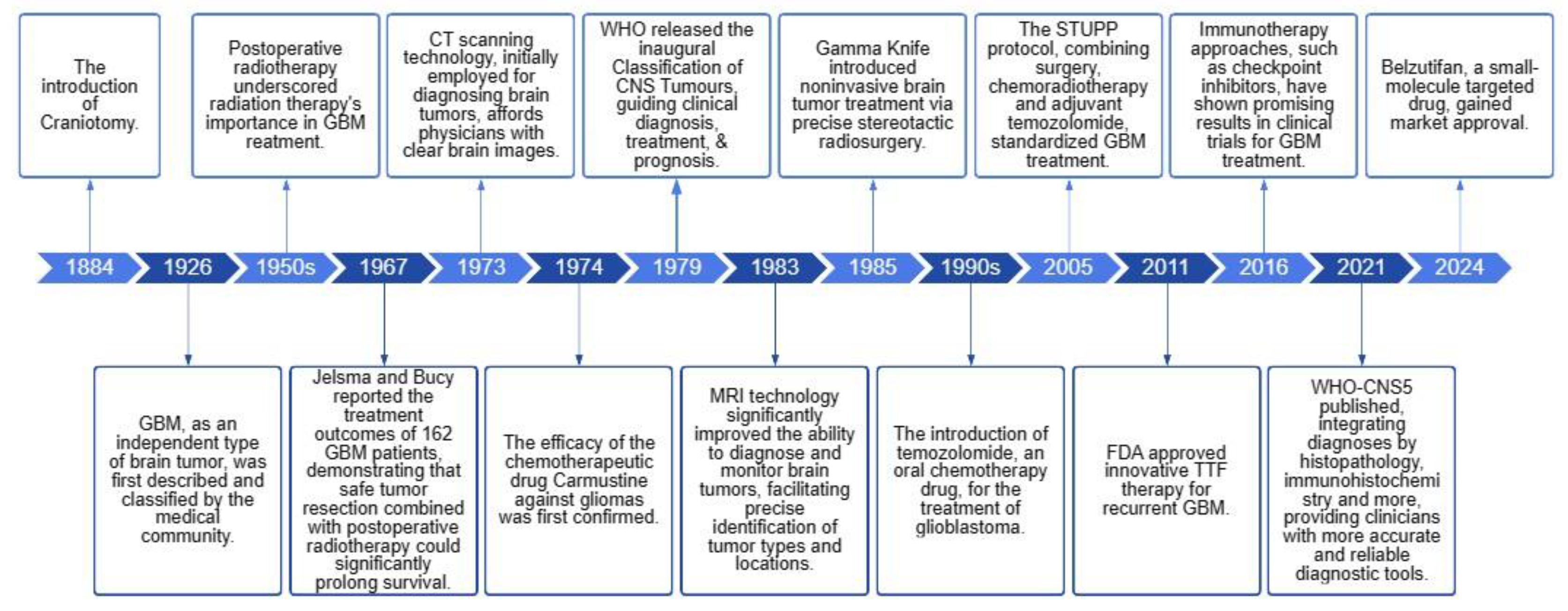
Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
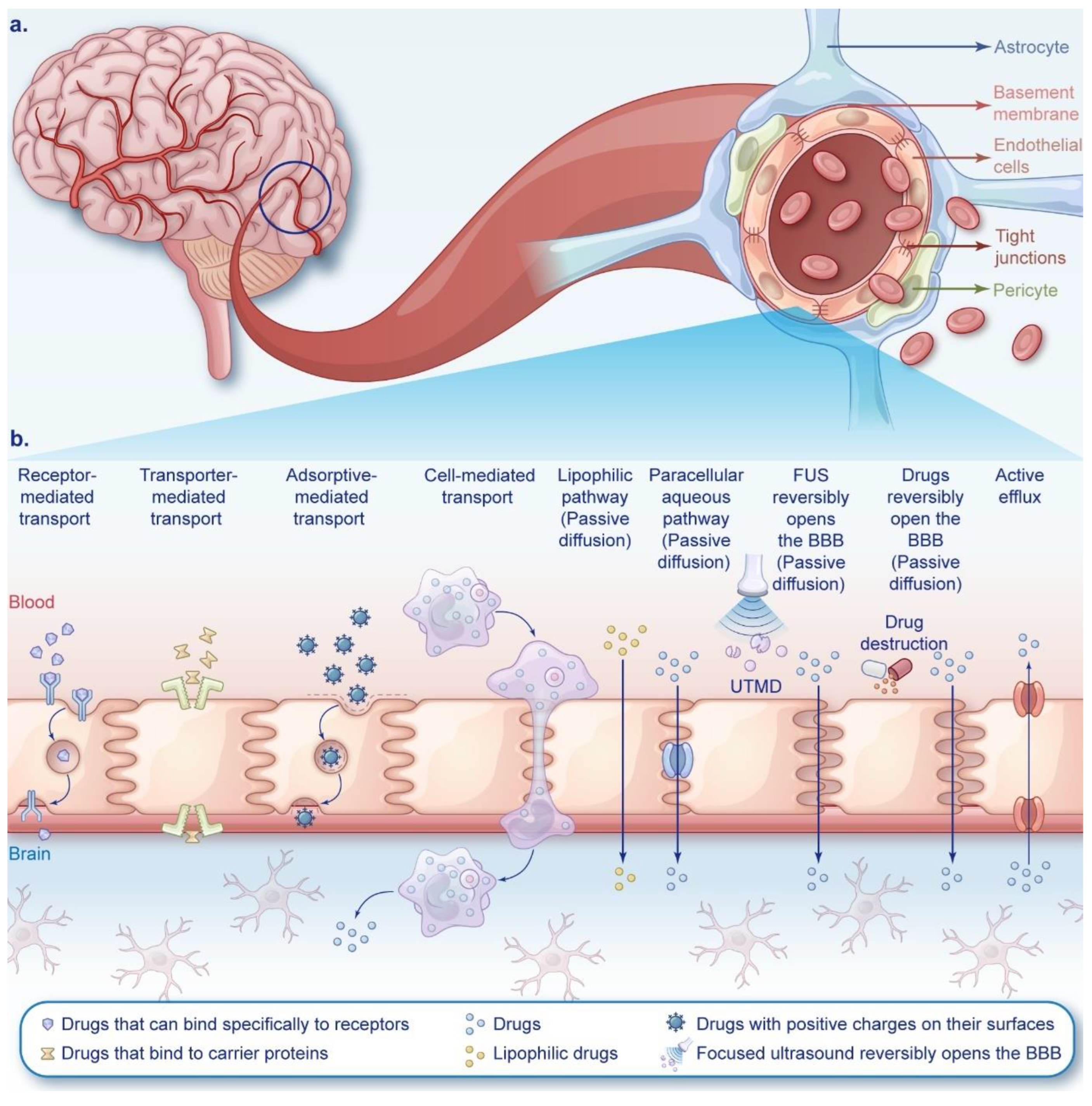
2. Strategies for NDDS to Cross the BBB
2.1. Receptor-Mediated Transport
2.1.1. Transferrin Receptors and Lactoferrin Receptors
2.1.2. Acetylcholine Receptors
2.1.3. Folate Receptors
2.1.4. Low-Density Lipoprotein Receptors
2.1.5. Epidermal Growth Factor Receptors
2.1.6. Human Insulin-like Growth Factor-1 Receptors
2.1.7. Integrins
2.1.8. CD13
2.1.9. Neuropilin-1
2.1.10. Heat Shock Protein 70
2.2. Transporter-Mediated Transport
2.2.1. Glucose Transporters
2.2.2. Choline Transporters
2.2.3. Amino Acid Transporters
2.2.4. Vitamin Transporters
2.2.5. Organic Cation Transporters
2.2.6. Organic Anion Transporters
2.2.7. Monocarboxylate Transporters
2.3. Adsorptive-Mediated Transport
2.3.1. Cationic Albumin
2.3.2. Cell-Penetrating Peptides
2.4. Cell-Mediated Transport
2.4.1. Erythrocytes
2.4.2. Leukocytes
2.4.3. Stem Cells
2.5. Passive Diffusion
3. Strategies for NDDS to Bypass the BBB
3.1. Intranasal Administration
3.2. Convection-Enhanced Delivery
3.3. Intracavitary/Intrathecal Drug Administration
4. Progress in Clinical Trials
5. Technical Challenges and New Strategies
- (1)
- Physical barrier". This encompasses the anatomical and functional features of BBB endothelial cells, forming a vital anatomical gateway for targeted brain drug delivery. The lipid bilayer membrane of these cells exhibits lipophilicity and hosts receptors, carrier proteins, and other components that regulate molecular trafficking from the bloodstream to brain tissue. High molecular weight drugs (>500 daltons) often fail to traverse this barrier [140]. Tight and adherens junctions between endothelial cells maintain BBB integrity, preventing unrestricted substance exchange.
- (2)
- "Transportation barrier". Functionally, the surface of the BBB endothelial cells is negatively charged,, impeding negatively charged compounds from entering neurons. Endothelial membranes express specific transporters regulating substrate influx/efflux, preventing unauthorized bloodstream substances from crossing [142]. Pericytes and astrocytes encapsulate BBB endothelial cells, creating resistance that allows only small molecules (e.g., water, gases, lipids) to diffuse passively. Large, charged, polar, hydrophilic molecules (amino acids, glucose, drugs) rely on luminal membrane transport proteins/receptors [137]. ATP-driven efflux pumps (P-glycoprotein) limit toxin/drug permeability, reducing CNS exposure [104,143], impacting drug efficacy, exacerbating side effects, and challenging drug action in brain tissue.
- (3)
- "Metabolic barrier". Various drug-metabolizing enzymes, such as CYP450 enzymes, have been documented within the endothelial cells of the brain [104].
6. Conclusions and Perspective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2016-2020. Neuro Oncol 2023, 25, iv1–iv99. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.; Gu, L.; Kang, X.; Li, J.; Song, Y.; Wang, Y.; Ma, W. Programmed cell death disrupts inflammatory tumor microenvironment (TME) and promotes glioblastoma evolution. Cell communication and signaling : CCS 2024, 22, 333. [Google Scholar] [CrossRef] [PubMed]
- Horbinski, C.; Berger, T.; Packer, R.J.; Wen, P.Y. Clinical implications of the 2021 edition of the WHO classification of central nervous system tumours. Nat Rev Neurol 2022, 18, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Smerdi, D.; Moutafi, M.; Kotsantis, I.; Stavrinou, L.C.; Psyrri, A. Overcoming Resistance to Temozolomide in Glioblastoma: A Scoping Review of Preclinical and Clinical Data. Life (Basel, Switzerland) 2024, 14. [Google Scholar] [CrossRef]
- Banks, W.A. From blood-brain barrier to blood-brain interface: new opportunities for CNS drug delivery. Nature reviews. Drug discovery 2016, 15, 275–292. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug discovery today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Pardridge, W.M. The blood-brain barrier: bottleneck in brain drug development. NeuroRx : the journal of the American Society for Experimental NeuroTherapeutics 2005, 2, 3–14. [Google Scholar] [CrossRef]
- Tiwari, S.B.; Amiji, M.M. A review of nanocarrier-based CNS delivery systems. Current drug delivery 2006, 3, 219–232. [Google Scholar] [CrossRef]
- Quader, S.; Kataoka, K.; Cabral, H. Nanomedicine for brain cancer. Adv Drug Deliv Rev 2022, 182, 114115. [Google Scholar] [CrossRef]
- Song, X.; Qian, H.; Yu, Y. Nanoparticles Mediated the Diagnosis and Therapy of Glioblastoma: Bypass or Cross the Blood-Brain Barrier. Small (Weinheim an der Bergstrasse, Germany) 2023, 19, e2302613. [Google Scholar] [CrossRef]
- Khan, I.; Baig, M.H.; Mahfooz, S.; Imran, M.A.; Khan, M.I.; Dong, J.J.; Cho, J.Y.; Hatiboglu, M.A. Nanomedicine for glioblastoma: Progress and future prospects. Semin Cancer Biol 2022, 86, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.C.; Morton, S.W.; Wyckoff, J.; Vu Han, T.L.; Hwang, M.K.; Maffa, A.; Balkanska-Sinclair, E.; Yaffe, M.B.; Floyd, S.R.; Hammond, P.T. Enhanced efficacy of combined temozolomide and bromodomain inhibitor therapy for gliomas using targeted nanoparticles. Nature communications 2018, 9, 1991. [Google Scholar] [CrossRef]
- Dong, M.; Liu, Y.; Liu, B.; Peng, J.; Tang, Y.; Lu, G.; Shi, H.; Zhu, F. Enhanced anti-glioma efficacy of biodegradable periodic mesoporous organosilica nanoparticles through target delivery of chemotherapeutics. Journal of materials science. Materials in medicine 2023, 34, 48. [Google Scholar] [CrossRef]
- Janjua, T.I.; Cao, Y.; Ahmed-Cox, A.; Raza, A.; Moniruzzaman, M.; Akhter, D.T.; Fletcher, N.L.; Kavallaris, M.; Thurecht, K.J.; Popat, A. Efficient delivery of Temozolomide using ultrasmall large-pore silica nanoparticles for glioblastoma. Journal of controlled release : official journal of the Controlled Release Society 2023, 357, 161–174. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Wang, H.; Zhang, K.; Liu, Y.; Li, Q.; Li, C.; Wen, Z.; Chen, Z. Biomimetic nanocarriers loaded with temozolomide by cloaking brain-targeting peptides for targeting drug delivery system to promote anticancer effects in glioblastoma cells. Heliyon 2024, 10, e28256. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wei, G.; Liu, B.; Wang, C.; Wang, J.; Lu, Y.; Cui, W.; Guo, H. Polyhedral Oligomeric Silsesquioxane-Based Nanoparticles for Efficient Chemotherapy of Glioblastoma. Small (Weinheim an der Bergstrasse, Germany) 2023, 19, e2207248. [Google Scholar] [CrossRef] [PubMed]
- Thirumurugan, S.; Dash, P.; Liu, X.; Tseng, Y.Y.; Huang, W.J.; Li, Y.; Zhao, G.; Lin, C.; Murugan, K.; Dhawan, U.; et al. Angiopep-2-decorated titanium-alloy core-shell magnetic nanoparticles for nanotheranostics and medical imaging. Nanoscale 2022, 14, 14789–14800. [Google Scholar] [CrossRef]
- He, C.; Zhang, Z.; Ding, Y.; Xue, K.; Wang, X.; Yang, R.; An, Y.; Liu, D.; Hu, C.; Tang, Q. LRP1-mediated pH-sensitive polymersomes facilitate combination therapy of glioblastoma in vitro and in vivo. Journal of nanobiotechnology 2021, 19, 29. [Google Scholar] [CrossRef]
- Banstola, A.; Duwa, R.; Emami, F.; Jeong, J.H.; Yook, S. Enhanced Caspase-Mediated Abrogation of Autophagy by Temozolomide-Loaded and Panitumumab-Conjugated Poly(lactic-co-glycolic acid) Nanoparticles in Epidermal Growth Factor Receptor Overexpressing Glioblastoma Cells. Molecular pharmaceutics 2020, 17, 4386–4400. [Google Scholar] [CrossRef]
- Alata, W.; Yogi, A.; Brunette, E.; Delaney, C.E.; van Faassen, H.; Hussack, G.; Iqbal, U.; Kemmerich, K.; Haqqani, A.S.; Moreno, M.J.; et al. Targeting insulin-like growth factor-1 receptor (IGF1R) for brain delivery of biologics. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2022, 36, e22208. [Google Scholar] [CrossRef]
- Vangala, V.; Nimmu, N.V.; Khalid, S.; Kuncha, M.; Sistla, R.; Banerjee, R.; Chaudhuri, A. Combating Glioblastoma by Codelivering the Small-Molecule Inhibitor of STAT3 and STAT3siRNA with α5β1 Integrin Receptor-Selective Liposomes. Molecular pharmaceutics 2020, 17, 1859–1874. [Google Scholar] [CrossRef]
- Lu, L.; Zhao, X.; Fu, T.; Li, K.; He, Y.; Luo, Z.; Dai, L.; Zeng, R.; Cai, K. An iRGD-conjugated prodrug micelle with blood-brain-barrier penetrability for anti-glioma therapy. Biomaterials 2020, 230, 119666. [Google Scholar] [CrossRef] [PubMed]
- Xie, R.; Wang, Y.; Tong, F.; Yang, W.; Lei, T.; Du, Y.; Wang, X.; Yang, Z.; Gong, T.; Shevtsov, M.; et al. Hsp70-Targeting and Size-Tunable Nanoparticles Combine with PD-1 Checkpoint Blockade to Treat Glioma. Small (Weinheim an der Bergstrasse, Germany) 2023, e2300570. [Google Scholar] [CrossRef]
- Zhou, J.; Meng, N.; Lu, L.; Lu, J.; Wu, S.; Ding, Y.; Wu, S.; Bao, Y.; Xu, Q.; Chen, R.; et al. A novel peptide-drug conjugate for glioma-targeted drug delivery. Journal of controlled release : official journal of the Controlled Release Society 2024, 369, 722–733. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, Y.; Xu, H.; Li, L.; Qian, F.; Wang, L.; Quan, A.; Ma, H.; Liu, H.; Yu, R. Cascade-Responsive 2-DG Nanocapsules Encapsulate aV-siCPT1C Conjugates to Inhibit Glioblastoma through Multiple Inhibition of Energy Metabolism. ACS applied materials & interfaces 2023, 15, 10356–10370. [Google Scholar] [CrossRef]
- Wang, H.; Chao, Y.; Zhao, H.; Zhou, X.; Zhang, F.; Zhang, Z.; Li, Z.; Pan, J.; Wang, J.; Chen, Q.; et al. Smart Nanomedicine to Enable Crossing Blood-Brain Barrier Delivery of Checkpoint Blockade Antibody for Immunotherapy of Glioma. ACS nano 2022, 16, 664–674. [Google Scholar] [CrossRef]
- Bhunia, S.; Vangala, V.; Bhattacharya, D.; Ravuri, H.G.; Kuncha, M.; Chakravarty, S.; Sistla, R.; Chaudhuri, A. Large Amino Acid Transporter 1 Selective Liposomes of l-DOPA Functionalized Amphiphile for Combating Glioblastoma. Molecular pharmaceutics 2017, 14, 3834–3847. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, Y.; Chen, Y.; Yang, Z.; Zhang, L.; Xiao, W.; Yang, J.; Guo, L.; Wu, Y. Dual-targeting for brain-specific liposomes drug delivery system: Synthesis and preliminary evaluation. Bioorganic & medicinal chemistry 2018, 26, 4677–4686. [Google Scholar] [CrossRef]
- Kou, L.; Hou, Y.; Yao, Q.; Guo, W.; Wang, G.; Wang, M.; Fu, Q.; He, Z.; Ganapathy, V.; Sun, J. L-Carnitine-conjugated nanoparticles to promote permeation across blood-brain barrier and to target glioma cells for drug delivery via the novel organic cation/carnitine transporter OCTN2. Artificial cells, nanomedicine, and biotechnology 2018, 46, 1605–1616. [Google Scholar] [CrossRef]
- Baklaushev, V.P.; Nukolova, N.N.; Khalansky, A.S.; Gurina, O.I.; Yusubalieva, G.M.; Grinenko, N.P.; Gubskiy, I.L.; Melnikov, P.A.; Kardashova, K.; Kabanov, A.V.; et al. Treatment of glioma by cisplatin-loaded nanogels conjugated with monoclonal antibodies against Cx43 and BSAT1. Drug delivery 2015, 22, 276–285. [Google Scholar] [CrossRef]
- Lu, L.; Wang, L.; Zhao, L.; Liao, J.; Zhao, C.; Xu, X.; Wang, F.; Zhang, X. A Novel Blood-Brain Barrier-Penetrating and Vascular-Targeting Chimeric Peptide Inhibits Glioma Angiogenesis. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Tian, J.; Wang, L.; Dong, S.; Fu, K.; Chen, S.; Liu, C. Efficient Delivery of Lomitapide using Hybrid Membrane-Coated Tetrahedral DNA Nanostructures for Glioblastoma Therapy. Adv Mater 2024, 36, e2311760. [Google Scholar] [CrossRef]
- Zou, Y.; Liu, Y.; Yang, Z.; Zhang, D.; Lu, Y.; Zheng, M.; Xue, X.; Geng, J.; Chung, R.; Shi, B. Effective and Targeted Human Orthotopic Glioblastoma Xenograft Therapy via a Multifunctional Biomimetic Nanomedicine. Adv Mater 2018, 30, e1803717. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Chen, Z.; Peng, Y.; Liu, C. IRGD-modified erythrocyte membrane biomimetic temozolomide nanodots for the treatment of glioblastoma. Nanotechnology 2024, 35. [Google Scholar] [CrossRef]
- Kim, G.B.; Aragon-Sanabria, V.; Randolph, L.; Jiang, H.; Reynolds, J.A.; Webb, B.S.; Madhankumar, A.; Lian, X.; Connor, J.R.; Yang, J.; et al. High-affinity mutant Interleukin-13 targeted CAR T cells enhance delivery of clickable biodegradable fluorescent nanoparticles to glioblastoma. Bioactive materials 2020, 5, 624–635. [Google Scholar] [CrossRef]
- Wang, J.; Tang, W.; Yang, M.; Yin, Y.; Li, H.; Hu, F.; Tang, L.; Ma, X.; Zhang, Y.; Wang, Y. Inflammatory tumor microenvironment responsive neutrophil exosomes-based drug delivery system for targeted glioma therapy. Biomaterials 2021, 273, 120784. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Cai, X.; Syahirah, R.; Yao, Y.; Xu, Y.; Jin, G.; Bhute, V.J.; Torregrosa-Allen, S.; Elzey, B.D.; Won, Y.Y.; et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nature communications 2023, 14, 2266. [Google Scholar] [CrossRef]
- Xiao, T.; He, M.; Xu, F.; Fan, Y.; Jia, B.; Shen, M.; Wang, H.; Shi, X. Macrophage Membrane-Camouflaged Responsive Polymer Nanogels Enable Magnetic Resonance Imaging-Guided Chemotherapy/Chemodynamic Therapy of Orthotopic Glioma. ACS nano 2021, 15, 20377–20390. [Google Scholar] [CrossRef]
- Ma, X.; Kuang, L.; Yin, Y.; Tang, L.; Zhang, Y.; Fan, Q.; Wang, B.; Dong, Z.; Wang, W.; Yin, T.; et al. Tumor-Antigen Activated Dendritic Cell Membrane-Coated Biomimetic Nanoparticles with Orchestrating Immune Responses Promote Therapeutic Efficacy against Glioma. ACS nano 2023, 17, 2341–2355. [Google Scholar] [CrossRef]
- Jiang, X.; Fitch, S.; Wang, C.; Wilson, C.; Li, J.; Grant, G.A.; Yang, F. Nanoparticle engineered TRAIL-overexpressing adipose-derived stem cells target and eradicate glioblastoma via intracranial delivery. Proceedings of the National Academy of Sciences of the United States of America 2016, 113, 13857–13862. [Google Scholar] [CrossRef]
- Malik, Y.S.; Sheikh, M.A.; Xing, Z.; Guo, Z.; Zhu, X.; Tian, H.; Chen, X. Polylysine-modified polyethylenimine polymer can generate genetically engineered mesenchymal stem cells for combinational suicidal gene therapy in glioblastoma. Acta biomaterialia 2018, 80, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Ismail, M.; Yang, W.; Li, Y.; Chai, T.; Zhang, D.; Du, Q.; Muhammad, P.; Hanif, S.; Zheng, M.; Shi, B. Targeted liposomes for combined delivery of artesunate and temozolomide to resistant glioblastoma. Biomaterials 2022, 287, 121608. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Mu, N.; Jia, B.; Guo, Q.; Pan, L.; Zhu, M.; Zhang, W.; Zhang, K.; Li, W.; Li, M.; et al. Targeting radiation-tolerant persister cells as a strategy for inhibiting radioresistance and recurrence in glioblastoma. Neuro Oncol 2022, 24, 1056–1070. [Google Scholar] [CrossRef]
- Ji, B.; Maeda, J.; Higuchi, M.; Inoue, K.; Akita, H.; Harashima, H.; Suhara, T. Pharmacokinetics and brain uptake of lactoferrin in rats. Life sciences 2006, 78, 851–855. [Google Scholar] [CrossRef]
- Kuplennik, N.; Lang, K.; Steinfeld, R.; Sosnik, A. Folate Receptor α-Modified Nanoparticles for Targeting of the Central Nervous System. ACS applied materials & interfaces 2019, 11, 39633–39647. [Google Scholar] [CrossRef]
- Neves, A.R.; Queiroz, J.F.; Weksler, B.; Romero, I.A.; Couraud, P.O.; Reis, S. Solid lipid nanoparticles as a vehicle for brain-targeted drug delivery: two new strategies of functionalization with apolipoprotein E. Nanotechnology 2015, 26, 495103. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, L.; Tian, H.; Wang, Z.; Liu, G.; Duan, X.; Guo, M.; Liu, J.; Zhang, W.; Nice, E.C.; et al. Drug Repurposing-Based Brain-Targeting Self-Assembly Nanoplatform Using Enhanced Ferroptosis against Glioblastoma. Small (Weinheim an der Bergstrasse, Germany) 2023, 19, e2303073. [Google Scholar] [CrossRef]
- Tashima, T. Brain Cancer Chemotherapy through a Delivery System across the Blood-Brain Barrier into the Brain Based on Receptor-Mediated Transcytosis Using Monoclonal Antibody Conjugates. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Yogi, A.; Hussack, G.; van Faassen, H.; Haqqani, A.S.; Delaney, C.E.; Brunette, E.; Sandhu, J.K.; Hewitt, M.; Sulea, T.; Kemmerich, K.; et al. Brain Delivery of IGF1R5, a Single-Domain Antibody Targeting Insulin-like Growth Factor-1 Receptor. Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Miura, Y.; Takenaka, T.; Toh, K.; Wu, S.; Nishihara, H.; Kano, M.R.; Ino, Y.; Nomoto, T.; Matsumoto, Y.; Koyama, H.; et al. Cyclic RGD-linked polymeric micelles for targeted delivery of platinum anticancer drugs to glioblastoma through the blood-brain tumor barrier. ACS nano 2013, 7, 8583–8592. [Google Scholar] [CrossRef]
- Quader, S.; Liu, X.; Chen, Y.; Mi, P.; Chida, T.; Ishii, T.; Miura, Y.; Nishiyama, N.; Cabral, H.; Kataoka, K. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. Journal of controlled release : official journal of the Controlled Release Society 2017, 258, 56–66. [Google Scholar] [CrossRef] [PubMed]
- An, S.; Jiang, X.; Shi, J.; He, X.; Li, J.; Guo, Y.; Zhang, Y.; Ma, H.; Lu, Y.; Jiang, C. Single-component self-assembled RNAi nanoparticles functionalized with tumor-targeting iNGR delivering abundant siRNA for efficient glioma therapy. Biomaterials 2015, 53, 330–340. [Google Scholar] [CrossRef]
- Huang, N.; Cheng, S.; Zhang, X.; Tian, Q.; Pi, J.; Tang, J.; Huang, Q.; Wang, F.; Chen, J.; Xie, Z.; et al. Efficacy of NGR peptide-modified PEGylated quantum dots for crossing the blood-brain barrier and targeted fluorescence imaging of glioma and tumor vasculature. Nanomedicine : nanotechnology, biology, and medicine 2017, 13, 83–93. [Google Scholar] [CrossRef]
- Wang, J.; Lei, Y.; Xie, C.; Lu, W.; Wagner, E.; Xie, Z.; Gao, J.; Zhang, X.; Yan, Z.; Liu, M. Retro-inverso CendR peptide-mediated polyethyleneimine for intracranial glioblastoma-targeting gene therapy. Bioconjugate chemistry 2014, 25, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Enerson, B.E.; Drewes, L.R. The rat blood-brain barrier transcriptome. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2006, 26, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Gyimesi, G.; Hediger, M.A. Transporter-Mediated Drug Delivery. Molecules 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Min, H.S.; Kim, H.J.; Naito, M.; Ogura, S.; Toh, K.; Hayashi, K.; Kim, B.S.; Fukushima, S.; Anraku, Y.; Miyata, K.; et al. Systemic Brain Delivery of Antisense Oligonucleotides across the Blood-Brain Barrier with a Glucose-Coated Polymeric Nanocarrier. Angew Chem Int Ed Engl 2020, 59, 8173–8180. [Google Scholar] [CrossRef]
- Anraku, Y.; Kuwahara, H.; Fukusato, Y.; Mizoguchi, A.; Ishii, T.; Nitta, K.; Matsumoto, Y.; Toh, K.; Miyata, K.; Uchida, S.; et al. Glycaemic control boosts glucosylated nanocarrier crossing the BBB into the brain. Nature communications 2017, 8, 1001. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Xie, C.; Zhang, M.; Ruan, H.; Wang, S.; Jiang, K.; Wang, F.; Zhan, C.; Lu, W.; et al. Nanodisk-based glioma-targeted drug delivery enabled by a stable glycopeptide. Journal of controlled release : official journal of the Controlled Release Society 2018, 284, 26–38. [Google Scholar] [CrossRef]
- Li, J.; Zhou, L.; Ye, D.; Huang, S.; Shao, K.; Huang, R.; Han, L.; Liu, Y.; Liu, S.; Ye, L.; et al. Choline-derivate-modified nanoparticles for brain-targeting gene delivery. Adv Mater 2011, 23, 4516–4520. [Google Scholar] [CrossRef]
- Li, J.; Guo, Y.; Kuang, Y.; An, S.; Ma, H.; Jiang, C. Choline transporter-targeting and co-delivery system for glioma therapy. Biomaterials 2013, 34, 9142–9148. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wu, Y.; Chen, N.; Wang, Q.; Wang, Y.; Li, Y.; Li, S.; Han, X.; Yang, E.; Tong, F.; et al. Early administration of MPC-n(IVIg) selectively accumulates in ischemic areas to protect inflammation-induced brain damage from ischemic stroke. Theranostics 2021, 11, 8197–8217. [Google Scholar] [CrossRef]
- Puris, E.; Gynther, M.; Auriola, S.; Huttunen, K.M. L-Type amino acid transporter 1 as a target for drug delivery. Pharm Res 2020, 37, 88. [Google Scholar] [CrossRef]
- Montaser, A.B.; Järvinen, J.; Löffler, S.; Huttunen, J.; Auriola, S.; Lehtonen, M.; Jalkanen, A.; Huttunen, K.M. L-Type Amino Acid Transporter 1 Enables the Efficient Brain Delivery of Small-Sized Prodrug across the Blood-Brain Barrier and into Human and Mouse Brain Parenchymal Cells. ACS chemical neuroscience 2020, 11, 4301–4315. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.Y.; Feng, M.M.; Yang, B.; Yan, Z.Y.; Wang, B.Q.; Bu, X.Y. Methylmercury-l-Cysteine targeting L-type amino acid transporter conjugate cytotoxicity on C6 glioma cells. Journal of biological regulators and homeostatic agents 2018, 32, 147–151. [Google Scholar] [PubMed]
- Salmaso, S.; Pappalardo, J.S.; Sawant, R.R.; Musacchio, T.; Rockwell, K.; Caliceti, P.; Torchilin, V.P. Targeting glioma cells in vitro with ascorbate-conjugated pharmaceutical nanocarriers. Bioconjugate chemistry 2009, 20, 2348–2355. [Google Scholar] [CrossRef]
- Quéléver, G.; Kachidian, P.; Melon, C.; Garino, C.; Laras, Y.; Pietrancosta, N.; Sheha, M.; Louis Kraus, J. Enhanced delivery of gamma-secretase inhibitor DAPT into the brain via an ascorbic acid mediated strategy. Organic & biomolecular chemistry 2005, 3, 2450–2457. [Google Scholar] [CrossRef]
- Kou, L.; Sun, R.; Ganapathy, V.; Yao, Q.; Chen, R. Recent advances in drug delivery via the organic cation/carnitine transporter 2 (OCTN2/SLC22A5). Expert opinion on therapeutic targets 2018, 22, 715–726. [Google Scholar] [CrossRef]
- Fink, M.A.; Paland, H.; Herzog, S.; Grube, M.; Vogelgesang, S.; Weitmann, K.; Bialke, A.; Hoffmann, W.; Rauch, B.H.; Schroeder, H.W.S.; et al. L-Carnitine-Mediated Tumor Cell Protection and Poor Patient Survival Associated with OCTN2 Overexpression in Glioblastoma Multiforme. Clinical cancer research : an official journal of the American Association for Cancer Research 2019, 25, 2874–2886. [Google Scholar] [CrossRef]
- Bakos, É.; Német, O.; Patik, I.; Kucsma, N.; Várady, G.; Szakács, G.; Özvegy-Laczka, C. A novel fluorescence-based functional assay for human OATP1A2 and OATP1C1 identifies interaction between third-generation P-gp inhibitors and OATP1A2. The FEBS journal 2020, 287, 2468–2485. [Google Scholar] [CrossRef]
- Higuchi, K.; Sivaprakasam, S.; Sennoune, S.R.; Ogura, J.; Bhutia, Y.D.; Rueda, R.; Pereira, S.L.; Ganapathy, V. A Proton-Coupled Transport System for β-Hydroxy-β-Methylbutyrate (HMB) in Blood-Brain Barrier Endothelial Cell Line hCMEC/D3. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Sadeghzadeh, M.; Wenzel, B.; Gündel, D.; Deuther-Conrad, W.; Toussaint, M.; Moldovan, R.P.; Fischer, S.; Ludwig, F.A.; Teodoro, R.; Jonnalagadda, S.; et al. Development of Novel Analogs of the Monocarboxylate Transporter Ligand FACH and Biological Validation of One Potential Radiotracer for Positron Emission Tomography (PET) Imaging. Molecules 2020, 25. [Google Scholar] [CrossRef]
- Huber, I.; Pandur, E.; Sipos, K.; Barna, L.; Harazin, A.; Deli, M.A.; Tyukodi, L.; Gulyás-Fekete, G.; Kulcsár, G.; Rozmer, Z. Novel cyclic C(5)-curcuminoids penetrating the blood-brain barrier: Design, synthesis and antiproliferative activity against astrocytoma and neuroblastoma cells. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences 2022, 173, 106184. [Google Scholar] [CrossRef] [PubMed]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS letters 1990, 268, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Sim, T.M.; Tarini, D.; Dheen, S.T.; Bay, B.H.; Srinivasan, D.K. Nanoparticle-Based Technology Approaches to the Management of Neurological Disorders. Int J Mol Sci 2020, 21. [Google Scholar] [CrossRef]
- Herce, H.D.; Schumacher, D.; Schneider, A.F.L.; Ludwig, A.K.; Mann, F.A.; Fillies, M.; Kasper, M.A.; Reinke, S.; Krause, E.; Leonhardt, H.; et al. Cell-permeable nanobodies for targeted immunolabelling and antigen manipulation in living cells. Nature chemistry 2017, 9, 762–771. [Google Scholar] [CrossRef]
- Hervé, F.; Ghinea, N.; Scherrmann, J.M. CNS delivery via adsorptive transcytosis. The AAPS journal 2008, 10, 455–472. [Google Scholar] [CrossRef]
- Lu, W.; Sun, Q.; Wan, J.; She, Z.; Jiang, X.G. Cationic albumin-conjugated pegylated nanoparticles allow gene delivery into brain tumors via intravenous administration. Cancer research 2006, 66, 11878–11887. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Sun, Y.; Dong, X. Transthyretin-Penetratin: A Potent Fusion Protein Inhibitor against Alzheimer's Amyloid-β Fibrillogenesis with High Blood Brain Barrier Crossing Capability. Bioconjugate chemistry 2024, 35, 419–431. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, P.; Ma, Z.; Lu, P.; Kebebe, D.; Liu, Z. Combination of cell-penetrating peptides with nanomaterials for the potential therapeutics of central nervous system disorders: a review. Journal of nanobiotechnology 2021, 19, 255. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, Q.; Chen, H.; Yuan, W.; Kuai, R.; Xie, F.; Zhang, L.; Wang, X.; Zhang, Z.; Liu, J.; et al. Comparison of four different peptides to enhance accumulation of liposomes into the brain. J Drug Target 2012, 20, 235–245. [Google Scholar] [CrossRef]
- Liu, L.; Guo, K.; Lu, J.; Venkatraman, S.S.; Luo, D.; Ng, K.C.; Ling, E.A.; Moochhala, S.; Yang, Y.Y. Biologically active core/shell nanoparticles self-assembled from cholesterol-terminated PEG-TAT for drug delivery across the blood-brain barrier. Biomaterials 2008, 29, 1509–1517. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Yu, Z.; Xu, T.; Wang, L.; Meng, N.; Jin, H.; Xu, B. Novel Nano-Drug Delivery System for Brain Tumor Treatment. Cells 2022, 11. [Google Scholar] [CrossRef]
- Ayer, M.; Klok, H.A. Cell-mediated delivery of synthetic nano- and microparticles. Journal of controlled release : official journal of the Controlled Release Society 2017, 259, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.; Javius-Jones, K.; Hong, S.; Park, H. Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform. Int J Nanomedicine 2023, 18, 509–525. [Google Scholar] [CrossRef] [PubMed]
- Stephan, M.T.; Irvine, D.J. Enhancing Cell therapies from the Outside In: Cell Surface Engineering Using Synthetic Nanomaterials. Nano Today 2011, 6, 309–325. [Google Scholar] [CrossRef]
- Yu, H.; Yang, Z.; Li, F.; Xu, L.; Sun, Y. Cell-mediated targeting drugs delivery systems. Drug delivery 2020, 27, 1425–1437. [Google Scholar] [CrossRef]
- He, H.; Ye, J.; Wang, Y.; Liu, Q.; Chung, H.S.; Kwon, Y.M.; Shin, M.C.; Lee, K.; Yang, V.C. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. Journal of controlled release : official journal of the Controlled Release Society 2014, 176, 123–132. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, T.; Li, N.; Gao, J. Cell membrane-based biomimetic vehicles for effective central nervous system target delivery: Insights and challenges. Journal of controlled release : official journal of the Controlled Release Society 2023, 360, 169–184. [Google Scholar] [CrossRef]
- Chai, Z.; Ran, D.; Lu, L.; Zhan, C.; Ruan, H.; Hu, X.; Xie, C.; Jiang, K.; Li, J.; Zhou, J.; et al. Ligand-Modified Cell Membrane Enables the Targeted Delivery of Drug Nanocrystals to Glioma. ACS nano 2019, 13, 5591–5601. [Google Scholar] [CrossRef]
- Karp, J.M.; Leng Teo, G.S. Mesenchymal stem cell homing: the devil is in the details. Cell stem cell 2009, 4, 206–216. [Google Scholar] [CrossRef] [PubMed]
- Elmadany, N.; Alhalabi, O.T.; Platten, M.; Bunse, L. Site-Specific Considerations on Engineered T Cells for Malignant Gliomas. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- Wang, D.; Starr, R.; Chang, W.C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Science translational medicine 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Sabbagh, A.; Beccaria, K.; Ling, X.; Marisetty, A.; Ott, M.; Caruso, H.; Barton, E.; Kong, L.Y.; Fang, D.; Latha, K.; et al. Opening of the Blood-Brain Barrier Using Low-Intensity Pulsed Ultrasound Enhances Responses to Immunotherapy in Preclinical Glioma Models. Clinical cancer research : an official journal of the American Association for Cancer Research 2021, 27, 4325–4337. [Google Scholar] [CrossRef]
- Chen, H.; Ji, J.; Zhang, L.; Chen, T.; Zhang, Y.; Zhang, F.; Wang, J.; Ke, Y. Inflammatory responsive neutrophil-like membrane-based drug delivery system for post-surgical glioblastoma therapy. Journal of controlled release : official journal of the Controlled Release Society 2023, 362, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Tong, H.I.; Kang, W.; Shi, Y.; Zhou, G.; Lu, Y. Physiological function and inflamed-brain migration of mouse monocyte-derived macrophages following cellular uptake of superparamagnetic iron oxide nanoparticles-Implication of macrophage-based drug delivery into the central nervous system. Int J Pharm 2016, 505, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.; Xiang, Y.; Liu, S.; Zhang, Y.; Wan, J.; Ci, Z.; Cui, M.; Shen, L.; Li, N.; Guan, Y. Macrophage membrane modified baicalin liposomes improve brain targeting for alleviating cerebral ischemia reperfusion injury. Nanomedicine : nanotechnology, biology, and medicine 2022, 43, 102547. [Google Scholar] [CrossRef]
- Yuan, X.; Hu, J.; Belladonna, M.L.; Black, K.L.; Yu, J.S. Interleukin-23-expressing bone marrow-derived neural stem-like cells exhibit antitumor activity against intracranial glioma. Cancer research 2006, 66, 2630–2638. [Google Scholar] [CrossRef]
- Li, M.; Sun, S.; Dangelmajer, S.; Zhang, Q.; Wang, J.; Hu, F.; Dong, F.; Kahlert, U.D.; Zhu, M.; Lei, T. Exploiting tumor-intrinsic signals to induce mesenchymal stem cell-mediated suicide gene therapy to fight malignant glioma. Stem cell research & therapy 2019, 10, 88. [Google Scholar] [CrossRef]
- Mangraviti, A.; Tzeng, S.Y.; Gullotti, D.; Kozielski, K.L.; Kim, J.E.; Seng, M.; Abbadi, S.; Schiapparelli, P.; Sarabia-Estrada, R.; Vescovi, A.; et al. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials 2016, 100, 53–66. [Google Scholar] [CrossRef]
- Mooney, R.; Weng, Y.; Tirughana-Sambandan, R.; Valenzuela, V.; Aramburo, S.; Garcia, E.; Li, Z.; Gutova, M.; Annala, A.J.; Berlin, J.M.; et al. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Future oncology (London, England) 2014, 10, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Pavon, L.F.; Sibov, T.T.; de Souza, A.V.; da Cruz, E.F.; Malheiros, S.M.F.; Cabral, F.R.; de Souza, J.G.; Boufleur, P.; de Oliveira, D.M.; de Toledo, S.R.C.; et al. Tropism of mesenchymal stem cell toward CD133(+) stem cell of glioblastoma in vitro and promote tumor proliferation in vivo. Stem cell research & therapy 2018, 9, 310. [Google Scholar] [CrossRef]
- Liu, X.; Tu, M.; Kelly, R.S.; Chen, C.; Smith, B.J. Development of a computational approach to predict blood-brain barrier permeability. Drug metabolism and disposition: the biological fate of chemicals 2004, 32, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Haumann, R.; Videira, J.C.; Kaspers, G.J.L.; van Vuurden, D.G.; Hulleman, E. Overview of Current Drug Delivery Methods Across the Blood-Brain Barrier for the Treatment of Primary Brain Tumors. CNS drugs 2020, 34, 1121–1131. [Google Scholar] [CrossRef] [PubMed]
- Radaram, B.; Pisaneschi, F.; Rao, Y.; Yang, P.; Piwnica-Worms, D.; Alauddin, M.M. Novel derivatives of anaplastic lymphoma kinase inhibitors: Synthesis, radiolabeling, and preliminary biological studies of fluoroethyl analogues of crizotinib, alectinib, and ceritinib. European journal of medicinal chemistry 2019, 182, 111571. [Google Scholar] [CrossRef]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Ye, D.; Zhang, X.; Yue, Y.; Raliya, R.; Biswas, P.; Taylor, S.; Tai, Y.C.; Rubin, J.B.; Liu, Y.; Chen, H. Focused ultrasound combined with microbubble-mediated intranasal delivery of gold nanoclusters to the brain. Journal of controlled release : official journal of the Controlled Release Society 2018, 286, 145–153. [Google Scholar] [CrossRef]
- Burgess, A.; Hynynen, K. Noninvasive and targeted drug delivery to the brain using focused ultrasound. ACS chemical neuroscience 2013, 4, 519–526. [Google Scholar] [CrossRef]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals (Basel, Switzerland) 2021, 14. [Google Scholar] [CrossRef]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T.; et al. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. Journal of controlled release : official journal of the Controlled Release Society 2016, 227, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, M.; Khan, M.W.; Zhai, G. Progress in brain targeting drug delivery system by nasal route. Journal of controlled release : official journal of the Controlled Release Society 2017, 268, 364–389. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, M.; Mathivet, T.; Wauthoz, N.; Rosière, R.; Garg, A.D.; Agostinis, P.; Mathieu, V.; Kiss, R.; Lefranc, F.; Boon, L.; et al. Sensitization of glioblastoma tumor micro-environment to chemo- and immunotherapy by Galectin-1 intranasal knock-down strategy. Scientific reports 2017, 7, 1217. [Google Scholar] [CrossRef]
- Chu, L.; Wang, A.; Ni, L.; Yan, X.; Song, Y.; Zhao, M.; Sun, K.; Mu, H.; Liu, S.; Wu, Z.; et al. Nose-to-brain delivery of temozolomide-loaded PLGA nanoparticles functionalized with anti-EPHA3 for glioblastoma targeting. Drug delivery 2018, 25, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Ramos, J.; Song, S.; Kong, X.; Foroutan, P.; Martinez, G.; Dominguez-Viqueria, W.; Mohapatra, S.; Mohapatra, S.; Haraszti, R.A.; Khvorova, A.; et al. Chitosan-Mangafodipir nanoparticles designed for intranasal delivery of siRNA and DNA to brain. Journal of drug delivery science and technology 2018, 43, 453–460. [Google Scholar] [CrossRef]
- Jahangiri, A.; Chin, A.T.; Flanigan, P.M.; Chen, R.; Bankiewicz, K.; Aghi, M.K. Convection-enhanced delivery in glioblastoma: a review of preclinical and clinical studies. Journal of neurosurgery 2017, 126, 191–200. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef]
- Desjardins, A.; Gromeier, M.; Herndon, J.E., 2nd; Beaubier, N.; Bolognesi, D.P.; Friedman, A.H.; Friedman, H.S.; McSherry, F.; Muscat, A.M.; Nair, S.; et al. Recurrent Glioblastoma Treated with Recombinant Poliovirus. The New England journal of medicine 2018, 379, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Séhédic, D.; Chourpa, I.; Tétaud, C.; Griveau, A.; Loussouarn, C.; Avril, S.; Legendre, C.; Lepareur, N.; Wion, D.; Hindré, F.; et al. Locoregional Confinement and Major Clinical Benefit of (188)Re-Loaded CXCR4-Targeted Nanocarriers in an Orthotopic Human to Mouse Model of Glioblastoma. Theranostics 2017, 7, 4517–4536. [Google Scholar] [CrossRef]
- Wang, J.L.; Barth, R.F.; Cavaliere, R.; Puduvalli, V.K.; Giglio, P.; Lonser, R.R.; Elder, J.B. Phase I trial of intracerebral convection-enhanced delivery of carboplatin for treatment of recurrent high-grade gliomas. PLoS One 2020, 15, e0244383. [Google Scholar] [CrossRef]
- Kunwar, S.; Chang, S.; Westphal, M.; Vogelbaum, M.; Sampson, J.; Barnett, G.; Shaffrey, M.; Ram, Z.; Piepmeier, J.; Prados, M.; et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol 2010, 12, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Fowler, M.J.; Cotter, J.D.; Knight, B.E.; Sevick-Muraca, E.M.; Sandberg, D.I.; Sirianni, R.W. Intrathecal drug delivery in the era of nanomedicine. Adv Drug Deliv Rev 2020, 165-166, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Pang, Y.; Li, L.; Pang, Y.; Zhang, J.; Wang, X.; Raes, G. Applications of nanobodies in brain diseases. Front Immunol 2022, 13, 978513. [Google Scholar] [CrossRef]
- Calias, P.; Banks, W.A.; Begley, D.; Scarpa, M.; Dickson, P. Intrathecal delivery of protein therapeutics to the brain: a critical reassessment. Pharmacology & therapeutics 2014, 144, 114–122. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®--the first FDA-approved nano-drug: lessons learned. Journal of controlled release : official journal of the Controlled Release Society 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Elinzano, H.; Toms, S.; Robison, J.; Mohler, A.; Carcieri, A.; Cielo, D.; Donnelly, J.; Disano, D.; Vatketich, J.; Baekey, J.; et al. Nanoliposomal Irinotecan and Metronomic Temozolomide for Patients With Recurrent Glioblastoma: BrUOG329, A Phase I Brown University Oncology Research Group Trial. American journal of clinical oncology 2021, 44, 49–52. [Google Scholar] [CrossRef]
- Clarke, J.L.; Molinaro, A.M.; Cabrera, J.R.; DeSilva, A.A.; Rabbitt, J.E.; Prey, J.; Drummond, D.C.; Kim, J.; Noble, C.; Fitzgerald, J.B.; et al. A phase 1 trial of intravenous liposomal irinotecan in patients with recurrent high-grade glioma. Cancer chemotherapy and pharmacology 2017, 79, 603–610. [Google Scholar] [CrossRef]
- Beier, C.P.; Schmid, C.; Gorlia, T.; Kleinletzenberger, C.; Beier, D.; Grauer, O.; Steinbrecher, A.; Hirschmann, B.; Brawanski, A.; Dietmaier, C.; et al. RNOP-09: pegylated liposomal doxorubicine and prolonged temozolomide in addition to radiotherapy in newly diagnosed glioblastoma--a phase II study. BMC cancer 2009, 9, 308. [Google Scholar] [CrossRef]
- Maier-Hauff, K.; Ulrich, F.; Nestler, D.; Niehoff, H.; Wust, P.; Thiesen, B.; Orawa, H.; Budach, V.; Jordan, A. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. Journal of neuro-oncology 2011, 103, 317–324. [Google Scholar] [CrossRef]
- Kumthekar, P.; Ko, C.H.; Paunesku, T.; Dixit, K.; Sonabend, A.M.; Bloch, O.; Tate, M.; Schwartz, M.; Zuckerman, L.; Lezon, R.; et al. A first-in-human phase 0 clinical study of RNA interference-based spherical nucleic acids in patients with recurrent glioblastoma. Science translational medicine 2021, 13. [Google Scholar] [CrossRef]
- Kasenda, B.; König, D.; Manni, M.; Ritschard, R.; Duthaler, U.; Bartoszek, E.; Bärenwaldt, A.; Deuster, S.; Hutter, G.; Cordier, D.; et al. Targeting immunoliposomes to EGFR-positive glioblastoma. ESMO open 2022, 7, 100365. [Google Scholar] [CrossRef]
- Thivat, E.; Casile, M.; Moreau, J.; Molnar, I.; Dufort, S.; Seddik, K.; Le Duc, G.; De Beaumont, O.; Loeffler, M.; Durando, X.; et al. Phase I/II study testing the combination of AGuIX nanoparticles with radiochemotherapy and concomitant temozolomide in patients with newly diagnosed glioblastoma (NANO-GBM trial protocol). BMC cancer 2023, 23, 344. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Walker, R.; MacDiarmid, J.; Brahmbhatt, H.; Anazodo, A.; McCowage, G.; Gifford, A.J.; Kavallaris, M.; Trahair, T.; Ziegler, D.S. A Phase 1 Study of Intravenous EGFR-ErbituxEDVsMIT in Children with Solid or CNS Tumours Expressing Epidermal Growth Factor Receptor. Targeted oncology 2024, 19, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Synold, T.W.; Badie, B.; Tirughana, R.; Lacey, S.F.; D'Apuzzo, M.; Metz, M.Z.; Najbauer, J.; Bedell, V.; Vo, T.; et al. Neural Stem Cell-Based Anticancer Gene Therapy: A First-in-Human Study in Recurrent High-Grade Glioma Patients. Clinical cancer research : an official journal of the American Association for Cancer Research 2017, 23, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Portnow, J.; Badie, B.; Suzette Blanchard, M.; Kilpatrick, J.; Tirughana, R.; Metz, M.; Mi, S.; Tran, V.; Ressler, J.; D'Apuzzo, M.; et al. Feasibility of intracerebrally administering multiple doses of genetically modified neural stem cells to locally produce chemotherapy in glioma patients. Cancer gene therapy 2021, 28, 294–306. [Google Scholar] [CrossRef]
- Fares, J.; Ahmed, A.U.; Ulasov, I.V.; Sonabend, A.M.; Miska, J.; Lee-Chang, C.; Balyasnikova, I.V.; Chandler, J.P.; Portnow, J.; Tate, M.C.; et al. Neural stem cell delivery of an oncolytic adenovirus in newly diagnosed malignant glioma: a first-in-human, phase 1, dose-escalation trial. Lancet Oncol 2021, 22, 1103–1114. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: structure, regulation, and drug delivery. Signal transduction and targeted therapy 2023, 8, 217. [Google Scholar] [CrossRef]
- Pardridge, W.M. CNS drug design based on principles of blood-brain barrier transport. Journal of neurochemistry 1998, 70, 1781–1792. [Google Scholar] [CrossRef]
- Song, X.; Qian, H.; Yu, Y. Nanoparticles Mediated the Diagnosis and Therapy of Glioblastoma: Bypass or Cross the Blood-Brain Barrier. Small (Weinheim an der Bergstrasse, Germany) 2023, e2302613. [Google Scholar] [CrossRef]
- Gao, H. Progress and perspectives on targeting nanoparticles for brain drug delivery. Acta Pharm Sin B 2016, 6, 268–286. [Google Scholar] [CrossRef]
- van Tellingen, O.; Yetkin-Arik, B.; de Gooijer, M.C.; Wesseling, P.; Wurdinger, T.; de Vries, H.E. Overcoming the blood-brain tumor barrier for effective glioblastoma treatment. Drug Resist Updat 2015, 19, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Li, J.Y.; Boado, R.J.; Pardridge, W.M. Blood-brain barrier genomics and cloning of a novel organic anion transporter. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 2008, 28, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.F. Importance of P-glycoprotein at blood-tissue barriers. Trends in pharmacological sciences 2004, 25, 423–429. [Google Scholar] [CrossRef]
- Peer, D.; Karp, J.M.; Hong, S.; Farokhzad, O.C.; Margalit, R.; Langer, R. Nanocarriers as an emerging platform for cancer therapy. Nature nanotechnology 2007, 2, 751–760. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Liu, Z.; Hirvonen, J.; Santos, H.A. Bridging the Knowledge of Different Worlds to Understand the Big Picture of Cancer Nanomedicines. Advanced healthcare materials 2018, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhang, X.; Huang, J.; Feng, K.; Zhang, Y.; Wu, J.; Ma, L.; Zhu, A.; Di, L. Bio-fabricated nanodrugs with chemo-immunotherapy to inhibit glioma proliferation and recurrence. Journal of controlled release : official journal of the Controlled Release Society 2023, 354, 572–587. [Google Scholar] [CrossRef]
- Zhan, Q.; Yi, K.; Cui, X.; Li, X.; Yang, S.; Wang, Q.; Fang, C.; Tan, Y.; Li, L.; Xu, C.; et al. Blood exosomes-based targeted delivery of cPLA2 siRNA and metformin to modulate glioblastoma energy metabolism for tailoring personalized therapy. Neuro Oncol 2022, 24, 1871–1883. [Google Scholar] [CrossRef]
| Strategies to cross the BBB | Targeting | NDDS | Drug | Combination therapy (if any) | Administration route | Administration model |
Safety Evaluation | Pharmacokinetics (PK) | Pharmacodynamics | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
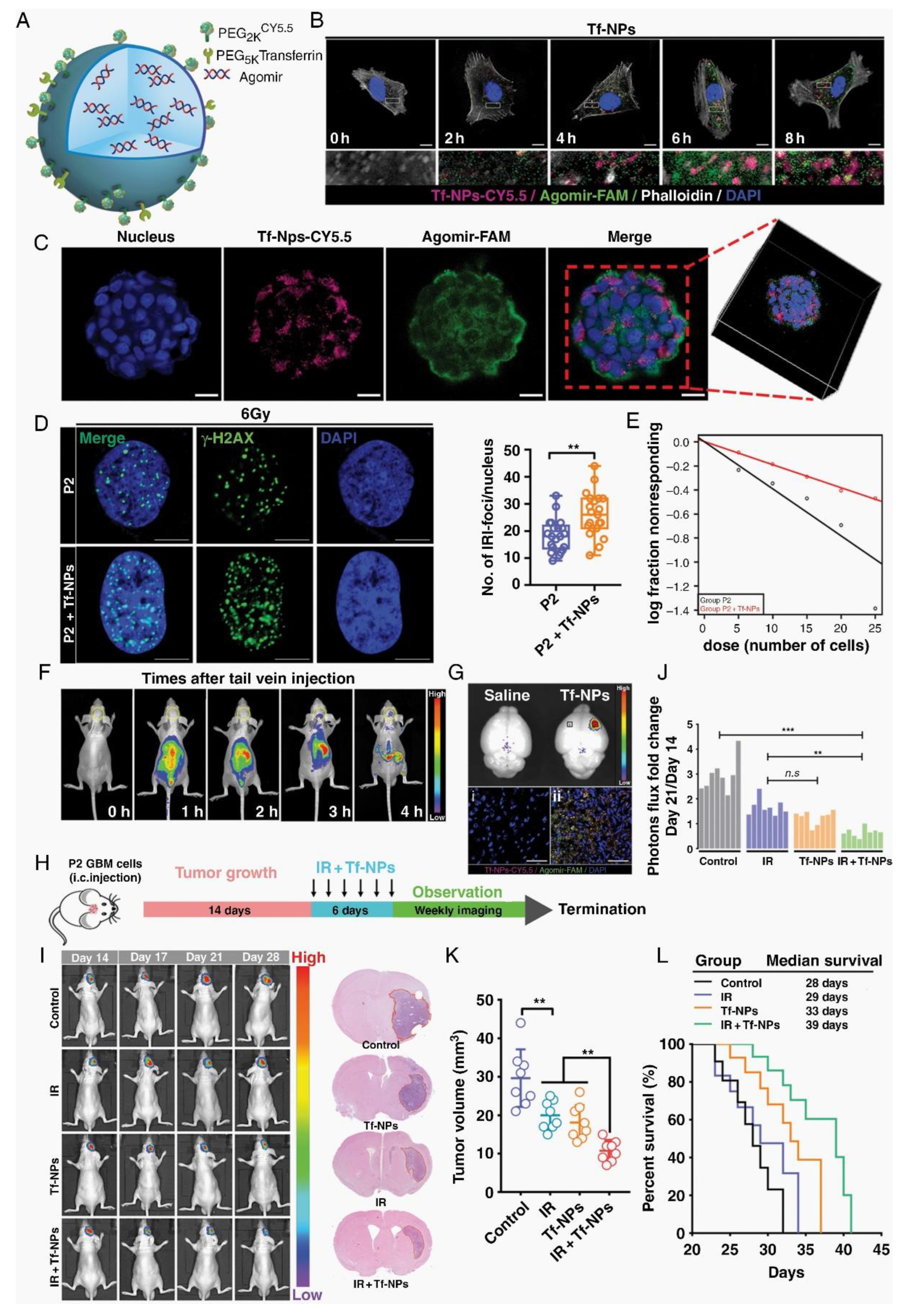
| RMT | Tf | Tf-NPs | TMZ | bromodomain inhibitor | tail vein injection | the human U87MG and murine GL261 glioma models | compare with the control group, mice treated with drug-loaded Tf-NPs maintained their body weight and body conditioning scores, and had significantly more stable WBC and PLT levels over the treatment course. | 24 h absorption efficiency was higher (13% vs > 1%). Accumulation and retention of Tf-NPs on the tumor surface, while non-functional NPs did not. NPs loaded with TMZ demonstrated an attenuated release profile with ~90% release by 48 h. | Tf-NPs bind to GBM tumors, enhance DNA damage & apoptosis, reduce tumor burden, improve survival, protect from systemic toxicity. | [12] |
| RMT | Lf | PMO-Lf@Dox | DOX | - | co-incubation | C6 cells | PMO concentration is 200 μg/mL, the cell survival rate is 90%,the hemolysis rate is< 2%. one week after tail vein injection of 60 mg/kg PMO, no obvious changes such as heart, liver, spleen, lung and kidney are seen. |
PMO-Lf@Dox-treated C6 cells showed significantly higher uptake after 24 hours than PMO@Dox-treated cells. | Lf-modified PMO enhances the inhibitory effect of Dox on C6 cells. | [13] |
| RMT | Lf | USLP-NH2-PEG-TMZ-Lf | TMZ | - | tail vein injection | transwell model with hCMEC/D3 cells; Balb/C mice |
Safety is evaluated by H&E staining of mouse organs (brain, kidney, lung, liver) post-nano-formulation IV injection, with no toxicity observed in 24 hours. | USLP-NH2-Cy5-PEG-Lf accumulated rapidly in the brain and reached its peak at 1 h after administration, and the USLP formula significantly reduces the external alignment. | In vitro apoptosis studies on GBM cell lines U87 and GL261 showed improved TMZ-induced apoptosis with USLP formulations compared to pure TMZ. | [14] |
| RMT | acetylcholine receptor | TMZ@RVG-Zein NPs | TMZ | - | co-incubation | U87 cell lines; BBB model with bEnd.3 cells | The TMZ@RVG-Zein NPs exhibited excellent stability, without causing significant side effects. | The TMZ@RVG-Zein NPs had an encapsulation efficiency (EE) of 77.9 ± 4.7% and a loading efficiency (LE) of 66.7 ± 2.9 mg/g. | The TMZ@RVG-Zein NPs have cytotoxic effects on U87 cells, induce apoptosis, and show enhanced cellular uptake compared to TMZ alone. | [15] |
| RMT | FR | iRPPFFA@TMZ | TMZ | - | tail vein injection | subcutaneous and orthotopic xenograft tumor models | The study evaluated the biocompatibility of POSS nanoparticles through a CCK-8 assay, hemolysis rate tests, and fluorescence imaging, showing low toxicity and effective cellular uptake for biomedical applications. | The multifunctional POSS nanoparticles demonstrated high stability with no significant fluorescence intensity change over 8 weeks, while the half-life of iRPPFFA nanoparticles was estimated at approximately 16 weeks. | In vivo studies showed that TMZ-loaded POSS nanoparticles significantly improved the survival of GBM-bearing mice, indicating enhanced therapeutic efficacy compared to monotherapy. | [16] |
| RMT | LRP-1 receptors | Ti@FeAu–Ang nanoparticles | Magnetic nanoparticles as therapeutic agents | - | tail vein injection | Rat GBM model | Preliminary safety analysis highlighted no toxicity to the hematological system after Ti@FeAu–Ang nanoparticle-induced hyperthermia treatment. Immunohistochemical analysis showed no significant organ damage and biological changes in vital organs such as the heart, liver, spleen, lungs, and kidneys. | The paper does not provide detailed pharmacokinetic data, but it does mention that the nanoparticles were prepared at various concentrations for the temperature elevation test and the in vivo assessment of tumor growth. | The Ti@FeAu–Ang nanoparticles demonstrated a temperature elevation of up to 12°C upon magnetic stimulation, indicating potential applications in MRI and hyperthermia-based cancer therapy. In vitro, the nanoparticles showed improved cytotoxicity up to 85% due to hyperthermia produced by a magnetic field. In vivo findings showed a 10-fold decrement in tumor volume compared to the control group. | [17] |
| RMT | LRP-1 receptors | Au-DOX@PO-ANG | DOX | radiotherapy | tail vein injection | U87-MG human GBM xenografts in nude mice | In vivo, blood biochemical indicators (CK-MB, AST, and Scr) were measured, and no significant pathological changes were observed in the main organs (heart, liver, spleen, lung, and kidney) of the Au-DOX@PO-ANG group compared to the PBS group. In vitro, the cytotoxicity of modified AuNPs was evaluated using CCK-8 assays, showing more than 80% cell viability at concentrations up to 10 mg/L, indicating non-toxicity to GBM cells. | The storage stability of the modified AuNPs in PBS (pH 7.4) at 4 °C showed little change in particle size over 4 weeks, indicating good storage ability and potential stability. | In vitro, the antitumor activity of Au-DOX@PO-ANG was evaluated using the CCK-8 assay, showing significant antitumor effects when combined with radiotherapy. In vivo, the therapeutic effect was observed through MRI, with the tumor volume rate of increase slowing in the Au-DOX@PO-ANG + RT group, and a significant increase in cell apoptosis was observed in this group, consistent with MRI data. | [18] |
| RMT | EGFR | PmAb-TMZ-PLGA-NPs | TMZ | Panitumumab | co-incubation | U-87MG and LN229 GBM cell lines. | In Vitro Cytotoxicity: Assessed using the Live/Dead assay kit and fluorescence-activated cell sorting (FACS). Immunoreactivity: Evaluated using EGFR-overexpressed U-87MG cells. |
In Vitro Drug Release: Studied using phosphate-buffered saline (PBS) at acidic (pH 5.0) and neutral (pH 7.4) conditions. Release Profile: Determined by UV-vis spectroscopy at various time points. |
Enhanced in U-87MG cells due to high EGFR expression compared to LN229 cells. | [19] |
| RMT | IGF1R | IGF1R4-mFc | Galanin Peptide | - | ISBP | Rat Model; Hargreaves Pain Model | No specific safety evaluation was described in detail. However, the use of sdAbs, which are known for their low immunogenicity and high solubility/stability, suggests potential safety advantages. | Brain Uptake: IGF1R4-mFc showed significant brain uptake compared to the negative control A20.1-mFc, with ~25% of the total amount accumulating in the brain parenchymal fraction post-ISBP. Linear Accumulation Plateau: The concentration curve for IGF1R4-mFc demonstrated a linear accumulation plateauing at approximately 400 µg (~1 µM), suggesting a saturable mechanism of transport. | Analgesic Effect: Systemic administration of IGF1R4-mFc fused with galanin induced a dose-dependent suppression of thermal hyperalgesia in the Hargreaves pain model, indicating pharmacological effectiveness of the brain-delivered cargo. | [20] |
| RMT | α5β1 integrin receptor | RGEK-lipopeptide containing coencapsulated STAT3siRNA and WP1066 | WP1066; STAT3siRNA | tail vein injection | intracranial orthotopic GL261 GBM model in C57BL/6J male mice | No Direct Safety Data: The document did not provide direct safety evaluation data. However, the use of integrin receptor-selective liposomes and their preferential accumulation in tumor tissue suggests potential for reduced systemic toxicity. | Preferential Accumulation: NIR-dye-labeled α5β1 integrin receptor-selective liposomes were found to accumulate preferentially in mouse brain tumor tissue after intravenous administration. Encapsulation Efficiency: Entrapment efficiency (%EE) for WP1066 was measured using analytical HPLC. |
Inhibition of GBM Growth: Coadministration of WP1066 and STAT3siRNA within RGDK-lipopeptide-based liposomes led to significant inhibition (>350% compared to untreated mice group) of orthotopically growing mouse GBM. | [21] | |
| RMT | αv β integrin and NRP-1 | CPT-S-S-PEG-iRGD@IR780 micelles | Camptothecin (CPT) | photodynamic therapy | tail vein injection | Intracranial orthotopic U87MG gliomas tumor model in Balb/c nude mice | The micelles were shown to be stable with controlled drug release under physiological conditions. Toxicity studies in vivo assessed mice's survival, body weight, and histopathology. | Information on the pharmacokinetic behavior of the micelles is not explicitly detailed in the abstract. However, the micelle design enables sustained release of CPT upon exposure to high glutathione levels in glioma cells. | The CPT-S-S-PEG-iRGD@IR780 micelles displayed significantly enhanced antitumor effects with laser irradiation, as compared to controls. Micelles with iRGD demonstrated favorable targeting ability to glioma cells and deep tumor penetration. | [22] |
| RMT | Hsp70 | D-A-DA/TPP | DOX | PD-1 Checkpoint Blockade | tail vein injection | C6-luc tumor-bearing mice | In vivo Toxicity Assessment: Safety evaluation is likely conducted through monitoring of animal health, blood chemistry, and tissue histology post-treatment. However, specific details are not provided. | Biodistribution and Clearance: The biodistribution and clearance of D-A-DA/TPP nanoparticles in vivo are not explicitly discussed in the excerpt. These would typically involve measuring nanoparticle concentrations in blood, tumor, and other organs over time. | Efficacy Evaluation: The efficacy of D-A-DA/TPP nanoparticles in inducing glioma apoptosis and prolonging median survival time is demonstrated through in vivo studies. Immune Response Activation: Combination with PD-1 checkpoint blockade is shown to further activate T cells and provoke an anti-tumor immune response. |
[23] |
| RMT | dopamine receptor and GRP78 receptor | pHA-AOHX-VAP-DOX | DOX | - | tail vein injection | intracranial U87 gliomabearing nude mice model | The maximum tolerated dose (MTD) was determined in healthy BALB/c mice. Hematoxylin-eosin (H&E) staining of major organs and blood samples were collected to measure blood routine and biochemical parameters. | The study evaluated the biodistribution of the conjugates in the tumor and normal tissues. The accumulation of DOX in the tumor site was assessed, and the conjugation with peptides reduced the accumulation of DOX in normal tissues. | The anti-GBM efficacy was evaluated by prolonging the survival time of mice and assessing tumor cell apoptosis through TUNEL staining. The inhibition of tumor angiogenesis was evaluated using CD31 immunofluorescence staining. | [24] |
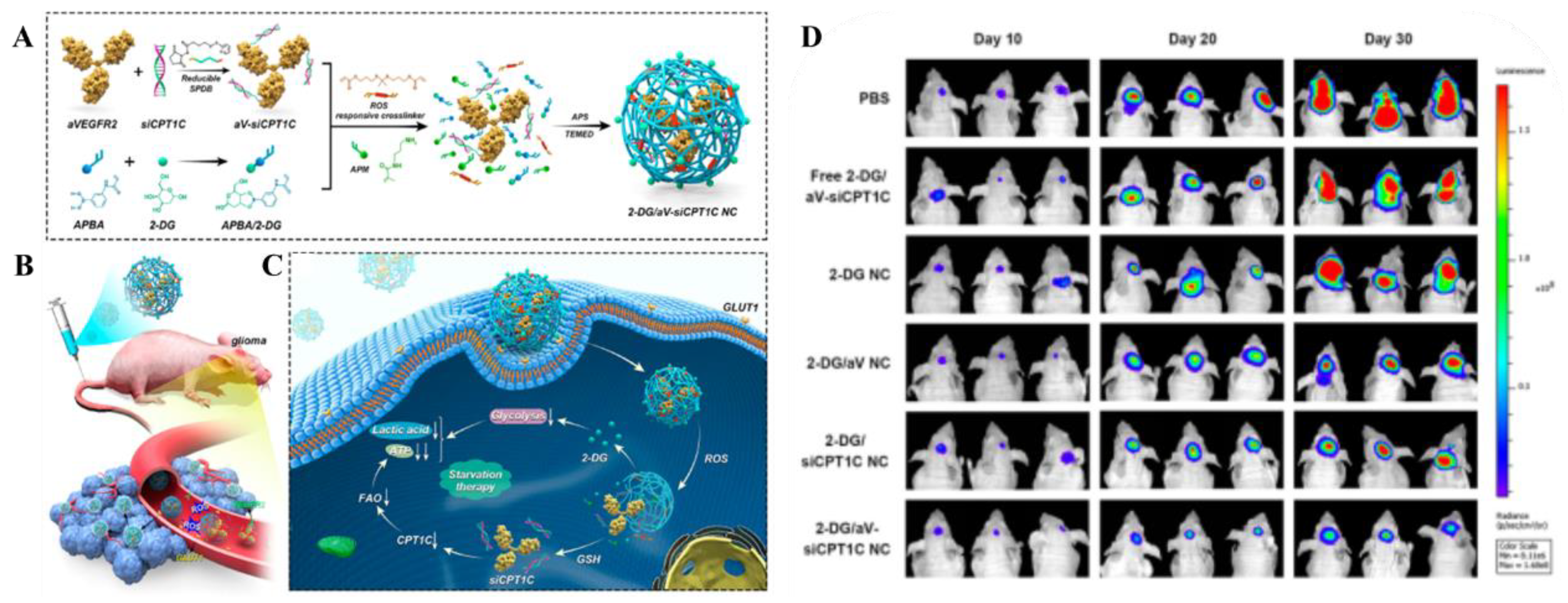
| TMT | GLUT1 | 2-DG/aV-siCPT1C NC | 2-DG | siCPT1C | tail vein injection | Orthotopic U87-Luci cells xenograft tumor model in BALB/c nude mice | The safety of the nanocapsules was evaluated through histopathologic studies and blood biochemical tests, including ALT, AST, BUN, and CREA levels. No significant toxicity or side effects were observed on normal cells and tissues. | The nanocapsules showed an extended half-life compared to free siRNA, with the 2-DG/aV-Cy5-siCPT1C NC having an elimination half-life (t1/2) of approximately 1.2 hours. | The nanocapsules effectively targeted GBM, inhibited energy metabolism, and showed a significant inhibitory effect on the growth of GBM. The combination of 2-DG, aV, and siCPT1C resulted in decreased lactic acid levels and reduced ATP production in tumor tissues, indicating effective metabolic pathway inhibition. | [25] |
| TMT | ChTs | pMPC-co-(anti-PD-L1-pPEGMA) | anti-PD-L1 | - | intravenous injection | LCPN orthotopic glioma tumor model | The safety and biocompatibility of the delivery system were evaluated through in vitro cytotoxicity studies using bEnd. 3 cells and LCPN cells, as well as histological examination of major organs from mice. | The PK of the Cy5.5-labeled IgG in the anti-PD-L1-MP-3 formulation was studied, showing prolonged blood circulation time compared to non-choline containing controls. | The system demonstrated pH-responsive protein release in vitro, with accelerated release at pH 6.0 simulating the acidic tumor microenvironment. In vivo studies showed significant tumor growth suppression and prolonged animal survival, indicating the activation of antitumor immune responses. | [26] |
| TMT | LAT1 | WP1066-loaded liposomes of Amphi-DOPA | wp1066 | DNA vaccine | tail vein injection | orthotopic GL261 tumor model in famale C57BL/6J mice | The safety and toxicity of the liposomal formulation are evaluated through in vivo serum toxicity profiles. No significant changes in biochemical and hematological parameters suggest the system is well-tolerated. | Preferential Accumulation in Brain Tissue: Intravenously administered NIR-dye labeled Amphi-DOPA liposomes showed preferential accumulation of the dye in brain tissue, indicating successful BBB penetration. | Enhanced Overall Survivability: WP1066-loaded Amphi-DOPA Liposomes Alone: Enhanced overall survivability of C57BL/6J mice bearing orthotopically established mouse GBM by ~60% compared to untreated mice. Combination Therapy: Further enhanced overall survivability (>300% compared to untreated mice) when combining WP1066-loaded Amphi-DOPA liposomes with in vivo DC-targeted DNA vaccination using a survivin-encoded DNA vaccine. |
[27] |
| TMT | SVCT2 | PTX-Glu-Vc-Lip | PTX | - | tail vein injection | intracranial C6 glioma bearing mice | The safety of the ligand-modified liposomes is demonstrated through hemolysis assays, showing no significant increase in hemocompatibility even at high concentrations of phospholipids. | The study evaluates the plasma concentration-time profiles and brain distribution of paclitaxel after intravenous injection of different liposome formulations. The pharmacokinetic parameters, including AUC(0-t), MRT, Tmax, Cmax, and t1/2, are reported for each formulation. | The cellular uptake of the liposomes is evaluated in GLUT1- and SVCT2-overexpressed C6 cells, showing higher uptake for the Glu-Vc-Lip compared to other formulations. The in vivo imaging of DiD-loaded liposomes demonstrates the targeting efficiency to the brain tumor site. | [28] |
| TMT | OCTN2 | LC-1000-PLGA NPs | PTX | - | tail vein injection | 2 D and 3 D tumor growth models using the glioma cell line T98G. | The specific dose is not explicitly mentioned in the provided text. However, the studies involve the use of different concentrations of paclitaxel in the in vitro cytotoxicity assays and varying lengths of PEG spacers in the nanoparticles. | Biodistribution: LC-PLGA NPs showed high accumulation in the brain as indicated by biodistribution and imaging assays in mice. In Vitro Release: Paclitaxel-loaded LC-PLGA NPs showed sustained release of paclitaxel compared to Taxol. | The pharmacodynamic evaluation includes in vitro cytotoxicity assays in T98G cells, demonstrating increased toxicity with LC-PLGA NPs compared to Taxol and paclitaxel-loaded PLGA NPs, and in vivo biodistribution studies showing enhanced brain accumulation of paclitaxel with LC-1000-PLGA NPs. | [29] |
| TMT | Cx43 and BSAT1 | Cx43-NG/CDDP and BSAT1-NG/CDDP | cisplatin | - | tail vein injection | intracranial implantation of rat GBM 101/8 in female Wistar rats | Safety is evaluated by monitoring body weight changes, general condition of the animals, and comparing the median survival rates of the different treatment groups. | The PK of the nanogels is assessed by monitoring the tumor volume changes over time using MRI and comparing the median survival times of the treated groups with the control group. | The antitumor efficacy of the targeted nanogels is evaluated by comparing the glioma volume and the survival rate of rats treated with targeted nanogels conjugated with specific mAbs against Cx43 and BSAT1 to those treated with non-targeted nanogels or free cisplatin. The study demonstrates significantly reduced tumor growth and increased lifespan in animals treated with targeted nanogels. | [30] |
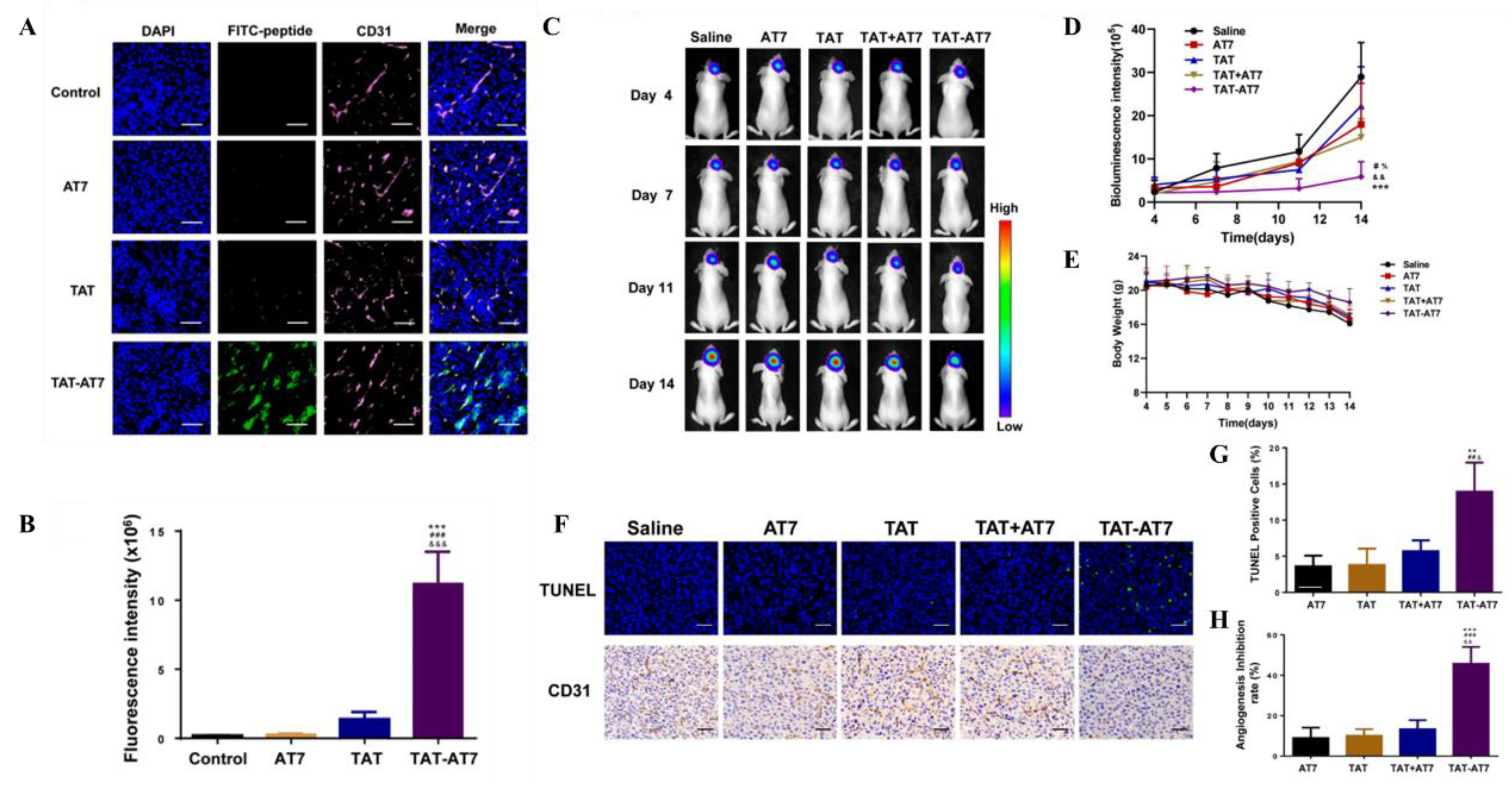
| AMT | TAT | TAT-AT7-modified PEI nanocomplex | secretory endostatin gene | - | intravenous injection | orthotopic U87-glioma-bearing nude mice model | TAT-AT7 showed no obvious hemolysis at concentrations ranging from 2.5 to 640 µmol/L, indicating good biosafety. | The study evaluated the cellular uptake of TAT-AT7 in bEnd3 cells (mouse brain microvascular endothelial cells) and its distribution in an intracranial glioma model, demonstrating high uptake efficiency and penetration capability. | TAT-AT7 exhibited significant inhibitory effects on HUVECs' proliferation, migration, invasion, and tubular structure formation. It also promoted apoptosis in HUVECs and inhibited zebrafish embryo angiogenesis. In vivo, TAT-AT7 significantly suppressed glioma growth, induced apoptosis of glioma cells, and inhibited angiogenesis. | [31] |
| CMT+ RMT | RF + active targeting | LMP tFNA NPs | LMP | - | tail vein injection | orthotopic U87-glioma-bearing nude mice model | In vitro and in vivo safety evaluations showed that RFA NPs had no significant cytotoxic effects on primary hepatocytes, astrocytes, or GBM cells, and no obvious immune side effects were observed in vivo. | Prolonged Circulation Time: Biomimetic NPs encapsulated with natural cell membranes prolonged the circulation time of the drug in vivo. Efficient BBB Permeability: The hybrid membrane coating facilitated efficient crossing of the BBB. |
Inhibition of GBM Growth: LMP loaded RFA NPs exhibited superior and specific anti-GBM activities in vitro and in vivo. Apoptosis/Pyroptosis Induction: LMP induced apoptosis and pyroptosis in GBM cells, reducing tumor growth. Reduced Off-Target Delivery: The RFA NPs demonstrated reduced off-target drug delivery, ensuring specificity. |
[32] |
| CMT | Active Targeting | Ang-RBCm@NM-(Dox/Lex) | Dox | Lexiscan (Lex) | Intravenous injection | orthotopic U87MG human GBM tumor-bearing nude mice | Cell Viability Studies: The nanomedicine was evaluated for cytotoxicity using in vitro cell viability assays. In vivo Toxicity Studies: While specific toxicity data are not detailed in the abstract, in vivo studies assessed the therapeutic efficacy and survival outcomes, which indirectly reflect safety. | The nanomedicine demonstrates a prolonged blood circulation time, with an elimination half-life (t1/2,β) of 9.3 hours for Ang-RBCm@NM-(Dox/Lex), which is longer than the RBCm@NM-(Dox/Lex) counterpart (t1/2,β = 7.8 hours). | Superior BBB Penetration: The angiopep-2 functionalization and Lex-mediated BBB opening facilitated superb penetration across the BBB. Effective Tumor Suppression: Treatment with Ang-RBCm@NM-(Dox/Lex) resulted in effective suppression of tumor growth and significantly improved median survival time in orthotopic U87MG human GBM tumor-bearing nude mice. |
[33] |
| CMT | Active Targeting | iRGD-EM:TNDs | TMZ | - | tail vein injection | orthotopic U87MG human GBM tumor-bearing nude mice | Safety is evaluated through hematoxylin and eosin (H&E) pathological staining of major organs, monitoring of body weight, and detection of IgE levels associated with hypersensitivity reactions. The results suggest low systemic toxicity and good biocompatibility of the iRGD-EM:TNDs. | The EM-coated nanodots demonstrate a longer elimination half-life, suggesting reduced degradation in vivo compared to traditional PEG stealth motifs. | The iRGD-EM:TNDs show enhanced cellular uptake, improved penetration in multicellular tumor spheroids, and increased transport ratios across the BBB in vitro and in vivo. The treatment with iRGD-EM:TNDs results in a 100% survival rate after 30 days post-tumor implantation and induces the highest level of cell apoptosis. | [34] |
| CMT | IL13Rα2 | T cells + BPLP-PLA-NPs (clicked) | DOX | CAR T cell therapy | tail vein injection | intracranial xenograft model using female immunodeficient nude mice | The safety evaluation includes assessing the cytotoxic effects of the nanoparticles and T cells on GBM cells in vitro and observing the behavior of T cells in vivo without causing observable side effects. | The study evaluates the retention of nanoparticles on T cells for at least 8 days, indicating the stability of the linkage for a suitable time window for in vivo delivery. | The system demonstrates enhanced cytotoxic effects in vitro with T cells clicked with doxorubicin-loaded nanoparticles compared to bare T cells. In vivo, T cells expressing TQM-13 serve as delivery shuttles for nanoparticles, significantly increasing the number of nanoparticles reaching brain tumors compared to nanoparticles alone. | [35] |
| CMT | inflammatory tumor microenvironment | NEs-Exos/DOX | DOX | - | intravenous injection | C6-Luc glioma-bearing mice models | The safety evaluation of the NEs-Exos/DOX system is not detailed in the provided text. The focus is on the efficacy of the system in crossing the BBB and targeting glioma cells. | The study does not provide specific pharmacokinetic data for the NEs-Exos/DOX system. However, it does mention that the NEs-Exos can rapidly penetrate the BBB and migrate into the brain, suggesting favorable pharmacokinetic properties for brain tumor targeting. | The pharmacodynamics of the NEs-Exos/DOX system are demonstrated through in vitro and in vivo assays, showing that the system can improve the anticancer efficacy of DOX, reduce mortality, and effectively suppress tumor growth while prolonging survival time in a glioma mouse model. | [36] |
| CMT | domain CLTX, and IgG4 hinge | CAR-neutrophils@RSiO2-TPZ | tirapazamine (TPZ) | - | intravenous injection | mouse xenograft model of GBM | Biocompatibility: CAR-neutrophils exhibited high biocompatibility with normal cells (SVG p12 glial cells, hPSCs, and hPSC-derived cells). Toxicity: Necrosis was not observed in major organs of experimental mice. However, concerns regarding off-target tissue toxicity or systemic toxicity were mentioned. | Nanoparticle delivery efficiency: CAR-neutrophils delivered >20% of administered nanodrugs to brain tumors compared to 1% by free nanodrugs. | In vitro cytotoxicity: CAR-neutrophils presented enhanced anti-tumor cytotoxicity compared to peripheral blood (PB) neutrophils. In vivo tumor growth inhibition: CAR-neutrophils loaded with TPZ-loaded SiO2 nanoparticles significantly inhibited tumor growth and prolonged animal survival in GBM xenograft models. Mechanism: Combination of CAR-enhanced direct cytolysis and chemotherapeutic-mediated tumor-killing via cellular uptake and glutathione (GSH)-induced degradation of nanoparticles within tumor cells. |
[37] |
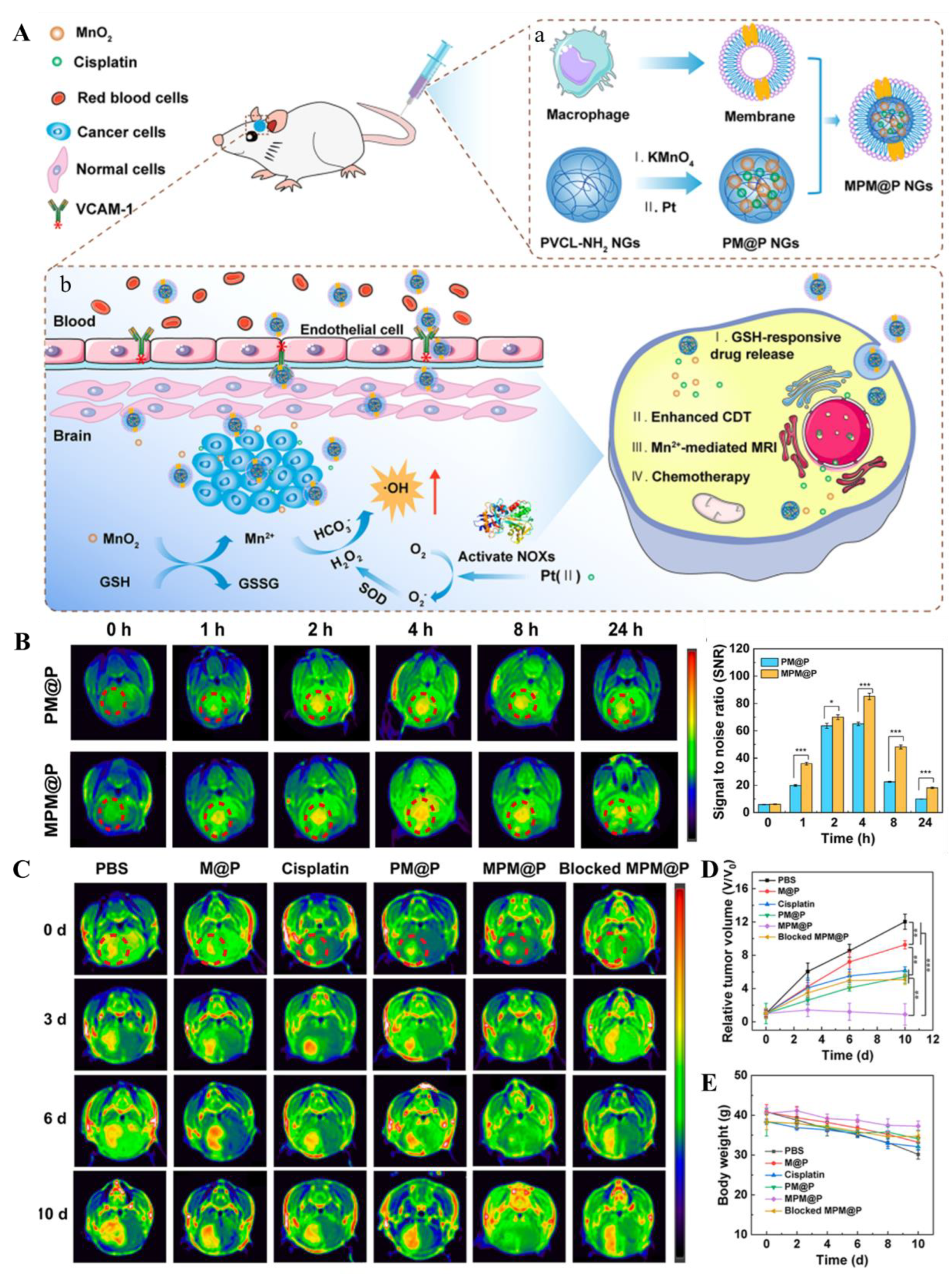
| CMT | Active Targeting | MPM@P NGs | cisplatin | chemodynamic therapy (CDT) | intravenous injection | orthotopic C6 glioma in a mouse model | The safety of the nanogels is evaluated through in vitro hemolysis assays, in vivo hematological indices, blood biochemical analysis, and histopathological examination of major organs. | The PK of the nanogels is assessed by tracking the platinum (Pt) content in blood after intravenous injection. The half-life of MPM@P NGs is determined to be longer than that of PM@P NGs without membrane coating, indicating improved blood circulation time. | The in vivo antitumor activity of the nanogels is evaluated by monitoring tumor volume changes using T1-weighted MR imaging. The MPM@P NGs demonstrate the smallest tumor size and most efficient therapeutic effect among all groups due to the combination of enhanced CDT and chemotherapy, as well as improved BBB crossing and glioma targeting ability. | [38] |
| CMT | Active Targeting | aDCM@PLGA/RAPA | RAPA | immunotherapeutic activation | tail vein injection | orthotopic C6 tumor model | Biocompatibility Tests: Studies investigating the biocompatibility and potential toxicity of aDCM@PLGA/RAPA in vivo and in vitro were mentioned but specific details were not provided. | Stability Studies: aDCM@PLGA/RAPA demonstrated good colloidal stability in PBS and plasma, suggesting the potential for prolonged circulation time in the blood. | Immune Activation: aDCM@PLGA/RAPA effectively activated T cells and NK cells, modifying the tumor microenvironment to an immune-supportive state. Antitumor Efficacy: The combined immunotherapeutic and chemotherapeutic effects led to significant inhibition of glioma growth and induced glial differentiation. | [39] |
| CMT | Active Targeting | nanoparticle-engineered TRAIL-expressing hADSCs | TRAIL | - | local intracranial delivery | mouse intracranial xenograft model of patient-derived GBM cells | Cell Viability: MTS assay revealed no significant change in viability of hADSCs transfected with nanoparticle-laden TRAIL DNA compared to controls, indicating safety. The in vivo safety is assessed by monitoring the survival and weight changes of the mice. | The PK of the TRAIL-overexpressing hADSCs is evaluated by observing the migration and infiltration of the cells towards GBM tumors in vivo and by measuring the levels of TRAIL protein expression in vivo. | The study demonstrates that TRAIL-overexpressing hADSCs induce significant apoptosis in GBM cells in vitro and in vivo, with negligible apoptotic activity in normal brain cells. The therapeutic effects include inhibition of tumor growth, extension of animal survival, and reduction of tumor mass and microsatellites occurrence. | [40] |
| CMT | Active Targeting | PEI-PLL-transfected MSCs | suicidal genes, namely, HSV-TK and TRAIL | ganciclovir | intratumoral injection | SD rats were used with C6 glioma cells injected intracranially | Cell Viability: Cell viability of MSCs transfected with the PEI-PLL copolymer was evaluated using the MTT assay. Cell viability was more than 90% at a pDNA/polymer ratio of 1:1.5 for the PEI-PLL copolymer. In vivo Toxicity: While not explicitly stated in the abstract or methods section, the use of nonviral vectors like PEI-PLL copolymer is generally considered safer than viral vectors due to their low immunogenicity and reduced risk of oncogenicity. |
The PK of the system is assessed by monitoring the survival rates and tumor growth in the animal model after treatment with the PEI-PLL-transfected MSCs. | The study demonstrates that the combination of HSV-TK and TRAIL genes in MSCs leads to a significant decrease in cell viability and an increase in apoptosis in glioma cells, both in vitro and in vivo. The reduction in cell proliferation markers Ki67 and angiogenesis marker VEGF, along with the TUNEL assay results, indicate the therapeutic effectiveness of the MSCs in inducing apoptosis in GBM cells. | [41] |
| CMT | Active Targeting | ApoE-ARTPC@TMZ | ART | TMZ | tail vein injection | orthotopic U251-TR GBM mouse model | Conducted in vivo experiments to assess the safety of the nanoplatform, including examination of body weight, blood cell counts (RBC, WBC, PLT), and histological examination of major organs and brain tissue. | Evaluated the circulation time of the liposomes in the bloodstream using FITC-Dex as a marker, showing prolonged circulation times for ApoE-ARTPC@FITC-Dex and ARTPC@FITC-Dex compared to free FITC-Dex. | Assessed the induction of apoptosis, DNA damage, and inhibition of MGMT expression and Wnt/β-catenin signaling. The combination therapy showed enhanced cytotoxicity, increased ROS generation, and significant induction of apoptosis in vivo. | [42] |
| Methods to overcome the BBB | Drug |
Combination therapy (if any) |
Research Phases | NDDS | Administration route |
Safety Evaluation | PK | Primary Efficacy Endpoints | ClinicalTrials.Gov Identifier | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Passive diffusion | DOX | TMZ + radiotherapy |
Phase Ⅱ | Caelyx™, PEG-Dox | Intravenous infusion + Oral Administration |
The treatment was well-tolerated, with most AEs classified as grade 1-2. However, some grade 3-4 AEs were also reported. | PK of PEG-Dox and Temozolomide were not specifically detailed in the document summary provided. However, the improved BBB penetration of PEG-Dox suggests enhanced PK compared to conventional doxorubicin. | Progression Free Survintravenous infusional at 12 Months (PFS-12): 30.2% in all patients. Median Overall Survival (mOS): 17.6 months in all patients including those from Phase I. Comparison to Historical Control: The endpoints did not differ significantly from the EORTC26981/NCIC-CE.3 data in a post-hoc statistical comparison. |
NCT00944801 | [128] |
| intratumoral injection | magnetic iron-oxide nanoparticles |
fractionated stereotactic radiotherapy | phase II | Magnetic Iron-Oxide Nanoparticles | Intratumoral Injection | Side Effects: Acute side effects during thermotherapy were classified according to the Common Toxicity Criteria (CTC) version 2.0. Common side effects included sweating (50.0%), general sensation of warmth (47.0%), and thermal stress with body temperature exceeding 38°C in 6 patients (9.1%). No serious complications were observed. | The study does not provide specific pharmacokinetic data. However, it mentions that no indication of iron being released from the intratumoral deposits or being metabolized was observed, suggesting the nanoparticles remained stable post-administration. | Overall Survival After First Tumor Recurrence (OS-2): The median OS-2 was 13.4 months (95% CI: 10.6–16.2 months) among the 59 patients with recurrent glioblastoma. Only tumor volume at study entry was significantly correlated with ensuing survival (P < 0.01). Overall Survival After Primary Tumor Diagnosis (OS-1): Median OS-1 was 23.2 months with a 95% confidence interval of 17.2–29.2 months. |
- | [129] |
| EPR | Irinotecan | - | phase I | nal-IRI | Intravenous infusion | The maximum tolerated dose (MTD) was determined for both WT and HT cohorts. Dose-limiting toxicities included diarrhea, dehydration, and fatigue. The study concluded that nal-IRI had no unexpected toxicities and that UGT1A1 genotype did not correlate with toxicity. | PK results were comparable to those seen in other PK studies of nal-IRI. The study analyzed PK parameters including maximum plasma concentrations (Cmax), areas under the plasma concentration-time curve (AUC0−t), and terminal half-life (t1/2) for total irinotecan, SN-38, and SN-38G. UGT1A1*28 genotype did not affect PK parameters. | The primary efficacy endpoint was PFS-6. The study reported PFS-6 as 2.9% for the intent-to-treat cohort, with a median PFS of 42 days and a median overall survival of 107 days. | - | [127] |
| Passive diffusion | Irinotecan | TMZ | phase I | nal-IRI | Intravenous infusion + Oral Administration | The study evaluates safety through monitoring dose-limiting toxicities (DLTs) which include grade 4 neutropenia, grade 3 diarrhea, hypokalemia, fatigue, anorexia, and other grade 3 or 4 nonhematologic toxicities. The MTD for nal-IRI was determined to be 50 mg/m^2 every 2 weeks with TMZ. | The study does not provide specific pharmacokinetic data. Enhanced BBB Penetration: Nanoliposomal encapsulation of irinotecan improved its ability to cross the BBB, as demonstrated in preclinical animal models. Tissue Analysis: In preclinical studies, nal-IRI showed a 10.9-fold increase in tumor area under the curve compared to free irinotecan and 35-fold selectivity for tumor versus normal tissue exposure. |
The primary efficacy endpoints are the assessment of response rate (complete or partial response as defined by Macdonald criteria) and PFS. The study was terminated after an interim analysis showed no activity (0% response rate) and a median PFS of 2 months. |
NCT03119064 | [126] |
| EPR + active transport | NU-0129 | - | phase 0 | siBcl2L12-SNAs | Intravenous infusion | The safety assessment revealed no significant treatment-related toxicities. The study monitored vital signs, blood chemistry, and adverse events in patients, with only two treatment-related severe adverse events (lymphopenia and hypophosphatemia) noted, which were considered 'possibly' related to the treatment. | PK analysis showed that siRNA was rapidly eliminated from plasma with a mean half-life of 0.09 hours, while gold (Au), used as a marker for the SNAs, had a much slower elimination with a mean half-life of 17 hours. The study also detailed the clearance rates and volume of distribution for both siRNA and Au. | The primary efficacy endpoints were the intratumoral accumulation of SNAs and the suppression of the Bcl2L12 gene. The study reported that NU-0129 uptake into glioma cells correlated with a significant reduction in tumor-associated Bcl2L12 protein expression. Additionally, the presence of Au in the tumor tissue was confirmed, indicating that the SNAs reached the patient tumors. | NCT03020017 | [130] |
| RMT | DOX | - | phase I | anti-EGFR ILs-dox | Intravenous infusion | The study reports safety data from the application of anti-EGFR ILs-dox in patients with relapsed glioblastoma. No grade 4 or 5 adverse events occurred. One case of severe pneumonitis was reported, which resolved with treatment. Other adverse events included febrile neutropenia in two patients, which was managed without sequelae. | The pharmacokinetic analysis showed that the mean plasma concentration of doxorubicin 24 hours after administration was 15,805 ng/ml. DOX concentrations in CSF were below 1 ng/ml in all patients, indicating that anti-EGFR ILs-dox do not cross the BBB at clinically relevant levels. However, significant levels of doxorubicin were detected in glioblastoma tissue 24 hours after application, suggesting that the disrupted BBB in high-grade gliomas may enable liposome delivery into tumor tissue. | The primary efficacy endpoints were the concentration of anti-EGFR ILs-dox in plasma, CSF, and glioblastoma tissue. The median PFS was 1.5 months, and the median OS was 8 months. One patient had a very long remission, suggesting that neoadjuvant administration may positively affect the outcome. | NCT03603379 | [131] |
| EPR | AGuIX nanoparticles |
standard of care for glioblastoma | phase I/Ⅱ | AGuIX nanoparticles | Intravenous infusion | Toxicity Assessment: DLT defined as any grade 3–4 NCI Common Terminology Criteria for Adverse Events (CTCAE) toxicity, except for alopecia, nausea, vomiting, or fever. Adverse Event Reporting: According to CTCAE (version 5.0). Neurological Status Evaluation: Clinical assessment and Mini-Mental State Examination (MMSE). |
PK parameters of AGuIX nanoparticles, including AUC, Tmax, and Cmax, will be measured on blood samples and urinary excretion during phase I of the study. | For phase I, the primary endpoint is the RP2D of AGuIX nanoparticles, with DLT defined as any grade 3–4 toxicity. For phase II, the primary endpoint is the 6-month progression-free survival rate, which will be estimated using the Kaplan–Meier method. | NCT04881032 | [132] |
| EPR + RMT | Mitoxantrone | - | phase I | EGFR-Erbitux EDVsMIT |
Intravenous infusion | The safety evaluation of EEDVsMit showed that it was well tolerated, with no dose-limiting toxicities observed. The most common drug-related adverse events were grade 1–2 fever, nausea, vomiting, rash, lymphopenia, and mildly deranged liver function tests. | The study does not provide detailed pharmacokinetic data within the summary. However, it mentions that the EDV selectively targets the cancer cell via the bispecific antibody and releases the cytotoxic drug into the tumor cell after macropinocytosis and breakdown in lysosomes. | The primary efficacy endpoints were safety and tolerability, with the study also aiming to preliminarily define the antitumor activity of mitoxantrone-containing EDVs. The best antitumor response observed was a mixed response in one patient, and all other patients had progressive disease. | NCT02687386 | [133] |
| CED | Irinotecan | - | phase I | nal-IRI | intratumoral injection | Safety is evaluated by monitoring adverse events, scheduled laboratory assessments, vital sign measurements, and physical examinations. Toxicities are graded according to the NCI CTCAEv4.0. | The study assesses the distribution of gadolinium, used in conjunction with real-time imaging, to model the drug distribution and evaluate the effectiveness of CED in delivering nal-IRI to the tumor site. | The primary efficacy endpoint is OS at 12 months (OS12), which will be considered evaluable for clinical efficacy. | NCT03086616 | - |
| CED | Irinotecan | - | phase I | Liposomal-Irinotecan | intratumoral injection | Safety is evaluated by monitoring DLTs within 30 days post-infusion, as well as any neurological or systemic grade-3 or higher toxicities. | PK measurements are taken at pre-dose, 1 day after drug administration, and 1 week post-op to estimate drug distribution and concentration in the brain. | The primary efficacy endpoints include the determination of the maximum tolerated dose and the assessment of PFS at 6 months and 10 years, as well as OS at 12 months and 10 years. | NCT 02022644 | - |
| Actively target | MT-201-GBM | standard radiation therapy and TMZ chemotherapy | phase I | MT-201-GBM | Intravenous administration | The study aims to assess the safety and tolerability of MT-201-GBM by measuring: Primary Safety Endpoint: Percentage of patients with a DLT during the DLT observation period within each dose level. Other Safety Measures: Changes in laboratory parameters (e.g., hemoglobin, creatinine, AST, bilirubin) and monitoring for adverse events. |
The study does not provide specific details on pharmacokinetic assessments. However, it does include immune response measurements, such as changes from baseline in IFN gamma, granzyme-B, and fluorospot, which may provide insights into the vaccine's biological activity. | The primary efficacy endpoints include the MTD of MT-201-GBM and immune response measurements after the second and third infusions compared to baseline. Secondary outcome measures include OS, PFS, and changes in immune response indicators. | NCT04741984 | - |
| Actively target and bypass the BBB | 5-FC | - | phase I | CD-NSCs | Intracranial Injection + Oral Administration | The study evaluated safety through the monitoring of adverse events, assessment of possible NSC migration into the systemic circulation, and testing for the presence of replication-competent retrovirus (RCR). No DLT was observed due to the CD-NSCs, and there was no development of anti-CD-NSC antibodies. | Intracerebral microdialysis was used to measure brain levels of 5-FC and 5-FU, showing CD-NSCs produced 5-FU locally in a 5-FC dose-dependent manner. Collected Blood Samples to assess systemic drug concentrations and perform immunologic correlative studies. | The primary efficacy endpoints included assessing the feasibility of treating recurrent high-grade glioma patients with intracranially administered CD-NSCs followed by oral 5-FC. Secondary objectives included assessing possible CDNSC immunogenicity, secondary tumorigenicity, and evaluating proof-of-concept regarding CD-NSC migration to tumor foci and localized conversion of 5-FC to 5-FU. | NCT01172964 | [134] |
| Actively target and bypass the BBB | 5-FC | Leucovorin | Phase I | CD-NSCs | Intracranial Injection | Safety is evaluated through monitoring of adverse events, assessment of possible NSC migration into the systemic circulation using qPCR, and testing for the presence of replication-competent retrovirus (RCR). No clinical signs of immunogenicity were observed, and only three patients developed anti-NSC antibodies. | Neuropharmacokinetic data were collected using intracerebral microdialysis to confirm continuous production of 5-FU in the brain during the course of 5-FC administration. The primary pharmacokinetic parameters of interest were the Cmax and AUC of 5-FC and 5-FU, measured in both dialysate samples and plasma. | The primary efficacy endpoints included assessing the feasibility of serially administering CD-NSCs and determining the recommended dosing for phase II testing. Secondary objectives included characterizing the relationship between intracerebral and systemic concentrations of 5-FC and 5-FU, assessing CD-NSC immunogenicity, and describing clinical activity. The best response observed was stable disease in three participants, with a range of 4 to 5 months. | NCT02015819 | [135] |
| Actively target and bypass the BBB | Irinotecan Hydrochloride | - | phase I | hCE1m6-NSCs | Intracranial administration + Intravenous administration |
Safety is evaluated through the grading of toxicities using the NCI CTCAE version 4.0, with specific attention to DLTs during the first treatment cycle. | PK data are collected via intracerebral microdialysis to measure SN-38 concentrations in the brain interstitium and plasma levels of irinotecan and SN-38. | The primary efficacy endpoints include the assessment of clinical benefit, defined as stable disease, partial response, or complete response, as measured by RANO criteria, for up to 6 months. | NCT02192359 | - |
| Actively target and bypass the BBB | Oncolytic Adenovirus (CRAd-S-pk7) | standard temozolomide chemotherapy and radiotherapy | phase I | NSC-CRAd-S-pk7 | Intracranial Injection | The trial demonstrated that the treatment was safe, with no formal dose-limiting toxicity reached. One patient developed viral meningitis (grade 3) due to an inadvertent injection into the lateral ventricle. | PK assessments were performed through medical history, imaging, blood samples, and chemistry values collected at screening and follow-up. The study did not detail specific pharmacokinetic parameters but focused on the safety and presence of the treatment. | The primary endpoints were safety and tolerability. Secondary endpoints included estimation of survival outcomes and evaluation of immune response correlation with clinical outcomes. The median progression-free survival was 9.05 months, and overall survival was 18.4 months. | NCT03072134 | [136] |
| CMT | Survivin DC cell injection | radiotherapy and TMZ chemotherapy | phase I | Survivin-loaded dendritic cell injection | Intradermal Injection + Intravenous Infusion | Safety is evaluated through the monitoring of adverse events that occur within 28 days after the first to the third dose, as judged by the investigator to be related to the study drug. DLT is a primary outcome measure. | Not specifically detailed in the document. However, the administration schedule (days 0, 14, and 28) and route of administration (ID and IV) indicate PK will be influenced by the absorption, distribution, metabolism, and excretion of the Survivin-loaded dendritic cells. | The primary efficacy endpoints include PFS measured over 2 years and OS also measured over 2 years. Secondary Outcome Measures include immune effect assessments such as Anti-Survivin antibody test, cytokine detection, and specific T-cell responses, as well as DC cell activity and in vivo processes, measured at specific time points. | NCT06524063 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





