Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
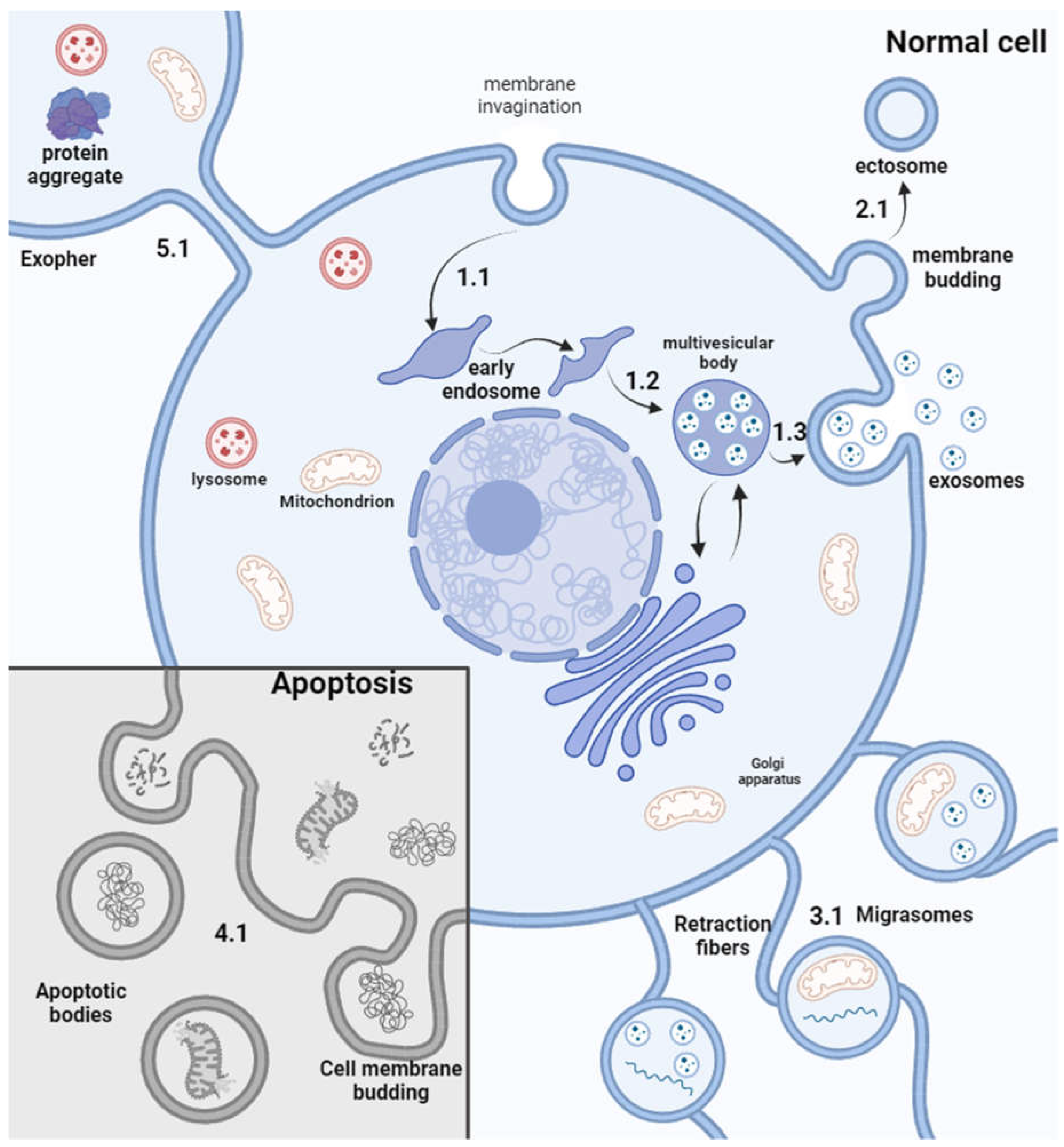
2. EV Preparation
3. Purification Methods
3.1. Size-Based Approaches
3.2. Precipitation-Based Methods
3.3. Affinity-Based Approaches
3.4. Chromatography Approaches
| Method | Principle | Scalability | Yield | EV Damage | Purity | Equipment requirement | Cost | Additional Pre/Post-steps | Time | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
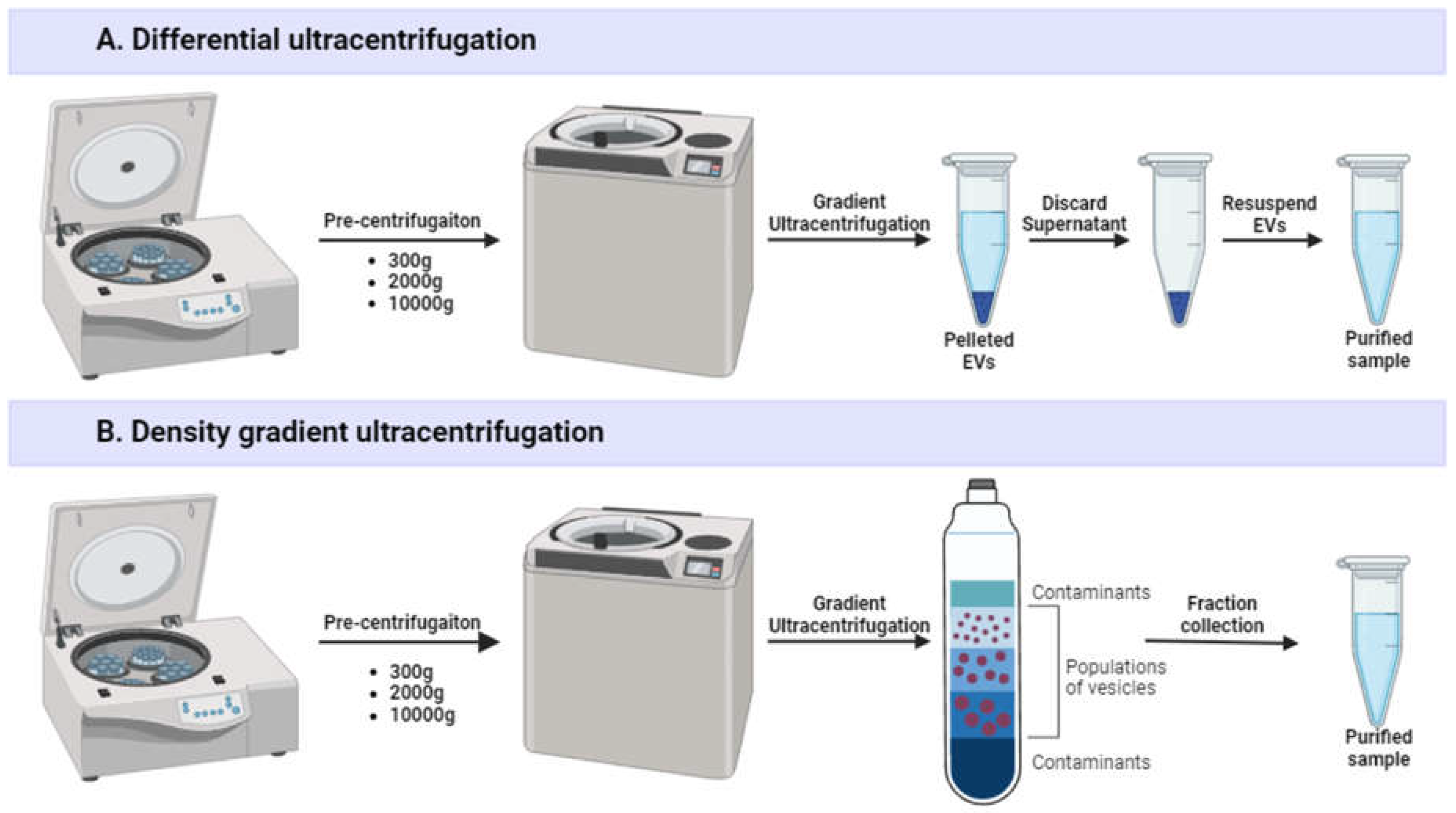
| Differential ultracentrifugation (dUC) | Serial UC steps | + | + | ↑↑↑ | ++ | +++ | + | No | ↑↑↑ | [19,21,22,23,24,25,26] |
| Density gradient ultracentrifugation (DGU) | Separation of EVs by density using gradient medium | + | + | ↑ | ++++ | +++ | ++ | Yes (media removal) | ↑↑↑ | [11,19,28] |
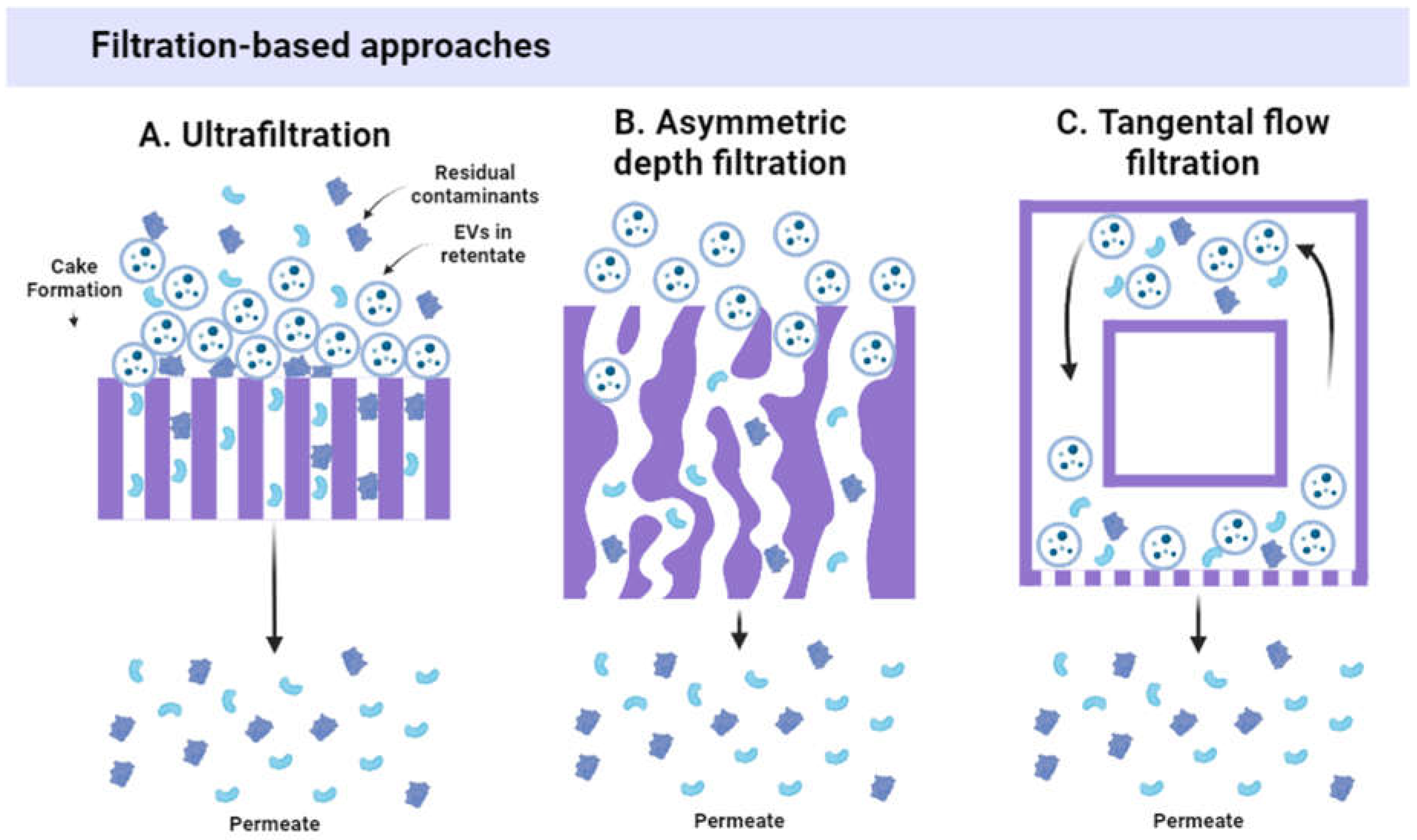
| Ultrafiltration (UF) | Filtration through semi-permeable membranes | +++ | +++ | ↑↑ | +++ | ++ | ++ | No | ↑/↑↑ | [11,19,30,31,32,33,34] |
| Asymmetric depth filtration (DF) | Filtration through porous medium | ++ | ++ | ↑ | +++ | ++ | ++ | No | ↑↑ | [35] |
| Tangential flow filtration (TFF) | Cross-flow filtration through membranes | ++++ | +++ | ↑ | +++ | ++ | ++ | No | ↑↑ | [11,15,36,37,38,39] |
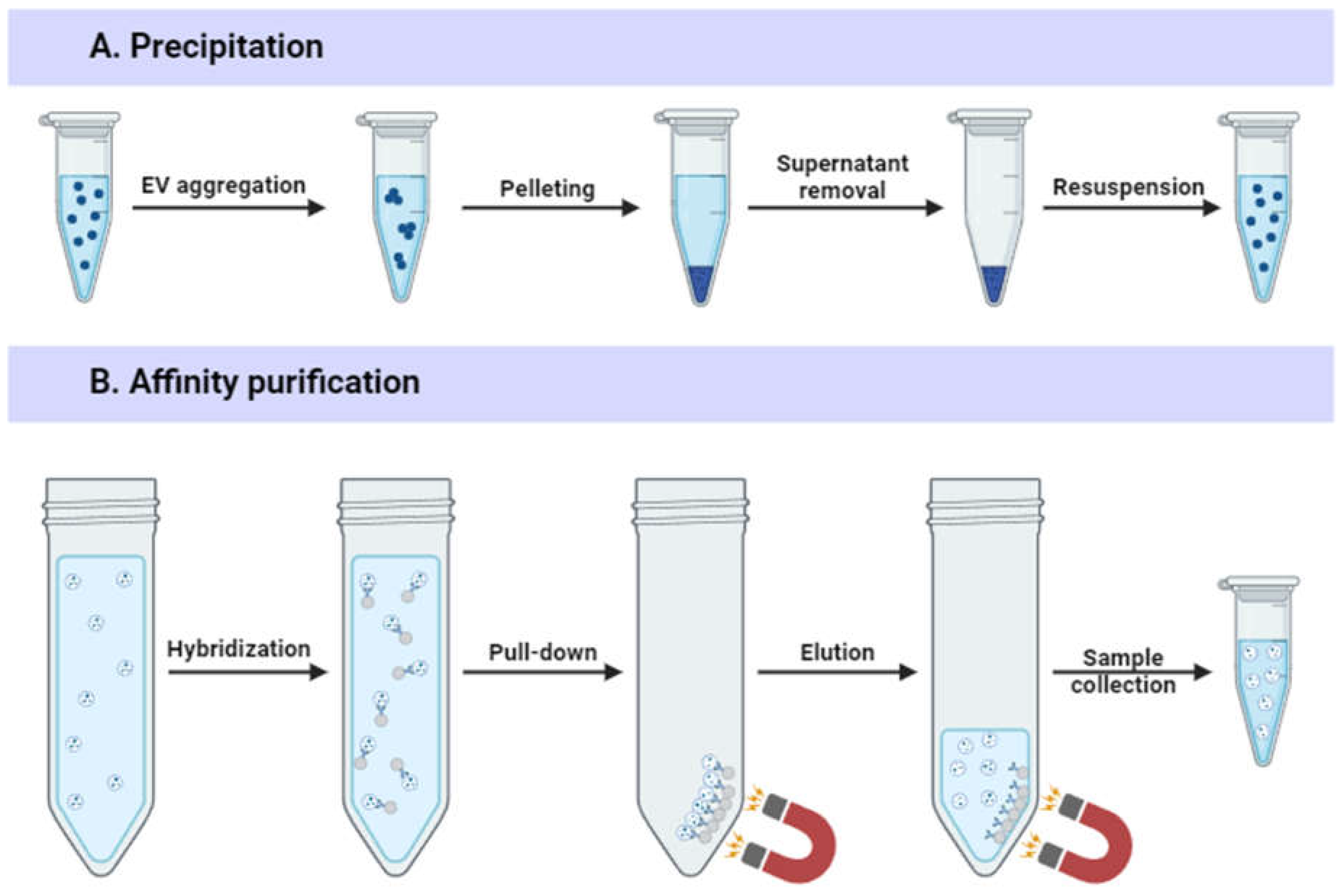
| Precipitation approaches | EV sedimentation using polymers | +++ | ++++ | ↑↑/↑↑↑ | + | + | ++ | Yes (polymer removal) | ↑↑↑ | [11,15,46,47,48] |
| Affinity-based isolation | EV capture via specific interactions with EV markers | +/++ | ++ | ↑/↑↑ | ++++ | ++ | +++ | No | ↑↑ | [33,50,51,52,53,55] |
| Size exclusion chromatography (SEC) | Separation by size through a bead-filled column | +++ (combined with UF/TFF) | ++ | ↑ | +++ | ++ | + | Yes (Pre-Concentration) | ↑↑ | [41,42,43,44,45] |
| Multimodal flowthrough chromatography (MFC) | Combination of size-exclusion and bind-elute chromatography | +++ (combined with pre-concentration) | ++ | ↑ | ++++ | ++ | ++ | Yes (Pre-Concentration) | ↑↑ | [57] |
| Anion-exchange chromatography (AIEX) | Binding of EVs to positively charged column | +++ | +++ | ↑↑ | ++ | ++ | ++ | Yes (Buffer exchange) | ↑↑↑ | [41,59,60,61] |
3.5. Challenges of EV Preparation
4. EV Cargo Loading Methods
4.1. Endogenous Loading
4.1.1. Cells Transfection/Transduction for RNA Loading
4.1.2. EV-Associated Motifs for RNA Loading
4.1.3. Interaction of RNA with Proteins Enriched within EV Membranes
4.1.4. RNA Enrichment on the Plasma Membrane
4.2. Endogenous Protein Loading
- 1)
- Fusing cargo to proteins enriched on EV membranes;
- 2)
- Using post-translational modifications of the cargo proteins;
- 3)
- Viral protein-assisted loading.
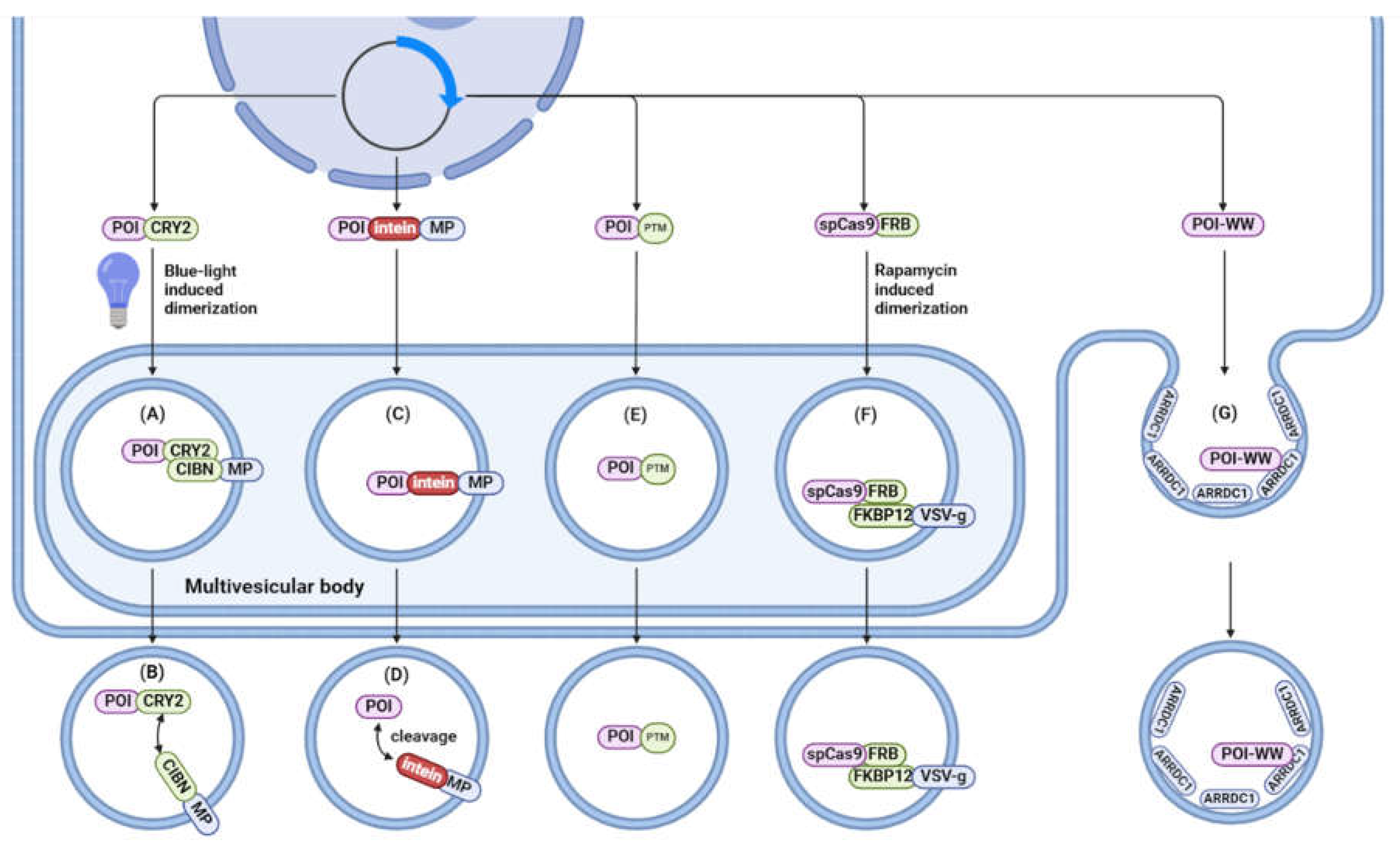
4.2.1. Fusion with Proteins Enriched within EV Membranes
4.2.2. Post-Translational Modifications of the Protein
4.2.3. Viral Protein-Assisted Loading
4.3. Exogenous Loading
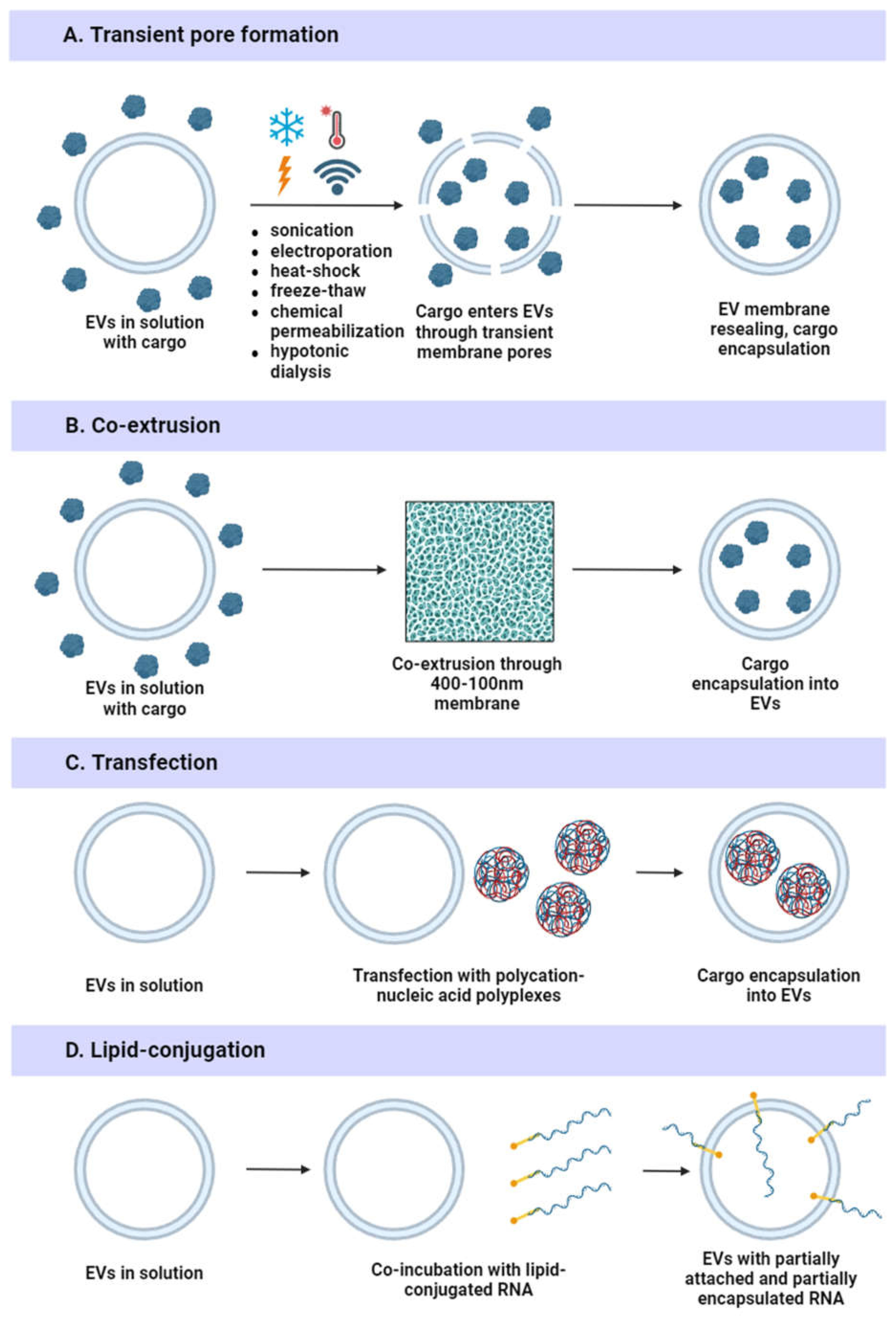
4.3.1. Physical Methods
4.3.2. Chemical Methods
5. Challenges of Loading Cargo into EVs
6. Surface Display of Functional Moieties on EVs
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ginini, L.; Billan, S.; Fridman, E.; Gil, Z. Insight into Extracellular Vesicle-Cell Communication: From Cell Recognition to Intracellular Fate. Cells 2022, 11, 1375. [Google Scholar] [CrossRef] [PubMed]
- Cecchin, R.; Troyer, Z.; Witwer, K.; Morris, K.V. Extracellular vesicles: The next generation in gene therapy delivery. Mol. Ther. 2023, 31, 1225–1230. [Google Scholar] [CrossRef]
- Banks, W.A.; Sharma, P.; Bullock, K.M.; Hansen, K.M.; Ludwig, N.; Whiteside, T.L. Transport of Extracellular Vesicles across the Blood-Brain Barrier: Brain Pharmacokinetics and Effects of Inflammation. Int. J. Mol. Sci. 2020, 21, 4407. [Google Scholar] [CrossRef]
- El Andaloussi, S.; Mäger, I.; Breakefield, X.O.; Wood, M.J.A. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat. Rev. Drug Discov. 2013, 12, 347–357. [Google Scholar] [CrossRef]
- Wei, Z.; Chen, Z.; Zhao, Y.; Fan, F.; Xiong, W.; Song, S.; Yin, Y.; Hu, J.; Yang, K.; Yang, L.; et al. Mononuclear phagocyte system blockade using extracellular vesicles modified with CD47 on membrane surface for myocardial infarction reperfusion injury treatment. Biomaterials 2021, 275, 121000. [Google Scholar] [CrossRef]
- Parodi, A.; Molinaro, R.; Sushnitha, M.; Evangelopoulos, M.; Martinez, J.O.; Arrighetti, N.; Corbo, C.; Tasciotti, E. Bio-inspired engineering of cell- and virus-like nanoparticles for drug delivery. Biomaterials 2017, 147, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.T.; Hosseini-Beheshti, E.; Afrose, D.; Ding, X.; Xia, B.; Grau, G.; Little, C.; McClements, L.; Li, J. Extracellular Vesicles from Mesenchymal Stromal Cells for the Treatment of Inflammation-Related Conditions. Int. J. Mol. Sci. 2021, 22, 3023. [Google Scholar] [CrossRef] [PubMed]
- Duong, A.; Parmar, G.; Kirkham, A.M.; Burger, D.; Allan, D.S. Registered clinical trials investigating treatment with cell-derived extracellular vesicles: a scoping review. Cytotherapy 2023, 25, 939–945. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular vesicles as a next-generation drug delivery platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Nieuwland, R.; Falcón-Pérez, J.M.; Théry, C.; Witwer, K.W. Rigor and standardization of extracellular vesicle research: Paving the road towards robustness. J. Extracell. Vesicles 2020, 10. [Google Scholar] [CrossRef]
- Brezgin, S.; Parodi, A.; Kostyusheva, A.; Ponomareva, N.; Lukashev, A.; Sokolova, D.; Pokrovsky, V.S.; Slatinskaya, O.; Maksimov, G.; Zamyatnin, A.A.; et al. Technological aspects of manufacturing and analytical control of biological nanoparticles. Biotechnol. Adv. 2023, 64, 108122. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Zhang, Q.; Franklin, J.L.; Coffey, R.J. Extracellular vesicles and nanoparticles: emerging complexities. Trends Cell Biol. 2023, 33, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, Y.; Peng, J.; Wu, D.; Zhao, X.; Cui, Y.; Chen, L.; Yan, X.; Du, Y.; Yu, L. Discovery of the migrasome, an organelle mediating release of cytoplasmic contents during cell migration. Cell Res. 2014, 25, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Melentijevic, I.; Toth, M.L.; Arnold, M.L.; Guasp, R.J.; Harinath, G.; Nguyen, K.C.; Taub, D.; Parker, J.A.; Neri, C.; Gabel, C.V.; et al. elegans neurons jettison protein aggregates and mitochondria under neurotoxic stress. Nature 2017, 542, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Colao, I.L.; Corteling, R.; Bracewell, D.; Wall, I. Manufacturing Exosomes: A Promising Therapeutic Platform. Trends Mol. Med. 2018, 24, 242–256. [Google Scholar] [CrossRef]
- EV-TRACK Consortium; Van Deun, J.; Mestdagh, P.; Agostinis, P.; Akay, Ö.; Anand, S.; Anckaert, J.; Martinez, Z.A.; Baetens, T.; Beghein, E.; et al. EV-TRACK: Transparent reporting and centralizing knowledge in extracellular vesicle research. Nat. Methods 2017, 14, 228–232. [Google Scholar] [CrossRef]
- Gardiner, C.; Di Vizio, D.; Sahoo, S.; Théry, C.; Witwer, K.W.; Wauben, M.; Hill, A.F. Techniques used for the isolation and characterization of extracellular vesicles: Results of a worldwide survey. J. Extracell. Vesicles 2016, 5, 32945. [Google Scholar] [CrossRef]
- Thery, C.; Clayton, A.; Amigorena, S.; Raposo, G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 3. [Google Scholar] [CrossRef]
- Konoshenko, M.Y.; Lekchnov, E.A.; Vlassov, A.V.; Laktionov, P.P. Isolation of Extracellular Vesicles: General Methodologies and Latest Trends. BioMed Res. Int. 2018, 2018, 8545347. [Google Scholar] [CrossRef]
- Zarovni, N.; Corrado, A.; Guazzi, P.; Zocco, D.; Lari, E.; Radano, G.; Muhhina, J.; Fondelli, C.; Gavrilova, J.; Chiesi, A. Integrated isolation and quantitative analysis of exosome shuttled proteins and nucleic acids using immunocapture approaches. Methods 2015, 87, 46–58. [Google Scholar] [CrossRef]
- Witwer, K.W.; Buzás, E.I.; Bemis, L.T.; Bora, A.; Lässer, C.; Lötvall, J.; Nolte-’t Hoen, E.N.; Piper, M.G.; Sivaraman, S.; Skog, J.; et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J. Extracell. Vesicles 2013, 2, 20360. [Google Scholar] [CrossRef] [PubMed]
- Linares, R.; Tan, S.; Gounou, C.; Arraud, N.; Brisson, A.R. High-speed centrifugation induces aggregation of extracellular vesicles. J. Extracell. Vesicles 2015, 4, 29509. [Google Scholar] [CrossRef] [PubMed]
- Lobb, R.j.; Becker, M.; Wen, S.W.; Wong, C.S.F.; Wiegmans, A.P.; Leimgruber, A.; Möller, A. Optimized exosome isolation protocol for cell culture supernatant and human plasma. J. Extracell. Vesicles 2015, 4, 27031. [Google Scholar] [CrossRef]
- Benayas, B.; Morales, J.; Egea, C.; Armisén, P.; Yáñez-Mó, M. Optimization of extracellular vesicle isolation and their separation from lipoproteins by size exclusion chromatography. J. Extracell. Biol. 2023, 2, e100. [Google Scholar] [CrossRef]
- Livshits, M.A.; Khomyakova, E.; Evtushenko, E.G.; Lazarev, V.N.; Kulemin, N.A.; Semina, S.E.; Generozov, E.V.; Govorun, V.M. Isolation of exosomes by differential centrifugation: Theoretical analysis of a commonly used protocol. Sci. Rep. 2015, 5, 17319–17319. [Google Scholar] [CrossRef] [PubMed]
- Lamparski, H.G.; Metha-Damani, A.; Yao, J.-Y.; Patel, S.; Hsu, D.-H.; Ruegg, C.; Le Pecq, J.-B. Production and characterization of clinical grade exosomes derived from dendritic cells. J. Immunol. Methods 2002, 270, 211–226. [Google Scholar] [CrossRef]
- Tauro, B.J.; Greening, D.W.; Mathias, R.A.; Ji, H.; Mathivanan, S.; Scott, A.M.; Simpson, R.J. Comparison of ultracentrifugation, density gradient separation, and immunoaffinity capture methods for isolating human colon cancer cell line LIM1863-derived exosomes. Methods 2012, 56, 293–304. [Google Scholar] [CrossRef]
- Onódi, Z.; Pelyhe, C.; Nagy, C.T.; Brenner, G.B.; Almási, L.; Kittel, M.; Manček-Keber, M.; Ferdinandy, P.; Buzás, E.I.; Giricz, Z. Isolation of High-Purity Extracellular Vesicles by the Combination of Iodixanol Density Gradient Ultracentrifugation and Bind-Elute Chromatography From Blood Plasma. Front. Physiol. 2018, 9, 1479. [Google Scholar] [CrossRef]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicles 2024, 13, e12404. [Google Scholar] [CrossRef]
- Sanz-Ros, J.; Mas-Bargues, C.; Romero-García, N.; Huete-Acevedo, J.; Dromant, M.; Borrás, C. Extracellular Vesicles as Therapeutic Resources in the Clinical Environment. Int. J. Mol. Sci. 2023, 24, 2344. [Google Scholar] [CrossRef]
- El Baradie, K.B.Y.; Nouh, M.; Iii, F.O.; Liu, Y.; Fulzele, S.; Eroglu, A.; Hamrick, M.W. Freeze-Dried Extracellular Vesicles From Adipose-Derived Stem Cells Prevent Hypoxia-Induced Muscle Cell Injury. Front. Cell Dev. Biol. 2020, 8, 181. [Google Scholar] [CrossRef]
- Benedikter, B.J.; Bouwman, F.G.; Vajen, T.; Heinzmann, A.C.A.; Grauls, G.; Mariman, E.C.; Wouters, E.F.M.; Savelkoul, P.H.; Lopez-Iglesias, C.; Koenen, R.R.; et al. Ultrafiltration combined with size exclusion chromatography efficiently isolates extracellular vesicles from cell culture media for compositional and functional studies. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Visan, K.S.; Wu, L.-Y.; Voss, S.; Wuethrich, A.; Möller, A. Status quo of Extracellular Vesicle isolation and detection methods for clinical utility. Semin. Cancer Biol. 2023, 88, 157–171. [Google Scholar] [CrossRef] [PubMed]
- Polyakov, Y.S.; Zydney, A.L. Ultrafiltration membrane performance: Effects of pore blockage/constriction. J. Membr. Sci. 2013, 434, 106–120. [Google Scholar] [CrossRef]
- Chernyshev, V.S.; Chuprov-Netochin, R.N.; Tsydenzhapova, E.; Svirshchevskaya, E.V.; Poltavtseva, R.A.; Merdalimova, A.; Yashchenok, A.; Keshelava, A.; Sorokin, K.; Keshelava, V.; et al. Asymmetric depth-filtration: A versatile and scalable method for high-yield isolation of extracellular vesicles with low contamination. J. Extracell. Vesicles 2022, 11, e12256. [Google Scholar] [CrossRef] [PubMed]
- Liangsupree, T.; Multia, E.; Riekkola, M.-L. Modern isolation and separation techniques for extracellular vesicles. J. Chromatogr. A 2020, 1636, 461773. [Google Scholar] [CrossRef] [PubMed]
- Busatto, S.; Vilanilam, G.; Ticer, T.; Lin, W.-L.; Dickson, D.W.; Shapiro, S.; Bergese, P.; Wolfram, J. Tangential Flow Filtration for Highly Efficient Concentration of Extracellular Vesicles from Large Volumes of Fluid. Cells 2018, 7, 273. [Google Scholar] [CrossRef]
- Visan, K.S.; Lobb, R.J.; Ham, S.; Lima, L.G.; Palma, C.; Edna, C.P.Z.; Wu, L.; Gowda, H.; Datta, K.K.; Hartel, G.; et al. Comparative analysis of tangential flow filtration and ultracentrifugation, both combined with subsequent size exclusion chromatography, for the isolation of small extracellular vesicles. J. Extracell. Vesicles 2022, 11, e12266. [Google Scholar] [CrossRef]
- Heath, N.; Grant, L.; De Oliveira, T.M.; Rowlinson, R.; Osteikoetxea, X.; Dekker, N.; Overman, R. Rapid isolation and enrichment of extracellular vesicle preparations using anion exchange chromatography. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Böing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.W.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef]
- Staubach, S.; Bauer, F.N.; Tertel, T.; Börger, V.; Stambouli, O.; Salzig, D.; Giebel, B. Scaled preparation of extracellular vesicles from conditioned media. Adv. Drug Deliv. Rev. 2021, 177, 113940. [Google Scholar] [CrossRef]
- Muller, L.; Hong, C.-S.; Stolz, D.B.; Watkins, S.C.; Whiteside, T.L. Isolation of biologically-active exosomes from human plasma. J. Immunol. Methods 2014, 411, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.D.; Shah, S. Methods of isolating extracellular vesicles impact down-stream analyses of their cargoes. Methods 2015, 87, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Lins, P.M.P.; Pirlet, E.; Szymonik, M.; Bronckaers, A.; Nelissen, I. Manufacture of extracellular vesicles derived from mesenchymal stromal cells. Trends Biotechnol. 2023, 41, 965–981. [Google Scholar] [CrossRef]
- Sidhom, K.; Obi, P.O.; Saleem, A. A Review of Exosomal Isolation Methods: Is Size Exclusion Chromatography the Best Option? Int. J. Mol. Sci. 2020, 21, 6466. [Google Scholar] [CrossRef]
- Wang, L.; Wang, D.; Ye, Z.; Xu, J. Engineering Extracellular Vesicles as Delivery Systems in Therapeutic Applications. Adv. Sci. 2023, 10, e2300552. [Google Scholar] [CrossRef]
- Ludwig, A.; De Miroschedji, K.; Doeppner, T.R.; Börger, V.; Ruesing, J.; Rebmann, V.; Durst, S.; Jansen, S.; Bremer, M.; Behrmann, E.; et al. Precipitation with polyethylene glycol followed by washing and pelleting by ultracentrifugation enriches extracellular vesicles from tissue culture supernatants in small and large scales. J. Extracell. Vesicles 2018, 7, 1528109. [Google Scholar] [CrossRef]
- Rekker, K.; Saare, M.; Roost, A.M.; Kubo, A.-L.; Zarovni, N.; Chiesi, A.; Salumets, A.; Peters, M. Comparison of serum exosome isolation methods for microRNA profiling. Clin. Biochem. 2014, 47, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, M.; Névo, N.; Jouve, M.; Valenzuela, J.I.; Maurin, M.; Verweij, F.J.; Palmulli, R.; Lankar, D.; Dingli, F.; Loew, D.; et al. Specificities of exosome versus small ectosome secretion revealed by live intracellular tracking of CD63 and CD9. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Li, P.; Kaslan, M.; Lee, S.H.; Yao, J.; Gao, Z. Progress in Exosome Isolation Techniques. Theranostics 2017, 7, 789–804. [Google Scholar] [CrossRef]
- Doyle, L.; Wang, M. Overview of Extracellular Vesicles, Their Origin, Composition, Purpose, and Methods for Exosome Isolation and Analysis. Cells 2019, 8, 727. [Google Scholar] [CrossRef]
- Karimi, N.; Dalirfardouei, R.; Dias, T.; Lötvall, J.; Lässer, C. Tetraspanins distinguish separate extracellular vesicle subpopulations in human serum and plasma – Contributions of platelet extracellular vesicles in plasma samples. J. Extracell. Vesicles 2022, 11, e12213. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Control. Release 2020, 329, 894–906. [Google Scholar] [CrossRef] [PubMed]
- De Voogt, W.S.; Frunt, R.; Leandro, R.M.; Triesscheijn, C.S.; Monica, B.; Paspali, I.; Tielemans, M.; Francois, J.J.J.M.; Seinen, C.W.; De Jong, O.G.; et al. EV-Elute: A Universal Platform for Enrichment of Functional Surface Marker-Defined Extracellular Vesicle Subpopulations (Preprint). BioRxiv 2023. [Google Scholar]
- Matsuzaka, Y.; Yashiro, R. Advances in Purification, Modification, and Application of Extracellular Vesicles for Novel Clinical Treatments. Membranes 2022, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Mao, J.; Barrero, R.A.; Wang, P.; Zhang, F.; Wang, T. Development of a CD63 Aptamer for Efficient Cancer Immunochemistry and Immunoaffinity-Based Exosome Isolation. Molecules 2020, 25, 5585. [Google Scholar] [CrossRef]
- Bonner, S.E.; van de Wakker, S.I.; Phillips, W.; Willms, E.; Sluijter, J.P.G.; Hill, A.F.; Wood, M.J.A.; Vader, P. Scalable purification of extracellular vesicles with high yield and purity using multimodal flowthrough chromatography. J. Extracell. Biol. 2024, 3, e138. [Google Scholar] [CrossRef]
- Hallal, S.; Tűzesi, G.E.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the extracellular vesicle surface for clinical molecular biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef]
- Seo, N.; Nakamura, J.; Kaneda, T.; Tateno, H.; Shimoda, A.; Ichiki, T.; Furukawa, K.; Hirabayashi, J.; Akiyoshi, K.; Shiku, H. Distinguishing functional exosomes and other extracellular vesicles as a nucleic acid cargo by the anion-exchange method. J. Extracell. Vesicles 2022, 11, e12205. [Google Scholar] [CrossRef]
- Notarangelo, M.; Zucal, C.; Modelska, A.; Pesce, I.; Scarduelli, G.; Potrich, C.; Lunelli, L.; Pederzolli, C.; Pavan, P.; la Marca, G.; et al. Ultrasensitive detection of cancer biomarkers by nickel-based isolation of polydisperse extracellular vesicles from blood. EBioMedicine 2019, 43, 114–126. [Google Scholar] [CrossRef]
- Koch, L.F.; Best, T.; Wüstenhagen, E.; Adrian, K.; Rammo, O.; Saul, M.J. Novel insights into the isolation of extracellular vesicles by anion exchange chromatography. Front. Bioeng. Biotechnol. 2024, 11, 1298892. [Google Scholar] [CrossRef] [PubMed]
- Stam, J.; Bartel, S.; Bischoff, R.; Wolters, J.C. Isolation of extracellular vesicles with combined enrichment methods. J. Chromatogr. B Analyt Technol. Biomed. Life Sci. 2021, 1169, 122604. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Nguyen, L.T.H.; Hickey, R.; Walters, N.; Wang, X.; Kwak, K.J.; Lee, L.J.; Palmer, A.F.; Reátegui, E. Immunomagnetic sequential ultrafiltration (iSUF) platform for enrichment and purification of extracellular vesicles from biofluids. Sci. Rep. 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Paganini, C.; Palmiero, U.C.; Pocsfalvi, G.; Touzet, N.; Bongiovanni, A.; Arosio, P. Scalable Production and Isolation of Extracellular Vesicles: Available Sources and Lessons from Current Industrial Bioprocesses. Biotechnol. J. 2019, 14, e1800528. [Google Scholar] [CrossRef]
- Vo, N.; Tran, C.; Tran, N.H.B.; Nguyen, N.T.; Nguyen, T.; Ho, D.T.K.; Nguyen, D.D.N.; Pham, T.; Nguyen, T.A.; Phan, H.T.N.; et al. A novel multi-stage enrichment workflow and comprehensive characterization for HEK293F-derived extracellular vesicles. J. Extracell. Vesicles 2024, 13, e12454. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Yuan, Y.; Xiong, Y.; Xu, H.; Pan, W.; Pan, H.; Zhu, Z. Microvesicles as drug delivery systems: A new frontier for bionic therapeutics in cancer. J. Drug Deliv. Sci. Technol. 2023, 79. [Google Scholar] [CrossRef]
- Johnston, J.; Stone, T.; Wang, Y. Biomaterial-enabled 3D cell culture technologies for extracellular vesicle manufacturing. Biomater. Sci. 2023, 11, 4055–4072. [Google Scholar] [CrossRef]
- Paolini, L.; Monguió-Tortajada, M.; Costa, M.; Antenucci, F.; Barilani, M.; Clos-Sansalvador, M.; Andrade, A.C.; Driedonks, T.A.P.; Giancaterino, S.; Kronstadt, S.M.; et al. Large-scale production of extracellular vesicles: Report on the “massivEVs” ISEV workshop. J. Extracell. Biol. 2022, 1. [Google Scholar] [CrossRef]
- Ng, C.Y.; Kee, L.T.; Al-Masawa, M.E.; Lee, Q.H.; Subramaniam, T.; Kok, D.; Ng, M.H.; Law, J.X. Scalable Production of Extracellular Vesicles and Its Therapeutic Values: A Review. Int. J. Mol. Sci. 2022, 23, 7986. [Google Scholar] [CrossRef]
- Goncalves, J.P.; Ghebosu, R.E.; Tan, X.N.S.; Iannotta, D.; Koifman, N.; Wolfram, J. Hyaluronic acid: An overlooked extracellular vesicle contaminant. J. Extracell. Vesicles 2023, 12, e12362. [Google Scholar] [CrossRef]
- Shelke, G.V.; Lässer, C.; Gho, Y.S.; Lötvall, J. Importance of exosome depletion protocols to eliminate functional and RNA-containing extracellular vesicles from fetal bovine serum. J. Extracell. Vesicles 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lee, Y.; Johansson, H.J.; Mäger, I.; Vader, P.; Nordin, J.Z.; Wiklander, O.P.B.; Lehtiö, J.; Wood, M.J.A.; EL Andaloussi, S. Serum-free culture alters the quantity and protein composition of neuroblastoma-derived extracellular vesicles. J. Extracell. Vesicles 2015, 4, 26883. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, S.; Mareschi, K.; Labanca, L.; Lucania, G.; Leone, M.; Sanavio, F.; Castello, L.; Rustichelli, D.; Signorino, E.; Gunetti, M.; et al. Inactivated human platelet lysate with psoralen: a new perspective for mesenchymal stromal cell production in Good Manufacturing Practice conditions. Cytotherapy 2014, 16, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Marinho, A.; Nunes, C.; Reis, S. Hyaluronic Acid: A Key Ingredient in the Therapy of Inflammation. Biomolecules 2021, 11, 1518. [Google Scholar] [CrossRef]
- van de Wakker, S.I.; Bauzá-Martinez, J.; Arceo, C.R.; Manjikian, H.; Blok, C.J.B.S.; Roefs, M.T.; Willms, E.; Maas, R.G.C.; Pronker, M.F.; de Jong, O.G.; et al. Size matters: Functional differences of small extracellular vesicle subpopulations in cardiac repair responses. J. Extracell. Vesicles 2024, 13, e12396. [Google Scholar] [CrossRef]
- Görgens, A.; Corso, G.; Hagey, D.W.; Wiklander, R.J.; Gustafsson, M.O.; Felldin, U.; Lee, Y.; Bostancioglu, R.B.; Sork, H.; Liang, X.; et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J. Extracell. Vesicles 2022, 11, e12238. [Google Scholar] [CrossRef]
- Trenkenschuh, E.; Richter, M.; Heinrich, E.; Koch, M.; Fuhrmann, G.; Friess, W. Enhancing the Stabilization Potential of Lyophilization for Extracellular Vesicles. Adv. Heal. Mater. 2021, 11, 2100538. [Google Scholar] [CrossRef]
- Susa, F.; Limongi, T.; Borgione, F.; Peiretti, S.; Vallino, M.; Cauda, V.; Pisano, R. Comparative Studies of Different Preservation Methods and Relative Freeze-Drying Formulations for Extracellular Vesicle Pharmaceutical Applications. ACS Biomater. Sci. Eng. 2023, 9, 5871–5885. [Google Scholar] [CrossRef]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical Translation of Extracellular Vesicles. Adv. Heal. Mater. 2023, 12, e2301010. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; De Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Jeske, R.; Liu, C.; Duke, L.; Castro, M.L.C.; Muok, L.; Arthur, P.; Singh, M.; Jung, S.; Sun, L.; Li, Y. Upscaling human mesenchymal stromal cell production in a novel vertical-wheel bioreactor enhances extracellular vesicle secretion and cargo profile. Bioact. Mater. 2023, 25, 732–747. [Google Scholar] [CrossRef] [PubMed]
- Mendt, M.; Kamerkar, S.; Sugimoto, H.; McAndrews, K.M.; Wu, C.-C.; Gagea, M.; Yang, S.; Blanko, E.V.R.; Peng, Q.; Ma, X.; et al. Generation and testing of clinical-grade exosomes for pancreatic cancer. J. Clin. Investig. 2018, 3. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Landry, M.P.; Moore, A.; Coreas, R. The protein corona from nanomedicine to environmental science. Nat. Rev. Mater. 2023, 8, 422–438. [Google Scholar] [CrossRef] [PubMed]
- Tenzer, S.; Docter, D.; Kuharev, J.; Musyanovych, A.; Fetz, V.; Hecht, R.; Schlenk, F.; Fischer, D.; Kiouptsi, K.; Reinhardt, C.; et al. Rapid formation of plasma protein corona critically affects nanoparticle pathophysiology. Nat. Nanotechnol. 2013, 8, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Corbo, C.; Molinaro, R.; Parodi, A.; Furman, N.E.T.; Salvatore, F.; Tasciotti, E. The Impact of Nanoparticle Protein Corona on Cytotoxicity, Immunotoxicity and Target Drug Delivery. Nanomedicine 2015, 11, 81–100. [Google Scholar] [CrossRef] [PubMed]
- Francia, V.; Schiffelers, R.M.; Cullis, P.R.; Witzigmann, D. The Biomolecular Corona of Lipid Nanoparticles for Gene Therapy. Bioconjugate Chem. 2020, 31, 2046–2059. [Google Scholar] [CrossRef]
- Corbo, C.; Molinaro, R.; Taraballi, F.; Furman, N.E.T.; Sherman, M.B.; Parodi, A.; Salvatore, F.; Tasciotti, E. Effects of the protein corona on liposome–liposome and liposome–cell interactions. Int. J. Nanomed. 2016, 3049–3063. [Google Scholar]
- Lima, T.; Bernfur, K.; Vilanova, M.; Cedervall, T. Understanding the Lipid and Protein Corona Formation on Different Sized Polymeric Nanoparticles. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Durán, N.; Silveira, C.P.; Durán, M.; Martinez, D.S.T. Silver nanoparticle protein corona and toxicity: a mini-review. J. Nanobiotechnology 2015, 13, 1–17. [Google Scholar] [CrossRef]
- Tóth, E.; Turiák, L.; Visnovitz, T.; Cserép, C.; Mázló, A.; Sódar, B.W.; Försönits, A.I.; Petővári, G.; Sebestyén, A.; Komlósi, Z.; et al. Formation of a protein corona on the surface of extracellular vesicles in blood plasma. J. Extracell. Vesicles 2021, 10, e12140. [CrossRef]
- Wolf, M.; Poupardin, R.W.; Ebner-Peking, P.; Andrade, A.C.; Blöchl, C.; Obermayer, A.; Gomes, F.G.; Vari, B.; Maeding, N.; Eminger, E.; et al. A functional corona around extracellular vesicles enhances angiogenesis, skin regeneration and immunomodulation. J. Extracell. Vesicles 2022, 11, e12207. [Google Scholar] [CrossRef] [PubMed]
- Haney, M.J.; Klyachko, N.L.; Zhao, Y.; Gupta, R.; Plotnikova, E.G.; He, Z.; Patel, T.; Piroyan, A.; Sokolsky, M.; Kabanov, A.V.; et al. Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J. Control. Release 2015, 207, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Godbout, K.; Lamothe, G.; Tremblay, J.P. CRISPR-Cas9 delivery strategies with engineered extracellular vesicles. Mol. Ther. - Nucleic Acids 2023, 34, 102040. [Google Scholar] [CrossRef]
- Walker, S.; Busatto, S.; Pham, A.; Tian, M.; Suh, A.; Carson, K.; Quintero, A.; Lafrence, M.; Malik, H.; Santana, M.X.; et al. Extracellular vesicle-based drug delivery systems for cancer treatment. Theranostics 2019, 9, 8001–8017. [Google Scholar] [CrossRef]
- Han, Y.; Jones, T.W.; Dutta, S.; Zhu, Y.; Wang, X.; Narayanan, S.P.; Fagan, S.C.; Zhang, D. Overview and Update on Methods for Cargo Loading into Extracellular Vesicles. Processes 2021, 9, 356. [Google Scholar] [CrossRef]
- Dimik, M.; Abeysinghe, P.; Logan, J.; Mitchell, M. The exosome: a review of current therapeutic roles and capabilities in human reproduction. Drug Deliv. Transl. Res. 2022, 13, 473–502. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, Q.; Sun, X.; Wang, Y. Recent Advancements in the Loading and Modification of Therapeutic Exosomes. Front. Bioeng. Biotechnol. 2020, 8. [Google Scholar] [CrossRef]
- Kenari, A.N.; Cheng, L.; Hill, A.F. Methods for loading therapeutics into extracellular vesicles and generating extracellular vesicles mimetic-nanovesicles. Methods 2020, 177, 103–113. [Google Scholar] [CrossRef]
- McCann, J.; Sosa-Miranda, C.D.; Guo, H.; Reshke, R.; Savard, A.; Buzatto, A.Z.; Taylor, J.A.; Li, L.; Gibbings, D.J. Contaminating transfection complexes can masquerade as small extracellular vesicles and impair their delivery of RNA. J. Extracell. Vesicles 2022, 11. [Google Scholar] [CrossRef]
- McConnell, R.E.; Youniss, M.; Gnanasambandam, B.; Shah, P.; Zhang, W.; Finn, J.D. Transfection reagent artefact likely accounts for some reports of extracellular vesicle function. J. Extracell. Vesicles 2022, 11. [Google Scholar] [CrossRef]
- Rädler, J.; Gupta, D.; Zickler, A.; EL Andaloussi, S. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol. Ther. 2023, 31, 1231–1250. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.; Baena-Lopez, L.A. Unpacking extracellular vesicles: RNA cargo loading and function. J. Extracell. Biol. 2022, 1. [Google Scholar] [CrossRef] [PubMed]
- Bolukbasi, M.F.; Mizrak, A.; Ozdener, G.B.; Madlener, S.; Ströbel, T.; Erkan, E.P.; Fan, J.-B.; O Breakefield, X.; Saydam, O. miR-1289 and “Zipcode”-like Sequence Enrich mRNAs in Microvesicles. Mol. Ther. - Nucleic Acids 2012, 1, e10. [Google Scholar] [CrossRef]
- Temoche-Diaz, M.M.; Shurtleff, M.J.; Nottingham, R.M.; Yao, J.; Fadadu, R.P.; Lambowitz, A.M.; Schekman, R.; States, U. Distinct mechanisms of microRNA sorting into cancer cell-derived extracellular vesicle subtypes. eLife 2019, 8. [Google Scholar] [CrossRef]
- Villarroya-Beltri, C.; Gutiérrez-Vázquez, C.; Sánchez-Cabo, F.; Pérez-Hernández, D.; Vázquez, J.; Martin-Cofreces, N.; Martinez-Herrera, D.J.; Pascual-Montano, A.; Mittelbrunn, M.; Sánchez-Madrid, F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013, 4, 2980. [Google Scholar] [CrossRef]
- Santangelo, L.; Giurato, G.; Cicchini, C.; Montaldo, C.; Mancone, C.; Tarallo, R.; Battistelli, C.; Alonzi, T.; Weisz, A.; Tripodi, M. The RNA-Binding Protein SYNCRIP Is a Component of the Hepatocyte Exosomal Machinery Controlling MicroRNA Sorting. Cell Rep. 2016, 17, 799–808. [Google Scholar] [CrossRef]
- Rufino-Ramos, D.; Albuquerque, P.R.; Leandro, K.; Carmona, V.; Martins, I.M.; Fernandes, R.; Henriques, C.; Lobo, D.; Faro, R.; Perfeito, R.; et al. Extracellular vesicle-based delivery of silencing sequences for the treatment of Machado-Joseph disease/spinocerebellar ataxia type 3. Mol. Ther. 2023, 31, 1275–1292. [Google Scholar] [CrossRef] [PubMed]
- Kuzembayeva, M.; Dilley, K.; Sardo, L.; Hu, W.-S. Life of psi: How full-length HIV-1 RNAs become packaged genomes in the viral particles. Virology 2014, 454-455, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Gee, P.; Lung, M.S.Y.; Okuzaki, Y.; Sasakawa, N.; Iguchi, T.; Makita, Y.; Hozumi, H.; Miura, Y.; Yang, L.F.; Iwasaki, M.; et al. Extracellular nanovesicles for packaging of CRISPR-Cas9 protein and sgRNA to induce therapeutic exon skipping. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Ivanov, A.V.; Valuev-Elliston, V.T.; Ivanova, O.N.; Kochetkov, S.N.; Starodubova, E.S.; Bartosch, B.; Isaguliants, M.G. Oxidative Stress during HIV Infection: Mechanisms and Consequences. Oxid. Med. Cell Longev. 2016, 2016, 8910396. [Google Scholar] [CrossRef]
- Hentze, M.W.; Castello, A.; Schwarzl, T.; Preiss, T. A brave new world of RNA-binding proteins. Nat. Rev. Mol. Cell Biol. 2018, 19, 327–341. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Sun, W.; Duan, Y.; Yang, G.; Yuan, L. In Vitro and in Vivo RNA Inhibition by CD9-HuR Functionalized Exosomes Encapsulated with miRNA or CRISPR/dCas. Nano Lett. 2018, 19, 19–28. [Google Scholar] [CrossRef]
- Hung, M.E.; Leonard, J.N. A platform for actively loading cargo RNA to elucidate limiting steps in EV-mediated delivery. J. Extracell. Vesicles 2016, 5, 31027. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Lyu, P.; Yoo, K.; Yadav, M.K.; Singh, R.; Atala, A.; Lu, B. Engineered extracellular vesicles as versatile ribonucleoprotein delivery vehicles for efficient and safe CRISPR genome editing. J. Extracell. Vesicles 2021, 10, e12076. [Google Scholar] [CrossRef]
- Kebaara, B.W.; Atkin, A.L. Long 3′-UTRs target wild-type mRNAs for nonsense-mediated mRNA decay in Saccharomyces cerevisiae. Nucleic Acids Res. 2009, 37, 2771–2778. [Google Scholar] [CrossRef]
- Li, W.; Maekiniemi, A.; Sato, H.; Osman, C.; Singer, R.H. An improved imaging system that corrects MS2-induced RNA destabilization. Nat. Methods 2022, 19, 1558–1562. [Google Scholar] [CrossRef]
- Zickler, A.M.; Liang, X.; De Luca, M.; Gupta, D.; Corso, G.; Errichelli, L.; Hean, J.; Kamei, N.; Niu, Z.; Zhou, G.; Roudi, S.; Nordin, J.; Castilla-Llorente, V.; Andaloussi, S.E.L. Novel endogenous engineering platform for robust loading and delivery of functional mRNA by extracellular vesicles. BioRxiv 5330, 2023.03.17.533081. Available online: https://www.biorxiv.org/content/10.1101/2023.03.17.533081v1%0Ahttps://www.biorxiv.org/content/10.1101/2023.03.17.533081v1.abstract%0Ahttps://www.biorxiv.org/content/10.1101/2023.03.17.533081v1%0Ahttps://www.biorxiv.org/content/10.1101/2023.03.17.533081v1.
- Kojima, R.; Bojar, D.; Rizzi, G.; Hamri, G.C.-E.; El-Baba, M.D.; Saxena, P.; Ausländer, S.; Tan, K.R.; Fussenegger, M. Designer exosomes produced by implanted cells intracerebrally deliver therapeutic cargo for Parkinson’s disease treatment. Nat. Commun. 2018, 9, 1305. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhang, X.; Xie, F.; Xu, B.; Xie, P.; Yang, T.; Shi, Q.; Zhang, C.-Y.; Zhang, Y.; Chen, J.; et al. An engineered exosome for delivering sgRNA:Cas9 ribonucleoprotein complex and genome editing in recipient cells. Biomater. Sci. 2020, 8, 2966–2976. [Google Scholar] [CrossRef]
- Osteikoetxea, X.; Silva, A.; Lázaro-Ibáñez, E.; Salmond, N.; Shatnyeva, O.; Stein, J.; Schick, J.; Wren, S.; Lindgren, J.; Firth, M.; et al. Engineered Cas9 extracellular vesicles as a novel gene editing tool. J. Extracell. Vesicles 2022, 11, e12225. [Google Scholar] [CrossRef]
- Huang, L.; Gu, N.; Zhang, X.; Wang, D. Light-Inducible Exosome-Based Vehicle for Endogenous RNA Loading and Delivery to Leukemia Cells. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- King, A.; Gottlieb, E.; Brooks, D.G.; Murphy, M.P.; Dunaief, J.L. Mitochondria-derived Reactive Oxygen Species Mediate Blue Light–induced Death of Retinal Pigment Epithelial Cells¶. Photochem. Photobiol. 2004, 79, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Zarifyan, D.; Goptar, I.; Chulanov, V. Orthologous CRISPR/Cas9 systems for specific and efficient degradation of covalently closed circular DNA of hepatitis B virus. Cell. Mol. Life Sci. 2019, 76, 1779–1794. [Google Scholar] [CrossRef]
- Kostyushev, D.; Brezgin, S.; Kostyusheva, A.; Ponomareva, N.; Bayurova, E.; Zakirova, N.; Kondrashova, A.; Goptar, I.; Nikiforova, A.; Sudina, A.; et al. Transient and tunable CRISPRa regulation of APOBEC/AID genes for targeting hepatitis B virus. Mol. Ther. - Nucleic Acids 2023, 32, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Ponomareva, N.; Zakirova, N.F.; Egorshina, A.; Yanvarev, D.V.; Bayurova, E.; Sudina, A.; Goptar, I.; et al. Depleting hepatitis B virus relaxed circular DNA is necessary for resolution of infection by CRISPR-Cas9. Mol. Ther. - Nucleic Acids 2023, 31, 482–493. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-S.; Li, Q.-C.; Yin, C.-Q.; Xue, W.; Song, C.-Q. Advances in CRISPR/Cas-based Gene Therapy in Human Genetic Diseases. Theranostics 2020, 10, 4374–4382. [Google Scholar] [CrossRef]
- Song, X.; Liu, C.; Wang, N.; Huang, H.; He, S.; Gong, C.; Wei, Y. Delivery of CRISPR/Cas systems for cancer gene therapy and immunotherapy. Adv. Drug Deliv. Rev. 2021, 168, 158–180. [Google Scholar] [CrossRef]
- Yim, N.; Ryu, S.-W.; Choi, K.; Lee, K.R.; Lee, S.; Choi, H.; Kim, J.; Shaker, M.R.; Sun, W.; Park, J.-H.; et al. Exosome engineering for efficient intracellular delivery of soluble proteins using optically reversible protein–protein interaction module. Nat. Commun. 2016, 7, 12277. [Google Scholar] [CrossRef]
- Liang, X.; Gupta, D.; Xie, J.; Van Wonterghem, E.; Van Hoecke, L.; Hean, J.; Niu, Z.; Wiklander, O.; Zheng, W.; Wiklander, R.J.; et al. Multimodal engineering of extracellular vesicles for efficient intracellular protein delivery. bioRxiv 2023. [Google Scholar] [CrossRef]
- Mangeot, P.-E.; Dollet, S.; Girard, M.; Ciancia, C.; Joly, S.; Peschanski, M.; Lotteau, V. Protein Transfer Into Human Cells by VSV-G-induced Nanovesicles. Mol. Ther. 2011, 19, 1656–1666. [Google Scholar] [CrossRef]
- Finkelshtein, D.; Werman, A.; Novick, D.; Barak, S.; Rubinstein, M. LDL receptor and its family members serve as the cellular receptors for vesicular stomatitis virus. Proc. Natl. Acad. Sci. 2013, 110, 7306–7311. [Google Scholar] [CrossRef] [PubMed]
- Campbell, L.A.; Coke, L.M.; Richie, C.T.; Fortuno, L.V.; Park, A.Y.; Harvey, B.K. Gesicle-Mediated Delivery of CRISPR/Cas9 Ribonucleoprotein Complex for Inactivating the HIV Provirus. Mol. Ther. 2019, 27, 151–163. [Google Scholar] [CrossRef] [PubMed]
- Kuate, S.; Stahl-Hennig, C.; Stoiber, H.; Nchinda, G.; Floto, A.; Franz, M.; Sauermann, U.; Bredl, S.; Deml, L.; Ignatius, R.; et al. Immunogenicity and efficacy of immunodeficiency virus-like particles pseudotyped with the G protein of vesicular stomatitis virus. Virology 2006, 351, 133–144. [Google Scholar] [CrossRef]
- Wang, Q.; Yu, J.; Kadungure, T.; Beyene, J.; Zhang, H.; Lu, Q. ARMMs as a versatile platform for intracellular delivery of macromolecules. Nat. Commun. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Silva, A.M.; Lázaro-Ibáñez, E.; Gunnarsson, A.; Dhande, A.; Daaboul, G.; Peacock, B.; Osteikoetxea, X.; Salmond, N.; Friis, K.P.; Shatnyeva, O.; et al. Quantification of protein cargo loading into engineered extracellular vesicles at single-vesicle and single-molecule resolution. J. Extracell. Vesicles 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Rädler, J.; Sork, H.; Niu, Z.; Roudi, S.; Bost, J.P.; Görgens, A.; Zhao, Y.; Mamand, D.R.; Liang, X.; et al. Identification of scaffold proteins for improved endogenous engineering of extracellular vesicles. Nat. Commun. 2023, 14, 1–14. [Google Scholar] [CrossRef]
- Dooley, K.; McConnell, R.E.; Xu, K.; Lewis, N.D.; Haupt, S.; Youniss, M.R.; Martin, S.; Sia, C.L.; McCoy, C.; Moniz, R.J.; et al. A versatile platform for generating engineered extracellular vesicles with defined therapeutic properties. Mol. Ther. 2021, 29, 1729–1743. [Google Scholar] [CrossRef]
- Wei, H.; Chen, Q.; Lin, L.; Sha, C.; Li, T.; Liu, Y.; Yin, X.; Xu, Y.; Chen, L.; Gao, W.; et al. Regulation of exosome production and cargo sorting. Int. J. Biol. Sci. 2021, 17, 163–177. [Google Scholar] [CrossRef]
- Whitley, J.A.; Kim, S.; Lou, L.; Ye, C.; Alsaidan, O.A.; Sulejmani, E.; Cai, J.; Desrochers, E.G.; Beharry, Z.; Rickman, C.B.; et al. Encapsulating Cas9 into extracellular vesicles by protein myristoylation. J. Extracell. Vesicles 2022, 11, e12196. [Google Scholar] [CrossRef]
- Cheng, Y.; Schorey, J.S. Targeting soluble proteins to exosomes using a ubiquitin tag. Biotechnol. Bioeng. 2015, 113, 1315–1324. [Google Scholar] [CrossRef]
- Carnino, J.M.; Ni, K.; Jin, Y. Post-translational Modification Regulates Formation and Cargo-Loading of Extracellular Vesicles. Front. Immunol. 2020, 11, 948. [Google Scholar] [CrossRef] [PubMed]
- Ilahibaks, N.F.; Ardisasmita, A.I.; Xie, S.; Gunnarsson, A.; Brealey, J.; Vader, P.; de Jong, O.G.; de Jager, S.; Dekker, N.; Peacock, B.; et al. TOP-EVs: Technology of Protein delivery through Extracellular Vesicles is a versatile platform for intracellular protein delivery. J. Control. Release 2023, 355, 579–592. [Google Scholar] [CrossRef]
- Ferreira, J.V.; Soares, A.d.R.; Ramalho, J.; Carvalho, C.M.; Cardoso, M.H.; Pintado, P.; Carvalho, A.S.; Beck, H.C.; Matthiesen, R.; Zuzarte, M.; et al. LAMP2A regulates the loading of proteins into exosomes. Sci. Adv. 2022, 8, eabm1140. [Google Scholar] [CrossRef] [PubMed]
- Mangeot, P.E.; Risson, V.; Fusil, F.; Marnef, A.; Laurent, E.; Blin, J.; Mournetas, V.; Massouridès, E.; Sohier, T.J.M.; Corbin, A.; et al. Genome editing in primary cells and in vivo using viral-derived Nanoblades loaded with Cas9-sgRNA ribonucleoproteins. Nat. Commun. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Megede, J.Z.; Chen, M.-C.; Doe, B.; Schaefer, M.; Greer, C.E.; Selby, M.; Otten, G.R.; Barnett, S.W. Increased Expression and Immunogenicity of Sequence-Modified Human Immunodeficiency Virus Type 1 gag Gene. J. Virol. 2000, 74, 2628–2635. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Guerrero, A.; Recalde, M.J.A.; Mangeot, P.E.; Costa, C.; Bernadin, O.; Périan, S.; Fusil, F.; Froment, G.; Martinez-Turtos, A.; Krug, A.; et al. Baboon Envelope Pseudotyped “Nanoblades” Carrying Cas9/gRNA Complexes Allow Efficient Genome Editing in Human T, B, and CD34+ Cells and Knock-in of AAV6-Encoded Donor DNA in CD34+ Cells. Front. Genome Ed. 2021, 3. [Google Scholar] [CrossRef]
- Tiroille, V.; Krug, A.; Bokobza, E.; Kahi, M.; Bulcaen, M.; Ensinck, M.M.; Geurts, M.H.; Hendriks, D.; Vermeulen, F.; Larbret, F.; et al. Nanoblades allow high-level genome editing in murine and human organoids. Mol. Ther. - Nucleic Acids 2023, 33, 57–74. [Google Scholar] [CrossRef]
- Kostyushev, D.; Kostyusheva, A.; Brezgin, S.; Smirnov, V.; Volchkova, E.; Lukashev, A.; Chulanov, V. Gene Editing by Extracellular Vesicles. Int. J. Mol. Sci. 2020, 21, 7362. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Zhong, J.; Pan, Q.; Zhou, T.; Ping, Y.; Liu, X. Exosome-mediated delivery of Cas9 ribonucleoprotein complexes for tissue-specific gene therapy of liver diseases. Sci. Adv. 2022, 8, eabp9435. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Stremersch, S.; Braeckmans, K.; De Smedt, S.C.; Hendrix, A.; Wood, M.J.A.; Schiffelers, R.M.; Raemdonck, K.; Vader, P. Electroporation-induced siRNA precipitation obscures the efficiency of siRNA loading into extracellular vesicles. J. Control. Release 2013, 172, 229–238. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Gudbergsson, J.M.; Skov, M.N.; Christiansen, G.; Gurevich, L.; Moos, T.; Duroux, M. Evaluation of electroporation-induced adverse effects on adipose-derived stem cell exosomes. Cytotechnology 2016, 68, 2125–2138. [Google Scholar] [CrossRef]
- Kimiz-Gebologlu, I.; Oncel, S.S. Exosomes: Large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J. Control. Release 2022, 347, 533–543. [Google Scholar] [CrossRef]
- Hood, J.L.; Scott, M.J.; Wickline, S.A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 2014, 448, 41–49. [Google Scholar] [CrossRef]
- Lennaárd, A.J.; Mamand, D.R.; Wiklander, R.J.; EL Andaloussi, S.; Wiklander, O.P.B. Optimised Electroporation for Loading of Extracellular Vesicles with Doxorubicin. Pharmaceutics 2021, 14, 38. [Google Scholar] [CrossRef]
- Peng, H.; Ji, W.; Zhao, R.; Yang, J.; Lu, Z.; Li, Y.; Zhang, X. Exosome: a significant nano-scale drug delivery carrier. J. Mater. Chem. B 2020, 8, 7591–7608. [Google Scholar] [CrossRef]
- Hettich, B.F.; Bader, J.J.; Leroux, J. Encapsulation of Hydrophilic Compounds in Small Extracellular Vesicles: Loading Capacity and Impact on Vesicle Functions. Adv. Heal. Mater. 2021, 11, 2100047. [Google Scholar] [CrossRef]
- Joshi, B.; Ortiz, D.; Zuhorn, I. Converting extracellular vesicles into nanomedicine: loading and unloading of cargo. Mater. Today Nano 2021, 16, 100148. [Google Scholar] [CrossRef]
- Zhang, D.; Lee, H.; Zhu, Z.; Minhas, J.K.; Jin, Y. Enrichment of selective miRNAs in exosomes and delivery of exosomal miRNAs in vitro and in vivo. Am. J. Physiol. Cell. Mol. Physiol. 2017, 312, L110–L121. [Google Scholar] [CrossRef]
- Lepock, J.R.; Frey, H.E.; Ritchie, K.P. Protein denaturation in intact hepatocytes and isolated cellular organelles during heat shock. J. Cell Biol. 1993, 122, 1267–1276. [Google Scholar] [CrossRef]
- Oshchepkova, A.; Zenkova, M.; Vlassov, V. Extracellular Vesicles for Therapeutic Nucleic Acid Delivery: Loading Strategies and Challenges. Int. J. Mol. Sci. 2023, 24, 7287. [Google Scholar] [CrossRef]
- Cao, E.; Chen, Y.; Cui, Z.; Foster, P.R. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol. Bioeng. 2003, 82, 684–690. [Google Scholar] [CrossRef]
- Xi, X.-M.; Chen, M.; Xia, S.-J.; Lu, R. Drug loading techniques for exosome-based drug delivery systems. An International Journal of Pharmaceutical Sciences 2021, 76, 61–67. [Google Scholar] [CrossRef]
- Danilushkina, A.A.; Emene, C.C.; Barlev, N.A.; Gomzikova, M.O. Strategies for Engineering of Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 13247. [Google Scholar] [CrossRef]
- de Abreu, R.C.; Ramos, C.V.; Becher, C.; Lino, M.; Jesus, C.; Martins, P.A.d.C.; Martins, P.A.T.; Moreno, M.J.; Fernandes, H.; Ferreira, L. Exogenous loading of miRNAs into small extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12111. [Google Scholar] [CrossRef]
- Roerig, J.; Schulz-Siegmund, M. Standardization Approaches for Extracellular Vesicle Loading with Oligonucleotides and Biologics. Small 2023, 19, e2301763. [Google Scholar] [CrossRef]
- Nordin, J.Z. Transfection reagents affect Extracellular Vesicle cargo transfer to recipient cells: The importance of appropriate controls in EV research. J. Extracell. Vesicles 2022, 11. [Google Scholar] [CrossRef]
- Komuro, H.; Aminova, S.; Lauro, K.; Harada, M. Advances of engineered extracellular vesicles-based therapeutics strategy. Sci. Technol. Adv. Mater. 2022, 23, 655–681. [Google Scholar] [CrossRef]
- Fuhrmann, G.; Serio, A.; Mazo, M.; Nair, R.; Stevens, M.M. Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J. Control. Release 2015, 205, 35–44. [Google Scholar] [CrossRef]
- Mayer, L.; Bally, M.; Cullis, P. Uptake of adriamycin into large unilamellar vesicles in response to a pH gradient. Biochim. et Biophys. Acta (BBA) - Biomembr. 1986, 857, 123–126. [Google Scholar] [CrossRef]
- Jeyaram, A.; Lamichhane, T.N.; Wang, S.; Zou, L.; Dahal, E.; Kronstadt, S.M.; Levy, D.; Parajuli, B.; Knudsen, D.R.; Chao, W.; et al. Enhanced Loading of Functional miRNA Cargo via pH Gradient Modification of Extracellular Vesicles. Mol. Ther. 2019, 28, 975–985. [Google Scholar] [CrossRef]
- Roerig, J.; Mitrach, F.; Schmid, M.; Hause, G.; Hacker, M.C.; Wölk, C.; Schulz-Siegmund, M. Synergistic siRNA Loading of Extracellular Vesicles Enables Functional Delivery into Cells. Small Methods 2022, 6, e2201001. [Google Scholar] [CrossRef]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Sutaria, D.S.; Badawi, M.; Phelps, M.A.; Schmittgen, T.D. Achieving the Promise of Therapeutic Extracellular Vesicles: The Devil is in Details of Therapeutic Loading. Pharm. Res. 2017, 34, 1053–1066. [Google Scholar] [CrossRef]
- Biscans, A.; Haraszti, R.A.; Echeverria, D.; Miller, R.; Didiot, M.-C.; Nikan, M.; Roux, L.; Aronin, N.; Khvorova, A. Hydrophobicity of Lipid-Conjugated siRNAs Predicts Productive Loading to Small Extracellular Vesicles. Mol. Ther. 2018, 26, 1520–1528. [Google Scholar] [CrossRef]
- Haraszti, R.A.; Miller, R.; Didiot, M.-C.; Biscans, A.; Alterman, J.F.; Hassler, M.R.; Roux, L.; Echeverria, D.; Sapp, E.; DiFiglia, M.; et al. Optimized Cholesterol-siRNA Chemistry Improves Productive Loading onto Extracellular Vesicles. Mol. Ther. 2018, 26, 1973–1982. [Google Scholar] [CrossRef]
- Didiot, M.-C.; Hall, L.M.; Coles, A.H.; A Haraszti, R.; Godinho, B.M.; Chase, K.; Sapp, E.; Ly, S.; Alterman, J.F.; Hassler, M.R.; et al. Exosome-mediated Delivery of Hydrophobically Modified siRNA for Huntingtin mRNA Silencing. Mol. Ther. 2016, 24, 1836–1847. [Google Scholar] [CrossRef]
- Tréton, G.; Sayer, C.; Schürz, M.; Jaritsch, M.; Müller, A.; Matea, C.-T.; Stanojlovic, V.; Melo-Benirschke, H.; Be, C.; Krembel, C.; et al. Quantitative and functional characterisation of extracellular vesicles after passive loading with hydrophobic or cholesterol-tagged small molecules. J. Control. Release 2023, 361, 694–716. [Google Scholar] [CrossRef]
- Meng, W.; He, C.; Hao, Y.; Wang, L.; Li, L.; Zhu, G. Prospects and challenges of extracellular vesicle-based drug delivery system: considering cell source. Drug Deliv. 2020, 27, 585–598. [Google Scholar] [CrossRef]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.; Gazeau, F. Engineering and loading therapeutic extracellular vesicles for clinical translation: A data reporting frame for comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- O’brien, K.; Breyne, K.; Ughetto, S.; Laurent, L.C.; Breakefield, X.O. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020, 21, 585–606. [Google Scholar] [CrossRef]
- Lamichhane, T.N.; Raiker, R.S.; Jay, S.M. Exogenous DNA Loading into Extracellular Vesicles via Electroporation is Size-Dependent and Enables Limited Gene Delivery. Mol. Pharm. 2015, 12, 3650–3657. [Google Scholar] [CrossRef]
- Alvarez-Erviti, L.; Seow, Y.; Yin, H.; Betts, C.; Lakhal, S.; Wood, M.J.A. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 2011, 29, 341–345. [Google Scholar] [CrossRef]
- Liu, Q.; Li, D.; Pan, X.; Liang, Y. Targeted therapy using engineered extracellular vesicles: principles and strategies for membrane modification. J. Nanobiotechnology 2023, 21, 1–28. [Google Scholar] [CrossRef]
- Wang, J.-H.; Forterre, A.V.; Zhao, J.; Frimannsson, D.O.; Delcayre, A.; Antes, T.J.; Efron, B.; Jeffrey, S.S.; Pegram, M.D.; Matin, A.C. Anti-HER2 scFv-Directed Extracellular Vesicle-Mediated mRNA-Based Gene Delivery Inhibits Growth of HER2-Positive Human Breast Tumor Xenografts by Prodrug Activation. Mol. Cancer Ther. 2018, 17, 1133–1142. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nishikawa, M.; Shinotsuka, H.; Matsui, Y.; Ohara, S.; Imai, T.; Takakura, Y. Visualization and in vivo tracking of the exosomes of murine melanoma B16-BL6 cells in mice after intravenous injection. J. Biotechnol. 2013, 165, 77–84. [Google Scholar] [CrossRef]
- Liang, X.; Niu, Z.; Galli, V.; Howe, N.; Zhao, Y.; Wiklander, O.P.B.; Zheng, W.; Wiklander, R.J.; Corso, G.; Davies, C.; et al. Extracellular vesicles engineered to bind albumin demonstrate extended circulation time and lymph node accumulation in mouse models. J. Extracell. Vesicles 2022, 11, e12248. [Google Scholar] [CrossRef]
- Zheng, W.; He, R.; Liang, X.; Roudi, S.; Bost, J.; Coly, P.; van Niel, G.; Andaloussi, S.E.L. Cell-specific targeting of extracellular vesicles through engineering the glycocalyx. J. Extracell. Vesicles 2022, 11, e12290. [Google Scholar] [CrossRef]
- Gao, X.; Ran, N.; Dong, X.; Zuo, B.; Yang, R.; Zhou, Q.; Moulton, H.M.; Seow, Y.; Yin, H. Anchor peptide captures, targets, and loads exosomes of diverse origins for diagnostics and therapy. Sci. Transl. Med. 2018, 10. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Tian, B.; Liu, J.; Yang, L.; Zeng, L.; Chen, T.; Hong, A.; Wang, X. Nucleolin-targeted Extracellular Vesicles as a Versatile Platform for Biologics Delivery to Breast Cancer. Theranostics 2017, 7, 1360–1372. [Google Scholar] [CrossRef]
- Pi, F.; Binzel, D.W.; Lee, T.J.; Li, Z.; Sun, M.; Rychahou, P.; Li, H.; Haque, F.; Wang, S.; Croce, C.M.; et al. Nanoparticle orientation to control RNA loading and ligand display on extracellular vesicles for cancer regression. Nat. Nanotechnol. 2017, 13, 82–89. [Google Scholar] [CrossRef]
- Zou, J.; Shi, M.; Liu, X.; Jin, C.; Xing, X.-J.; Qiu, L.; Tan, W. Aptamer-Functionalized Exosomes: Elucidating the Cellular Uptake Mechanism and the Potential for Cancer-Targeted Chemotherapy. Anal. Chem. 2019, 91, 2425–2430. [Google Scholar] [CrossRef]
- Kooijmans, S.A.A.; Aleza, C.G.; Roffler, S.R.; van Solinge, W.W.; Vader, P.; Schiffelers, R.M. Display of GPI-anchored anti-EGFR nanobodies on extracellular vesicles promotes tumour cell targeting. J. Extracell. Vesicles 2016, 5, 31053. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Zhang, H.-X.; He, C.-P.; Fan, S.; Zhu, Y.-L.; Qi, C.; Huang, N.-P.; Xiao, Z.-D.; Lu, Z.-H.; Tannous, B.A.; et al. Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 2017, 150, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Ocansey, D.K.W.; Xu, W.; Qian, H. Extracellular vesicles: A bright star of nanomedicine. Biomaterials 2020, 269, 120467. [Google Scholar] [CrossRef] [PubMed]
- Hung, M.E.; Leonard, J.N. Stabilization of Exosome-targeting Peptides via Engineered Glycosylation. J. Biol. Chem. 2015, 290, 8166–8172. [Google Scholar] [CrossRef]
- Witwer, K.W. On your MARCKS, get set, deliver: Engineering extracellular vesicles. Mol. Ther. 2021, 29, 1664–1665. [Google Scholar] [CrossRef]
- Vader, P.; Mol, E.A.; Pasterkamp, G.; Schiffelers, R.M. Extracellular vesicles for drug delivery. Adv. Drug Deliv. Rev. 2016, 106, 148–156. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





