Submitted:
01 October 2024
Posted:
02 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results and Discussion
Identification of LOX10 and HPL in Thylakoid Membranes of Maize Chloroplasts
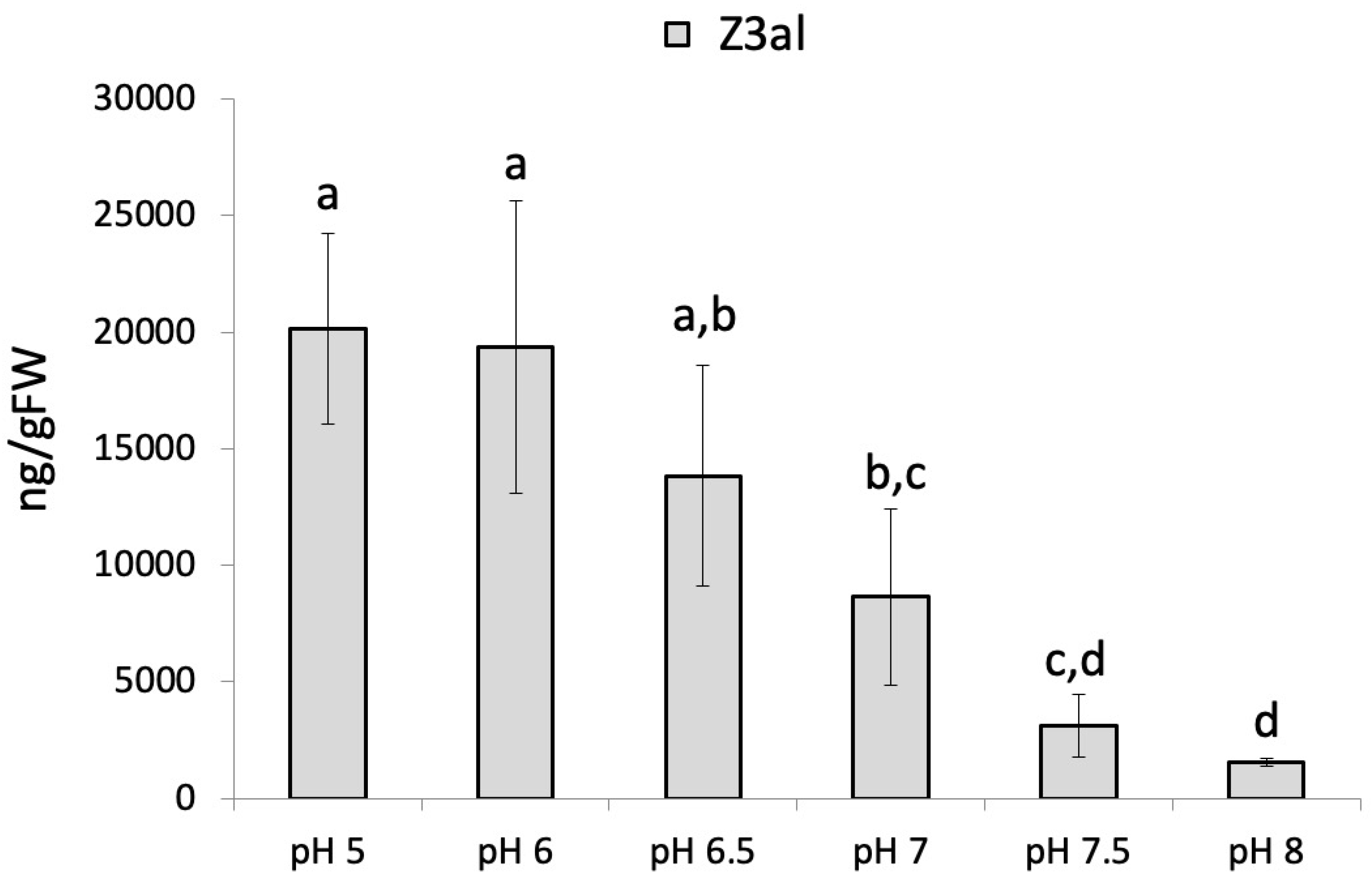
Z3al Production Strongly Depends on the pH of the Environment
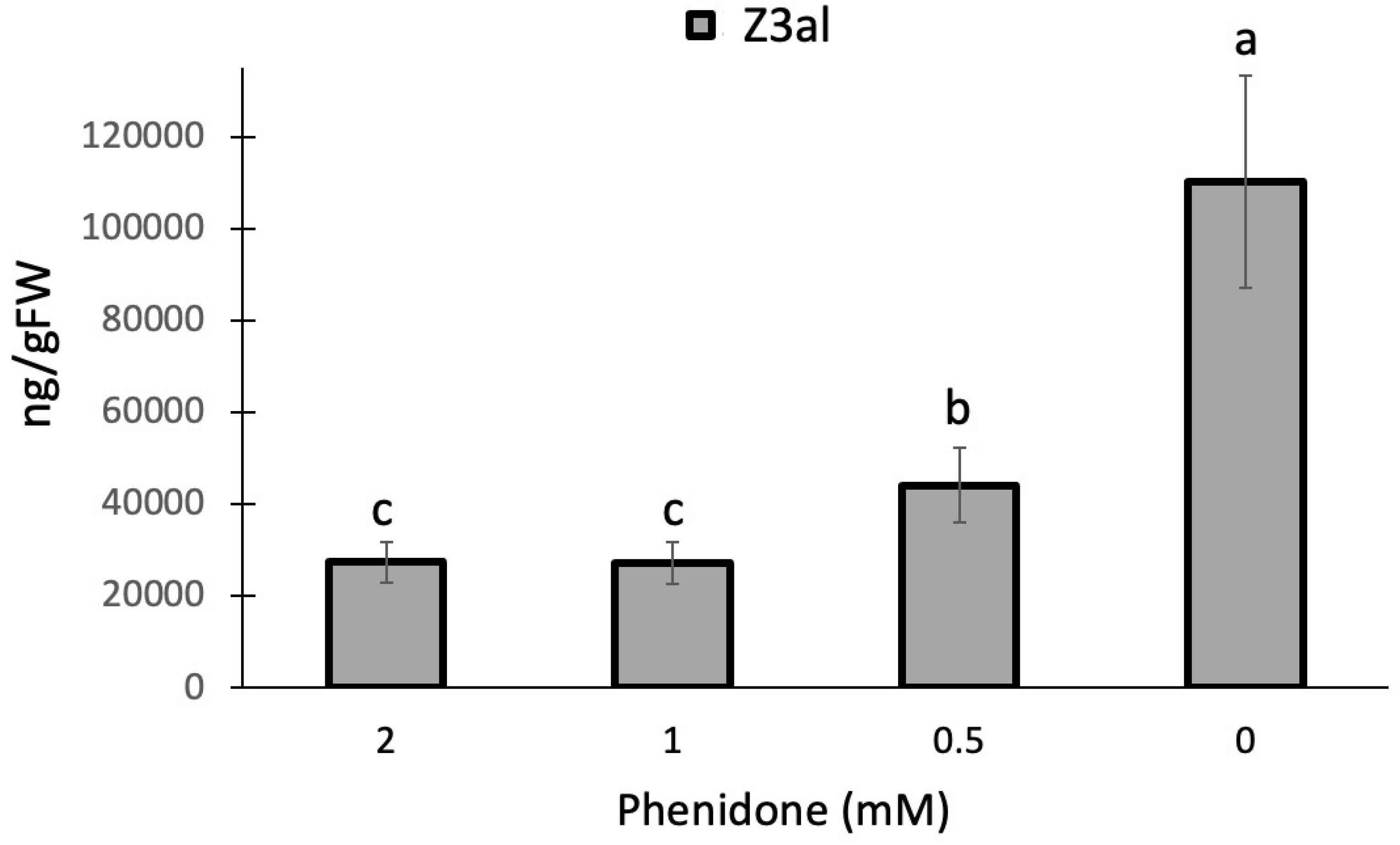
Phenidone is an Effective Inhibitor of Z3al Production
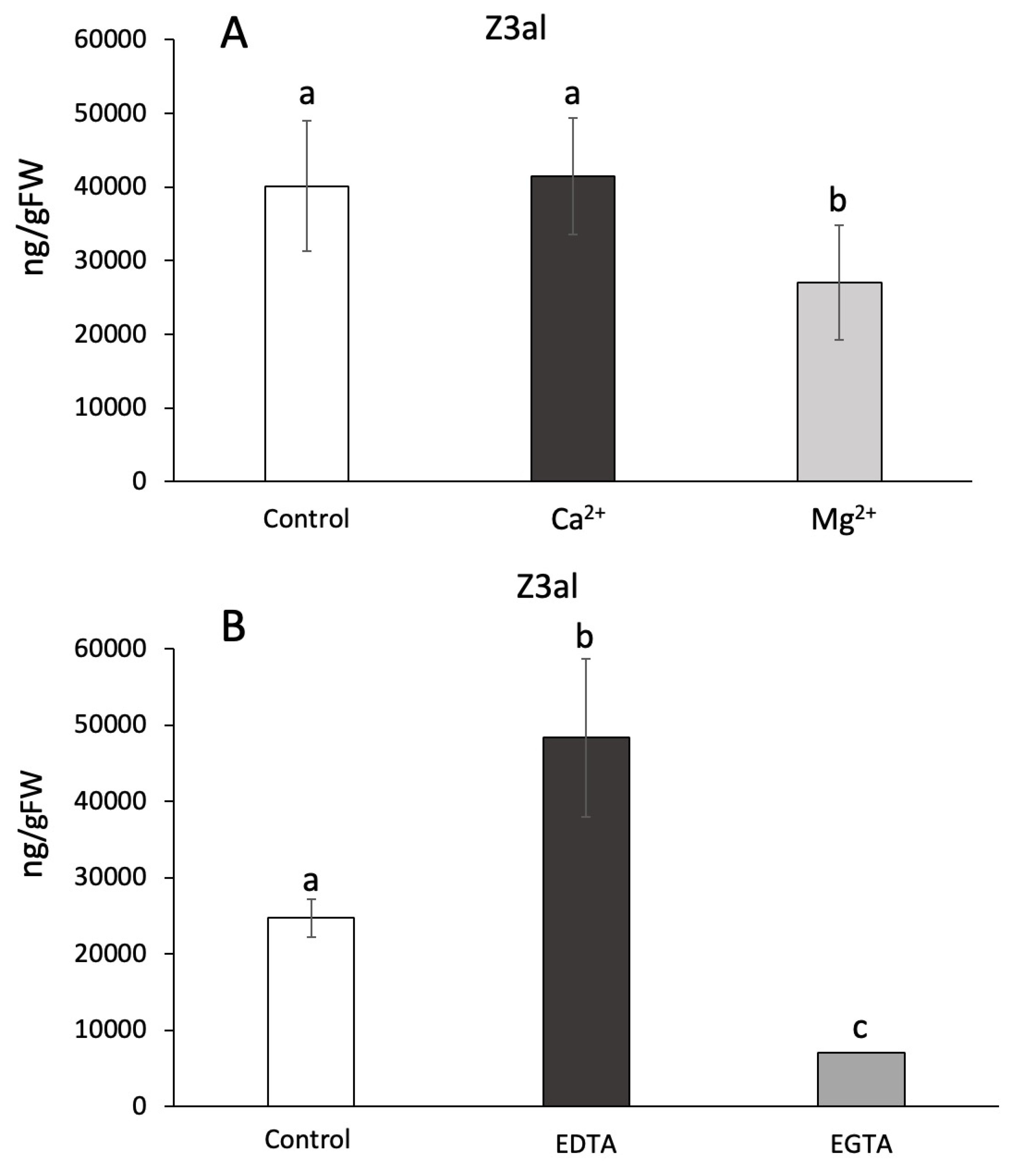
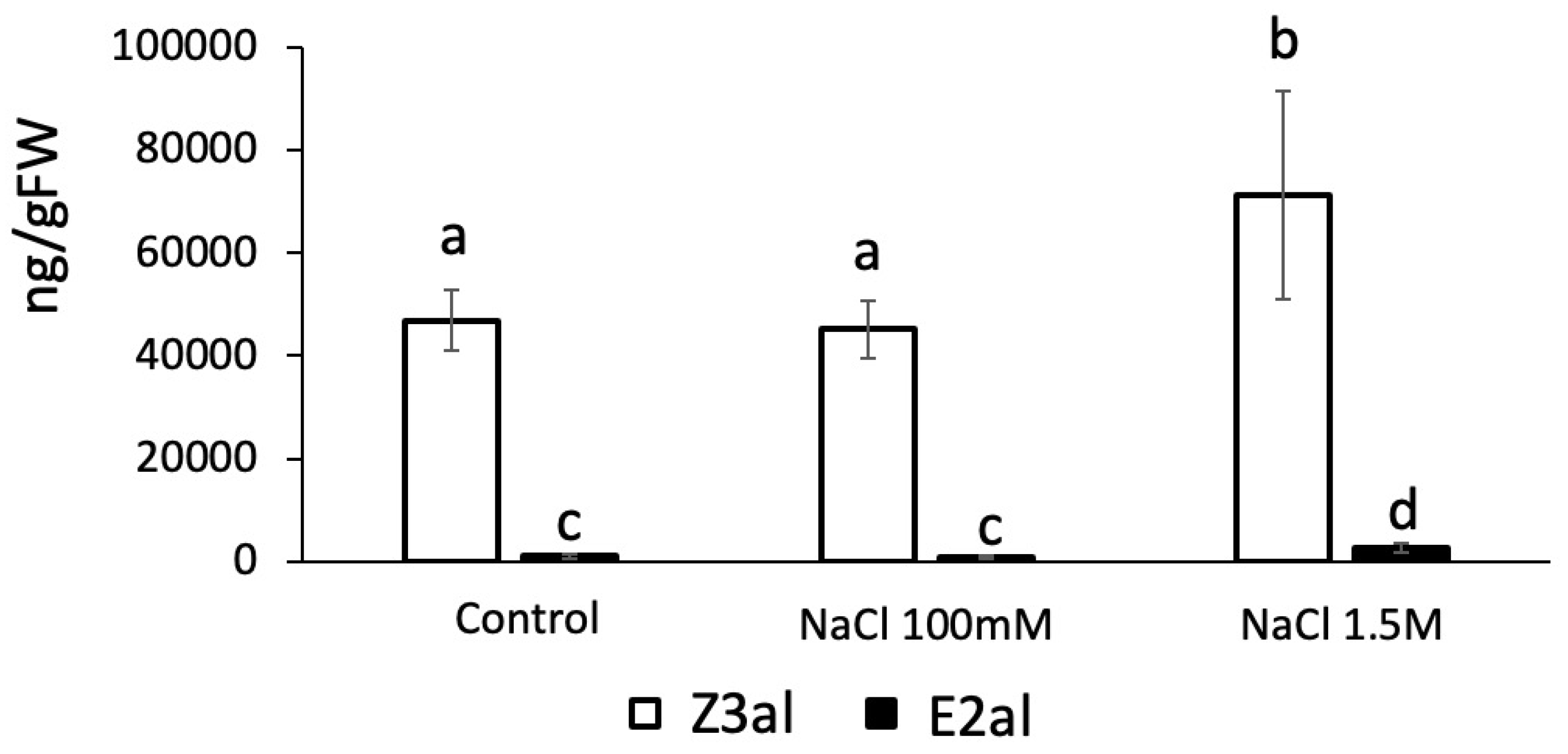
Effect of Ions on Z-3-Hexenal Production in Maize
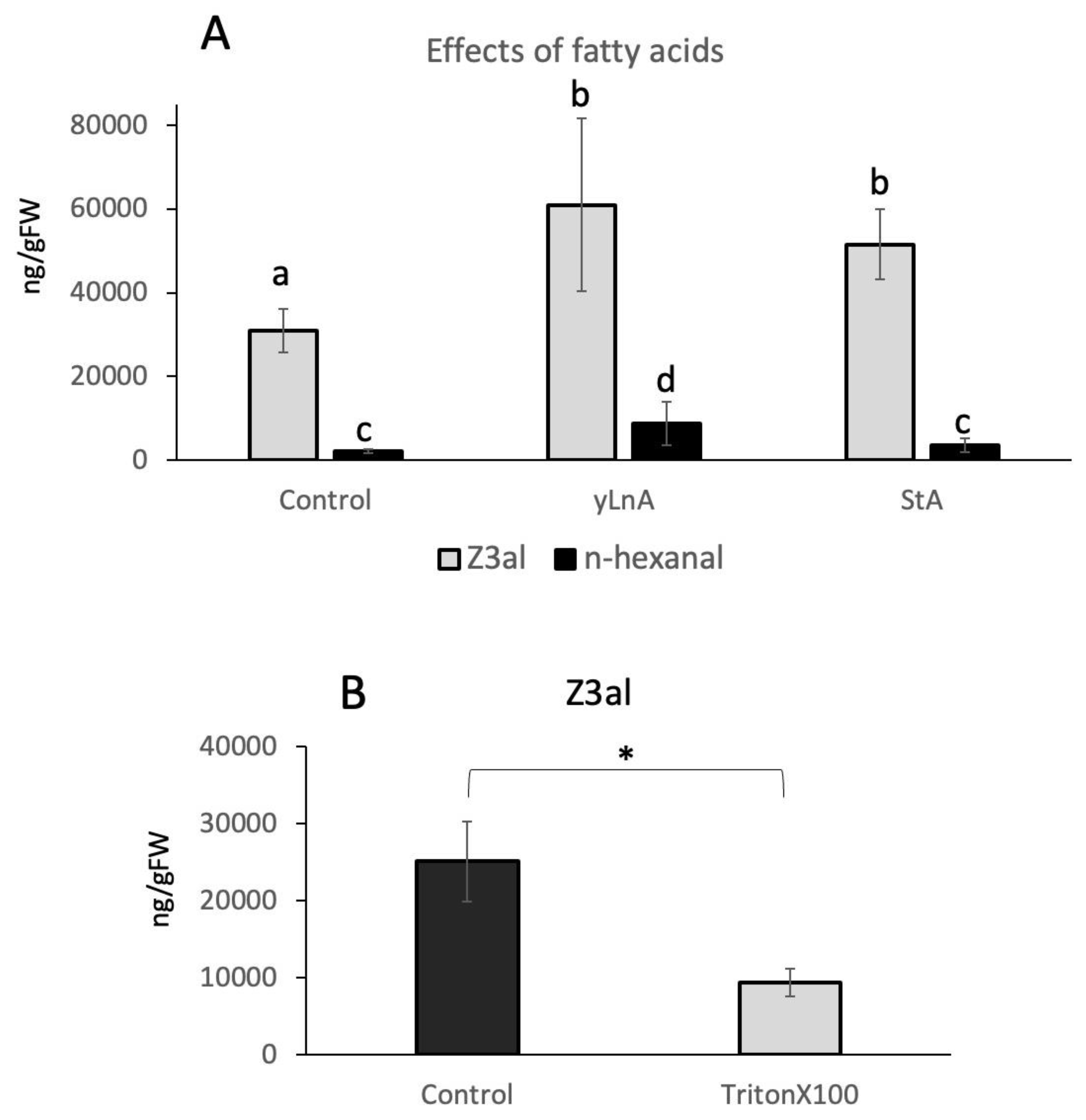
Effects of Fatty Acids and Detergent on GLV Production
3. Summary
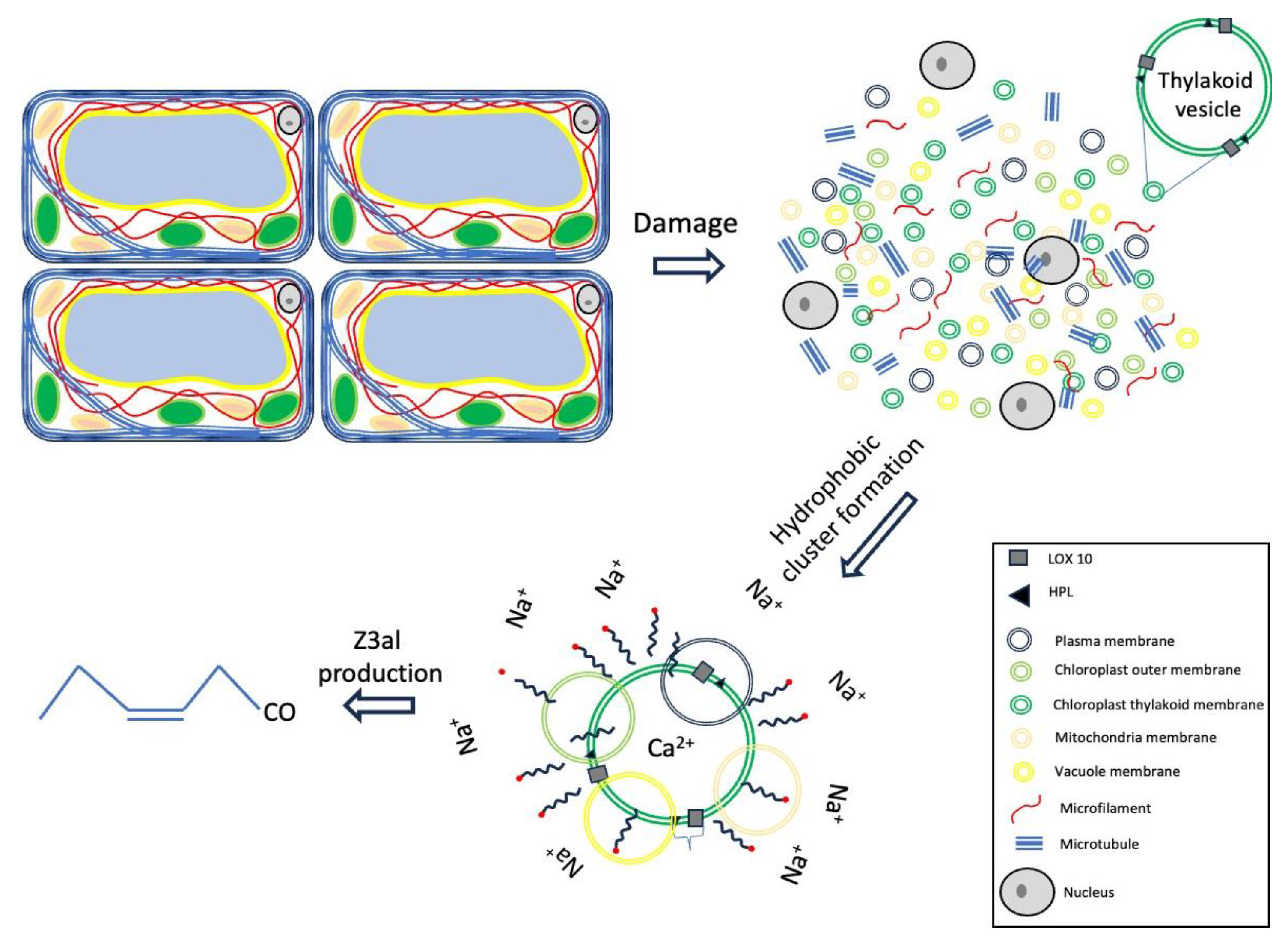
4. Materials and Methods
Chemicals
Plant Material
5. Experimental Setups
Chloroplast Isolation and Thylakoid Enrichment
Mass Spectrometer Analysis of Thylakoid Samples
GLV Assays
GLV Analysis
Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Curtius, T.; Franzen, H. Über die chemischen Bestandteile grüner Pflanzen. Über den Blätteraldehyd. Liebigs Ann. Chem 1912, 390, 89–121. [Google Scholar] [CrossRef]
- Matsui, K.; Engelberth, J. Green leaf volatiles-the forefront of plant responses against biotic attack. Plant Cell Physiol. 2022, 63, 1378–1390. [Google Scholar] [CrossRef] [PubMed]
- Scala, A.; Allmann, S.; Mirabella, R.; Haring, M.A.; Schuurink, R.C. Green leaf volatiles: A plant’s multifunctional weapon against herbivores and pathogens. Int. J. Mol. Sci. 2013, 14, 17781–17811. [Google Scholar] [CrossRef] [PubMed]
- Engelberth, J. Green Leaf Volatiles: A new player in the protection against abiotic stresses? Int. J. Mol. Sci. 2024, 25, 9471. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, A. The biogeneration of green odour by green leaves. Phytochem. 1993, 34, 1201–1218. [Google Scholar] [CrossRef]
- Matsui, K. Green leaf volatiles: Hydroperoxide lyase pathway of oxylipin metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef]
- Nakashima, A.; von Reuss, S.H.; Tasaka, H.; Nomura, M.; Mochizuki, S.; Iijima, Y.; Aoki, K.; Shibata, D.; Boland, W.; Takabayashi, J.; Matsui, K. Traumatin- and dinortraumatin-containing galactolipids in Arabidopsis. J. Biol. Chem 2013, 288, 26078–26088. [Google Scholar] [CrossRef]
- Zimmerman, D.C.; Coudron, C.A. Identification of traumatin, a wound hormone, as 12-oxo-trans-10-dodecenoic acid. Plant Physiol. 1979, 63, 536–541. [Google Scholar] [CrossRef]
- Engelberth, M.; Engelberth, J. Variability in the capacity to produce damage-induced aldehyde green leaf volatiles among different plant species provides novel insights into biosynthetic diversity. Plants 2020, 9, 213. [Google Scholar] [CrossRef]
- Kunishima, M.; Yamauchi, Y.; Mizutani, M.; Kuse, M.; Takikawa, H.; Sugimoto, Y. Identification of (Z)-3:(E)-2-hexenal isomerase essential to the production of the leaf aldehyde in plants. J Biol. Chem. 2016, 291, 14023–14033. [Google Scholar] [CrossRef]
- Spyropoulou, E.A.; Dekker, H.L.; Steemers, L.; van Maarseveen, J.H.; de Koster, C.G.; Haring, M.A.; Schuurink, R.C.; Allmann, S. Identification and Characterization of (3Z):(2E)-Hexenal Isomerases from Cucumber. Front. Plant Sci. 2017, 8, 1342. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K.; Sugimoto, K.; Mano, J.; Ozawa, R.; Takabayashi, J. Differential metabolism of green leaf volatiles in injured and intact parts of a wounded leaf meet distinct ecophysiological requirements. PLoS ONE 3643, 7, e36433. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.J.; Chambers, J.Q.; Holm, J.; Jardine, A.B.; Fontes, C.G.; Zorzanelli, R.F.; Meyers, K.T.; Fernandez de Souza, V.; Garcia, S.; Giminez, B.O.; Piva, L.R.; Higuchi, N.; Artaxo, P.; Martin, S.; Manzi, A.O. Green leaf volatile emissions during high temperature and drought stress in a Central Amazon rainforest. Plants 2015, 4, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Jardine, K.; Barron-Gafford, G.A.; Norman, J.P.; Abrell, L.; Monson, R.K.; Meyers, K.T; Pavao-Zuckerman, M.; Dontsova, K.; Kleist, E.; Werner, C.; Huxman, T.E. Green leaf volatiles and oxygenated metabolite emission bursts from mesquite branches following light–dark transitions. Photosynth. Res. 2012, 113, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, K.; Khan, Z.R.; Pickett, J.A.; Toshova, T.; Wadhams, L.J. Diel periodicity in the production of green leaf volatiles by wild and cultivated host plants of stemborer moths, Chilo partellus and Busseola fusca. J. Chem. Ecol. 2006, 32, 565–577. [Google Scholar] [CrossRef]
- Röse, U.S.R.; Tumlinson, J.H. Systemic induction of volatile release in cotton: How specific is the signal to herbivory? Planta 2005, 222, 327–335. [Google Scholar] [CrossRef]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicon) plant-to-plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef]
- Aratani, Y.; Uemura, T.; Hagihara, T.; Matsui, K.; Toyota, M. Green leaf volatile sensory calcium transduction in Arabidopsis. Nat. Commun. 2023, 14, 6236. [Google Scholar] [CrossRef]
- Engelberth, J.; Contreras, C.F.; Dalvi, C.; Li, T.; Engelberth, M. Early transcriptome analyses of Z-3-hexenol-treated Zea mays revealed distinct transcriptional networks and anti-herbivore defense potential of green leaf volatiles. PLoS ONE 2013, 8, e77465. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kunishima, M.; Mizutani, M.; Sugimotot, Y. Reactive short-chain leaf volatiles act as powerful inducers of abiotic stress-related gene expression. Sci. Rep. 2015, 5, 8030. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Matsuda, A.; Matsuura, N.; Mizutani, M.; Sugimoto, Y. Transcriptome analysis of Arabidopsis thaliana with green leaf volatiles: possible role of green leaf volatiles as self-made damage-associated patterns. J. Pestic. Sci 2018, 43, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Tanarsuwongkul, S.; Fisher, K.W.; Mullis, B.T.; Negi, H.; Roberts, J.; Tomlin, F.; Wang, Q.; Stratmann, J.W. Green leaf volatiles co-opt proteins involved in molecular pattern signalling in plant cells. Plant Cell Environ. 2024, 47, 928–946. [Google Scholar] [CrossRef] [PubMed]
- Ohgami, S.; Ono, E.; Horiwaka, M.; Murata, J.; Totsuka, K.; Toyonaga, H.; Ohba, Y.; Dohra, H.; Asai, T.; Matsui, K.; Mizutani, M.; Watanabe, N.; Ohnishi, T. Volatile glycosylation in tea plants: sequential glycosylations for the biosynthesis of aroma b-primeverosides are catalyzed by two Camellia sinensis glycosyltransferases. Plant Physiol 2015, 168, 464–477. [Google Scholar] [CrossRef] [PubMed]
- Cofer, T.M.; Erb, M.; Tumlinson, J.H. The Arabidopsis thaliana carboxylesterase AtCXE12 converts volatile (Z)-3-hexenyl acetate to (Z)-3-hexenol. bioRxiv 2023. [Google Scholar]
- Mano, J.; Kanameda, S.; Kuramitsu, R.; Matsuura, N.; Yamauchi, Y. Detoxification of Reactive Carbonyl Species by Glutathione Transferase Tau Isozymes. Front. Plant Sci. 2019, 24, 487. [Google Scholar] [CrossRef]
- Engelberth, J.; Engelberth, M. Developmental stages affect the capacity to produce aldehyde green leaf volatiles in Zea mays and Vigna radiata. Plants 2022, 11, 526. [Google Scholar] [CrossRef]
- Christensen, S.A.; Nemchenko, A.; Borrego, E.; Murray, I.; Sobhy, I.S.; Bosak, L.; DeBlasio, S.; Erb, M.; Robert, C.A.M.; Vaughn, K.A.; Herrfurth, C.; Tumlinson, J., Feussner; Jackso, D.; Turlings, T.C.J.; Engelberth, J.; Nansen, C.; Meeley, R.; Kolomiets, M.V. The maize lipoxygenase, ZmLOX10, mediates green leaf volatile, jasmonate and herbivore-induced plant volatile production for defense against insect attack. Plant J 2013, 74, 59–73. [Google Scholar] [CrossRef]
- Yactayo-Chang, J.P.; Hunter, C.T.; Alborn, H.T.; Christensen, S.A.; Block, A.K. Production of the Green Leaf Volatile (Z)-3-Hexenal by a Zea maysHydroperoxide Lyase. Plants 2022, 11, 2201. [Google Scholar] [CrossRef]
- Nemchenko, A.; Kunze, S.; Feussner, I.; Kolomiets, M. Duplicate maize 13-lipoxygenase genes are differentially regulated by circadian rhythm, cold stress, wounding, pathogen infection, and hormonal treatments. J. Exp. Bot. 2006, 57, 3767–3779. [Google Scholar] [CrossRef]
- Farmaki, T.; Sanmartin, M.; Jiminez, P.; Paneque, M.; Sanz, C.; Vancanneyt, G.; Leon, J.; Sanchez-Serrano, J.J. Differential distribution of the lipoxygenase pathway enzymes within potato chloroplasts. J. Exp. Bot. 2007, 58, 555–568. [Google Scholar] [CrossRef]
- Blee, E.; Joyard, J. Envelope membranes from spinach chloroplasts are a site of metabolism of fatty acid hydroperoxides. Plant Physiol. 1996, 110, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, J.E.; Itoh, A.; Howe, G.A. Tomato allene oxide synthase and fatty acid hydroperoxide lyase, two cytochrome P450s involved in oxylipins metabolism, are targeted to different membranes of chloroplast envelope. Plant Physiol. 2001, 125, 306–317. [Google Scholar] [CrossRef] [PubMed]
- Rustgi, S.; Springer, A.; Kang, C.; von Wettstein, D.; Reinbothe, C.; Reinbothe, S.; Pollmann, S. Allene oxide synthase and hydroperoxide lyase, two non-canonical cytochrome p450s in Arabidopsis thaliana and their different roles in plant defense. Int. J. Mol. Sci. 2019, 20, 3064. [Google Scholar] [CrossRef] [PubMed]
- Demchenko, K.; Zdyb, A.; Feussner, I.; Pawlowski, K. Analysis of the subcellular localization of lipoxygenase in legume and actinorhizal nodules. Plant. Biol. 2012, 14, 46–63. [Google Scholar] [CrossRef]
- Zhang, C.; Cao, S.; Jin, Y.; Chen, Q.; Xing, Q.; Qi, H. Melon 13-lipoxygenase CmLOX18 may be involved in C6 volatile biosynthesis in fruit. Sci. Rep. 2017, 7, 2816. [Google Scholar]
- Weichert, H.; Kolbe, A.; Kraus, A.; Wasternack, C.; Feussner, I. Metabolic profiling of oxylipins in germinating cucumber seedlings—Lipoxygenase-dependent degradation of triacylglycerols and biosynthesis of volatile aldehydes. Planta 2002, 215, 612–619. [Google Scholar] [CrossRef]
- Ishiguro, S.; Kawai-Oda, A.; Ueda, J.; Nishida, I.; Okada, K. The DEFECTIVE IN ANTHER DEHISCIENCE gene encodes a novel phospholipase A1 catalyzing the initial step of jasmonic acid biosynthesis, which synchronizes pollen maturation, anther dehiscence, and flower opening in Arabidopsis. Plant Cell 2001, 13, 2191–2209. [Google Scholar] [CrossRef]
- Matsui, K. Properties and structures of fatty acid hydroperoxide lyase. Belg. J. Bot. 1998, 131, 50–62. [Google Scholar]
- Hornostaj, A.R.; Robinson, D.S. Purification of hydroperoxide lyase from cucumber. Food Chem. 1999, 66, 173–180. [Google Scholar] [CrossRef]
- De Domenico, S.; Tsesmetzis, N.; Di Sansebastiano, G.P.; Hughes, R.K.; Casey, R.; Santino, A. Subcellular localization of Medicago truncatula 9/13-hydroperoxide lyase reveal a new localization pattern and activation mechanism for CYP74C enzymes. BMC Plant Biol. 2007, 7. [Google Scholar] [CrossRef]
- Savchenko, T.; Pearse, I.S.; Ignatia, L.; Karban, R.; Dehesh, K. Insect herbivores selectively suppress the HPL branch of the oxylipin pathway in host plants. Plant J. 2012, 73, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Dehesh, K. Insect herbivores selectively mute GLV production in plants. Plant Signal. Behav. 2013, 8, e24136. [Google Scholar] [CrossRef] [PubMed]
- Takai, H.; Ozawa, R.; Takabayashi, J.; Fujii, S.; Arai, K.; Ichiki, R.T.; Koeduka, T.; Dohra, H.; Ohnishi, T.; Taketazu, S.; et al. Silkworms suppress the release of green leaf volatiles by mulberry leaves with an enzyme from their spinnerets. Sci. Rep. 2018, 8, 11942. [Google Scholar] [CrossRef]
- Jones, A.C.; Seidl-Adams, I.; Engelberth, J.; Hunter, C.T.; Alborn, H.; Tumlinson, J.H. Herbivorous caterpillars can utilize three different mechanisms to alter green leaf volatile emissions. Environ. Entomol. 2019, 48, 419–425. [Google Scholar] [CrossRef]
- Jones, A.; Cofer, T.M.; Engelberth, J.; Tumlinson, J.H. Herbivorous caterpillars and the green leaf volatile (GLV) quandary. J. Chem. Ecol. 2022, 48, 337–345. [Google Scholar] [CrossRef]
- Engelberth, J. Selective inhibition of jasmonic acid accumulation by a small α, β-unsaturated carbonyl and phenidone reveals different modes of octadecanoid signalling activation in response to insect elicitors and green leaf volatiles in Zea mays. BMC Res. Notes 2011, 3, 377. [Google Scholar] [CrossRef]
- Bruinsma, M.; van Broekhoven, S.; Poelman, E.H.; Posthumus, M.A.; Müller, M.J.; van Loon, J.J.; Dicke, M. Inhibition of lip-oxygenase affects induction of both direct and indirect plant defences against herbivorous insects. Oecologia 2010, 162, 393–404. [Google Scholar] [CrossRef]
- Agut, B.; Gamir, J.; Jacas, J.A.; Hurtado, M.; Flors, V. Different metabolic and genetic responses in citrus may explain relative susceptibility to Tetranychus urticae. Pest Manag. Sci. 2014, 70, 1728–41. [Google Scholar] [CrossRef]
- Engelberth, J.; Koch, T.; Schüler, G.; Bachmann, N.; Rechtenbach, J.; Boland, W. Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 2001, 125, 369–77. [Google Scholar] [CrossRef]
- Pare, P.W.; Tumlinson, J.H. De Novo Biosynthesis of Volatiles Induced by Insect Herbivory in Cotton Plants. Plant Physiol. 1997, 114, 1161–1167. [Google Scholar] [CrossRef]
- Ogunola, O.F.; Hawkins, L.K.; Mylroie, E.; Kolomiets, M.V.; Borrego, E.; Tang, J.D.; Williams, W.P.; Warburton, M.L. Characterization of the maize lipoxygenase gene family in relation to aflatoxin accumulation resistance. PLoS One 2017, 12, e0181265. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, S.; Matsui, K. Green leaf volatile-burst in Arabidopsis is governed by galactolipid oxygenation by a lipoxygenase that is under control of calcium ion. Biochem. Biophys. Res. Comm. 2018, 505, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Newcomer, M.E.; Brash, A.R. The structural basis for specificity in lipoxygenase catalysis. Protein Sci 2014, 24, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Jethva, D.; Shruthi, P.; Patel, S.; Karbhari, N.; Savaliya, N. Comparison of Magnesium by EDTA and EGTA as chelating agent. Int. J. Clinical Biochem. 2022, 7, 3. [Google Scholar]
- Holleman, A. F.; Wiberg, E. Inorganic Chemistry; Academic Press: San Diego, 2001. [Google Scholar]
- Hu, X.; Dong, Q.; Yang, J.; Zhang, Y. Recognizing metal and acid radical ion binding sites by integrating ab initio modeling with template-based transferals. Bioinformatics 2016, 32, 3260–3269. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sato, K.; Kinbara, K. Calcium-induced reversible assembly of phosphorylated amphiphile within lipid bilayer membranes. Chem. Commun. 2021, 57, 4106–4109. [Google Scholar] [CrossRef]
- Bhattacharya, O.; Ortiz, I.; Walling, L.L. Methodology: an optimized, high yield tomato leaf chloroplast isolation and stroma extraction protocol for proteomics analyses and identification of chloroplast co-localizing proteins. Plant Methods 2020, 16, 131. [Google Scholar] [CrossRef]
- Bouchnak, I.; Moyet, L.; Salvi, D.; Kuntz, M.; Rolland, N. Preparation of Chloroplast Sub- compartments from Arabidopsis for the Analysis of Protein Localization by Immunoblotting or Proteomics. JoVE 2018, 58581. [Google Scholar] [CrossRef]
| Name | Protein View (NCBIprot) |
emPAI | Identified sequences (1-letter code) |
Mr |
|---|---|---|---|---|
| Lipoxygenase 2.3, chloroplastic [Zea mays] (LOX10) | PWZ26982.1 | 0.45 | QLTFGATTLR FEVPEMIER SKLDPEVYGPAESAITK YTMEINALAR GEDGELELTIK SDEAVAADPELR DEPWWPVLDTR NMPVEEGGPGEEMEK |
102005 |
| Linolenate hydroperoxide lyase, chloroplastic [Zea mays] (HPL) | PWZ25671.1 | 0.4 | TFAMDLLHR ASVGAMLDAVDAEFGKDDGSDK EGMPLVR DPEVFERPEEFVPER YDDFEVEGTSFTK |
55519 |
| Phospholipase A(1) DAD1, chloroplastic [Zea mays] (PLA1) |
PWZ29609.1 | 0.09 | AVSFGGPRVGNVAFR | 43169 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





