1. Introduction
Sarcopenia, characterized by gradual and progressive loss of skeletal muscle mass and strength, is a critical concern in aging populations [
1,
2]. As a syndrome directly associated with physical frailty, disabilities, and increased mortality [
3,
4,
5,
6], understanding and quantifying its impact on the overall quality of life (QoL) are important for both clinical and therapeutic strategies [
7]. The progressive nature of sarcopenia not only impairs physical capability but also affects the overall well-being and independence of older adults. It often leads to a cascade of negative health outcomes, including an increased risk of falls, fractures, and hospitalization, as well as a decline in mental health due to reduced social interactions and increased feelings of depression [
8,
9]. These results highlight the need for early identification and intervention to mitigate the adverse effects of sarcopenia on QoL.
To gain comprehensive insights into the requirements of older adults and those with sarcopenia, a judicious evaluation of their QoL using a suitable and validated questionnaire is imperative [
10]. In this regard, the SarQoL
® questionnaire emerges as a specialized instrument designed to specifically evaluate the QoL dimensions affected by sarcopenia [
11,
12]. With its meticulous consideration of 55 items encompassing seven domains related to health-related QoL, the SarQoL
® questionnaire provides comprehensive and multiple domains of QoL, including physical, mental, and social aspects, thereby offering a holistic view of the impact sarcopenia has on individuals [
12]. Its emphasis on musculoskeletal health highlights its capability as a reliable screening tool for identifying older adults at an increased risk of sarcopenia [
13]. Additionally, recent literature has highlighted the importance of QoL assessment in sarcopenia, indicating that a diminished QoL is a consistent outcome for those affected by this condition [
7]. The SarQoL
® questionnaire has been validated in various languages, demonstrating its versatility and sensitivity in detecting QoL impairments specific to sarcopenic sufferers [
11,
12,
14,
15,
16]. This broad applicability underscores the necessity of incorporating QoL measures into routine sarcopenia assessments to guide clinical management and intervention strategies [
17].
Furthermore, the operational definitions of sarcopenia have evolved, incorporating not only muscle mass and strength but also muscle function, as recommended by leading gerontological societies [
18]. These definitions emphasize the relevance of implementing precise and reliable tools, such as the SarQoL, to evaluate outcomes and interventions. However, although these tools are available, their implementation in diverse populations and settings often reveals variability in QoL outcomes, suggesting a need for continued research on threshold values and cutoff points that can effectively signal the presence of sarcopenia across different demographic groups [
19]. The Korean older adult population, like many others, is facing a growing prevalence of sarcopenia due to an aging society. However, the cultural and lifestyle factors unique to this population necessitate localized validation of screening tools such as the SarQoL
® questionnaire. Previous studies have validated the SarQoL-K and demonstrated its applicability in the Korean context [
20]. Building on this foundation, the present study sought to further refine the use of the SarQoL
® questionnaire by identifying a specific cutoff point that can effectively identify individuals with sarcopenia among community-dwelling Korean older adults.
Thus, this study aimed to evaluate the association between the SarQoL overall score and the presence of sarcopenia and to determine a specific cut-off point for the SarQoL® questionnaire that can serve as an effective screening tool based on the revised Asian Working Group for Sarcopenia (AWGS) consensus criteria among Korean older adults.
2. Materials and Methods
2.1. Ethical Consideration
This research is a cross-sectional cohort study that adhered to the principles stated in the Declaration of Helsinki and received ethical approval approved by the institutional review board of Gachon University (1044396-202204-HR-082-02). All the participants provided written informed consent and signed the consent form before participating in the study. Additionally, the present manuscript was prepared in accordance with the recent version of the Standards for Reporting Diagnostic Accuracy (STARD) guideline [
21].
2.2. Participants
A convenience sample of older adults who visited community centers in metropolitan areas of South Korea from February to June 2023 was used. Of the 664 adults aged 65 years and older, 496 completed the SarQoL® questionnaire. Individuals with a body mass index (BMI) greater than 30 kg/m², those for whom calculating the appendicular skeletal muscle mass (ASM) was not appropriate, and those who had difficulties following the measurement instructions were excluded. Consequently, 451 older adults were included in the final analysis.
2.3. Measurements
The Inbody 120 (Inbody Corp., Seoul, Korea) was used for ASM, which was calculated using the following equation: ASM (kg) = 0.244 × body weight (kg) + 7.8 × height (m) + 6.6 × gender (1 for men and 0 for women) − 0.098 × age (years) + race (0 for whites, 1.4 for blacks, and −1.2 for Asians) − 3.3 [
22]. Handgrip strength was measured using a Smedley-type handheld dynamometer (Fabrication Enterprises Inc., Elmsford, NY, USA). Participants were instructed to exert maximal effort for 5 s with both dominant and non-dominant hands, and two trials were performed, with the best performance recorded for analysis. The short physical performance battery (SPPB) protocol was employed to assess physical performance, comprising three domains: balance, gait speed, and chair stand tests [
23]. Based on the collected data, sarcopenia was diagnosed according to the AWGS criteria, considering participants with low muscle mass (ASM/ht
2 < 7.0 kg/m
2 for men and < 5.7 kg/m
2 for women) and low muscle strength (grip strength < 28 kg for men and < 18 kg for women), and/or low physical performance (SPPB < 9). The AWGS criteria were used as the reference standard [
24] in this study because of their status as the current consensus criteria and their applicability to samples recruited in South Korea.
The paper-based Korean version of the SarQoL
® questionnaire was used as the index test. The positivity of the index tests and cutoff points were defined using the receiver operating characteristic (ROC) curve. The Korean version of the SarQoL
® questionnaire comprises 22 questions, which include a total of 53 items, as some of the questions are structured in a matrix format. The SarQoL
® questionnaire generates an overall score derived from seven distinct domain scores: D1 (physical and mental health), D2 (locomotion), D3 (body composition), D4 (functionality), D5 (activities of daily living), D6 (leisure activities), and D7 (fears). Higher scores indicate better QoL. This version of the SarQoL
® questionnaire was used to assess an overall QoL, which is scored from 0 to 100 points based on the questionnaire responses [
20,
25]. The overall QoL score was calculated using a specialized MS Access database developed specifically for this purpose and can be obtained from the official website,
www.sarqol.org. A lower score indicates lower QoL and, consequently, a greater likelihood of sarcopenia-related disabilities [
25].
To compare the performance of the SarQoL questionnaire with a widely used screening tool for sarcopenia, the SARC-F questionnaire was administered [
26]. The SARC-F consists of 5 questions that assess strength, assistance in walking, rising from a chair, climbing stairs, and falling. Each item is scored from 0 to 2, resulting in a total score ranging from 0 to 10, where a score of ≥ 4 suggests the need for further examination for sarcopenia. SARC-F was developed to detect sarcopenia, and its performance has been widely evaluated [
26].
The reference and index tests were completed by the participant during a single visit.
2.4. Statistical Analysis
Statistical analyses were performed using Statistical Product and Service Solutions (IBM Corp., Armonk, NY, USA), version 22.0, for Windows and MedCalculator software (MedCalc Software Ltd., Ostend, Belgium). The Kolmogorov-Smirnov test was used to test the normal distribution of the collected data. Binary logistic regression was used to evaluate the predictive probability of the SarQoL for sarcopenia diagnosis. Pearson’s chi-square test for cross-tabulation was conducted to assess the sensitivity and specificity. The receiver operating characteristic (ROC) curve was plotted with sensitivity on the vertical axis and 100-specificity on the horizontal axis. The optimal cutoff point for the overall SarQoL score was determined using the Youden index [
27]. The area under the curve (AUC), representing diagnostic accuracy, was calculated using ROC curve analysis. An AUC value greater than 0.9 indicates high accuracy, 0.7–0.9 moderate accuracy, 0.5–0.7 low accuracy, and 0.5 a chance result [
28]. Additionally, the DeLong method was used to compare the diagnostic abilities of the SarQoL
® questionnaire and the SARC-F questionnaire. An independent t-test was conducted to compare sarcopenia diagnostic indicators and SarQoL scores between the groups. Statistical significance was set at 0.05.
3. Results
All participants were assessed for sarcopenia using the AWGS criteria, and 125 (27.7%) of them were diagnosed with sarcopenia. The sarcopenic participants were older than those not diagnosed as sarcopenic (82.4 ± 6.0 vs. 75.8 ± 6.1 years,
p < 0.001). The general characteristics of the study participants are presented in
Table 1.
Significant positive correlations were found between the SarQoL and sarcopenia diagnostic indicators, including ASM (r = 0.272, p < 0.001), grip strength (r = 0.503, p < 0.001), and SPPB (r = 0.546, p < 0.001). Binary logistic regression showed that SarQoL score was significantly associated with sarcopenia (OR: 0.937; 95% CI: 0.923–0.952), indicating that an increase of one unit in SarQoL score decreased the probability of sarcopenia by approximately 6.5%.
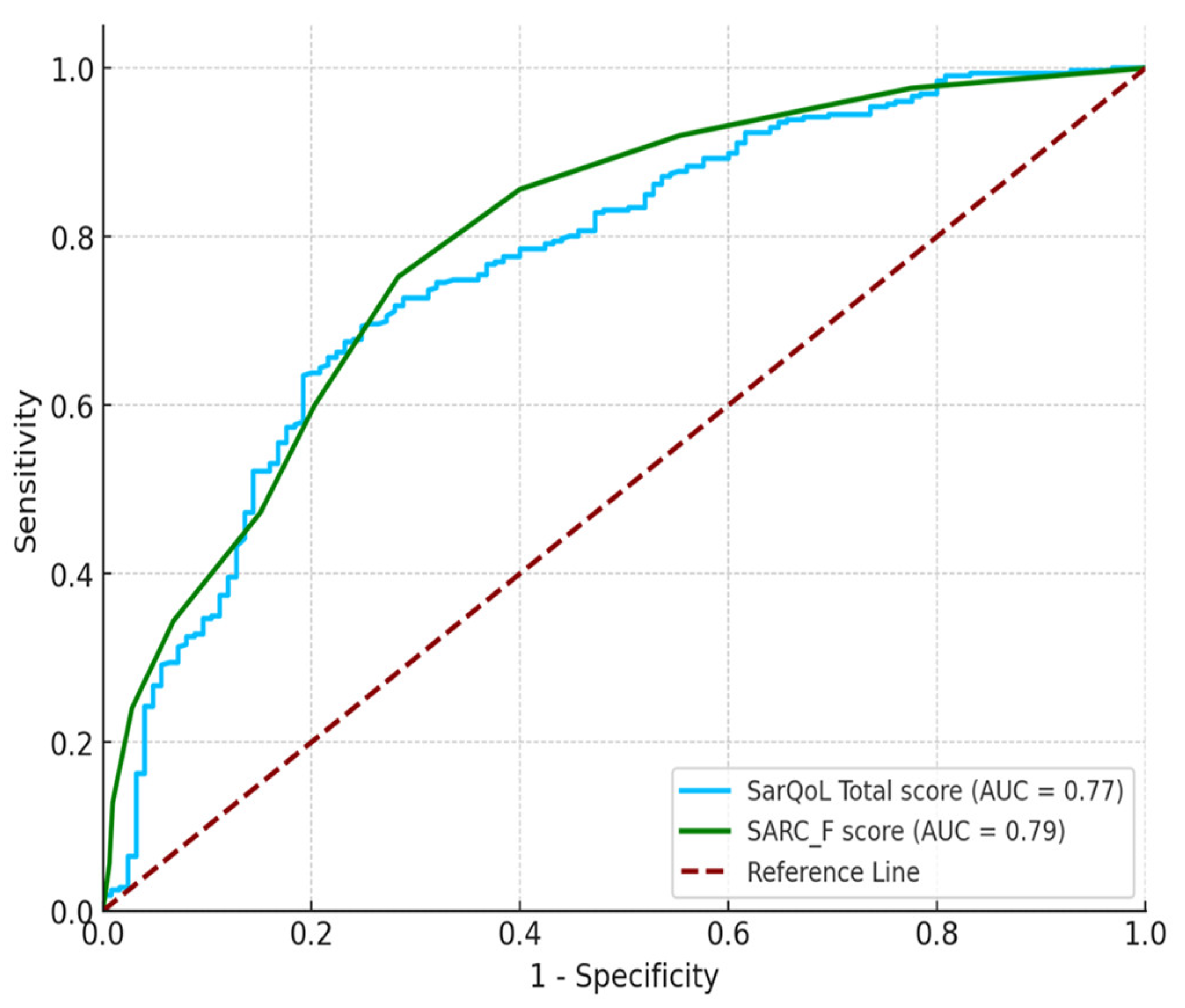
The cutoff point of the SarQoL overall score as a criterion for predicting sarcopenia had significant results (
p < 0.001), with an AUC of 0.768 (95% CI: 0.715 to 0.817). The Youden index was maximized at ≤ 58.5 points for the overall SarQoL score (Jc = 0.445, Se = 0.693, and Sp = 0.752), indicating good accuracy.
Table 2 presents the index test results of the reference standards for the diagnosis of sarcopenia. A significant relationship was observed between the index and reference standard tests (
p < 0.001). The AUC for the SARC-F was 0.794 (95% CI: 0.748 to 0.835).
Table 3 describes the differences between the diagnostic abilities of the SarQoL (AUC=0.768) and SARC-F (AUC=0.794). No significant difference was found in AUC values between the SarQoL and SARC-F (
p > 0.05) (
Figure 1).
Table 4 illustrates the differences in the SarQoL scores between participants with and without sarcopenia. Participants diagnosed with sarcopenia had significantly lower overall SarQoL scores (50.5 ± 15.8) compared to those without sarcopenia (66.5 ± 15.4), with a
p-value of < 0.001. This trend was consistent across all seven domains of the SarQoL
® questionnaire. For instance, in the “Physical and Mental Health” domain, sarcopenic participants scored 48.1 ± 16.7, significantly lower than the 60.5 ± 17.2 score of non-sarcopenic participants. Similarly, in the “Locomotion” domain, sarcopenic participants scored 45.2 ± 21.9, compared to 61.9 ± 23.4 in those without sarcopenia. Other domains such as “Body Composition,” “Functionality,” “Activities of Daily Living,” “Leisure Activities,” and “Fears” all displayed statistically significant lower scores in sarcopenic participants, underscoring the profound impact of sarcopenia on QoL across various aspects.
4. Discussion
This study substantiated the validity and reliability of the SarQoL
® questionnaire as an essential tool for assessing QoL among Korean community-dwelling older adults with sarcopenia. Consistent with prior research [
17,
29,
30], our findings underscore the significant correlation between sarcopenia severity and diminished QoL, thereby reinforcing the need for specialized tools such as the SarQoL in clinical settings.
The SarQoL
® questionnaire, with a cut-off value of ≤ 58.5, demonstrated good diagnostic accuracy, as evidenced by an AUC of 0.768, offering a sensitivity of 69.3% and specificity of 75.2%. This finding aligns with those of previous studies, such as Beaudart et al. and Geerinck et al., who also reported a strong discriminative ability of the SarQoL score in identifying sarcopenic individuals [
17,
30]. For instance, Geerinck et al. identified a cut-off point of ≤ 52.4 in a European cohort, resulting in an AUC of 0.771, sensitivity of 64.7%, and specificity of 80.5%, similar to our findings, albeit with slight variations likely because of population differences [
30].
In this study, the SarQoL® was compared with the SARC-F, a well-known sarcopenia screening tool. Although the SARC-F displayed a slightly higher AUC (0.794) than the SarQoL®, the difference was not statistically significant, suggesting that both tools were comparably effective in detecting sarcopenia in this population. Importantly, SarQoL® offers additional value by assessing the broader impact of sarcopenia on QoL, which SARC-F does not capture. This makes the SarQoL® a more comprehensive tool, particularly valuable in contexts in which understanding the overall well-being of individuals with sarcopenia is crucial.
A key strength of this study lies in establishing a cut-off value tailored specifically to the Korean older adult population, marking the first such effort in an Asian cohort. While previous research has primarily focused on European populations [
30], our study reflects the unique cultural and lifestyle factors of Korean older adults. A validation study by Yoo et al. also confirmed the robust psychometric properties of the SarQoL-K, supporting its applicability across different cultural contexts. By providing a population-specific threshold, our study enhances the clinical utility of SarQoL
® in Korea and underscores the importance of considering demographic and cultural differences in clinical research.
The strong association between the SarQoL scores and sarcopenia severity highlights the multifaceted impact of sarcopenia in older adults. Sarcopenia, characterized by the loss of muscle mass and strength, directly impairs physical mobility, leading to reduced independence and diminished mental well-being [
31,
32]. The resultant psychosocial effects, such as decreased social interactions and heightened depression, further contribute to the lower QoL observed in sarcopenic individuals [
33]. These findings underscore the importance of addressing both physical and psychological aspects of sarcopenia in clinical practice. The SarQoL
® questionnaire’s holistic approach, which includes mental and social dimensions alongside physical health, aligns with the shift toward patient-centered care in geriatric medicine [
34]. Early identification of sarcopenia using the SarQoL can facilitate timely interventions that not only aim to preserve muscle function but also enhance overall QoL [
34]. This is particularly crucial, as sarcopenia often leads to a cascade of health declines, including an increased risk of falls, fractures, and hospitalization. Therefore, integrating SarQoL assessments into routine screening could play a pivotal role in improving the health outcomes of older adults.
Given the increasing prevalence of sarcopenia among the aging population, this research is crucial for enabling early and accurate identification and intervention [
18]. While traditional diagnostic methods focus on physical metrics such as muscle mass and strength, the SarQoL
® questionnaire offers a more holistic perspective by integrating mental and social aspects with physical health [
25]. By establishing a specific cutoff score, our study introduces a practical tool that can be seamlessly integrated into clinical practice to enhance early screening and management of sarcopenia. Early detection is critical, as timely interventions can prevent further decline in muscle function and QoL, thereby reducing the risk of associated disabilities. The consistently lower SarQoL
® scores across multiple domains in participants with sarcopenia demonstrated the questionnaire’s ability to capture the broad-ranging effects of sarcopenia, offering insights beyond traditional physical assessments.
Despite its strengths, this study also outlined some limitations. The cross-sectional design limits our ability to infer causality between low SarQoL® scores and the presence of sarcopenia. Longitudinal studies are needed to establish the predictive validity of SarQoL® over time. Additionally, our study sample was recruited from community centers in metropolitan areas, which may have limited the external validity of our findings. The sample may not represent the broader older adult population in rural areas or those who are less mobile and less likely to participate in such studies.
In summary, this study highlights the importance of culturally tailored tools in clinical practice and suggests that the SarQoL® questionnaire, with its cut-off, can play a pivotal role in the early screening and management of sarcopenia in Korean older adult populations.
5. Conclusions
This study advances the field of sarcopenia research by providing a validated tool that enhances early screening and comprehensive assessment of sarcopenia. The SarQoL® questionnaire, with its established cut-off point, has the potential to significantly improve clinical outcomes by facilitating earlier and more accurate identification of sarcopenia, leading to timely, targeted interventions. Future research should continue to validate and expand these findings to ensure that the SarQoL® questionnaire becomes an integral part of sarcopenia management across diverse global populations.
Author Contributions
J. K. and H. L. designed the study and conducted data collection. H. L. analyzed the extracted data and wrote the first draft of the manuscript. J. K. contributed to data discussions and critically reviewed the manuscript. All the authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2022S1A5C2A07090938).
Institutional Review Board Statement
This research received ethical approval from the Institutional Review Board of Gachon University (1044396-202204-HR-082-02).
Informed Consent Statement
Informed consent was obtained from all the participants involved in the study.
Data Availability Statement
The datasets generated in this study are available from the corresponding author upon request.
Acknowledgments
We would like to express our sincere gratitude to all the personnel at the Institute of Human Convergence Health Science for their support in data collection.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yanai, K., et al., MicroRNAs in Sarcopenia: A Systematic Review. Front Med (Lausanne), 2020. 7: p. 180. [CrossRef]
- Chianca, V., et al., Sarcopenia: imaging assessment and clinical application. Abdom Radiol (NY), 2022. 47(9): p. 3205-3216. [CrossRef]
- Zhang, T., J.K. Cheng, and Y.M. Hu, Gut microbiota as a promising therapeutic target for age-related sarcopenia. Ageing Res Rev, 2022. 81: p. 101739. [CrossRef]
- Paez, H.G., C.R. Pitzer, and S.E. Alway, Age-Related Dysfunction in Proteostasis and Cellular Quality Control in the Development of Sarcopenia. Cells, 2023. 12(2). [CrossRef]
- Al Saedi, A., et al., Lipid metabolism in sarcopenia. Bone, 2022. 164: p. 116539. [CrossRef]
- Purnamasari, D., et al., Sarcopenia and Chronic Complications of Type 2 Diabetes Mellitus. Rev Diabet Stud, 2022. 18(3): p. 157-165. [CrossRef]
- Beaudart, C., et al., Sarcopenia and health-related quality of life: A systematic review and meta-analysis. J Cachexia Sarcopenia Muscle, 2023. 14(3): p. 1228-1243. [CrossRef]
- Fonfría-Vivas, R., et al., Assessing quality of life with SarQol is useful in screening for sarcopenia and sarcopenic obesity in older women. Aging Clin Exp Res, 2023. [CrossRef]
- Iacob, S., et al., Assessment of Sarcopenia Related Quality of Life Using SarQoL® Questionnaire in Patients With Liver Cirrhosis. Front Nutr, 2022. 9: p. 774044. [CrossRef]
- Tsekoura, M., et al., Sarcopenia and Its Impact on Quality of Life. Adv Exp Med Biol, 2017. 987: p. 213-218. [CrossRef]
- Pap, Z., et al., Evaluation of the sarcopenia quality of life (SarQoL) questionnaire in community dwelling outpatient postmenopausal hungarian women. BMC Musculoskelet Disord, 2023. 24(1): p. 331. [CrossRef]
- Beaudart, C., et al., Measuring health-related quality of life in sarcopenia: summary of the SarQoL psychometric properties. Aging Clin Exp Res, 2023. 35(8): p. 1581-1593. [CrossRef]
- Shafiee, G., et al., Development of a Simple and Practical Screening Tool for Detection of Sarcopenia in Older People: The Bushehr Elderly Health Program. Front Med (Lausanne), 2021. 8: p. 655759. [CrossRef]
- Tsekoura, M., et al., Cross cultural adaptation of the Greek sarcopenia quality of life (SarQoL) questionnaire. Disabil Rehabil, 2020. 42(7): p. 1006-1012. [CrossRef]
- Alekna, V., et al., Validation of the Lithuanian version of sarcopenia-specific quality of life questionnaire (SarQoL(®)). Eur Geriatr Med, 2019. 10(5): p. 761-767. [CrossRef]
- Lee, S.C., et al., Translation and validation of the Taiwanese SarQoL, a quality of life questionnaire specific to sarcopenia. J Formos Med Assoc, 2023. 122(3): p. 249-257. [CrossRef]
- Beaudart, C., et al., Quality of life in sarcopenia measured with the SarQoL®: impact of the use of different diagnosis definitions. Aging Clin Exp Res, 2018. 30(4): p. 307-313. [CrossRef]
- Alhmly, H.F. and R.A. Fielding, A Critical Review of Current Worldwide Definitions of Sarcopenia. Calcified Tissue International, 2024. 114(1): p. 74-81. [CrossRef]
- Soares, L.A., et al., Accuracy of handgrip and respiratory muscle strength in identifying sarcopenia in older, community-dwelling, Brazilian women. Sci Rep, 2023. 13(1): p. 1553. [CrossRef]
- Yoo, J.I., et al., Translation and validation of the Korean version of the Sarcopenia Quality of Life (SarQoL-K®) questionnaire and applicability with the SARC-F screening tool. Qual Life Res, 2021. 30(2): p. 603-611. [CrossRef]
- Cohen, J.F., et al., STARD 2015 guidelines for reporting diagnostic accuracy studies: explanation and elaboration. BMJ Open, 2016. 6(11): p. e012799. [CrossRef]
- Lee, R.C., et al., Total-body skeletal muscle mass: development and cross-validation of anthropometric prediction models. Am J Clin Nutr, 2000. 72(3): p. 796-803. [CrossRef]
- Welch, S.A., et al., The Short Physical Performance Battery (SPPB): A Quick and Useful Tool for Fall Risk Stratification Among Older Primary Care Patients. J Am Med Dir Assoc, 2021. 22(8): p. 1646-1651. [CrossRef]
- Chen, L.K., et al., Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Dir Assoc, 2020. 21(3): p. 300-307.e2. [CrossRef]
- Beaudart, C., et al., Development of a self-administrated quality of life questionnaire for sarcopenia in elderly subjects: the SarQoL. Age Ageing, 2015. 44(6): p. 960-6. [CrossRef]
- Malmstrom, T.K., et al., SARC-F: a symptom score to predict persons with sarcopenia at risk for poor functional outcomes. J Cachexia Sarcopenia Muscle, 2016. 7(1): p. 28-36. [CrossRef]
- Hajian-Tilaki, K., Receiver Operating Characteristic (ROC) Curve Analysis for Medical Diagnostic Test Evaluation. Caspian J Intern Med, 2013. 4(2): p. 627-35. [PubMed]
- Swets, J.A., Measuring the accuracy of diagnostic systems. Science, 1988. 240(4857): p. 1285-93. [CrossRef]
- Beaudart, C., et al., Validation of the SarQoL®, a specific health-related quality of life questionnaire for Sarcopenia. J Cachexia Sarcopenia Muscle, 2017. 8(2): p. 238-244. [CrossRef]
- Geerinck, A., et al., Assessment of the performance of the SarQoL(®) questionnaire in screening for sarcopenia in older people. Aging Clin Exp Res, 2021. 33(8): p. 2149-2155. [CrossRef]
- Bottoni, A., et al., Sarcopenia: an overview and analysis of molecular mechanisms. Nutrire, 2019. 44(1): p. 6. [CrossRef]
- Marzetti, E., et al., Sarcopenia: an overview. Aging Clinical and Experimental Research, 2017. 29(1): p. 11-17. [CrossRef]
- Nipp, R.D., et al., Sarcopenia Is Associated with Quality of Life and Depression in Patients with Advanced Cancer. The Oncologist, 2017. 23(1): p. 97-104. [CrossRef]
- Beaudart, C., et al., Measuring health-related quality of life in sarcopenia: summary of the SarQoL psychometric properties. Aging Clinical and Experimental Research, 2023. 35(8): p. 1581-1593. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).