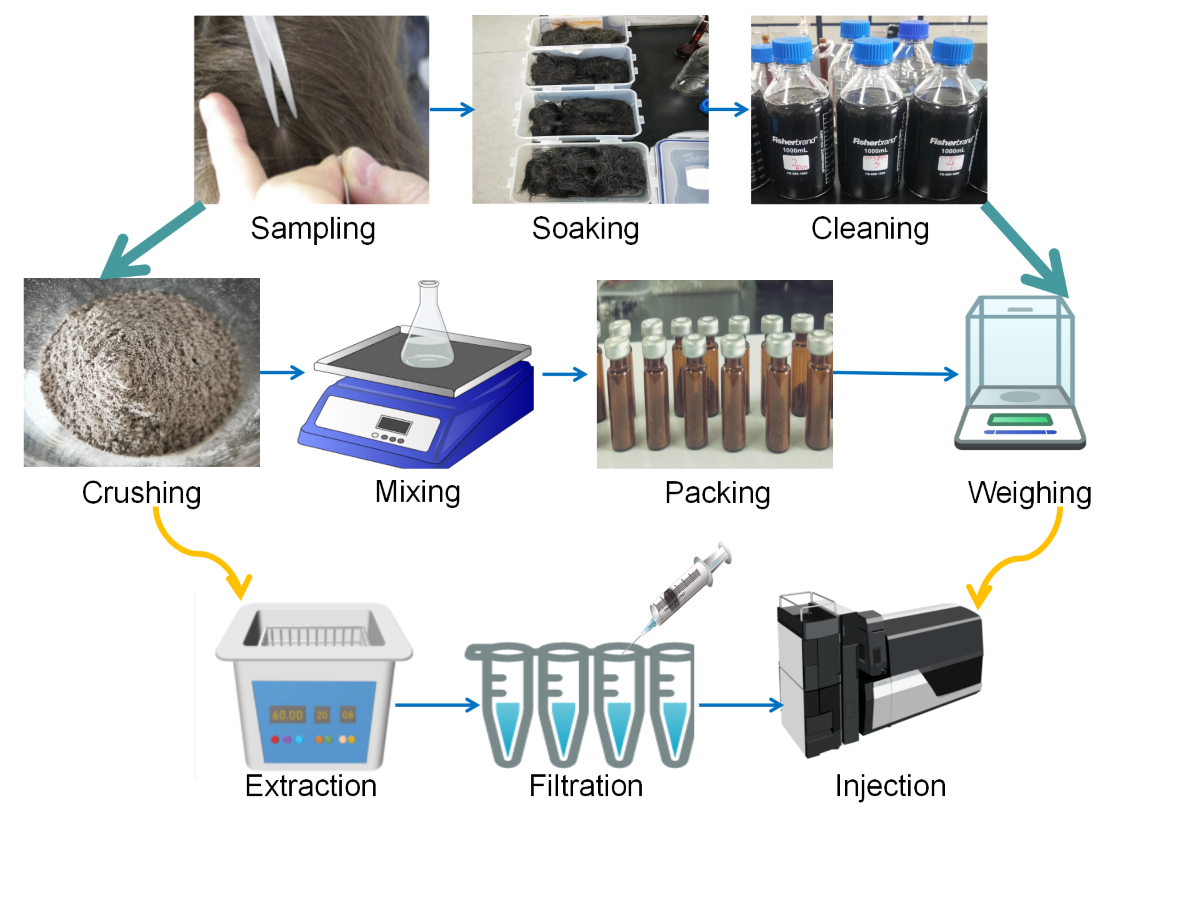
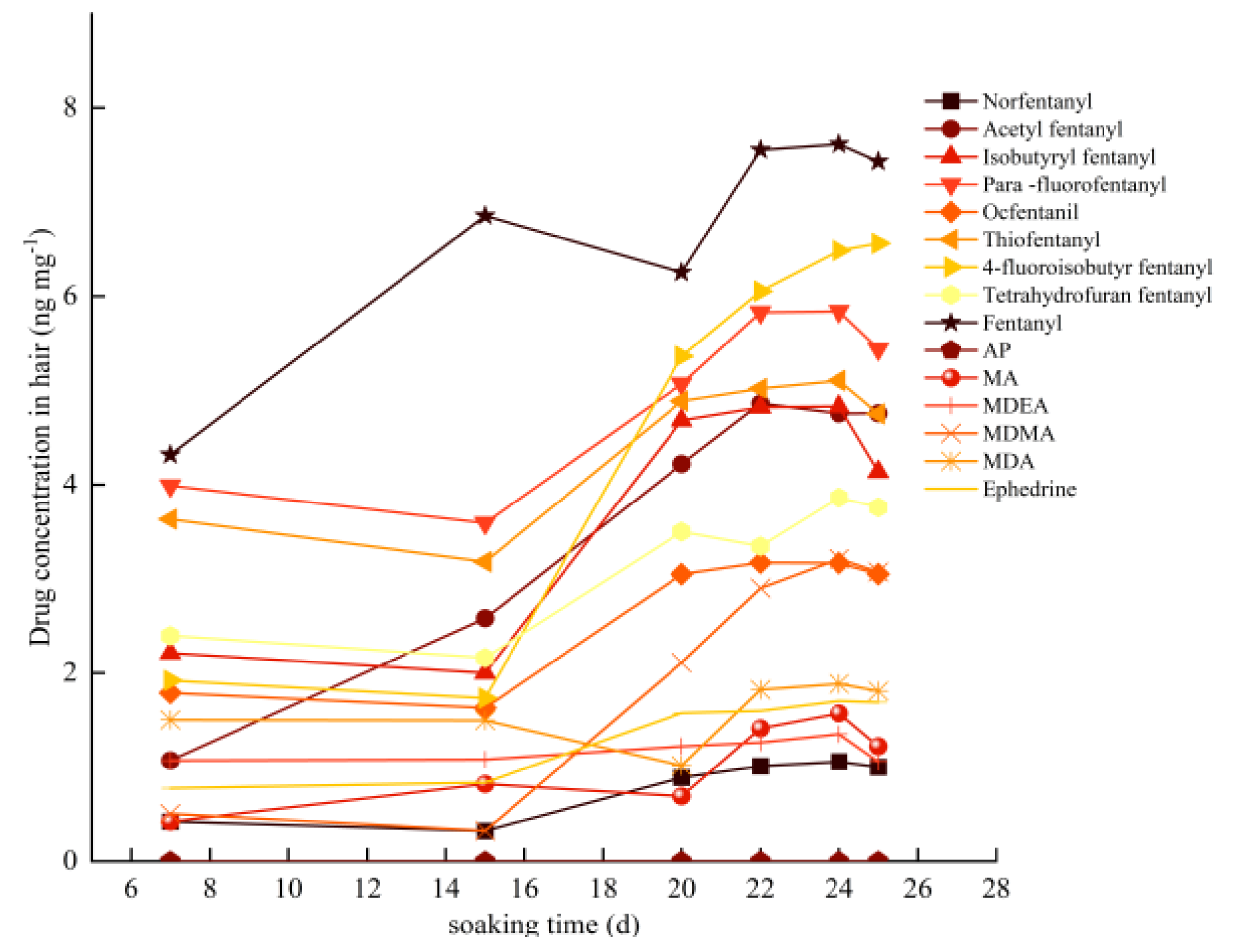
2.2. Optimization of Sample Pretreatment Conditionsres
Based on relevant literature and the structural characteristics of drugs, an experiment was conducted to explore the effects of acid hydrolysis, alkaline hydrolysis, and methanol ultrasonic hydrolysis on drug concentration in hair. The results showed that acid hydrolysis resulted in a higher drug concentration in hair as compared to alkaline hydrolysis and methanol ultrasonic extraction. To determine the reasonable experimental factors and levels, a single-factor variable method combined with response surface analysis was used to investigate the effects of different extraction temperatures, extraction times, liquid-to-material ratios, and hydrochloric acid concentrations on drug concentration. Response surface methodology is a statistical method that is useful for solving problems that involve multiple factors. This method compensates for the limitations of orthogonal experiments, which can only combine factor levels and are unable to optimize the optimal process. In a recent experiment, Box Benhnken response surface methodology was used to optimize the ultrasound extraction conditions of 15 drugs in hair and establish a mathematical model. This experiment was based on single-factor experiments. An experiment with four factors and three levels was designed using Design Expert13.0 software to explore the optimal conditions for extracting drugs from hair. The evaluation criteria were based on the drug concentration in the hair. Extraction temperature, extraction time, liquid-to-material ratio, and hydrochloric acid concentration were used as independent variables. Taking methamphetamine as an example, the quadratic multiple regression equation between the fitted drug concentration (ng mg-1) and extraction temperature (a), extraction time (b), liquid-to-material ratio (c), and hydrochloric acid concentration (d) are drug concentration (ng mg-1) =1.56-0.2153a+0.0629b-0.0903c+0.0844d-0.0762ab+0.0209ac+0.0669ad-0.0273bc+ 0.0641bd+ 0.2007cd-0.2592a2-0.3245b2+ 0.0469c2+0.0710d2.
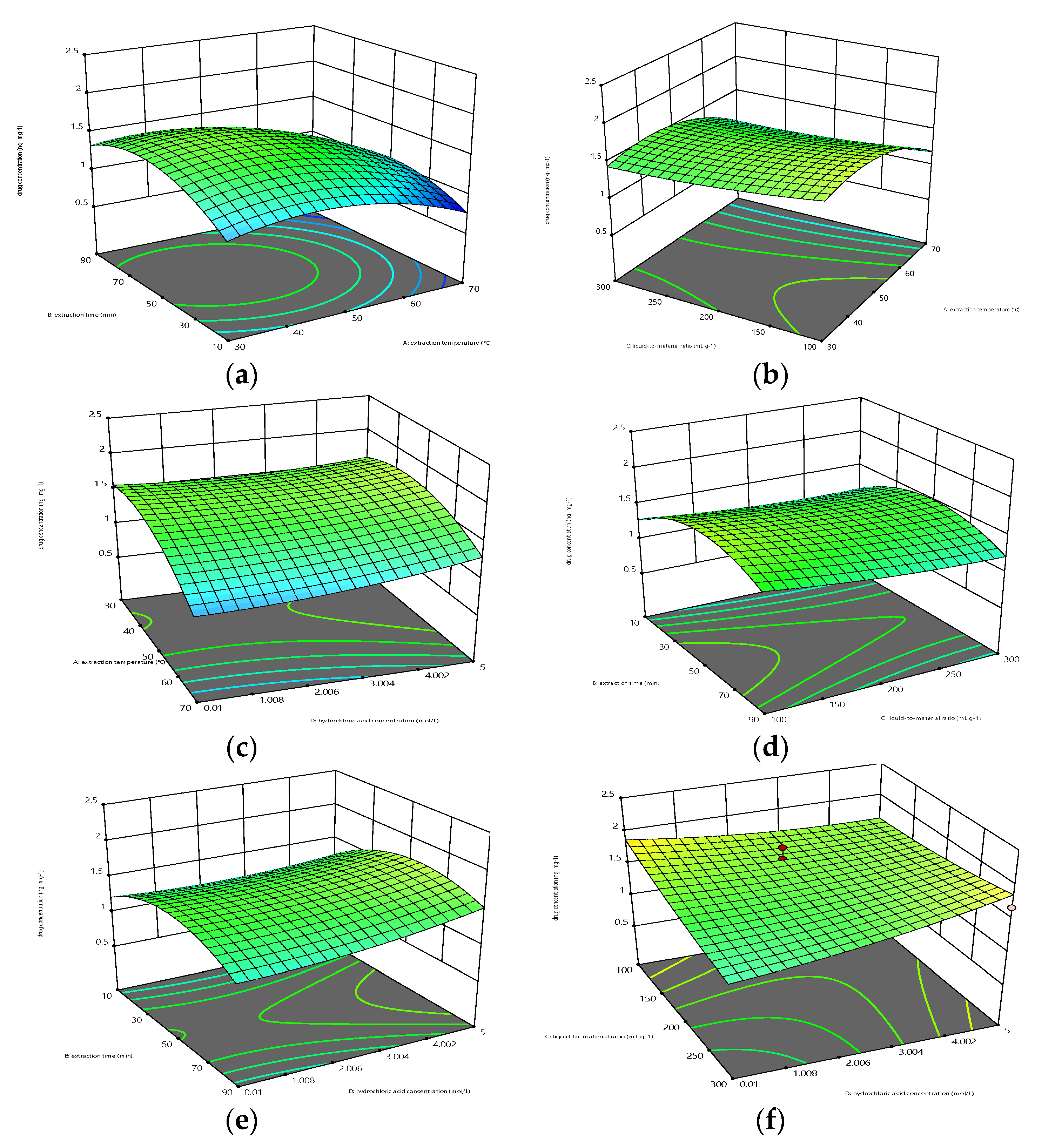
The Model F-value of 3.97 implies the model is significant. There is only a 0.73% chance that an F-value this large could occur due to noise. P-values less than 0.0500 indicate model terms are significant. In this case, A, CD, A², and B² are significant model terms. The impact of various factors on the extraction amount of 15 drugs in hair is extraction temperature (a) ˃ liquid-to-material ratio (c) ˃ hydrochloric acid concentration (d)˃ extraction time (b).
Response surface and contour maps can visually represent how different factors interact with the response value. The contour lines illustrate the magnitude of the interaction between two factors. A circular or irregular shape indicates that the interaction between two factors is not significant and has no promoting effect, while an ellipse indicates that the interaction has a significant promoting effect[
27].
It is evident from
Figure 2 (a) that the amount of drug extracted is affected by the extraction temperature and time. The liquid-to-material ratio is fixed at 200 mL g
-1, and the hydrochloric acid concentration is fixed at 2.5 mol L
-1. The trend of the extraction amount increases and then decreases as temperature and time increase. The steeper slope of the surface corresponding to temperature extraction compared to extraction time indicates that temperature has a greater impact on drug extraction than time. The contour lines are elliptical in shape, indicating a significant interaction between extraction temperature and extraction time. There is a promoting effect between the two factors, which is consistent with the results of the variance analysis of the AB term in the regression equation.
Figure 2 (b) shows that the amount of drug extracted is impacted by the extraction temperature and liquid-to-material ratio. The extraction time is fixed at 50 min, and the hydrochloric acid concentration is fixed at 2.5 mol L
-1. The amount of drug extracted shows an increasing trend initially, followed by a decrease, as the extraction temperature and liquid-to-material ratio are increased. The slope of the surface corresponding to the extraction temperature is steeper than that of the liquid-to-material ratio, indicating that the influence of temperature on drug extraction is greater than that of the liquid-to-material ratio. The contour line is not a complete ellipse, indicating that there is no interaction between extraction temperature and liquid-to-material ratio. This is consistent with the analysis of variance results of the AC term in the regression equation.
Figure 2 (c) shows the effects of extraction temperature and hydrochloric acid concentration on the amount of drug extraction. The slope of the curve corresponding to the concentration of hydrochloric acid rises and sharply decreases, indicating that a high concentration of hydrochloric acid will lead to a decrease in the amount of drug extracted. The contour lines show irregular shapes, indicating that there is no interaction between extraction temperature and hydrochloric acid concentration. This is consistent with the results of the analysis of variance for the AD term in the regression equation.
Figure 2 (d) shows the effect of extraction time and liquid-to-material ratio on the extraction of drug content. The slope of the surface corresponding to the liquid-to-material ratio is steeper than the extraction time, indicating that the impact of the liquid-to-material ratio on drug extraction is greater than the extraction time. The shape of the contour line indicates that the interaction between extraction time and the liquid-to-material ratio is not significant, and there is no promoting effect between the two factors, which is consistent with the results of the variance analysis of the BC term in the regression equation.
Figure 2 (e) shows the effect of extraction time and hydrochloric acid concentration on the content of extracted drugs. The slope of the curve corresponding to hydrochloric acid concentration is steeper than the extraction time, indicating that the impact of hydrochloric acid concentration on drug extraction is greater than that of extraction time. The shape of the contour line indicates that there is no promoting effect between the two factors, which is consistent with the results of the variance analysis of the BD term in the regression equation.
Figure 2 (f) illustrates how the content of extracted drugs is affected by liquid-to-material ratio and hydrochloric acid concentration. The elliptical contour line shows a significant interaction between the liquid-to-material ratio and hydrochloric acid concentration, consistent with the analysis of variance for the CD term in the regression equation.
When using Design Expert 13.0 software to predict the extraction temperature of 38℃, extraction time of 54 minutes, the liquid-to-material ratio of 100:1 (mL g-1), and hydrochloric acid concentration of 0.01 mol L-1, the drug concentration is the highest. Under this condition, the predicted value of MA is 1.973 ng mg-1. According to the actual operating conditions, the optimal extraction process was revised to an extraction temperature of 40℃, extraction time of 50 min, liquid-to-material ratio of 100:1 (mL g-1), and hydrochloric acid concentration of 0.01 mol L-1. The measured value of MA obtained is 1.915 ng mg-1. Verified the effectiveness of the response surface model. Therefore, the optimal extraction conditions for 15 drugs in the hair are an extraction temperature of 40℃, an extraction time of 50 minutes, a liquid-to-material ratio of 100:1 (mL g-1), and a hydrochloric acid concentration of 0.01 mol L-1.
When using Design Expert 13.0 software to predict the extraction temperature of 38℃, extraction time of 54 minutes, the liquid-to-material ratio of 100:1 (mL g-1), and hydrochloric acid concentration of 0.01 mol L-1, the drug concentration is the highest. Under this condition, the predicted value of MA is 1.973 ng mg-1. According to the actual operating conditions, the optimal extraction process was revised to an extraction temperature of 40℃, extraction time of 50 min, liquid-to-material ratio of 100:1 (mL g-1), and hydrochloric acid concentration of 0.01 mol L-1. The measured value of MA obtained is 1.915 ng mg-1. Verified the effectiveness of the response surface model. Therefore, the optimal extraction conditions for 15 drugs in the hair are an extraction temperature of 40℃, an extraction time of 50 minutes, a liquid-to-material ratio of 100:1 (mL g-1), and a hydrochloric acid concentration of 0.01 mol L-1.
2.3. Method Validation
LOD and LOQ: Add a series of low-concentration mixed standard solutions of 15 drugs to blank hair for LC-MS/MS and GC-MS/MS analysis. The LOD value was considered the concentration value giving an S/N>3 for at least three diagnosticians for each substance, while the LOQ was the minimum concentration giving an S/N>10 for at least three diagnosticions. The results showed that the detection limit of LC-MS/MS was 0.05 pg mg-1~5.0 pg mg-1, and the quantification limit was 0.25 pg mg-1~20.0 pg mg-1, indicating good sensitivity. In the GC-MS/MS method, the detection limit is 0.02 ng mg-1~0.08 ng mg-1, and the quantification limit is 0.08 ng mg-1~0.20 ng mg-1. The LOD and the LOQ of both methods meet the requirements for detecting hair-poisoning products.
Linearity and Range: Prepare a series of 6 standard solutions with different concentrations, and add an equal amount of methamphetamine D5 internal standard solution to the standard solution to form a mixed standard solution. Add blank hair samples for analysis according to pre-processing methods, and use the internal standard working curve method for quantitative analysis. Measure each concentration level three times in parallel. Draw a standard curve using the mass concentration (x) of the drug as the x-axis and the peak area ratio (y) of the drug to methamphetamine D5 as the y-axis. The results showed that in LC-MS/MS, there was a good linear relationship among 15 drugs within the concentration range of 10.0-200.0 pg mg-1, with a correlation coefficient>0.999. In GC-MS/MS, 15 drugs showed good linear relationships within the concentration range of 0.4 ng mg-1~4.5 ng mg-1, with a correlation coefficient>0.999. Both analysis methods meet the requirements of quantitative analysis.
Intraday and daytime precision experiments: Prepare hair extract and perform quantitative analysis using LC-MS/MS and GC-MS/MS. Measure 6 consecutive injections and calculate the intra-day precision. The results showed that in LC-MS/MS, RSDs<4% (n=6). Continuous measurement for 5 days, with a daytime precision of RSDs<6.0% (n=30). In GC-MS/MS, RSDs<6% (n=6). Continuous measurement for 5 days, with daytime precision RSDs<10% (n=30). The established LC-MS/MS and GC-MS/MS methods have good repeatability and meet the analysis requirements.
Stability experiment: Prepare hair extracts and inject samples at different time intervals (1, 24, 48, 72, and 96 hours) after preparation to evaluate hair sample stability. The results showed that in LC-MS/MS, RSDs<6%, and GC-MS/MS, RSDs<10%. The prepared hair extract exhibits good stability within 96 hours at room temperature.
Recovery rate and matrix effect: Accurately weigh 9 blank hair samples, add 15 drugs at low, medium, and high concentration levels, and prepare 3 parallel portions for each concentration. Determine the recovery rate using LOCTRL, MEDCTRL, and HICTRL (n=3). Quantitative analysis was conducted using LC-MS/MS and GC-MS/MS, and recovery rates were calculated at different concentrations using regression equations. The results showed that the average recovery rates of LOCTRL, MEDCTRL, and HICTRL for 15 drugs in LC-MS/MS were 96.04%-110.8%, 84.46%-104.3%, 88.69%-100.7%, and RSD ≤ 10.2%. The average recovery rates of LOCTRL, MEDCTRL, and HICTRL in GC-MS/MS were 96.84%-116.4%, 85.50%-102.1%, and 86.14%-112.9%, respectively, with RSDs<9%. This indicates that the method has high accuracy.
Evaluate the matrix effect by analyzing the ratio of peak areas obtained from low, medium, and high concentration standard solutions in blank hair matrix and pure methanol solution. The results indicate that the matrix effect in LC-MS/MS and GC-MS/MS ranges from 80% to 120%, with an RSD<15%, which meets the analysis requirements.
2.4. Quantitative Analysis
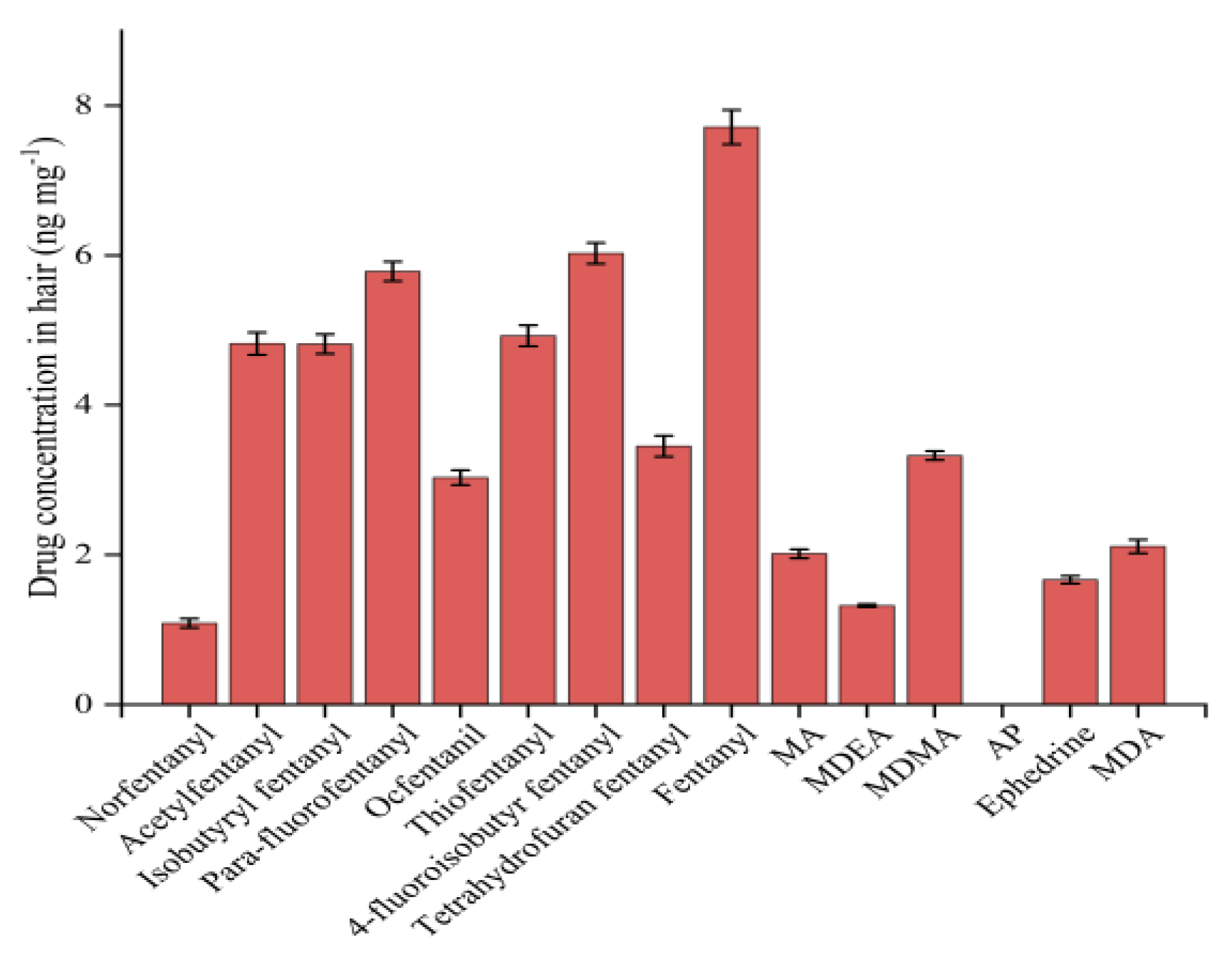
LC-MS/MS: From the 200 bottles of hair samples, randomly select 11 bottles and measure each sample 3 times. The results showed that the content of 15 drugs in the prepared hair samples was 1.012 ng mg-1~7.830 ng mg-1. Norfentanyl: (1.012±0.03) ng mg-1; Acetylfentanyl: (4.788±0.04) ng mg-1; Isobutyryl fentanyl: (4.826±0.02) ng mg-1; P-Fluorofentanyl: (5.830±0.03) ng mg-1; Ocfentanyl: (3.188±0.05) ng mg-1; Thiofentanyl: (5.010±0.04) ng mg-1; 4-fluoroisobutyryl fentanyl: (6.303±0.03) ng mg-1; Tetrahydrofuran fentanyl: (3.444±0.01) ng mg-1; Fentanyl: (7.830±0.03) ng mg-1; MA: (1.789±0.023) ng mg-1; MDEA: (1.321±0.01) ng mg-1; MDMA: (3.284±0.03) ng mg-1; Ephedrine: (1.667±0.03) ng mg-1; MDA: (1.938±0.04) ng mg-1; Amphetamine was not detected.
GC-MS/MS: Randomly select 11 bottles from the package of 200 hair samples and measure each bottle thrice. The results showed that the content of 15 drugs in the prepared hair samples was 1.087 ng mg-1~7.712 ng mg-1. Norfentanyl: (1.087±0.06) ng mg-1; Acetylfentanyl: (4.821±0.15) ng mg-1; Isobutyryl fentanyl: (4.816±0.13) ng mg-1; P-Fluorofentanyl: (5.786±0.13) ng mg-1; Ocfentanyl: 3.031±0.10 ng mg-1; Thiofentanyl: (4.925±0.14) ng mg-1; 4-fluoroisobutyryl fentanyl: (6.028±0.14) ng mg-1; Tetrahydrofuran fentanyl: (3.451±0.14) ng mg-1; Fentanyl: (7.712±0.23) ng mg-1; MA: (2.015±0.06) ng mg-1; MDEA: (1.322±0.02) ng mg-1; MDMA: (3.325±0.06) ng mg-1; Ephedrine: (1.667±0.05) ng mg-1; MDA: (2.112±0.09) ng mg-1; Amphetamine was not detected.
The results showed that in LC-MS/MS and GC-MS/MS, Fmeasured<F0.05 (10,22)=2.30. The prepared hair sample has good uniformity. The concentrations of 15 drugs in the prepared hair samples were 1.050 ng mg-1~7.771 ng mg-1, with RSDs<7.0%.
Table 1.
Comparison of GC-MS/MS and LC-MS/MS results (ng mg-1).
Table 1.
Comparison of GC-MS/MS and LC-MS/MS results (ng mg-1).
| Drug name |
LC-MS/MS |
GC-MS/MS |
ME/% |
Mean |
| Mean |
RSD/% |
Mean |
RSD/% |
| Norfentanyl |
1.012 |
1.6 |
1.087 |
3.7 |
-3.8 |
1.050 |
| Acetylfentanyl |
4.788 |
0.6 |
4.821 |
2.0 |
-1.6 |
4.805 |
| Isobutyryl fentanyl |
4.826 |
0.3 |
4.816 |
1.9 |
0.5 |
4.821 |
| P-Fluorofentanyl |
5.830 |
0.2 |
5.786 |
1.6 |
2.2 |
5.808 |
| Ocfentanyl |
3.188 |
1.1 |
3.031 |
2.2 |
7.9 |
3.110 |
| Thiofentanyl |
5.010 |
0.6 |
4.925 |
2.0 |
4.3 |
4.968 |
| 4-fluoroisobutyryl fentanyl |
6.303 |
0.4 |
6.028 |
1.7 |
13.8 |
6.166 |
| Tetrahydrofuran fentanyl |
3.444 |
0.2 |
3.451 |
3.0 |
-0.3 |
3.448 |
| Fentanyl |
7.830 |
0.3 |
7.712 |
1.9 |
5.9 |
7.771 |
| AP |
- |
- |
- |
- |
- |
- |
| MA |
1.789 |
1.3 |
2.015 |
2.0 |
-11.3 |
1.902 |
| MDEA |
1.321 |
0.3 |
1.322 |
1.2 |
-0.05 |
1.322 |
| MDMA |
3.284 |
0.7 |
3.325 |
1.2 |
-2.1 |
3.305 |
| Ephedrine |
1.667 |
1.3 |
1.667 |
1.6 |
-0.02 |
1.667 |
| MDA |
1.938 |
1.6 |
2.112 |
2.7 |
-8.7 |
2.025 |
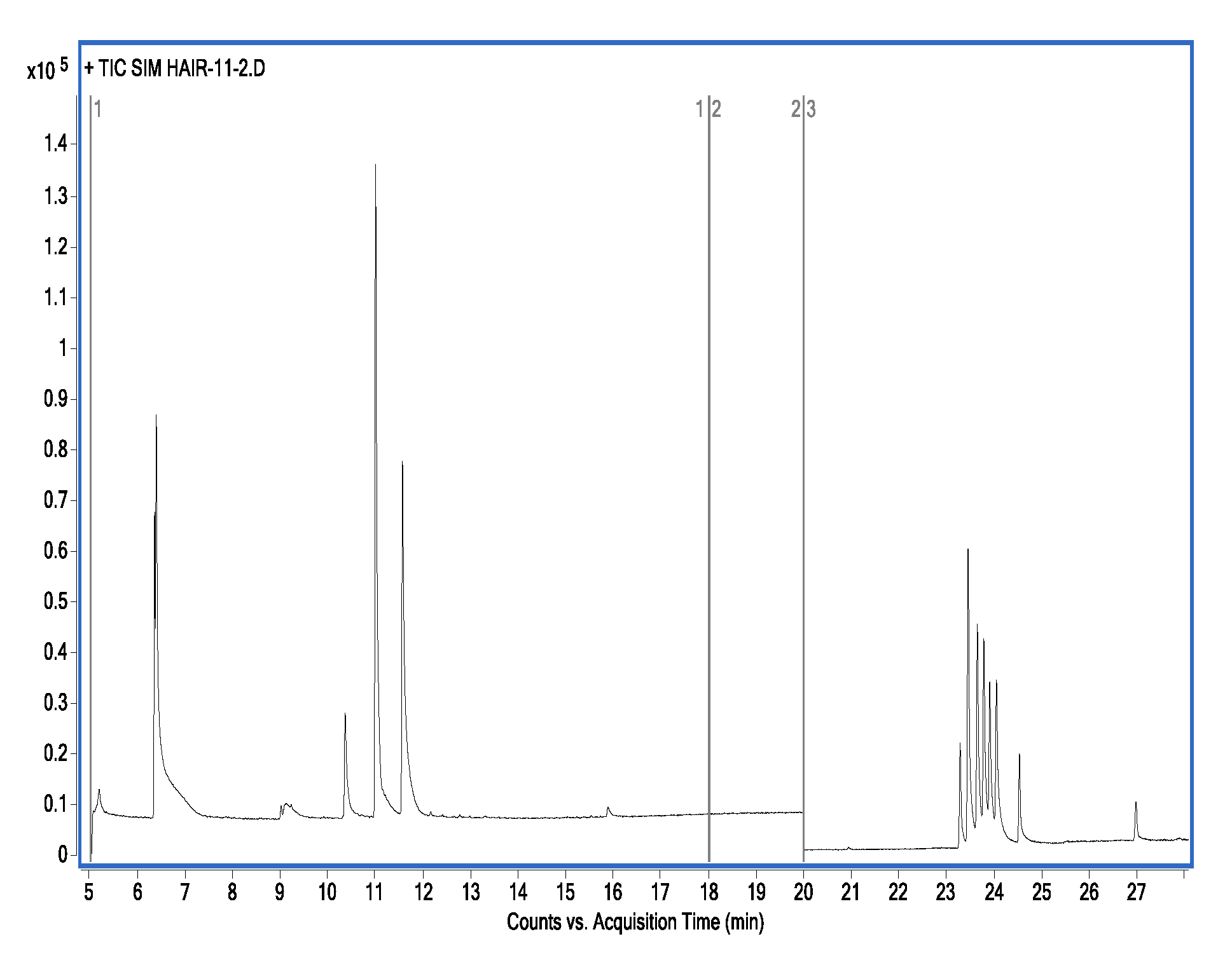
Figure 3.
GC-MS/MS chromatograms of 15 drugs in hair.
Figure 3.
GC-MS/MS chromatograms of 15 drugs in hair.
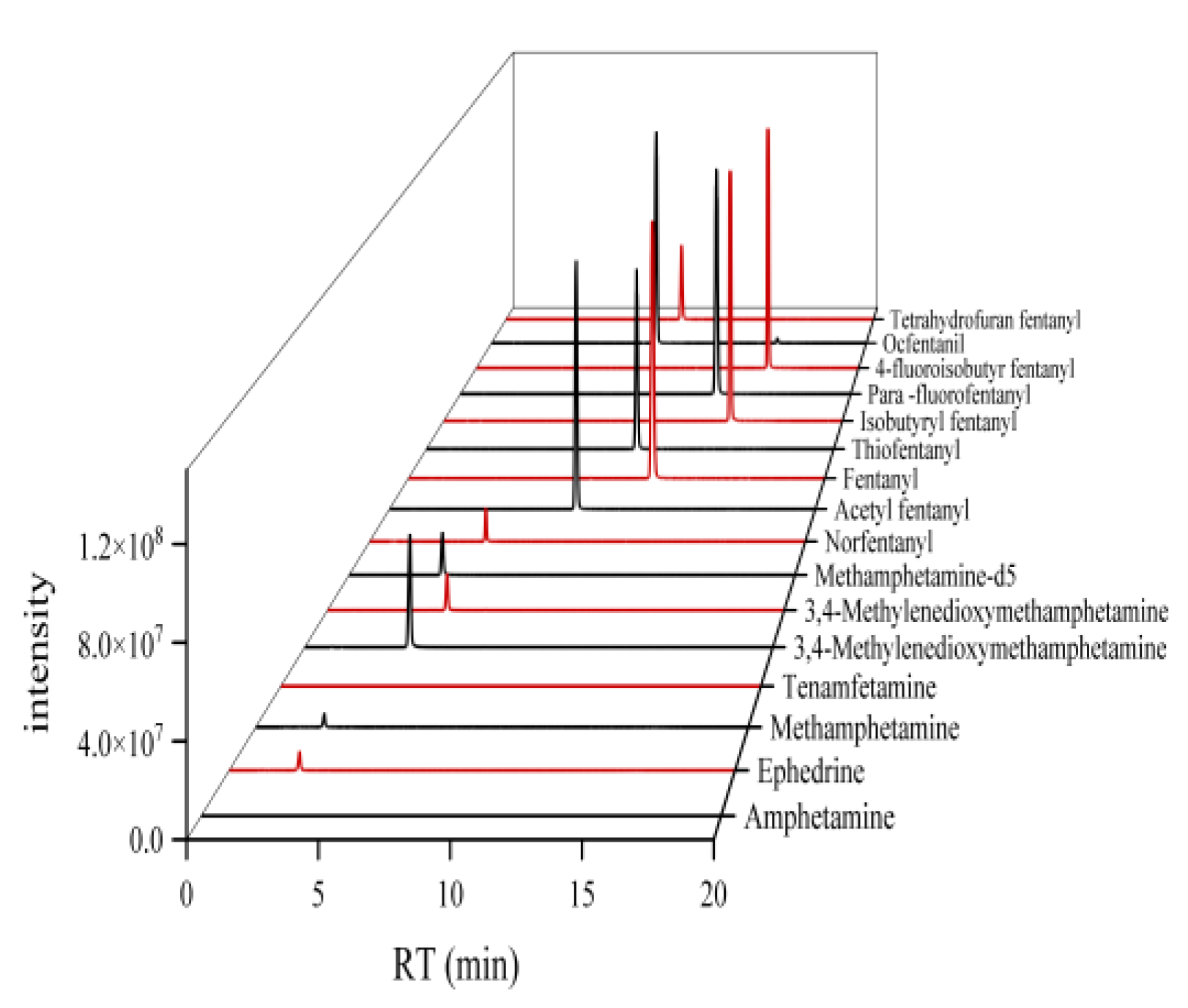
Figure 4.
LC-MS/MS chromatograms of 15 drugs in hair.
Figure 4.
LC-MS/MS chromatograms of 15 drugs in hair.
Figure 5.
Quantitative results for 15 drugs (GC-MS/MS).
Figure 5.
Quantitative results for 15 drugs (GC-MS/MS).
Figure 6.
Quantitative results for 15 drugs (LC-MS/MS).
Figure 6.
Quantitative results for 15 drugs (LC-MS/MS).