Submitted:
02 September 2024
Posted:
03 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Measurements
2.2.1. Socio-Demographic Information
2.2.3. Anthropometric Measurements
2.2.3. Lifestyle Factors
Nutrient Intakes Estimate
Sun Exposure Behaviors
Physical Activity Assessment
Laboratory Assessment
Biomarkers Cut-Off Points Definition
Statistical Analysis
Logistic Regression
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kinyoki, D.; Osgood-Zimmerman, A.E.; Bhattacharjee, N.V.; Local Burden of Disease Anaemia Collaborators; Schaeffer, L.E.; Lazzar-Atwood, A.; Lu, D.; Ewald, S.B.; Donkers, K.M.; Letourneau, I.D.; et al. Anemia Prevalence in Women of Reproductive Age in Low- and Middle-Income Countries between 2000 and 2018. Nat. Med. 2021, 27, 1761–1782. [Google Scholar] [CrossRef] [PubMed]
- Cui, A.; Zhang, T.; Xiao, P.; Fan, Z.; Wang, H.; Zhuang, Y. Global and Regional Prevalence of Vitamin D Deficiency in Population-Based Studies from 2000 to 2022: A Pooled Analysis of 7.9 Million Participants. Front. Nutr. 2023, 10, 1070808. [Google Scholar] [CrossRef] [PubMed]
- Horton, S.; Ross, J. The Economics of Iron Deficiency. Food Policy 2003, 28, 51–75. [Google Scholar] [CrossRef]
- Heath, A.; Kim, I.; Hodge, A.; English, D.; Muller, D. Vitamin D Status and Mortality: A Systematic Review of Observational Studies. Int. J. Environ. Res. Public. Health 2019, 16, 383. [Google Scholar] [CrossRef]
- Liu, Z.; Sun, R.; Li, J.; Cheng, W.; Li, L. Relations of Anemia With the All-Cause Mortality and Cardiovascular Mortality in General Population: A Meta-Analysis. Am. J. Med. Sci. 2019, 358, 191–199. [Google Scholar] [CrossRef]
- Wagner, C.L.; Hollis, B.W. The Implications of Vitamin D Status During Pregnancy on Mother and Her Developing Child. Front. Endocrinol. 2018, 9, 500. [Google Scholar] [CrossRef]
- Smith, E.R.; Shankar, A.H.; Wu, L.S.-F.; Aboud, S.; Adu-Afarwuah, S.; Ali, H.; Agustina, R.; Arifeen, S.; Ashorn, P.; Bhutta, Z.A.; et al. Modifiers of the Effect of Maternal Multiple Micronutrient Supplementation on Stillbirth, Birth Outcomes, and Infant Mortality: A Meta-Analysis of Individual Patient Data from 17 Randomised Trials in Low-Income and Middle-Income Countries. Lancet Glob. Health 2017, 5, e1090–e1100. [Google Scholar] [CrossRef]
- WHO. Global nutrition targets 2025: anaemia policy brief (WHO/NMH/NHD/14.4). Geneva: World Health Organization; 2014.
- WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and Mineral Nutrition Information System. Geneva, World Health Organization, 2011 (WHO/NMH/NHD/MNM/11.1) available online on:http://www.who.int/vmnis/indicators/haemoglobin. pdf, accessed on 22may2021).
- World Health Organization Nutritional Anaemias: Tools for Effective Prevention and Control; World Health Organization: Geneva, 2017; ISBN 978-92-4-151306-7.
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef]
- Holick, M.F. The Vitamin D Deficiency Pandemic: Approaches for Diagnosis, Treatment and Prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef]
- Holick, M.F. Ultraviolet B Radiation: The Vitamin D Connection. Adv. Exp. Med. Biol. 2017, 996, 137–154. [Google Scholar] [CrossRef]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Endocrine Society Evaluation, Treatment, and Prevention of Vitamin D Deficiency: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Fleet, J.C.; Shapses, S.A. Vitamin D. In Present Knowledge in Nutrition; Elsevier, 2020; pp. 93–114. ISBN 978-0-323-66162-1. [Google Scholar]
- Dimitrov, V.; Barbier, C.; Ismailova, A.; Wang, Y.; Dmowski, K.; Salehi-Tabar, R.; Memari, B.; Groulx-Boivin, E.; White, J.H. Vitamin D-Regulated Gene Expression Profiles: Species-Specificity and Cell-Specific Effects on Metabolism and Immunity. Endocrinology 2021, 162, bqaa218. [Google Scholar] [CrossRef] [PubMed]
- Ciepiela, P.; Dulęba, A.J.; Kowaleczko, E.; Chełstowski, K.; Kurzawa, R. Vitamin D as a Follicular Marker of Human Oocyte Quality and a Serum Marker of in Vitro Fertilization Outcome. J. Assist. Reprod. Genet. 2018, 35, 1265–1276. [Google Scholar] [CrossRef]
- Pérez-Fernandez, R.; Alonso, M.; Segura, C.; Muñoz, I.; Garcia-Caballero, T.; Diéguez, C. Vitamin D Receptor Gene Expression in Human Pituitary Gland. Life Sci. 1996, 60, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W. Vitamin D Receptor: New Assignments for an Already Busy Receptor. Endocrinology 2006, 147, 5542–5548. [Google Scholar] [CrossRef]
- Aucella, F.; Scalzulli, R.P.; Gatta, G.; Vigilante, M.; Carella, A.M.; Stallone, C. Calcitriol Increases Burst-Forming Unit-Erythroid Proliferation in Chronic Renal Failure. A Synergistic Effect with r-HuEpo. Nephron Clin. Pract. 2003, 95, c121–127. [Google Scholar] [CrossRef]
- Alon, D.B.; Chaimovitz, C.; Dvilansky, A.; Lugassy, G.; Douvdevani, A.; Shany, S.; Nathan, I. Novel Role of 1,25(OH)(2)D(3) in Induction of Erythroid Progenitor Cell Proliferation. Exp. Hematol. 2002, 30, 403–409. [Google Scholar] [CrossRef]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hepcidin and Iron Homeostasis. Biochim. Biophys. Acta BBA - Mol. Cell Res. 2012, 1823, 1434–1443. [Google Scholar] [CrossRef]
- Zughaier, S.M.; Alvarez, J.A.; Sloan, J.H.; Konrad, R.J.; Tangpricha, V. The Role of Vitamin D in Regulating the Iron-Hepcidin-Ferroportin Axis in Monocytes. J. Clin. Transl. Endocrinol. 2014, 1, e19–e25. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and Anemia: Insights into an Emerging Association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Mei, G.; Zhou, F.; Kong, B.; Chen, L.; Chen, H.; Wang, L.; Tang, Y.; Yao, P. Vitamin D Decreases Pancreatic Iron Overload in Type 2 Diabetes through the NF-κB-DMT1 Pathway. J. Nutr. Biochem. 2022, 99, 108870. [Google Scholar] [CrossRef] [PubMed]
- Shawki, A.; Knight, P.B.; Maliken, B.D.; Niespodzany, E.J.; Mackenzie, B. H+-Coupled Divalent Metal-Ion Transporter-1. In Current Topics in Membranes; Elsevier, 2012; Vol. 70, pp. 169–214. ISBN 978-0-12-394316-3. [Google Scholar]
- Fleet, J.C.; Aldea, D.; Chen, L.; Christakos, S.; Verzi, M. Regulatory Domains Controlling High Intestinal Vitamin D Receptor Gene Expression Are Conserved in Mouse and Human. J. Biol. Chem. 2022, 298, 101616. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surała, O.; Orysiak, J.; Szczepańska, B.; Witek, K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients 2018, 10, 167. [Google Scholar] [CrossRef]
- Sim, J.J.; Lac, P.T.; Liu, I.L.A.; Meguerditchian, S.O.; Kumar, V.A.; Kujubu, D.A.; Rasgon, S.A. Vitamin D Deficiency and Anemia: A Cross-Sectional Study. Ann. Hematol. 2010, 89, 447–452. [Google Scholar] [CrossRef]
- Liu, T.; Zhong, S.; Liu, L.; Liu, S.; Li, X.; Zhou, T.; Zhang, J. Vitamin D Deficiency and the Risk of Anemia: A Meta-Analysis of Observational Studies. Ren. Fail. 2015, 37, 929–934. [Google Scholar] [CrossRef]
- Blanco-Rojo, R.; Pérez-Granados, A.M.; Toxqui, L.; Zazo, P.; De La Piedra, C.; Vaquero, M.P. Relationship between Vitamin D Deficiency, Bone Remodelling and Iron Status in Iron-Deficient Young Women Consuming an Iron-Fortified Food. Eur. J. Nutr. 2013, 52, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Mogire, R.M.; Muriuki, J.M.; Morovat, A.; Mentzer, A.J.; Webb, E.L.; Kimita, W.; Ndungu, F.M.; Macharia, A.W.; Cutland, C.L.; Sirima, S.B.; et al. Vitamin D Deficiency and Its Association with Iron Deficiency in African Children. Nutrients 2022, 14, 1372. [Google Scholar] [CrossRef]
- Soepnel, L.M.; Mabetha, K.; Draper, C.E.; Silubonde, T.M.; Smuts, C.M.; Pettifor, J.M.; Norris, S.A. A Cross-Sectional Study of the Associations between Biomarkers of Vitamin D, Iron Status, and Hemoglobin in South African Women of Reproductive Age: The Healthy Life Trajectories Initiative, South Africa. Curr. Dev. Nutr. 2023, 7, 100072. [Google Scholar] [CrossRef]
- Arabi, S.M.; Ranjbar, G.; Bahrami, L.S.; Vafa, M.; Norouzy, A. The Effect of Vitamin D Supplementation on Hemoglobin Concentration: A Systematic Review and Meta-Analysis. Nutr. J. 2020, 19, 11. [Google Scholar] [CrossRef]
- Azizi-Soleiman, F.; Vafa, M.; Abiri, B.; Safavi, M. Effects of Iron on Vitamin D Metabolism: A Systematic Review. Int. J. Prev. Med. 2016, 7, 126. [Google Scholar] [CrossRef] [PubMed]
- Toxqui, L.; Pérez-Granados, A.M.; Blanco-Rojo, R.; Wright, I.; González-Vizcayno, C.; Vaquero, M.P. Effects of an Iron or Iron and Vitamin D–Fortified Flavored Skim Milk on Iron Metabolism: A Randomized Controlled Double-Blind Trial in Iron-Deficient Women. J. Am. Coll. Nutr. 2013, 32, 312–320. [Google Scholar] [CrossRef]
- Heldenberg, D.; Tenenbaum, G.; Weisman, Y. Effect of Iron on Serum 25-Hydroxyvitamin D and 24,25-Dihydroxyvitamin D Concentrations. Am. J. Clin. Nutr. 1992, 56, 533–536. [Google Scholar] [CrossRef]
- Ministère de la Santé, M. La Lutte Contre Les Troubles Dus Aux Carences En Micronutriments. Situation et Perspectives. Rapport Ministère de La Santé 2003.
- PNN, Enquête Nationale sur la Nutrition, Diversité alimentaire, Carence en Fer, Carence en Vitamine A, Carence en Iode. 2019. Available online: https://www.sante.gov.ma/Documents/2022/07/rapport%20ENN%202019-2020%20ajout%20preface%20(1).pdf (accessed on 2 January 2022).
- Programme national de nutrition. Available online: https://www.sante.gov.ma/Documents/2019/06/Programme%20National%20de%20Nutrition.pdf (accessed on 21 April 2021).
- Glenn, D. Israel. Determining Sample Size 1. University of Florida IFAS Extension. 2012, pp. 1–5. Available online: http://Edis.Ifas.Ufl.Edu (accessed on 12 january 2021).
- Chaparro, C.M.; Suchdev, P.S. Anemia Epidemiology, Pathophysiology, and Etiology in Low- and Middle-Income Countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chen, W.; Li, D.; Yin, X.; Zhang, X.; Olsen, N.; Zheng, S.G. Vitamin D and Chronic Diseases. Aging Dis. 2017, 8, 346. [Google Scholar] [CrossRef]
- de Souza, S.S.; Camargos, A.F.; Ferreira, M.C.F.; de Assis Nunes Pereira, F.; de Rezende, C.P.; Araújo, C.A.A.; Silva Filho, A.L. Hemoglobin Levels Predict Quality of Life in Women with Heavy Menstrual Bleeding. Arch. Gynecol. Obstet. 2010, 281, 895–900. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Schmidt, J.; Kisters, K. Important Drug-Micronutrient Interactions: A Selection for Clinical Practice. Crit. Rev. Food Sci. Nutr. 2020, 60, 257–275. [Google Scholar] [CrossRef]
- Kalus, U.; Pruss, A.; Wodarra, J.; Kiesewetter, H.; Salama, A.; Radtke, H. Influence of Blood Donation on Levels of Water-soluble Vitamins. Transfus. Med. 2008, 18, 360–365. [Google Scholar] [CrossRef]
- WHO Consultation on Obesity (1997: Geneva, Switzerland)World Health Organization. Division of Noncommunicable Diseases & World Health Organization. Programme of Nutrition, Family and Reproductive Health. (1998) Obesity : Preventing and Managing the Global Epidemic : Report of a WHO Consultation on Obesity, Geneva, 3-5 June 1997.
- Zouine, N.; Lhilali, I.; Menouni, A.; Godderis, L.; El Midaoui, A.; El Jaafari, S.; Zegzouti Filali, Y. Development and Validation of Vitamin D- Food Frequency Questionnaire for Moroccan Women of Reproductive Age: Use of the Sun Exposure Score and the Method of Triad’s Model. Nutrients 2023, 15, 796. [Google Scholar] [CrossRef]
- Neve J Aliments et Préparations Typiques de La Population Marocaine: Outil Pour Estimer La Consommation Alimentaire. 2008.
- The French Agency for Food, Environmental and Occupationnal Health Safty (ANSES) Ciqual French Food Composition 2017.
- U.S. Department of Agriculture, Agricultural Research Service USDA Food and Nutrient Database for Dietary Studies 2015-2016. 2018.
- Skolmowska, D.; Głąbska, D. Analysis of Heme and Non-Heme Iron Intake and Iron Dietary Sources in Adolescent Menstruating Females in a National Polish Sample. Nutrients 2019, 11, 1049. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, B.; Schönfeldt, H.C.; Hall, N. Total and Haem Iron Content Lean Meat Cuts and the Contribution to the Diet. Food Chem. 2016, 193, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S, discussion 1229S-1231S. [Google Scholar] [CrossRef] [PubMed]
- Vitamin and Mineral Requirements in Human Nutrition; Weltgesundheitsorganisation, Ed.; 2. ed.; Geneva, 2004; ISBN 978-92-4-154612-6.
- Lhilali, I.; Zouine, N.; Menouni, A.; Godderis, L.; Kestemont, M.-P.; El Midaoui, A.; El Jaafari, S.; Filali-Zegzouti, Y. Sun Exposure Score and Vitamin D Levels in Moroccan Women of Childbearing Age. Nutrients 2023, 15, 688. [Google Scholar] [CrossRef] [PubMed]
- Forde, C. Scoring the International Physical Activity Questionnaire (IPAQ); University of Dublin: Dublin, Ireland, 2018; p. 3. [Google Scholar]
- Craig, C.; Marshall, A.; Sjostrom, M.; et al. International physical activity questionnaire-short form. J Am Coll Health 2017, 65, 492–501. [Google Scholar]
- Nairz, M.; Theurl, I.; Wolf, D.; Weiss, G. Iron Deficiency or Anemia of Inflammation?: Differential Diagnosis and Mechanisms of Anemia of Inflammation. Wien. Med. Wochenschr. 2016, 166, 411–423. [Google Scholar] [CrossRef]
- World Health Organization WHO Guideline on Use of Ferritin Concentrations to Assess Iron Status in Individuals and Populations; World Health Organization: Geneva, 2020; ISBN 978-92-4-000012-4.
- Bouillon, R.; Van Schoor, N.M.; Gielen, E.; Boonen, S.; Mathieu, C.; Vanderschueren, D.; Lips, P. Optimal Vitamin D Status: A Critical Analysis on the Basis of Evidence-Based Medicine. J. Clin. Endocrinol. Metab. 2013, 98, E1283–E1304. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D Status: Measurement, Interpretation, and Clinical Application. Ann. Epidemiol. 2009, 19, 73–78. [Google Scholar] [CrossRef]
- Bouillon, R.; Manousaki, D.; Rosen, C.; Trajanoska, K.; Rivadeneira, F.; Richards, J.B. The Health Effects of Vitamin D Supplementation: Evidence from Human Studies. Nat. Rev. Endocrinol. 2022, 18, 96–110. [Google Scholar] [CrossRef]
- Levey, A.S. A More Accurate Method To Estimate Glomerular Filtration Rate from Serum Creatinine: A New Prediction Equation. Ann. Intern. Med. 1999, 130, 461. [Google Scholar] [CrossRef]
- Hosmer, D.W.; Lemeshow, S.; Sturdivant, R.X. Applied Logistic Regression; Wiley Series in Probability and Statistics; 1st ed.; Wiley, 2013; ISBN 978-0-470-58247-3.
- Zhou, X.; Obuchowski, N.A.; McClish, D.K. Statistical Methods in Diagnostic Medicine; Wiley Series in Probability and Statistics; 1st ed.; Wiley, 2011; ISBN 978-0-470-18314-4.
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef] [PubMed]
- Várbíró, S.; Takács, I.; Tűű, L.; Nas, K.; Sziva, R.E.; Hetthéssy, J.R.; Török, M. Effects of Vitamin D on Fertility, Pregnancy and Polycystic Ovary Syndrome—A Review. Nutrients 2022, 14, 1649. [Google Scholar] [CrossRef] [PubMed]
- Binkley, N.; Bikle, D.D.; Dawson-Hughes, B.; Plum, L.; Sempos, C.; DeLuca, H.F. Nonskeletal Effects of Vitamin D. In Principles of Bone Biology; Elsevier, 2020; pp. 757–774 ISBN 978-0-12-814841-9.
- Szarpak, L.; Feduniw, S.; Pruc, M.; Ciebiera, M.; Cander, B.; Rahnama-Hezavah, M.; Szarpak, Ł. The Vitamin D Serum Levels in Pregnant Women Affected by COVID-19: A Systematic Review and Meta-Analysis. Nutrients 2023, 15, 2588. [Google Scholar] [CrossRef] [PubMed]
- Estébanez, N.; Gómez-Acebo, I.; Palazuelos, C.; Llorca, J.; Dierssen-Sotos, T. Vitamin D Exposure and Risk of Breast Cancer: A Meta-Analysis. Sci. Rep. 2018, 8, 9039. [Google Scholar] [CrossRef] [PubMed]
- Osanai, M.; Lee, G.-H. CYP24A1-Induced Vitamin D Insufficiency Promotes Breast Cancer Growth. Oncol. Rep. 2016, 36, 2755–2762. [Google Scholar] [CrossRef]
- Suh, Y.J.; Lee, J.E.; Lee, D.H.; Yi, H.G.; Lee, M.H.; Kim, C.S.; Nah, J.W.; Kim, S.K. Prevalence and Relationships of Iron Deficiency Anemia with Blood Cadmium and Vitamin D Levels in Korean Women. J. Korean Med. Sci. 2016, 31, 25. [Google Scholar] [CrossRef]
- Shin, J.Y.; Shim, J.Y. Low Vitamin D Levels Increase Anemia Risk in Korean Women. Clin. Chim. Acta 2013, 421, 177–180. [Google Scholar] [CrossRef]
- Seong, J.M.; Park, C.E.; Gi, M.Y.; Cha, J.A.; Moon, A.E.; Lee, J.H.; Sung, H.H.; Lim, J.H.; Oh, S.H.; Chung, C.H.; et al. Gender Difference in the Relationship between Anemia and Vitamin D in Korean Adults: The Fifth Korea National Health and Nutrition Examination Survey. J. Clin. Biochem. Nutr. 2021, 69, 299–304. [Google Scholar] [CrossRef]
- Thomas, C.E.; Guillet, R.; Queenan, R.A.; Cooper, E.M.; Kent, T.R.; Pressman, E.K.; Vermeylen, F.M.; Roberson, M.S.; O’Brien, K.O. Vitamin D Status Is Inversely Associated with Anemia and Serum Erythropoietin during Pregnancy. Am. J. Clin. Nutr. 2015, 102, 1088–1095. [Google Scholar] [CrossRef]
- Bacchetta, J.; Zaritsky, J.J.; Sea, J.L.; Chun, R.F.; Lisse, T.S.; Zavala, K.; Nayak, A.; Wesseling-Perry, K.; Westerman, M.; Hollis, B.W.; et al. Suppression of Iron-Regulatory Hepcidin by Vitamin D. J. Am. Soc. Nephrol. 2014, 25, 564–572. [Google Scholar] [CrossRef]
- Smith, E.M.; Alvarez, J.A.; Kearns, M.D.; Hao, L.; Sloan, J.H.; Konrad, R.J.; Ziegler, T.R.; Zughaier, S.M.; Tangpricha, V. High-Dose Vitamin D3 Reduces Circulating Hepcidin Concentrations: A Pilot, Randomized, Double-Blind, Placebo-Controlled Trial in Healthy Adults. Clin. Nutr. Edinb. Scotl. 2017, 36, 980–985. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Wang, J.; Hu, W.; Song, X.; Yuan, D.; Yan, X. The Association between Standardized Serum 25-Hydroxyvitamin D Concentration and Risk of Anemia: A Population-Based Cross-Sectional Study. Int. J. Clin. Pract. 2022, 2022, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, A.; von Hurst, P.R.; Beck, K.L.; Mazahery, H.; Lim, K.; Badenhorst, C.E. Relationship between Vitamin D, Iron, and Hepcidin in Premenopausal Females, Potentially Confounded by Ethnicity. Eur. J. Nutr. 2023, 62, 3361–3368. [Google Scholar] [CrossRef]
- Michalski, E.S.; Nguyen, P.H.; Gonzalez-Casanova, I.; Nguyen, S.V.; Martorell, R.; Tangpricha, V.; Ramakrishnan, U. Serum 25-Hydroxyvitamin D but Not Dietary Vitamin D Intake Is Associated with Hemoglobin in Women of Reproductive Age in Rural Northern Vietnam. J. Clin. Transl. Endocrinol. 2017, 8, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Alvarez, J.A.; Martin, G.S.; Zughaier, S.M.; Ziegler, T.R.; Tangpricha, V. Vitamin D Deficiency Is Associated with Anaemia among African Americans in a US Cohort. Br. J. Nutr. 2015, 113, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, A.; Lemire, M.; Lévesque, B.; Ayotte, P. Determinants of Iron Deficiency and Anemia among Nunavimmiut: Results from the Qanuilirpitaa? 2017 Nunavik Health Survey. Can. J. Public Health Rev. Can. Sante Publique 2024, 115, 152–167. [Google Scholar] [CrossRef]
- Slominski, A.T.; Kim, T.-K.; Li, W.; Yi, A.-K.; Postlethwaite, A.; Tuckey, R.C. The Role of CYP11A1 in the Production of Vitamin D Metabolites and Their Role in the Regulation of Epidermal Functions. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt A, 28–39. [Google Scholar] [CrossRef]
- Hou, Y.; Zhang, S.; Wang, L.; Li, J.; Qu, G.; He, J.; Rong, H.; Ji, H.; Liu, S. Estrogen Regulates Iron Homeostasis through Governing Hepatic Hepcidin Expression via an Estrogen Response Element. Gene 2012, 511, 398–403. [Google Scholar] [CrossRef]
- Velarde, M.C. Mitochondrial and Sex Steroid Hormone Crosstalk during Aging. Longev. Heal. 2014, 3, 2. [Google Scholar] [CrossRef]
- Toxqui, L.; Vaquero, M. Chronic Iron Deficiency as an Emerging Risk Factor for Osteoporosis: A Hypothesis. Nutrients 2015, 7, 2324–2344. [Google Scholar] [CrossRef]
- Pike, J.W.; Meyer, M.B. The Vitamin D Receptor: New Paradigms for the Regulation of Gene Expression by 1,25-Dihydroxyvitamin D3. Endocrinol. Metab. Clin. North Am. 2010, 39, 255–269. [Google Scholar] [CrossRef] [PubMed]
- Pasricha, S.-R.; Drakesmith, H.; Black, J.; Hipgrave, D.; Biggs, B.-A. Control of Iron Deficiency Anemia in Low- and Middle-Income Countries. Blood 2013, 121, 2607–2617. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Dolce, A.; Celenza, G.; Grandone, E.; Perilli, M.G.; Siragusa, S.; Carta, G.; Orecchioni, A.; Mariani, G. Iron-Dependent Erythropoiesis in Women with Excessive Menstrual Blood Losses and Women with Normal Menses. Ann. Hematol. 2014, 93, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, T.H. Iron Requirements in Pregnancy and Strategies to Meet Them. Am. J. Clin. Nutr. 2000, 72, 257S–264S. [Google Scholar] [CrossRef]
- Hurrell, R.; Egli, I. Iron Bioavailability and Dietary Reference Values. Am. J. Clin. Nutr. 2010, 91, 1461S–1467S. [Google Scholar] [CrossRef]
- Milman, N.T. Dietary Iron Intake in Women of Reproductive Age in Europe: A Review of 49 Studies from 29 Countries in the Period 1993–2015. J. Nutr. Metab. 2019, 2019, 1–13. [Google Scholar] [CrossRef]
- Spiro, A.; Buttriss, J.L. Vitamin D : An Overview of Vitamin D Status and Intake in E Urope. Nutr. Bull. 2014, 39, 322–350. [Google Scholar] [CrossRef]
- Benhammou, S.; Heras-González, L.; Ibáñez-Peinado, D.; Barceló, C.; Hamdan, M.; Rivas, A.; Mariscal-Arcas, M.; Olea-Serrano, F.; Monteagudo, C. Comparison of Mediterranean Diet Compliance between European and Non-European Populations in the Mediterranean Basin. Appetite 2016, 107, 521–526. [Google Scholar] [CrossRef]
- Masella, R.; Malorni, W. Gender-Related Differences in Dietary Habits. Clin. Manag. Issues 2017, 11. [Google Scholar] [CrossRef]
- High Commission for Planning. National Survey on Household Consumption and Expenditure. [Internet]. Rabat(Morocco): High Commission for Plannig, 2013/2014. Available from: Https://Www.Hcp.Ma/Downloads/Enquete-Nationale-Sur-La-Consommation-et-Les-Depenses-Des-Menages_t21181.Html (Accessed 20 April 2020).
- Zijp, I.M.; Korver, O.; Tijburg, L.B.M. Effect of Tea and Other Dietary Factors on Iron Absorption. Crit. Rev. Food Sci. Nutr. 2000, 40, 371–398. [Google Scholar] [CrossRef]
- Thankachan, P.; Walczyk, T.; Muthayya, S.; Kurpad, A.V.; Hurrell, R.F. Iron Absorption in Young Indian Women: The Interaction of Iron Status with the Influence of Tea and Ascorbic Acid. Am. J. Clin. Nutr. 2008, 87, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Skolmowska, D.; Głąbska, D.; Kołota, A.; Guzek, D. Effectiveness of Dietary Interventions to Treat Iron-Deficiency Anemia in Women: A Systematic Review of Randomized Controlled Trials. Nutrients 2022, 14, 2724. [Google Scholar] [CrossRef] [PubMed]
- Mawer, E.B.; Backhouse, J.; Holman, C.A.; Lumb, G.A.; Stanbury, S.W. The Distribution and Storage of Vitamin D and Its Metabolites in Human Tissues. Clin. Sci. 1972, 43, 413–431. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Menzie, C.M.; Yanoff, L.B.; Denkinger, B.I.; McHugh, T.; Sebring, N.G.; Calis, K.A.; Yanovski, J.A. Obesity-Related Hypoferremia Is Not Explained by Differences in Reported Intake of Heme and Nonheme Iron or Intake of Dietary Factors That Can Affect Iron Absorption. J. Am. Diet. Assoc. 2008, 108, 145–148. [Google Scholar] [CrossRef]
- Frelut, M.-L.; Girardet, J.-P.; Bocquet, A.; Briend, A.; Chouraqui, J.-P.; Darmaun, D.; Dupont, C.; Feillet, F.; Hankard, R.; Rozé, J.-C.; et al. Impact of Obesity on Biomarkers of Iron and Vitamin D Status in Children and Adolescents: The Risk of Misinterpretation. Arch. Pédiatrie 2018, 25, 3–5. [Google Scholar] [CrossRef]
- Alshwaiyat, N.; Ahmad, A.; Wan Hassan, W.M.R.; Al-jamal, H. Association between Obesity and Iron Deficiency (Review). Exp. Ther. Med. 2021, 22, 1268. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased Bioavailability of Vitamin D in Obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef]
- Santana, K.V.D.S.D.; Oliver, S.L.; Mendes, M.M.; Lanham-New, S.; Charlton, K.E.; Ribeiro, H. Association between Vitamin D Status and Lifestyle Factors in Brazilian Women: Implications of Sun Exposure Levels, Diet, and Health. eClinicalMedicine 2022, 47, 101400. [Google Scholar] [CrossRef]
- Bustamante, M.; Hernandez-Ferrer, C.; Sarria, Y.; Harrison, G.I.; Nonell, L.; Kang, W.; Friedländer, M.R.; Estivill, X.; González, J.R.; Nieuwenhuijsen, M.; et al. The Acute Effects of Ultraviolet Radiation on the Blood Transcriptome Are Independent of Plasma 25OHD3. Environ. Res. 2017, 159, 239–248. [Google Scholar] [CrossRef]
- Kift, R.; Rhodes, L.E.; Farrar, M.D.; Webb, A.R. Is Sunlight Exposure Enough to Avoid Wintertime Vitamin D Deficiency in United Kingdom Population Groups? Int. J. Environ. Res. Public. Health 2018, 15, 1624. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Vitamin D Deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Maeda, S.S.; Saraiva, G.L.; Hayashi, L.F.; Cendoroglo, M.S.; Ramos, L.R.; Corrêa, M. de P.; Henrique de Mesquita, C.; Lazaretti-Castro, M. Seasonal Variation in the Serum 25-Hydroxyvitamin D Levels of Young and Elderly Active and Inactive Adults in São Paulo, Brazil: The São PAulo Vitamin D Evaluation Study (SPADES). Dermatoendocrinol. 2013, 5, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Damian, M.-T.; Vulturar, R.; Login, C.C.; Damian, L.; Chis, A.; Bojan, A. Anemia in Sports: A Narrative Review. Life Basel Switz. 2021, 11, 987. [Google Scholar] [CrossRef] [PubMed]
- Brownlie, T.; Utermohlen, V.; Hinton, P.S.; Haas, J.D. Tissue Iron Deficiency without Anemia Impairs Adaptation in Endurance Capacity after Aerobic Training in Previously Untrained Women. Am. J. Clin. Nutr. 2004, 79, 437–443. [Google Scholar] [CrossRef]
- Haas, J.D.; Brownlie, T. Iron Deficiency and Reduced Work Capacity: A Critical Review of the Research to Determine a Causal Relationship. J. Nutr. 2001, 131, 676S–690S. [Google Scholar] [CrossRef]
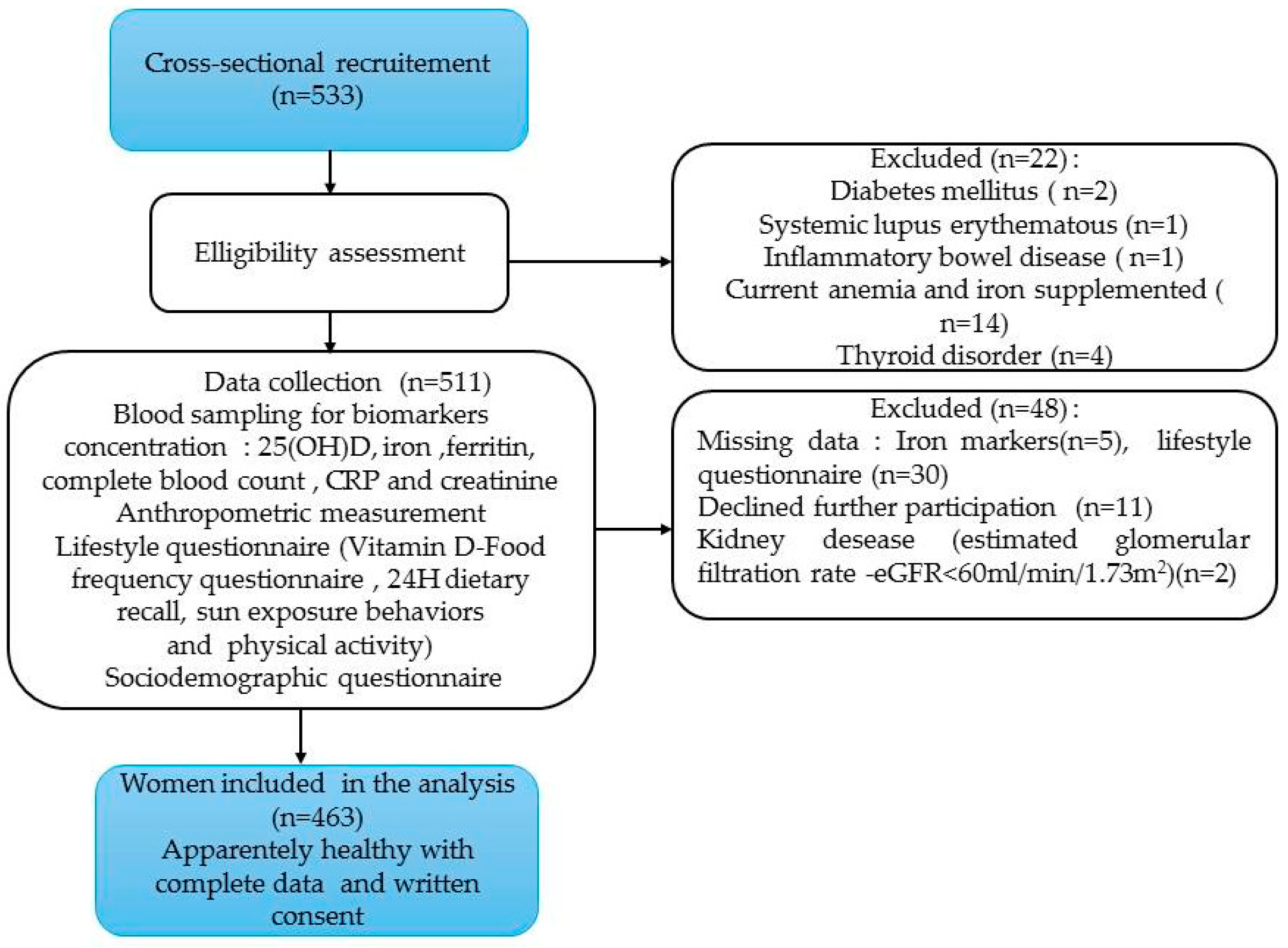
| Characteristics | All participant (n=463) |
Anemic (n=108) |
Non anemic (n =355) |
p-value1 |
|---|---|---|---|---|
| Median (IQR) or n (%) | ||||
| Anemia status(HB<12g/dl) | 463 (100) | 108(23.3) | 355(76.6) | <0.001 |
| Socio-demographic characteristics; | ||||
| Age, years | 29.0(11.0) | 28.0(10.0) | 29.0(11.0) | 0.639 |
| Education Illiterate ≤10 years (secondary-college) ≥11years(university or higher ) |
84(18.1) 222(47.9) 157(33.9) |
20(18.5) 56(51.9) 32(29.6) |
64(18.0) 166(46.8) 125(35.2) |
0.259 |
| Marital status Married Unmarried |
292(63.1) 171(36.9) |
65(60.2) 43(39.8) |
227(63.9) 128(36.1) |
0.479 |
| Employment Yes No |
201(43.4) 262(56.6) |
44(40.7) 64(59.3) |
157(44.2) 198(55.8) |
0.522 |
| Localization Urban Rural |
299(64.6) 164(35.4) |
60(55.6) 48(44.4) |
239(67.3) 116(32.7) |
0.025 |
| Anthropometric measures; BMI, Kg/m2 |
26.5(6.4) |
27.6(7.0) |
25.8(6.9) |
0.001 |
| BMI classification Underweight(<18kg/m2) Normal(<25kg/m2) Overweight (25 to<30 kg/m2) Obesity (≥30kg/m2) |
6(1.3) 173(37.4) 176(38.0) 108(23.3) |
1(0.9) 29(26.9) 43(39.8) 35(32.4) |
5(1.4) 144(40.6) 133(37.5) 73(20.6) |
0.024 |
| Lifestyle factors; PAC score, MET min/week |
1597.6(1892.4) | 1358.4(1178.8) |
1778.2(1765.0) |
<0.0001 |
| PAC levels (%) Low Moderate High |
53(11.5) 373(80.7) 36(7.8) |
29(12.3) 75(86.4) 3(2.8) |
24(6.8) 298(83.9) 33(9.3) |
<0.0001 |
| Dietary intake Energy, Kcal/day |
1678.4(651.3) |
1748.1(758.3) |
1652.6(596.8) |
0.295 |
| Vitamin D intake, µg/day | 2.7(2.74) | 2.3(2.3) | 2.8(2.76) | 0.035 |
| Vitamin D intake categories; Low (<5 µg /day) Adequate (≥5 µg /day) |
376(81.2) 87(18.8) |
91(84.3) 17(15.7) |
285(80.3) 70(19.7) |
0.352 |
| Iron intake, mg/day | 11.4(6.1) | 9.2(6.4) |
11.5(5.1) | 0.011 |
| Iron intake categories; Low (<(58.8 mg /day) Adequate (≥58.8 mg /day) |
463(100) 0(0.0) |
108(23.3) 0(0.0) |
355(76.7) 0(0.0) |
- |
| Heminic iron intake, mg/day | 0.5(0.2) | 0.4(0.3) | 0.5(0.2) | 0.051 |
| Non-Heminic iron intake, mg/day | 11.0(5.3) | 8.4(6.5) |
10.1(5.1) |
0.012 |
| Sun exposure behaviors; Sunscore (SES), median (IQR) Sunscore categories (%) Insufficient-Moderate Sufficient-High |
15.5(8.5) 247(53.3) 216(46.7) |
14.4(6.9) 60(55.6) 48(44.4) |
15.9(10.5) 156(43.9) 199(56.1) |
0.060 0.034 |
| Saison (%) Summer-spring Autun-winther |
281(60.69) 182 (39.30) |
72 (15.55%) 36 (7.78%) |
209 (45.14%) 146 (31.53%) |
0.180 |
| Parameters | All participant (n=463) |
25(OH)D (ng/ml) cut-off points | P-value 1 | ||
|---|---|---|---|---|---|
| 20 (n=334) |
20-30 (n=82) |
>30 (n=47) |
|||
| Median (IQR) or n(%) | |||||
|
25(OH)D (ng/mL) Cut-off points, <12ng/ml 12-20ng/ml 20-30ng/ml >30ng/ml |
14.4(10.8) 99(29.7) 334(72.1) 82(17.1) 47(10.2) |
11.7(5.6) | 24.3(4.7) | 34.9(12.2) | <0.001 |
| BC parameters; | |||||
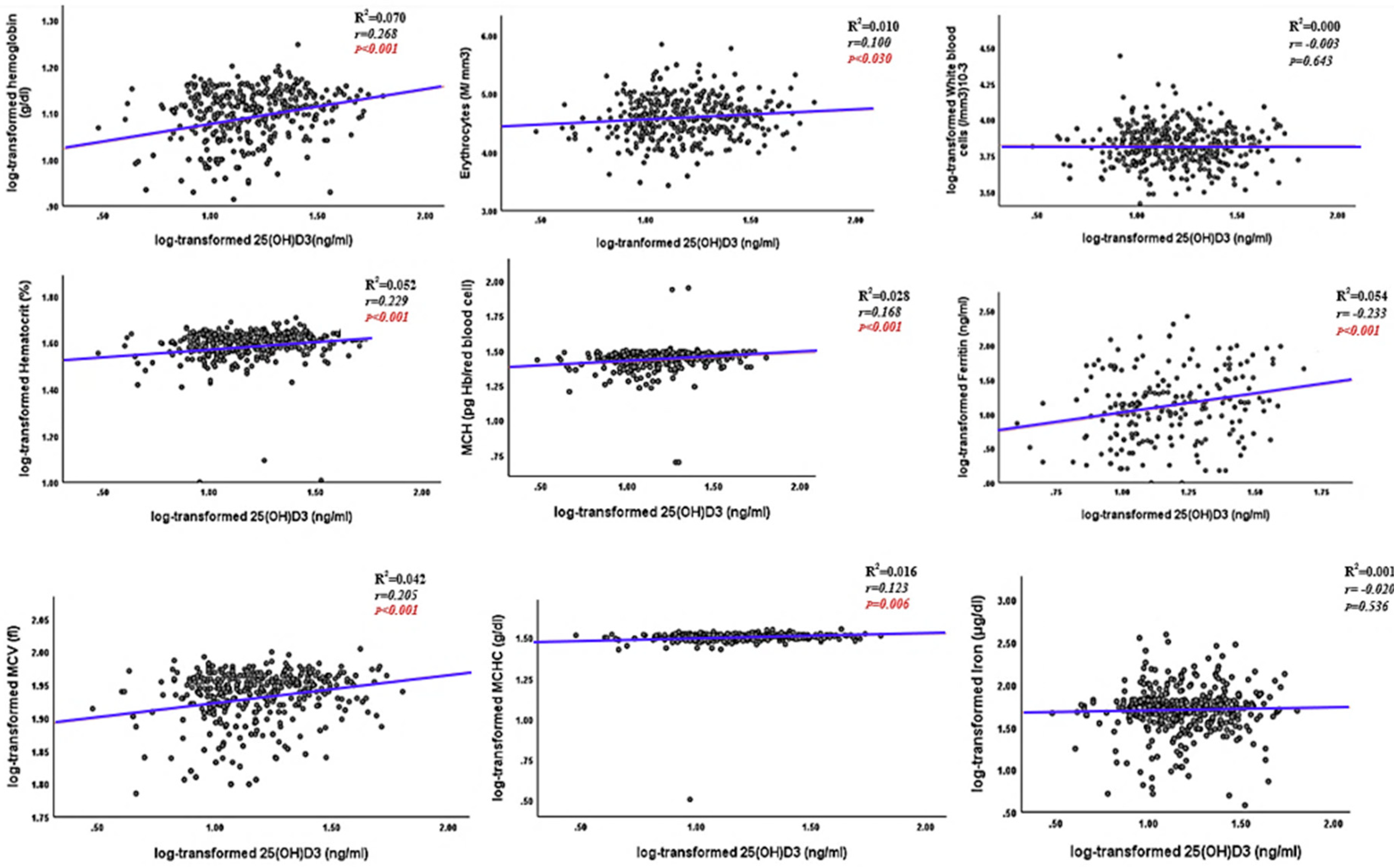
| Hemoglobin (g/dl) | 12.8(2.2) | 12.2(3.0) | 13.4(1.5) | 13.1(1.4) | <0.001 |
| Erythrocytes (M/ mm3) | 4.5(0.5) | 4.4(0.5) | 4.6(0.4) | 4.5(0.4) | 0.005 |
| Hematocrit (%) | 40.0(5.7) | 38.1(6.8) | 41.5(5.4) | 40.1(3.8) | <0.001 |
| MCV (fl) | 88.0(8.0) | 86.3(9.7) | 89.2(6.2) | 90.0(7.0) | <0.001 |
| MCH (pg Hb/erythrocytes ) | 28.0(3.4) | 27.3(4.8) | 29.0(2.1) | 29.0(2.0) | <0.001 |
| MCHC (g/dl) | 32.1(1.4) | 31.7(2.0) | 32.3(1.2) | 32.2(1.0) | <0.001 |
| White blood cells (/mm3)10-3 | 2.1(0.07) | 6.7 (2.6) | 6.4(3.1) | 6.6(2.8) | 0.643 |
|
Iron markers. Ferritin (µg/l) |
13.4 (20.8) |
11.4(15.5) |
14.8(29.4) |
32.8(44.9) |
0.002 |
| Iron (μg/dl) | 55.5(50.5) | 51.1(49.8) | 53.0(47.8) | 77.3(60.6) | 0.604 |
|
Renal fonction Plasma creatinine (mg/dl) |
0.7(0.1) |
0.6(0.1) |
0.7(0.1) |
0.7(0.1) |
0.231 |
| eGFR (ml/min/1.73 m2) | 196.9(96.16) | 184.5(93.7) | 215.4(94.0) | 204.8(86.3) | 0.010 |
|
Inflammatory marker CRP (mg/dl) |
3.75(4.57) |
3.8(4.3) |
3.1(4.9) |
4.1(6.7) |
0.366 |
| Inflammation present (CRP>5) Yes No |
101(21.8) 362(78.2) |
81(24.3) 253(75.7) |
12(14.6) 70(85.4) |
8(17.0) 39(17.0) |
0.118 |
| All Participant (n=463) |
25(OH)D (ng/ml) cut-off points | P-value1 | |||
|---|---|---|---|---|---|
| <20 (n=334) |
20-30 (n=82) |
>30 (n=47) |
|||
| n(%) | |||||
| Anemia status (HB<12mg/dl) Non anemic Anemic |
355 (76.7) 108(23.3) |
233(65.6) 101(93.5) |
46(13.0) 6(5.6) |
76(21.4) 1(11.0) |
<0.001 |
| Anemia severity (n=108) Milde to moderat(10.9<Hb<12g/dl) Severe (HB <8g/dl) |
101(93.51) 7(6.48) |
94(93.1) 7(100) |
6(5.6) 0(0.0) |
1(0.6) 0(0.0) |
0.771 |
| Iron deficiency No Yes |
331(71.5) 132(27.6) |
225(68.0) 109(82.6) |
43 (19.0) 19(14.4) |
63(13.0) 4(3.0) |
0.001 |
| Iron deficiency anemia No Yes |
409(88.3) 54(11.7) |
285(69.7) 49(90.7) |
77(18.8) 5(9.3) |
47(11.5) 0(0.0) |
0.003 |
| Unadjusted model | Adjusted model | |||||
|---|---|---|---|---|---|---|
| Β(SE) | OR (95% CI) | P-value | β(SE) | OR (95% CI) | P-value | |
| Outcome: Anemia | ||||||
|
Vitamin D status Sufficient-insufficient (>20 ng/mL) Deficient (<20 ng/mL) |
2.17(0.43) |
Reference 8.79(4.06,23.00) |
<0.001 | 1.97(0.45) |
Reference 7.17(3.19, 19.28) |
<0.001 |
| Physical activity intensity Moderate to high Low |
1.67(0.31) | Reference 5.35(2.88,9.91) |
<0.001 | 1.76(0.34) | Reference 5.80(2.96, 11.62) |
<0.001 |
| ID No Yes |
1.54 (0.23) |
Reference 4.70 (2.95, 7.49) |
<0.001 |
1.61(0.26) |
Reference 5.02(2.99,8.45) |
<0.001 |
| Localization Urbain Rural |
0.50 (0.22) |
Reference 1.64 ((1.06,2.55) |
0.026 |
0.57(0.25) |
Reference 1.77(1.07,2.94) |
0.021 |
| Sunscore categories (%): Sufficient to high SES Insufficient to moderate SES |
0.46 (0.22) |
Reference 1.59( 1.03, 2.46) |
0.034 |
0.31( 0.25) |
Reference 1.33(0.82,2.17) |
0.221 |
| Iron intake(mg/day) | -0.07(0.02) | 0.93 (0.88-0.98) | 0.008 | -0.05 (0.02) | 0.94 (0.88, 0.99) | 0.0419 |
| Outcome: Iron deficiency | ||||||
|
Vitamin D status Sufficient-insufficient (>20 ng/mL) |
Reference |
Reference |
||||
| Deficient (<20 ng/mL) | 0.75(0.25) | 2.12(1.28, 3.50) | 0.003 | 0.75(0.26 ) | 2.20(1.32, 3.77) | 0.007 |
| Age (years) | -0.04(0.01) | 0.95(0.92,0.98) | 0.009 |
-0.04(0.01) |
0.95(0.92,0.98) | 0.012 |
| Non heminic iron intake(mg/day) | -0.04(0.02) | 0.95(0.90,1.00) | 0.084 | -0.05(0.02) | 0.95(−1.10,1.00) | 0.037 |
| Education Illetrat Literate |
-0.38(0.18) |
Reference 0.67(0.46,0.97) |
0.037 |
-0.30(0.14) |
Reference 0.74(0.54,0.99) |
0.069 |
| Outcome: Iron deficiency anemia | ||||||
| Vitamin D status Sufficient -insufficient (>20ng/ml) |
Reference |
Reference |
||||
| Deficient (<20 ng/mL) | 1.42(0.48) |
4.16(1.62,10.71) |
0.003 | 1.41(0.48) | 4.10(1.73,12.08) | 0.004 |
| Iron intake (mg/day) | -0.08(0.03) | 0.92 (0.86, 0.98) | 0.013 | -0.08(0.03) | 0.91(0.85, 0.99) | 0.030 |
| Localization Rural Urban |
0.60(0.29) |
Reference 1.82(1.02,3.23) |
0.039 |
0.55(0.30) |
Reference 1.74(0.96, 3.15) |
0.084 |
| Education Illiterate Literate |
-0.397(0.20) |
Reference 0.67(0.44,1.00) |
0.052 |
-0.38(0.21) |
Reference 0.68(0.44,1.03) |
0.070 |
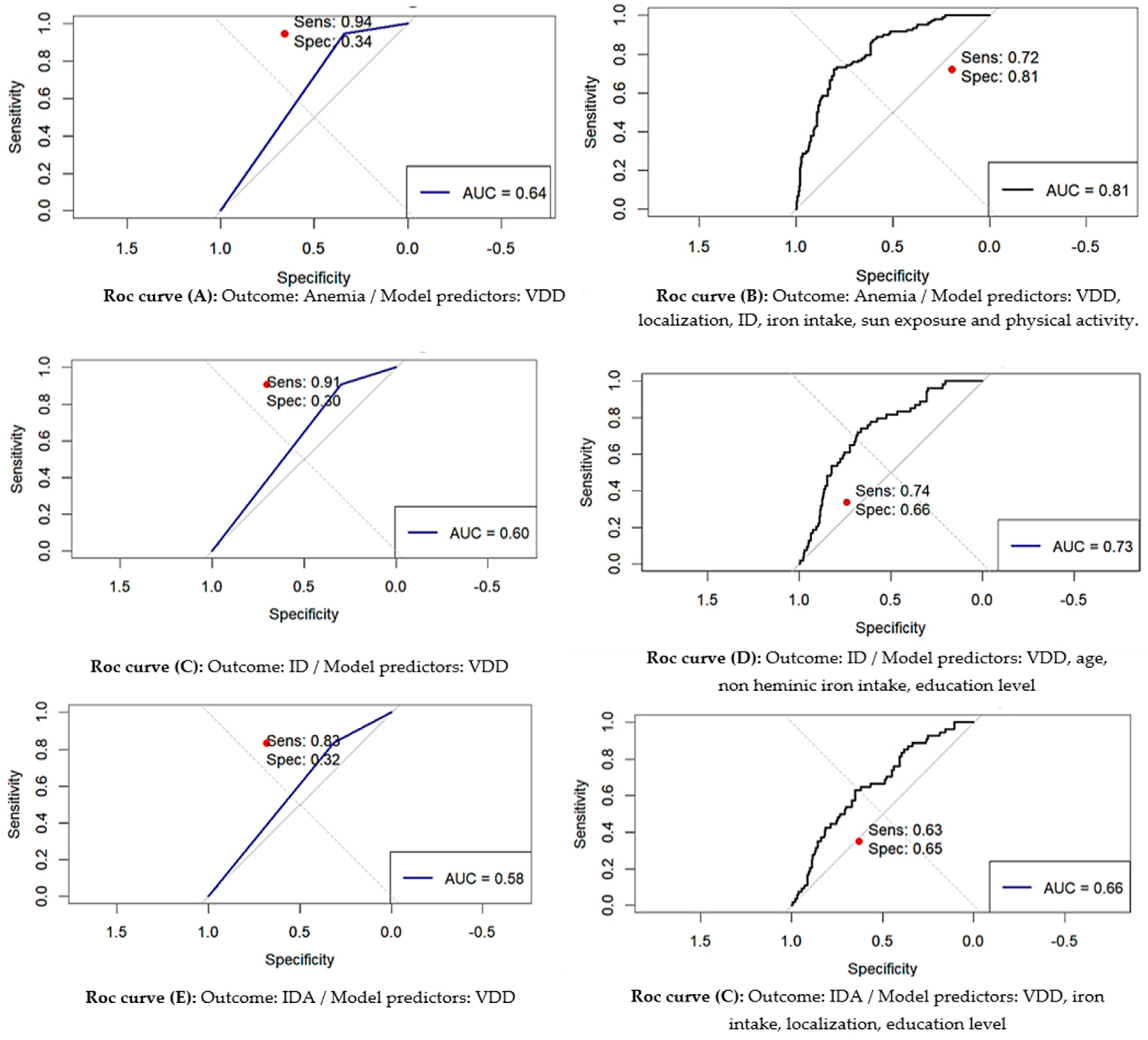
| Outcome | Predictors | AUC | 95% CI for AUC | p-value for AUC | Optimal Threshold | Youden Index | Optimal Sensitivity |
Optimal Specificity |
|---|---|---|---|---|---|---|---|---|
| Anemia | VDD | 0.643 | 0.610-0.676 | < 0.001 | 0.175 | 0.285 | 0.944 | 0.341 |
| VDD, localization, ID, iron intake and physical activity, sun exposure |
0.813 |
0.768-0.855 |
< 0.001 |
0.269 |
0.528 |
0.722 |
0.806 |
|
| ID | VDD | 0.575 | 0.535-0.616 | < 0.001 | 0.250 | 0.151 | 0.833 | 0.317 |
| VDD, age, non heminic iron intake, education level | 0.654 | 0.604-0.704 |
< 0.001 |
0.233 |
0.243 |
0.634 |
0.648 |
|
| IDA | VDD | 0.603 | 0.557-0.644 | < 0.001 | 0.093 | 0.206 | 0.907 | 0.298 |
| VDD, iron intake, localization, education level. | 0.712 | 0.643-0.776 | < 0.001 |
0.123 |
0.389 |
0.741 |
0.648 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





