1. Introduction
Chinese Baijiu, one of the six major distilled spirits globally, is renowned for its distinctive flavor profile (H. Liu & Sun, 2018(Z. Ren, Chen, Q., Tang, T., Huang, Z., 2024). The phrase “Qu is the bone of Baijiu” highlights the critical role of Qu in the Baijiu brewing process. High-quality Baijiu production relies heavily on Daqu (Deng, Zheng, Huang, Huang, Ye, & Luo, 2023), a key fermentation starter. Daqu is made from wheat that is first crushed, mixed with water, pressed into brick blocks, and then cultured and fermented under controlled temperature and humidity conditions (Z. Ren, Xie, J., Tang, T., Huang, Z., 2024). Following fermentation, Daqu is air-dried and stored as brick-shaped blocks (H. Liu & Sun, 2018). Based on peak fermentation temperatures, Daqu is categorized into four types: high-temperature Daqu, medium-high-temperature Daqu (MT-Daqu), medium-temperature Daqu, and low-temperature Daqu. Currently, MT-Daqu is widely used as a fermentation starter in the production of SAB. In 2023, SAB production saw revenues exceeding $40 billion, with over 2.5 million tons of MT-Daqu utilized. Sichuan is the largest SAB-producing region, accounting for over 60% of both production and sales, followed by Jiangsu, Anhui, Henan, and other regions.
Daqu contains a diverse array of microorganisms, enzymes, and compounds that are crucial for the saccharification and fermentation of grains, thus earning its designation as a traditional Baijiu fermentation starter (J. Liu, Chen, Fan, Huang, & Han, 2018; Z. Ren, Liu, L., Tang, T., Huang, K., & Huang, Z., 2024). The manufacture of Daqu involves microbial enrichment, culture, and screening, with the environmental conditions during production playing a direct role in shaping the microbial community and the overall quality of Daqu (Zhou, 2024). Key environmental factors influencing the fermentation of MT-Daqu include temperature, acidity, oxygen, carbon dioxide, humidity, moisture, and other conditions. Temperature and acidity are the primary factors affecting bacterial community succession, while temperature and humidity are crucial for fungal community succession during MT-Daqu fermentation (S. Ma, et al., 2022; Zhu, et al., 2022). Additionally, studies have demonstrated that biological heat is a significant environmental driver influencing the microbial community in MT-Daqu. The peak temperature is a critical control parameter and one of the most important microbial indicators in the MT-Daqu fermentation process (Xiao, et al., 2017).
Moreover, enzyme activity is a critical index for evaluating the quality of MT-Daqu. The “General Methods of Analysis for Daqu” (QB/T 4257-2011) includes four key evaluation indexes for enzyme activity(Tang, 2014). Multi-omics studies have shown that MT-Daqu is rich in enzymes, making it a major source of enzyme resources, especially those that function under extreme conditions. These enzymes are crucial for the saccharification and fermentation processes of grains (He, et al., 2023; Y. Liu, Tang, T.-X., Pei, X.-Q., Zhang, C., Wu, Z.-L., 2014). Previous research has provided an in-depth analysis of the production process, enzymes, and microorganisms involved in MT-Daqu, laying a theoretical foundation for optimizing preparation conditions and control parameters. Additionally, the current production process of MT-Daqu is well described (Figure S1A). However, while considerable attention has been given to the process, microorganisms, and enzymes in MT-Daqu, there remains a gap in research concerning its substance composition, particularly the composition of Qu-aroma in MT-Daqu.
Qu-aroma is a critical quality index for Daqu, as outlined in the Chinese industry standard QB/T 4257–2011, and is also a key component of Baijiu aroma, as specified in the Chinese national standard GB/T 10781.4-2024. In the production of SAB, MT-Daqu, grains, and fermented grains are mixed and fermented together before distillation to produce the base Baijiu. As a result, Qu-aroma is imparted to the final product. A study employing headspace solid-phase microextraction (HS-SPME) combined with gas chromatography-mass spectrometry (GC-MS) identified 139 volatile compounds in the flavor profile of MT-Daqu. These include 36 esters, 22 alcohols, 14 aldehydes, 12 nitrogenous compounds, 9 terpenes, 8 phenols and furans, 7 acids and ketones, 4 lactones, 3 sulfur compounds, and 9 others (Wang, et al., 2022). Further research on MT-Daqu aroma has identified four primary aroma categories: fermentation aroma, material aroma, aging aroma, and others. These encompass 10 secondary aromas and 52 tertiary aromas, with 37 aroma-active volatile compounds identified using thin-film (TF)-SPME-GC-O-MS (Yang, et al., 2024). These studies have provided a detailed characterization of the composition and aroma characteristics of Qu-aroma in MT-Daqu.
Despite these advances, geographical and environmental differences in MT-Daqu production have been noted, leading to variations in the structure and potential functions of microbial communities and volatile compounds in MT-Daqu samples from different regions (Shiyuan Ma, et al., 2022). This suggests that Qu-aroma in MT-Daqu may differ across regions, yet there remains a lack of comprehensive research on regional variations in the Qu-aroma of MT-Daqu.
In the present study, MT-Daqu samples were collected from four SAB-producing areas in Sichuan and three Nongxiangxing Baijiu-producing areas outside Sichuan. Qu-aroma in MT-Daqu was analyzed using both artificial and electronic noses, alongside GC-MS for the qualitative and quantitative analysis of volatile components. Additionally, GC-O was utilized to identify aroma-active compounds contributing to Qu-aroma across different regions. Recombination experiments were also conducted to evaluate regional differences in Qu-aroma among MT-Daqu samples. This study provides a comprehensive understanding of Qu-aroma variations in MT-Daqu from diverse SAB-producing areas and offers theoretical insights to enhance Qu-aroma in MT-Daqu across different production regions.
2. Materials and Methods
2.1. Sample Collection
Samples were collected from seven SAB-producing areas: Yibin (Y1-5), Luzhou (L1-5), Suining (S1-4), Mianyang (M1-4), Bozhou (B1-4), Sanmenxia (SM1-4), and Zhoukou (Z1-4), with detailed location information provided in Figure S1B. Each sample weighed at least 5 kg. Of this, 200 g were stored in liquid nitrogen for volatile compound analysis, while the remaining samples were used for E-nose analysis and sensory evaluation.
2.2. Chemicals
Chemicals used in this study were categorized into four groups based on their properties and semi-quantitative content.
Group A: ethyl butyrate, ethyl heptanoate, ethyl isovalerate, ethyl valerate, ethyl caproate, ethyl phenylacetate, ethyl palmitate, phenethyl acetate, 3-methyl-1-butanol, butyric acid, 3-methylbutanoic acid, caproic acid, furfuryl alcohol, phenethyl alcohol, benzyl alcohol. These were purchased from ANPEL Laboratory Technologies Co., Ltd. (Shanghai, China) with a purity greater than 99%.
Group B: 1-heptanol, 2-ethylhexanol, ethyl benzoate, 1-octanol, 1-octen-3-ol, phenylethylene, and I-caryophyllene. These were obtained from ALADDIN Chemical Reagent Co., Ltd. (Shanghai, China) with a purity greater than 98%.
Group C: phenylethylene, γ-nonanolactone, 4-ethylguaiacol, butyraldehyde, hexanal and 1-nonanal (≥98% purity), 3-methylbutanal, 2,6-dimethylpyrazine (≥97% purity), tetramethylpyrazine (≥97% purity), phenylacetaldehyde, γ-butyrolactone, and furfural (≥95% purity). These were purchased from Sichuan Victory Biological Technology Co., Ltd. (Chengdu, China).
Group D: guaiacol, (e)-2-nonenal, (e)-2-octenal, 2,3-dimethyl-5-ethylpyrazine, 4-hydroxy-4-methoxystyrene, 2,3,5-trimethylpyrazine, benzaldehyde (≥98% purity), and (e)-2,4-decadienal (≥95% purity). These were also obtained from Sichuan Victory Biological Technology Co., Ltd. (Chengdu, China).
Additionally, n-alkanes (C7-C30), the internal standards n-butyl acetate, and 2-acetylpyridine were sourced from Tan-Mo Technologies Co., Ltd. (Beijing, China). Liquid nitrogen and nitrogen gas (99.999% purity) were provided by Chengdu Xinyuan Chemical Co., Ltd. (Chengdu, China).
2.3. Sensory Evaluation
Sensory evaluation was performed following the Chinese standard GB/T 16291.1-2012 and the industry-specific MT-Daqu evaluation method. Twelve evaluators, all experienced in Daqu flavor analysis, were recruited from Sichuan University of Science and Engineering (Yibin, Sichuan, China). The group comprised 7 males and 5 females, with an average age of 34 years, each having over 5 years of experience in sensory evaluation of Daqu. The evaluators were assessed for their odor perception, sample discrimination ability, consistency, and repeatability in a standard food odor evaluation laboratory, using methods described in previous studies (Duan, et al., 2024). As the evaluators were already familiar with Daqu aroma, no additional training in sensory analysis or aroma identification was required. However, they were introduced to the establishment and use of evaluation scales.
For the sensory evaluation, MT-Daqu samples were portioned into 20 g servings, with at least 10 servings per sample. Each sample was assigned a 3-digit random identifier. During each evaluation session, samples were randomly selected based on these identifiers. The evaluators initially conducted a blind assessment of the 7 MT-Daqu samples, describing both the category and intensity of the aroma, following previously established methods (Yu, et al., 2023).
Data on the MT-Daqu aroma were then compiled and organized by categorizing the aroma compounds into hierarchical levels. Sensory descriptors were selected and developed using methods outlined in previous studies (Yu, et al., 2023), and evaluators re-evaluated the samples to confirm the completeness of flavor identification and the accuracy of classification.
Finally, based on the primary aroma categories identified, the evaluators rated the aroma intensity of 30 samples using a 100-point scale. The sensory evaluation was conducted blindly, with evaluators unaware of specific sample details. Each sample was evaluated in triplicate, and the results were averaged for statistical analysis.
2.4. Electronic Nose Analysis
An iNose E-nose (iSenso, USA) was utilized to evaluate the Qu-aroma characteristics of MT-Daqu samples. In brief, 20 g of each sample was placed in a 150-mL glass vial with a rubber stopper, then incubated in a 50 °C water bath for 30 minutes to equilibrate. Following the manufacturer’s instructions, the device was calibrated with zero gas, and the sensors were reset three times. Gas samples were drawn from the headspace of the vials at a flow rate of 200 mL/min, with a sampling time of 6 seconds and a detection time of 80 seconds. The E-nose system featured 20 sensors; for this study, 14 sensors were selected, as detailed in Table S1. The maximum value and maximum slope of the sensor responses were analyzed. A radar plot was created to visualize flavor intensity, and principal component analysis (PCA) was employed to assess differences among the samples.
2.5. Extraction of Volatile Compounds
2.5.1. Solid-Phase Microextraction (SPME)
Volatile compounds from MT-Daqu samples were extracted following a method (Wang, et al., 2022) with minor modifications. Briefly, 5 g of the sample was placed in a 40-mL centrifuge tube with 20 mL of ultrapure water and subjected to ultrasonic treatment for 1 hour. The mixture was then soaked for 12 hours before centrifugation at 7,000 rpm for 5 minutes. The supernatant was discarded, and the loss of extract was calculated by weighing the remaining supernatant and comparing it to the initial weight of ultrapure water added. The data were used to convert the volatile compounds. Next, 5 mL of the supernatant and a stir bar were transferred to a 15-mL glass vial, and 1 μL of 2-acetylpyridine (2.12 μg/μL) was added as an internal standard. The vial was sealed and placed in a 50 °C water bath, stirring at 180 rpm for 30 minutes. SPME fibers (DVB/CAR/PDMS, 50/30 μm, Supelco, Bellefonte, PA, USA) were then inserted into the headspace vial to adsorb volatile compounds for 30 minutes. After adsorption, the fibers were inserted into the GC inlet for 5 minutes to release the adsorbed volatile compounds, which were subsequently analyzed qualitatively and semi-quantitatively.
2.5.2. Liquid-Liquid Extraction (LLE)
Volatile compounds from MT-Daqu were extracted as described in section 2.5.1, with the extract volume being recorded. The LLE method used, with minor modifications, was adapted from a published paper (Zhao, et al., 2018). In this method, n-hexane was added at a ratio of 1:1.5 to the extract volume. The organic phase was collected after two rounds of extraction. This organic phase was then dried over anhydrous Na2SO4 and concentrated to 1000 μL using a low-temperature vacuum concentrator (RayKol, Xiamen, China). The concentrated extract was immediately analyzed using GC-MS/MS for precise quantification of volatile flavor compounds in MT-Daqu. Additionally, the extract was subjected to aroma extraction dilution analysis (AEDA) to identify key aroma-active compounds in MT-Daqu.
2.6. Identification of Volatile Compounds
Volatile compounds in MT-Daqu were analyzed using a headspace solid-phase microextraction gas chromatography-mass spectrometry/olfactometry (HS-SPME-GC-MS/O) system, consisting of an Agilent 8890 GC coupled with a 7250 MS (Santa Clara, CA, USA) and an olfactometer (ODP C300, Gerstel, Germany). The GC-MS conditions followed the protocols established in previous experimental protocols (Deng, Zheng, Huang, Huang, Ye, & Luo, 2023). The shunt ratio between MS and ODP was 1:1, with the ODP transmission line and olfactory port temperatures set to 250 °C and 200 °C, respectively. GC-O analysis was conducted by three experienced evaluators, who had been trained to identify 40 volatile compounds at concentrations five times their odor thresholds in water prior to the experiment. Each MT-Daqu sample was analyzed in triplicate. Volatile compounds were categorized based on their mass spectra, referencing the NIST 20 mass spectral library database. Retention indices (RIs) were calculated using the retention times of n-alkanes (C7-C30) under the same GC-MS conditions. Final identification of the volatile compounds in MT-Daqu was achieved by correlating standard, odor, RI, and mass spectra data.
2.7. Quantitative Analysis
2.7.1. Semi-Quantitative Analysis
Semi-quantitative analysis of volatile compounds in MT-Daqu was conducted using HS-SPME-GC-MS, following a method described elsewhere (Yu, et al., 2023). To perform this analysis, internal standards-volatile compounds that are absent from MT-Daqu but have known concentrations-were added to the samples. The concentrations of volatile compounds were calculated based on the peak areas of these internal standards in the GC-MS spectrum, without correcting for differences among the volatile compounds. In this study, n-butyl acetate and 2-acetylpyridine were chosen as internal standards for the quantification process.
2.7.2. Accurate Quantitative Analysis
LLE-GC-MS/MS was employed for the quantitative analysis of 46 aroma-active volatile compounds in MT-Daqu. Mixed standard solutions of these compounds were prepared according to the groupings described in section 2.1. For Groups A and B, n-butyl acetate was used as the internal standard, while 2-acetylpyridine was used for Groups C and D. Each group of mixed standards was prepared at seven different concentration gradients.
Sampling was performed in shunt mode with a 10:1 ratio, and GC-MS analysis (7890 GC, 7100D Mass, Agilent Technologies, CA) was conducted under the same experimental conditions. Multiple reaction monitoring (MRM) parameters used in this study are detailed in Table S2. Quantification of the volatile compounds in MT-Daqu was achieved using the internal standard method, with concentrations calculated from a quantitative curve generated using the linear least-squares method.
2.8. Key aroma-Active Compounds Analysis
2.8.1. Odor Activity Value (OAV)
The OAV of volatile compounds in MT-Daqu was calculated to assess the concentration of aroma-active compounds relative to their respective odor thresholds in water, following a method described elsewhere (Yu, et al., 2024). The odor thresholds used in this study are based on values reported in a previous study (van Gemert, 2011). OAV values for aroma-active compounds in each sample group were computed according to the specified grouping requirements.
2.8.2. Aroma Extraction Dilution Analysis (AEDA)
Based on the OAV values, samples with the highest total OAV for aroma-active compounds within each group were selected for AEDA according to a method described in a previous study (Zhao, et al., 2018). The volatile compound extracts from MT-Daqu were initially diluted 5-fold and then further diluted in 2-fold steps for gas chromatography-olfactometry (GC-O) analysis under the previously described conditions. The maximum dilution factor represented the flavor dilution (FD) of each compound, with each dilution corresponding to its respective FD factor. Three replicates were performed for each sample, with a higher FD factor indicating a greater contribution of the compound to the overall aroma of the sample.
2.8.3. Aroma Recombination Experiments
Since wheat is the primary raw material used in MT-Daqu preparation, wheat flour was employed as a substrate for aroma activity recombination experiments (Yu, et al., 2023). Based on the quantitative analysis of volatile compounds in MT-Daqu samples, aroma-active compounds with OAVs greater than 1, excluding those contributing to the raw material aroma, were selected and added to the wheat flour model to simulate sample aroma. Sensory evaluation and E-nose data analysis were conducted to compare the aroma profiles before and after recombination. Differences in the content of key aroma-active volatile compounds in each sample group informed the addition of specific aroma compounds, with sensory and E-nose analyses performed to assess changes in aroma profiles post-recombination.
2.9. Statistical Analysis
The content of volatile compounds and aroma scores in MT-
Daqu samples were expressed as means ± standard deviations (SD). Differences among sample groups were assessed using Duncan’s ANOVA, conducted with SPSS v.14.0 (SPSS Inc., Chicago, IL, USA). Aroma score differences were visualized using GraphPad Prism 8 (GraphPad Software, Boston, USA). Principal component analysis (PCA), partial least squares discriminant analysis (PLS-DA), and visualizations such as complex heatmaps, radar maps, UpSet Venn diagrams, and Sankey diagrams were generated using RStudio software (
https://posit.co/download/rstudio-server/).
3. Results
3.1. Sensory Evaluation of MT-Daqu Samples
A sensory evaluation was conducted on 30 MT-
Daqu samples from 7 SAB-producing regions. The
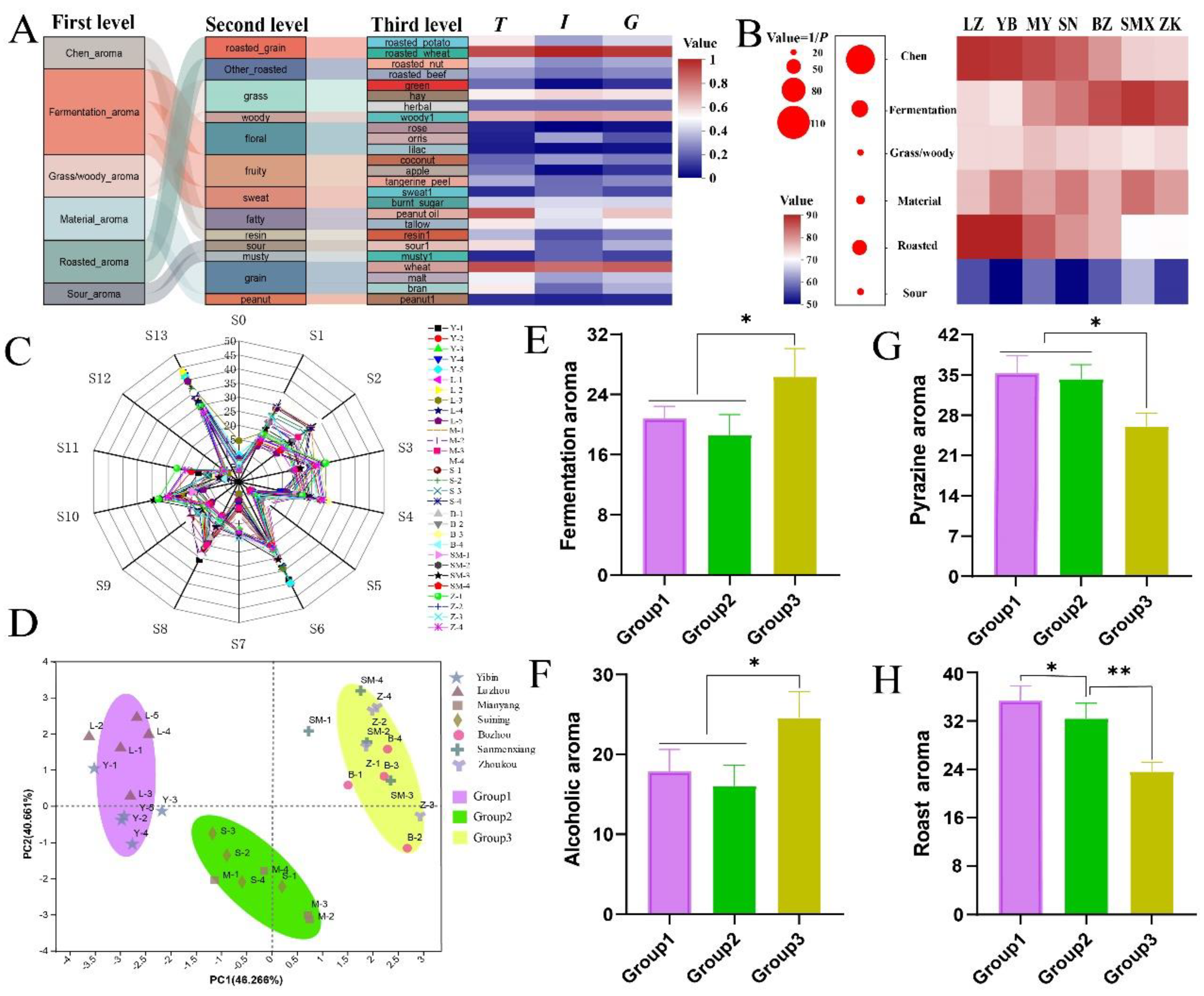
Qu-aroma was categorized into three hierarchical levels. The first level included seven primary aromas: Chen aroma, fermentation aroma, grass/woody aroma, material aroma, roasted aroma, and sour aroma. The second and third levels included 13 and 25 additional aromas, respectively. The relationships among these hierarchical levels of
Qu-aroma in MT-
Daqu samples are illustrated in
Figure 1A. The roasted wheat aroma was the most frequently detected and intense across all MT-
Daqu samples, followed by fatty and grain aromas. In contrast, rose and lilac aromas were observed with the lowest frequency and intensity. The sensory evaluation revealed that Chen aroma and roasted aroma were the predominant characteristics, with fermentation aroma serving as a secondary note.
A heatmap illustrating the sensory evaluation scores for MT-Daqu samples from various regions was created, with statistical differences assessed using Duncan’s ANOVA (Figure 1B). With the exception of the sour aroma, which received an average score of approximately 60, all other Qu-aroma categories had scores exceeding 70. Significant regional differences were observed in the Chen aroma (P < 0.01), as well as in the roasted aroma and fermentation aroma (P < 0.05). Specifically, MT-Daqu samples from Sichuan displayed significantly higher scores for Chen and roasted aromas compared to samples from other regions (P < 0.05). Conversely, Sichuan MT-Daqu samples had notably lower scores for fermentation aroma (P < 0.05).
E-nose analysis utilizing 14 sensors was performed to further elucidate the
Qu-aroma characteristics of MT-
Daqu samples. A radar map, constructed from the aroma scores provided by the sensors, highlighted the overall aroma profile across the 30 samples, revealing notable similarities (
Figure 1C). Sensor S13, which detects pyrazines and lactone compounds, recorded the highest scores, followed by Sensor S6, which detects fatty and oil compounds. Conversely, Sensors S0, S5, S11, and S12, which detect ammonia, hydrogen, hydrogen sulfide, and sulfide, respectively, exhibited low scores. Principal Component Analysis (PCA) of the sensor data (
Figure 1D) showed that the first principal component (PC1) and the second principal component (PC2) accounted for 46.27% and 40.67% of the total variance, respectively. The PCA score plots revealed three distinct clusters of samples: Group 1, comprising MT-
Daqu samples from Luzhou and Yibin; Group 2, consisting of samples from Mianyang and Suining; and Group 3, including samples from Bozhou, Sanmenxia, and Zhoukou. Statistical analysis indicated that Group 3 samples had significantly higher fermentation aroma (S1, S3, and S10 averaged) compared to the other groups (P < 0.05). Similarly, the alcohol aroma (S3) followed this pattern (
Figures 1E and F).
In contrast, Groups 1 and 2 exhibited significantly higher levels of pyrazine aroma (S13) and roasted aroma (S4) compared to Group 3 (P < 0.05). Group 1, in particular, demonstrated a significantly higher roasted aroma than Group 2 (P < 0.05) (Figures 1G and H). Overall, the combined sensory evaluation and E-nose analysis reveal that Sichuan MT-Daqu samples have lower fermentation aroma but higher roasted aroma compared to samples from other regions.
3.2. Identification and Semi-Quantitative Analysis of Volatile Compounds in MT-Daqu Samples Using HS-SPME-GC- MS/O
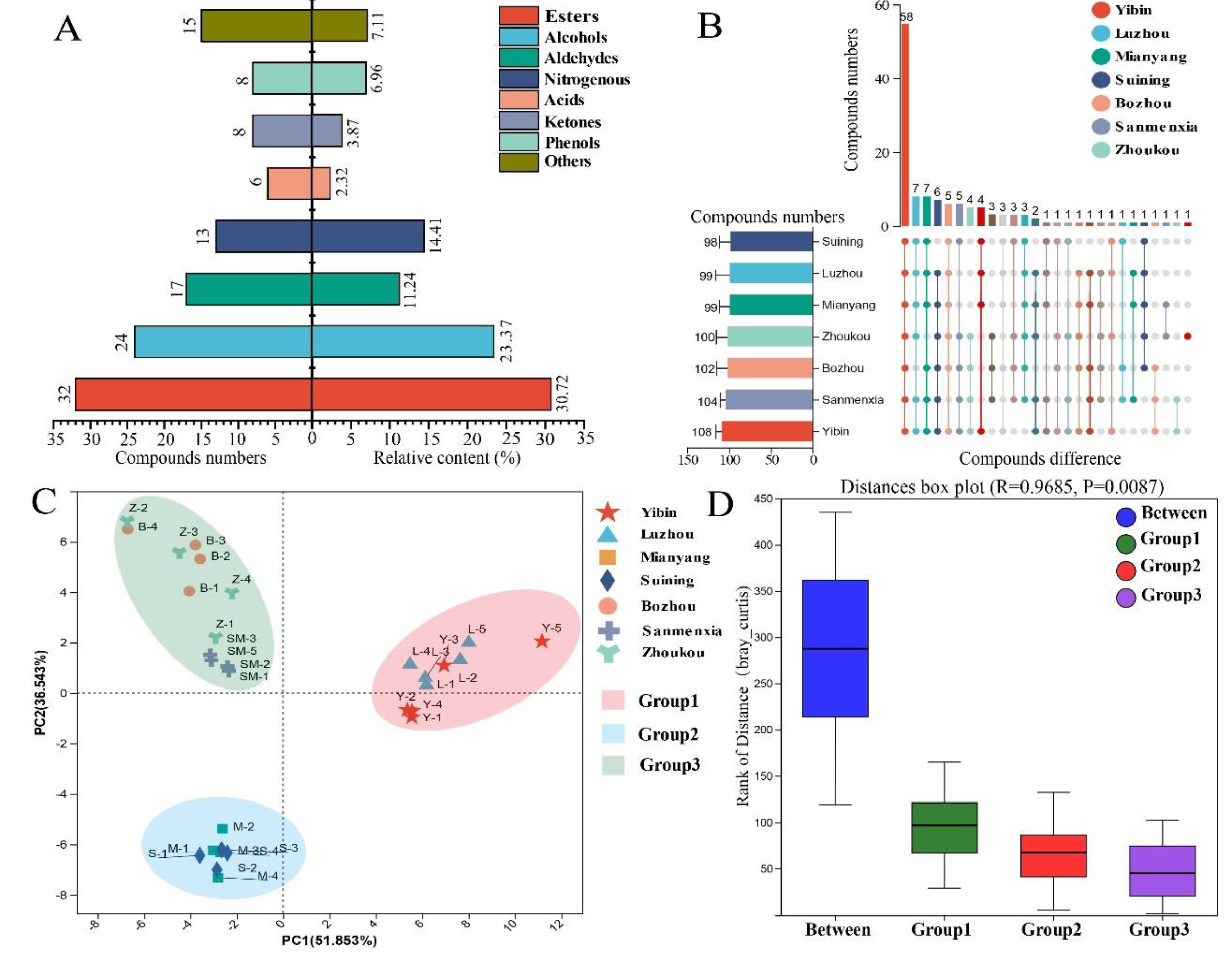
Subsequently, the volatile compounds in MT-
Daqu samples from seven SAB producing regions were analyzed using HS-SPME-GC-MS/O. A total of 123 volatile compounds were identified through a combined analysis of standards, odors, RI, and mass spectra results (
Table S2). The identified volatile compounds were categorized as follows: 32 esters (30.72%), 24 alcohols (23.37%), 17 aldehydes (11.24%), 13 nitrogenous compounds (14.41%), 6 acids (2.32%), 8 ketones (3.87%), 8 phenols (6.96%), and 15 other components (7.11%) (
Figure 2A).
An UpSet Venn diagram was constructed to visualize the similarities and differences in volatile compounds across different regions (Figure 2B). There were no significant differences in the total number of volatile compounds among regions (P > 0.05), with 58 volatile compounds being common to all MT-Daqu samples. Notably, limonene was detected exclusively in MT-Daqu samples from the Zhoukou region.
To further explore regional differences, Principal Component Analysis (PCA) based on semi-quantitative results of volatile compounds was performed. PC1 accounted for 51.85% and PC2 for 36.54% of the variance. PCA score plots indicated that MT-Daqu samples clustered into three distinct categories, which aligned with the grouping criteria observed in the E-nose analysis (Figure 2C).
To validate these groupings, ANOSIM analysis based on Bray-Curtis dissimilarity was conducted with 999 permutations (
Figure 2D). The results demonstrated significantly higher among-group differences compared to within-group differences (R = 0.9685, P < 0.01), confirming the validity of the grouping criteria. Furthermore, GC-O analysis identified 46 aroma-active compounds among the 123 volatile compounds in MT-
Daqu (
Table 1). Of these, 32 were common across all samples. Although qualitative and semi-quantitative analyses indicated only minor differences in the number of identified volatile compounds across MT-
Daqu samples from various regions, significant variations were observed in the content of specific volatile compounds, highlighting the need for further investigation into the absolute concentrations of
Qu-aroma-active compounds.
3.3. Quantitative Analysis of Volatile Aroma-Active Compounds in MT-Daqu
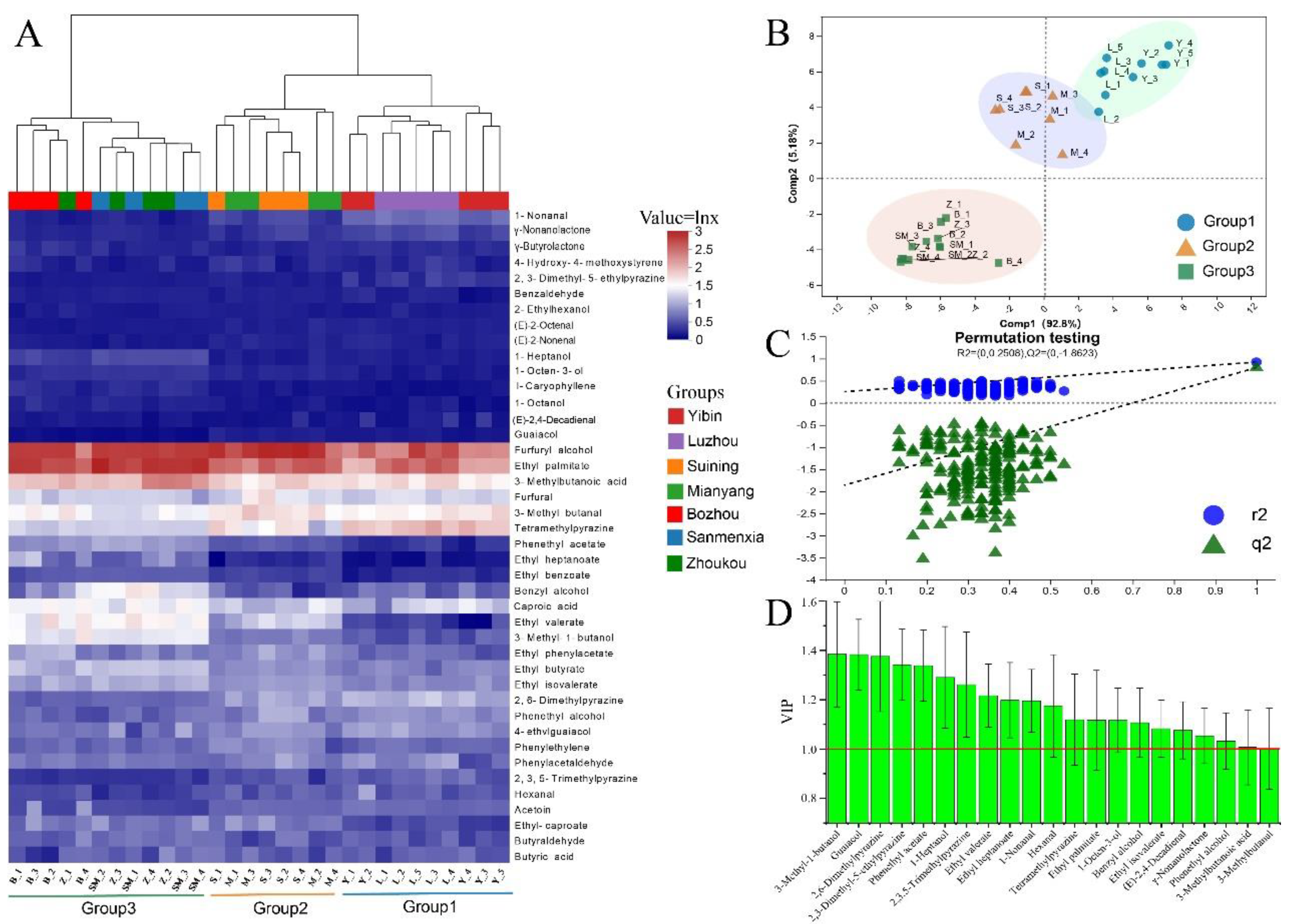
LLE-GC-MS/MS was used to accurately quantify 46 aroma-active compounds in MT-
Daqu samples. A cluster heatmap, based on the concentrations of these aroma-active compounds, is presented in
Figure 3A. Among the identified compounds, furfuryl alcohol exhibited the highest average concentration at 13.12 mg/kg, followed by ethyl palmitate (5.35 mg/kg), 3-methylbutanoic acid (3.73 mg/kg), tetramethylpyrazine (3.71 mg/kg), and 3-methylbutanal (4.35 mg/kg). Hierarchical clustering using Bray-Curtis dissimilarity revealed three distinct groups: Group 1, which included samples from Luzhou and Yibin; Group 2, which comprised samples from Suining and Mianyang; and Group 3, which consisted of samples from the remaining regions. This clustering pattern is consistent with previous analyses, and subsequent analyses focused on these three groups.
To further investigate the differences in aroma-active substances among the three groups, PLS-DA analysis was conducted. The results are shown in Figure 3B. The PLS-DA model demonstrated an excellent fit, with R2X, R2Y, and Q2 values of 0.709, 0.989, and 0.956, respectively. The first component (Comp 1) accounted for 92.8% of the variance in the samples, while Comp 2 accounted for 5.18%. The PLS-DA score plots clearly separated the three MT-Daqu groups, although Groups 1 and 2 were found to be relatively close to each other. Permutation tests (n=200) confirmed the quality of the model fit, with R2 = 0.2508 and Q2 = -1.8623. The negative values for Q2 indicate that the model fit the data well (Figure 3C).
Using Variable Importance in Projection (VIP) values greater than 1 to screen for key volatile compounds, 21 aroma-active compounds were identified as markers for discriminating the three sample groups (Figure 3D). These included 5 esters (i.e., phenethyl acetate, ethyl valerate, ethyl heptanoate, ethyl palmitate, and ethyl isovalerate), 5 alcohols (i.e., 3-methyl-1-butanol, 1-heptanol, 1-octen-3-ol, benzyl alcohol, and phenethyl alcohol), 5 aldehydes (i.e., 1-nonanal, γ-nonanolactone, 3-methylbutanal, hexanal, and (E)-2,4-decadienal), 4 nitrogenous components (i.e., 2,6-dimethylpyrazine, 2,3,5-trimethylpyrazine, tetramethylpyrazine, and 2,3-dimethyl-5-ethylpyrazine), and 2 other compounds (i.e., 3-methylbutanoic acid and guaiacol). The quantitative analysis highlighted significant differences in the levels of these aroma-active compounds between MT-Daqu samples from Sichuan and those from other regions, as well as among samples from various SAB-producing areas within Sichuan.
3.4. Analysis of Key Aroma-Active Compounds in MT-Daqu from Different Regions
Based on the results of sensory evaluation, E-nose analysis, and aroma-active compounds analyses, the 46 identified aroma-active compounds in MT-Daqu were classified into several aroma categories: fermentation aroma (14 compounds), Chen aroma (6 compounds), roasted aroma (5 compounds), sour aroma (3 compounds), material aroma (6 compounds), and grass/woody aroma (7 compounds).
MT-Daqu samples were categorized into three groups, as previously determined in section 3.3. The average content of aroma-active compounds within each group was calculated, and differences between groups were assessed using ANOVA.
Additionally, OAVs of aroma-active compounds were calculated based on their odor thresholds in water. One representative sample from each group was selected for AEDA to determine the FD factors for the 46 aroma-active compounds (Table 1). Significant differences in the content of 10 aroma-active compounds were observed among the three groups (P < 0.05), with 12 aroma-active compounds showing significant differences between Sichuan province samples and those from other regions (P < 0.05). Notably, except for furfuryl alcohol and furfural, these differences were found in aroma-active compounds with VIP > 1. The OAVs of 3-methylbutanal, which contributes to malt aroma, were highest in Group 3, reaching 3302.66. Among fermentation aromas, ethyl isovalerate had the highest OAVs, reaching 1509.32 in Group 3. Other compounds such as ethyl valerate, ethyl heptanoate, and ethyl caproate also showed higher OAVs in Group 3 compared to Groups 1 and 2.
In contrast, the OAVs of compounds such as 1-nonanal, (E)-2-nonenal, and (E)-2,4-decadienal, which contribute to Chen aroma, were higher in Group 1 and Group 2 compared to Group 3. Additionally, the OAVs of 2,3-dimethyl-5-ethylpyrazine, which contributes to roasted aroma, were highest in Group 1, followed by Group 2 and Group 3. The FD factors of the 46 aroma-active compounds for Y-2, S-3, and B-3 samples, as determined by the AEDA experiment, were similar to those of OAVs in MT-Daqu samples from different regions.
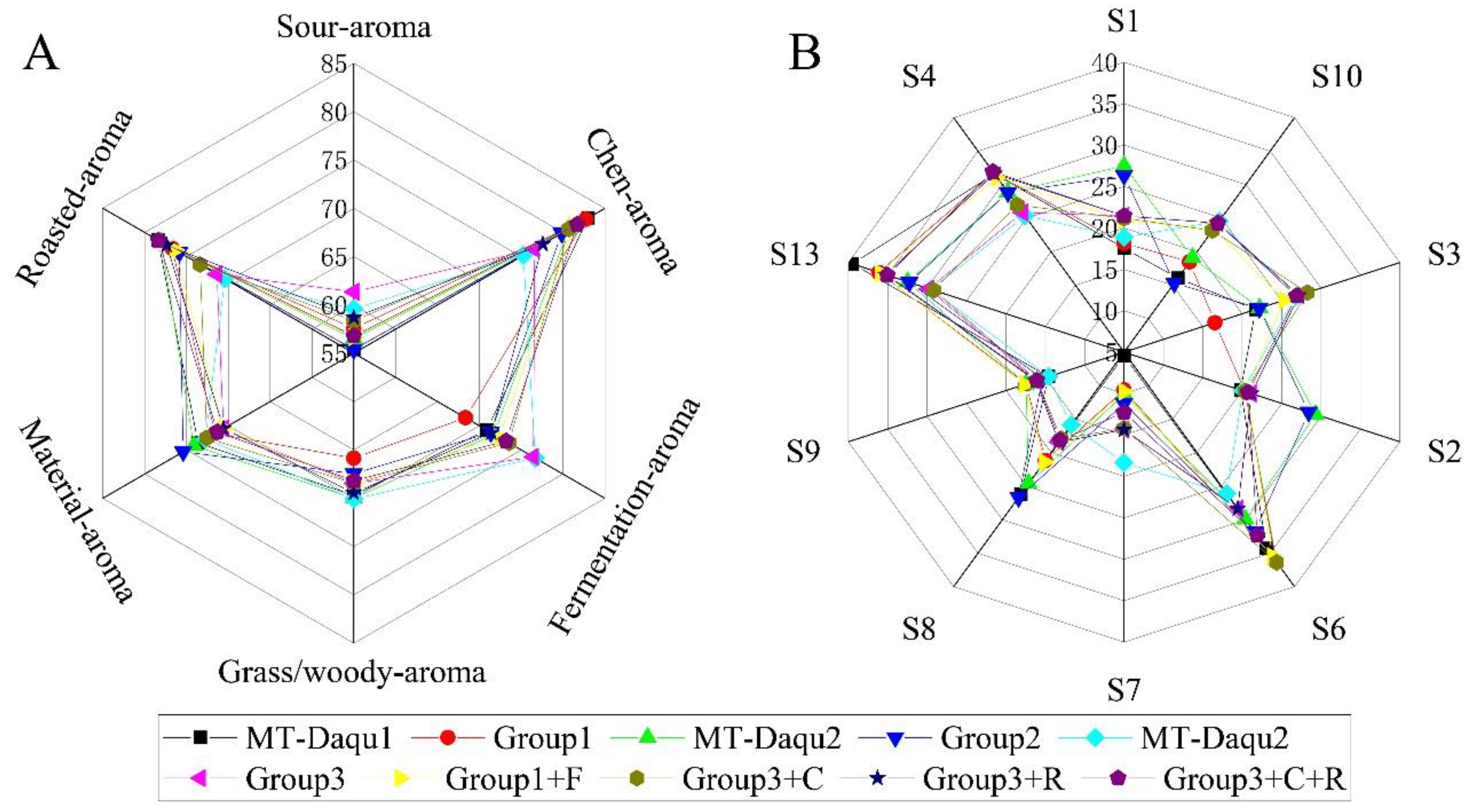
Based on the clustering pattern of samples and the content of aroma-active compounds, recombinant experiments (Group 1, Group 2, and Group 3) were recombinant experiments were performed using samples from Group 1, Group 2, and Group 3 to create combined models (MT-Daqu 1, MT-Daqu 2, and MT-Daqu 3). The average scores for these recombination models were analyzed through sensory evaluation (
Figure 4A) and E-nose analysis (
Figure 4B). The results revealed that the aroma profiles of the recombinant models closely matched those of the original samples, demonstrating that the key aroma-active compounds in MT-
Daqu samples were accurately characterized.
Based on the differences observed in the content of aroma-active compounds, OAVs, and FD factors in MT-Daqu samples, three groups of components were selected for recombination experiments: 9 fermentation-aroma compounds, 4 Chen-aroma compounds, and 4 roasted-aroma compounds (Table 1). The addition of these three groups of aroma-active compounds improved the scores for their respective aroma categories in the recombinant models (Figure 4A and B), confirming that these compounds are key contributors to the aroma profile of MT-Daqu. Notably, Chen aroma and roasted aroma were more pronounced in Group 1 samples compared to Group 3, whereas fermentation aroma compounds were more prominent in Group 3.
Further experiments involved adding partial fermentation aroma compounds to Group 1 (Group 1+F), adding partial Chen aroma to Group 3 (Group 3+C), and adding partial roasted aroma to Group 3 (Group 3+R). The specific added contents are detailed in
Table 2. The aroma components and their contents were significantly different in Group 1 and Group 3. Aroma evaluation after recombination revealed that the fermentation aroma score for the Group 1+F model was lower than that of Group 3. Additionally, the fermentation aroma scores for Group 3+C, Group 3+R, and Group 3+C+R decreased, suggesting that Chen aroma and roasted aroma compounds may mask the fermentation aroma in MT-
Daqu.
4. Discussion
The importance of Daqu in Baijiu brewing is well-established, with its role as a saccharifying agent and fermentation starter being critical to the production process (W. H. Liu, et al., 2023; Yang, et al., 2024). Recently, leading Baijiu producers have increasingly focused on the impact of Qu-aroma on the final aroma profile of Baijiu. The Chinese standard GB/T 10781.4-2024 has formally recognized Qu-aroma as a key metric for evaluating Baijiu aroma, attributing Qu-aroma to the fermentation process involving Daqu (Chen, et al., 2024).
In the present study, 30 MT-Daqu samples from 7 SAB-producing regions in China were analyzed, identifying 7 primary aromas contributing to the Qu-aroma of MT-Daqu: Chen aroma, fermentation aroma, grass/woody aroma, material aroma, roasted aroma, and sour aroma. These primary aromas were further subdivided into 25 specific sub-aromas. While these findings are consistent with existing literature, there are some differences in classification compared to previous studies (Yang, et al., 2024). Notably, other aromas such as unpleasant, rancid/cheesy, and fermented odors have been reported in past research. However, the sensory evaluators in this study found these aromas were not consistently present across all MT-Daqu samples and therefore did not consider them characteristic of Qu-aroma, despite their occasional detection.
In this study, a total of 123 volatile compounds were identified in MT-Daqu samples from various regions, with 46 of these compounds found to be aroma-active. This finding contrasts with previous reports that identified 111 and 90 volatile compounds in MT-Daqu (Wang, et al., 2022; Yang, et al., 2024). The higher number of identified compounds in the present study can be attributed to more extensive sample coverage and representativeness. Moreover, the use of improved sample pretreatment methods and advanced detection equipment likely contributed to a more thorough analysis of the Qu-aroma compounds in MT-Daqu samples.
From an aroma-active perspective, the fermentation aroma category was the most diverse, encompassing 14 compounds primarily including alcohols and esters. However, sensory evaluation scores for fermentation aroma were lower compared to those for Chen aroma and roasted aroma categories. This discrepancy can be attributed to two main factors: (i) several fermentation aroma-active compounds, such as 1-octanol, γ-nonanolactone, and benzyl alcohol, have low OAVs and FD factors; and (ii) the potential masking effect of Chen aroma and roasted aroma on fermentation aroma. This masking effect has been documented in previous studies, which explored the factors influencing aroma release of ester alcohols in Baijiu (Niu, Yao, Xiao, Zhu, Zhu, & Chen, 2018; Qin, et al., 2024).
Chen aroma is a significant component of MT-Daqu, primarily composed of fatty and oil aldehydes. Key aroma-active compounds associated with Chen aroma include hexanal, 1-nonanal, (E)-2-nonenal, (E)-2,4-decadienal, and butyraldehyde, which aligns with previous research on Chen aroma compounds in MT-Daqu (Yang, et al., 2024). Roasted aroma comprises five aroma-active compounds, including three pyrazines, 4-hydroxy-4-methoxystyrene, and 3-methyl-1-butanol. Pyrazines are crucial to roasted aroma (Deng, Zheng, Huang, Huang, Ye, & Luo, 2023; Yang, et al., 2024), formed during the high-temperature fermentation (55-60 °C) of wheat flour over extended periods (8-15 days), which facilitate Maillard reactions. Moreover, microorganisms such as Bacillus contribute to the synthesis of pyrazines (Y. Liu, et al., 2023).
The sour aroma, primarily attributed to 3-methylbutanoic acid, is carefully controlled in MT-Daqu production. High-quality MT-Daqu is expected to have minimal sour aroma, which is reflected in the low sour taste scores in sensory evaluations. Material aroma, which includes compounds such as 3-methylbutanal, γ-butyrolactone, and furfuryl alcohol, likely originates from the wheat or auxiliary materials used in MT-Daqu preparation (Yang, et al., 2024). Grass/woody aroma, characterized by compounds like 1-heptanol, 2-ethylhexanol, phenylacetaldehyde, 2,3,5-trimethylpyrazine (grass aroma), I-caryophyllene, and guaiacol (wood aroma), has lower OAVs and FD factors, resulting in lower sensory evaluation scores. These aromas may also originate from the auxiliary tools and instruments used during MT-Daqu production.
This study provides a detailed analysis of the Qu-aroma of MT-Daqu by examining the composition and characteristics of aroma-active compounds. The findings offer valuable insights into the distinct features of the Qu-aroma and lay the groundwork for further research on how it impacts the overall aroma of SAB. The study also assessed regional differences in Qu-aroma characteristics and aroma-active compounds, highlighting significant variations across different production areas. For instance, MT-Daqu samples from Sichuan were found to have higher scores for Chen aroma and roasted aroma compared to samples from other regions, whereas the fermentation aroma showed the opposite trend. Additionally, six major aroma-active compounds-ethyl isovalerate, ethyl heptanoate, hexanal, 1-nonanal, (E)-2,4-decadienal, and 2,3-dimethyl-5-ethylpyrazine-were consistently identified across all MT-Daqu samples. Despite significant regional differences in the content of certain aroma compounds (P < 0.05), their overall contribution to the aroma was not markedly different. For example, compounds like 3-methyl-1-butanol, ethyl palmitate, and tetramethylpyrazine, while varying in content, did not significantly alter the overall aroma profile, potentially due to their high aroma thresholds (Yu, et al., 2024).
The observed differences in aroma-active compounds among MT-Daqu samples from various regions effectively account for the variations in Qu-aroma characteristics. These regional differences in Qu-aroma result from a combination of factors, including environmental conditions and production parameters. While some of the aroma in MT-Daqu originates directly from raw materials and tools-such as material aroma and grass/woody aroma-the majority of aroma compounds undergo transformations through chemical reactions and microbial interactions. Previous research has demonstrated that the microbial community structure of MT-Daqu varies by region, with environmental conditions being a key driver of microbial evolution (S. Ma, et al., 2022; Shiyuan Ma, et al., 2022). These microbial differences largely contribute to the variations in volatile compounds found in MT-Daqu. For example, yeast has been shown to significantly influence the production of alcohols and esters (Li, Xu, Sam, Li, Hu, & Tao, 2024), while Bacillus is crucial for synthesizing nitrogen compounds (Y. Liu, et al., 2023). Additionally, environmental conditions impact the chemical reactions in MT-Daqu. Changes in temperature and humidity, for instance, can affect Maillard reaction and fatty acid oxidation processes, thereby influencing the formation of Chen aroma and roasted aroma compounds in MT-Daqu.
To better understand the regional differences in the Qu-aroma of MT-Daqu from various producing areas, future research should focus on the production conditions and mechanisms influencing Qu-aroma development. Investigating these factors will provide valuable insights for optimizing production processes with a flavor-oriented approach, ultimately enhancing the quality of MT-Daqu.
5. Conclusions
In this study, the aroma composition and key aroma-active compounds of MT-Daqu from seven different production areas were thoroughly analyzed, revealing significant regional variations in the aroma profiles of MT-Daqu. The Qu-aroma of MT-Daqu samples was characterized using seven broad categories and twenty-five specific aroma descriptors. A total of 123 volatile compounds were identified, 46 of which were recognized as aroma-active. Six major aroma-active compounds were found to significantly influence the aroma differences among MT-Daqu samples. This study underscores the regional variations in the Qu-aroma of MT-Daqu and offers insights that could guide flavor-oriented optimization of the production process to enhance MT-Daqu’s quality.
CRediT authorship contribution statement
Jie Deng: Data curation, Visualization, Writing-original draft, Writing-review & editing. Jia Zheng:Resources, Writing-review & editing,. Dan Huang: Project administration, Supervision, Writing-review & editing. Guangbin Ye: Methodology, Writing-review & editing. Huibo Luo: Conceptualization, Supervision, Writing-review & editing, Resources.
Acknowledgments
This study was financially supported by the Sichuan Province Science and Technology Program (grant 2022YFS0547), and Wuliangye Industry-University-Research Cooperation Project (grant CXY2022R006).
Conflict of interest declarations
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Chen, L., Zhao, Y., Chen, X., Zhang, Y., Li, H., Zhao, D., Wang, B., Ye, X., Sun, B., & Sun, J. (2024). Peanut Pairing Baijiu: To Enhance Retronasal Aroma Intensity while Reducing Baijiu Aftertaste. J Agric Food Chem, 72(26), 14851-14864.
- Deng, J., Zheng, J., Huang, D., Huang, Z., Ye, G., & Luo, H. (2023). Characterization of physicochemical properties, volatile compounds and microbial community structure in four types of Daqu. Lwt, 184, 115064. [CrossRef]
- Duan, J., Cheng, W., Lv, S., Deng, W., Hu, X., Li, H., Sun, J., Zheng, F., & Sun, B. (2024). Characterization of key aroma compounds in soy sauce flavor baijiu by molecular sensory science combined with aroma active compounds reverse verification method. Food Chem, 443, 138487. [CrossRef]
- He, M., Jin, Y., Liu, M., Yang, G., Zhou, R., Zhao, J., & Wu, C. (2023). Metaproteomic investigation of enzyme profile in daqu used for the production of Nongxiangxing baijiu. Int J Food Microbiol, 400, 110250. [CrossRef]
- Li, Y., Xu, L., Sam, F. E., Li, A., Hu, K., & Tao, Y. (2024). Improving aromatic higher alcohol acetates in wines by co-fermentation of Pichia kluyveri and Saccharomyces cerevisiae: growth interaction and amino acid competition. J Sci Food Agric, 104(11), 6875-6883. [CrossRef]
- Liu, H., & Sun, B. (2018). Effect of Fermentation Processing on the Flavor of Baijiu. J Agric Food Chem, 66(22), 5425-5432. [CrossRef]
- Liu, J., Chen, J., Fan, Y., Huang, X., & Han, B. (2018). Biochemical characterisation and dominance of different hydrolases in different types of Daqu - a Chinese industrial fermentation starter. J Sci Food Agric, 98(1), 113-121. [CrossRef]
- Liu, W. H., Chai, L. J., Wang, H. M., Lu, Z. M., Zhang, X. J., Xiao, C., Wang, S. T., Shen, C. H., Shi, J. S., & Xu, Z. H. (2023). Bacteria and filamentous fungi running a relay race in Daqu fermentation enable macromolecular degradation and flavor substance formation. Int J Food Microbiol, 390, 110118. [CrossRef]
- Liu, Y., Li, M., Hong, X., Li, H., Huang, R., Han, S., Hou, J., & Pan, C. (2023). Screening and identification of high yield tetramethylpyrazine strains in Nongxiangxing liquor Daqu and study on the mechanism of tetramethylpyrazine production. J Sci Food Agric, 103(14), 6849-6860. [CrossRef]
- Liu, Y., Tang, T.-X., Pei, X.-Q., Zhang, C., Wu, Z.-L. (2014). Identification of ketone reductase ChKRED20 from the genome of Chryseobacterium sp. CA49 for highly efficient anti-Prelog reduction of 3,5-bis(trifluoromethyl)acetophenone. J. Mol. Catal. B: Enzym., 102, 1-8. [CrossRef]
- Ma, S., Luo, H., Zhao, D., Qiao, Z., Zheng, J., An, M., & Huang, D. (2022). Environmental factors and interactions among microorganisms drive microbial community succession during fermentation of Nongxiangxing daqu. Bioresour Technol, 345, 126549. [CrossRef]
- Ma, S., Shang, Z., Chen, J., Shen, Y., Li, Z., Huang, D., & Luo, H. (2022). Differences in structure, volatile metabolites, and functions of microbial communities in Nongxiangxing daqu from different production areas. Lwt, 166, 113784. [CrossRef]
- Niu, Y., Yao, Z., Xiao, Z., Zhu, G., Zhu, J., & Chen, J. (2018). Sensory evaluation of the synergism among ester odorants in light aroma-type liquor by odor threshold, aroma intensity and flash GC electronic nose. Food Res Int, 113, 102-114. [CrossRef]
- Qin, D., Lv, S., Shen, Y., Shi, J., Jiang, Y., Cheng, W., Wang, D., Li, H., Zhang, Y., Cheng, H., Ye, X., & Sun, B. (2024). Decoding the key compounds responsible for the empty cup aroma of soy sauce aroma type baijiu. Food Chem, 434, 137466. [CrossRef]
- Ren, Z., Chen, Q., Tang, T., Huang, Z. (2024). Unraveling the water source and formation process of Huangshui in solid-state fermentation. Food Sci. Biotechnol, 1-11. [CrossRef]
- Ren, Z., Liu, L., Tang, T., Huang, K., & Huang, Z. (2024). Effectively Increase the L(+)-Isomer Proportion of Ethyl Lactate in Baijiu by Isolating and Applying L(+)-Lactic Acid-Producing Bacteria. Preprints. Preprints, . [CrossRef]
- Ren, Z., Xie, J., Tang, T., Huang, Z. (2024). Short-chain carboxylates facilitate the counting of yeasts in Sub-high temperature Daqu. Polish J. Microbiol, . [CrossRef]
- Tang, T.-X., Liu, Y., Wu, Z.-L. (2014). Characterization of a robust anti-Prelog short-chain dehydrogenase/reductase ChKRED20 from Chryseobacterium sp. CA49. J. Mol. Catal. B: Enzym., 105, 82-88. [CrossRef]
- van Gemert, L. J. (2011). Compilations of odour threshold values in air, water and other media (Second Enlarged and Revised Edittion): Oliemans Punter & Partners BV.
- Wang, Z., Wang, S., Liao, P., Chen, L., Sun, J., Sun, B., Zhao, D., Wang, B., & Li, H. (2022). HS-SPME Combined with GC-MS/O to Analyze the Flavor of Strong Aroma Baijiu Daqu. Foods, 11(1), 116. [CrossRef]
- Xiao, C., Lu, Z. M., Zhang, X. J., Wang, S. T., Ao, L., Shen, C. H., Shi, J. S., & Xu, Z. H. (2017). Bio-Heat Is a Key Environmental Driver Shaping the Microbial Community of Medium-Temperature Daqu. Appl Environ Microbiol, 83(23). [CrossRef]
- Yang, S.-B., Fu, J.-J., He, J.-H., Zhang, X.-J., Chai, L.-J., Shi, J.-S., Wang, S.-T., Zhang, S.-Y., Shen, C.-H., Lu, Z.-M., & Xu, Z.-H. (2024). Decoding the Qu-aroma of medium-temperature Daqu starter by volatilomics, aroma recombination, omission studies and sensory analysis. Food Chem, 457, 140186. [CrossRef]
- Yu, M., Li, T., Wan, S., Song, H., Zhang, Y., Raza, A., Wang, C., Wang, H., & Wang, H. (2023). Sensory-directed establishment of sensory wheel and characterization of key aroma-active compounds for spicy tallow hot pot seasoning. Food Chem, 405, 134904. [CrossRef]
- Yu, M., Zhuang, L., Xie, Q., Li, T., Song, H., Wang, L., Li, K., Jiang, S., Zhang, Y., & Zheng, C. (2024). Influences of human milk proteins on the release of human milk odors: Non-covalent interactions between α-lactalbumin and key odor skeleton compounds. Food Hydrocolloids, 155, 110235eleton compounds. [CrossRef]
- Zhao, D., Shi, D., Sun, J., Li, A., Sun, B., Zhao, M., Chen, F., Sun, X., Li, H., Huang, M., & Zheng, F. (2018). Characterization of key aroma compounds in Gujinggong Chinese Baijiu by gas chromatography-olfactometry, quantitative measurements, and sensory evaluation. Food Res Int, 105, 616-627.
- Zhou, L., Tang, T., Deng, D., Wang, Y., & Pei, D. (2024). Isolation and Electrochemical Analysis of a Facultative Anaerobic Electrogenic Strain sp. SQ-1. Polish Journal of Microbiology, , 1-11. [CrossRef]
- Zhu, M., Zheng, J., Xie, J., Zhao, D., Qiao, Z. W., Huang, D., & Luo, H. B. (2022). Effects of environmental factors on the microbial community changes during medium-high temperature Daqu manufacturing. Food Res Int, 153, 110955. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).