1. Introduction
Down syndrome (DS), characterized by the presence of an extra copy of chromosome 21, is the most common chromosomal abnormality among live-born infants [
1]. Individuals with DS are at increased risk for various co-occurring health conditions in gastrointestinal, neurological, sensory, metabolic, endocrine and respiratory systems, and at increased risk of mortality compared to those without DS [
2,
3]. The National Institute of Health Investigation of Co-occuring conditions across the Lifespan to Understand Down syndrome (NIH INCLUDE) project, launched in 2018, calls for the assembly and study of large populations of individuals with DS to follow the development of the condition over time and to fully characterize condition traits at different stages of development [
4].
Administrative data provide a time- and cost-savings approach to gather information on a large number of children over time. Individuals of interest can be identified using International Classification of Diseases (ICD) diagnosis codes. Because ICD codes are collected for billing rather than research purposes, ICD-based diagnoses are subject to biases that threaten their clinical validity. Codes that are reimbursed well are more likely to be included in a patient’s record compared with those that are not [
5,
6]. Further, codes that represent disease conditions that function often as comorbidities rather than the primary reasons for clinical encounters are less likely to show up on an individual’s record, particularly in disease conditions that are defined by vague clinical criteria [
7,
8,
9]. Indeed, a previous study identifying individuals with DS using ICD-9 codes specific to DS observed only moderate sensitivity (87%) and positive predictive value (PPV: 79%) [
10].
The objective of this study was to develop an algorithm using birth certificates and/or claims data (ICD codes) to identify individuals with DS, and validate the algorithm using manual chart review. If an accurate algorithm is identified, it can be used to identify individuals with DS for future research studies using administrative data.
2. Materials and Methods
2.1. Study Design Cohort
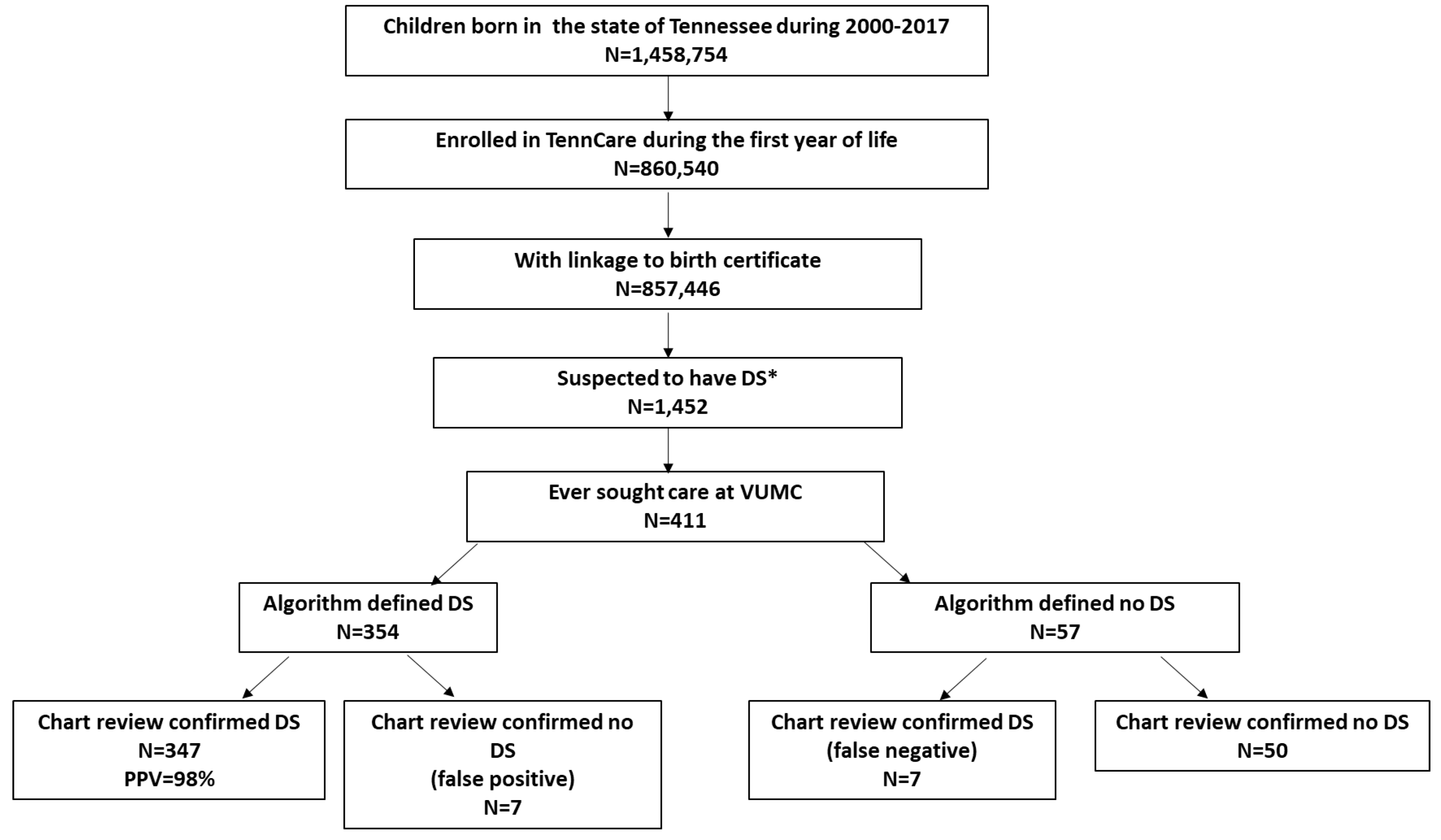
We used data from a state Medicaid program (Tennessee Medicaid Program, TennCare) and identified children born between 2000-2017 who were continuously enrolled in TennCare during their first year of life and whose records were linked to their birth certificates. We then identified children suspected of having DS because they either had DS recorded on their birth certificate or they had at least one ICD diagnostic code of DS (ICD-9: 758.0; ICD-10: Q90) within any of the diagnostic fields during healthcare encounters up to age six. Children were followed to age six years, death, disenrollment, or the end of study (December 31, 2020), whichever came first. We restricted this cohort to those who ever sought care at Vanderbilt University Medical Center (VUMC), a comprehensive academic medical center in the United States in order to perform a manual chart review of their records (n=411, 28.3%) (
Figure 1). The diagnosis of DS was confirmed through manual chart review of the electronic medical records using a structured chart abstraction form, and was the gold standard we used to validate our algorithm. For children with chart review confirmed DS, type of DS, timing of first diagnosis (prenatal vs. postnatal), and method of diagnosis were determined. The study protocol was approved by the VUMC and Tennessee Department of Health Institutional Review Boards.
2.2. Algorithm Defining DS
We developed an algorithm for DS that used birth certificate data and/or ICD diagnosis codes (ICD-9: 758.0; ICD-10: Q90). A new birth certificate format was adopted in 2004. Prior to 2004, there was a single check box to indicate DS. From 2004 onward, there were separate indications for karyotype confirmed and karyotype pending DS.
Children were defined as having DS if they met one of the following criteria:
Having birth certificate indication for “karyotype confirmed” DS
Having birth certificate indication for “karyotype pending” DS or just DS if test type was not specified (i.e., prior to 2004) and having at least two healthcare encounters for DS during the first six years of life
Having at least three healthcare encounters for DS during the first six years of life, with the first and last encounter separated by at least 30 days
2.3. Statistical Analysis
Demographic characteristics of the study population were described using median (interquartile range: IQR) for continuous variables and frequency and proportion for categorical variables.
Among individuals whom the algorithm defined as having DS, we calculated the positive predictive value (PPV), the proportion of the study population whose DS was confirmed by manual abstract review. Among individuals whom the algorithm defined as not having DS, we calculated the proportion who did not have DS according to manual abstract review. The corresponding 95% confidence intervals (CIs) were calculated using Wilson’s formula [
11]. All analyses were performed using R software version 4.3.1 (R foundation for statistical computing, Vienna, Austria).
3. Results
There were 857,446 born 2000-2017 who were continuously enrolled in TennCare during their first year of life with a linkage to the birth certificate. Of these, 1452 were suspected to have DS by having either an indication for DS on their birth certificate or an ICD diagnosis code for DS during their first six years of life.
Of the 1452 children with suspected DS, 411 ever sought care at VUMC (
Figure 1). All 411 children with suspected DS had at least one ICD diagnosis code for DS, and 24.8% (n=102) had DS coded on the birth certificate (
Table 1). These children had predominantly singleton births (98.3%) and older siblings at the time of birth (69.7%). Fifty-five percent of the children were assigned male at birth. The median gestational age was 38 weeks (IQR 36, 39) and the median birthweight was 2920 grams (IQR 2495, 3280). A large proportion of these children had congenital heart disease (83.5%) (
Table 2).
Among these 411 children with suspected DS, our algorithm determined that 354 (86.1%) had DS by meeting at least one of the criteria (
Figure 1).
Table 3 presented the detailed number of children determined as having DS by meeting each combination of the criteria defined in the algorithm. Of the 102 children who had DS coded on the birth certificate, 101 (99.0%) were determined as having DS by meeting either criterion 1 or criterion 2. Three hundred and fifty children met criterion 3 (3+ ICD diagnoses for DS criterion). These children composed 98.9% of the population identified by the algorithm.
When compared to the gold standard of manual medical chart review, 347 out of the 354 algorithm-defined DS children had their DS confirmed, for a PPV of 98.0% (95% CI: 96.0%-99.0%). Examination of criterion-specific PPV suggested that the 101 children with an birth certificate indication and met criterion 1 or 2 had DS confirmed by chart review (PPV=100%). Among the 253 children who exclusively met the 3+ ICD diagnoses for DS criterion (criterion 3), 97.2% (95%CI: 94.4%-98.7%) had DS confirmed by chart review (
Table 3). Of the 57 children who did not satisfy any of the algorithm’s criteria for DS, 87.7% (95% CI: 76.8%-93.9%) were true negatives based on chart review (
Figure 1). There were a total of seven children whom the algorithm incorrectly identified as having DS (false positives) and seven children who were incorrectly identified as not having DS (false negatives) (
Figure 1,
Table 3). The median number of ICD billing codes for DS which appeared in the medical records was 4 (range: 3, 14) and 1 (range: 1, 2) over 6.00 (range 4.00, 6.00) and 4.71 (range 1.23, 5.25) years for the false positives and false negative children, respectively. The corresponding median age when an ICD code for DS first appeared on each child’s billing record was 111 days (range 11, 1673) for the false positives and 97 days (range 0 [at the birth hospitalization], 1079) for the false negative children.
Among children whose DS was confirmed by medical chart review, we further determined type of DS, diagnosis method, and timing of first DS diagnosis (prenatal vs. postnatal). Of the 283 children whose type of DS was known, 266 (94.0%) had Trisomy 21 nondisjunction (
Table 4). Among the 293 children whose timing of first diagnosis was known, 28.3% (n=83) were diagnosed prior to delivery. Though not statistically significant (trend test p=0.16), the proportion of children whose DS was confirmed prenatally increased over time: 27.5% (born 2000-2004), 23.5% (born 2005-2009), 28.4% (born 2010-2014), and 34.8% (born 2015-2017). Further examination found that 38.6% (n=32) of children who were diagnosed prior to delivery had DS indicated on the birth certificate. Karyotyping was the most common method used to diagnose DS, followed by amniocentesis/chorionic villus sampling and fluorescence in situ hybridization (
Table 4). About 4% of children (n=13) had non-invasive prenatal testing for DS. A close examination of the 57 children whose chart review confirmed no DS suggested that majority of them had or were suspected to have some sort of chromosomal abnormality (73.4%) and had congenital heart disease (73.7%).
4. Discussion
Using birth certificate data and ICD diagnosis data, we developed an algorithm to accurately determine children with DS. Of the 411 children who were suspected to have DS and sought care at VUMC, our algorithm defined 86.1% as having DS. Manual chart review confirmed that the algorithm is accurate in differentiating suspected DS children with and without DS, with 98.0% and 87.3% accuracy, respectively, in determining those truly with DS and those truly without DS.
We found that birth certificate as a source in DS case identification was less sensitive. Only approximately a quarter of children with suspected DS had the condition coded on the birth certificate. The birth certificate is typically filled out 24 to 48 hours after birth, making a postnatal diagnosis of DS unlikely to be fully confirmed at the time of record filling [
12]. Among children whose condition was known prior to delivery, less than 40% had DS coded on the birth certificate. This may reflect a more careful practice allowing physicians with a questioning attitude if testing was done with maternal blood test or only mild DS signs noted. On the other hand, in this cohort of children with suspected DS, a DS indication on the birth certificate had high predictive value. In our study, only one out of 102 subjects had the indication wrong on his/her birth certificate. By requesting either karyotype confirmed DS (criterion 1) or two more ICD coded visits in addition to karyotype pending DS (or simply DS if no test indicated) (criterion 2), our algorithm had correctly identified all 101 children whose DS confirmed by chart review (PPV=100%). It is important for future research to be aware of the strength and limitations of using birth certificate indication in DS case identification.
Our finding that not all children with an ICD code for DS truly had DS is consistent with previous literature, and highlights that there are some inaccuracies within the administrative billing data such that billing codes can be inappropriately assigned to patient encounters [
10]. The majority of the children with an inappropriate indication and/or billing(s) for DS have or were suspected to have a different chromosomal abnormality. Is it important to note that consistent wrong indications for DS is not common in practice. By requiring repeated coding across independent healthcare encounters, our algorithm significantly reduced misclassification. We further required a minimum one-month interval between the first and last ICD-coded encounters, when ICD coding was used alone, to ensure there were at least two independent healthcare visits that were likely for different health issues/episodes with codes for DS.
An important contribution is the finding that ICD coding alone can be an efficient and valid approach in DS case identification. Almost all children our algorithm defined as having DS satisfied the 3+ ICD coding criteria, and overwhelmingly were confirmed to have DS by chart review. As birth certificate data is not always available in research settings using administrative and electronic medical records data, an algorithm with a stand-alone ICD coding criterion allows broad application.
Despite the widespread availability of prenatal testing for DS, only 28% of children with DS were identified prenatally over the study period. The proportion with prenatal diagnosis among live-born children with DS increased slightly over time. As our study included children who were alive until at least age one (per study inclusion criteria of continuous enrollment during the first year of life), the low proportion of children with DS whose condition was diagnosed prenatally may not directly reflect the overall prenatal testing rate, as diagnosing resulting in pregnancy terminations and infant death are not included in the study population. In a systematic review, it was estimated that 67% to 85% of individuals with a positive prenatal diagnosis of DS terminate their pregnancy in the US [
13]. Second, approximately 5% of infants born with DS die during infancy and thus were not be included in our study population [
14].
Our study has several limitations. We developed and applied our algorithm to a study population of children who have regular and frequent health care visits. DS as a comorbidity may be more likely to be coded during well-child visits in children with DS with fewer comorbidities than among children with DS with significant comorbidities and specialty care and more frequent disease-driven clinical encounters. Requirement of at least three independent indications/visits for DS could miss children with DS with fewer comorbidities and requirements for clinical care. This would be particularly true when children were followed within a limited time period. Of the seven children with chart review confirmed DS whom the algorithm failed to identify, the median follow up time was 4.71 years, 1.28 years shorter than those of children whom algorithm defined as having DS. We also restricted our study population to those who sought care at a single academic medical center, which may not be generalizable in other settings. Indeed, a comparison of a similar cohort of children born and enrolled in the Medicaid in the same time period, but never sought care at the medical center (i.e. 1452-411= 1041,
Figure 1) suggested that although all of them had at least one healthcare visit with ICD codes for DS, children with suspected DS who never sought care at VUMC were less likely to have an indication of DS on the birth certificate and less likely to be defined as having DS by the algorithm (P<.001) (
Table S1). Children with suspected DS who sought care at VUMC were more likely to have congenital heart disease and had older, more educated mothers. These mothers were more likely to be married and less likely to smoke during pregnancy (
Table S2). Lastly, our study population of children with suspected DS had, by design, a high likelihood of DS than the general population. When applied to a general population, the algorithm may not perform as well. The algorithm may not identify children with DS if they have limited healthcare encounters and/or have healthcare encounters not frequently coded for DS.
Authors should discuss the results and how they can be interpreted from the perspective of previous studies and of the working hypotheses. The findings and their implications should be discussed in the broadest context possible. Future research directions may also be highlighted.
5. Conclusions
In conclusion, we developed an algorithm that used data from birth certificates and ICD codes and had a high PPV for identifying children with DS. Application of this algorithm will provide a reliable and efficient approach to identify and study individuals with DS, and the algorithm remains accurate for researchers who do not have access to birth certificate data and use ICD codes alone.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on:
Preprints.org, Table S1: Children who were suspected of having DS by an indication for DS on the birth certificate and/or with at least one ICD diagnosis for DS, born 2000-2017, enrolled in TennCare during infancy, and ever/never sought care at VUMC; Table S2: Maternal and infant characteristics of children with suspected DS, born 2000-2017, enrolled in TennCare during infancy, and ever/never sought care at VUMC.
Author Contributions
Conceptualization, P.W., T.V.H.; methodology, L.A., A.C.M., and R.L.L.; software, H.N., T.D.; validation, L.A., K.M.B., and A.C.M.; formal analysis, H.N.; investigation, L.A., K.M.B., and P.W.; resources, P.W.; data curation, T.D.; writing—original draft preparation, L.A., H.N., and P.W.; writing—review and editing, K.M.B., A.C.M., R.L.L., T.D., C.A.R., T.G., B.M.S., and T.V.H.; visualization, H.N., C.A.R.; supervision, P.W.; project administration, P.W.; funding acquisition, P.W., T.V.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institutes of Health (grant number R01 AI143710 to PW, R01 AI136526 to TVH, K01 HL161257 which supports BMS, and TL1 TR002244 which supports LA).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Vanderbilt University (approval no. 182243) and the Tennessee Department of Health (approval no. 2019-0110) for studies involving humans. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Informed Consent Statement
Patient consent was waived as it was deemed as a secondary data analysis for which consent was not required.
Data Availability Statement
The data to support the findings of this study were from the Division of TennCare in the Tennessee Department of Finance and Administration and from medical records for healthcare encounters at Vanderbilt University Medical Center. De-identified data are available on request from the corresponding author, with the approvals from TennCare and VUMC Institutional Review Board.
Acknowledgments
We sincerely thank the Division of TennCare within the Tennessee Department of Finance and Administration and the Tennessee Department of Health, Office of Policy, Planning & Assessment for providing the study data.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Stallings, E.B.; Isenburg, J.L.; Rutkowski, R.E.; Kirby, R.S.; Nembhard, W.N.; Sandidge, T.; Villavicencio, S.; Nguyen, H.H.; McMahon, D.M.; Nestoridi, E.; Pabst, L.J.; National Birth Defects Prevention, N. National population-based estimates for major birth defects, 2016-2020. Birth Defects Res 2024, 116, e2301. [Google Scholar] [CrossRef] [PubMed]
- Antonarakis, S.E.; Skotko, B.G.; Rafii, M.S.; Strydom, A.; Pape, S.E.; Bianchi, D.W.; Sherman, S.L.; Reeves, R.H. Down syndrome. Nat Rev Dis Primers 2020, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.J.; Trotter, T.; Santoro, S.L.; Christensen, C.; Grout, R.W.; Council On, G.; Burke, L.W.; Berry, S.A.; Geleske, T.A.; Holm, I.; Hopkin, R.J.; Introne, W.J.; Lyons, M.J.; Monteil, D.C.; Scheuerle, A.; Stoler, J.M.; Vergano, S.A.; Chen, E.; Hamid, R.; Downs, S.M.; Grout, R.W.; Cunniff, C.; Parisi, M.A.; Ralston, S.J.; Scott, J.A.; Shapira, S.K.; Spire, P. Health Supervision for Children and Adolescents With Down Syndrome. Pediatrics 2022, 149. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Health. https://www.nih.gov/include-project (April 3).
- Quan, H.; Parsons, G.A.; Ghali, W.A. Validity of procedure codes in International Classification of Diseases, 9th revision, clinical modification administrative data. Med Care 2004, 42, 801–809. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Austin, P. Administrative database research has unique characteristics that can risk biased results. J Clin Epidemiol 2012, 65, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lash, T.L.; Mor, V.; Wieland, D.; Ferrucci, L.; Satariano, W.; Silliman, R.A. Methodology, design, and analytic techniques to address measurement of comorbid disease. J Gerontol A Biol Sci Med Sci 2007, 62, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.S.; Chan, B.K.; Schembri, M.E.; Rainwater, J.A. Can administrative data be used to compare postoperative complication rates across hospitals? Med Care 2002, 40, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Romano, P.S.; Schembri, M.E.; Rainwater, J.A. Can administrative data be used to ascertain clinically significant postoperative complications? Am J Med Qual 2002, 17, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Jensen, K.M.; Cooke, C.R.; Davis, M.M. Fidelity of administrative data when researching Down syndrome. Med Care 2014, 52, e52–e57. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.D.; Cai, T.T.; DasGupta, A.; Agresti, A.; Coull, B.A.; Casella, G.; Corcoran, C.; Mehta, C.; Ghosh, M.; Santner, T.J.; Brown, L.D.; Cai, T.T.; DasGupta, A. Interval estimation for a binomial proportion - Comment - Rejoinder. Stat Sci 2001, 16, 101–133. [Google Scholar] [CrossRef]
- Northam, S.; Polancich, S.; Restrepo, E. Birth certificate methods in five hospitals. Public Health Nurs 2003, 20, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Natoli, J.L.; Ackerman, D.L.; McDermott, S.; Edwards, J.G. Prenatal diagnosis of Down syndrome: a systematic review of termination rates (1995-2011). Prenat Diagn 2012, 32, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; Kucik, J.E.; Correa, A. Causes of death and case fatality rates among infants with down syndrome in metropolitan Atlanta. Birth Defects Res A Clin Mol Teratol 2007, 79, 775–780. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).