Submitted:
02 September 2024
Posted:
04 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Fruits with maximum global production and their byproducts
3. Toxicological qualities of citrus fruit byproducts
3.1. Oranges
3.2. Pomelos
3.3. Mandarin
3.4. Lemon
3.5. Grape
4. Toxicological qualities of tropical fruit byproducts
4.1. Avocado
4.2. Pineapple
4.3. Banana
4.4. Papaya
4.5. Watermelon
4.6. Melon
5. Fruit berries (strawberries, blackberries, cranberries and raspberries)
5.1. Blueberries
5.2. Strawberries
6. Toxicological qualities of other fruits byproducts
6.1. Cherry
6.2. Apple
6.3. Mango
6.4. Plum
6.5. Peaches
6.6. Apricot
| Commodity | Heavy metals (mg/kg) | References | ||||||
| As | Cd | Cr | Co | Ni | Pb | |||
| Apple | Peel Seed Pomace |
<1 | 0.57-3.8 | <1 | <1 | [32, 62, 97] | ||
| Apricot | Kernel Pomace |
0.1-6 |
2.7-35.7 | [108] | ||||
| Avocados | Seeds | 0.57-2.29 | 0.00 | 0.00 | [62, 63] | |||
| Bananas | Peels | <0.0001 | 0.0013-0.18 | 1.42-4.04 | 0.4-47.2 | 0.0038-0.64 | [30, 60, 61] | |
| Blueberries | Pomace | 0.011 | 0.242 | 0.08 | 0.592 | 0.73 | [90] | |
| Grape | Peel/skin/ pomace |
<0.5 | 0.18-2.41 | <0.5 | 0.021-1.11 | [29, 31, 32] | ||
| Lemons | Peels byproducts |
0.004 | 0.00047- 0.25 | 1.04 | 0.038 | 0.973-1.24 | 0.0188- 0.22 | [29, 30, 34] |
| Limes | Byproducts | ND | 0.003 | 0.073 | 1.678 | 0.128 | [34] | |
| Mangoes | 0.33 | 28.0 | [60] | |||||
| Orange | Peel Seed |
<0.5 | 1.04–4.14 |
0.015 | 0.05-2.36 | 0.01-1.75 |
[29, 30, 32, 34, 41] | |
| Papayas | Peel Seed |
0.0287-0.03 | 0.0027-0.00685 | 0.278-7.36 | 0.4-219 | 0.246-13.2 | 0.03-0.044 | [60, 77, 78] |
| Peaches | 0.17-1.38 | <0.10 | [107] | |||||
| Pineapples | Peels | <0.0001 | 0.0074 | 8.77 | 70.3 | 0.0027 | [60, 61] | |
| Plums | Peels | 1.2 | ND | 2.8 | ND | [105, 106] | ||
| Kernels | <0.1 | 0.13 | 0.2 | 1.7 | 0.13 | |||
| Pomelos | Peels | 1.36x10-3 | 0.0296 | [29] | ||||
| Raspberries | Pomace | 0.0084 | 0.116 | 0.073 | 0.762 | 0.047 | [90] | |
| Strawberry | Pomace | 0.0078 | <0.01 |
0.00336 | 0.0011 | [93, 94] | ||
| Mandarins | 0.00062 | 0.020 | [33] | |||||
| Watermelons | Peel Seed |
0.008-0.1 | 4.65 | 0.06-0.09 | [62, 82] | |||
| Commodity | Mycotoxins (mg/kg) |
Toxicant organic compounds (mg/kg) |
Anti-nutritional contaminants (mg/kg) |
Fungicide/Pesticide residues (mg/kg) |
References | ||
|---|---|---|---|---|---|---|---|
| Apple | Peel/skin Seed Pomace |
AOH-3-S = 1.4-10.8x10-3 AME-3-S = 1.7-10x10-3 |
Naphthaleneacetic acid = 0.433 Amygdalin = 1000-4000 |
Oxalates = 890.7 Hydrogen cyanides = 960.4 Alkaloids = 79.9 Phytates = 14.2 |
Acetamiprid = 72-81 |
[47, 98, 99, 101, 102] | |
| Apricots | Seeds | Aflatoxin B1 and B2 = 0.0017- 22.451 | Amygdalin = 52,000 | Tannins = 1564.4 Phytic acid = 1171.5 Oxalates = 156 |
[104, 109, 110] | ||
| Avocados | Seeds | Tannins = 7.6 Alkaloids = 54 Phytates = 4.4 Oxalates = 44 |
[63] | ||||
| Bananas | Peels | Phytate = 2.11 - 9270 Alkaloids = 0.45 - 5.45 Oxalate = 20 - 8280 Glycosides 149020 Tannin = 900 |
Chlorpyrifos = 0.11–0.8 methiocarb = 0.014-0.183 |
[73, 74] | |||
| Blueberries | Pomace | Chlorpyrifos-methyl = 4.27x10-3 thiametoxan 5.15x10-3 azoxytrobin 0.187 |
[91, 92] | ||||
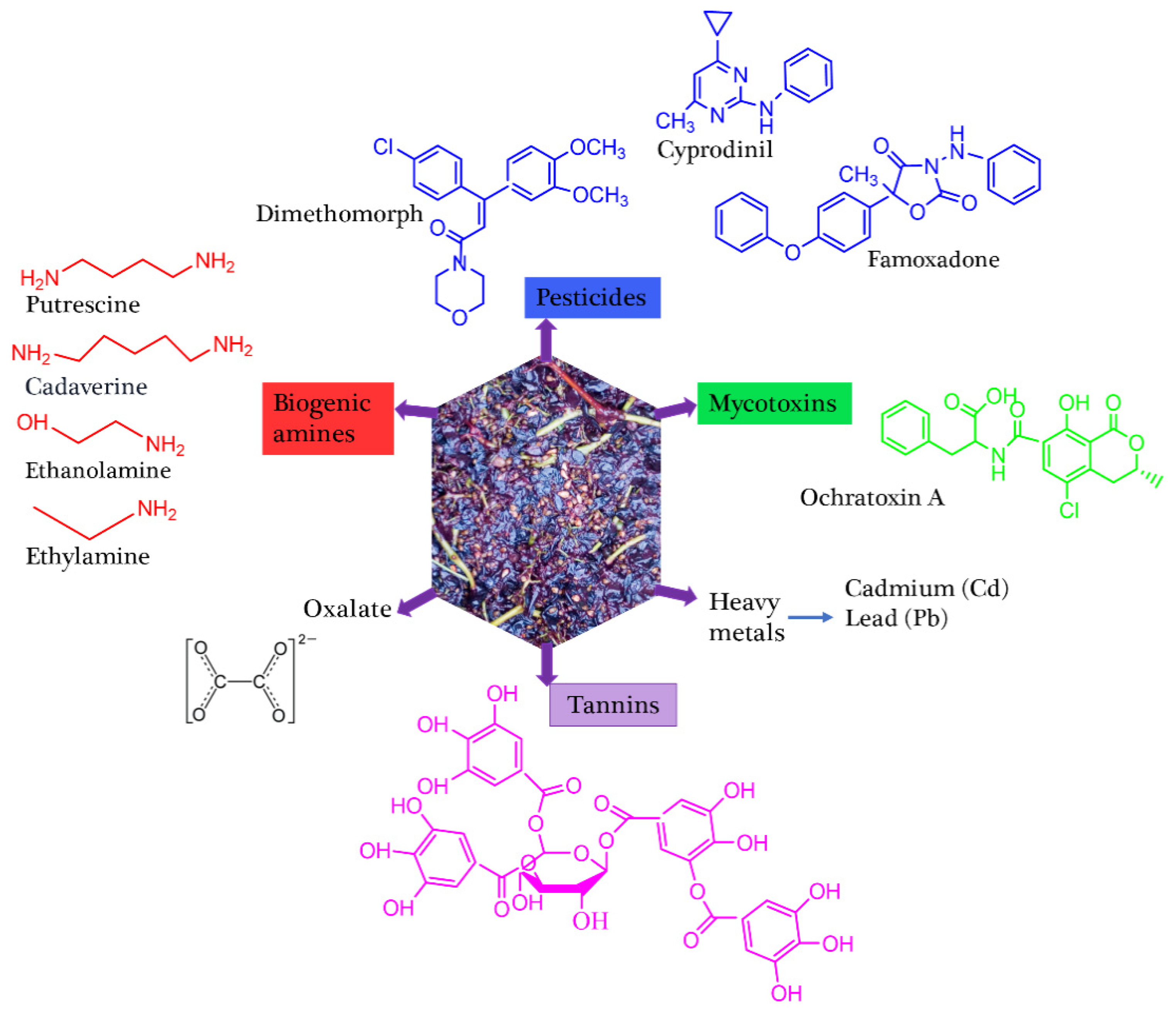
| Grapes | Peel/skin Pomace |
Ochratoxin A = 0.1-0.32x10-3 | Oxalate = 0.6-0.7 Tannins = 0.274-0.41 |
Cyprodinil = 1.07-1.94 dimethomorph = 0.56-2.73 Feamoxadone = 1.55 |
[29, 31, 36] | ||
| Lemons | Peels/Pomace | Oxalate = 0.4-0.5 Tannins = 0.28 |
Pyriproxyfen = 0.039 Fludioxonil = 0.008 Propiconazole = 0.008 Pyrimethanil = 3.8 |
[29, 34, 36] | |||
| Limes | Pomace | Fludioxonil = 0.009 Flutriafol = 0.11 Propiconazole = 0.008 Imazalil = 1.49 Tebuconazole = 0.076 |
[34] | ||||
| Mangoes | Putrescine = 0.9 | Phytic acid = 254.8 Oxalate = 724 Tannin = 153x103 |
[76, 103, 111] | ||||
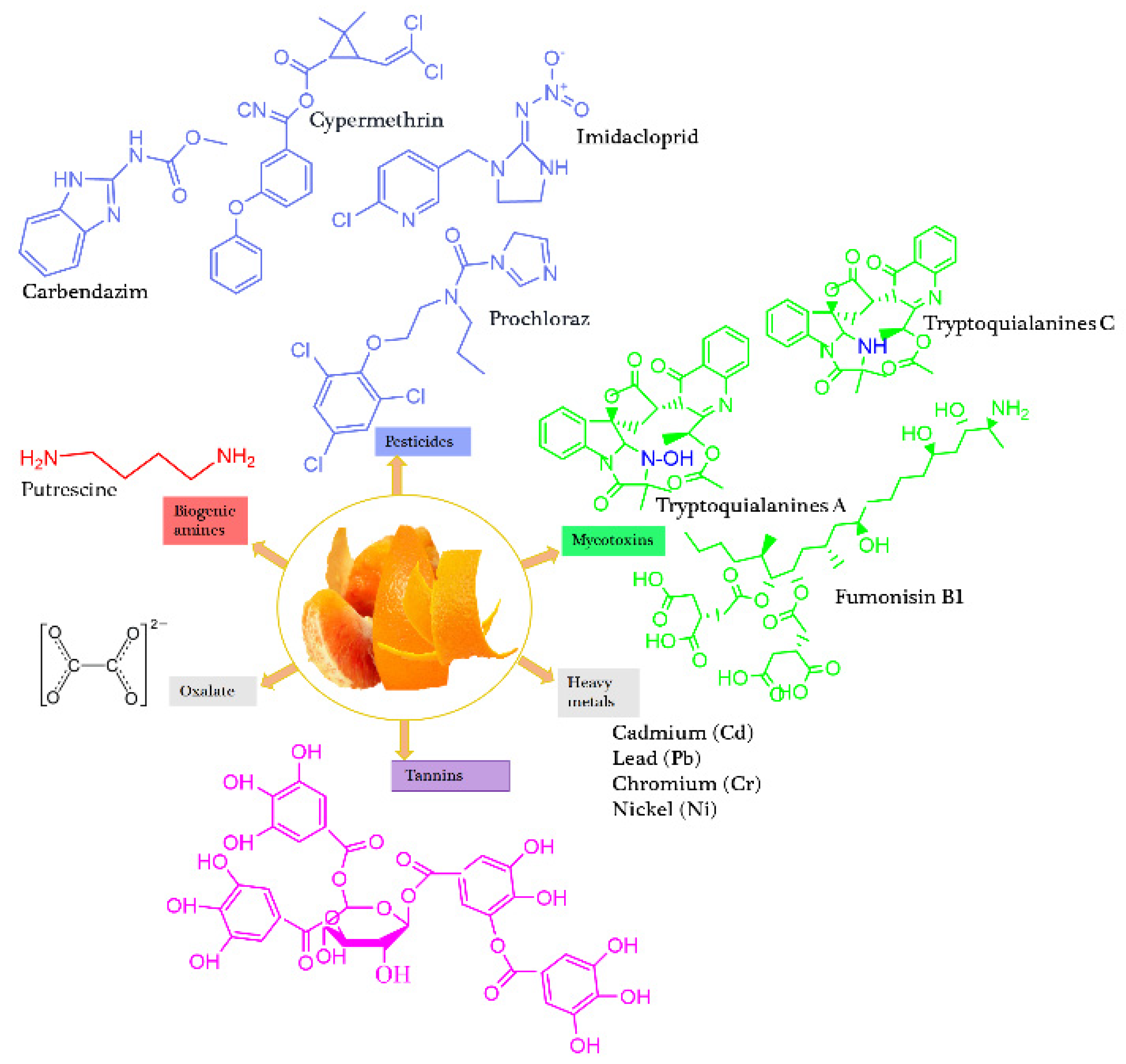
| Orange | Peel Pomace |
tryptoquialanine A = 248.1 tryptoquialanine C = 375.80 |
Putrescine = 11.34–151.1 | Tannins = 0.228 Oxalates = 1.2-997.8 Hydrogen cyanides = 397.9 Alkaloids = 54.4 Phytates = 23.4 |
Etoxazole = 0.010~0.637 Imidacloprid = 162.16 Carbendazim = 372.1 Abamectin = 0.261 Cypermethrin = 495.6 Prochloraz = 8.11.7 |
[29, 36, 44, 45, 47, 51, 111] | |
| Papayas | Peel | Putrescine = 5.3–19.3 | Tannin = 17.6-500 Oxalate = 0.6 Phytate = 0.6 |
[76, 79, 111] | |||
| Peaches | Seeds | Putrescine = 1.82–2.02 | Tannins = 5137.6 Phytic acid = 2126.3 Oxalates = 385.9 HCN = 372 |
[104, 111] | |||
| Pears | Putrescine = 23.6–24.2 | [111] | |||||
| Pineapples | Pineapple shell Pomace |
Fusarium = 250 Aflatoxin B2 = 0.008 x10-3 Aflatoxin G1 = 0.013-0.033 x10-3 Ochratoxin A = 0.051 x10-3 |
Putrescine =1.39–7.96 | Oxalates = 0.4-1290.6 Hydrogen cyanides = 715 Alkaloids = 161.9 Phytates = 19.9 |
Carbaryl = 0.262 Carbofuran = 14.3 Fenobucarb = 0.01 Isoprocarb = 0.313 Propachlor = 0.015 |
[36, 47, 67-69, 111] | |
| Pomelos | Peels | Tannins = 0.315 | Total pesticides = 0.216 | [29] | |||
| Strawberry | Pomace | Putrescine = 2.04–6.42 bis-2-ethylhexyl phthalate (DEHP) = 0.25 diisobutyl phthalate (DIBP) = 0.283 and dibutyl phthalate (DBP) and 0.222 |
Total pesticides = 2.143 Procymidone = 0.7 acetamiprid = 0.212 boscalid = 0.745 carbendazim = 0.13 |
[94, 95, 111] | |||
| Tangerines | Peels | Alternariol = 0.003 - 0.017 | [34] | ||||
| Watermelons | Peel | Phytate = 9900 Tannin = 32x105 Oxalate = 2130 Hydrogen cyanides = 1210.2 Alkaloids = 100.9 |
Dimethoate = 1730 | [47, 83, 86] | |||
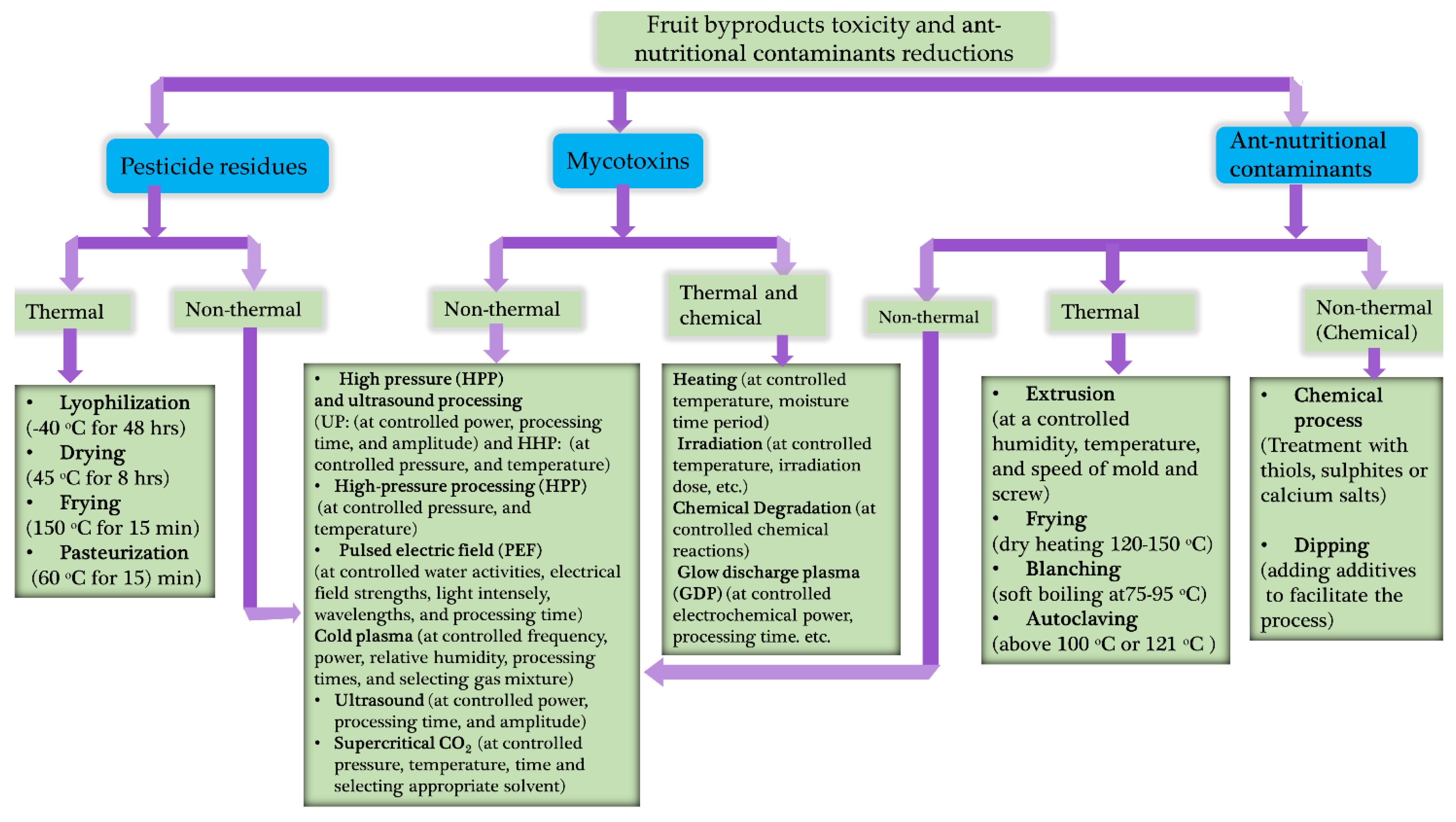
7. Current trends on fruit byproduct toxicant reduction
8. Novel functional foods from fruit byproducts freed from toxication
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pushparaj, K., A. Meyyazhagan, M. Pappuswamy, A. Mousavi Khaneghah, W.-C. Liu, and B. Balasubramanian, Occurrence, identification, and decontamination of potential mycotoxins in fruits and fruit by-products. Food Frontiers. 2023. 4(1): p. 32-46. [CrossRef]
- Gebrekidan, A., Y. Weldegebriel, A. Hadera, and B. Van der Bruggen, Toxicological assessment of heavy metals accumulated in vegetables and fruits grown in Ginfel river near Sheba Tannery, Tigray, Northern Ethiopia. Ecotoxicology and Environmental Safety. 2013. 95: p. 171-178. [CrossRef]
- Abuzed Sadee, B. and R. Jameel Ali, Determination of heavy metals in edible vegetables and a human health risk assessment. Environmental Nanotechnology, Monitoring & Management. 2023. 19: p. 100761. [CrossRef]
- Nepal, B. and K. J. Stine, Glycoalkaloids: Structure, Properties, and Interactions with Model Membrane Systems. Processes. 2019. 7(8): p. 513.
- Park, B.K., S.H. Kwon, M.S. Yeom, K.S. Joo, and M.J. Heo, Detection of pesticide residues and risk assessment from the local fruits and vegetables in Incheon, Korea. Scientific Reports. 2022. 12(1): p. 9613. [CrossRef]
- Salgado, N., M.A. Silva, M.E. Figueira, H.S. Costa, and T.G. Albuquerque, Oxalate in Foods: Extraction Conditions, Analytical Methods, Occurrence, and Health Implications. Foods. 2023. 12(17): p. 3201.
- Liu, B., G. Han, Z. Zhang, R. Liu, C. Jiang, S. Wang, and M.-Y. Han, Shell Thickness-Dependent Raman Enhancement for Rapid Identification and Detection of Pesticide Residues at Fruit Peels. Analytical Chemistry. 2012. 84(1): p. 255-261. [CrossRef]
- Uddin, R., M.U. Thakur, M.Z. Uddin, and G.M.R. Islam, Study of nitrate levels in fruits and vegetables to assess the potential health risks in Bangladesh. Scientific Reports. 2021. 11(1): p. 4704. [CrossRef]
- Santos, C.M.d., C.M.P.d. Abreu, J.M. Freire, E.d.R. Queiroz, and M.M. Mendonça, Chemical characterization of the flour of peel and seed from two papaya cultivars. Food Science and Technology. 2014. 34(2): p. 353-357.
- WHO, Health impacts of chemicals, W.H.O. (WHO), Editor. 2015, World Health Organization (WHO): Geneva, Switzerland.
- Kuppusamy, S., K. Venkateswarlu, and M. Megharaj, Evaluation of nineteen food wastes for essential and toxic elements. International Journal of Recycling of Organic Waste in Agriculture. 2017. 6(4): p. 367-373.
- Hong, C., Y. Jia, X. Yang, Z. He, and P. Stoffella, Assessing lead thresholds for phytotoxicity and potential dietary toxicity in selected vegetable crops. Bulletin of environmental contamination and toxicology. 2008. 80(4): p. 356-361.
- Oyeyinka, B.O. and A.J. Afolayan, Comparative Evaluation of the Nutritive, Mineral, and Antinutritive Composition of Musa sinensis L.(Banana) and Musa paradisiaca L.(Plantain) Fruit Compartments. Plants. 2019. 8(12): p. 598.
- Silva, M.A., T.G. Albuquerque, R.C. Alves, M.B.P.P. Oliveira, and H.S. Costa, Melon (Cucumis melo L.) by-products: Potential food ingredients for novel functional foods? Trends in Food Science & Technology. 2020. 98: p. 181-189. [CrossRef]
- Shendge, A.K., S. Panja, T. Basu, N.B. Ghate, and N. Mandal, Ameliorating effects of white mulberry on iron-overload-induced oxidative stress and liver fibrosis in Swiss albino mice. Food and Chemical Toxicology. 2021. 156: p. 112520. [CrossRef]
- Erukainure, O.L., N.Z. Msomi, B.K. Beseni, V.F. Salau, O.M. Ijomone, N.A. Koorbanally, and M.S. Islam, Cola nitida infusion modulates cardiometabolic activities linked to cardiomyopathy in diabetic rats. Food and Chemical Toxicology. 2021. 154: p. 112335. [CrossRef]
- Dhanisha, S.S., S. Drishya, and C. Guruvayoorappan, Pithecellobium dulce induces apoptosis and reduce tumor burden in experimental animals via regulating pro-inflammatory cytokines and anti-apoptotic gene expression. Food and Chemical Toxicology. 2022. 161: p. 112816. [CrossRef]
- Inanc, M.E., S. Gungor, D. Yeni, F. Avdatek, V. Ipek, R. Turkmen, O. Corum, H. Karaca, and A. Ata, Protective role of the dried white mulberry extract on the reproductive damage and fertility in rats treated with carmustine. Food and Chemical Toxicology. 2022. 163: p. 112979. [CrossRef]
- FAOSTAT, Food and Agricultural Data, FAO, Editor. 2022, FAOSTAT DOI: https://www.fao.org/faostat/en/#data.
- Jiménez-Moreno, N., I. Esparza, F. Bimbela, L.M. Gandía, and C. Ancín-Azpilicueta, Valorization of selected fruit and vegetable wastes as bioactive compounds: Opportunities and challenges. Critical Reviews in Environmental Science and Technology. 2020. 50(20): p. 2061-2108. [CrossRef]
- Hasan, M.M., M.R. Islam, A.R. Haque, M.R. Kabir, K.J. Khushe, and S.M.K. Hasan, Trends and challenges of fruit by-products utilization: insights into safety, sensory, and benefits of the use for the development of innovative healthy food: a review. Bioresources and Bioprocessing. 2024. 11(1): p. 10. [CrossRef]
- Vilas-Boas, A.A., M. Pintado, and A.L.S. Oliveira Natural Bioactive Compounds from Food Waste: Toxicity and Safety Concerns. Foods, 2021. 10,. [CrossRef]
- Gomes, S., B. Vieira, C. Barbosa, and R. Pinheiro, Evaluation of mature banana peel flour on physical, chemical, and texture properties of a gluten-free Rissol. Journal of Food Processing and Preservation. 2022. 46(8): p. e14441. [CrossRef]
- Teshome, E., T.A. Teka, R. Nandasiri, J.R. Rout, D.V. Harouna, T. Astatkie, and M.M. Urugo, Fruit by-products and their industrial applications for nutritional benefits and health promotion: a comprehensive review. Sustainability. 2023. 15(10): p. 7840.
- Yu, J. and M. Ahmedna, Functional components of grape pomace: their composition, biological properties and potential applications. International Journal of Food Science & Technology. 2013. 48(2): p. 221-237. [CrossRef]
- Choonut, A., M. Saejong, and K. Sangkharak, The Production of Ethanol and Hydrogen from Pineapple Peel by Saccharomyces Cerevisiae and Enterobacter Aerogenes. Energy Procedia. 2014. 52: p. 242-249. [CrossRef]
- Czech, A., E. Zarycka, D. Yanovych, Z. Zasadna, I. Grzegorczyk, and S. Kłys, Mineral Content of the Pulp and Peel of Various Citrus Fruit Cultivars. Biological Trace Element Research. 2020. 193(2): p. 555-563. [CrossRef]
- Sagar, N.A., S. Pareek, S. Sharma, E.M. Yahia, and M.G. Lobo, Fruit and Vegetable Waste: Bioactive Compounds, Their Extraction, and Possible Utilization. Comprehensive Reviews in Food Science and Food Safety. 2018. 17(3): p. 512-531. [CrossRef]
- Czech, A., A. Malik, B. Sosnowska, and P. Domaradzki, Bioactive Substances, Heavy Metals, and Antioxidant Activity in Whole Fruit, Peel, and Pulp of Citrus Fruits. International Journal of Food Science. 2021. 2021(1): p. 6662259. [CrossRef]
- Saleem, M. and M.T. Saeed, Potential application of waste fruit peels (orange, yellow lemon and banana) as wide range natural antimicrobial agent. Journal of King Saud University - Science. 2020. 32(1): p. 805-810. [CrossRef]
- Moncalvo, A., L. Marinoni, R. Dordoni, G. Duserm Garrido, V. Lavelli, and G. Spigno, Waste grape skins: evaluation of safety aspects for the production of functional powders and extracts for the food sector. Food Additives & Contaminants: Part A. 2016. 33(7): p. 1116-1126. [CrossRef]
- Bożym, M., I. Florczak, P. Zdanowska, J. Wojdalski, and M. Klimkiewicz, An analysis of metal concentrations in food wastes for biogas production. Renewable Energy. 2015. 77: p. 467-472. [CrossRef]
- Neshovska, H., Determination of heavy metal content (CD and PB) in citrus feed raw material. 2023.
- Mateus, A.R.S., S. Mariño-Cortegoso, S.C. Barros, R. Sendón, L. Barbosa, A. Pena, and A. Sanches-Silva, Citrus by-products: A dual assessment of antioxidant properties and food contaminants towards circular economy. Innovative Food Science & Emerging Technologies. 2024. 95: p. 103737. [CrossRef]
- Commission, E., Commission regulation (EU) 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing regulation (EC), in no 1881/2006, O.J.o.t.E. Union, Editor. 2023 p. 103–157.
- Nagarajaiah, S.B. and J. Prakash, Chemical Composition and Bioactivity of Pomace from Selected Fruits. International Journal of Fruit Science. 2016. 16(4): p. 423-443. [CrossRef]
- Soares Mateus, A.R., S. Barros, A. Pena, and A. Sanches-Silva, Chapter Two - The potential of citrus by-products in the development of functional food and active packaging, in Advances in Food and Nutrition Research, E. Capanoglu, M.D. Navarro-Hortal, T.Y. Forbes-Hernández, and M. Battino, Editors. 2023, Academic Press. p. 41-90 DOI:Â . [CrossRef]
- Socas-Rodríguez, B., J.A. Mendiola, M.Á. Rodríguez-Delgado, E. Ibáñez, and A. Cifuentes, Safety assessment of citrus and olive by-products using a sustainable methodology based on natural deep eutectic solvents. Journal of Chromatography A. 2022. 1669: p. 462922. [CrossRef]
- European Food Safety, A., L. Carrasco Cabrera, and P. Medina Pastor, The 2020 European Union report on pesticide residues in food. EFSA Journal. 2022. 20(3): p. e07215. [CrossRef]
- Liu, Y., E. Heying, and S.A. Tanumihardjo, History, Global Distribution, and Nutritional Importance of Citrus Fruits. Comprehensive Reviews in Food Science and Food Safety. 2012. 11(6): p. 530-545. [CrossRef]
- Simeon, E.O., N.S. Amamilom, and I.W. Azuka, Metal assessment and phytochemical screening of orange fruit (Citrus sinensis) seeds and peels. Journal of Pharmacognosy and Phytochemistry. 2018. 7(3): p. 709-714.
- Benayad, O., M. Bouhrim, S. Tiji, L. Kharchoufa, M. Addi, S. Drouet, C. Hano, J.M. Lorenzo, H. Bendaha, M. Bnouham, and M. Mimouni Phytochemical Profile, α-Glucosidase, and α-Amylase Inhibition Potential and Toxicity Evaluation of Extracts from Citrus aurantium (L) Peel, a Valuable By-Product from Northeastern Morocco. Biomolecules, 2021. 11,. [CrossRef]
- Costa, C.A.R.A., T.C. Cury, B.O. Cassettari, R.K. Takahira, J.C. Flório, and M. Costa, Citrus aurantium L. essential oil exhibits anxiolytic-like activity mediated by 5-HT1A-receptors and reduces cholesterol after repeated oral treatment. BMC Complementary and Alternative Medicine. 2013. 13(1): p. 42. [CrossRef]
- Li, Y., B. Jiao, Q. Zhao, C. Wang, Y. Gong, Y. Zhang, and W. Chen, Effect of commercial processing on pesticide residues in orange products. European Food Research and Technology. 2012. 234(3): p. 449-456. [CrossRef]
- de Vilhena Araújo, É., P.H. Vendramini, J.H. Costa, M.N. Eberlin, C.C. Montagner, and T.P. Fill, Determination of tryptoquialanines A and C produced by Penicillium digitatum in oranges: Are we safe? Food Chemistry. 2019. 301: p. 125285. [CrossRef]
- Teixeira, F., B.A. Santos, G. Nunes, J.M. Soares, L.A. Amaral, G.H. Souza, J.T. Resende, B. Menegassi, B.P. Rafacho, K. Schwarz, E.F. Santos, and D. Novello Addition of Orange Peel in Orange Jam: Evaluation of Sensory, Physicochemical, and Nutritional Characteristics. Molecules, 2020. 25,. [CrossRef]
- Romelle, F.D., A. Rani, and R.S. Manohar, Chemical composition of some selected fruit peels. European Journal of Food Science and Technology. 2016. 4(4): p. 12-21.
- Phuong, T.N.Q., P. Van Hung, and N.T.L. Phi, Extraction of flavonoids in pomelos’ peels using Box-Behnken response surface design and their biological activities. Vietnam Journal of Science, Technology and Engineering. 2021. 63(2): p. 52-57.
- Li, X., S. Song, F. Wei, X. Huang, Y. Guo, and T. Zhang, Occurrence, distribution, and translocation of legacy and current-use pesticides in pomelo orchards in South China. Science of The Total Environment. 2024. 913: p. 169674. [CrossRef]
- Pu, S.-m., R.-h. Liang, J. Chen, C.-m. Liu, C.-j. Xu, M.-s. Chen, and J. Chen, Characterization and evaluation of Majia pomelo seed oil: A novel industrial by-product. Food Chemistry Advances. 2022. 1: p. 100051. [CrossRef]
- Wang, Z., J. Pang, C. Liao, Q. Zhang, and D. Sun, Determination of etoxazole in different parts of citrus fruit and its potential dietary exposure risk assessment. Chemosphere. 2021. 268: p. 128832. [CrossRef]
- Rossi, R.C., S.R. da Rosa, P. Weimer, J.G. Lisbôa Moura, V.R. de Oliveira, and J. de Castilhos, Assessment of compounds and cytotoxicity of Citrus deliciosa Tenore essential oils: From an underexploited by-product to a rich source of high-value bioactive compounds. Food Bioscience. 2020. 38: p. 100779. [CrossRef]
- Bhuia, M.S., M.A. Aktar, R. Chowdhury, J. Ferdous, M.A. Rahman, M.S.A. Hasan, and M.T. Islam, Therapeutic potentials of ononin with mechanistic insights: A comprehensive review. Food Bioscience. 2023. 56: p. 103302. [CrossRef]
- Martínez-Zamora, L., M. Cano-Lamadrid, F. Artés-Hernández, and N. Castillejo Flavonoid Extracts from Lemon By-Products as a Functional Ingredient for New Foods: A Systematic Review. Foods, 2023. 12,. [CrossRef]
- Lubinska-Szczygeł, M., A. Kuczyńska-Łażewska, M. Rutkowska, Ż. Polkowska, E. Katrich, and S. Gorinstein Determination of the Major By-Products of Citrus hystrix Peel and Their Characteristics in the Context of Utilization in the Industry. Molecules, 2023. 28,. [CrossRef]
- Georganas, A., E. Giamouri, A.C. Pappas, E. Zoidis, M. Goliomytis, and P. Simitzis Utilization of Agro-Industrial By-Products for Sustainable Poultry Production. Sustainability, 2023. 15,. [CrossRef]
- Lluís, L., M. Muñoz, M. Rosa Nogués, V. Sánchez-Martos, M. Romeu, M. Giralt, J. Valls, and R. Solà, Toxicology evaluation of a procyanidin-rich extract from grape skins and seeds. Food and Chemical Toxicology. 2011. 49(6): p. 1450-1454. [CrossRef]
- Sano, A., Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food and Chemical Toxicology. 2017. 108: p. 519-523. [CrossRef]
- Heber, D., N.P. Seeram, H. Wyatt, S.M. Henning, Y. Zhang, L.G. Ogden, M. Dreher, and J.O. Hill, Safety and Antioxidant Activity of a Pomegranate Ellagitannin-Enriched Polyphenol Dietary Supplement in Overweight Individuals with Increased Waist Size. Journal of Agricultural and Food Chemistry. 2007. 55(24): p. 10050-10054. [CrossRef]
- de Matuoka e Chiocchetti, G., E.A. De Nadai Fernandes, M.A. Bacchi, R.A. Pazim, S.R.V. Sarriés, and T.M. Tomé, Mineral composition of fruit by-products evaluated by neutron activation analysis. Journal of Radioanalytical and Nuclear Chemistry. 2013. 297(3): p. 399-404. [CrossRef]
- Aboul-Enein, A.M., Z.A. Salama, A.A. Gaafar, H.F. Aly, F. Abou-Elella, and H.A. Ahmed, Identification of phenolic compounds from banana peel (Musa paradaisica L.) as antioxidant and antimicrobial agents. Journal of chemical and pharmaceutical research. 2016. 8(4): p. 46-55.
- Hassan, M., A. Belanche, E. Jiménez, I. Rivelli, A.I. Martín-García, A. Margolles, and D.R. Yáñez-Ruiz, Evaluation of the nutritional value and presence of minerals and pesticides residues in agro-industrial by-products to replace conventional ingredients of small ruminant diets. Small Ruminant Research. 2023. 229: p. 107117. [CrossRef]
- OKOYE, O.O.F.N.C., Comparative Study of the Constituents of the Fruits Pulps and Seeds of Canarium ovatum, Persea americana and Dacryodes edulis. Jordan Journal of Chemistry (JJC). 2017. 12(2): p. 113-125.
- García-Vargas, M.C., M.D. Contreras, and E. Castro Avocado-Derived Biomass as a Source of Bioenergy and Bioproducts. Applied Sciences, 2020. 10,. [CrossRef]
- Tremocoldi, M.A., P.L. Rosalen, M. Franchin, A.P. Massarioli, C. Denny, E.R. Daiuto, J.A.R. Paschoal, P.S. Melo, and S.M. de Alencar, Exploration of avocado by-products as natural sources of bioactive compounds. PLoS One. 2018. 13(2).
- Yusof, Y., S.A. Yahya, and A. Adam, Novel Technology for Sustainable Pineapple Leaf Fibers Productions. Procedia CIRP. 2015. 26: p. 756-760. [CrossRef]
- Santos, D.I., C.F. Martins, R.A. Amaral, L. Brito, J.A. Saraiva, A.A. Vicente, and M. Moldão-Martins Pineapple (Ananas comosus L.) By-Products Valorization: Novel Bio Ingredients for Functional Foods. Molecules, 2021. 26,. [CrossRef]
- Stępień, Ł., G. Koczyk, and A. Waśkiewicz, Diversity of Fusarium species and mycotoxins contaminating pineapple. Journal of Applied Genetics. 2013. 54(3): p. 367-380. [CrossRef]
- Wanwimolruk, C., S. Wanwimolruk, K. Kuaykaimuk, J. Buddhaprom, P. Saenserm, and S. Soikham, Food Safety of Thailand’s Pineapples, Bananas, and Dragon Fruits from Pesticide Contamination: a Study Using GC-MS Analysis. Philippine Journal of Science. 2022. 151(6B): p. 2315-2326.
- Šeremet, D., K. Durgo, S. Jokić, A. Huđek, A. Vojvodić Cebin, A. Mandura, J. Jurasović, and D. Komes, Valorization of Banana and Red Beetroot Peels: Determination of Basic Macrocomponent Composition, Application of Novel Extraction Methodology and Assessment of Biological Activity In Vitro. Sustainability. 2020. 12(11): p. 4539.
- Xie, L., Q. Yang, Y. Wu, J. Xiao, H. Qu, Y. Jiang, and T. Li, Fumonisin B1 Biosynthesis Is Associated with Oxidative Stress and Plays an Important Role in Fusarium proliferatum Infection on Banana Fruit. Journal of Agricultural and Food Chemistry. 2023. 71(13): p. 5372-5381. [CrossRef]
- Xie, L., Y. Wu, Y. Wang, Y. Jiang, B. Yang, X. Duan, and T. Li, Fumonisin B1 induced aggressiveness and infection mechanism of Fusarium proliferatum on banana fruit. Environmental Pollution. 2021. 288: p. 117793. [CrossRef]
- Gomes, H.d.O., J.M.C. Menezes, J.G.M. da Costa, H.D.M. Coutinho, R.N.P. Teixeira, and R.F. do Nascimento, Evaluating the presence of pesticides in bananas: An integrative review. Ecotoxicology and Environmental Safety. 2020. 189: p. 110016. [CrossRef]
- Mohd Zaini, H., J. Roslan, S. Saallah, E. Munsu, N.S. Sulaiman, and W. Pindi, Banana peels as a bioactive ingredient and its potential application in the food industry. Journal of Functional Foods. 2022. 92: p. 105054. [CrossRef]
- Ozabor, P., A. Ojokoh, A. Wahab, and O. Aramide, Effect of fermentation on the proximate and antinutrient composition of banana peels. Int J Biotechnol. 2020. 9(2): p. 105-17.
- Melesse, A., H. Steingass, M. Schollenberger, and M. Rodehutscord, Component composition, in vitro gas and methane production profiles of fruit by-products and leaves of root crops. The Journal of Agricultural Science. 2018. 156(7): p. 949-958. [CrossRef]
- Kumar, S.S., G.T. V, K. K, and M. John, Antioxidant potential and mineral elemental profiling of young and mature fruit and leaf of Carica papaya L. cultivar 'Red Lady'. Journal of Trace Elements and Minerals. 2024. 9: p. 100166. [CrossRef]
- Vinha, A.F., A.S.G. Costa, L. Espírito Santo, D.M. Ferreira, C. Sousa, E. Pinto, A. Almeida, and M.B.P.P. Oliveira High-Value Compounds in Papaya By-Products (Carica papaya L. var. Formosa and Aliança): Potential Sustainable Use and Exploitation. Plants, 2024. 13,. [CrossRef]
- Ibrahim, S., E.D. Inelo, and M.O. Eke, Physico-chemical, alveograph and anti-nutritional properties of breads formulated from wheat and pawpaw (Carica papaya) seed flour blends. Asian Food Science Journal. 2021. 20(3): p. 72-85.
- Marfo, E., O. Oke, and O. Afolabi, Chemical composition of papaya (Carica papaya) seeds. Food Chemistry. 1986. 22(4): p. 259-266.
- Capossio, J.P., M.P. Fabani, M.C. Román, X. Zhang, J. Baeyens, R. Rodriguez, and G. Mazza Zero-Waste Watermelon Production through Nontraditional Rind Flour: Multiobjective Optimization of the Fabrication Process. Processes, 2022. 10,. [CrossRef]
- Falade, O.S., I.O. Otemuyiwa, A.S. Adekunle, S.A. Adewusi, and O. Oluwasefunmi, Nutrient composition of watermelon (Citrullis lanatus (Thunb.) Matsum. &Nakai) and egusi melon (Citrullus colocynthis (L.) Schrad.) seeds. Agriculturae Conspectus Scientificus. 2020. 85(1): p. 43-49.
- Wanwimolruk, S., O. Kanchanamayoon, S. Boonpangrak, and V. Prachayasittikul, Food safety in Thailand 1: it is safe to eat watermelon and durian in Thailand. Environmental Health and Preventive Medicine. 2015. 20(3): p. 204-215. [CrossRef]
- Zia, S., M.R. Khan, M.A. Shabbir, and R.M. Aadil, An update on functional, nutraceutical and industrial applications of watermelon by-products: A comprehensive review. Trends in Food Science & Technology. 2021. 114: p. 275-291. [CrossRef]
- Zia, S., M.R. Khan, R.M. Aadil, and I.G. Medina-Meza, Bioactive Recovery from Watermelon Rind Waste Using Ultrasound-Assisted Extraction. ACS Food Science & Technology. 2024. 4(3): p. 687-699. [CrossRef]
- Jyothi lakshmi, A. and P. Kaul, Nutritional potential, bioaccessibility of minerals and functionality of watermelon (Citrullus vulgaris) seeds. LWT - Food Science and Technology. 2011. 44(8): p. 1821-1826. [CrossRef]
- Rolim, P.M., L.M.A.J. Seabra, and G.R. de Macedo, Melon By-Products: Biopotential in Human Health and Food Processing. Food Reviews International. 2020. 36(1): p. 15-38. [CrossRef]
- De Laurentiis, V., S. Corrado, and S. Sala, Quantifying household waste of fresh fruit and vegetables in the EU. Waste Management. 2018. 77: p. 238-251. [CrossRef]
- Juan, C., J. Mañes, G. Font, and A. Juan-García, Determination of mycotoxins in fruit berry by-products using QuEChERS extraction method. LWT. 2017. 86: p. 344-351. [CrossRef]
- Shotyk, W., Trace elements in wild berries from reclaimed lands: Biomonitors of contamination by atmospheric dust. Ecological Indicators. 2020. 110: p. 105960. [CrossRef]
- Dorosh, O., V.C. Fernandes, C. Delerue-Matos, and M.M. Moreira Blueberry Pruning Wastes: From an Undervalued Agricultural Residue to a Safe and Valuable Source of Antioxidant Compounds for the Food Industry. Foods, 2024. 13,. [CrossRef]
- Milinčić, D.D., U.D. Vojinović, A.Ž. Kostić, M.B. Pešić, B.D. Špirović Trifunović, D.V. Brkić, M.Ž. Stević, M.O. Kojić, and N.S. Stanisavljević, In vitro assessment of pesticide residues bioaccessibility in conventionally grown blueberries as affected by complex food matrix. Chemosphere. 2020. 252: p. 126568. [CrossRef]
- Tozzi, F., G. Renella, C. Macci, G. Masciandaro, C. Gonnelli, I. Colzi, L. Giagnoni, S. Pecchioli, S. Nin, and E. Giordani, Agronomic performance and food safety of strawberry cultivated on a remediated sediment. Science of The Total Environment. 2021. 796: p. 148803. [CrossRef]
- Shao, W.-C., Y.-Y. Zang, H.-Y. Ma, Y. Ling, and Z.-P. Kai, Concentrations and Related Health Risk Assessment of Pesticides, Phthalates, and Heavy Metals in Strawberries from Shanghai, China. Journal of Food Protection. 2021. 84(12): p. 2116-2122. [CrossRef]
- Sójka, M., A. Miszczak, P. Sikorski, K. Zagibajło, E. Karlińska, and M. Kosmala, Pesticide residue levels in strawberry processing by-products that are rich in ellagitannins and an assessment of their dietary risk to consumers. NFS Journal. 2015. 1: p. 31-37. [CrossRef]
- Mateus, A.R.S., S.C. Barros, S.M. Cortegoso, R. Sendón, L. Barbosa-Pereira, K. Khwaldia, G. Pataro, G. Ferrari, M. Breniaux, R. Ghidossi, A. Pena, and A. Sanches-Silva, Potential of fruit seeds: Exploring bioactives and ensuring food safety for sustainable management of food waste. Food Chemistry: X. 2024. 23: p. 101718. [CrossRef]
- Wang, Q., J. Liu, and S. Cheng, Heavy metals in apple orchard soils and fruits and their health risks in Liaodong Peninsula, Northeast China. Environmental Monitoring and Assessment. 2014. 187(1): p. 4178. [CrossRef]
- Bolarinwa, I.F., C. Orfila, and M.R.A. Morgan, Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chemistry. 2015. 170: p. 437-442. [CrossRef]
- Skinner, R.C., J.C. Gigliotti, K.-M. Ku, and J.C. Tou, A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutrition Reviews. 2018. 76(12): p. 893-909. [CrossRef]
- Lyu, F., S.F. Luiz, D.R. Azeredo, A.G. Cruz, S. Ajlouni, and C.S. Ranadheera Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes, 2020. 8,. [CrossRef]
- Pavicich, M.A., M. De Boevre, A. Vidal, F. Iturmendi, H. Mikula, B. Warth, D. Marko, S. De Saeger, and A. Patriarca, Fate of free and modified Alternaria mycotoxins during the production of apple concentrates. Food Control. 2020. 118: p. 107388. [CrossRef]
- Hrynko, I., P. Kaczyński, M. Pietruszyńska, and B. Łozowicka, The effect of food thermal processes on the residue concentration of systemic and non-systemic pesticides in apples. Food Control. 2023. 143: p. 109267. [CrossRef]
- Madalageri, D.M., P. Bharati, and U. Kage, Physicochemical properties, nutritional and antinutritional composition of pulp and peel of three mango varieties. Int. J. Educ. Sci. Res. 2017. 7: p. 81-94.
- Sorour, M., A.-H. Mehanni, S.M. Hussein, and M.A. Mustafa, Utilization of Treated Seed Kernel Flours of Some Fruits in Biscuit Manufacture. European Journal of Nutrition & Food Safety. 2022: p. 1-13.
- Mohammadi-Moghaddam, T., A. Firoozzare, M. Kariminejad, M. Sorahi, and Z. Tavakoli, Black plum peel as a useful by-product for the production of new foods: chemical, textural, and sensory characteristics of Halva Masghati. International Journal of Food Properties. 2020. 23(1): p. 2005-2019. [CrossRef]
- Akter, S., M.E. Netzel, M.T. Fletcher, U. Tinggi, and Y. Sultanbawa Chemical and Nutritional Composition of Terminalia ferdinandiana (Kakadu Plum) Kernels: A Novel Nutrition Source. Foods, 2018. 7,. [CrossRef]
- Mihaylova, D., A. Popova, I. Desseva, N. Petkova, M. Stoyanova, R. Vrancheva, A. Slavov, A. Slavchev, and A. Lante Comparative Study of Early- and Mid-Ripening Peach (Prunus persica L.) Varieties: Biological Activity, Macro-, and Micro- Nutrient Profile. Foods, 2021. 10,. [CrossRef]
- Tareen, A.K., M.A. Panezai, A. Sajjad, J.K. Achakzai, A.M. Kakar, and N.Y. Khan, Comparative analysis of antioxidant activity, toxicity, and mineral composition of kernel and pomace of apricot (Prunus armeniaca L.) grown in Balochistan, Pakistan. Saudi Journal of Biological Sciences. 2021. 28(5): p. 2830-2839. [CrossRef]
- Kolesar, E., E. Tvrda, M. Halenar, M. Schneidgenova, L. Chrastinova, L. Ondruska, R. Jurcik, A. Kovacik, E. Kovacikova, P. Massanyi, and A. Kolesarova, Assessment of rabbit spermatozoa characteristics after amygdalin and apricot seeds exposure in vivo. Toxicology Reports. 2018. 5: p. 679-686. [CrossRef]
- Zivoli, R., L. Gambacorta, L. Piemontese, and M. Solfrizzo Reduction of Aflatoxins in Apricot Kernels by Electronic and Manual Color Sorting. Toxins, 2016. 8,. [CrossRef]
- Sánchez-Pérez, S., O. Comas-Basté, J. Rabell-González, M.T. Veciana-Nogués, M.L. Latorre-Moratalla, and M.C. Vidal-Carou Biogenic Amines in Plant-Origin Foods: Are they Frequently Underestimated in Low-Histamine Diets? Foods, 2018. 7,. [CrossRef]
- Cámara, M.A., S. Cermeño, G. Martínez, and J. Oliva, Removal residues of pesticides in apricot, peach and orange processed and dietary exposure assessment. Food Chemistry. 2020. 325: p. 126936. [CrossRef]
- Campagnollo, F.B., K.C. Ganev, A.M. Khaneghah, J.B. Portela, A.G. Cruz, D. Granato, C.H. Corassin, C.A.F. Oliveira, and A.S. Sant'Ana, The occurrence and effect of unit operations for dairy products processing on the fate of aflatoxin M1: A review. Food Control. 2016. 68: p. 310-329. [CrossRef]
- Khaneghah, A.M., Y. Fakhri, L. Abdi, C.F.S.C. Coppa, L.T. Franco, and C.A.F. de Oliveira, The concentration and prevalence of ochratoxin A in coffee and coffee-based products: A global systematic review, meta-analysis and meta-regression. Fungal Biology. 2019. 123(8): p. 611-617. [CrossRef]
- Nabizadeh, S., N. Shariatifar, E. Shokoohi, S. Shoeibi, M. Gavahian, Y. Fakhri, A. Azari, and A. Mousavi Khaneghah, Prevalence and probabilistic health risk assessment of aflatoxins B1, B2, G1, and G2 in Iranian edible oils. Environmental Science and Pollution Research. 2018. 25(35): p. 35562-35570. [CrossRef]
- Bhat, R.V., Human health problems associated with current agricultural food production. Asia Pacific journal of clinical nutrition. 2008. 17.
- Gomiero, T., Food quality assessment in organic vs. conventional agricultural produce: Findings and issues. Applied Soil Ecology. 2018. 123: p. 714-728. [CrossRef]
- González, N., M. Marquès, M. Nadal, and J.L. Domingo, Occurrence of environmental pollutants in foodstuffs: A review of organic vs. conventional food. Food and Chemical Toxicology. 2019. 125: p. 370-375. [CrossRef]
- Adebo, O.A., T. Molelekoa, R. Makhuvele, J.A. Adebiyi, A.B. Oyedeji, S. Gbashi, M.A. Adefisoye, O.M. Ogundele, and P.B. Njobeh, A review on novel non-thermal food processing techniques for mycotoxin reduction. International Journal of Food Science & Technology. 2021. 56(1): p. 13-27. [CrossRef]
- Agriopoulou, S., E. Stamatelopoulou, and T. Varzakas Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods, 2020. 9,. [CrossRef]
- Jouany, J.P., Methods for preventing, decontaminating and minimizing the toxicity of mycotoxins in feeds. Animal Feed Science and Technology. 2007. 137(3): p. 342-362. [CrossRef]
- Wang, P., Q. Yao, X. Meng, X. Yang, X. Wang, Q. Lu, and A. Liu, Effective protective agents against organ toxicity of deoxynivalenol and their detoxification mechanisms: A review. Food and Chemical Toxicology. 2023. 182: p. 114121. [CrossRef]
- Fu, W., C. Dai, Z. Ma, Q. Li, D. Lan, C. Sun, X. Wu, J. Li, and S. Wang, Enhanced glutathione production protects against zearalenone-induced oxidative stress and ferroptosis in female reproductive system. Food and Chemical Toxicology. 2024. 185: p. 114462. [CrossRef]
- Zhang, Y., K.-X. Cao, Q.-J. Niu, J. Deng, L. Zhao, M.M. Khalil, N.A. Karrow, K. Kuča, and L.-H. Sun, Alpha-class glutathione S-transferases involved in the detoxification of aflatoxin B1 in ducklings. Food and Chemical Toxicology. 2023. 174: p. 113682. [CrossRef]
- Prakash, B., P.P. Singh, V. Gupta, and T.S. Raghuvanshi, Essential oils as green promising alternatives to chemical preservatives for agri-food products: New insight into molecular mechanism, toxicity assessment, and safety profile. Food and Chemical Toxicology. 2024. 183: p. 114241. [CrossRef]
- Singh, P.P., A.K. Jaiswal, T.S. Raghuvanshi, and B. Prakash, Insights into the antimicrobial efficacy of Coleus aromaticus essential oil against food-borne microbes: Biochemical and molecular simulation approaches. Food and Chemical Toxicology. 2023. 182: p. 114111. [CrossRef]
- Nie, T., Q. Wu, M. Long, W. Wu, and K. Kuca, New insight into mycotoxins and bacterial toxins: Toxicity assessment, molecular mechanism and food safety (preface to the special issue of food and chemical toxicology on the outcomes of Myco & bacterial toxin). Food and Chemical Toxicology. 2024. 188: p. 114655. [CrossRef]
- Pathak, V.M., V.K. Verma, B.S. Rawat, B. Kaur, N. Babu, A. Sharma, S. Dewali, M. Yadav, R. Kumari, S. Singh, A. Mohapatra, V. Pandey, N. Rana, and J.M. Cunill, Current status of pesticide effects on environment, human health and it’s eco-friendly management as bioremediation: A comprehensive review. Frontiers in Microbiology. 2022. 13.
- Mir, S.A., B.N. Dar, M.M. Mir, S.A. Sofi, M.A. Shah, T. Sidiq, K.V. Sunooj, A.M. Hamdani, and A. Mousavi Khaneghah, Current strategies for the reduction of pesticide residues in food products. Journal of Food Composition and Analysis. 2022. 106: p. 104274. [CrossRef]
- Munir, S., A. Azeem, M. Sikandar Zaman, and M. Zia Ul Haq, From field to table: Ensuring food safety by reducing pesticide residues in food. Science of The Total Environment. 2024. 922: p. 171382. [CrossRef]
- Leskovac, A. and S. Petrović Pesticide Use and Degradation Strategies: Food Safety, Challenges and Perspectives. Foods, 2023. 12,. [CrossRef]
- Pecenka, J.R., L.L. Ingwell, R.E. Foster, C.H. Krupke, and I. Kaplan, IPM reduces insecticide applications by 95% while maintaining or enhancing crop yields through wild pollinator conservation. Proceedings of the National Academy of Sciences. 2021. 118(44): p. e2108429118.
- Pop, C., R. Suharoschi, and O.L. Pop Dietary Fiber and Prebiotic Compounds in Fruits and Vegetables Food Waste. Sustainability, 2021. 13,. [CrossRef]
- Najar, I.N., P. Sharma, R. Das, S. Tamang, K. Mondal, N. Thakur, S.G. Gandhi, and V. Kumar, From waste management to circular economy: Leveraging thermophiles for sustainable growth and global resource optimization. Journal of Environmental Management. 2024. 360: p. 121136. [CrossRef]
- Antranikian, G. and W.R. Streit, Microorganisms harbor keys to a circular bioeconomy making them useful tools in fighting plastic pollution and rising CO2 levels. Extremophiles. 2022. 26(1): p. 10. [CrossRef]
- Najar, I.N., M.T. Sherpa, S. Das, S. Das, and N. Thakur, Microbial ecology of two hot springs of Sikkim: Predominate population and geochemistry. Science of The Total Environment. 2018. 637-638: p. 730-745. [CrossRef]
- Najar, I.N., M.T. Sherpa, S. Das, and N. Thakur, Bacterial diversity and functional metagenomics expounding the diversity of xenobiotics, stress, defense and CRISPR gene ontology providing eco-efficiency to Himalayan Hot Springs. Functional & Integrative Genomics. 2020. 20(4): p. 479-496. [CrossRef]
- Banerjee, A., S. Sarkar, T. Govil, P. González-Faune, G. Cabrera-Barjas, R. Bandopadhyay, D.R. Salem, and R.K. Sani, Extremophilic Exopolysaccharides: Biotechnologies and Wastewater Remediation. Frontiers in Microbiology. 2021. 12.
- Mehta, R., P. Singhal, H. Singh, D. Damle, and A.K. Sharma, Insight into thermophiles and their wide-spectrum applications. 3 Biotech. 2016. 6(1): p. 81. [CrossRef]
- Turner, P., G. Mamo, and E.N. Karlsson, Potential and utilization of thermophiles and thermostable enzymes in biorefining. Microbial Cell Factories. 2007. 6(1): p. 9. [CrossRef]
- Navina, B.K., N.K. Velmurugan, P. Senthil Kumar, G. Rangasamy, J. Palanivelu, P. Thamarai, A.S. Vickram, A. Saravanan, and A. Shakoor, Fungal bioremediation approaches for the removal of toxic pollutants: Mechanistic understanding for biorefinery applications. Chemosphere. 2024. 350: p. 141123. [CrossRef]
- Bhattacharya, A., D. Gola, P. Dey, and A. Malik, Synergistic and Antagonistic Effects on Metal Bioremediation with Increasing Metal Complexity in a Hexa-metal Environment by Aspergillus fumigatus. International Journal of Environmental Research. 2020. 14(6): p. 761-770. [CrossRef]
- Kalia, S., A. Bhattacharya, S.K. Prajapati, and A. Malik, Utilization of starch effluent from a textile industry as a fungal growth supplement for enhanced α-amylase production for industrial application. Chemosphere. 2021. 279: p. 130554. [CrossRef]
- Khatua, S., J. Simal-Gandara, and K. Acharya, Myco-remediation of plastic pollution: current knowledge and future prospects. Biodegradation. 2024. 35(3): p. 249-279. [CrossRef]
- Debnath, P., P. Dey, A. Chanda, and T. Bhakta, A Survey on Pineapple and its medicinal value. Scholars Academic Journal of Pharmacy. 2012. 1(1): p. 24-29.
- Ketnawa, S., P. Chaiwut, and S. Rawdkuen, Pineapple wastes: A potential source for bromelain extraction. Food and Bioproducts Processing. 2012. 90(3): p. 385-391. [CrossRef]
- Banerjee, S., V. Ranganathan, A. Patti, and A. Arora, Valorisation of pineapple wastes for food and therapeutic applications. Trends in Food Science & Technology. 2018. 82: p. 60-70. [CrossRef]
- de Toledo, N.M.V., L.P. Nunes, P.P.M. da Silva, M.H.F. Spoto, and S.G. Canniatti-Brazaca, Influence of pineapple, apple and melon by-products on cookies: physicochemical and sensory aspects. International Journal of Food Science & Technology. 2017. 52(5): p. 1185-1192. [CrossRef]
- Roda, A. and M. Lambri, Food uses of pineapple waste and by-products: a review. International Journal of Food Science & Technology. 2019. 54(4): p. 1009-1017. [CrossRef]
- Plazzotta, S., R. Ibarz, L. Manzocco, and O. Martín-Belloso, Optimizing the antioxidant biocompound recovery from peach waste extraction assisted by ultrasounds or microwaves. Ultrasonics Sonochemistry. 2020. 63: p. 104954. [CrossRef]
- Amariz, A., M.A.C.d. Lima, and R.E. Alves, Quality and antioxidant potential of byproducts from refining of fruit pulp. Food Science and Technology. 2018. 38: p. 203-209.
- Esparza, I., N. Jiménez-Moreno, F. Bimbela, C. Ancín-Azpilicueta, and L.M. Gandía, Fruit and vegetable waste management: Conventional and emerging approaches. Journal of Environmental Management. 2020. 265: p. 110510. [CrossRef]
- Rudke, C.R.M., A.A.F. Zielinski, and S.R.S. Ferreira, From Biorefinery to Food Product Design: Peach (Prunus persica) By-Products Deserve Attention. Food and Bioprocess Technology. 2023. 16(6): p. 1197-1215. [CrossRef]
- Pérez-Jiménez, J., S. Arranz, and F. Saura-Calixto, Proanthocyanidin content in foods is largely underestimated in the literature data: An approach to quantification of the missing proanthocyanidins. Food Research International. 2009. 42(10): p. 1381-1388. [CrossRef]
- Pérez-Jiménez, J. and F. Saura-Calixto, Fruit peels as sources of non-extractable polyphenols or macromolecular antioxidants: Analysis and nutritional implications. Food Research International. 2018. 111: p. 148-152. [CrossRef]
- Rodríguez-González, S., I.F. Pérez-Ramírez, D.M. Amaya-Cruz, M.A. Gallegos-Corona, M. Ramos-Gomez, O. Mora, and R. Reynoso-Camacho, Polyphenol-rich peach (Prunus persica L.) by-product exerts a greater beneficial effect than dietary fiber-rich by-product on insulin resistance and hepatic steatosis in obese rats. Journal of Functional Foods. 2018. 45: p. 58-66. [CrossRef]
- Mihaylova, D., A. Popova, I. Desseva, I. Dincheva, and Y. Tumbarski Valorization of Peels of Eight Peach Varieties: GC–MS Profile, Free and Bound Phenolics and Corresponding Biological Activities. Antioxidants, 2023. 12,. [CrossRef]
- Gamboa-Santos, J., A.C. Soria, T. Fornari, M. Villamiel, and A. Montilla, Optimisation of convective drying of carrots using selected processing and quality indicators. International Journal of Food Science & Technology. 2013. 48(10): p. 1998-2006. [CrossRef]
- García-Aparicio, M.d.P., F. Castro-Rubio, and M.L. Marina, Unlocking peach juice byproduct potential in food waste biorefineries: Phenolic compounds profile, antioxidant capacity and fermentable sugars. Bioresource Technology. 2024. 396: p. 130441. [CrossRef]
- Choi, Y.-S., Y.-B. Kim, K.-E. Hwang, D.-H. Song, Y.-K. Ham, H.-W. Kim, J.-M. Sung, and C.-J. Kim, Effect of apple pomace fiber and pork fat levels on quality characteristics of uncured, reduced-fat chicken sausages. Poultry Science. 2016. 95(6): p. 1465-1471. [CrossRef]
- Huc-Mathis, D., C. Journet, N. Fayolle, and V. Bosc, Emulsifying properties of food by-products: Valorizing apple pomace and oat bran. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2019. 568: p. 84-91. [CrossRef]
- Perussello, C.A., Z. Zhang, A. Marzocchella, and B.K. Tiwari, Valorization of Apple Pomace by Extraction of Valuable Compounds. Comprehensive Reviews in Food Science and Food Safety. 2017. 16(5): p. 776-796. [CrossRef]
- Ayala-Zavala, J.F., V. Vega-Vega, C. Rosas-Domínguez, H. Palafox-Carlos, J.A. Villa-Rodriguez, M.W. Siddiqui, J.E. Dávila-Aviña, and G.A. González-Aguilar, Agro-industrial potential of exotic fruit byproducts as a source of food additives. Food Research International. 2011. 44(7): p. 1866-1874. [CrossRef]
- Rotta, E.M., D.R. de Morais, P.B.F. Biondo, V.J. dos Santos, M. Matsushita, and J.V. Visentainer, Use of avocado peel (Persea americana) in tea formulation: a functional product containing phenolic compounds with antioxidant activity. Acta Scientiarum. Technology. 2016. 38(1): p. 23-29.
- Bankar, A., B. Joshi, A.R. Kumar, and S. Zinjarde, Banana peel extract mediated novel route for the synthesis of silver nanoparticles. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2010. 368(1): p. 58-63. [CrossRef]
- Vu, H.T., C.J. Scarlett, and Q.V. Vuong, Phenolic compounds within banana peel and their potential uses: A review. Journal of Functional Foods. 2018. 40: p. 238-248. [CrossRef]
- Satari, B. and K. Karimi, Citrus processing wastes: Environmental impacts, recent advances, and future perspectives in total valorization. Resources, Conservation and Recycling. 2018. 129: p. 153-167. [CrossRef]
- Espitia, P.J.P., W.-X. Du, R.d.J. Avena-Bustillos, N.d.F.F. Soares, and T.H. McHugh, Edible films from pectin: Physical-mechanical and antimicrobial properties - A review. Food Hydrocolloids. 2014. 35: p. 287-296. [CrossRef]
- Mainente, F., A. Menin, A. Alberton, G. Zoccatelli, and C. Rizzi, Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders. International Journal of Food Science & Technology. 2019. 54(4): p. 952-958. [CrossRef]
- Acun, S. and H. Gül, Effects of grape pomace and grape seed flours on cookie quality. Quality Assurance and Safety of Crops & Foods. 2014. 6(1): p. 81-88.
- Mattos, G.N., R.V. Tonon, A.A.L. Furtado, and L.M.C. Cabral, Grape by-product extracts against microbial proliferation and lipid oxidation: a review. Journal of the Science of Food and Agriculture. 2017. 97(4): p. 1055-1064. [CrossRef]
- Ajila, C.M., M. Aalami, K. Leelavathi, and U.J.S.P. Rao, Mango peel powder: A potential source of antioxidant and dietary fiber in macaroni preparations. Innovative Food Science & Emerging Technologies. 2010. 11(1): p. 219-224. [CrossRef]
- Ashoush, I.S. and M.G.E. Gadallah, Utilization of mango peels and seed kernels powders as sources of phytochemicals in biscuit. 2011.
- Adilah, A.N., B. Jamilah, M.A. Noranizan, and Z.A.N. Hanani, Utilization of mango peel extracts on the biodegradable films for active packaging. Food Packaging and Shelf Life. 2018. 16: p. 1-7. [CrossRef]
- Torres-León, C., A.A. Vicente, M.L. Flores-López, R. Rojas, L. Serna-Cock, O.B. Alvarez-Pérez, and C.N. Aguilar, Edible films and coatings based on mango (var. Ataulfo) by-products to improve gas transfer rate of peach. LWT. 2018. 97: p. 624-631. [CrossRef]
- Kodagoda, K. and R. Marapana, Development of non-alcoholic wines from the wastes of Mauritius pineapple variety and its physicochemical properties. 2017.
- Arshad, Z.I.M., A. Amid, F. Yusof, I. Jaswir, K. Ahmad, and S.P. Loke, Bromelain: an overview of industrial application and purification strategies. Applied Microbiology and Biotechnology. 2014. 98(17): p. 7283-7297. [CrossRef]
- Gonzalez, J., W. Donoso, N. Sandoval, M. Reyes, P. Gonzalez, M. Gajardo, E. Morales, A. Neira, I. Razmilic, J.A. Yuri, and R. Moore-Carrasco, Apple Peel Supplemented Diet Reduces Parameters of Metabolic Syndrome and Atherogenic Progression in ApoE−/− Mice. Evidence-Based Complementary and Alternative Medicine. 2015. 2015(1): p. 918384. [CrossRef]
- Tremocoldi, M.A., P.L. Rosalen, M. Franchin, A.P. Massarioli, C. Denny, É.R. Daiuto, J.A.R. Paschoal, P.S. Melo, and S.M.d. Alencar, Exploration of avocado by-products as natural sources of bioactive compounds. PloS one. 2018. 13(2): p. e0192577.
- Araújo, R.G., R.M. Rodriguez-Jasso, H.A. Ruiz, M.M.E. Pintado, and C.N. Aguilar, Avocado by-products: Nutritional and functional properties. Trends in Food Science & Technology. 2018. 80: p. 51-60. [CrossRef]
- Yu, X., H. Lin, Y. Wang, W. Lv, S. Zhang, Y. Qian, X. Deng, N. Feng, H. Yu, and B. Qian, d-limonene exhibits antitumor activity by inducing autophagy and apoptosis in lung cancer. OncoTargets and Therapy. 2018. 11(null): p. 1833-1847. [CrossRef]
- Miller, J.A., P.A. Thompson, I.A. Hakim, H.H.S. Chow, and C.A. Thomson, d-Limonene: a bioactive food component from citrus and evidence for a potential role in breast cancer prevention and treatment. Oncology Reviews. 2011. 5(1): p. 31-42. [CrossRef]
- Burton-Freeman, B.M., A.K. Sandhu, and I. Edirisinghe, Mangos and their bioactive components: adding variety to the fruit plate for health. Food & Function. 2017. 8(9): p. 3010-3032. [CrossRef]
- Maxwell, E.G., N.J. Belshaw, K.W. Waldron, and V.J. Morris, Pectin – An emerging new bioactive food polysaccharide. Trends in Food Science & Technology. 2012. 24(2): p. 64-73. [CrossRef]
- Wang, X., L. Gao, H. Lin, J. Song, J. Wang, Y. Yin, J. Zhao, X. Xu, Z. Li, and L. Li, Mangiferin prevents diabetic nephropathy progression and protects podocyte function via autophagy in diabetic rat glomeruli. European Journal of Pharmacology. 2018. 824: p. 170-178. [CrossRef]
- Nowicka, P. and A. Wojdyło, Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. European Food Research and Technology. 2019. 245(5): p. 1123-1136. [CrossRef]

| Fruit byproducts | Functional foods | References |
|---|---|---|
| Apple pomace | dietary fiber source in baked foods, chicken-meat-based sausages, and yogurt products, stabilizer for oil-water emulsions | [160-162] |
| Avocado by-product and avocado peels | antioxidants, antimicrobials, food additives (colorants, flavorings, and thickening agents), functional beverage formulation | [163, 164] |
| Banana peel | Antioxidant, antibacterial, antifungal activity, blood sugar reduction, lowering of cholesterol, anti-angiogenic activity and neuro-protective effect, synthesis of bio-inspired silver nanoparticles | [165, 166] |
| Citrus peel | Source of molasses, pectin, oil, and limone, thickener, emulsifier, and stabilizer in many foods, pectin being used as a polymeric matrix for edible films for active food pack by-product | [167, 168] |
| Grape pomace | Grape pomace powders contained in meat and fish derivatives, fiber in bakery products, oil from grape seed | [169-171] |
| Mango peel | Antioxidant and dietary fiber in macaroni, sources of phytochemicals in biscuits, edible films | [172-175] |
| Pineapple peel, core and stem | Pineapple peel can be used as a nutrient in fermentation processes being a rich source of sugar, core can be used in pineapple juice concentrates, vinegar, and wine production, pineapple stem contains bromelain enzyme and its extraction can be used as a meat tenderizer, bread dough improver. | [176, 177] |
| fruit byproducts | Medicinal and pharmaceutical exploitation | References |
|---|---|---|
| Apple peel | Reduces metabolic syndrome and atherogenic progression | [178] |
| Avocado peel | inhibitor for the inflammation mediator nitric oxide by a possible reduction of free radicals during inflammation, anticancer, antidiabetic, and antihypertensive effects | [179, 180] |
| Banana peel | antioxidant, antibacterial, antifungal activity, reduce blood sugar, lower cholesterol, and show anti-angiogenic activity and neuro-protective effect, silver nanoparticles, which are used as antimicrobials to pathogenic fungi |
[165, 181] |
| Citrus pulp and seed | Therapeutic effect on lung cancer in mice and breast cancer in mice and rats shown by D-limonene. | [181, 182] |
| Mango | Anti-inflammatory and antioxidative properties during obesity, diabetes, CVD, and skin cancer in vivo studies, reduction of carcinogenesis | [183-185] |
| Peach kernel | Phenols, carotenoids, and cyanogenic glycosides of peach kernel possess antidiabetic, antioxidative, and anti-aging properties | [186] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





