Submitted:
01 September 2024
Posted:
03 September 2024
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
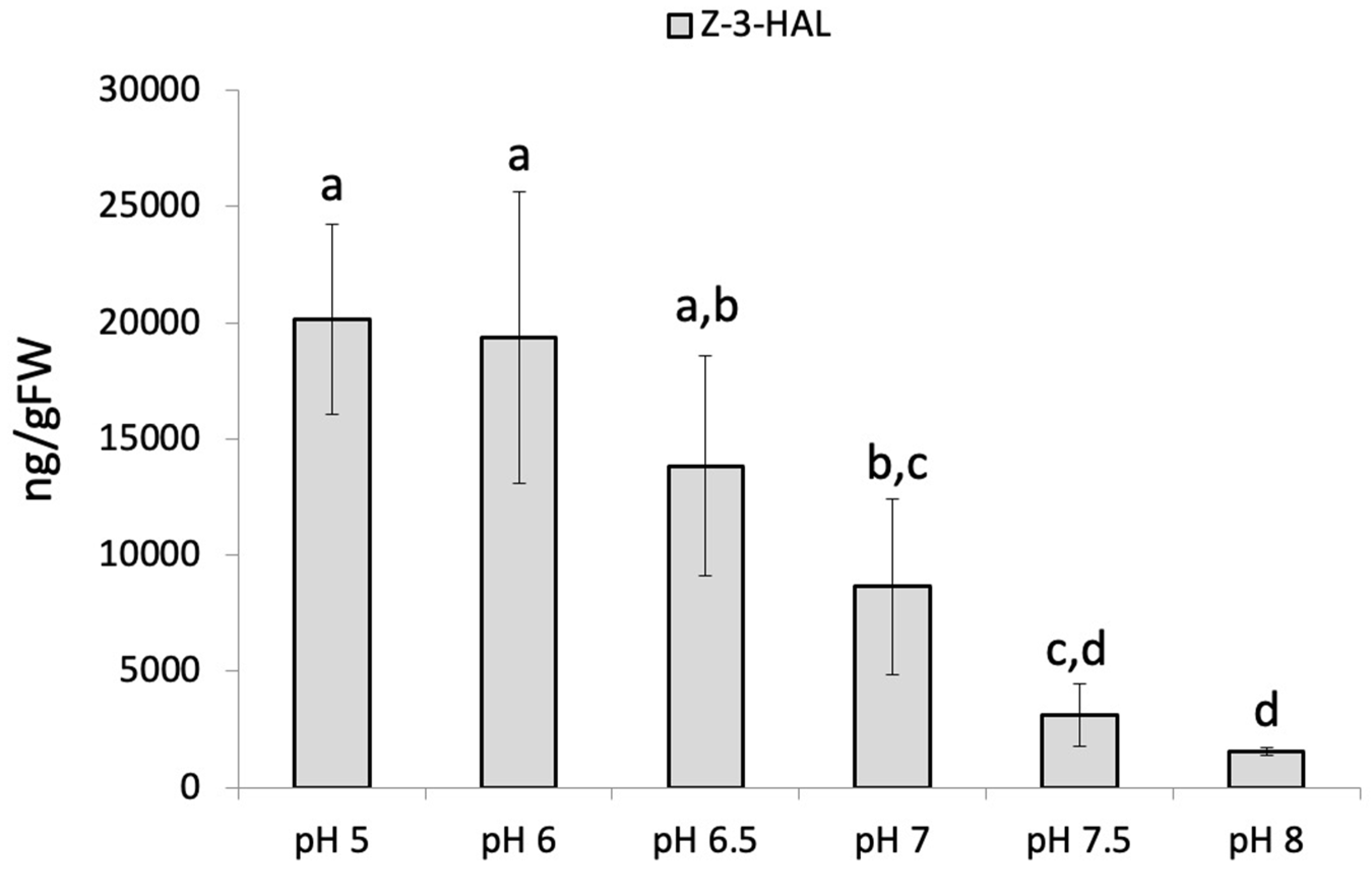
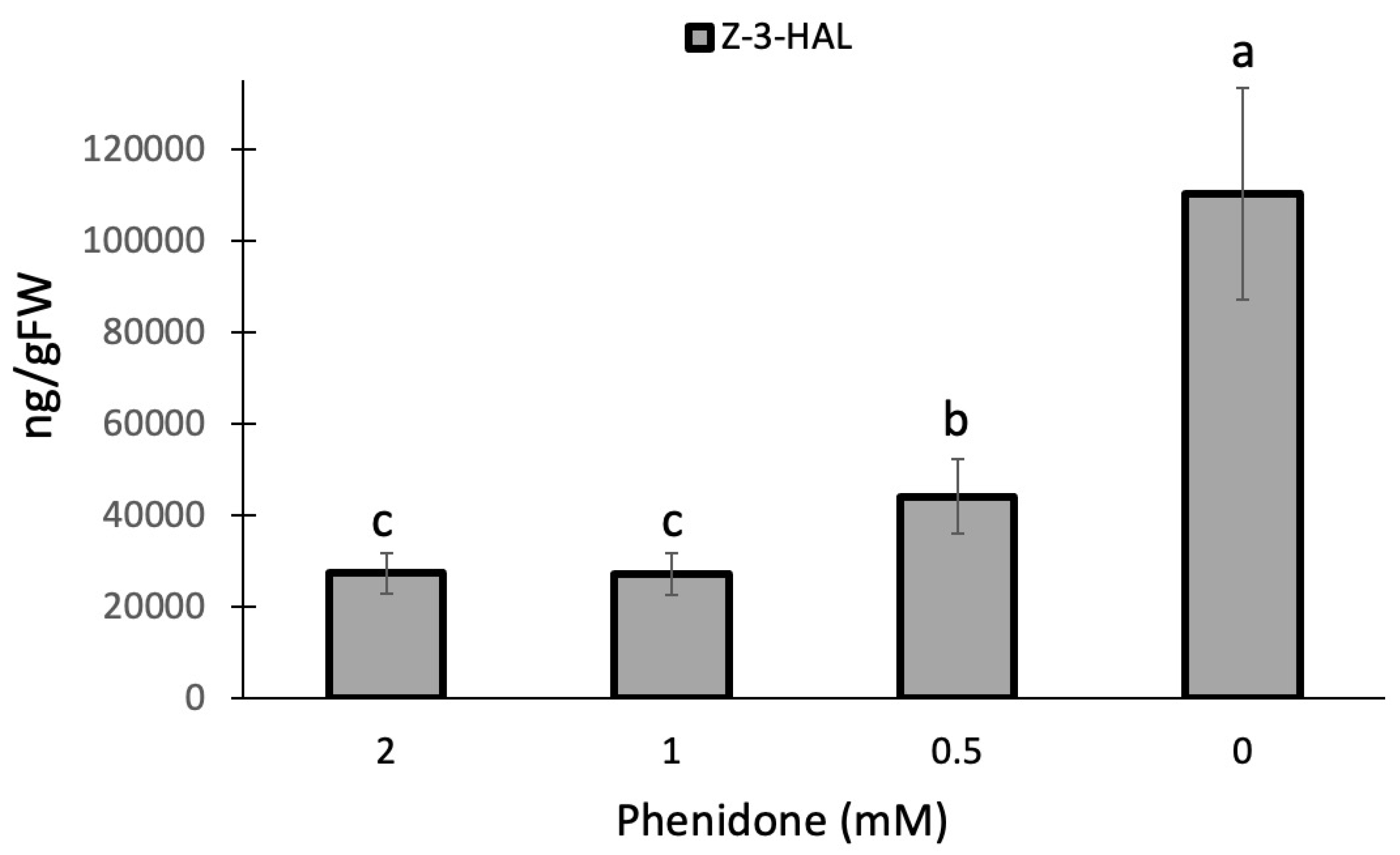
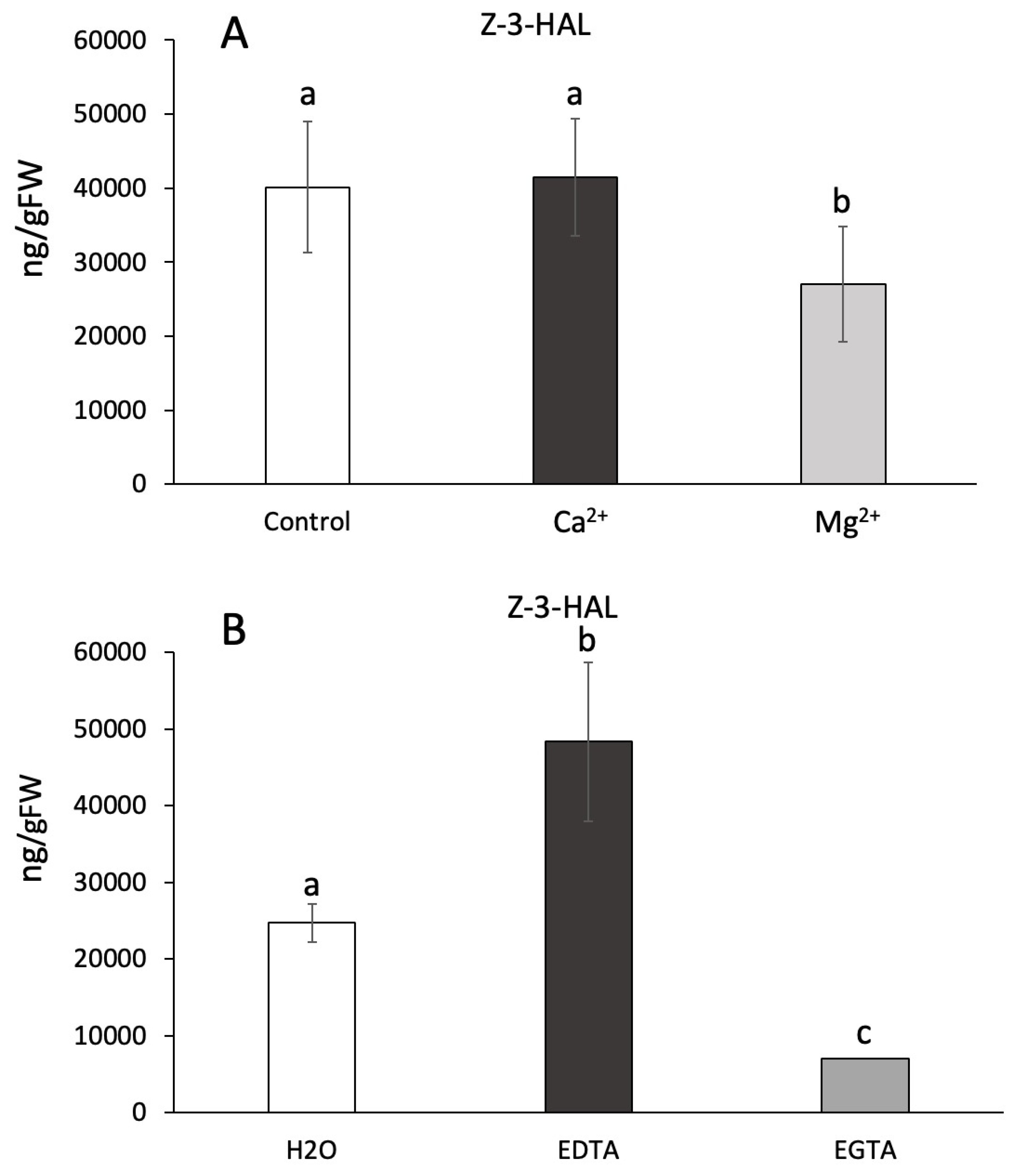
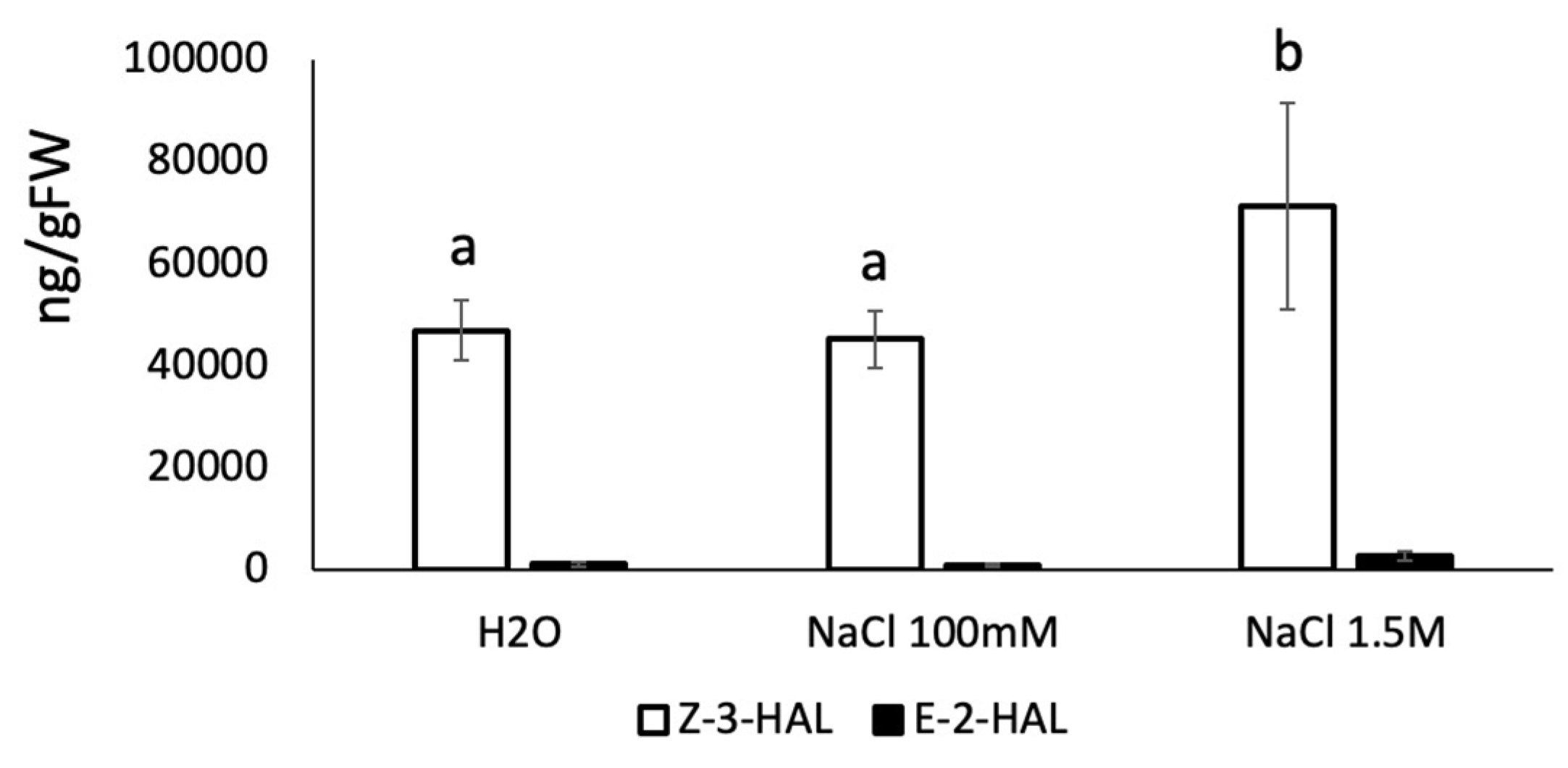
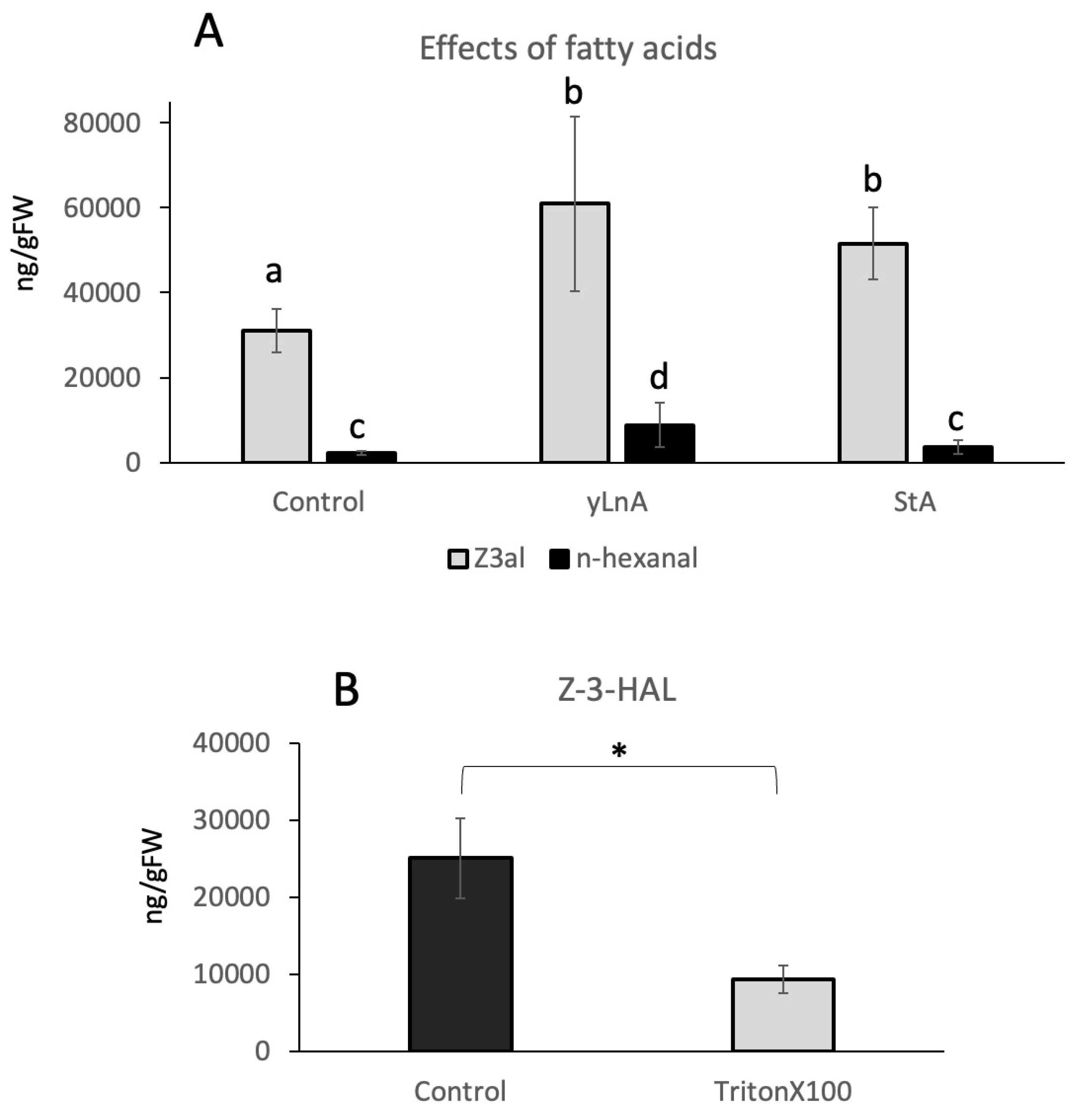
2. Results and Discussion
3. Summary
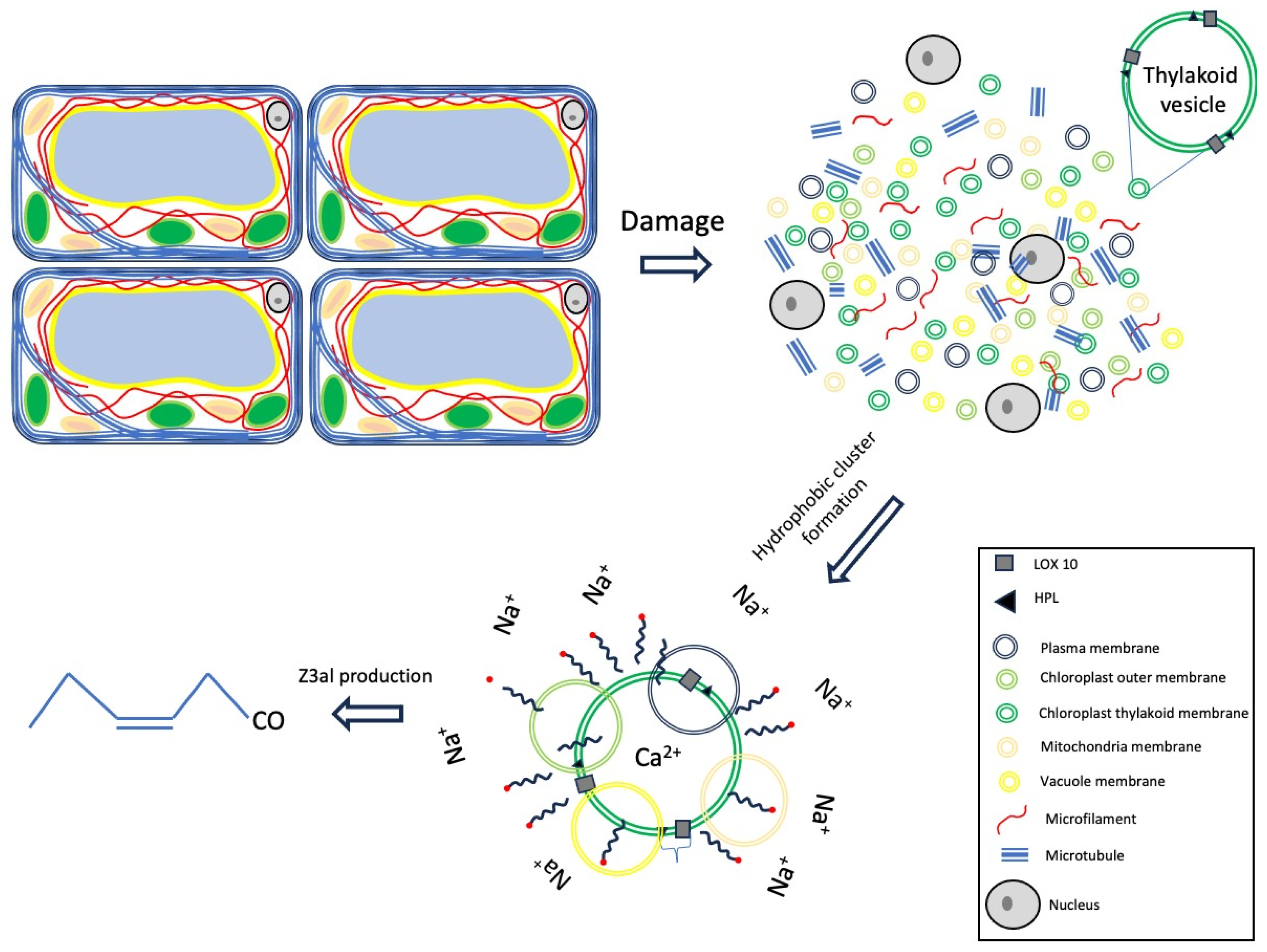
4. Materials and Methods
4.1. Experimental Setups
Author Contributions
Funding
Conflicts of Interest
References
- Curtius, T.; Franzen, H. Über die chemischen Bestandteile grüner Pflanzen. Über den Blätteraldehyd. Liebigs Ann. Chem. 1912, 390, 89–121. [Google Scholar] [CrossRef]
- Matsui, K.; Engelberth, J. Green leaf volatiles-the forefront of plant responses against biotic attack. Plant Cell Physiol 2022, 63, 1378–1390. [Google Scholar] [CrossRef]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781. [Google Scholar] [CrossRef]
- Engelberth, J. Green Leaf Volatiles: A New Player in the Protection against Abiotic Stresses? Int. J. Mol. Sci. 2024, 25, 9471. [Google Scholar] [CrossRef]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochemistry 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Nakashima, A.; von Reuss, S.H.; Tasaka, H.; Nomura, M.; Mochizuki, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Boland, W.; Takabayashi, J.; Matsui, K. Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis. J. Biol. Chem. 2013, 288, 26078–26088. [Google Scholar] [CrossRef]
- Zimmerman, D.C.; Coudron, C.A. Identification of traumatin, a wound hormone, as 12-oxo-trans-10-dodecenoic acid. Plant Physiol 1979, 63, 536–541. [Google Scholar] [CrossRef]
- Engelberth, M.; Engelberth, J. Variability in the capacity to produce damage-induced aldehyde green leaf volatiles among different plant species provides novel insights into biosynthetic diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerase essential to the production of the leaf aldehyde in plants. J Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and Characterization of (3Z):(2E)-Hexenal Isomerases from Cucumber. Front Plant Sci 2017, 8, 1342. [Google Scholar] [CrossRef]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolism of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 2012, 7, e36433. [Google Scholar] [CrossRef]
- Jardine, K.J.; Chambers, J.Q.; Holm, J.; Jardine, A.B.; Fontes, C.G.; Zorzanelli, R.F.; Meyers, K.T.; Fernandez de Souza, V.; Garcia, S.; Giminez, B.O.; et al. Green leaf volatile emissions during high temperature and drought stress in a Central Amazon rainforest. Plants 2015, 4, 678–690. [Google Scholar] [CrossRef]
- Jardine, K.; Barron-Gafford, G.A.; Norman, J.P.; Abrell, L.; Monson, R.K.; Meyers, K.T.; Pavao-Zuckerman, M.; Dontsova, K.; Kleist, E.; Werner, C.; Huxman, T.E. Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light–dark transitions. Photosynth Res 2012, 113, 321–333. [Google Scholar] [CrossRef]
- Chamberlain, K.; Khan, Z.R.; Pickett, J.A.; Toshova, T.; Wadhams, L.J. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 2006, 32, 565–577. [Google Scholar] [CrossRef]
- Röse, U.S.R.; Tumlinson, J.H. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta 2005, 222, 327–335. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci 2012, 196, 93–100. [Google Scholar] [CrossRef]
- Aratani, Y.; Uemura, T.; Hagihara, T.; Matsui, K.; Toyota, M. Green leaf volatile sensory calcium transduction in Arabidopsis. Nat Commun 2023, 14, 6236. [Google Scholar] [CrossRef]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early transcriptome analyses of Z-3-hexenol-treated Zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. PLoS ONE 2013, 8, e77465. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimotot, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci Rep 2015, 5, 8030. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Matsuda, A.; Matsuura, N.; Mizutani, M.; Sugimoto, Y. Transcriptome analysis of Arabidopsis thaliana with green leaf volatiles: Possible role of green leaf volatiles as self-made damage-associated patterns. J. Pestic. Sci. 2018, 43, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tanarsuwongkul, S.; Fisher, K.W.; Mullis, B.T.; Negi, H.; Roberts, J.; Tomlin, F.; Wang, Q.; Stratmann, J.W. Green leaf volatiles co-opt proteins involved in molecular pattern signalling in plant cells. Plant Cell Environ 2024, 47, 928–946. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, S.; Ono, E.; Horiwaka, M.; Murata, J.; Totsuka, K.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Matsui, K.; Mizutani, M.; Watanabe, N.; Ohnishi, T. Volatile glycosylation in tea plants: Sequential glycosylations for the biosynthesis of aroma b-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol. 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Cofer, T.M.; Erb, M.; Tumlinson, J.H. The Arabidopsis thaliana carboxylesterase AtCXE12 converts volatile (Z)-3-hexenyl acetate to (Z)-3-hexenol. bioRxiv 2023. [Google Scholar]
- Mano, J.; Kanameda, S.; Kuramitsu, R.; Matsuura, N.; Yamauchi, Y. Detoxification of Reactive Carbonyl Species by Glutathione Transferase Tau Isozymes. Front Plant Sci 2019, 10, 487. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J.; Engelberth, M. Developmental stages affect the capacity to produce aldehyde green leaf volatiles in Zea mays and Vigna radiata. Plants 2022, 11, 526. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.M.; Vaughn, K.A.; Herrfurth, C.; Tumlinson, J.; Feussner, I.; Jackso, D.; Turlings, T.C.J.; Engelberth, J.; Nansen, C.; Meeley, R.; Kolomiets, M.V. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J. 2013, 74, 59–73. [Google Scholar] [CrossRef]
- 28. Yactayo-Chang, J.P.; Hunter, C.T.; Alborn, H.T.; Christensen, S.A.; Block, A.K. Production of the Green Leaf Volatile (Z)-3-Hexenal by a Zea maysHydroperoxide Lyase. Plants 2022, 11, 2201. [Google Scholar] [CrossRef]
- Nemchenko, A.; Kunze, S.; Feussner, I.; Kolomiets, M. Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J Exp Bot 2006, 57, 3767–3779. [Google Scholar] [CrossRef]
- Farmaki, T.; Sanmartin, M.; Jiminez, P.; Paneque, M.; Sanz, C.; Vancanneyt, G.; Leon, J.; Sanchez-Serrano, J.J. Differential distribution of the lipoxygenase pathway enzymes within potato chloroplasts. J. Exp. Bot. 2007, 58, 555–568. [Google Scholar] [CrossRef]
- Blee, E.; Joyard, J. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol. 1996, 110, 445–454. [Google Scholar] [CrossRef]
- Froehlich, J.E.; Itoh, A.; Howe, G.A. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipins metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol. 2001, 125, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.; Springer, A.; Kang, C.; von Wettstein, D.; Reinbothe, C.; Reinbothe, S.; Pollmann, S. Allene oxide synthase and hydroperoxide lyase, two non-canonical cytochrome p450s in Arabidopsis thaliana and their different roles in plant defense. Int. J. Mol. Sci. 2019, 20, 3064. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, K.; Zdyb, A.; Feussner, I.; Pawlowski, K. Analysis of the subcellular localization of lipoxygenase in legume and actinorhizal nodules. Plant. Biol. 2012, 14, 46–63. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, S.; Jin, Y.; Chen, Q.; Xing, Q.; Qi, H. Melon 13-lipoxygenase CmLOX18 may be involved in C6 volatile biosynthesis in fruit. Sci. Rep. 2017, 7, 2816. [Google Scholar]
- Weichert, H.; Kolbe, A.; Kraus, A.; Wasternack, C.; Feussner, I. Metabolic profiling of oxylipins in germinating cucumber seedlings—Lipoxygenase-dependent degradation of triacylglycerols and biosynthesis of volatile aldehydes. Planta 2002, 215, 612–619. [Google Scholar] [CrossRef]
- Ishiguro, S.; Kawai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 2001, 13, 2191–2209. [Google Scholar] [CrossRef]
- Matsui, K. Properties and structures of fatty acid hydroperoxide lyase. Belg. J. Bot. 1998, 131, 50–62. [Google Scholar]
- Hornostaj, A.R.; Robinson, D.S. Purification of hydroperoxide lyase from cucumber. Food Chem. 1999, 66, 173–180. [Google Scholar] [CrossRef]
- De Domenico, S.; Tsesmetzis, N.; Di Sansebastiano, G.P.; Hughes, R.K.; Casey, R.; Santino, A. Subcellular localization of Medicago truncatula 9/13-hydroperoxide lyase reveal a new localization pattern and activation mechanism for CYP74C enzymes. BMC Plant Biol. 2007, 7, 58. [Google Scholar] [CrossRef]
- Savchenko, T.; Pearse, I.S.; Ignatia, L.; Karban, R.; Dehesh, K. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J. 2012, 73, 653–662. [Google Scholar] [CrossRef]
- Savchenko, T.; Dehesh, K. Insect herbivores selectively mute GLV production in plants. Plant Signal. Behav. 2013, 8, e24136. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Ozawa, R.; Takabayashi, J.; Fujii, S.; Arai, K.; Ichiki, R.T.; Koeduka, T.; Dohra, H.; Ohnishi, T.; Taketazu, S.; et al. Silkworms suppress the release of green leaf volatiles by mulberry leaves with an enzyme from their spinnerets. Sci. Rep. 2018, 8, 11942. [Google Scholar] [CrossRef]
- Jones, A.C.; Seidl-Adams, I.; Engelberth, J.; Hunter, C.T.; Alborn, H.; Tumlinson, J.H. Herbivorous caterpillars can utilize three different mechanisms to alter green leaf volatile emissions. Environ. Entomol. 2019, 48, 419–425. [Google Scholar] [CrossRef]
- Jones, A.; Cofer, T.M.; Engelberth, J.; Tumlinson, J.H. (2022) Herbivorous caterpillars and the green leaf volatile (GLV) quandary. J Chem Ecol 2022, 48, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Selective inhibition of jasmonic acid accumulation by a small α, β-unsaturated carbonyl and phenidone reveals different modes of octadecanoid signalling activation in response to insect elicitors and green leaf volatiles in Zea mays. BMC Res Notes 2011, 4, 377. [Google Scholar] [CrossRef] [PubMed]
- Bruinsma, M.; van Broekhoven, S.; Poelman, E.H.; Posthumus, M.A.; Müller, M.J.; van Loon, J.J.; Dicke, M. Inhibition of lipoxygenase affects induction of both direct and indirect plant defences against herbivorous insects. Oecologia 2010, 162, 393–404. [Google Scholar] [CrossRef]
- Agut, B.; Gamir, J.; Jacas, J.A.; Hurtado, M.; Flors, V. Different metabolic and genetic responses in citrus may explain relative susceptibility to Tetranychus urticae. Pest Manag Sci 2014, 70, 1728–1741. [Google Scholar] [CrossRef]
- Engelberth, J.; Koch, T.; Schüler, G.; Bachmann, N.; Rechtenbach, J.; Boland, W. Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol 2001, 125, 369–377. [Google Scholar] [CrossRef]
- Pare, P.W.; Tumlinson, J.H. De Novo Biosynthesis of Volatiles Induced by Insect Herbivory in Cotton Plants. Plant Physiol 1997, 114, 1161–1167. [Google Scholar] [CrossRef]
- Ogunola, O.F.; Hawkins, L.K.; Mylroie, E.; Kolomiets, M.V.; Borrego, E.; Tang, J.D.; Williams, W.P.; Warburton, M.L. Characterization of the maize lipoxygenase gene family in relation to aflatoxin accumulation resistance. PLoS ONE 2017, 12, e0181265. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Matsui, K. Green leaf volatile-burst in Arabidopsis is governed by galactolipid oxygenation by a lipoxygenase that is under control of calcium ion. Biochem. Biophys. Res. Comm. 2018, 505, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci 2014, 24, 298–309. [Google Scholar] [CrossRef]
- Jethva, D.; Shruthi, P.; Patel, S.; Karbhari, N.; Savaliya, N. Comparison of Magnesium by EDTA and EGTA as chelating agent. Int J Clinical Biochem 2022, 7, 3. [Google Scholar]
- Holleman, A.F.; Wiberg, E. (2001). Inorganic Chemistry. San Diego: Academic Press.
- Hu, X.; Dong, Q.; Yang, J.; Zhang, Y. Recognizing metal and acid radical ion binding sites by integrating ab initio modeling with template-based transferals. Bioinformatics 2016, 32, 3260–3269. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sato, K.; Kinbara, K. Calcium-induced reversible assembly of phosphorylated amphiphile within lipid bilayer membranes. Chem Commun 2021, 57, 4106–4109. [Google Scholar] [CrossRef]
- Bhattacharya, O.; Ortiz, I.; Walling, L.L. Methodology: An optimized, high yield tomato leaf chloroplast isolation and stroma extraction protocol for proteomics analyses and identification of chloroplast co-localizing proteins. Plant Methods 2020, 16, 131. [Google Scholar] [CrossRef]
- Bouchnak, I.; Moyet, L.; Salvi, D.; Kuntz, M.; Rolland, N. Preparation of Chloroplast Sub- compartments from Arabidopsis for the Analysis of Protein Localization by Immunoblotting or Proteomics. JoVE 2018. [Google Scholar] [CrossRef]
- Engelberth, M.; Engelberth, J. Variability in the capacity to produce damage-induced aldehyde green leaf volatiles among different plant species provides novel insights into biosynthetic diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Developmental stages affect the capacity to produce aldehyde green leaf volatiles in Zea mays and Vigna radiata. Plants 2022, 11, 526. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




