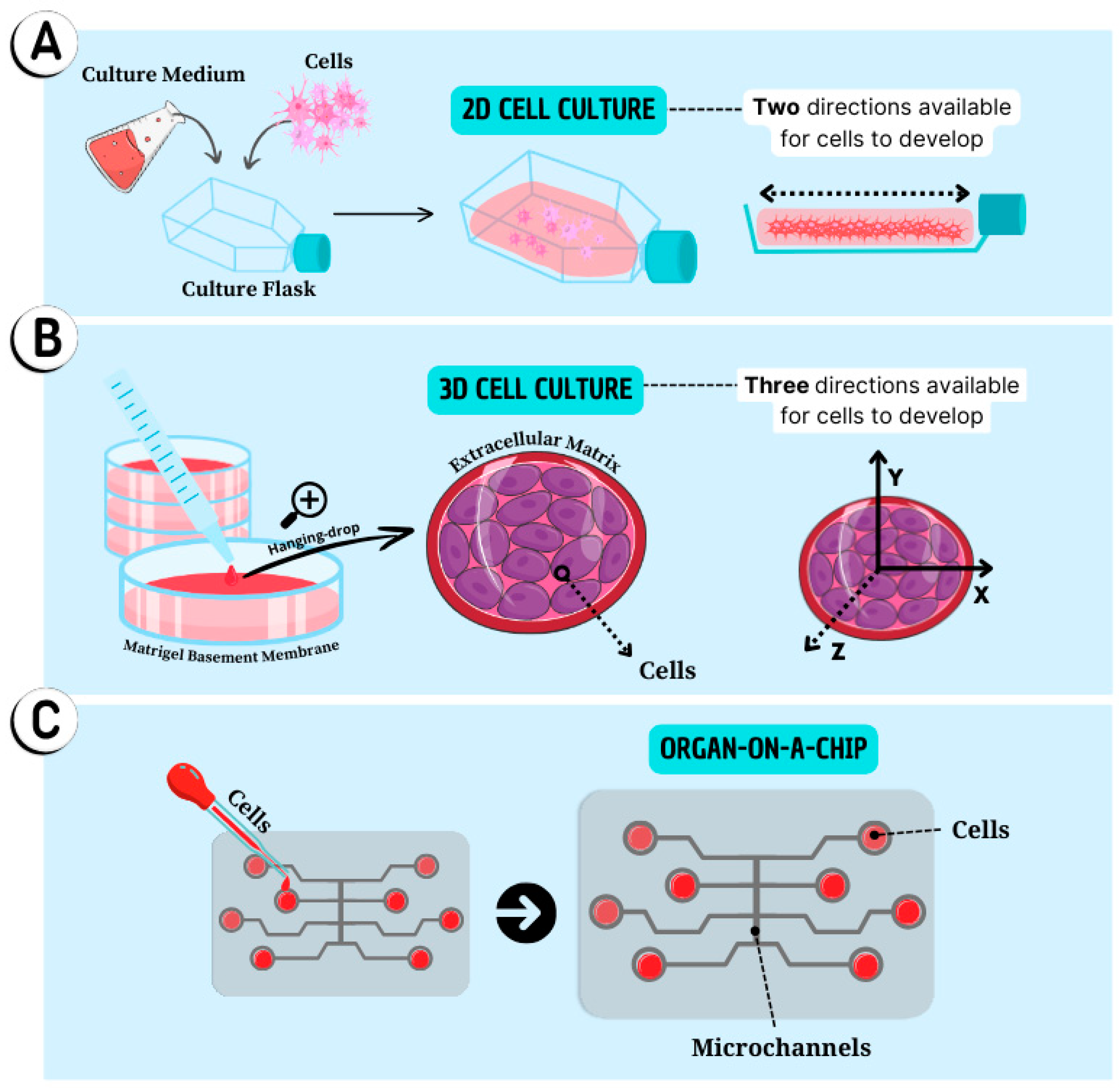
2. Two-Dimensional (2D) Cell Cultures
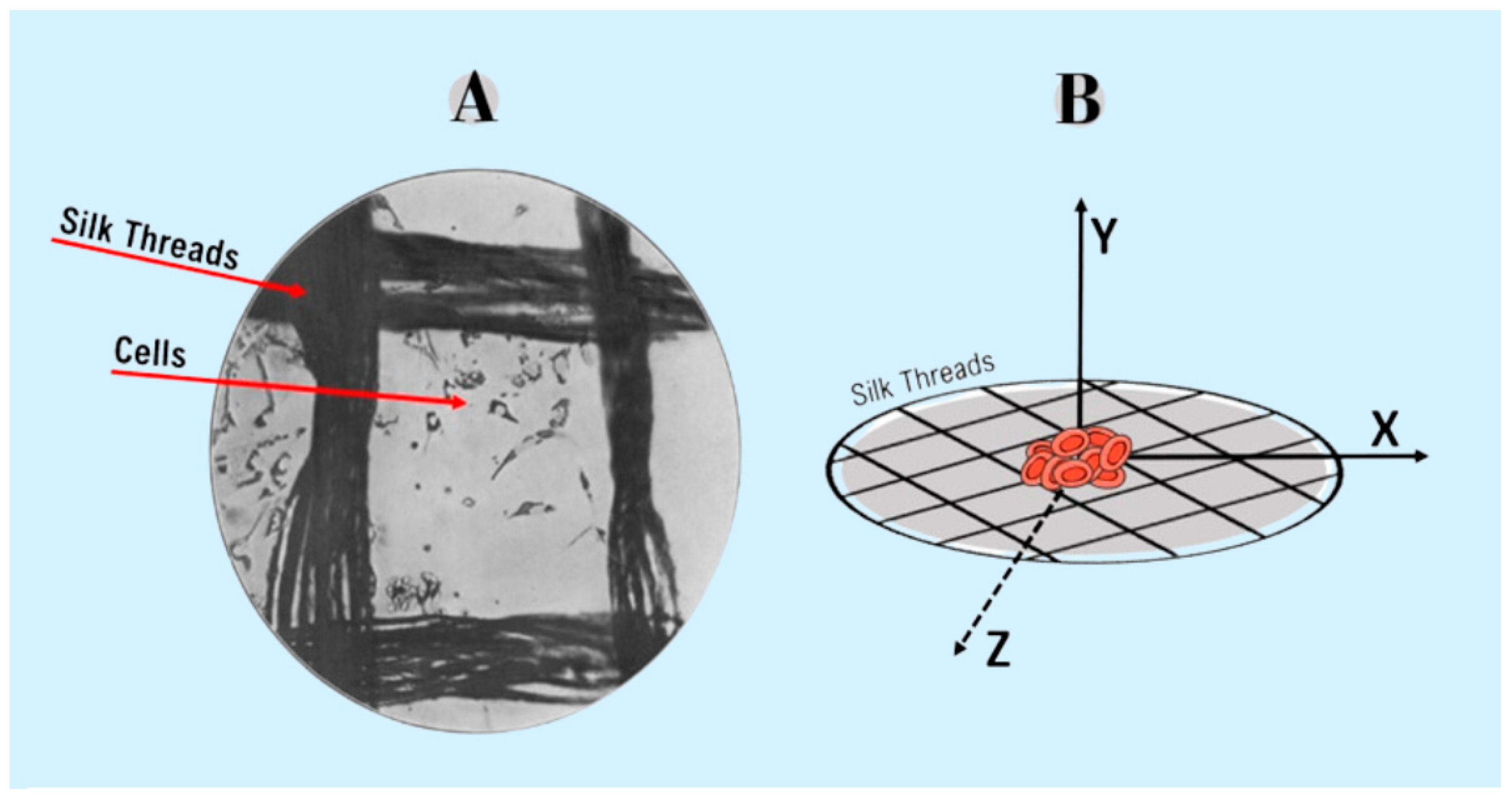
3. Three-Dimensional (3D) Cell Culture: Spheroids and Organoids
4. Organs-on-a-Chip
5. New Perspectives for Cell Culture Methodologies
6. Conclusion Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [CrossRef]
- Jedrzejczak-Silicka, M. History of Cell Culture. In New Insights into Cell Culture Technology; IntechOpen, 2017. Available online: https://www.intechopen.com/chapters/53566 (accessed on 29 August 2023).
- Sander, K. Landmarks in Developmental Biology 1883–1924: Historical Essays from Roux’s Archives; Springer Berlin Heidelberg: Berlin, Heidelberg, 1997. Available online: http://link.springer.com/10.1007/978-3-642-60492-8 (accessed on 30 August 2024).
- Loeb, L. The production of deciduomata and the relation between the ovaries and the formation of the decidua. J. Am. Med. Assoc. 1908, L, 23, 1897.
- Loeb, L. The Influence of certain Bacteria on the Coagulation of the Blood. J. Med. Res. 1903, 10, 3, 407–419.
- Loeb, L. Wounds of the pregnant uterus. Proc. Soc. Exp. Biol. Med. 1906, 4, 1, 93–94. [CrossRef]
- Loeb, L. The cyclic changes in the ovary of the guinea pig. J. Morphol. 1911, 2, 1, 37–70. [CrossRef]
- Loeb, L. On Transplantation of tumors. J. Med. Res. 1901, 6, 1, 28–38.
- Loeb, L. Further Investigations in Transplantation of tumors. J. Med. Res. 1902, 8, 1, 44–73.
- Loeb, L. On the Blood Lymph Cells and inflammatory Processes of Limulus. J. Med. Res. 1902, 7, 1, 145–158.
- Harrison, R.G. Observations on the living developing nerve fiber. Proc. Soc. Exp. Biol. Med. 1906, 4, 1, 140–143.
- Taylor, M.W. A History of Cell Culture. In Viruses and Man: A History of Interactions; Springer International Publishing: Cham, 2014, 41–52. Available online: https://doi.org/10.1007/978-3-319-07758-1_3 (accessed on 30 April 2021).
- Rodríguez-Hernandez, C.; García, S.E.; Olvera-Sandoval, C.; Ramírez Castillo, F.; Loera Muro, A.; González, F.; Guerrero-Barrera, A. Cell culture: History, Development and Prospects. International Journal of Current Research and Academic Review. 2014, 2, 188-200.
- Malinin, T.I. Remembering Alexis Carrel and Charles A. Lindbergh. Tex. Heart Inst. J. 1996, 23, 1, 28–35.
- Carrel, A.; Burrows, M.T. Cultivation of tissues in vitro and its technique. J. Exp. Med. 1911, 13, 3, 387–396. [CrossRef]
- Carrel, A.; Burrows, M.T. An addition to the technique of the cultivation of tissues in vitro. J. Exp. Med. 1911, 14, 3, 244–247. [CrossRef]
- Carrel, A.; Lindbergh, C.A. The culture of Whole Organs. Science 1935, 81, 2112, 621–623. [CrossRef]
- Caprio, N.D.; Burdick, J.A. Engineered biomaterials to guide spheroid formation, function, and fabrication into 3D tissue constructs. Acta Biomater. 2022, S1742-7061, 22, 00620-1.
- Nguyen, R.; Da Won Bae, S.; Qiao, L.; George, J. Developing liver organoids from induced pluripotent stem cells (iPSCs): An alternative source of organoid generation for liver cancer research. Cancer Lett. 2021, 508, 13–17. [CrossRef]
- Chen, G.; Liu, W.; Yan, B. Breast Cancer MCF-7 Cell Spheroid Culture for Drug Discovery and Development. J. Cancer Ther. 2022, 13, 3, 117–130. [CrossRef]
- da Silva da Costa, F.A.; Soares, M.R.; Malagutti-Ferreira, M.J.; da Silva, G.R.; Lívero, F.A.R.; Ribeiro-Paes, J.T. Three-Dimensional Cell Cultures as a Research Platform in Lung Diseases and COVID-19. Tissue Eng. Regen. Med. 2021, 18, 5, 735–745. [CrossRef]
- Hospodiuk-Karwowski, M.; Chi, K.; Pritchard, J.; Catchmark, J.M. Vascularized pancreas-on-a-chip device produced using a printable simulated extracellular matrix. Biomed. Mater. 2022, 17, 6, 065006. [CrossRef]
- Lucey, B.P.; Nelson-Rees, W.A.; Hutchins, G.M. Henrietta Lacks, HeLa Cells, and Cell Culture Contamination. Arch. Pathol. Lab. Med. 2009, 133, 9, 1463–1467.
- Byrnes, W.M. Ernest Everett Just, Johannes Holtfreter, and the origin of certain concepts in embryo morphogenesis. Mol. Reprod. Dev. 2009, 76, 10, 912–921.
- Holtfreter, J. A study of the mechanics of gastrulation. J. Exp. Zool. 1944, 95, 2, 171–212.
- Holtfreter, J. Neural induction in explants which have passed through a sublethal cytolysis. J. Exp. Zool. 1947, 106, 2, 197–222. [CrossRef]
- Moscona, A.; Moscona, H. The dissociation and aggregation of cells from organ rudiments of the early chick embryo. J. Anat. 1952, 86, 3, 287–301.
- Moscona, A. The development in vitro of chimeric aggregates of dissociated embryonic chick and mouse cells. Proc. Natl. Acad. Sci. USA 1957, 43, 1, 184–194. [CrossRef]
- Moscona, A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp. Cell Res. 1961, 22, 455–475. [CrossRef]
- Hoffman, R.M. 3D Sponge-Matrix Histoculture: An Overview. Methods Mol. Biol. 2018, 1760, 11–17.
- Leighton, J. The growth patterns of some transplantable animal tumors in sponge matrix tissue culture. J. Natl. Cancer Inst. 1954, 15, 2, 275–293. [CrossRef]
- McCulloch, E.A.; Till, J.E. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat. Res. 1960, 13, 1, 115–135. [CrossRef]
- Till, J.E.; McCulloch, E.A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat. Res. 1961, 14, 2, 213–222. [CrossRef]
- Becker, A.J.; McCulloch, E.A.; Till, J.E. Cytological demonstration of the clonal nature of spleen colonies derived from transplanted mouse marrow cells. Nature 1963, 197, 4866, 452–454. [CrossRef]
- Siminovitch, L.; McCulloch, E.A.; Till, J.E. The distribution of colony-forming cells among spleen colonies. J. Cell. Comp. Physiol. 1963, 62, 3, 327–336. [CrossRef]
- Friedenstein, A.J. Osteogenetic activity of transplanted transitional epithelium. Acta Anat. Basel. 1961, 45, 31–59. [CrossRef]
- Friedenstein, A.J.; Piatetzky-Shapiro, I.I.; Petrakova, K.V. Osteogenesis in transplants of bone marrow cells. J. Embryol. Exp. Morphol. 1966, 16, 381–390.
- Friedenstein, A.J.; Latzinik, N.V.; Gorskaya, Y.F.; Luria, E.A.; Moskvina, I.L. Stromal cells responsible for transferring the microenvironment of the hemopoietic tissues. Transplantation 1978, 9, 3, 267–274.
- Friedenstein, A.J.; Deriglazova, U.F.; Kulagina, N.N.; Panasuk, A.F.; Rudakowa, S.F.; Luriá, E.A; Ruadkow, I.A. Precursors for fibroblasts in different populations of hematopoietic cells as detected by the in vitro colony assay method. Exp. Hematol. 1974, 2, 83–92.
- Owen, M.E.; Cave, J.; Joyner, C.J. Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J. Cell Sci. 1987, 87, 731–738. [CrossRef]
- Triffitt, J.T. The Collaborative Spark That Ignited the Field of Stromal Stem Cell Biology. Bioengineering 2024, 11, 652. [CrossRef]
- Friedenstein, A.J.; Chailakhjan, R.K.; Lalykina, K.S. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970, 3, 393–403. [CrossRef]
- Friedenstein, A.J.; Chailakhyan, R.K.; Gerasimov, U.V. Bone marrow osteogenic stem cells: In vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987, 20, 3, 263–272. [CrossRef]
- Owen, M. Marrow stromal stem cells. J. Cell Sci. Suppl. 1988, 10, 63–76.
- Owen, M.; Friedenstein, A.J. Stromal stem cells: Marrow-derived osteogenic precursors. Ciba Found. Symp. 1988, 136, 42–60.
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [CrossRef]
- Horwitz, E.M.; Le Blanc, K.; Dominici, M.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Deans, R.J.; Krause, D.S.; Keating, A. Clarification of the Nomenclature for MSC: The International Society for Cellular Therapy Position Statement. Cytotherapy 2005, 7, 5, 393–395. [CrossRef]
- Steinberg, M.S. The Problem of Adhesive Selectivity in Cellular Interactions. Proc. Natl. Acad. Sci. USA 1964, 52, 1, 94–100.
- Evans, M. Origin of mouse embryonal carcinoma cells and the possibility of their direct isolation into tissue culture. J. Reprod. Fertil. 1981, 62, 625–631.
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 5819, 154–156. [CrossRef]
- Martin, G.R. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl. Acad. Sci. USA, 1981, 78 12, 7634–7638. [CrossRef]
- Bradley, A.; Evans, M.; Kaufman, M.H.; Robertson, E. Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 1984, 309, 255–256. [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science, 1998, 282, 5391, 1145–1147. [CrossRef]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131(5), 861–872. [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 4, 663–676.
- Yu, J.; Vodyanik, M.; Smuga-Otto, K.; Frane, J.; Antosiewicz-Bourget, J.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; Slukvin, I.I.; Thomson, J.A. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [CrossRef]
- Aasen, T.; Raya, A.; Barrero, M.J.; Garreta, E.; Consiglio, A.; Gonzalez, F.; Vassena, R.; Bilić, J.; Pekarik, V.; Tiscornia, G.; Edel, M.; Boué, S.; Izpisúa Belmonte, J.C. Efficient and rapid generation of induced pluripotent stem cells from human keratinocytes. Nat. Biotechnol. 2008, 26, 11, 1276–1284. [CrossRef]
- Loh, Y.H.; Agarwal, S.; Park, I.H.; Urbach, A.; Huo, H.; Heffner, G.C.; Kim, K.; Miller, J.D.; Ng, K.; Daley, GQ. Generation of induced pluripotent stem cells from human blood. Blood 2009, 113, 22, 5476–5479. [CrossRef]
- Haase, A.; Olmer, R.; Schwanke, K.; Wunderlich, S.; Merkert, S.; Hess, C.; Zweigerdt, R.; Gruh, I.; Meyer, J.; Wagner, S.; Maier, L.S.; Han, D.W.; Glage, S.; Miller, K.; Fischer, P.; Schöler, H.R.; Martin, U. Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell 2009, 5, 4, 434–441. [CrossRef]
- Cai, J.; Li, W.; Su, H.; Qin, D.; Yang, J.; Zhu, F.; Xu, J.; He, W.; Guo, X.; Labuda, K.; Peterbauer, A.; Wolbank, S.; Zhong, M.; Li, Z.; Wu, W.; So, K.F.; Redl, H.; Zeng, L.; Esteban, M.A.; Pei D. Generation of human induced pluripotent stem cells from umbilical cord matrix and amniotic membrane mesenchymal cells. J. Biol. Chem. 2010, 285, 11227–11234. [CrossRef]
- Yan, X.; et al. Induction of pluripotent stem cells from human third molar mesenchymal stromal cells. J. Biol. Chem. 2010, 285(38), 29270–29278.
- Zhou, T.; Benda, C.; Duzinger, S.; Huang,Y.; Li, X.; Li, Y.; Guo, X.; Cao, G.; Chen, S.; Hao, L.; Chan, Y.; Kwong-Man, Ng.; Ho, J.C.; Wieser, M.; Wu, J.; Redl, H.; Tse, H.F.; Grillari, J.; Grillari-Voglauer, R. Generation of induced pluripotent stem cells from urine. J. Am. Soc. Nephrol. 2011, 22(7), 1221–1228. [CrossRef]
- Zhou, T.; Benda, C.; Dunzinger, S.; Huang, Y.; Ho, J.C.; Yang, J.; Wang, Y.; Zhang, Y.; Zhuang, Q.; Li, Y.; Bao, X.; Tse, H.F.; Grillari, J.; Grillari-Voglauer, R.; Pei, D.; Esteban, M.A. Generation of human induced pluripotent stem cells from urine samples. Nat. Protoc,. 2012, 7, 12, 2080–2089. [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 12, 745–754. [CrossRef]
- Yao, T.; Asayama, Y. Animal-cell culture media: History, characteristics, and current issues. Reprod. Med. Biol. 2017, 16, 2, 99–117.
- Bartfeld, S.; Clevers, H. Stem cell-derived organoids and their application for medical research and patient treatment. J. Mol. Med. 2017, 95, 7, 729–738. [CrossRef]
- Huch, M. Building stomach in a dish. Nat. Cell Biol. 2015, 17, 8, 966–967. [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture?. Front. Mol. Biosci. 2020, 7. [CrossRef]
- Lam, M.T.; Longaker, M.T. Comparison of several attachment methods for human iPS, embryonic, and adipose-derived stem cells for tissue engineering. J. Tissue Eng. Regen. Med. 2012, 6, 3, s80–s86. [CrossRef]
- Arruda de Faria, C.; Silva Júnior, W.A.; Caetano Andrade Coelho, K.B.; Bassi, M.; Colombari, E.; Zanette, D.L; Ribeiro-Paes, JT. Mesenchymal stromal cells-based therapy in a murine model of elastase-induced emphysema: Simvastatin as a potential adjuvant in cellular homing. Pulm. Pharmacol. Ther. 2021, 70, 102075. [CrossRef]
- Longhini-dos-Santos, N.; Barbosa-de-Oliveira, V.A.; Stessuk, T.; Sakalem, M.E.; Ribeiro-Paes, J.T. Cell therapy decreases inflammation and improves the morphology of the lung parenchyma in a murine model of cigarette smoke-induced emphysema. Int. J. New Technol. Res. 2018, 4, 1, 263154. [CrossRef]
- Passanha, F.R.; Geuens, T.; Konig, S.; van Blitterswijk, C.A.; LaPointe, V.L.S. Cell culture dimensionality influences mesenchymal stem cell fate through cadherin-2 and cadherin-11. Biomaterials 2020, 254, 120127. [CrossRef]
- Cui, T.; Liu, W.; Yu, C.; Ren, J.; Li, Y.; Shi, X.; Li, Q.; Zhang, J. Protective effects of allicin on acute myocardial infarction in rats via hydrogen sulfide-mediated regulation of coronary arterial vasomotor function and myocardial calcium transport. Front. Pharmacol. 2022, 752244. [CrossRef]
- Gillet, J.P.; Varma, S.; Gottesman, M.M. The clinical relevance of cancer cell lines. J. Natl. Cancer Inst. 2013, 105, 7, 452–458. [CrossRef]
- Imamura, Y.; Mukohara, T.; Shimono, Y.; Funakoshi, Y.; Chayahara, N.; Toyoda, M.; Kiyota, N.; Takao, S.; Kono, S.; Nakatsura, T.; Minami, H. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol. Rep. 2015, 33,4, 1837–1843.
- Mukherjee, S.G.; O'Claonadh, N.; Casey, A.; Chambers, G. Comparative in vitro cytotoxicity study of silver nanoparticle on two mammalian cell lines. Toxicol. In Vitro 2012, 26, 2, 238–251. [CrossRef]
- Zhou, H.M.; Shen, Y.; Wang, Z.J.; Li, L.; Zheng, Y.F.; Häkkinen, L.; Haapasalo, M. In vitro cytotoxicity evaluation of a novel root repair material. J. Endod. 2013, 39, 4, 478–483. [CrossRef]
- Tannenbaum, J.; Bennett, B.T. Russell and Burch’s 3Rs then and now: The need for clarity in definition and purpose. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 2, 120–132.
- Gusson-Zanetoni, J.P.; da Silva, J.S.G.M.; Picão, T.B.; Cardin, L.T.; Prates, J.; Sousa, S.O. Effect of Piper cubeba total extract and isolated lignans on head and neck cancer cell lines and normal fibroblasts. J. Pharmacol. Sci. 2022, 148, 1, 93–102. [CrossRef]
- Kamaruddin, M.S.H.; Chong, G.H.; Mohd Daud, N.; Putra, N.R.; Md Salleh, L.; Suleiman, N. Bioactivities and green advanced extraction technologies of ginger oleoresin extracts: A review. Food Res. Int. 2023, 164, 112283. [CrossRef]
- Popovici, V.; Matei, E.; Cozaru, G.C.; Bucur, L.; Gîrd, C.E.; Schröder, V.; Ozon, E.A.; Musuc, A.M.; Mitu, M.A.; Atkinson, I.. In Vitro Anticancer Activity of Mucoadhesive Oral Films Loaded with Usnea barbata (L.) F. H. Wigg Dry Acetone Extract, with Potential Applications in Oral Squamous Cell Carcinoma Complementary Therapy. Antioxidants 2022, 11, 1934. [CrossRef]
- Miki, Y.; Ono, K.; Hata, S.; Suzuki, T.; Kumamoto, H.; Sasano, H. The advantages of co-culture over mono cell culture in simulating in vivo environment. J. Steroid Biochem. Mol. Biol. 2012, 131, 3, 68–75. [CrossRef]
- Liu, R.; Meng, X.; Yu, X.; Wang, G.; Dong, Z.; Zhou, Z.; Qi, M.; Yu, X.; Ji, T.; Wang, F.. From 2D to 3D Co-Culture Systems: A Review of Co-Culture Models to Study the Neural Cells Interaction. Int. J. Mol. Sci. 2022, 23, 21, 13116. [CrossRef]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 4, 8, 911. [CrossRef]
- Cheaito, K.; Bahmad, H.F.; Jalloul, H.; Hadadeh, O.; Msheik, H.; El-Hajj, A.; Mukherji, D.; Al-Sayegh, M.; Abou-Kheir, W. Epidermal Growth Factor Is Essential for the Maintenance of Novel Prostate Epithelial Cells Isolated From Patient-Derived Organoids. Front. Cell Dev. Biol. 2020, 29, 8, 571677. [CrossRef]
- Wang, Z.; Wang, L.; Su, X.; Pu, J.; Jiang, M.; He, B. Rational transplant timing and dose of mesenchymal stromal cells in patients with acute myocardial infarction: a meta-analysis of randomized controlled trials. Stem Cell Res. Ther. 2017, 1, 21. [CrossRef]
- Geurts, M.H.; van der Vaart, J.; Beumer, J.; Clevers, H. The Organoid Platform: Promises and Challenges as Tools in the Fight against COVID-19. Stem Cell Rep. 2021, 16, 3, 412–8. [CrossRef]
- Alhaque, S.; Themis, M.; Rashidi, H. Three-dimensional cell culture: from evolution to revolution. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 1750, 20170216. [CrossRef]
- Bose, S.; Clevers, H.; Shen, X. Promises and Challenges of Organoid-Guided Precision Medicine. Med 2021, 2, 9, 1011–26. [CrossRef]
- Fetah, K.; Tebon, P.; Goudie, M.J.; Eichenbaum, J.; Ren, L.; Barros, N.; Rohollah, N.; Samad, A.; Nureddin, A.; Mehmet, R. D.; Ali,K. The emergence of 3D bioprinting in organ-on-chip systems. Prog. Biomed. Eng. 2019, 1, 1, 012001.
- van der Vaart, J.; Clevers, H. Airway organoids as models of human disease. J. Intern. Med. 2021, 289, 5, 604–13. [CrossRef]
- Sun, X.Y.; Ju, X.C.; Li, Y.; Zeng, P.M.; Wu, J.; Zhou, Y.Y.; Shen, L.B.; Dong, J.; Chen, Y.J.; Luo, Z.G. Generation of vascularized brain organoids to study neurovascular interactions. eLife, 2022, 4, 11, e76707. [CrossRef]
- Peng, L.; Gao, L.; Wu, X.; Fan, Y.; Liu, M.; Chen, J.; Song, J.; Kong, J.; Dong, Y.; Li, B.; Liu, A.; Bao, F. Lung Organoids as Model to Study SARS-CoV-2 Infection. Cells 2022, 11, 17, 2758. [CrossRef]
- Marrero, D.; Pujol-Vila, F.; Vera, D.; Gabriel, G.; Illa, X.; Elizalde-Torrent, A.; Alvarez, M.; Villa, R. Gut-on-a-chip: Mimicking and monitoring the human intestine. Biosens. Bioelectron. 2021, 181, 113156. [CrossRef]
- Criscione, J.; Rezaei, Z.; Hernandez Cantu, C.M.; Murphy, S.; Shin, S.R.; Kim, D.H. Heart-on-a-chip platforms and biosensor integration for disease modeling and phenotypic drug screening. Biosens. Bioelectron. 2023, 220, 114840. [CrossRef]
- Ko, J.; Park, D.; Lee, S.; Gumuscu, B.; Jeon, N.L. Engineering Organ-on-a-Chip to Accelerate Translational Research. Micromachines 2022, 13, 8, 1200. [CrossRef]
- Telles-Silva, K.A.; Pacheco, L.; Komatsu, S.; Chianca, F.; Caires-Júnior, L.C.; Araujo, B.H.S., Goulart, E.; Zatz, M.Applied Hepatic Bioengineering: Modeling the Human Liver Using Organoid and Liver-on-a-Chip Technologies. Front. Bioeng. Biotechnol. 2022, 10, 845360. [CrossRef]
- Gaitán-Salvatella, I.; López-Villegas, E.O.; González-Alva, P.; Susate-Olmos, F.; Álvarez-Pérez, M.A. Case Report: Formation of 3D Osteoblast Spheroid Under Magnetic Levitation for Bone Tissue Engineering. Front. Mol. Biosci. 2021, 8, 672518. [CrossRef]
- Baarsma, H.A.; Van der Veen, C.H.T.J.; Lobee, D.; Mones, N.; Oosterhout, E.; Cattani-Cavalieri, I. Epithelial 3D-spheroids as a tool to study air pollutant-induced lung pathology. SLAS Discov. 2022, 27, 3, 185–90. [CrossRef]
- Ryu, N.E.; Lee, S.H.; Park, H. Spheroid Culture System Methods and Applications for Mesenchymal Stem Cells. Cells 2019, 8, 12, 1620. [CrossRef]
- Rauth, S.; Karmakar, S.; Batra, S.K.; Ponnusamy, M.P. Recent advances in organoid development and applications in disease modeling. Biochim. Biophys. Acta BBA - Rev. Cancer, 2021, 1875, 2, 188527. [CrossRef]
- Long, C.; Wang, J.; Gan, W.; Qin, X.; Yang, R.; Chen, X. Therapeutic potential of exosomes from adipose-derived stem cells in chronic wound healing. Front. Surg. 2022, 9, 1030288. [CrossRef]
- He, C.; Lu, D.; Lin, Z.; Chen, H.; Li, H.; Yang, X.; Yang, M.; Wang, K.; Wei, X.; Zheng, S.; Xu, X. . Liver Organoids, Novel and Promising Modalities for Exploring and Repairing Liver Injury. Stem Cell Rev. Rep. 2023, 19, 2, 345–57. [CrossRef]
- Chen, J.; Na, F. Organoid technology and applications in lung diseases: Models, mechanism research and therapy opportunities. Front. Bioeng. Biotechnol. 2022, 10, 1066869. [CrossRef]
- Grimm, D.; Schulz, H.; Krüger, M.; Cortés-Sánchez, J.L.; Egli, M.; Kraus, A. The Fight against Cancer by Microgravity: The Multicellular Spheroid as a Metastasis Model. Int. J. Mol. Sci. 2022, 23, 6, 3073. [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 5, 402–20. [CrossRef]
- Alzamil, L.; Nikolakopoulou, K.; Turco, M.Y. Organoid systems to study the human female reproductive tract and pregnancy. Cell Death Differ. 2021, 28, 1, 35–51. [CrossRef]
- Brassard, J.A.; Nikolaev, M.; Hübscher, T.; Hofer, M.; Lutolf, M.P. Recapitulating macro-scale tissue self-organization through organoid bioprinting. Nat Mater. 2021, 20,1, 22–9. [CrossRef]
- Miller, A.J.; Dye, B.R.; Ferrer-Torres, D.; Hill, D.R.;Overeem, A.W., Shea, L.D.; Spence, J.R. Generation of lung organoids from human pluripotent stem cells in vitro. Nat Protoc. 2019, 14, 2, 518–40. [CrossRef]
- Sutherland, R.M.; Inch, W.R.; McCredie, J.A.; Kruuv, J. A Multi-Component Radiation Survival Curve Using an in vitro Tumour Model. Int J Radiat Biol Relat Stud Phys Chem Med. 1970, 18, 5, 491–95. [CrossRef]
- Sutherland, R.M.; McCredie, J.A.; Inch, W.R. Growth of Multicell Spheroids in Tissue Culture as a Model of Nodular Carcinomas. J Natl Cancer Inst. 1971, 46, 1, 113–20. [CrossRef]
- Hamburger, A.W.; Salmon, S.E. Primary Bioassay of Human Tumor Stem Cells. Science. 1977, 197, 4302, 461–63. [CrossRef]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A Murine Tumor Producing a Matrix of Basement Membrane. J Exp Med. 1977, 145, 1, 204–20. [CrossRef]
- Garreta, E.; Kamm, R.D.; Chuva de Sousa Lopes, S.M.; Lancaster, M.A.; Weiss, R.; Trepat, X.; Hyun, I.; Montserrat, N. Rethinking organoid technology through bioengineering. Nat Mater. 2021, 20, 2, 145–55. [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell. 2016, 165, 7, 1586–97. [CrossRef]
- Jeong, Y.; Tin, A.; Irudayaraj, J. Flipped Well-Plate Hanging-Drop Technique for Growing Three-Dimensional Tumors. Front Bioeng Biotechnol. 2022, 4,10, 898699. [CrossRef]
- Kotze, L.A.; Beltran, C.G.G.; Lang, D.; Loxton, A.G.; Cooper, S.; Meiring, M.; Koegelenberg, C.F.N.; Allwood, B.W.; Malherbe, S.T.; Hiemstra, A.M.; Glanzmann, B.; Kinnear, C.; Walzl, G.; du Plessis, N. Establishment of a Patient-Derived, Magnetic Levitation-Based, Three-Dimensional Spheroid Granuloma Model for Human Tuberculosis. mSphere. 2021, 6, 4. [CrossRef]
- Rodoplu, D.; Matahum, J.S.; Hsu, C.H. A microfluidic hanging drop-based spheroid co-culture platform for probing tumor angiogenesis. Lab Chip. 2022, 22, 7, 1275–85. [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R. M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.M.; Bankson, J.A.; Gelovani, J.G.; Killian, T.C.; Arap, W.; Pasqualini, R. Three-dimensional tissue culture based on magnetic cell levitation. Nat Nanotechnol. 2010, 5,4, 291–6. [CrossRef]
- Goulart, E.; Caires-Junior, L.C.; de Telles-Silva, K.A.; Araujo, B.H.S.; Rocco, S.A.; Sforca, M.; Kobayashi, G.S.; Musso, C.M.; Assoni, A.F.; Oliveira, D.; Caldini, E.; Raia, S.; Lelkes, P.I.; Zatz, M. 3D bioprinting of liver spheroids derived from human induced pluripotent stem cells sustain liver function and viability in vitro. Biofabrication. 2019, 12, 1, 015010. [CrossRef]
- Bruns J.; Zustiak, S.P. Hydrogel-Based Spheroid Models of Glioblastoma for Drug Screening Applications. Mo Med. 2021, 118, 4, 346–51.
- Guillaume, O.; Kopinski-Grünwald, O.; Weisgrab, G.; Baumgartner, T.; Arslan, A.; Whitmore K.; Van Vlierberghe, S.; Ovsianikov, A. Hybrid spheroid microscaffolds as modular tissue units to build macro-tissue assemblies for tissue engineering. Acta Biomater, 2023, 15, 165, 72-85. [CrossRef]
- Huang, Z.; Yu, P.; Tang, J. Characterization of Triple-Negative Breast Cancer MDA-MB-231 Cell Spheroid Model. OncoTargets Ther. 2020, 13, 5395–405. [CrossRef]
- Decarli, M.C.; Vidigal De Castro, M.; Adami Nogueira, J.; Harue, T.; Nagahara, M.; Buzatto Westin, C.; Leite, R.; de Oliveira, A.; Da Silva, J.V.; Moroni, L.; Mota, C.; Moraes, Â.M. Development of a Device Useful to Reproducibly Produce Large Quantities of Viable and Uniform Stem Cell Spheroids with Controlled Diameters. Biomaterials Advances. 2022, 135, 112685.
- Marsee, A.; Roos, F.J.M.; Verstegen, M.M.A.; Organoid Consortium H.P.B.; Gehart. H; de Koning, E.; Lemaigre, F.; Forbes, S.J.; Peng, W.C.; Huch, M.; Takebe, T.; Vallier, L.; Clevers, H.; van der Laan, L.J.W.; Spee, B. Building consensus on definition and nomenclature of hepatic, pancreatic, and biliary organoids. Cell Stem Cell. 2021, 28, 5, 816–32. [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and potential in organoid research. Nat Rev Genet. 2018, 19,11, 671–87. [CrossRef]
- Zhao, Z.; Chen, X.; Dowbaj, A.M.; Sljukic, A.; Bratlie, K.; Lin, L.; Li, F.S. E.; Gowri, M. B.; Zhaowei, C.; Soragni, A.; Meritxell, H.; Zenga, Y. A.; Wang, Q.; Yu, H. Organoids. Nat Rev Methods Primer. 2022, 2,1, 1–21.
- Sakalem, M.E.; Ribeiro-Paes, J.T. New methodologies for old problems: tridimensional gastrointestinal organoids and guts-on-a-chip. J Coloproctology Rio. 2018, 38, 90–3. [CrossRef]
- Shahabipour, F.; Ashammakhi, N.; Oskuee, R.K.; Bonakdar, S.; Hoffman, T.; Shokrgozar, M.A.; Khademhosseini, A. Key components of engineering vascularized 3-dimensional bioprinted bone constructs. Transl Res., 2020, 216, 57–76. [CrossRef]
- Schutgens, F.; Clevers, H. Human Organoids: Tools for Understanding Biology and Treating Diseases. Annu Rev Pathol Mech Dis. 2020, 15, 1. 211–34. [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv Healthc Mater. 2017, 6, 17, 1700175.
- Kim, W.; Gwon, Y.; Park, S.; Kim, H.; Kim, J. Therapeutic strategies of three-dimensional stem cell spheroids and organoids for tissue repair and regeneration. Bioact Mater. 2023, 19, 50–74. [CrossRef]
- Lee, S.Y.; Koo, I.S.; Hwang, H.J.; Lee, D.W. In Vitro three-dimensional (3D) cell culture tools for spheroid and organoid models. SLAS Discov. 2023, 28, 4, 119–37. [CrossRef]
- Fang, Z.; Li, P.; Du, F.; Shang, L.; Li, L. The role of organoids in cancer research. Exp Hematol Oncol. 2023, 12, 1, 69. [CrossRef]
- LeSavage, B.L.; Suhar, R.A.; Broguiere, N.; Lutolf, M.P.; Heilshorn, S.C. Next-generation cancer organoids. Nat Mater. 2022, 21, 2, 143–59. [CrossRef]
- Wei, J.; Zhang, W.; Zhao, B. Human liver organoid: modeling liver steatosis and beyond. Cell Regen. 2023, 12, 1, 17. [CrossRef]
- Nuciforo, S.; Heim, M.H. Organoids to model liver disease. JHEP Rep. 2021,3, 1, 100198. [CrossRef]
- Bouwmeester, M.C.; Bernal, P.N.; Oosterhoff, L.A.; van Wolferen, M.E.; Lehmann, V.; Vermaas, M.; Buchholz, M.B.; Peiffer, Q.C.; Malda, J.; van der Laan, L.J.W.; Kramer, N.I.; Schneeberger, K.; Levato, R.; Spee, B. Bioprinting of Human Liver-Derived Epithelial Organoids for Toxicity Studies. Macromol Biosci. 2021, 21, 12, 2100327. [CrossRef]
- Takebe, T.; Sekine, K.; Enomura, M.; Koike, H.; Kimura, M.; Ogaeri, T.; Zhang, R.R.; Ueno, Y.; Zheng, Y.W.; Koike, N.; Aoyama, S.; Adachi, Y.; Taniguchi, H. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature. 2013, 499, 7459, 481–4.
- Eichmüller, O.L.; Knoblich, J.A. Human Cerebral Organoids — a New Tool for Clinical Neurology Research. Nat Rev Neurol. 2022, 18, 11, 661–80. [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.A.; Wenzel, D.; Bicknell, L.S.; Hurles M. E.; Homfray, T.; Penninger, M. P.; Jackson, P. A.; Knoblich, A. J. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature. 2013, 501, 7467, 373–79. [CrossRef]
- Lancaster, M.A.; Knoblich, J.A. Generation of cerebral organoids from human pluripotent stem cells. Nat Protoc. 2014, 9, 10, 2329–40. [CrossRef]
- Dumont, S.; Jan, Z.; Heremans, R.; Van Gorp, T.; Vergote, I.; Timmerman D. Organoids of epithelial ovarian cancer as an emerging preclinical in vitro tool: a review. J Ovarian Res. 2019, 12, 1, 105. [CrossRef]
- Liu, H.D.; Xia, B.R.; Jin, M.Z.; Lou, G. Organoid of ovarian cancer: genomic analysis and drug screening. Clin Transl Oncol. 2020, 22, 8, 1240–51. [CrossRef]
- Hai, J.; Zhang, H.; Zhou, J.; Wu, Z.; Chen, T.; Papadopoulos, E.; Dowling, C.M.; Pyon, V.; Pan, Y.; Liu, J.B.; Bronson, R.T.; Silver, H.; Lizotte, P.H.; Deng, J.; Campbell, J.D.; Sholl, L.M.; Ng, C.; Tsao, M.S.; Thakurdin, C.; Bass, A.J.; Wong, K.K. Generation of genetically engineered mouse lung organoid models for squamous cell lung cancers allows for the study of combinatorial immunotherapy. Clin Cancer Res Off J Am Assoc Cancer Res. 2020, 26, 13, 3431–42.
- Reid, J.A.; Mollica, P.A.; Bruno, R.D.; Sachs, P.C. Consistent and reproducible cultures of large-scale 3D mammary epithelial structures using an accessible bioprinting platform. Breast Cancer Res. 2018, 20, 1, 122.
- Sprangers, J.; Zaalberg, I.C.; Maurice, M.M. Organoid-based modeling of intestinal development, regeneration, and repair. Cell Death Differ. 2021, 28, 1, 95–107. [CrossRef]
- Salgado, A.J.; Oliveira, J.M.; Martins, A.; Teixeira, F.G.; Silva, N.A.; Neves, N.M.; Sousa, N.; Reis, R.L. Tissue engineering and regenerative medicine. Int Rev Neurobiol. 2013, 108,1-33. [CrossRef]
- Tang, X.Y.; Wu, S.; Wang, D.; Chu, C.; Hong, Y.; Tao, M.; Hu, H.; Xu, M.; Guo, X.; Liu, Y. Human organoids in basic research and clinical applications. Signal Transduct Target Ther. 2022, 7, 1, 1–17. [CrossRef]
- Gilazieva, Z.; Ponomarev, A.; Rutland, C.; Rizvanov, A.; Solovyeva, V. Promising applications of tumor spheroids and organoids for personalized medicine. Cancers. 2020, 12, 10, 2727. [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat Rev Mater. 2021, 6, 5, 402–20. [CrossRef]
- Voges, H.K.; Foster, S.R.; Reynolds, L.; Parker, B.L; Devilée, L.; Quaife-Ryan, G.A.; Fortuna, P.R.J.; Mathieson, E.; Fitzsimmons, R.; Lor, M.; Batho, C.; Reid, J.; Pocock, M.; Friedman, C.E.; Mizikovsky, D.; Francois, M.; Palpant, N.J.; Needham, E.J.; Peralta, M.; Monte-Nieto, G.D.; Jones, L.K.; Smyth, I.M.; Mehdiabadi, N.R; Bolk, F.; Janbandhu, V.; Yao, E.; Harvey, R.P.; Chong, J.J.H.; Elliott, D.A; Stanley, E.G.; Wiszniak, S.; Schwarz, Q.; James, D.E.; Mills, R.J.; Porrello, E.R.; Hudson, J.E. Vascular cells improve functionality of human cardiac organoids. Cell Rep. 2023, 42, 5, 112322. [CrossRef]
- Salewskij, K.; Penninger, J.M. Blood vessel organoids for development and disease. Circ Res. 2023, 132, 4, 498–510. [CrossRef]
- Shelton, SE. Vascular microphysiological systems. Curr Opin Hematol. 2024, 31, 3, 155–61. [CrossRef]
- Varani, J.; McClintock, S.D.; Aslam, M.N. Organoid culture to study epithelial cell differentiation and barrier formation in the colon: bridging the gap between monolayer cell culture and human subject research. Vitro Cell Dev Biol - Anim. 2021, 57, 2, 174–90. [CrossRef]
- Shpichka, A.; Bikmulina, P.; Peshkova, M.; Kosheleva, N.; Zurina, I.; Zahmatkesh, E.; Khoshdel-Rad, N.; Lipina, M.; Golubeva, E.; Butnaru, D.; Svistunov, A.; Vosough, M.; Timashev, P. Engineering a model to study viral infections: bioprinting, microfluidics, and organoids to defeat coronavirus disease 2019 (COVID-19). Int J Bioprinting. 2020, 6,4, 302. [CrossRef]
- Xing, Y.;Liu, J.; Guo, X.; Liu, H.; Zeng, W.; Wang, Y.; Zhang, C.; Lu, Y.; He, D.; Ma, S.; He, Y.; Xing, X.H. Engineering organoid microfluidic system for biomedical and health engineering: a review. Chin J Chem Eng. 2021, 30, 244–54. [CrossRef]
- Silva da Costa, F.A.; Soares, M.R.; Malagutti-Ferreira, M.J.; Silva, G.R.; Lívero, F.A.D.R.; Ribeiro-Paes, J.T. Three-dimensional cell cultures as a research platform in lung diseases and COVID-19. Tissue Eng Regen Med. 2021, 18, 5, 735–45. [CrossRef]
- Li, Z.; Hui, J.; Yang, P.; Mao, H. Microfluidic organ-on-a-chip system for disease modeling and drug development. Biosensors. 2022, 12, 6, 370. [CrossRef]
- Huh, D.; Matthews, B.D.; Mammoto, A; Montoya-Zavala, M.; Hsin, H.Y.; Ingber, D.E. Reconstituting organ-level lung functions on a chip. Science. 2010, 328, 5986, 1662–8. [CrossRef]
- Huh, D.; Hamilton, G.A.; Ingber, D.E. From 3D cell culture to organs-on-chips. Trends Cell Biol. 2011, 21, 12, 745–54. [CrossRef]
- Mathur, A.; Loskill, P.; Shao, K.; Huebsch, N.; Hong, S.; Marcus, S.G.; Marks, N.; Mandegar, M.; Conklin, B.R.; Lee, L.P.; Healy, K.E. Human iPSC-based cardiac microphysiological system for drug screening applications. Sci Rep. 2015, 5, 1, 8883. [CrossRef]
- Kilic, O.; Pamies, D.; Lavell, E.; Schiapparelli, P.; Feng, Y.; Hartung, T.; Bal-Price, A.; Hogberg, H.T.; Quinones-Hinojosa, A.; Guerrero-Cazares, H.; Levchenko, A . Brain-on-a-chip model enables analysis of human neuronal differentiation and chemotaxis. Lab Chip. 2016, 16, 21, 4152–62. [CrossRef]
- Weber, E.J.; Lidberg, K.A.; Wang, L.; Bammler, T.K.; MacDonald, J.W.; Li, M.J.; Redhair, M.; Atkins, W.M.; Tran, C.; Hines, K.M.; Herron, J.; Xu, L.; Monteiro, M.B.; Ramm, S.; Vaidya, V.; Vaara, M.; Vaara, T.; Himmelfarb, J.; Kelly, E.J. Human kidney on a chip assessment of polymyxin antibiotic nephrotoxicity. JCI Insight. 2018, 3, 24. [CrossRef]
- Jang, K.J.; Otieno, M.A.; Ronxhi, J.; Lim, H. K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; Nawroth, J.; Simic, D.; Lam, W.; Singer, M.; Barale, E.; Singh, B.; Sonee, M.; Streeter, A.J.; Manthey, C.; Jones, B.; Srivastava, A.; Andersson, L.C.; Williams, D.; Park, H.; Barrile, R.; Sliz, J.; Herland, A.; Haney, S.; Karalis, K.; Ingber, D.E.; Hamilton, G.A. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci Transl Med. 2019, 11, 517. [CrossRef]
- Mun, K.; Arora, K.; Huang, Y.; Yang, F.; Yarlagadda, S.; Ramananda, Y.; Abu-El-Haija, M.; Palermo, J.J.; Appakalai, B.N.; Nathan, J.D.; Naren, A.P. Patient-derived pancreas-on-a-chip to model cystic fibrosis-related disorders. Nat Commun. 2019, 10, 1, 3124. [CrossRef]
| Cell Source | Cell Lineage | Species | Factors | Methods | Reference |
|---|---|---|---|---|---|
| Embryonic/Adult Fibroblasts | MEF and TTF cells | Mice | Oct3/4, Sox2, c-Myc, and Klf4 | Retroviral vector transduction; Plat-E cells line expansion | [55] |
| Adult Fibroblasts | MSC derived from human OCT4 knock-in ES cells | Human | Oct4, Sox2, NANOG, and Lin28 | Lentiviral vector transduction 293FT cell line expansion | [56] |
| Keratinocytes | Keratinocytes foreskin derived | Human | Oct4, Sox2, c-Myc, and Klf4 | Retroviral MSCVpuro vector transduction; Phoenix Amphotropic cell line expansion | [57] |
| Peripheral Blood Cells | CD34+ mobilized human peripheral blood cells | Human | Oct4, Sox2, Klf4 and c-Myc | Retroviral vector transduction; 293T cells line expansion | [58] |
| Cord Blood Cells |
Human cord blood (CB)-derived endothelial cells (ECs) |
Human | Oct4, Sox2, NANOG, and Lin28 | Lentiviral vector Addgene transduction | [59] |
| Amniotic Membrane MSC | MSC placenta derived | Human | Sox2, Klf4, Oct4, and c-Myc | pMX-based retroviruses transduction | [60] |
| Dental MSC | MSC Human third molars derived | Human | Oct3/4, Sox2, c-Myc, and Klf4 | EcoRI site of the pMXs-based retroviruses transduction | [61] |
| Urine Cells | RPTE cells | Human | Oct4, Sox2, Klf4, and c-Myc | Adgene retroviral vector transduction, HEK293T cells expansion | [62,63] |
| Animal Model | 2D Cell Culture | 3D Cell Culture | Organ-on-a-chip | |
|---|---|---|---|---|
| Ease of Maintenance | + | +++ | +++ | ++ |
| Recapitulation of Biology Development | +++ | N/A | ++ | +++ |
| Length of Experiments | ++ | +++ | +++ | +++ |
| Genetic Engineering | + | +++ | +++ | +++ |
| Physiological Complexity | +++ | N/A | + | ++ |
| Relative Cost | + | +++ | ++ | ++ |
| Recapitulation of Human Physiology | ++ | + | +++ | +++ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





