1. Introduction
Cystic fibrosis related diabetes (CFRD) is a specific extra-pulmonary complication associated with increased morbidity and mortality in the cystic fibrosis (CF) population. Among the extrapulmonary complications of CF patients, CFRD is by far the most common affecting about 20% of adolescents and 50% of adults. [
1] It is considered to be a major complication, especially as life expectancy of CF patients increases. CFRD is a unique form of diabetes, most frequently diagnosed in young adults and the prevalence increases with age. It is characterized by an insidious onset, therefore annual screening from the age of 10 is recommended with oral glucose tolerance testing. [
2] The etiology of CFRD seems to be linked to the partial loss or dysfunction of pancreatic islets, leading to insulin deficiency and to insulin resistance caused by chronic baseline inflammation, which flares during infectious exacerbations. [
3] The etiology of insulin secretion impairment is also linked to a direct effect of CF transmembrane conductance regulator (CFTR) protein, expressed on the beta cell surface. [
4] It is well known that hyperglycemia and insulin insufficiency have a great impact on clinical outcomes in CF patients. Decline of pulmonary function and of nutritional status begin about 2-4 years prior the diagnosis of CFRD. [
5] This deterioration is believed to be associated with protein catabolism due to insulin insufficiency. As a matter of a fact the worsening of pulmonary function is directly associated to the severity of insulin insufficiency. [
6] Furthermore another important aspect concerns poor nutritional status, highlighted by weight loss and a decline in body mass index (BMI). [
1] As forementioned CFRD is strongly associated with decline of pulmonary function, increased frequency of exacerbations and poor nutritional status, therefore worse prognosis. [
7,
8]
CFTR modulators, a group of innovative drugs for the treatment of CF, have revolutionized the prognosis of these patients: their role is to correct and potentiate defects in the channel function. [
9] Various combinations of these modulators are available: the most recent formulation available is a triple combination therapy of elexacaftor/tezacaftor/ivacaftor (ETI) approved for patients with at least one copy of F508del variation. [
10]
The impact of ETI therapy on pulmonary function and nutritional status has been widely studied and confirmed, whereas there are only few studies regarding the possible outcomes on glycemic control and insulin requirement in patients affected by CFRD.
Grancini et al. demonstrated a significant reduction of glycated hemoglobin A1c (HbA1c) values after 6 months of ETI therapy but unchanged insulin requirement. [
9] In A large Danish study including 321 CF patients showed a HbA1c decline over 12 months of ETI treatment, not associated with a change in insulin usage in the sub-cohort of 26 CFRD patients. [
11] Lurquin et al. showed a significant decrease of insulin requirement during follow-up in 17 patients undergoing CFTR modulator therapy with either ETI or tezacaftor/ivacaftor, despite an increase in HbA1c levels. [
12] Recently, Cohen et al. demonstrated a decrease of 120-min Oral Glucose Tolerance Test (OGTT) value after the initiation of CFTR modulators therapy. [
13]
As stated by Salazar-Barragan et al.’s systematic review most studies had small sample sizes and therefore their power to demonstrate the effectiveness of ETI is limited. [
14] As modulator therapies’ influence on CFRD and metabolism is becoming clearer, our goal is to evaluate whether ETI is effective on glycemic control and insulin requirement or not in a larger cohort of CFRD patients.
The primary aim is to study how HbA1c levels change in adult and pediatric patients affected by cystic fibrosis-related diabetes after one-year of elexacaftor/tezacaftor/ivacaftor therapy. The secondary aim is to study the improvement of total daily insulin dose, pulmonary function and metabolism in this population.
2. Materials and Methods
A retrospective single-center observational study was conducted at the Regional Cystic Fibrosis Centre and the Regional Diabetology Centre of IRCCS Istituto Giannina Gaslini. All patients with insulin dependent CFRD who started ETI therapy were included. Patients who started insulin therapy after initiating ETI were excluded. The observational period was divided in Time 0 (T0 - ETI treatment initiation), Time 1 (T1 – 3 months of ETI therapy), Time 2 (T2 – 6 months of therapy) and finally Time 3 (T3 - 12 months of therapy). The number of pulmonary exacerbations and hospitalizations, during the year before initiating ETI, were collected, as well as previous use of CFTR modulators, six-minute-walking test (6MWT) and chloride sweat test data during the 6 months prior ETI initiation (pre-T0). Demographic (age, sex, ethnicity) and clinical data were collected. CFTR genotype, age of CFRD diagnosis, insulin therapy duration, age of ETI initiation and insulin regimen were collected at T0. At T0, T1, T2 and T3 we compared weight, body mass index (BMI), forced expiratory volume in the first second (FEV1), forced vital capacity (FVC), total daily dose of insulin (TDD), basal dose of insulin, bolus dose of insulin, TDD per kilogram and glycated hemoglobin (HbA1c) values. At T0 and T3 we compared cystic fibrosis questionnaire (CFQ-R). At pre-T0 and T2 we compared 6MWT and chloride sweat test data; and at pre-T0 and T3 we compared the number of pulmonary exacerbations and hospitalizations. The same data were consequently subanalyzed according to the different CFTR genotypes (Group 1 - subjects F508del/F508del vs Group 2 - subjects F508del/any other CF-causing mutation).
2.1. Statistical Methods
Data are described as mean and standard deviation (SD) or median and range for continuous variables, and as absolute and relative frequencies for categorical variables. Comparisons between T0, T1, T2 and T3 to examine continuous variables were performed using Paired Wilcoxon test. P values ≤ 0.05 were considered statistically significant, and all P values were based on two tailed tests. Statistical analysis was performed using SPSS for Windows (SPSS Inc, Chicago, Illinois USA). Data were extracted from electronic medical records.
This study was conducted in accordance with the Helsinki Declaration. According to Italian legislation, the study did not need ethical approval, as it was a purely observational retrospective study on twenty-eight collected anonymous data. Furthermore, it was not possible to request informed consent for participation in the study, given the nature of the study. In any case, consent to completely anonymous use of clinical data for research/epidemiological purposes is requested by the clinical routine at the time of admission/diagnostic procedure.
3. Results
Twenty-eight patients (16 males vs 12 females; 57.1% vs 42.9%) affected by CF and diagnosed with CFRD undergoing insulin therapy followed at our regional CF Centre were included, with median age of 33 years (SD 13.26, minimum age 12 years, maximum age 56 years) at the start of ETI therapy. Three pediatric patients (10.7% of the cohort) were included in the study (one patient aged 12, two aged 15). 16 patients presented with genotype F508del in homozygosity (Group 1) and the leftover 12 patients had genotype F508del in heterozygosity (Group 2). The type of CFTR modulator therapy used before ETI was lumacaftor/ivacaftor in 12 patients, tezacaftor/ivacaftor in 2 patients; the remaining 14 patients had not taken any modulator therapy prior ETI. We analyzed the insulin regimen of each patient: 16 patients used only basal insulin, whereas 12 patients followed basal-bolus scheme. Demographic and clinical characteristics are shown in
Table 1.
The main results are shown in detail in
Table 2.
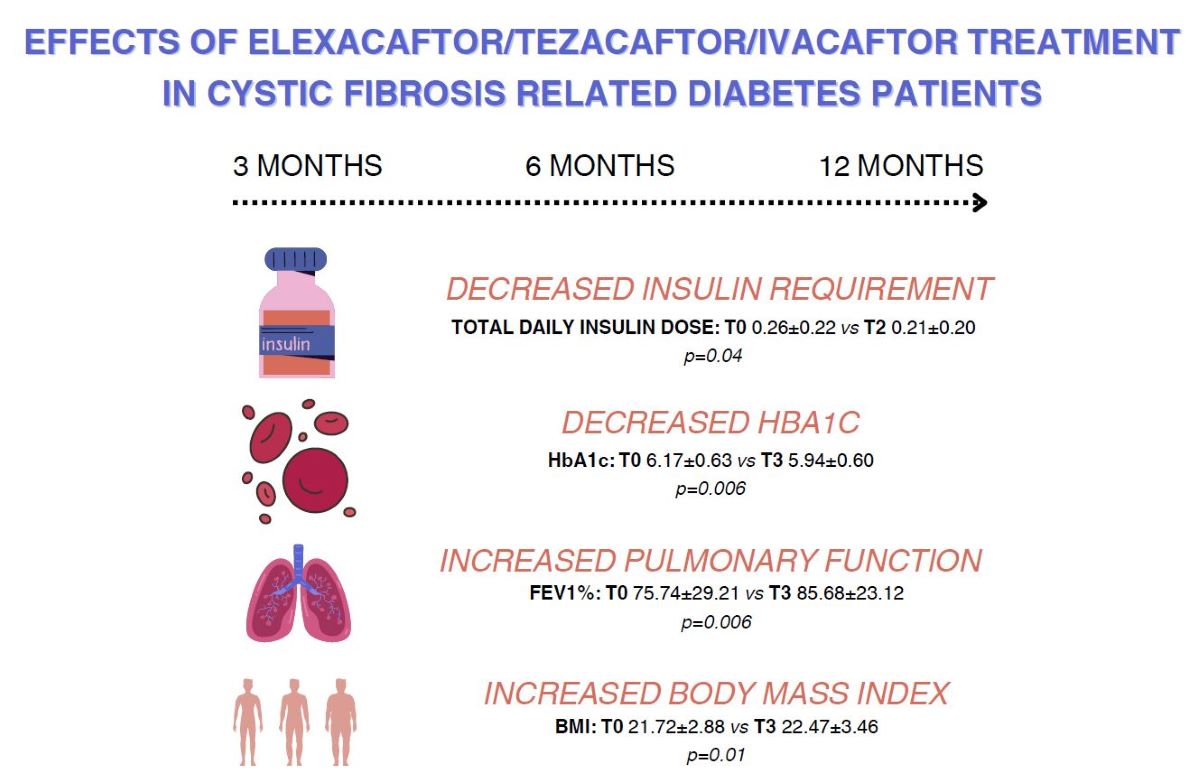
The TDD (IU) and HbA1c significantly decreased at T1 and T2, but not at T3. Results of chloride sweat test (mmol/L) as expected also decreased significantly. The number of hospitalizations (n) and pulmonary exacerbations significantly decreased over the study period. All pulmonary function parameters ameliorated significantly at 3, 6 and 12 months, in particular the parameter FEV1%. Nutritional status significantly improved over the observational period; BMI (kg/m2) increased significantly. The psychological status of our patients was assessed via CFQ-R and appeared to improve significantly in the first year of ETI treatment.
The results of the subanalysis based on diverse genotypes are illustrated in detail as
Supplementary Material.
The total daily dose of insulin (IU) in patients belonging to group 1 decreased significantly only at T0 vs T2 and HbA1c (%) decreased significantly only at T0 vs T1. In group 2 HbA1c decreased significantly at T0 vs T2 and T0 vs T3. BMI improved significantly in both groups. As for pulmonary function all spirometry parameters were significantly increased at 3, 6 and 12 months in group 1.Patients in group 2 showed significantly higher FEV1 (ml) at 3 months and 6 months.
4. Discussion
To the best of our knowledge this is the first retrospective observational study that demonstrates both improvement in glycemic control (by means of HbA1c) and insulin requirement in insulin dependent CFRD patients, both adult and pediatric, after one year of only ETI treatment.
Our results regarding decreased HbA1c values are concordant with the only other study that demonstrates glycemic control improvement in patients with CFRD under ETI treatment. [
9] Evidence supporting our findings in patients undergoing CFTR modulator therapy up until now has been contrasting or inconclusive, highlighting only favorable trends of glycemic control in patients with CFRD or impaired glucose intolerance [
15,
16] or no improvement at all [
17]. In particular Volkova et al. suggest that the prevalence of CFRD was lower in patients who received only ivacaftor, therefore underlining the efficacy of the sole use of a single CFTR modulator, as well as Tsabari et al. who examinate only a cohort wo was administered ivacaftor. [
15,
16] Our results highlight the innovative amelioration in glycemic control while using the triple combined modulator therapy. A major use of continuous glucose monitoring in our patients affected by CFRD would allow us to consolidate our data in the future.
Many studies have demonstrated ETI’s superiority compared to other modulators for various CF-measures such as FEV1, sweat chloride levels, BMI and pulmonary exacerbations. [
18,
19] Clear glycemic and metabolic outcomes regarding ETI treatment are less studied and yet to be demonstrated. Scully et al. were among the first to demonstrate potential improvements of glycemic control thanks to ETI treatment, but over 3-11 months in a mixed population (patients with and without CFRD). [
7] These results are contrasting with Crow et al. who demonstrated no statistically significant changes in measures of glycemia, HbA1c, nor in basal insulin requirements after 6 months of ETI treatment were detected [
20]; as opposed to our finding in which both HbA1c and insulin requirement were statistically lower after ETI treatment. The physiopathological mechanism behind the amelioration of glycemic control is still unclear and the explanation is likely to be multifactorial including better intestinal absorption and nutritional status, and decreased inflammatory state, enhanced beta cell function alongside improved insulin sensitivity. [
9]
We observed a greater reduction of insulin requirement in the first three months of ETI treatment, followed by a minor reduction compared to T0 (slighter increase compared to T1). This “plateau” reached by our patients could be due to the concomitant weight gain or increased appetite, therefore a slight adjustment to insulin daily dose per kg and possibly a higher adherence to CFRD treatment. More studies are needed to confirm a potentiated effect of ETI treatment in the first months that could explain the major reduction at the beginning of the treatment. HbA1c levels at all three times were significantly lower than T0, despite a slight increase at T3 compared to T2 supporting the minor increase of insulin requirement. Further studies and longer periods of observation are necessary to better understand this phenomenon.
The cohort’s pulmonary function improved and all parameters assessing clinical and pulmonary improvements such as spirometry, number of pulmonary exacerbations and of hospitalizations significantly ameliorated in agreement with previous published studies, as well as nutritional status and BMI [
21,
22,
23,
24,
25,
26]
Because depression and anxiety are frequently diagnosed in patients withCF and associated with a lower quality of life and a lower adherence to airway clearance treatment we have evaluated patients’ mental health status as an important outcome. Indeed, mental health appeared to improve significantly in our patients once beginning ETI treatment according to CFQ-R data, in agreement with other studies. [
27,
28]
We subanalyzed our data based on two different genotypes, F508del in homozygosity and in heterozygosity. Pulmonary function always improved constantly and significantly in patients who had F508del in homozygosity while group 2 patients showed only a minor improvement. These data are explained by the fact that patients with hetrozygous F508del have a better FEV1 and a smaller margin of improvement. The extremely significant increase of BMI our results are in line with other studies in which weight gain appears to be higher in patients with F508del in heterozygosity. [
29,
30] As for insulin requirement and glycemic control our results appears contrasting between the 2 groups where group 2 did not show any reduction in insulin requirements after one year of treatment despite a significant reduction in HbA1c values at 6 and 12 months. Group 1, on the other hand, significantly decreased the amount of insulin required which proved smaller significant only at T2, accompanied by a smaller decrease in HbA1c values at T1.
This study has some limitations including the retrospective nature where some data are missing at visits at 3-6-12 months post-ETI treatment initiation and the relatively small number of patients. A strength of the study is that it showed that ETI therapy both significantly improved glycemic control and significantly decreased insulin requirement in CFRD patients. Further studies with a larger population and a longer follow-up period are needed to better understand the trends in insulin requirements in CFRD patients using ETI treatment and to appropriately adjust insulin therapy during their follow-up.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Pulmonary function and metabolic changes in patients affected by cystic fibrosis related diabetes with genotype F508del/F508del before and after treatment with elexacaftor/tezacaftor/ivacaftor. Pre-T0: 1 year before; T0: treatment initiation; T1: 3 months after; T2: 6 months after; T3 1 year after. FEV1: forced expiratory volume in the first second; FVC forced vital capacity; HBA1c: glycated hemoglobin. Table S2: Pulmonary function and metabolic changes in patients affected by cystic fibrosis related diabetes with genotype F508del/any other CFTR mutation before and after treatment with elexacaftor/tezacaftor/ivacaftor. Pre-T0: 1 year before; T0: treatment initiation; T1: 3 months after; T2: 6 months after; T3 1 year after. FEV1: forced expiratory volume in the first second; FVC forced vital capacity; HBA1c: glycated hemoglobin.
Author Contributions
C.C., F.C., M.B., R.C., N.M. and G.d.A. were involved in conceptualization, design, and conduction of the study, M.F.S, M.S., G.S. retrieved data, M.G.C analyzed the data of the results, M.F.S. and M.B. wrote the first draft of the manuscript, N.M., M.M., R.C. edited, reviewed, and approved the final version of the manuscript.
Informed Consent Statement
This study was conducted in accordance with the Helsinki Declaration. According to Italian legislation, the study did not need ethical approval, as it was a purely observational retrospective study on routinely collected anonymous data. Furthermore, it was not possible to request informed consent for participation in the study, given the nature of the study.
Data Availability Statement
The datasets generated during and/or analyzed in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Moran A, Pekow P, Grover P, Zorn M, Slovis B, Pilewski J, Tullis et al. Cystic Fibrosis Related Diabetes Therapy Study Group. Insulin therapy to improve BMI in cystic fibrosis-related diabetes without fasting hyperglycemia: results of the cystic fibrosis related diabetes therapy trial. Diabetes Care. 1: 2009 Oct;32(10), 2009.
- Granados, A.; Chan, C.L.; Ode, K.L.; Moheet, A.; Moran, A.; Holl, R. Cystic fibrosis related diabetes: Pathophysiology, screening and diagnosis. J. Cyst. Fibros. 2019, 18, S3–S9. [Google Scholar] [CrossRef]
- Gottlieb, P.A.; Yu, L.; Babu, S.; Wenzlau, J.; Bellin, M.; Frohnert, B.I.; Moran, A. No Relation Between Cystic Fibrosis–Related Diabetes and Type 1 Diabetes Autoimmunity. Diabetes Care 2012, 35, e57–e57. [Google Scholar] [CrossRef] [PubMed]
- Ntimbane, T.; Mailhot, G.; Spahis, S.; Rabasa-Lhoret, R.; Kleme, M.-L.; Melloul, D.; Brochiero, E.; Berthiaume, Y.; Levy, E. CFTR silencing in pancreatic β-cells reveals a functional impact on glucose-stimulated insulin secretion and oxidative stress response. Am. J. Physiol. Metab. 2016, 310, E200–E212. [Google Scholar] [CrossRef]
- Lanng, S.; Thorsteinsson, B.; Nerup, J.; Koch, C. Influence of the development of diabetes mellitus on clinical status in patients with cystic fibrosis. Eur. J. Pediatr. 1992, 151, 684–687. [Google Scholar] [CrossRef]
- Burgess, J.C.; Bridges, N.; Banya, W.; Gyi, K.M.; Hodson, M.E.; Bilton, D.; Simmonds, N.J. HbA1c as a screening tool for cystic fibrosis related diabetes. J. Cyst. Fibros. 2015, 15, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Scully, K.J.; Marchetti, P.; Sawicki, G.S.; Uluer, A.; Cernadas, M.; Cagnina, R.E.; Kennedy, J.C.; Putman, M.S. The effect of elexacaftor/tezacaftor/ivacaftor (ETI) on glycemia in adults with cystic fibrosis. J. Cyst. Fibros. 2021, 21, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Norris, A.W.; Ode, K.L.; Merjaneh, L.; Sanda, S.; Yi, Y.; Sun, X.; Engelhardt, J.F.; Hull, R.L. Survival in a bad neighborhood: pancreatic islets in cystic fibrosis. J. Endocrinol. 2019, 241, R35–R50. [Google Scholar] [CrossRef]
- Grancini V, Gramegna A, Zazzeron L, Alicandro G, Porcaro LL, Piedepalumbo F et al. Effects of elexacaftor / tezacaftor / ivacaftor triple combination therapy on glycaemic control and body composition in patients with cystic fibrosis-related diabetes. Diabetes Metab. 2023 Sep;49(5):101466.
- Taylor-Cousar JL, Mall MA, Ramsey BW, McKone EF, Tullis E, Marigowda G et al. Clinical development of triple-combination CFTR modulators for cystic fibrosis patients with one or two F508delalleles. ERJ Open Res. 2019 Jun 17;5(2), 2019.
- Nielsen BU, Olsen MF, Mabuza Mathiesen IH, Pressler T, Ritz C, Katzenstein TL at al. Decline in HbA1c during the first year of elexacaftor/tezacaftor/ivacaftor treatment in the Danish cystic fibrosis cohort: Short title: Decline in HbA1c after elexacaftor/tezacaftor/ivacaftor treatment. J Cyst Fibros. 2024 Jan;23(1):103-108. [CrossRef]
- Lurquin, F.; Gohy, S.; Hermans, M.P.; Preumont, V. Combined CFTR modulator therapies are linked with anabolic benefits and insulin-sparing in cystic fibrosis-related diabetes. J. Clin. Transl. Endocrinol. 2023, 33, 100320. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.; Mass, A.; Reiter, J.; Zangen, D.H.; Cohen-Cymberknoh, M. Long-Term therapy with CFTR modulators consistently improves glucose metabolism in adolescents and adults with Cystic Fibrosis. Respir. Med. 2024, 228, 107664. [Google Scholar] [CrossRef]
- Salazar-Barragan, M.; Taub, D.R. The Effects of Elexacaftor, Tezacaftor, and Ivacaftor (ETI) on Blood Glucose in Patients With Cystic Fibrosis: A Systematic Review. Cureus 2023, 15, e41697. [Google Scholar] [CrossRef]
- Volkova, N.; Moy, K.; Evans, J.; Campbell, D.; Tian, S.; Simard, C.; Higgins, M.; Konstan, M.W.; Sawicki, G.S.; Elbert, A.; et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J. Cyst. Fibros. 2019, 19, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Tsabari, R.; Elyashar, H.I.; Cymberknowh, M.C.; Breuer, O.; Armoni, S.; Livnat, G.; Kerem, E.; Zangen, D.H. CFTR potentiator therapy ameliorates impaired insulin secretion in CF patients with a gating mutation. J. Cyst. Fibros. 2015, 15, e25–e27. [Google Scholar] [CrossRef] [PubMed]
- Colombo, C.; Foppiani, A.; Bisogno, A.; Gambazza, S.; Daccò, V.; Nazzari, E.; Leone, A.; Giana, A.; Mari, A.; Battezzati, A. Lumacaftor/ivacaftor in cystic fibrosis: effects on glucose metabolism and insulin secretion. J. Endocrinol. Investig. 2021, 44, 2213–2218. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.N.; Rabih, A.M.; Niaj, A.; Raman, A.; Uprety, M.; Calero, M.J.; Villanueva, M.R.B.; Joshaghani, N.; Villa, N.; Badla, O.; et al. Newly Discovered Cutting-Edge Triple Combination Cystic Fibrosis Therapy: A Systematic Review. Cureus 2022, 14, e29359. [Google Scholar] [CrossRef]
- Zaher, A.; ElSaygh, J.; Elsori, D.; ElSaygh, H.; Sanni, A. A Review of Trikafta: Triple Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Modulator Therapy. Cureus 2021, 13, e16144. [Google Scholar] [CrossRef] [PubMed]
- Crow H, Bengtson C, Shi X, Graves L 3rd, Anabtawi A. CGM patterns in adults with cystic fibrosis-related diabetes before and after elexacaftor-tezacaftor-ivacaftor therapy. J Clin Transl Endocrinol. 1: 2022 Oct 1;30, 2022.
- Carnovale V, Iacotucci P, Terlizzi V, Colangelo C, Ferrillo L, Pepe A et al. Elexacaftor/Tezacaftor/Ivacaftor in Patients with Cystic Fibrosis Homozygous for the F508delMutation and Advanced Lung Disease: A 48-Week Observational Study. J Clin Med. 2022 Feb 16;11(4):1021.
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Regard, L.; Martin, C.; Burnet, E.; Da Silva, J.; Burgel, P.-R. CFTR Modulators in People with Cystic Fibrosis: Real-World Evidence in France. Cells 2022, 11, 1769. [Google Scholar] [CrossRef]
- Walter, E.; Bass, J.L. The Effect of Elexacaftor/Tezacaftor/Ivacaftor on Hospitalizations and Intravenous Antibiotic Use. Perm. J. 2022, 26, 73–79. [Google Scholar] [CrossRef]
- Caley, L.; Jarosz-Griffiths, H.; Smith, L.; Gale, L.; Barrett, J.; Kinsey, L.; Davey, V.; Nash, M.; Jones, A.; Whitehouse, J.; et al. Body mass index and nutritional intake following Elexacaftor/Tezacaftor/Ivacaftor modulator therapy in adults with cystic fibrosis. J. Cyst. Fibros. 2023, 22, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Taelman, V.; Declercq, D.; Van Biervliet, S.; Weygaerde, Y.V.; Lapauw, B.; Van Braeckel, E. Effect of 18 months elexacaftor-tezacaftor-ivacaftor on body mass index and glycemic control in adults with cystic fibrosis. Clin. Nutr. ESPEN 2023, 58, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Tervo, J.P.; DiMango, E.; Gudis, D.A.; Keating, C.; Zhang, Y.; Leu, C.; Altman, K.; Vilarello, B.; Jacobson, P.; Overdevest, J.B. Olfaction, body mass index, and quality of life with cystic fibrosis combination therapy. Int. Forum Allergy Rhinol. 2023, 13, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Piehler, L.; Thalemann, R.; Lehmann, C.; Thee, S.; Röhmel, J.; Syunyaeva, Z.; Stahl, M.; Mall, M.A.; Graeber, S.Y. Effects of elexacaftor/tezacaftor/ivacaftor therapy on mental health of patients with cystic fibrosis. Front. Pharmacol. 2023, 14. [Google Scholar] [CrossRef] [PubMed]
- Kos, R.; Neerincx, A.H.; Fenn, D.W.; Brinkman, P.; Lub, R.; Vonk, S.E.M.; Roukema, J.; Reijers, M.H.; Terheggen-Lagro, S.W.J.; Altenburg, J.; et al. Real-life efficacy and safety of elexacaftor/tezacaftor/ivacaftor on severe cystic fibrosis lung disease patients. Pharmacol. Res. Perspect. 2022, 10, e01015. [Google Scholar] [CrossRef] [PubMed]
- Griese, M.; Costa, S.; Linnemann, R.W.; Mall, M.A.; McKone, E.F.; Polineni, D.; Quon, B.S.; Ringshausen, F.C.; Taylor-Cousar, J.L.; Withers, N.J.; et al. Safety and Efficacy of Elexacaftor/Tezacaftor/Ivacaftor for 24 Weeks or Longer in People with Cystic Fibrosis and One or More F508del Alleles: Interim Results of an Open-Label Phase 3 Clinical Trial. Am. J. Respir. Crit. Care Med. 2021, 203, 381–385. [Google Scholar] [CrossRef] [PubMed]
Table 1.
Demographic and clinical characteristics.
Table 1.
Demographic and clinical characteristics.
| Age (years) |
|
|
| Mean |
33.5 |
|
| Median |
33.0 |
|
| Standard Deviation |
± 13.26 |
|
| |
Frequency (N) |
Percentage (%) |
| Sex |
Total = 28 |
|
| Female |
12 |
42.9 |
| Male |
16 |
57.1 |
| |
|
|
| Genotype |
Total = 28 |
|
| F508del/F508del (homozygosis) |
16 |
57.1 |
| F508del/other (heteroyzygosis) |
12 |
42.9 |
| |
|
|
| Previous use of CFTR-modulators |
Total = 28 |
|
| None |
14 |
50.0 |
| Lumacaftor/Ivacaftor |
12 |
42.9 |
| Tezacaftor/Ivacaftor |
2 |
7.1 |
| |
|
|
| Insulin Regimen |
Total = 28 |
|
| Once Daily Basal Insulin |
16 |
57.1 |
| Basal-Bolus |
12 |
42.9 |
Table 2.
Pulmonary function and metabolic changes in patients affected by cystic fibrosis related diabetes before and after treatment with elexacaftor/tezacaftor/ivacaftor. Pre-T0: 1 year before; T0: treatment initiation; T1: 3 months after; T2: 6 months after; T3 1 year after. FEV1: forced expiratory volume in the first second; FVC forced vital capacity; HBA1c: glycated hemoglobin; CFQR: cystic fibrosis questionnaire revised.
Table 2.
Pulmonary function and metabolic changes in patients affected by cystic fibrosis related diabetes before and after treatment with elexacaftor/tezacaftor/ivacaftor. Pre-T0: 1 year before; T0: treatment initiation; T1: 3 months after; T2: 6 months after; T3 1 year after. FEV1: forced expiratory volume in the first second; FVC forced vital capacity; HBA1c: glycated hemoglobin; CFQR: cystic fibrosis questionnaire revised.
| |
N |
Pre-T0 |
N |
T0 |
T1 |
T0 vs T1 |
N |
T2 |
Pre-T0 vs T2 |
N |
T3 |
T0 vs T3 |
| Hospitalizations |
28 |
0.86±0.89 |
|
|
|
|
|
|
|
28 |
0.32±0.48 |
0.003 |
| Pulmonary exacerbations |
28 |
1.64±1.59 |
|
|
|
|
|
|
|
28 |
0.46±0.79 |
0.002 |
| Six minute walking test |
19 |
574.18±68.04 |
|
|
|
|
|
|
|
19 |
572.75±92.99 |
0.71 |
| Chloride sweat test |
15 |
118.53±22.21 |
|
|
|
|
|
|
|
15 |
49.93±17.81 |
0.001 |
| Body mass index |
|
|
26 |
21.72±2.88 |
22.53±3.11 |
0.0001 |
26 |
22.48±3.02 |
0.0001 |
25 |
22.47±3.46 |
0.006 |
| Fev1 |
|
|
27 |
2420.0±1038.4 |
2782.2±946.6 |
0.0001 |
27 |
2774.8±991.7 |
0.0001 |
25 |
2771.6±967.1 |
0.001 |
| Fvc |
|
|
27 |
3267.4±1043.3 |
3640.0±899.1 |
0.001 |
27 |
3694.8±959.8 |
0.0001 |
25 |
3671.5±938.7 |
0.001 |
| Fev1% |
|
|
27 |
75.74±29.21 |
85.44±23.70 |
0.001 |
27 |
85.37±25.40 |
0.002 |
25 |
85.68±23.12 |
0.01 |
| Total daily insulin dose |
|
|
27 |
0.26±0.22 |
0.18±0.18 |
0.01 |
25 |
0.21±0.20 |
0.04 |
23 |
0.21±0.22 |
0.10 |
| Hba1c |
|
|
23 |
6.17±0.63 |
5.94±0.58 |
0.001 |
26 |
5.89±0.59 |
0.004 |
23 |
5.94±0.60 |
0.006 |
| Cfqr |
|
|
26 |
64.63±7.59 |
|
|
26 |
71.74±4.12 |
0.0001 |
15 |
70.13±4.84 |
0.01 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).